Abstract
Aim
This study assessed whether increased amino acid and energy intake in preterm infants during the first week of life was associated with improved neurodevelopment at the corrected age (CA) of 24 months.
Methods
We evaluated preterm infants from two consecutive cohorts in 2004 (Cohort 1) and 2005 (Cohort 2) with different nutritional intakes in the Netherlands. Nutritional intake and growth were recorded until week 5 and after discharge. Neurodevelopment was determined using the Bayley Scales of Infant Development – Second Edition at a CA of 24 months.
Results
Compared to Cohort 1 (n = 56), Cohort 2 (n = 56) received higher nutritional intake during week 1 (p < 0.001). The weight gain in Cohort 2 was higher until week 5, especially among boys (p < 0.002). The mean Mental Developmental Index (MDI) scores did not differ, but Cohort 2 was associated with an increased chance of having an MDI ≥ 85, with an odds ratio of 6.4 and 95% confidence interval (CI) of 1.5–27.4, among all girls with a higher protein intake (5.3, 1.2–23.3). The Psychomotor Developmental Index increased with increasing nutritional intake, especially among boys (β‐coefficient 3.1, 95% CI 0.2–6.0).
Conclusion
Higher nutritional intake was associated with different improvements in growth and neurodevelopment in boys and girls.
Keywords: Gender, Growth velocity, Mental Developmental Index, Protein, Psychomotor Developmental Index
Abbreviations
- BSDI‐II
Bayley Scales of Infant Development – Second Edition
- CA
Corrected age
- CLD
Chronic lung disease
- GA
Gestational age
- HC
Head circumference
- IRDS
Infant respiratory distress syndrome
- MDI
Mental Developmental Index
- PDA
Patent ductus arteriosus
- PDI
Psychomotor Developmental Index
- PN
Parenteral nutrition
- SDS
Standard deviation score
- VLBW infants
Very low birthweight infants
Key notes.
This study assessed whether increased amino acid and energy intake in preterm infants during the first week of life was associated with improved neurodevelopment at the corrected age of 24 months.
We evaluated preterm infants from two consecutive cohorts with different nutritional intakes in the Netherlands – 56 in 2004 and 56 in 2005.
Higher nutritional intake was associated with different improvements in growth and neurodevelopment in boys and girls.
Introduction
In addition to promoting growth similar to intra‐uterine foetal growth, adequate functional development is the main goal of nutritional supplementation for preterm infants 1. Postnatal growth has been associated with neurodevelopmental outcomes in very low birthweight (VLBW) infants 2, 3. In addition to postnatal weight gain, head growth may serve as a predictor of brain growth and therefore of neurodevelopmental outcomes 4. Follow‐up studies of children born preterm have indicated that subnormal head growth predicted poorer intelligence quotient scores at three and eight years of age 5, 6. Poindexter et al. 7 associated low early amino acid intake with increased risks of having a small‐for‐age head circumference (HC) at 18 months of age, specifically in boys.
In a retrospective study, Stephens et al. 8 determined that higher nutritional intake in the first week of life was associated with higher Mental Developmental Index (MDI) scores and related this increase to higher protein and energy intakes. Van den Akker et al. 9 suggested that the effects of nutritional interventions in the first few days following birth may be gender specific. VLBW boys, but not girls, were affected by low amino acid and energy intakes when assessed at a corrected age (CA) of 24 months. Previously, we demonstrated that increased amino acid and energy intakes during the first week of life were associated with a statistically significant short‐term improvement in weight gain in VLBW infants 10. A secondary analysis of these data revealed that this was mainly based on improvement in male infants.
In this study, we investigated the neurodevelopment of the surviving infants of the original cohorts in relation to early nutritional intake. We hypothesised that nutritional supplementation during the first week would not only improve weight gain in the early postnatal period, but would also lead to improved head growth and neurodevelopmental outcomes at a CA of 24 months, specifically in boys.
Methods
This study evaluated the long‐term outcome data of a previously described prospective cohort study that was conducted during two consecutive years, 2004 (Cohort 1) and 2005 (Cohort 2), to evaluate a change in the composition of standard parenteral nutrition (PN) 10. The study was approved by the local ethics committee. The cohort characteristics, the nutritional protocol and its modification and the short‐term results were described in detail in the previous paper 10. The original study included preterm infants born before 34 weeks of gestation who were admitted to our tertiary neonatal intensive care unit at the Radboud university medical center in the Netherlands on the first day of life on the assumption that PN would be needed for at least five days. Infants with major congenital malformations or asphyxia were excluded from the study. During both time periods, no major changes in clinical practice occurred and all infants received nutrition according to standard institutional protocols. The two cohorts primarily differed in PN intake which consisted of standard components, with higher amounts of amino acids and carbohydrates for Cohort 2. According to the new protocol, Cohort 2 achieved full PN two days earlier than Cohort 1, at postnatal day 4 versus 6, respectively 10.
The intake of all nutrients via both PN and enteral feeding, as well as growth characteristics, was recorded daily during the first two weeks, weekly until week 5 and at term‐CA. Based on the information in the patient charts, the mean daily weight gain was calculated weekly for the first five weeks, according to Patel's formula 11.
This study included the surviving participants of the original cohorts who received standard follow‐up care provided for VLBW infants born prior to 32 weeks of gestation or with a birthweight of <1500 g. In accordance with the national follow‐up programme, anthropometric data were recorded at the CAs of six months, 12 and 24 months. The head circumference standard deviation score (HC SDS) was calculated using the Swedish growth reference for preterm infants and the Dutch reference of the nationwide growth study from term‐CA onwards 12, 13. SDSs for weight and length were calculated using the Dutch reference 12. In addition, a neurodevelopmental assessment using the Bayley Scales of Infant Development – Second Edition (BSID‐II) was performed at 24 months of CA, resulting in MDI and Psychomotor Developmental Index (PDI). Scores of 85 or above were categorised as normal outcomes, whereas scores between 70 and 85 reflected moderate impairment and scores below 70 indicated severe impairment. 14 We assumed the diagnosis of normal neurodevelopmental outcome as clinically relevant and therefore dichotomised the MDI and PDI scores with a cut‐off score of ≥85 reflecting normal outcome and score of below 85 reflecting neurodevelopmental impairment.
The original cohort study was powered to assess differences in postnatal growth. As this study only evaluated the surviving infants of these cohorts, no power calculation was carried out. The statistical analyses were performed using IBM SPSS statistics, version 22.0 for Windows (IBM SPSS Inc., Chicago, IL, USA). As the values of the continuous variables were normally distributed, mean differences with 95% confidence intervals (95% CI) were calculated between the two cohorts. For dichotomous data, odds ratios (OR) with 95% CIs were calculated. Subsequently, linear regression analyses were performed to assess the effects on the continuous MDI and PDI scores of infants belonging to Cohort 1 or 2. These analyses were also used for protein and energy intake in week 1, separately and simultaneously, adjusted for a potential confounder set that included gender, gestational age (GA), birthweight, maternal education, infant respiratory distress syndrome (IRDS), days on ventilator, indomethacin treatment and late‐onset sepsis. This confounder set was reduced if the model was unstable or when interpretation issues called for greater precision, by manually excluding those variables that did not change the effect estimate by more than 10% when they were excluded. Logistic regression analyses were performed for the dichotomous outcomes MDI ≥ 85 and PDI ≥ 85. All analyses were performed for the total study population and separately for the subgroups of boys and girls.
Results
The original Cohort 1 comprised 68 infants (38 girls), while Cohort 2 comprised 79 infants (37 girls). The detailed cohort characteristics have previously been reported 10. For this study, we evaluated all 112 surviving children who visited the routine follow‐up at the CA of 24 months. A further six infants had died, two from Cohort 1 and four from Cohort 2, while three and nine infants from the two respective cohorts were not invited, according to the criteria of the follow‐up programme, and 7 and 10 infants did not participate in the 24‐month follow‐up (Fig. 1).
Figure 1.
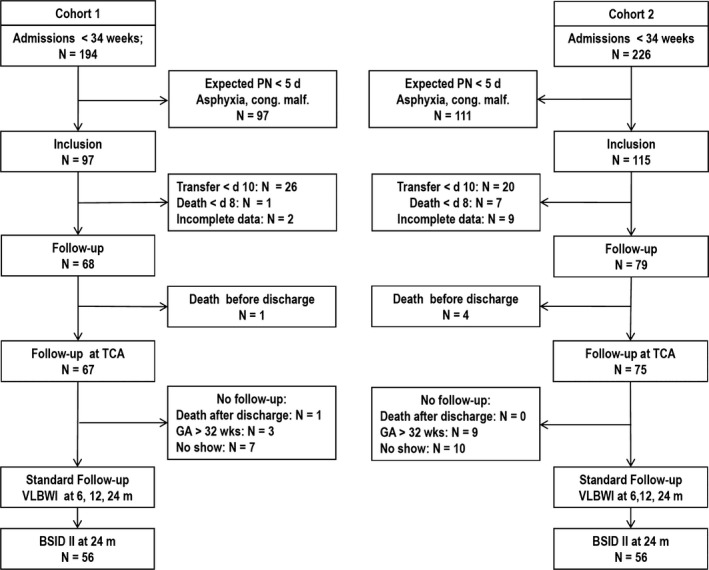
Consort diagram. PN = parenteral nutrition; VLBWI = very low birthweight infants (GA < 32 weeks or birthweight < 1500 g; BSID‐II = Bayley Scales of Infant Development – Second Edition; GA = gestational age.
Cohort characteristics
The cohort characteristics for the participating children, and the potential determinants of the outcomes, are presented in Table 1. Mean GA and birthweight did not differ between the two cohorts, but the duration of mechanical ventilation was 1.4 days shorter for infants in Cohort 2. Concerning nutritional intake, Cohort 2 received 3.3 (95% CI 2.0–4.6) g/kg/week more protein than Cohort 1 and had a 92 (54–131) kcal/kg/week higher energy intake during the first week. These differences were slightly smaller during the second week.
Table 1.
Cohort characteristics and potential determinants of the outcomes
| Cohort 1 (n = 56), mean (SD) | Cohort 2 (n = 56), mean (SD) | Mean difference (C2−C1) | 95% CI | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Gestational age in weeksa | 29.3 (2.2) | 29.6 (1.8) | 0.3 | −0.4 to 1.1 |
| Birthweight in gramsa | 1124 (290) | 1153 (330) | 29 | −87 to 146 |
| Head circumference of week 1 in cm | 26.5 (2.2) | 26.7 (1.8) | −0.3 | −1.1 to 0.6 |
| Days of mechanical ventilationa | 3.8 (4.3) | 2.4 (3.5) | −1.4 | −2.9 to 0.1 |
| Nutritional intake | ||||
| Protein week 1 (g/kg/week) | 14.7 (3.4) | 18.0 (3.6) | 3.3 | 2.0 to 4.6 |
| Protein week 2 (g/kg/week) | 21.1 (5.0) | 22.8 (4.8) | 1.8 | −0.1 to 3.6 |
| Energy week 1 (kcal/kg/week) | 513 (94) | 605 (111) | 92 | 54 to 131 |
| Energy week 2 (kcal/kg/week) | 710 (173) | 777 (165) | 68 | 5 to 131 |
| Cohort 1 (n = 56), number (%) | Cohort 2 (n = 56), number (%) | OR (C1 versus C2) | 95% CI | |
|---|---|---|---|---|
| Potential determinants of the outcomes | ||||
| Gender, femalea | 32 (57) | 27 (48) | 1.4 | 0.7 to 3.0 |
| IUGR | 9 (16) | 11 (20) | 0.8 | 0.3 to 2.1 |
| IRDSa | 38 (68) | 33 (59) | 1.5 | 0.7 to 3.2 |
| CLD | 10 (18) | 6 (11) | 1.8 | 0.6 to 5.4 |
| PDA | 28 (50) | 17 (30) | 2.3 | 1.1 to 5.0 |
| PDA treatmenta | 25 (45) | 12 (21) | 3.0 | 1.3 to 6.8 |
| LOSa | 26 (46) | 26 (46) | 1.0 | 0.5 to 2.1 |
| NEC > 1b | 3 (5) | 5 (9) | 0.6 | 0.1 to 2.5 |
| IVH grade 3/4 | 3 (5) | 1 (2) | 3.1 | 0.3 to 30.9 |
| Maternal educationa low/high (missing) | 10/37 (9) | 8/37 (11) | 1.3 | 0.4 to 3.5 |
C1 = Cohort 1; C2 = Cohort 2; OR = Odds ratio; 95% CI = 95% Confidence interval; IUGR = Intra‐uterine growth retardation defined as a birthweight < 10th percentile; IRDS = Infant respiratory distress syndrome; CLD = Chronic lung disease defined as supplemental oxygen for >28 days; PDA = Patent ductus arteriosus diagnosed by echocardiography; PDA treatment = Including up to three courses of indomethacin and, or, surgical ligation; LOS = Late‐onset sepsis defined by positive blood culture and clinical signs of infection occurring more than 72 hours after birth; NEC = Necrotising enterocolitis defined by Bell's stage > 1b; IVH = Intraventricular haemorrhage; Low maternal education = ≤10 years of education.
Included in potential confounder set in multivariable analyses (IUGR, CLD and PDA not included because of large overlap with other variables and NEC and IVH not included because of small numbers).
Only small differences were seen between the two cohorts with regard to gender, the numbers of infants with prematurity‐related morbidities and maternal education. The only exception was patent ductus arteriosus (PDA), and its treatment, which occurred more frequently among infants in Cohort 1. Independent of cohort status, male infants seemed to have had an increased risk of IRDS and chronic lung disease (CLD) compared to female infants (OR 2.5, 95% CI 1.1–5.6 and 3.1, 1.0–9.6, respectively).
Growth and neurodevelopmental outcomes
The outcome parameters postnatal growth velocity, HC SDSs and BSID‐II scores are presented in Table 2. The infants in Cohort 2 exhibited a higher mean daily weight gain from birth through to week 5 compared to Cohort 1, with a mean difference of 1.8 (95% CI 0.7–3.0) g/kg/day that was mainly due to a 3.1 (1.3–4.8) g/kg/day higher weight gain in male infants. The HC SDS in week one was lower in Cohort 2 than Cohort 1, but at the CA of six months, the HC SDS was normal compared to the Dutch population for both cohorts. This means that infants in Cohort 2 achieved a greater catch‐up growth for HC (0.6 SDS; 0.2–0.9) compared to Cohort 1. At the CA of 24 months, the weight, length and HC were not different between the cohorts and within the normal range compared to the reference population.
Table 2.
Postnatal growth and neurodevelopmental outcomes
| Cohort 1 (n = 56) | Cohort 2 (n = 56) | Mean difference (C2−C1) | 95% CI | |
|---|---|---|---|---|
| Growth velocity from birth through week 5 [g/kg/day; mean (SD)] | ||||
| Total cohort | 9.4 (3.0) | 11.3 (2.9) | 1.8 | 0.7 to 3.0 |
| Girls | 10.0 (2.7) | 10.7 (2.9) | 0.7 | −0.8 to 2.2 |
| Boys | 8.8 (3.4) | 11.8 (2.9) | 3.1 | 1.3 to 4.8 |
| Head circumference [standard deviation score; mean (SD)] | ||||
| Week 1 | −1.0 (1.1) | −1.6 (1.0) | −0.5 | −1.0 to −0.1 |
| Month 6 | −0.1 (1.3) | 0.05 (0.9) | 0.2 | −0.3 to 0.6 |
| Month 6–week 1* | 1.1 (0.7) | 1.7 (0.8) | 0.6 | 0.2 to 0.9 |
| Anthropometry at CA of 24 months [standard deviation score; mean (SD)] | ||||
| Head circumference | −0.2 (1.2) | −0.2 (1.0) | 0.0 | −0.4 to 0.4 |
| Weight | −0.7 (1.0) | −0.6 (0.9) | 0.1 | −0.8 to 0.4 |
| Length | −0.5 (1.0) | −0.4 (0.9) | 0.2 | −0.8 to 0.6 |
| BSID‐II [continuous scores; mean (SD)] | ||||
| MDI total cohort | 98 (13) | 99 (14) | 1.1 | −4.4 to 6.6 |
| Girls | 103 (10) | 103 (12) | −0.4 | −6.4 to 5.5 |
| Boys | 90 (14) | 96 (16) | 6.0 | −3.5 to 15.4 |
| PDI total cohort | 84 (16) | 87 (17) | 3.1 | −3.4 to 9.5 |
| Girls | 88 (16) | 90 (17) | 1.7 | −7.2 to 10.6 |
| Boys | 79 (16) | 84 (17) | 5.7 | −3.6 to 15.0 |
| OR (C 1 versus C 2) | 95% CI | |||
| BSID‐II [dichotomous; number (%)] | ||||
| MDI < 85 (n) | 16 (29) | 9 (16) | 2.1 | 0.9 to 5.4 |
| MDI ≥ 85 (n) | 39 (71) | 47 (84) | ||
| PDI < 85 (n) | 33 (59) | 30 (54) | 1.2 | 0.6 to 2.6 |
| PDI ≥ 85 (n) | 23 (41) | 26 (46) | ||
C1 = Cohort 1; C2 = Cohort 2; OR = Odds ratio; 95% CI = 95% Confidence interval; Month 6–week 1* = Change in SDS from birth until six months of corrected age (CA); BSID‐II = Bayley Scores of Infant Development – Second Edition at 24 months CA 14; MDI = Mental Developmental Index (n = 45/56); PDI = Psychomotor Developmental Index (n = 54/54); MDI and PDI < 85 = Moderately to severely impaired mental or psychomotor development, including not being able to perform the BSID‐II.
Growth velocity (n = 54/54) calculated using the exponential formula described by Patel et al. 11. Head circumference standard deviation score (HC SDS) calculated using the Swedish growth reference for preterm infants and the Dutch reference of the nationwide growth study at six months CA (week 1 n = 45/42; month 6 = 52/51; month 6–week 1 n = 46/40) 12, 13. Weight and length SDSs were calculated using the Dutch reference 12.
In Cohort 1, 10 infants could not be tested for MDI adequately, because they were unable to complete the tests or were easily distracted. As their parents reported concerns regarding their behaviour, and they all had PDI scores of <70, these infants were categorised into the group with an MDI of <85. One infant refused to cooperate with MDI testing and was excluded from the analysis. The mean MDI and PDI scores were not different between the two cohorts, either in the total group or in the subgroups of boys and girls. However, the girls in both cohorts achieved higher scores than the boys, especially in Cohort 1 with a mean difference MDI of 12.7 (95% CI 5.2–20.2), PDI of 9.4 (0.8–18.0). In Cohort 2, the differences between boys and girls were smaller, with a mean difference for MDI of 6.3 (95% CI 1.2–13.8) and 5.5 (−4.1 to 14.9) for PDI.
The children in Cohort 1 also seemed to be at increased risk of having an MDI below 85 (OR 2.1, 95% CI 0.9–5.4) compared to Cohort 2. Subgroup analyses by MDI revealed that children with an MDI below 85 had a lower mean protein intake during the first week of life compared to children with an MDI of ≥85: 14.9 versus 16.8 g/kg/week with a mean difference of 1.8 (95% CI 0.1–3.5). Furthermore, an MDI below 85 seemed to be associated with having had a PDA (OR 1.9, 95% CI 0.8–4.7), IRDS (2.2, 0.8–6.0) or CLD (3.3, 1.1–10.1) and with ≤10 years of maternal education (4.0, 1.3–13.8). For children with a PDI below 85, the same associations were seen but with slightly lower effect estimates: PDI < 85 PDA (OR 1.6, 95% CI 0.8–3.8), IRDS (2.2, 1.0–4.8), CLD (2.6, 0.8–8.8) and maternal education (2.5, 0.8–7.6). Compared to girls, boys seemed to have increased risks of having an MDI below 85 (OR 5.1, 95% CI 1.8–14.0) and a PDI below 85 (1.9, 0.9–4.0).
Effect of early nutrition on neurodevelopmental scores and outcomes
Table 3 presents the results of the linear regression analysis of the effects of early nutritional intake on the continuous neurodevelopmental outcome scores adjusted for confounders. Table 4 presents the results of the logistic regression analysis of the chance to achieve a normal neurodevelopmental outcome in relation to early nutritional intake adjusted for confounders. The different nutritional protocols in Cohorts 1 and Cohort 2 did not influence the mean MDI scores, either in the total study population or in the subgroups of boys and girls. However, a 1 g/kg higher intake of protein adjusted for energy in week one was associated with an increase in the PDI score of 2.4 (95% CI 0.6–4.3), especially among the boys (3.1, 0.2–6.0). This led to an approximately 1.5 higher chance of having a PDI ≥ 85 per g/kg higher protein intake (OR 1.4, 95% CI 1.1–1.8), and this was also more pronounced among the boys (Table 4). Cohort 2 was associated with a markedly higher chance of having an MDI ≥ 85 in the total study population (6.4; 1.5–27.4), as well as for boys and girls separately. Among girls, a positive influence of protein intake on the chance of having an MDI ≥ 85, with or without an adjustment for energy intake, was also observed (5.3, 1.2–23.3 and 2.8, 1.1–6.8, respectively).
Table 3.
Effects of early nutritional intake on neurodevelopmental outcome scores
| MDI scorea | PDI scorea | |||||
|---|---|---|---|---|---|---|
| Crude β | Adjusted β | 95% CI | Crude β | Adjusted β | 95% CI | |
| Total population (n = 112) | ||||||
| Cohort 2 | 1.12 | 2.56 | −2.58 to 7.70 | 3.07 | 1.78 | −4.52 to 8.09 |
|
Protein week 1 and Energy week 1 |
−0.17 and 0.01 |
0.37 and −0.01 |
−1.33 to 2.08 and −0.06 to 0.05 |
2.46 and −0.05 |
2.44
b and −0.06 |
0.55 to 4.32 and −0.12 to 0.00 |
| Protein week 1 | 0.12 | 0.18 | −0.68 to 1.04 | 1.16 | 0.80 | −0.22 to 1.83 |
| Energy week 1 | 0.01 | 0.00 | −0.03 to 0.03 | 0.03 | 0.01 | −0.03 to 0.04 |
| Boys (n = 53) | ||||||
| Cohort 2 | 5.98 | 6.79 | −2.71 to 16.28 | 5.69 | 2.47 | −7.68 to 12.61 |
|
Protein week 1 and Energy week 1 |
0.21 and 0.03 |
0.52 and 0.01 |
−2.41 to 3.44 and −0.10 to 0.11 |
2.98 and −0.05 |
3.08
c and −0.04 |
0.19 to 5.98 and −0.15 to 0.06 |
| Protein week 1 | 0.88 | 0.64 | −0.89 to 2.18 | 1.74 | 1.26 | −0.44 to 2.97 |
| Energy week 1 | 0.03 | 0.02 | −0.34 to 0.08 | 0.05 | 0.02 | −0.04 to 0.08 |
| Girls (n = 59) | ||||||
| Cohort 2 | −0.41 | −0.57 | −6.87 to 5.72 | 1.70 | −0.97 | −10.21 to 8.28 |
|
Protein week 1 and Energy week 1 |
−0.41 and −0.00 |
0.06 and −0.05 |
−2.14 to 2.26 and −0.08 to 0.05 |
2.03 and −0.05 |
1.38 and −0.04 |
−1.67 to 4.42 and −0.13 to 0.05 |
| Protein week 1 | −0.44 | −0.38 | −1.43 to 0.68 | 0.72 | 0.09 | −1.38 to 1.56 |
| Energy week 1 | −0.01 | −0.01 | −0.04 to 0.02 | 0.01 | −0.01 | −0.05 to 0.04 |
MDI = Mental Developmental Index; PDI = Psychomotor Developmental Index; 95% CI = 95% Confidence interval.
Bold numbers = indicative of an effect. Linear regression effect sizes were estimated for four separate situations: (i) being a member of Cohort 2, (ii) the combination of protein and energy intake in week 1, (iii) protein intake in week 1 alone and (iv) energy intake in week 1 alone.
All analyses were adjusted for the potential confounder set: gender, gestational age, birthweight, maternal education, infant respiratory distress syndrome, days on ventilator, indomethacin treatment and late‐onset sepsis, unless otherwise indicated.
Adjusted for gender, maternal education and days on ventilator only.
Adjusted for maternal education only.
Table 4.
Effect of early nutrition on neurodevelopment
| MDI score ≥ 85a | PDI score ≥ 85a | |||||
|---|---|---|---|---|---|---|
| Crude OR | Adjusted OR | 95% CI | Crude OR | Adjusted OR | 95% CI | |
| Total population (n = 112) | ||||||
| Cohort 2 | 2.14 | 6.41 | 1.50 to 27.40 | 1.24 | 1.05 | 0.46 to 2.42 |
|
Protein week 1 and Energy week 1 |
1.12 and 1.00 |
1.16 and 1.00 |
0.80 to 1.69 and 0.99 to 1.01 |
1.34 and 0.99 |
1.38
b and 0.99 |
1.07 to 1.79 and 0.98 to 1.00 |
| Protein week 1 | 1.14 | 1.17 | 0.96 to 1.43 | 1.09 | 1.02 | 0.89 to 1.17 |
| Energy week 1 | 1.00 | 1.00 | 1.00 to 1.01 | 1.00 | 1.00 | 0.99 to 1.00 |
| Boys (n = 53) | ||||||
| Cohort 2 | 2.41 | 6.32 c | 1.03 to 38.88 | 1.71 | 0.73 | 0.17 to 3.2 |
|
Protein week 1 and Energy week 1 |
1.06 and 1.00 |
1.10 and 0.99 |
0.66 to 1.85 and 0.98 to 1.01 |
1.50 and 1.00 |
1.77
d and 0.98 |
0.99 to 3.15 and 0.96 to 1.00 |
| Protein week 1 | 1.11 | 0.92 | 0.70 to 1.21 | 1.16 | 1.00 | 0.79 to 1.26 |
| Energy week 1 | 1.00 | 1.00 | 0.99 to 1.01 | 1.00 | 1.00 | 0.99 to 1.00 |
| Girls (n = 59) | ||||||
| Cohort 2 | 4.82 | 6.10b | 0.58 to 64.50 | 1.08 | 0.80 | 0.25 to 2.57 |
|
Protein week 1 and Energy week 1 |
1.27 and 1.00 |
5.33
e and 0.98 |
1.22 to 23.28 and 0.95 to 1.01 |
1.28 and 0.99 |
1.26 and 0.99 |
0.84 to 1.88 and 0.98 to 1.00 |
| Protein week 1 | 1.23 | 2.75 e | 1.10 to 6.83 | 1.05 | 0.98 | 0.82 to 1.17 |
| Energy week 1 | 1.01 | 1.02 | 1.00 to 1.03 | 1.00 | 1.00 | 0.99 to 1.00 |
MDI = Mental Developmental Index; PDI = Psychomotor Developmental Index; OR = Odds ratio; 95% CI = 95% Confidence interval.
Bold numbers = indicative of an effect. Scores ≥ 85 reflect normal neurodevelopment. Logistic regression odds ratios were analysed for four independent variables (i) being a member of Cohort 2, (ii) the combination of protein and energy intake in week 1, (iii) protein intake in week 1 alone and (iv) energy intake in week 1 alone.
All analyses were adjusted for the potential confounder set: gender, gestational age (GA), birthweight, maternal education, infant respiratory distress syndrome (IRDS), days on ventilator, indomethacin treatment and late‐onset sepsis, unless otherwise indicated.
Adjusted for maternal education only.
Adjusted for GA, birthweight, maternal education, indomethacin treatment and late‐onset sepsis.
§Adjusted for maternal education, IRDS, days on ventilator, indomethacin treatment and late‐onset sepsis.
¶Adjusted for maternal education and days on ventilator only.
Discussion
Our evaluation of two cohorts at the CA of 24 months indicated that infants who received more protein and energy during the first week of life demonstrated a short‐term improvement in postnatal weight gain, especially among male infants, while catch‐up growth in HC seemed to be improved as well. The mean BSID‐II scores were not different between the two cohorts, and the MDI scores did not seem to be influenced by the nutritional protocol. However, we found differences in the subgroups of children, namely boys and girls. Children in Cohort 2 were more likely to have an MDI ≥ 85, which was clearly associated with a higher protein intake among girls, while higher protein intake had a positive effect on the PDI score among boys.
While HC growth has been related to neurodevelopmental outcome, two randomised trials demonstrated that impaired head growth was associated with the persistence of cumulative protein and energy deficits and that postnatal abnormal head growth was ameliorated via the optimisation of nutritional intake, especially parenteral intake 15, 16. The improved catch‐up in HC we saw with improved nutrition was in accordance with the results of the above‐mentioned trials.
A systematic review evaluated the effects of increased nutritional intake in the neonatal period on neurodevelopmental outcomes in VLBW infants, and the results were comparable to our cohorts 17. This review evaluated 15 studies with regard to differences in neurodevelopmental scores and survival without impairment. The relationship between increased nutrition and neurodevelopmental outcomes remained unclear, probably due to the variety of nutritional interventions and neurodevelopmental outcome measures. In contrast, we not only compared the differences between the cohorts, but also evaluated the mental developmental and psychomotor indices in relation to the individual nutritional intake of the first week, adjusting for a number of confounders of neurodevelopment and evaluated subgroups of children. In general, preterm infants have been shown to be at risk for adverse neurodevelopmental outcomes associated with socio‐economic status and gender, as well as with a number of clinical factors that may be related to each other 18. Therefore, it is difficult to evaluate the effect of a single determinant, such as nutritional intake. In our study, for instance, the diagnosis and treatment of PDA seemed to be important risk factors for adverse outcome, but children with a PDA also had a lower protein and energy intake during the first 2 weeks compared to children without PDA. However, by including treatment for PDA in our confounder set, we were able to estimate the effects of protein and energy intake independent of PDA and other determinants of neurodevelopmental outcomes in preterm‐born children. The same holds true for the other relevant confounders included in our analyses. However, some residual confounding by maternal education may still have been present in our results due to the relatively large number of missing values for this variable.
There is no consensus on whether improvements in postnatal weight gain and neurodevelopment are a result of a higher intake of amino acids 2, 3, 19. A Cochrane review found no evidence that high doses of amino acids had a positive effect on neurological outcomes 20. In contrast, van den Akker et al. 9 presented the long‐term outcome results of a randomised trial that evaluated the early administration of amino acids and indicated that the intervention group, especially the male infants who received amino acids immediately after birth, experienced fewer disabilities at the CA of 24 months. Vlaardingerbroek et al. 21 demonstrated that high levels of amino acids and nonprotein energy sources were needed to achieve an anabolic state in VLBW infants. Our findings are in accordance with the latter study because Cohort 2 received not only more amino acids, but also an increase in energy 9.
Gender seemed to be an important factor that determined the consequences of nutritional interventions, both in our study on short‐term growth effects, and in other studies 9, 10, 22, 23, 24. While the general male disadvantage regarding mortality, morbidity and neurodevelopmental outcomes among VLBW infants is well established, the above‐mentioned nutritional intervention studies found that higher intake was specifically advantageous for male infants 25, 26. Male vulnerability in outcomes has been related to higher perinatal testosterone and cortisol concentrations and an impaired adaptive response to stress that may have an influence on brain development 27, 28. Also, studies have demonstrated gender‐specific differences in early brain maturation 29, 30. All of this may indicate different requirements of nutritional intake for male and female infants in the early postnatal period, and this could explain the different effects on neurodevelopmental outcomes for boys and girls that we observed.
In accordance with the study of van den Akker et al. 9, our results indicate that both male and female infants were vulnerable to nutritional deficits, but may have had different needs. Even short‐term interventions may have significant effects for male and female infants, either inducing harm if the nutritional intake is inadequate or preventing poor neurological outcomes if the nutritional needs are met.
In addition to the strengths mentioned above, this study also had some limitations. First, the patients were not randomly assigned to a treatment, but were recruited over two time periods. However, all patients received nutrition according to a standardised protocol, the data were recorded in the same standardised manner, and the results were corrected for disparities in patient characteristics and morbidities. Second, although we improved the nutritional intake in Cohort 2, these infants did still not receive the intake recommended by guidelines issued after the two study periods 1, 10. This factor may have attenuated our results, for both boys and girls. Furthermore, this study was not designed and powered to evaluate gender differences. Nevertheless, our data are consistent with recent studies and thus indicate that it may be necessary to develop different nutritional strategies for specific subgroups of preterm infants.
Conclusion
This study showed that an increase in the intake of amino acids and energy during the first week of life was associated with improved short‐term weight gain, possibly improved catch‐up of head growth and several specific long‐term neurodevelopmental outcomes among preterm‐born boys and girls at the CA of two years. Our findings indicate a need for adequately powered and randomised studies to evaluate gender‐specific nutritional needs in preterm infants.
Conflict of interest
Authors declare no financial activities relevant to this study; JBvG declares financial activities outside the submitted work. He received honoraria from HiPP GmbH & Co and is currently receiving grants from Danone and Mead Johnson Nutrition. He has also received payments for lectures, including service on a speaker's bureau, the Nestle Nutrition Institute, Baxter, Danone and Nutricia Nederland NV. Finally, he has received royalties from Reed Elsevier.
Funding
No funding.
References
- 1. Agostoni C, Buonocore G, Carnielli VP, De CM, Darmaun D, Decsi T, et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr 2010; 50: 85–91. [DOI] [PubMed] [Google Scholar]
- 2. Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006; 117: 1253–61. [DOI] [PubMed] [Google Scholar]
- 3. Franz AR, Pohlandt F, Bode H, Mihatsch WA, Sander S, Kron M, et al. Intrauterine, early neonatal, and postdischarge growth and neurodevelopmental outcome at 5.4 years in extremely preterm infants after intensive neonatal nutritional support. Pediatrics 2009; 123: e101–9. [DOI] [PubMed] [Google Scholar]
- 4. Cheong JL, Hunt RW, Anderson PJ, Howard K, Thompson DK, Wang HX, et al. Head growth in preterm infants: correlation with magnetic resonance imaging and neurodevelopmental outcome. Pediatrics 2008; 121: e1534–40. [DOI] [PubMed] [Google Scholar]
- 5. Hack M, Breslau N, Weissman B, Aram D, Klein N, Borawski E. Effect of very low birth weight and subnormal head size on cognitive abilities at school age. N Engl J Med 1991; 325: 231–7. [DOI] [PubMed] [Google Scholar]
- 6. Cooke RW, Foulder‐Hughes L. Growth impairment in the very preterm and cognitive and motor performance at 7 years. Arch Dis Child 2003; 88: 482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Poindexter BB, Langer JC, Dusick AM, Ehrenkranz RA. Early provision of parenteral amino acids in extremely low birth weight infants: relation to growth and neurodevelopmental outcome. J Pediatr 2006; 148: 300–5. [DOI] [PubMed] [Google Scholar]
- 8. Stephens BE, Walden RV, Gargus RA, Tucker R, McKinley L, Mance M, et al. First‐week protein and energy intakes are associated with 18‐month developmental outcomes in extremely low birth weight infants. Pediatrics 2009; 123: 1337–43. [DOI] [PubMed] [Google Scholar]
- 9. van den Akker CH, te Braake FW, Weisglas‐Kuperus N, van Goudoever JB. Observational outcome results following a randomized controlled trial of early amino acid administration in preterm infants. J Pediatr Gastroenterol Nutr 2014; 59: 714–9. [DOI] [PubMed] [Google Scholar]
- 10. Christmann V, Visser R, Engelkes M, de Grauw A, van Goudoever J, van Heijst A. The enigma to achieve normal postnatal growth in preterm infants – using parenteral or enteral nutrition? Acta Paediatr 2013; 102: 471–9. [DOI] [PubMed] [Google Scholar]
- 11. Patel AL, Engstrom JL, Meier PP, Kimura RE. Accuracy of methods for calculating postnatal growth velocity for extremely low birth weight infants. Pediatrics 2005; 116: 1466–73. [DOI] [PubMed] [Google Scholar]
- 12. Fredriks AM, van Buuren S, Burgmeijer RJ, Meulmeester JF, Beuker RJ, Brugman E, et al. Continuing positive secular growth change in The Netherlands 1955–1997. Pediatr Res 2000; 47: 316–23. [DOI] [PubMed] [Google Scholar]
- 13. Niklasson A, Albertsson‐Wikland K. Continuous growth reference from 24th week of gestation to 24 months by gender. BMC Pediatr 2008; 8: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bayley N. Bayley Scales of Infant Development‐II. 2nd ed San Antonio, TX: Harcourt Brace & Company, 1993. [Google Scholar]
- 15. Tan M, Abernethy L, Cooke R. Improving head growth in preterm infants–a randomised controlled trial II: MRI and developmental outcomes in the first year. Arch Dis Child Fetal Neonatal Ed 2008; 93: F342–6. [DOI] [PubMed] [Google Scholar]
- 16. Morgan C, McGowan P, Herwitker S, Hart AE, Turner MA. Postnatal head growth in preterm infants: a randomized controlled parenteral nutrition study. Pediatrics 2014; 133: e120–8. [DOI] [PubMed] [Google Scholar]
- 17. Chan SH, Johnson MJ, Leaf AA, Vollmer B. Nutrition and neurodevelopmental outcomes in preterm infants: a systematic review. Acta Paediatr 2016; 105: 587–99. [DOI] [PubMed] [Google Scholar]
- 18. Leijon I. Factors of importance for neurodevelopment in preterm infants. Acta Paediatr 2010; 99: 642–4. [DOI] [PubMed] [Google Scholar]
- 19. Sammallahti S, Pyhala R, Lahti M, Lahti J, Pesonen AK, Heinonen K, et al. Infant growth after preterm birth and neurocognitive abilities in young adulthood. J Pediatr 2014; 165: 1109–15.e3. [DOI] [PubMed] [Google Scholar]
- 20. Trivedi A, Sinn JK. Early versus late administration of amino acids in preterm infants receiving parenteral nutrition. Cochrane Database Syst Rev 2013; 7: CD008771. [DOI] [PubMed] [Google Scholar]
- 21. Vlaardingerbroek H, Roelants JA, Rook D, Dorst K, Schierbeek H, Vermes A, et al. Adaptive regulation of amino acid metabolism on early parenteral lipid and high‐dose amino acid administration in VLBW infants – a randomized, controlled trial. Clin Nutr 2014; 33: 982–90. [DOI] [PubMed] [Google Scholar]
- 22. Lucas A, Fewtrell MS, Morley R, Singhal A, Abbott RA, Isaacs E, et al. Randomized trial of nutrient‐enriched formula versus standard formula for postdischarge preterm infants. Pediatrics 2001; 108: 703–11. [DOI] [PubMed] [Google Scholar]
- 23. Isaacs EB, Fischl BR, Quinn BT, Chong WK, Gadian DG, Lucas A. Impact of breast milk on intelligence quotient, brain size, and white matter development. Pediatr Res 2010; 67: 357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frondas‐Chauty A, Simon L, Branger B, Gascoin G, Flamant C, Ancel PY, et al. Early growth and neurodevelopmental outcome in very preterm infants: impact of gender. Arch Dis Child Fetal Neonatal Ed 2014; 99: F366–72. [DOI] [PubMed] [Google Scholar]
- 25. Hintz SR, Kendrick DE, Vohr BR, Kenneth Poole W, Higgins RD. Gender differences in neurodevelopmental outcomes among extremely preterm, extremely‐low‐birthweight infants. Acta Paediatr 2006; 95: 1239–48. [DOI] [PubMed] [Google Scholar]
- 26. Peacock JL, Marston L, Marlow N, Calvert SA, Greenough A. Neonatal and infant outcome in boys and girls born very prematurely. Pediatr Res 2012; 71: 305–10. [DOI] [PubMed] [Google Scholar]
- 27. Cho JI, Carlo WA, Su X, McCormick KL. Associations between salivary testosterone and cortisol levels and neonatal health and growth outcomes. Early Hum Dev 2012; 88: 789–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wainstock T, Shoham‐Vardi I, Glasser S, Anteby E, Lerner‐Geva L. Fetal sex modifies effects of prenatal stress exposure and adverse birth outcomes. Stress 2015; 18: 49–56. [DOI] [PubMed] [Google Scholar]
- 29. Vasileiadis GT, Thompson RT, Han VK, Gelman N. Females follow a more “compact” early human brain development model than males. A case‐control study of preterm neonates. Pediatr Res 2009; 66: 551–5. [DOI] [PubMed] [Google Scholar]
- 30. Skiold B, Alexandrou G, Padilla N, Blennow M, Vollmer B, Aden U. Sex differences in outcome and associations with neonatal brain morphology in extremely preterm children. J Pediatr 2014; 164: 1012–8. [DOI] [PubMed] [Google Scholar]


