Abstract
Background
The consensus documents published to date on hereditary angioedema with C1 inhibitor deficiency (C1‐INH‐HAE) have focused on adult patients. Many of the previous recommendations have not been adapted to pediatric patients. We intended to produce consensus recommendations for the diagnosis and management of pediatric patients with C1‐INH‐HAE.
Methods
During an expert panel meeting that took place during the 9th C1 Inhibitor Deficiency Workshop in Budapest, 2015 (www.haenet.hu), pediatric data were presented and discussed and a consensus was developed by voting.
Results
The symptoms of C1‐INH‐HAE often present in childhood. Differential diagnosis can be difficult as abdominal pain is common in pediatric C1‐INH‐HAE, but also commonly occurs in the general pediatric population. The early onset of symptoms may predict a more severe subsequent course of the disease. Before the age of 1 year, C1‐INH levels may be lower than in adults; therefore, it is advisable to confirm the diagnosis after the age of one year. All neonates/infants with an affected C1‐INH‐HAE family member should be screened for C1‐INH deficiency. Pediatric patients should always carry a C1‐INH‐HAE information card and medicine for emergency use. The regulatory approval status of the drugs for prophylaxis and for acute treatment is different in each country. Plasma‐derived C1‐INH, recombinant C1‐INH, and ecallantide are the only agents licensed for the acute treatment of pediatric patients. Clinical trials are underway with additional drugs. It is recommended to follow up patients in an HAE comprehensive care center.
Conclusions
The pediatric‐focused international consensus for the diagnosis and management of C1‐INH‐HAE patients was created.
Keywords: C1 inhibitor deficiency, diagnosis, hereditary angioedema, management, pediatric
Abbreviations
- AAs
attenuated androgens
- ACEIs
angiotensin‐converting enzyme inhibitors
- C1‐INH
C1 inhibitor
- C1‐INH‐HAE
hereditary angioedema with C1 inhibitor deficiency
- C1q
subunit of the first complement component
- C3
third complement component
- C4
fourth complement component
- CH50
total hemolytic complement
- EMA
European Medicines Agency
- Factor XII
coagulation factor XII
- FDA
Food and Drug Administration
- FFP
fresh frozen plasma
- HAE
hereditary angioedema
- HAWK
Hereditary Angioedema International Working Group
- HRQoL
health‐related quality of life
- LTP
long‐term prophylaxis
- pdC1‐INHBe
Berinert®
- pdC1‐INHCi
Cinryze®
- pdC1‐INH
human plasma‐derived C1 inhibitor
- QoL
quality of life
- RCTs
randomized controlled trials
- rhC1‐INH
recombinant human C1 inhibitor
- SDP
solvent detergent plasma
- STP
short‐term prophylaxis
- TA
tranexamic acid
- UAE
upper airway edema
Hereditary angioedema with C1 inhibitor deficiency (C1‐INH‐HAE) is a rare autosomal dominant disorder due to either deficiency (type I, 85% of cases) or dysfunction (type II, 15% of cases) of the serine protease inhibitor (serpin) C1 inhibitor (C1‐INH). A less common form of hereditary angioedema has a positive family history, but normal C1‐INH protein quantity and function: In some cases, the disease appears to be related to factor F12 gene defects (FXII‐HAE), while in most cases, the cause of this form of angioedema remains unknown (U‐HAE). This consensus addresses only C1‐INH‐HAE in the pediatric ages of birth until 18th birthday. Angioedema is due to the leakage of plasma from postcapillary venules mediated by the unregulated generation of bradykinin 1. C1‐INH‐HAE is characterized by recurrent attacks of nonpruritic, nonpitting subcutaneous, and/or submucosal angioedema that can affect any part of the body. Publications on clinical manifestations combining pediatric and adult patients show that skin involvement is the most frequent location of the edema (91% of patients) followed in frequency by abdominal attacks (73%) and upper airway edema (48%) 2. Viewing per‐episode, nearly all episodes consisted of skin swellings and abdominal attacks (96.5%). Per‐episode, laryngeal events are rare (0.9%), but potentially life threatening 3. One‐third of patients may develop an erythematous, nonpruritic rash, erythema marginatum, which might precede or accompany angioedema, although it can also occur independently 4. Sudden swellings of the gastrointestinal mucosa are common and often associated with severe debilitating abdominal pains. In one‐quarter of patients, severe abdominal pain may be the initial symptom. Acute abdominal pain mimics acute abdomen and may lead to unnecessary abdominal surgery. Edema involving the submucosa of the upper airways may cause airway obstruction and without treatment may lead to suffocation and death. The reported age of onset of attacks varies from 4.4 to 18 years with mean age of first attack at the age of ten 3, 5, 6, 7, 8, 9, 10, 11, 12, 13. Early onset of symptoms may predict a more severe course of disease 3, 14, 15. HAE attacks usually become more severe at puberty particularly in females and swellings may occur for the first time with the introduction of estrogen‐containing medications 16, 17. The diagnosis of C1‐INH‐HAE is often delayed for years because of the rarity of the disease and of the fact that its symptoms overlap with those of other forms of angioedema. The time between the onset of symptoms and diagnosis averages 8.5 years 18. The diagnosis of C1‐INH‐HAE type II may be limited by the availability of testing for functional C1‐INH level. In patients without a positive family history or with C1‐INH‐HAE type II, delay in diagnosis is usually longer 6, 9, 11, 12, 18, 19, 20. The utility of antifibrinolytics and androgens in C1‐INH‐HAE prophylaxis and plasma‐derived C1‐INH (pdC1‐INH) in replacement therapy have long been recognized. In recent years, other novel therapies have become available with efficacy proven by double‐blind studies mostly conducted in adults. International consensus publications on HAE have mostly been relevant to adult C1‐INH‐HAE 21, 22, 23. Pediatric‐focused international consensus for the diagnosis and management of C1‐INH‐HAE patients has not been previously published. This report presents international consensus for the diagnosis and management of C1‐INH‐HAE in the pediatric age group.
Methods
Bibliographic search
Data sources
A PubMed search (last updated December 31, 2015) was performed using the following key words: hereditary angioedema, C1 inhibitor, C1 inhibitor deficiency, pediatrics, adolescence, children, diagnosis, treatment, consensus, guidelines; additional titles from the reference lists of published articles in English language; additional data from abstracts known to the authors.
Discussion
An expert panel meeting and Round Table discussion took place during the 9th C1 Inhibitor Deficiency Workshop in Budapest on May 30, 2015 (www.haenet.hu). Data were presented followed by discussion and consensus was determined by voting.
Evidence level
The levels of evidence to support the views expressed in this document will be indicated in accordance with the U.S. Preventive Services Task Force Guidelines for ranking evidence on the effectiveness of treatments or screening, U.S. Preventive Services Task Force, August 1989 (Guide to clinical preventive services: Report of the U.S. Preventive Task Force. DIANE Publishing. p. 24. ISBN 9781568062976) (Table 1).
Table 1.
Levels of evidence (U.S. Preventive Services Task Force for ranking evidence about the effectiveness of treatments or screening)
| Levels | Description |
|---|---|
| I | Evidence obtained from at least one properly designed randomized controlled trial. |
| II‐1 | Evidence obtained from well‐designed controlled trials without randomization. |
| II‐2 | Evidence obtained from well‐designed cohort or case–control analytic studies, preferably from more than one center or research group. |
| II‐3 | Evidence obtained from multiple time series with or without the intervention. |
| Dramatic results in uncontrolled trials might also be regarded as this type of evidence. | |
| III | Opinions of respected authorities, based on clinical experience |
| Descriptive studies | |
| Reports of expert committees |
Results
Clinical symptoms
Similar to adults, clinical events in pediatric patients with C1‐INH‐HAE are characterized by recurrent subcutaneous and/or submucosal edematous episodes without wheals or pruritus, and if untreated, the edema may persist for 1 to 5 days before resolving spontaneously 24.
Onset of symptoms
In C1‐INH‐HAE, attacks may occur at any age after birth, but in utero angioedema symptoms have not been reported. The presence of a fetus with C1‐INH‐HAE may affect the number of maternal attacks 25, 26. The nature of C1‐INH transport across the placental barrier is unclear, but likely requires active transport. Although C1‐INH deficiency is present at birth, clinical symptoms are rare during infancy. Newborns may experience erythema marginatum as a prodromal symptom, but rarely swelling 4, 27. The reported age of onset of attacks varies from 4.4 to 18 years with mean age of first attack at the age of ten 3, 5, 6, 7, 8, 9, 10, 11, 12, 13. Colic may be an unrecognized symptom of C1‐INH‐HAE in infancy 28, 29, 30. Early onset of symptoms may predict a more severe subsequent course of disease 3, 14, 15.
Frequency and severity of symptoms
The frequency and severity of the symptoms exhibit a substantial inter‐ and intraindividual variation. Symptoms often worsen during puberty, particularly in females 3, 14, 31, 32. Onset of symptoms may occur with the introduction of estrogen‐containing medications for acne or birth control 17. The role of puberty in boys is less obvious.
Trigger factors
A multitude of factors may trigger edematous episodes in C1‐INH‐HAE at any age 33. In children, most attacks occur without a clear trigger. However, the most common attack triggers include mechanical trauma, mental stress, and airway infections 14, 34. Although dental eruption is not a frequent trigger for angioedema attacks, it could act as a provoking factor in some children 34. In adolescent girls, menstruation and ovulation are additional triggers 31. Certain medicines (such as estrogen‐containing oral contraceptives, angiotensin‐converting enzyme inhibitors, ACEIs) can trigger attacks 35, 36.
Location of symptoms
Subcutaneous edema
Subcutaneous edema of the extremities is often the earliest and most common swelling site in pediatric patients 3, 5, 14, 37, 38. Subcutaneous swelling is a common cause of school absenteeism and may affect a child's progress in school and participation in sports and other daily activities 14, 34.
Submucosal edema
Bowel
Bowel wall edema and related symptoms of colicky abdominal pain, nausea, vomiting, and postattack watery diarrhea are common (80–90%) in the pediatric patient population 3, 5, 14, 37. As abdominal pain is frequent in the general pediatric population, the wide differential diagnosis must always be considered including acute appendicitis, mesenteric lymphadenitis, intussusception, partial malrotation with intestinal torsion, Meckel's diverticulum, polycystic ovaries, ovarian or testicular torsion, intestinal hemorrhage or infarction, recurrent peritonitis of familial Mediterranean fever, and other abdominal diseases. Afflicted patients are often admitted to a surgical department for observation and at times subjected to an unnecessary operation. Abdominal ultrasound or CT scan may be performed to help exclude acute surgical abdominal disease 29, 30, 39, 40, 41, 42, 43, 44. Abdominal ultrasound may be a sensitive, rapid, and noninvasive differential diagnostic modality in patients with known C1‐INH‐HAE to help differentiate acute appendicitis and monitor response to event intervention with C1‐INH‐HAE therapeutic agents 43, 45, 46. Clinical and ultrasound response to specific C1‐INH‐HAE therapeutic medications helps differentiate C1‐INH‐HAE from non‐C1‐INH‐HAE‐related abdominal events. Standard biochemical and hematological blood tests are often not helpful in abdominal attacks to discriminate C1‐INH‐HAE from non‐C1‐INH‐HAE events. Neutrophilia may occur secondary to an HAE attack 47, 48, 49. Low C4 and low C1‐INH functional levels during an abdominal attack might be retrospectively helpful in confirming that abdominal symptoms are related to C1‐INH‐HAE. Commonly, the results of these tests are not available in time to be of help during the acute event.
Upper airway edema (UAE)
It usually first occurs between 11 and 45 years of age, with the mean age of 26. The earliest laryngeal edema recorded has been 3 years of age 14, 50. Although UAE is usually not the first presenting symptom of C1‐INH‐HAE, it may be the first presenting event and this first event may be fatal 50, 51. Death from asphyxiation may occur at any age with mean age at asphyxiation of 40.6 years (range: 9–78 years). Death by asphyxiation is less common in pediatric C1‐INH‐HAE patients 50, 51, 52. Inspection of the larynx is more difficult in young patients and it takes less swelling to asphyxiate in small children because of the smaller upper airway diameter 53, 54, 55. The differential diagnosis in pediatrics includes allergic food reactions, croup, pseudocroup, foreign body aspiration, and acute epiglottitis. For this reason, airway protection is the main task for the emergency department even when specific therapy for C1‐INH‐HAE is not available or it is not promptly administered 56, 57.
Other locations
Edema can occur at any site including the urinary bladder, urethra, genitalia, kidneys, muscles, joints, pericardial or pleural spaces and can be associated with neurological symptoms associated with headache, transient visual disturbances, and migraine‐like symptoms in pediatrics 3, 14, 58.
Prodromal symptoms
Of pediatric patients with C1‐INH‐HAE, 42% to 58% experience prodromal symptoms including erythema marginatum (a map‐like rash on the skin; reported from newborn onward) 4, 6, 14. Skin lesions with a similar appearance may develop in viral and bacterial infections and autoinflammatory diseases including rheumatoid diseases and periodic fever syndromes. The rash may be misdiagnosed as urticaria and C1‐INH‐HAE patients with erythema marginatum have a longer diagnostic delay 27, 59, 60, 61.
Concomitant disease
A higher incidence of concomitant celiac disease has been observed in C1‐INH‐HAE pediatric patients. In celiac pediatric HAE patients, celiac dietary restriction may reduce abdominal symptoms 62.
Diagnosis
Prenatal
Prenatal diagnosis may be considered when a disease‐causing mutation has been detected in a C1‐INH‐HAE family. If the family would consider pregnancy termination with the diagnosis of an affected fetus and varying with local ethical restrictions, then prenatal diagnosis of C1‐INH‐HAE may be achieved by chorionic villous sampling or amniocentesis 63. C1‐INH‐HAE has a highly variable disease severity within and between families with poor correlation between gene defect and clinical severity. Advances in therapy have significantly improved the health‐related quality of life (HRQoL) of patients. Therefore, the decision whether to perform prenatal diagnosis should be made by the parents following appropriate counseling and the careful evaluation of benefits and risks. Preimplantation diagnosis and implantation of unaffected fetuses is under consideration in some jurisdictions. No mutation can be detected in the C1‐INH (SERPING1) gene in 8–10% of C1‐INH‐HAE 64, 65, 66, 67.
Postnatal
Blood laboratory testing
Blood testing to diagnose C1‐INH‐HAE in pediatrics is similar to adults 21. Low functional C1‐INH with low C4 suggests C1‐INH‐HAE at all ages, but requires confirmation. When accompanied by a low antigenic C1‐INH level, then C1‐INH‐HAE type I is possible. If low C4 and low functional C1‐INH are associated with normal or elevated antigenic C1‐INH levels, then C1‐INH‐HAE type II is likely. These testings should be repeated to confirm the diagnosis of C1‐INH‐HAE 68.
Acquired angioedema with C1‐INH deficiency (antibody or C1‐INH consumption‐mediated or B‐cell dyscrasia settings) is usually seen only in adults and is unlikely under 40 years of age. Therefore, C1q is usually not indicated for testing in the pediatric ages. C2, C3, and CH50 testing are not indicated for C1‐INH‐HAE diagnosis at any age. Some immunoregulatory disorders and congenital complement deficiencies other than C1‐INH‐HAE should be kept in mind, however, and further complement investigations may be carried out as clinically indicated particularly if negative family history. C1‐INH‐HAE‐like events have been seen in congenital C4 deficiencies or early‐onset lupus‐like disorders, and in these cases, testing the other complement components may be indicated 69.
In families with known C1‐INH‐HAE, first‐degree relatives, whether symptomatic or asymptomatic, should be screened with C1‐INH (preferably functional) and C4 levels at earliest convenience. The first swelling may be upper airway and may be fatal and come on without warning (Fig. 1A).
Figure 1.
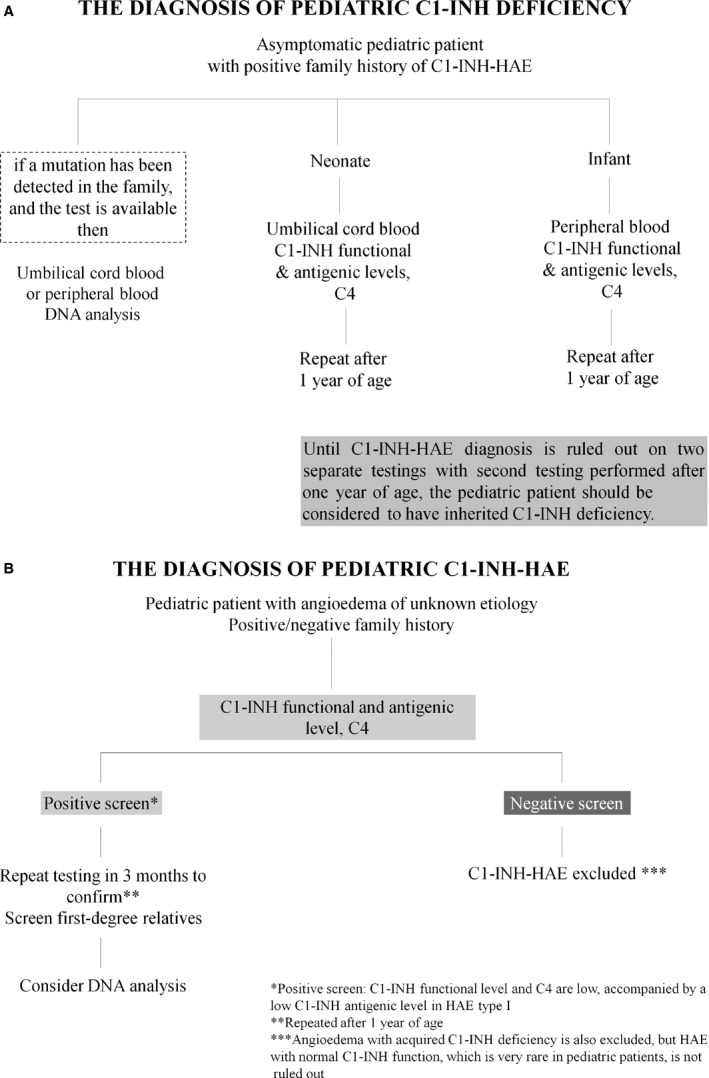
The diagnosis of C1‐INH deficiency in families with known C1‐INH‐HAE (A) and the diagnosis of C1‐INH‐HAE in pediatric patients with angioedema of unknown etiology (B).
Genetic testing
Genetic testing is not required to confirm the diagnosis of C1‐INH‐HAE unless prenatal testing is considered or in rare cases where a differential diagnosis is required in newborns and infants. Genetic testing may be helpful bearing in mind that not all of the mutations detected by routine genetic testing are undoubtedly disease causing 65. The detection of disease‐associated mutations requires a meticulous analysis of the gene and, possibly, the genetic testing of other affected and disease‐free family members. When genetic testing is available and a known family mutation is detected, then DNA analysis from cord blood or peripheral blood is sufficient to establish the diagnosis (Fig. 1A).
Diagnosis under the age of one year
Asymptomatic newborns or infants with a family history of C1‐INH‐HAE should be considered to have hereditary C1‐INH deficiency until the diagnosis is ruled out. C1‐INH levels are normal or even elevated from ages of one to five years compared to adults 70, but before the age of 1 year, the antigenic and functional C1‐INH levels may be lower than in adults, with the lowest levels in umbilical cord blood 71, 72. Both antigenic and functional C1‐INH cord blood levels correspond to 70% and 61.8% of adult normal values increasing to normal adult levels by the age of one year 71, 72. Moreover, neonatal serum complement levels are influenced by birth weight and gestational age 71, 73, 74. In newborns and infants aged less than 1 year, both C1‐INH antigenic level and functional activity are low in the patients with C1‐INH‐HAE type I and are within normal range in non‐C1‐INH‐HAE patients 68, 72. However, under one year of age, C4 levels are frequently low in non‐C1‐INH‐HAE patients as well. Therefore, testing for C1‐INH antigenic and functional levels are helpful to diagnose C1‐INH‐HAE regardless of the age of the patient, but low C4 levels under one year of age are not diagnostic for C1‐INH‐HAE 68.
If C1‐INH antigenic and functional levels are normal in a newborn or infant, the diagnosis of C1‐INH‐HAE is unlikely but confirmation after the age of one year is advisable. If functional and/or antigenic C1‐INH levels are low in a newborn or infant with suspected C1‐INH‐HAE, then we suggest repeating the testing after the age of one year. A final diagnosis requires at least two matching HAE screening results with the second test performed after one year of age 72, 75. If the familial gene is known, then C1‐INH‐HAE diagnosis in a newborn or infant can be helped by genetic testing (Fig. 1A).
Diagnostic testing if patient history suggestive of C1‐INH‐HAE, but negative family history
Negative family history does not rule out C1‐INH‐HAE. Clinical suspicion of C1‐INH‐HAE‐like symptoms at any age is an indication for screening regardless of the presence or absence of family history. C1‐INH‐HAE screening includes functional and antigenic C1‐INH levels and C4. If screening is suggestive of C1‐INH‐HAE, a second test should be performed to confirm the diagnosis. If C1‐INH‐HAE is suggested by testing, then all first‐degree relatives in the ascending line should be screened (including symptom‐free individuals). As with many autosomal dominant disorders, 25% of cases may be a de novo mutation which may then be passed onto future descendants 76. SERPING1 gene sequencing may be helpful to confirm the C1‐INH‐HAE in this setting 64, 66. If screening is negative for C1‐INH deficiency, angioedema with acquired C1‐INH deficiency is also excluded, but HAE with normal C1‐INH function, which is very rare in pediatric patients, is not ruled out (Fig 1B).
Management
Diagnosis and management of C1‐INH‐HAE are best achieved through comprehensive care clinics (level III evidence).
Education and counseling
Education of patients and their family members, family physicians, and consultant specialists including pediatricians with respect to diagnosis and therapy of C1‐INH‐HAE is the cornerstone of successful management of C1‐INH‐HAE in all age groups, but especially in pediatrics (level III evidence) 14, 22, 77, 78, 79. Parents should be provided with comprehensible information on specific characteristics of C1‐INH‐HAE and on management options for all age groups at the time of diagnosis and with each follow‐up comprehensive care HAE clinic visit. Furthermore, distance communication options should be made available including telephone and Internet access to the clinic 14. It is important that teachers and responsible child care workers receive detailed written information on the disease 14, 22. Because young children might not be able to correctly describe their condition, they should always carry a multilingual C1‐INH‐HAE identification and information card containing a description of emergency procedures along with acute treatment products for emergency use (see below for acute treatment options). Alert devices, including identifying wrist or neck bands with emergency contact information, should also be considered 22, 80. A detailed individual action and treatment plan should be provided to the families. Self‐ or assisted treatment techniques should be discussed and training programs for these offered 7, 22, 81.
Primary prevention
Avoidance of C1‐INH‐HAE triggers
As described in Section Trigger factors above, some medications may trigger C1‐INH‐HAE events including ACEIs and estrogen‐containing oral contraceptives. These agents should be avoided in C1‐INH‐HAE patients of all ages whenever possible 14, 31, 33, 35, 36. In some cases, attacks can be prevented through counseling, lifestyle changes, and by avoiding triggering factors, most specifically contact sports and other activities involving physical tissue trauma. Although breastfeeding is known to confer protection against numerous diseases, it does not decrease nor prevent C1‐INH‐HAE and its symptoms 82. Immunizations are usually recommended for pediatrics with C1‐INH‐HAE and we suggest the usual schedule for vaccination. The aim of C1‐INH‐HAE management at all ages should be to normalize activities and lifestyle whenever possible. With the availability of modern effective therapeutic and prophylactic interventions, patients should be encouraged to lead as normal a lifestyle as possible. There is no recommendation for specific activity avoidance 34.
Genetic management approaches
Gene therapy at various levels and genetic corrective interventions are under study, but not yet available. Preselection of unaffected embryos for implantation is under consideration in some jurisdictions 63.
Drug treatment
Prophylaxis
As indicated above, prophylaxis begins with identification and elimination or avoidance of precipitating factors, if possible 34, 38. Therapeutic prophylaxis usually includes either short‐term prophylaxis (STP) before events that are at an increased risk of precipitating an attack or long‐term prophylaxis (LTP), which would be used to prevent attacks long term. So far, no randomized controlled trials (RCTs) on prophylactic treatment restricted to the pediatric population have been conducted. Few pediatric patients have been included in RCTs (no level I evidence) leaving most pediatric prophylaxis level III evidence 14, 21, 22, 34, 38, 78, 83, 84, 85.
Short‐term prophylaxis
As in adults, indications for STP in pediatrics include patient‐specific triggers, medical and dental procedures 85. For most ‘minor interventions’, the recommendation is to choose on‐demand treatment if a swelling event is precipitated rather than prophylaxis, provided that a licensed on‐demand medication is immediately available in the case of emergency (level III evidence). For interventions that involve airway manipulation or that might lead to tissue swelling, prophylaxis with a dose of 15 to 30 units per kg pdC1‐INH (Berinert® [pdC1‐INHBe]) concentrate is recommended. There are no studies supporting appropriate timing of the STP nor consensus on the recommended maximum dose (1000 units versus 15 to 30 units/kg) (level III evidence) 14, 21, 22, 34, 78, 83, 84, 85. STP with pdC1‐INH recommendations varies from during procedure or one or more hours before the procedure trying to give as close to the procedure as possible. If licensed on‐demand acute treatment medication is not available with planned procedures, the following treatment options are recommend for STP: oral attenuated androgens (AAs), mainly danazol 2.5 to 10 mg/kg/day, mean dose suggestion 5 mg/kg/day (maximum 600 mg daily) (stanozolol and oxandrolone being used less often); or antifibrinolytics like tranexamic acid (TA) 20 to 50 mg/kg/day split into 2 or 3 doses with a maximum of 3 to 6 g/day, considering dose adjustment for renal impairment (epsilon aminocaproic acid is used less often). Prophylaxis should start (at least) 5 days before and be continued for 2 days postprocedure (level III evidence). As prophylaxis may fail, effective on‐demand treatment should be available whenever possible (level III evidence) 14, 21, 22, 34, 78, 83, 84, 85. In emergency situations and when licensed on‐demand therapies are not available, 10 ml/kg of solvent detergent plasma (SDP) (safer than fresh frozen plasma (FFP)) may be used prophylactically pre‐ or perioperatively or on‐demand (level III evidence).
Long‐term prophylaxis
As with adults, indications and options for long‐term prophylaxis (LTP) are controversial for pediatric C1‐INH‐HAE patients. LTP should be considered to minimize the impact of C1‐INH‐HAE on patients’ QoL. Agents for LTP include antifibrinolytics (tranexamic acid, TA; epsilon aminocaproic acid), AAs (danazol, stanozolol, oxandrolone), and pdC1‐INH.
Most consider TA to be the agent of choice for LTP in pediatrics, but TA is contraindicated for patients with a history of thromboembolism or a known thrombophilia defect (level III evidence). Patients with a family history of known thrombophilia defect should be screened for the defect before receiving LTP with TA (although the occurrence of thrombotic events is very rare). There are few data regarding the appropriate dose of TA with 20 to 50 mg/kg/day split into 2 or 3 doses with a maximum of 3 to 6 g/day mainly used for LTP (dose adjustment for renal impairment; level III evidence). We recommend starting at the lower dose and increasing as needed to suppress events. When antifibrinolytics fail to achieve the desired improvement or if they are contraindicated or not tolerated, then most recommend pdC1‐INH for LTP (level III evidence).
AAs are usually not considered for LTP in pediatrics prior to Tanner Stage V. After Tanner Stage V, AAs may be used trying to achieve the minimum effective dose. Danazol has been used effectively in pediatrics at doses of 2.5 to 5 mg/kg/day (200 mg daily should not be exceeded). Treatment should start at 2.5 mg/kg/day and increase slowly every 2 weeks until symptom suppression or the maximum tolerated or maximum recommended dose is reached. AA administration requires careful safety monitoring 14, 34. Dosage for oxandrolone has not been established for pediatrics, although some suggest that this is the preferred androgen for pediatric patients. The initial dose for adults is 2.5 mg three times daily and the lowest dose to control the attacks should be reached. Church JA reported the use of 0.1 mg/kg/day in a child and virilizing effects were seen. The drug has to be formulated so decreasing concentrations could be tried (level III evidence) 86, 87.
PdC1‐INH may be the safest LTP approach (level III evidence) and recommended over AA LTP where possible.
LTP does not necessarily mean uninterrupted medication for life. As events change (e.g., changes in stressors or hormonal fluxes), a step‐up, stabilize, step‐down, or intermittent approach to LTP may be a consideration. In general, intermittent LTP may be appropriate in some patients, while others may require continuous LTP (level III evidence). We recommend a pdC1‐INH LTP dose of 10 to 20 units per kg per dose once or twice weekly with an initial maximum dose of 1000 units (level III evidence). The safety and effectiveness of pdC1‐INH has not been established in pediatrics. Three of the 24 subjects in the randomized, placebo‐controlled, cross‐over, routine prophylaxis trial with pdC1‐INH (Cinryze® [pdC1‐INHCi]) were under the age of 18 years (9, 14, and 16 years of age). Data on pediatric IV pdC1‐INH LTP dose and frequency are very limited and we are awaiting the results of ongoing pediatric pdC1‐INHCi study and the results of controlled clinical trials of subcutaneous pdC1‐INH in preventing HAE attacks in this age group. Combination LTP approaches including intermittent LTP or combination LTP agents (e.g., TA plus pdC1‐INH at various doses and frequencies) need further consideration. To date, safety, efficacy, and tolerability of pdC1‐INH appear to be similar in pediatric and adult patients (level III evidence), but approval age and indication of various pdC1‐INH concentrates vary by jurisdiction 14, 21, 22, 34, 78, 83, 84, 85, 88, 89.
Acute treatment
All swelling events are eligible for acute treatment (level III evidence) 90.
Upper airway swellings should always receive acute treatment as early as possible followed by immediate assessment in the emergency room. Clinical trials suggest that earlier treatment shortens attack duration and improve treatment outcomes (level III evidence) 91, 92, 93, 94.
Every patient with C1‐INH‐HAE should be considered for home therapy and self‐/caregiver administration training. This can be facilitated through peer‐to‐peer encouragement and training at summer camps with pediatric patients of varied ages or by in‐home nurse training (level I evidence).
Level I evidence for acute treatment of C1‐INH‐HAE has been reviewed for pdC1‐INHBe, pdC1‐INHCi, recombinant human C1‐INH (rhC1‐INH) (Rhucin/Ruconest®), kallikrein inhibitor ecallantide (Kalbitor®), and bradykinin B2 receptor antagonist icatibant (Firazyr®) 85, 91, 92, 95, 96, 97, 98, 99. Unfortunately, these treatments have been licensed mainly for adults with pediatric licensing pending and ages for licenses varying by jurisdiction. At present, pdC1‐INH, rhC1‐INH and ecallantide (12 years and up; in Europe and USA, pdC1‐INHBe is licensed for all age groups) are the only agents licensed for pediatric acute treatment 14, 21, 24, 34, 100. There are few reports of use of pdC1‐INH in very young children and babies 101, 102.
Plasma‐derived C1‐INH concentrates
The plasma‐derived C1‐INH concentrates (pdC1‐INHBe and pdC1‐INHCi) are both approved for C1‐INH‐HAE acute treatment in pediatric patients in Europe by the EMA (European Medicines Agency) with doses of 20 units per kg for pdC1‐INHBe (pdC1‐INHBe is approved by the EMA and FDA [Food and Drug Administration] for all ages and licensed for home/self therapy) and 1000 units for pdC1‐INHCi (pdC1‐INHCi is approved in Europe for ages of 12 years and older by the EMA; not approved for acute treatment of HAE attacks in USA). PdC1‐INHCi is approved by the FDA and EMA for prophylaxis for adolescents and adults and is licensed for home/self‐therapy. In Brazil, pdC1‐INHBe is approved for home/self‐therapy and for pediatric and adult use 95, 99, 103, 104, 105, 106, 107.
Ecallantide
Ecallantide is licensed by the FDA for the acute treatment of HAE attacks in patients with C1‐INH‐HAE at the age of 12 years and older (ecallantide is not licensed in Europe). It is administered subcutaneously as a 30 mg dose 97. Hypersensitivity, including anaphylaxis, is a known risk of ecallantide treatment and occurs in 3% of treatments; no deaths are reported 108. Because of the anaphylaxis risk, this drug should be administered only by a healthcare professional who has medical knowledge in the management of anaphylaxis.
Recombinant human C1‐INH
rhC1‐INH is licensed by the FDA and EMA for the acute treatment of C1‐INH‐HAE for the patients aged 13 and older 96, 109. An open‐label treatment study with rhC1‐INH in a pediatric population (2–13 years) is ongoing. The dose is 50 units per kg and is given by intravenous injection.
Icatibant
Icatibant is licensed for acute treatment (including home/self‐treatment) of C1‐INH‐HAE for ages 18 years or older by the FDA, EMA, ANVISA (Brazil) and other Latin American countries (Table 2) 91, 92. Icatibant is not licensed for pediatric use, but a clinical trial in pediatric patients is ongoing.
Table 2.
Therapeutic options—license status
| Drug | Registration | Indication | Age/Groups | a | ||||
|---|---|---|---|---|---|---|---|---|
| Acute treatment | Prophylaxis | Home therapy | ||||||
| STP | LTP | Childrenb | Adolescencec | |||||
| pdC1‐INH (Berinert®) | Europe | ✓ | ✓ | — | ✓ | ✓ | ✓ | i.v. |
| USA | ✓ | — | — | ✓ | ✓ | ✓ | ||
| Latin America (Brazil, Argentina, Mexico, Colombia, Chile, Puerto Rico) | ✓ | ✓ | — | ✓ | ✓ | ✓ | i.v. | |
| Australia | ✓ | — | — | ✓ | — | ✓ | i.v. | |
| Canada | ✓ | — | — | — | — | ✓ | i.v. | |
| Israel | ✓ | ✓ | — | ✓ | ✓ | ✓ | i.v. | |
| Japan | ✓ | ✓ | — | ✓ | — | ✓ | i.v. | |
| South Korea | ✓ | ✓ | — | ✓ | ✓ | ✓ | i.v. | |
| pdC1‐INH (Cinryze®) | Europe | ✓ | ✓ | ✓ | ✓ | Trial ongoing | ✓ | i.v. |
| USA | — | ✓ | ✓ | ✓ | Trial ongoing | ✓ | ||
| Latin America | — | — | — | — | — | — | i.v. | |
| Australia | — | ✓ | ✓ | ✓ | — | — | i.v. | |
| Canada | — | ✓ | ✓ | ✓ | — | — | i.v. | |
| Israel | ✓ | ✓ | ✓ | ✓ | — | — | i.v. | |
| rhC1‐INH (Ruconest®) | Europe | ✓ | — | — | — | Trial ongoing | ✓ | i.v. |
| USA | ✓ | — | — | — | Trial ongoing | ✓ | ||
| Latin America | — | — | — | — | — | — | ||
| Icatibant (Firazyr®) | Europe | ✓ | — | — | ✓ | Trial ongoing | Trial ongoing | s.c. |
| USA | ✓ | — | — | ✓ | Trial ongoing | Trial ongoing | ||
| Latin America (Brazil, Argentina, Mexico, Colombia) | ✓ | — | — | ✓ | — | — | s.c. | |
| Australia | ✓ | — | — | ✓ | Trial ongoing | Trial ongoing | s.c. | |
| Canada | ✓ | — | — | ✓ | — | — | s.c. | |
| Israel | ✓ | — | — | ✓ | Trial ongoing | Trial ongoing | s.c. | |
| Kuwait | ✓ | — | — | ✓ | — | — | s.c. | |
| South Africa | ✓ | — | — | ✓ | — | — | s.c. | |
| Attenuated androgensd | Europe | — | ✓ | ✓ | — | — | ✓ | Oral |
| USA | — | — | ✓ | — | — | — | ||
| Latin America (Brazil, Argentina, Mexico, Colombia) | — | — | ✓ | ✓ | — | — | Oral | |
| Australia | — | — | ✓ | — | — | — | Oral | |
| Tranexamic acid (Cyklokapron®; Transamin®; Hemoblock®) | Europe | — | — | ✓ | — | ✓ | ✓ | Oral |
| USA | — | — | ✓ | — | ✓ | ✓ | Oral | |
| Canada | — | — | ✓ | — | ✓ | ✓ | Oral | |
| Australia | — | — | ✓ | — | ✓ | ✓ | Oral | |
| Latin America (Brazil, Argentina, Mexico, Colombia) | ✓ | — | — | ✓ | — | — | Oral | |
| Ecallantide (Kalbitor®) | Europe | — | — | — | — | — | — | s.c. |
| USA | ✓ | — | — | — | — | ✓ | ||
| Latin America | — | — | — | — | — | — | ||
i.v., intravenous; s.c., subcutaneous.
Children aged 0 to ≤12 years.
Adolescents aged 12 to ≤18 years.
Attenuated androgens not licensed in Germany, Austria, and Switzerland.
pdC1‐INH, human plasma‐derived C1‐INH; rhC1‐INH, recombinant human C1‐INH.
Plasma
If licensed on‐demand acute treatment medication is not available or not accessible, 10 ml/kg of plasma may be used on‐demand—solvent detergent plasma is preferred over fresh frozen plasma for safety reduction of risk of transfusion transmitted diseases (level III evidence).
Therapeutic options and the license status are summarized in Table 2.
Home‐based treatment
Home therapy for hemophilia has been in use for more than 40 years 85, 110. Home‐based acute treatment and prophylaxis of C1‐INH‐HAE has been recommended for all ages in many consensus documents 22, 84, 85, 111, 112.
Formal approval of various agents for home therapy varies by jurisdiction. Ecallantide, SDP, and FFP are not recommended for self‐therapy because of a small risk of anaphylaxis; however, in‐home therapy by a nurse trained in the treatment of anaphylaxis is an option for ecallantide 22, 90, 111, 112, 113.
Investigators have examined barriers to self‐therapy from the perspective of the nurse 114 and physician 111, 112, 114 and more recently from the patient perspective 114, 115, 116. All three components of the healthcare system agree that self‐care/self‐home treatment is preferred despite these barriers.
Patients who do not perform self‐treatment tend to overestimate the difficulties of training and of becoming proficient in self‐treatment 116. In contrast, patients who already perform self‐treatment are more confident in their training and their ability to apply both subcutaneous and intravenous injections 116. Although many physicians consider multiple training appointments necessary 112, the majority of patients performing self‐treatment reported that it took them only one or two sessions to feel competent enough for self‐administration.
Confidence is a large factor in patient's adherence to treatment and feeling of independence. One of the many benefits of self‐treatment therapy is greater freedom to live a normal life at home, at school or work, or while traveling, leading to improved overall QoL 116, 117, 118, 119.
Comprehensive care centers and follow‐up
We recommend following up the patient and family unit at least once per year in an HAE comprehensive care center by a consultant pediatrician C1‐INH‐HAE specialist with access to endocrinology and psychology consultation if needed. For patients on LTP who require closer monitoring, we suggest a monitoring schedule of every three to six months. As with other chronic illnesses, close attention should be paid to growth and development 34. At these visits, the patient diary, outpatient records, discharge summaries, and possible treatment‐emergent adverse events should be reviewed to assess the disease severity and treatment tolerability and to develop or adjust the treatment and prophylaxis strategies. Patients on AA should see an endocrinologist at each visit. Recommendations for adverse event screening while on LTP with AA or TA are similar for pediatric patients as for adults described in recent consensus documents. Between visits, comprehensive care clinic support should be made available via telephone or e‐mail. The exchange of information should be maintained with the family practitioner and/or pediatrician 21, 22. The analysis of HRQoL outcomes at follow‐up visits may help in evaluating therapeutic effectiveness; but it has to be kept in mind that QoL questionnaires currently available for use in C1‐INH‐HAE have been validated only in patients over 17 years of age 120. An adaptation of HAE‐QoL to pediatrics is planned.
International variation in availability of healthcare options and levels of healthcare services
The knowledge about C1‐INH‐HAE diagnosis and therapy, especially in pediatric patients, is still limited, particularly in developing countries. A recent survey about C1‐INH‐HAE in Latin America and the unavailability of data and medications in Latin America as in most African and Asian countries certainly influence the choice of therapy in these countries 11. AAs have been used in pediatrics in many developing countries because of the cost and lack of alternative medications although they are not recommended in the guidelines nor before Tanner Stage V development. In light of this, QoL, morbidity, and the possibility of mortality need to be carefully balanced against the adverse effects of AAs when making the decision to prescribe androgens to pediatric patients 11, 121. Due to the rareness of the disease, emergency departments (EDs) are often unaware of the protocols for treating C1‐INH‐HAE attacks, particularly in pediatrics. Establishing an effective approach to pediatric C1‐INH‐HAE has been a challenge.
A recent publication reported that the average age at the diagnosis of 25 pediatric patients evaluated in the USA was 7.2 years for patients mostly with a known positive family history 5. In Brazil, the mean age at the diagnosis was 8.3 ± 5.1 years with 94% of 50 patients (<18 years old) being symptomatic (ASG, personal communication presented in the 7th Budapest Workshop) 12.
Even though the patients had a known family history of C1‐INH‐HAE and testing for C1‐INH‐HAE is generally recommended at an age of 1 year in this setting, a diagnosis in these patients was only established after several years 5. Unnecessary procedures are frequently reported in pediatric patients with C1‐INH‐HAE 122. Zilberberg et al. 118 evaluated emergency department (ED) visits of C1‐INH‐HAE patients in the United States in 2006 and 2007. During these two years, half of the 221 pediatric patients (<18 years old) had to be hospitalized due to a C1‐INH‐HAE attack. Because no drugs for attacks had been approved by the FDA at that time, and only FFP was available for attacks, this study could reflect the situation of patients with established C1‐INH‐HAE diagnosis in countries where attack therapy is not available as in most of Latin American, Asian, or African countries. In addition, we should consider the high cost of being treated in the ED in comparison with self‐treatment at home 106.
Estimation of the economic burden associated with C1‐INH‐HAE is difficult and must reflect the costs for medical interventions including hospital and outpatient care, prophylactic and acute therapeutic medications, and also absenteeism of the parents and/or caregivers from work and school absenteeism The true cost of the disease from medications alone in developed countries is frequently in many hundred of thousands of US dollars per year. The cost of the disease in developing countries without specific medications for C1‐INH‐HAE is often excessive absenteeism, significant morbidity, failure to maintain employment, and higher risk of mortality 119, 123.
With the help of a parent or a guardian, pediatric patients have successfully administered pdC1‐INH concentrate, with faster initiation of treatment, less time to symptom relief, and fewer days of hospitalization and days lost from school. In addition, even at a young age, pediatric patients can be taught to safely administer intravenous and subcutaneous therapy as is obvious from data from hemophilic patients 90.
Conclusions
Phase III clinical trials are needed in the pediatric populations so that drug treatments for prophylaxis and acute therapy are approved for all ages. New drug protocols should include pediatric age patients for all rare diseases and use these data to power and develop clinical trials specifically for pediatrics. The future appears that medications will be delivered prophylactically by the subcutaneous and oral routes, which will reduce the stress of frequent intravenous injections. Long‐term follow‐up programs are essential in pediatric patients as these cohorts represent unique populations at risk for adverse events given the growth phases and developmental changes in this population. International registries for pediatric patients with C1‐INH‐HAE disease will facilitate safety and efficacy data and allow earlier detection of long‐term adverse event and benefits of specific interventions. In summary, more therapeutic trials, data on dosing by weight, databases, and data to support self‐administration programs are needed to further the science and clinical care of the pediatric population with C1‐INH‐HAE.
Funding
The international consensus on the diagnosis and management of pediatric patients with hereditary angioedema with C1 inhibitor deficiency (C1‐INH‐HAE) was arrived at during the 9th C1 Inhibitor Deficiency Workshop held on May 28–31, 2015, Budapest, Hungary. This meeting was funded by unrestricted educational grants from Biocryst, CSL Behring, Dyax, Pharming NV, Shire Pharmaceuticals, and Swedish Orphan Biovitrum. Publication of this manuscript was sponsored by HAEi—International Patient Organization for C1 Inhibitor Deficiencies.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgment
We would like to thank the executive committee of the patient organization HAE International (HAEi) for approving this manuscript.
Appendix : HAWK (Hereditary Angioedema International Working Group)
Werner Aberer, Austria; Sladjana Andrejevic, Serbia; Emel Aygoeren‐Pürsün, Germany; Alena Banerji, USA; Noemi‐Anna Bara, Romania; Murat Bas, Germany; Jonathan Bernstein, USA; Stephen Betschel, Canada; Janne Björkander, Sweden; Isabelle Boccon‐Gibod, France; Laurence Bouillet, France; Maria Bova, Italy; Henrik Halle Boysen, Denmark; Manuel Branco‐Ferreira, Portugal; Anette Bygum, Denmark; Teresa Caballero, Spain; Mauro Cancian, Italy; Anthony Castaldo, USA; Sandra Christiansen, USA; Marco Cicardi, Italy; Christian Drouet, France; Jose Fabiani, Argentina; Mark Gompels, UK; Maria Teresa Gonzalez‐Quevedo, Spain; Jimmy Gooi, UK; Richard Gower, USA; Nihal Mete Gökmen, Turkey; Vesna Grivcheva‐Panovska, Macedonia; Mar Guilarte, Spain; Okan Gülbahar, Turkey; Erik Hack, The Netherlands; Roman Hakl, Czech Republic; György Harmat, Hungary; Miloš Jeseňák, Slovakia; Stephen Jolles, UK; Allen Kaplan, USA; Connie Katelaris, Australia; Mitja Kosnik, Slovenia; Kinga Viktória Kőhalmi, Hungary; Iris Leibovich, Israel; Marcel Levi, The Netherlands; Henry Li, USA; Hilary J. Longhurst, UK; William Lumry, USA; Markus Magerl, Germany; Alejandro Malbran, Argentina; Ludovic Martin, France; Marcus Maurer, Germany; Enikő Mihály, Romania; Dumitru Moldovan, Romania; Mariana Murdjeva, Bulgaria; Imola Beatrix Nagy, Hungary; Erik W. Nielsen, Norway; Sandra Nieto, Mexico; Patrik Nordenfelt, Sweden; Kristine Obtulowitzc, Poland; Maria Pedrosa, Spain; Grzegorz Porębski, Poland; Nieves Prior, Spain; Avner Reshef, Israel; Marc A. Riedl, USA; Bernd Rosenkranz, South Africa; Peter Schmid‐Grendelmeier, Switzerland; Spath Péter, Switzerland; Matthaios Speletas, Greece; Maria Staevska, Bulgaria; Marcin Stobiecki, Poland; Massimo Triggiani, Italy; Nóra Veszeli, Hungary; Walter Wuillemin, Switzerland; Zhi Yu Xiang, China; Beverley Yamamoto, Japan; Bruce Zuraw, USA.
Farkas H, Martinez‐Saguer I, Bork K, Bowen T, Craig T, Frank M, Germenis AE, Grumach AS, Luczay A, Varga L, Zanichelli A, on behalf of HAWK . International consensus on the diagnosis and management of pediatric patients with hereditary angioedema with C1 inhibitor deficiency. Allergy 2017; 72:300–313.
Edited by: Werner Aberer
All authors equally contributed to this work.
References
- 1. Kaplan AP. Bradykinin and the pathogenesis of hereditary angioedema. World Allergy Organ J 2011;4:73–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Agostoni A, Cicardi M. Hereditary and acquired C1‐inhibitor deficiency: biological and clinical characteristics in 235 patients. Medicine (Baltimore) 1992;71:206–215. [DOI] [PubMed] [Google Scholar]
- 3. Bork K, Meng G, Staubach P, Hardt J. Hereditary angioedema: new findings concerning symptoms, affected organs, and course. Am J Med 2006;119:267–274. [DOI] [PubMed] [Google Scholar]
- 4. Martinez‐Saguer I, Farkas H. Erythema marginatum as an early symptom of hereditary angioedema: case report of 2 newborns. Pediatrics 2016;137:e20152411. [DOI] [PubMed] [Google Scholar]
- 5. Bennett G, Craig T. Hereditary angioedema with a focus on the child. Allergy Asthma Proc 2015;36:70–73. [DOI] [PubMed] [Google Scholar]
- 6. Bygum A. Hereditary angio‐oedema in Denmark: a nationwide survey. Br J Dermatol 2009;161:1153–1158. [DOI] [PubMed] [Google Scholar]
- 7. Caballero T. Angio‐oedema due to hereditary C1 inhibitor deficiency in children. Allergol Immunopathol (Madr) 2013;41:45–53. [DOI] [PubMed] [Google Scholar]
- 8. Ohsawa I, Honda D, Nagamachi S, Hisada A, Shimamoto M, Inoshita H et al. Clinical manifestations, diagnosis, and treatment of hereditary angioedema: survey data from 94 physicians in Japan. Ann Allergy Asthma Immunol 2015;114:492–498. [DOI] [PubMed] [Google Scholar]
- 9. Bouillet L, Launay D, Fain O, Boccon‐Gibod I, Laurent J, Martin L et al. Hereditary angioedema with C1 inhibitor deficiency: clinical presentation and quality of life of 193 French patients. Ann Allergy Asthma Immunol 2013;111:290–294. [DOI] [PubMed] [Google Scholar]
- 10. Ren HL, Zhang HY. Clinical features of hereditary angioedema: analysis of 133 cases. Zhonghua Yi Xue Za Zhi 2007;87:2772–2776. [PubMed] [Google Scholar]
- 11. Fabiani J, Valle SO, Olivares M, Nieto S, Landeros EH, Ginaca A et al. Improving C1 inhibitor deficiency (type 1 and type 2 hereditary angioedema) in Latin America. J Investig Allergol Clin Immunol 2014;24:445–447. [PubMed] [Google Scholar]
- 12. Grumach AS, Valle SO, Toledo E, de Moraes Vasconcelos D, Villela MM, Mansour E et al. Hereditary angioedema: first report of the Brazilian registry and challenges. J Eur Acad Dermatol Venereol 2013;27:338–344. [DOI] [PubMed] [Google Scholar]
- 13. Gomez‐Traseira C, Perez‐Fernandez E, Lopez‐Serrano MC, Garcia‐Ara MC, Pedrosa M, Lopez‐Trascasa M et al. Clinical pattern and acute and long‐term management of hereditary angioedema due to C1‐esterase inhibitor deficiency. J Investig Allergol Clin Immunol 2015;25:358–364. [PubMed] [Google Scholar]
- 14. Farkas H. Pediatric hereditary angioedema due to C1‐inhibitor deficiency. Allergy Asthma Clin Immunol 2010;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martinez‐Saguer I, Graff J, Rusicke E, Aygören‐Pürsün E, Klingebiel T, Kreuz W. Does early clinical manifestation of hereditary angioedema (HAE) influence the clinical course of the disease? J Allergy Clin Immunol Pract 2013;131:AB30. [Google Scholar]
- 16. Sanhueza PI. Contraception in hereditary angioedema. Fertil Steril 2008;90:21–22. [DOI] [PubMed] [Google Scholar]
- 17. Bouillet L, Ponard D, Drouet C, Jullien D, Massot C. Angioedema and oral contraception. Dermatology 2003;206:106–109. [DOI] [PubMed] [Google Scholar]
- 18. Zanichelli A, Magerl M, Longhurst H, Fabien V, Maurer M. Hereditary angioedema with C1 inhibitor deficiency: delay in diagnosis in Europe. Allergy Asthma Clin Immunol 2013;9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zanichelli A, Arcoleo F, Barca MP, Borrelli P, Bova M, Cancian M et al. A nationwide survey of hereditary angioedema due to C1 inhibitor deficiency in Italy. Orphanet J Rare Dis 2015;10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roche O, Blanch A, Caballero T, Sastre N, Callejo D, Lopez‐Trascasa M. Hereditary angioedema due to C1 inhibitor deficiency: patient registry and approach to the prevalence in Spain. Ann Allergy Asthma Immunol 2005;94:498–503. [DOI] [PubMed] [Google Scholar]
- 21. Bowen T, Cicardi M, Farkas H, Bork K, Longhurst HJ, Zuraw B et al. 2010 International consensus algorithm for the diagnosis, therapy and management of hereditary angioedema. Allergy Asthma Clin Immunol 2010;6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Craig T, Aygoren‐Pursun E, Bork K, Bowen T, Boysen H, Farkas H et al. WAO guideline for the management of hereditary angioedema. World Allergy Organ J 2012;5:182–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cicardi M, Aberer W, Banerji A, Bas M, Bernstein JA, Bork K et al. Classification, diagnosis, and approach to treatment for angioedema: consensus report from the Hereditary Angioedema International Working Group. Allergy 2014;69:602–616. [DOI] [PubMed] [Google Scholar]
- 24. Agostoni A, Aygoren‐Pursun E, Binkley KE, Blanch A, Bork K, Bouillet L et al. Hereditary and acquired angioedema: problems and progress: proceedings of the third C1 esterase inhibitor deficiency workshop and beyond. J Allergy Clin Immunol 2004;114(3 Suppl):S51–S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Czaller I, Visy B, Csuka D, Fust G, Toth F, Farkas H. The natural history of hereditary angioedema and the impact of treatment with human C1‐inhibitor concentrate during pregnancy: a long‐term survey. Eur J Obstet Gynecol Reprod Biol 2010;152:44–49. [DOI] [PubMed] [Google Scholar]
- 26. Martinez‐Saguer I, Rusicke E, Aygoren‐Pursun E, Heller C, Klingebiel T, Kreuz W. Characterization of acute hereditary angioedema attacks during pregnancy and breast‐feeding and their treatment with C1 inhibitor concentrate. Am J Obstet Gynecol 2010;203:1–7. [DOI] [PubMed] [Google Scholar]
- 27. Farkas H, Harmat G, Fay A, Fekete B, Karadi I, Visy B et al. Erythema marginatum preceding an acute oedematous attack of hereditary angioneurotic oedema. Acta Derm Venereol 2001;81:376–377. [DOI] [PubMed] [Google Scholar]
- 28. McCollough M, Sharieff GQ. Abdominal pain in children. Pediatr Clin North Am 2006;53:107–137. [DOI] [PubMed] [Google Scholar]
- 29. Kim JH, Kang HS, Han KH, Kim SH, Shin KS, Lee MS et al. Systemic classification for a new diagnostic approach to acute abdominal pain in children. Pediatr Gastroenterol Hepatol Nutr 2014;17:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Janardhanan D, Nair S, Subramanian TS. Recurrent abdominal pain due to hereditary angioedema. Indian J Pediatr 2007;74:83–84. [DOI] [PubMed] [Google Scholar]
- 31. Bouillet L, Longhurst H, Boccon‐Gibod I, Bork K, Bucher C, Bygum A et al. Disease expression in women with hereditary angioedema. Am J Obstet Gynecol 2008;199:1–4. [DOI] [PubMed] [Google Scholar]
- 32. Visy B, Fust G, Varga L, Szendei G, Takacs E, Karadi I et al. Sex hormones in hereditary angioneurotic oedema. Clin Endocrinol (Oxf) 2004;60:508–515. [DOI] [PubMed] [Google Scholar]
- 33. Zotter Z, Csuka D, Szabo E, Czaller I, Nebenfuhrer Z, Temesszentandrasi G et al. The influence of trigger factors on hereditary angioedema due to C1‐inhibitor deficiency. Orphanet J Rare Dis 2014;9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Farkas H, Varga L, Szeplaki G, Visy B, Harmat G, Bowen T. Management of hereditary angioedema in pediatric patients. Pediatrics 2007;120:713–722. [DOI] [PubMed] [Google Scholar]
- 35. Assadi FK, Wang HE, Lawless S, McKay CP, Hopp L, Fattori D. Angiotensin converting enzyme inhibitor‐induced angioedema: a report of two cases. Pediatr Nephrol 1999;13:917–919. [DOI] [PubMed] [Google Scholar]
- 36. Quintana EC, Attia MW. Angiotensin‐converting enzyme inhibitor angioedema in a pediatric patient: a case report and discussion. Pediatr Emerg Care 2001;17:438–440. [DOI] [PubMed] [Google Scholar]
- 37. Nanda MK, Elenburg S, Bernstein JA, Assa'ad AH. Clinical features of pediatric hereditary angioedema. J Allergy Clin Immunol Pract 2015;3:392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Farkas H, Harmat G, Fust G, Varga L, Visy B. Clinical management of hereditary angio‐oedema in children. Pediatr Allergy Immunol 2002;13:153–161. [DOI] [PubMed] [Google Scholar]
- 39. Lindecken KD, Adolph M, Paterakis S. Pedicle torsion, hemorrhagic ovarian infarct. A rare cause of pediatric acute abdomen. Zentralbl Chir 1991;116:679–682. [PubMed] [Google Scholar]
- 40. Foix‐L'Helias L, Weiss L, Mollet‐Boudjemline A, Fallik D, Trioche‐Eberschweiler P, Labrune P. Recurring acute abdominal pains in an adolescent as the presenting manifestations of hereditary angioneurotic oedema. Acta Paediatr 2005;94:1158–1161. [DOI] [PubMed] [Google Scholar]
- 41. Sanchez A, Ecochard A, Maestracci M, Rodiere M. Hereditary angioedema causing colocolic intussusception. Arch Pediatr 2008;15:271–274. [DOI] [PubMed] [Google Scholar]
- 42. Pritzker HA, Levin TL, Weinberg G. Recurrent colocolic intussusception in a child with hereditary angioneurotic edema: reduction by air enema. J Pediatr Surg 2004;39:1144–1146. [DOI] [PubMed] [Google Scholar]
- 43. Farkas H, Harmat G, Fekete B, Karadi I, Visy B, Varga L. Acute abdominal attack of hereditary angioneurotic oedema associated with ultrasound abnormalities suggestive of acute hepatitis. Acta Paediatr 2002;91:971–974. [DOI] [PubMed] [Google Scholar]
- 44. Berkun Y, Eisenstein E, Ben‐Chetrit E. FMF – clinical features, new treatments and the role of genetic modifiers: a critical digest of the 2010–2012 literature. Clin Exp Rheumatol 2012;30(3 Suppl):S90–S95. [PubMed] [Google Scholar]
- 45. Farkas H, Harmat G, Kaposi PN, Karadi I, Fekete B, Fust G et al. Ultrasonography in the diagnosis and monitoring of ascites in acute abdominal attacks of hereditary angioneurotic oedema. Eur J Gastroenterol Hepatol 2001;13:1225–1230. [DOI] [PubMed] [Google Scholar]
- 46. Dinkel HP, Maroske J, Schrod L. Sonographic appearances of the abdominal manifestations of hereditary angioedema. Pediatr Radiol 2001;31:296–298. [DOI] [PubMed] [Google Scholar]
- 47. Cugno M, Zanichelli A, Bellatorre AG, Griffini S, Cicardi M. Plasma biomarkers of acute attacks in patients with angioedema due to C1‐inhibitor deficiency. Allergy 2009;64:254–257. [DOI] [PubMed] [Google Scholar]
- 48. Zotter Z, Csuka D, Varga L, Füst G, Farkas H. WBC elevation and the resulting neutrophilia characterize hereditary angioedema attacks. Angioedema 2010;1:10–16. [Google Scholar]
- 49. Veszeli N, Csuka D, Zotter Z, Imreh E, Jozsi M, Benedek S et al. Neutrophil activation during attacks in patients with hereditary angioedema due to C1‐inhibitor deficiency. Orphanet J Rare Dis 2015;10:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bork K, Hardt J, Schicketanz KH, Ressel N. Clinical studies of sudden upper airway obstruction in patients with hereditary angioedema due to C1 esterase inhibitor deficiency. Arch Intern Med 2003;163:1229–1235. [DOI] [PubMed] [Google Scholar]
- 51. Bork K, Siedlecki K, Bosch S, Schopf RE, Kreuz W. Asphyxiation by laryngeal edema in patients with hereditary angioedema. Mayo Clin Proc 2000;75:349–354. [DOI] [PubMed] [Google Scholar]
- 52. Bork K, Hardt J, Witzke G. Fatal laryngeal attacks and mortality in hereditary angioedema due to C1‐INH deficiency. J Allergy Clin Immunol 2012;130:692–697. [DOI] [PubMed] [Google Scholar]
- 53. Shah UK, Jacobs IN. Pediatric angioedema: ten years’ experience. Arch Otolaryngol Head Neck Surg 1999;125:791–795. [DOI] [PubMed] [Google Scholar]
- 54. Doherty G. Acute and chronic airway obstruction in children. Anaesth Intensive Care Med 2009;10:191–195. [Google Scholar]
- 55. Farkas H. Management of upper airway edema caused by hereditary angioedema. Allergy Asthma Clin Immunol 2010;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. El‐Hachem C, Amiour M, Guillot M, Laurent J. Hereditary angioneurotic edema: a case report in a 3‐year‐old child. Arch Pediatr 2005;12:1232–1236. [DOI] [PubMed] [Google Scholar]
- 57. O'Bier A, Muniz AE, Foster RL. Hereditary angioedema presenting as epiglottitis. Pediatr Emerg Care 2005;21:27–30. [DOI] [PubMed] [Google Scholar]
- 58. Altorjai P, Visy B, Kormos ZS, Harmat G, Fekete F, Farkas H. Pericardiac effusion complicating an acute abdominal attack of hereditary angioneurotic edema. Am J Case Rep 2008;9:CR233–CR236. [Google Scholar]
- 59. Hubiche T, Boralevi F, Jouvencel P, Taieb A, Leaute‐Labreze C. Reticular erythema signalling the onset of episodes of hereditary angioedema in a child. Ann Dermatol Venereol 2005;132:249–251. [DOI] [PubMed] [Google Scholar]
- 60. Rasmussen ER, Valente de Freitas P, Bygum A. Urticaria and prodromal symptoms including erythema marginatum in Danish patients with hereditary angioedema. Acta Derm Venereol 2015;96:373–376. [DOI] [PubMed] [Google Scholar]
- 61. Magerl M, Doumoulakis G, Kalkounou I, Weller K, Church MK, Kreuz W et al. Characterization of prodromal symptoms in a large population of patients with hereditary angio‐oedema. Clin Exp Dermatol 2014;39:298–303. [DOI] [PubMed] [Google Scholar]
- 62. Csuka D, Kelemen Z, Czaller I, Molnar K, Fust G, Varga L et al. Association of celiac disease and hereditary angioedema due to C1‐inhibitor deficiency. Screening patients with hereditary angioedema for celiac disease: is it worth the effort? Eur J Gastroenterol Hepatol 2011;23:238–244. [DOI] [PubMed] [Google Scholar]
- 63. Caballero T, Farkas H, Bouillet L, Bowen T, Gompel A, Fagerberg C et al. International consensus and practical guidelines on the gynecologic and obstetric management of female patients with hereditary angioedema caused by C1 inhibitor deficiency. J Allergy Clin Immunol 2012;129:308–320. [DOI] [PubMed] [Google Scholar]
- 64. Pappalardo E, Caccia S, Suffritti C, Tordai A, Zingale LC, Cicardi M. Mutation screening of C1 inhibitor gene in 108 unrelated families with hereditary angioedema: functional and structural correlates. Mol Immunol 2008;45:3536–3544. [DOI] [PubMed] [Google Scholar]
- 65. Speletas M, Szilagyi A, Psarros F, Moldovan D, Magerl M, Kompoti M et al. Hereditary angioedema: molecular and clinical differences among European populations. J Allergy Clin Immunol 2015;135:570–573. [DOI] [PubMed] [Google Scholar]
- 66. Kalmar L, Bors A, Farkas H, Vas S, Fandl B, Varga L et al. Mutation screening of the C1 inhibitor gene among Hungarian patients with hereditary angioedema. Hum Mutat 2003;22:498. [DOI] [PubMed] [Google Scholar]
- 67. Bautista‐Llacer R, Alberola TM, Vendrell X, Fernandez E, Perez‐Alonso M. Case report: first successful application of preimplantation genetic diagnosis for hereditary angiooedema. Reprod Biomed Online 2010;21:658–662. [DOI] [PubMed] [Google Scholar]
- 68. Pedrosa M, Phillips‐Angles E, Lopez‐Lera A, Lopez‐Trascasa M, Caballero T. Complement study versus CINH gene testing for the diagnosis of type I hereditary angioedema in children. J Clin Immunol 2016;36:16–18. [DOI] [PubMed] [Google Scholar]
- 69. Castelli R, Zanichelli A, Cicardi M, Cugno M. Acquired C1‐inhibitor deficiency and lymphoproliferative disorders: a tight relationship. Crit Rev Oncol Hematol 2013;87:323–332. [DOI] [PubMed] [Google Scholar]
- 70. Andrew M, Vegh P, Johnston M, Bowker J, Ofosu F, Mitchell L. Maturation of the hemostatic system during childhood. Blood 1992;80:1998–2005. [PubMed] [Google Scholar]
- 71. Grumach AS, Ceccon ME, Rutz R, Fertig A, Kirschfink M. Complement profile in neonates of different gestational ages. Scand J Immunol 2014;79:276–281. [DOI] [PubMed] [Google Scholar]
- 72. Nielsen EW, Johansen HT, Holt J, Mollnes TE. C1 inhibitor and diagnosis of hereditary angioedema in newborns. Pediatr Res 1994;35:184–187. [DOI] [PubMed] [Google Scholar]
- 73. Nurnberger W, Stannigel H, Muntel V, Michelmann I, Wahn V, Gobel U. In‐vivo activation of the 4th component of the complement system (C4) in premature and term infants with generalized bacterial infections. Klin Padiatr 1990;202:141–146. [DOI] [PubMed] [Google Scholar]
- 74. Hogasen AK, Overlie I, Hansen TW, Abrahamsen TG, Finne PH, Hogasen K. The analysis of the complement activation product SC5 b‐9 is applicable in neonates in spite of their profound C9 deficiency. J Perinat Med 2000;28:39–48. [DOI] [PubMed] [Google Scholar]
- 75. Roach B, Kim Y, Jerome E, Michael AF. Influence of age and sex on serum complement components in children. Am J Dis Child 1981;135:918–920. [DOI] [PubMed] [Google Scholar]
- 76. Pappalardo E, Cicardi M, Duponchel C, Carugati A, Choquet S, Agostoni A et al. Frequent de novo mutations and exon deletions in the C1 inhibitor gene of patients with angioedema. J Allergy Clin Immunol 2000;106:1147–1154. [DOI] [PubMed] [Google Scholar]
- 77. Caballero T, Baeza ML, Cabanas R, Campos A, Cimbollek S, Gomez‐Traseira C et al. Consensus statement on the diagnosis, management, and treatment of angioedema mediated by bradykinin. Part II. Treatment, follow‐up, and special situations. J Investig Allergol Clin Immunol 2011;21:422–441. [PubMed] [Google Scholar]
- 78. Bowen T, Cicardi M, Bork K, Zuraw B, Frank M, Ritchie B et al. Hereditary angiodema: a current state‐of‐the‐art review, VII: Canadian Hungarian 2007 International Consensus Algorithm for the Diagnosis, Therapy, and Management of Hereditary Angioedema. Ann Allergy Asthma Immunol 2008;100(1 Suppl):S30–S40. [DOI] [PubMed] [Google Scholar]
- 79. MacGinnitie AJ. Pediatric hereditary angioedema. Pediatr Allergy Immunol 2014;25:420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ebo DG, Verweij MM, De Knop KJ, Hagendorens MM, Bridts CH, De Clerck LS et al. Hereditary angioedema in childhood: an approach to management. Paediatr Drugs 2010;12:257–268. [DOI] [PubMed] [Google Scholar]
- 81. Kreuz W, Rusicke E, Martinez‐Saguer I, Aygoren‐Pursun E, Heller C, Klingebiel T. Home therapy with intravenous human C1‐inhibitor in children and adolescents with hereditary angioedema. Transfusion 2012;52:100–107. [DOI] [PubMed] [Google Scholar]
- 82. Kelemen Z, Visy B, Csuka D, Czaller I, Fust G, Farkas H. Abdominal symptoms of hereditary angioedema and early weaning. Eur J Clin Nutr 2010;64:1025–1027. [DOI] [PubMed] [Google Scholar]
- 83. Wahn V, Aberer W, Eberl W, Fasshauer M, Kuhne T, Kurnik K et al. Hereditary angioedema (HAE) in children and adolescents – a consensus on therapeutic strategies. Eur J Pediatr 2012;171:1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zuraw BL, Banerji A, Bernstein JA, Busse PJ, Christiansen SC, Davis‐Lorton M et al. US Hereditary Angioedema Association Medical Advisory Board 2013 recommendations for the management of hereditary angioedema due to C1 inhibitor deficiency. J Allergy Clin Immunol Pract 2013;1:458–467. [DOI] [PubMed] [Google Scholar]
- 85. Betschel S, Badiou J, Binkley K, Hebert J, Kanani A, Keith P et al. Canadian hereditary angioedema guideline. Allergy Asthma Clin Immunol 2014;10:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chagas KDN, Arruk VG, Andrade ME, Vasconcelos DDM, Kirschfink M, Duarte AJ et al. Therapeutic approach of hereditary angioedema. Rev Assoc Med Bras 2004;50:314–319. [DOI] [PubMed] [Google Scholar]
- 87. Church JA. Oxandrolone treatment of childhood hereditary angioedema. Ann Allergy Asthma Immunol 2004;92:377–378. [DOI] [PubMed] [Google Scholar]
- 88. Agostoni A, Marasini B, Cicardi M, Martignoni GC. Intermittent therapy with danazol in hereditary angioedema. Lancet 1978;1:453. [DOI] [PubMed] [Google Scholar]
- 89. Bowen T. Hereditary angioedema: beyond international consensus – circa December 2010 – The Canadian Society of Allergy and Clinical Immunology Dr. David McCourtie Lecture. Allergy Asthma Clin Immunol 2011;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Longhurst HJ, Farkas H, Craig T, Aygoren‐Pursun E, Bethune C, Bjorkander J et al. HAE international home therapy consensus document. Allergy Asthma Clin Immunol 2010;6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Cicardi M, Banerji A, Bracho F, Malbran A, Rosenkranz B, Riedl M et al. Icatibant, a new bradykinin‐receptor antagonist, in hereditary angioedema. N Engl J Med 2010;363:532–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lumry WR, Li HH, Levy RJ, Potter PC, Farkas H, Moldovan D et al. Randomized placebo‐controlled trial of the bradykinin B(2) receptor antagonist icatibant for the treatment of acute attacks of hereditary angioedema: the FAST‐3 trial. Ann Allergy Asthma Immunol 2011;107:529–537. [DOI] [PubMed] [Google Scholar]
- 93. Craig TJ, Rojavin MA, Machnig T, Keinecke HO, Bernstein JA. Effect of time to treatment on response to C1 esterase inhibitor concentrate for hereditary angioedema attacks. Ann Allergy Asthma Immunol 2013;111:211–215. [DOI] [PubMed] [Google Scholar]
- 94. Cicardi M, Bork K, Caballero T, Craig T, Li HH, Longhurst H et al. Evidence‐based recommendations for the therapeutic management of angioedema owing to hereditary C1 inhibitor deficiency: consensus report of an International Working Group. Allergy 2012;67:147–157. [DOI] [PubMed] [Google Scholar]
- 95. Zuraw BL, Busse PJ, White M, Jacobs J, Lumry W, Baker J et al. Nanofiltered C1 inhibitor concentrate for treatment of hereditary angioedema. N Engl J Med 2010;363:513–522. [DOI] [PubMed] [Google Scholar]
- 96. Zuraw B, Cicardi M, Levy RJ, Nuijens JH, Relan A, Visscher S et al. Recombinant human C1‐inhibitor for the treatment of acute angioedema attacks in patients with hereditary angioedema. J Allergy Clin Immunol 2010;126:821–827. [DOI] [PubMed] [Google Scholar]
- 97. Cicardi M, Levy RJ, McNeil DL, Li HH, Sheffer AL, Campion M et al. Ecallantide for the treatment of acute attacks in hereditary angioedema. N Engl J Med 2010;363:523–531. [DOI] [PubMed] [Google Scholar]
- 98. Levy RJ, Lumry WR, McNeil DL, Li HH, Campion M, Horn PT et al. EDEMA4: a phase 3, double‐blind study of subcutaneous ecallantide treatment for acute attacks of hereditary angioedema. Ann Allergy Asthma Immunol 2010;104:523–529. [DOI] [PubMed] [Google Scholar]
- 99. Craig TJ, Levy RJ, Wasserman RL, Bewtra AK, Hurewitz D, Obtulowicz K et al. Efficacy of human C1 esterase inhibitor concentrate compared with placebo in acute hereditary angioedema attacks. J Allergy Clin Immunol 2009;124:801–808. [DOI] [PubMed] [Google Scholar]
- 100. Gompels MM, Lock RJ, Abinun M, Bethune CA, Davies G, Grattan C et al. C1 inhibitor deficiency: consensus document. Clin Exp Immunol 2005;139:379–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Martinez‐Saguer I, Rusicke E, Aygoren‐Pursun E, von Hentig N, Klingebiel T, Kreuz W. Pharmacokinetic analysis of human plasma‐derived pasteurized C1‐inhibitor concentrate in adults and children with hereditary angioedema: a prospective study. Transfusion 2010;50:354–360. [DOI] [PubMed] [Google Scholar]
- 102. Aygoren‐Pursun E, Martinez‐Saguer I, Rusicke E, Klingebiel T, Kreuz W. On demand treatment and home therapy of hereditary angioedema in Germany – the Frankfurt experience. Allergy Asthma Clin Immunol 2010;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wasserman RL, Levy RJ, Bewtra AK, Hurewitz D, Craig TJ, Kiessling PC et al. Prospective study of C1 esterase inhibitor in the treatment of successive acute abdominal and facial hereditary angioedema attacks. Ann Allergy Asthma Immunol 2011;106:62–68. [DOI] [PubMed] [Google Scholar]
- 104. Riedl MA, Hurewitz DS, Levy R, Busse PJ, Fitts D, Kalfus I. Nanofiltered C1 esterase inhibitor (human) for the treatment of acute attacks of hereditary angioedema: an open‐label trial. Ann Allergy Asthma Immunol 2012;108:49–53. [DOI] [PubMed] [Google Scholar]
- 105. Kruger R, Dahlinger N, Magerl M, von Bernuth H, Wahn V. Daily subcutaneous administration of human C1 inhibitor in a child with hereditary angioedema type 1. Pediatr Allergy Immunol 2015;27:223–224. [DOI] [PubMed] [Google Scholar]
- 106. Petraroli A, Squeglia V, Di Paola N, Barbarino A, Bova M, Spano R et al. Home therapy with plasma‐derived C1 inhibitor: a strategy to improve clinical outcomes and costs in hereditary angioedema. Int Arch Allergy Immunol 2015;166:259–266. [DOI] [PubMed] [Google Scholar]
- 107. Farkas H, Csuka D, Zotter Z, Szabo E, Czaller I, Varga L et al. Treatment of attacks with plasma‐derived C1‐inhibitor concentrate in pediatric hereditary angioedema patients. J Allergy Clin Immunol 2013;131:909–911. [DOI] [PubMed] [Google Scholar]
- 108. Craig TJ, Li HH, Riedl M, Bernstein JA, Lumry WR, MacGinnitie AJ et al. Characterization of anaphylaxis after ecallantide treatment of hereditary angioedema attacks. J Allergy Clin Immunol Pract 2015;3:206–212. [DOI] [PubMed] [Google Scholar]
- 109. Li HH, Moldovan D, Bernstein JA, Reshef A, Porebski G, Stobiecki M et al. Recombinant human‐C1 inhibitor is effective and safe for repeat hereditary angioedema attacks. J Allergy Clin Immunol Pract 2015;3:417–423. [DOI] [PubMed] [Google Scholar]
- 110. Strawczynski H, Stachewitsch A, Morgenstern G, Shaw ME. Delivery of care to hemophilic children: home care versus hospitalization. Pediatrics 1973;51:986–991. [PubMed] [Google Scholar]
- 111. Craig TJ. Recent advances in hereditary angioedema self‐administration treatment: summary of an International Hereditary Angioedema Expert Meeting. Int Arch Allergy Immunol 2013;161(Suppl 1):26–27. [DOI] [PubMed] [Google Scholar]
- 112. Cicardi M, Craig TJ, Martinez‐Saguer I, Hebert J, Longhurst HJ. Review of recent guidelines and consensus statements on hereditary angioedema therapy with focus on self‐administration. Int Arch Allergy Immunol 2013;161(Suppl 1):3–9. [DOI] [PubMed] [Google Scholar]
- 113. Riedl M. Hereditary angioedema therapies in the United States: movement toward an international treatment consensus. Clin Ther 2012;34:623–630. [DOI] [PubMed] [Google Scholar]
- 114. Tuong LA, Olivieri K, Craig TJ. Barriers to self‐administered therapy for hereditary angioedema. Allergy Asthma Proc 2014;35:250–254. [DOI] [PubMed] [Google Scholar]
- 115. Fouche AS, Saunders EF, Craig T. Depression and anxiety in patients with hereditary angioedema. Ann Allergy Asthma Immunol 2014;112:371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wang A, Fouche A, Craig TJ. Patients perception of self‐administrated medication in the treatment of hereditary angioedema. Ann Allergy Asthma Immunol 2015;115:120–125. [DOI] [PubMed] [Google Scholar]
- 117. Caballero T, Sala‐Cunill A, Cancian M, Craig TJ, Neri S, Keith PK et al. Current status of implementation of self‐administration training in various regions of Europe, Canada and the USA in the management of hereditary angioedema. Int Arch Allergy Immunol 2013;161(Suppl 1):10–16. [DOI] [PubMed] [Google Scholar]
- 118. Zilberberg MD, Nathanson BH, Jacobsen T, Tillotson G. Descriptive epidemiology of hereditary angioedema emergency department visits in the United States, 2006–2007. Allergy Asthma Proc 2011;32:390–394. [DOI] [PubMed] [Google Scholar]
- 119. Wilson DA, Bork K, Shea EP, Rentz AM, Blaustein MB, Pullman WE. Economic costs associated with acute attacks and long‐term management of hereditary angioedema. Ann Allergy Asthma Immunol 2010;104:314–320. [DOI] [PubMed] [Google Scholar]
- 120. Prior N, Remor E, Gomez‐Traseira C, Lopez‐Serrano C, Cabanas R, Contreras J et al. Development of a disease‐specific quality of life questionnaire for adult patients with hereditary angioedema due to C1 inhibitor deficiency (HAE‐QoL): Spanish multi‐centre research project. Health Qual Life Outcomes 2012;10:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Farkas H, Harmat G, Gyeney L, Fust G, Varga L. Danazol therapy for hereditary angio‐oedema in children. Lancet 1999;354:1031–1032. [DOI] [PubMed] [Google Scholar]
- 122. Bork K, Staubach P, Eckardt AJ, Hardt J. Symptoms, course, and complications of abdominal attacks in hereditary angioedema due to C1 inhibitor deficiency. Am J Gastroenterol 2006;101:619–627. [DOI] [PubMed] [Google Scholar]
- 123. Aygoren‐Pursun E, Bygum A, Beusterien K, Hautamaki E, Sisic Z, Wait S et al. Socioeconomic burden of hereditary angioedema: results from the hereditary angioedema burden of illness study in Europe. Orphanet J Rare Dis 2014;9:99. [DOI] [PMC free article] [PubMed] [Google Scholar]


