Summary
Background
Overt or subclinical hypothyroidism is a common finding in adult populations affected by non‐alcoholic fatty liver disease (NAFLD). Currently, there are only sparse data available on the association of thyroid dysfunction and NAFLD in obese children and adolescents.
Objective
The study aims to investigate the association of thyroid function test values with NAFLD and metabolic risk factors in a population of obese children and adolescents.
Methods
A total of 332 overweight and obese children and adolescents (170 girls) aged between 10 and 19 years were analysed. Subjects underwent ultrasound examination of the liver. Thyroid function was evaluated by laboratory determination of thyroid‐stimulating hormone (TSH), total triiodothyronine (T3) and total thyroxine levels. All included subjects were either euthyroid or had subclinical hypothyroidism (TSH > 4 μU mL−1, normal thyroxine). Further metabolic profiling included the determination of lipid status, insulin and liver function tests. Anthropometric parameters body mass index, waist and hip circumference were documented.
Results
The prevalence of hepatic steatosis was 29.8%. Subjects with NAFLD had significantly higher TSH levels than those without (p = 0.0007). After dividing TSH values into quartiles, both univariate and multivariate analyses (adjusted for age, body mass index–standard deviation scores and stage of puberty) showed a significant association with hepatic steatosis (p < 0.05).
Conclusion
Taking possible variables into consideration, our results show that there is a significant association between hepatic steatosis and the TSH levels in obese children and adolescents. Mild thyroid dysfunction may therefore have a role in determining an unfavourable metabolic profile in obese children and adolescents.
Keywords: Hypothyroidism, NAFLD, paediatric, thyroid dysfunction
Introduction
The increasing prevalence of overweight and obesity means that non‐alcoholic fatty liver disease (NAFLD), with an estimated prevalence of 20–30%. Rising prevalence rates of NAFLD go hand‐in‐hand with marked increases in obesity prevalence in childhood and adolescence over the past decades 1.
Available epidemiologic data from Europe demonstrates that NAFLD in childhood and adolescence is a problem of global scale 2. In the USA, data from the population‐based National Health and Nutrition Examination Survey III show that approximately 3% of adolescents are affected by elevated alanine aminotransferase (ALT) serum levels 3. In an autopsy study, Schwimmer et al. reported an average prevalence of steatosis hepatis of 9.6% in children and adolescents in the USA, which increased with age from 0.7% in the preschool age group to 17.3% in older adolescents 4. Main predictors for NAFLD in childhood and adolescence identified in these studies comprised obesity, older age and male gender. The central role of overweight and obesity as risk factor for paediatric NAFLD is underscored by cohort studies in obese children and adolescents reporting prevalence rates ranging from 10% to 77% 2, 5, 6.
The spectrum of disease ranges from simple steatosis (fatty liver) through nonalcoholic steatohepatitis (NASH) to cirrhosis of the liver. While the prognosis for simple steatosis is favourable, with a benign course, steatohepatitis tends to progress, or may even result in hepatic malignancy 1, 2, 3, 4, 5, 6, 7.
As the development of NAFLD and NASH may play a pivotal role in determining the risk for type 2 diabetes and associated metabolic disease, there has been considerable scientific interest in identifying potential risk factors and surrogate markers for NAFLD in recent years 8. Thyroid hormone status is a key regulator of energy metabolism, while adverse alterations of body composition, lipid status, cardiac function/blood pressure and various non‐traditional cardiovascular risk factors are associated with various degrees of thyroid dysfunction, ranging from subclinical to overt hypothyroidism 9. Elevated levels of thyroid‐stimulating hormone (TSH) are a common finding in populations of obese children and adolescents 10. At present, it remains less clear as to whether the frequently observed hyperthyrotropinemia is a secondary consequence of obesity or whether it has an independent role in the pathogenesis of obesity, dyslipidemia 11 and insulin resistance 12. Interestingly, hypothyroidism and NAFLD are characterized by very similar metabolic features, particularly with regard to dyslipidemia, decreased fatty acid oxidation, increased hepatic lipid peroxidation 13 and insulin resistance 14. It therefore seems plausible to hypothesize a role for thyroid dysfunction in the pathogenesis of NAFLD 15. A few studies have recently shown a relationship between altered thyroid function test values and NAFLD in both adults 16 and children 17, 18.
In a retrospective study of 332 overweight or obese children and adolescents, our aim was to address the hypothesis that there is an association, independent of possible confounders, between thyroid hormone levels and the risk of occurrence and severity (as determined by ultrasound) of NAFLD in obese children and adolescents.
Methods
Study population
In a prospective cross‐sectional study carried out at the Center for Pediatric and Adolescent Medicine, Department of Internal Medicine I, University of Ulm, and the Hochried Hospital between February 2000 and Mai 2001, data were collected from 617 children and adolescents who were participating in a weight reduction programme at the Hochried Pediatric Medical Center in Murnau. Inclusion criteria for the present study were age between 8 and 20 years, informed consent of the patient or parent or guardian and a fasting period of at least 5 h prior to testing. Exclusion criteria were long‐term medication (including L‐thyroxine, iodide or thyrostatic agents), a history of increased regular alcohol consumption and a lack of lab test results or anthropometric parameters in the database. Out of the total of n = 617 children and adolescents in the database, 332 patients were included in the study after applying these criteria. Subjects who had missing data (n = 144), invalid laboratory values (n = 2), regularly drank alcohol (n = 17), were taking regular medication (including thyroid drugs) (n = 141) or whose age was outside the defined range (n = 30) were excluded. Patients with elevated ALT, aspartate aminotransferase (AST) or gamma‐glutamyltransferase (GGT) levels were screened of hepatitis B, hepatitis C and haemochromatosis. The screening tests were negative in all subjects. The study was approved by the Ethics Committee of the Faculty of Medicine, University of Ulm.
Investigation
The history of each subject was checked for age, gender, nationality, previous illnesses, regular medication, diets, smoking and alcohol consumption.
Physical data that were extracted included the body weight in kilogrammes (kg) (fasting, wearing only underclothes) and height in centimetres (cm) (standing, without shoes), corrected in each case to one decimal place; blood pressure in millimetres of mercury (mmHg) (three measurements were taken when the patient was sitting down, with the mean calculated from the last two measurements) and abdominal girth in centimetres measured in the horizontal plane at the waist and hips. The values obtained were used to calculate the waist‐to‐hip ratio, body mass index (BMI) and the BMI–standard deviation scores (BMI‐SDS) based on German reference values 19 using Cole's least mean square (LMS) method 20. The stage of puberty (P1–P5) was determined in accordance with Tanner's scale 21, and the subjects were divided into a prepubertal group (Tanner 1), an intrapubertal group (Tanner 2–3) and a postpubertal group (Tanner 4–5).
After a 5‐h fasting period, ultrasound scanning of the liver in three planes was performed by a single experienced examiner (>50 000 examinations). The subject lay supine with the right arm above the head. Scans were performed in deep inspiration and with a relaxed abdominal wall. A Versa Plus scanner (Siemens, Erlangen, Germany) equipped with a 3.5 MHz multi‐frequency convex array probe was used for the examinations. Venous (20 mL) and capillary (50 μL) blood samples were taken after the subject had not eaten for 12 h. Fasting blood glucose was measured in the capillary sample. An LP 700 analyser was used to determine glucose by GOD‐PAP (glucose oxidase ‐ phenol aminophenazone), cholesterol and high‐density lipoprotein with CHOD‐PAP (cholesterol oxidase ‐ phenol aminophenazone), low‐density lipoprotein from the total cholesterol value as well as cholesterol by a precipitation method and triglycerides with GPO‐PAP (glycerol‐3‐phosphate oxidase ‐ phenol aminophenazone). Insulin was measured with a double‐antibody radioimmunoassay. AST, ALT and GGT were determined with the Dimension RxL system (Dade Behring, Eschborn, Germany) 2using standard methods for transaminases (DuPont, Delaware, USA). The reference ranges were as follows: ALT, 0–23 U L−1 (men) and 0–19 U L−1 (women); AST, 0–19 U L−1 (men) and 0–15 U L−1 (women); GGT, up to 14 years of age <17 U L−1 and over 14 years of age <23 U L−1 (men) and <19 U L−1 (women). The thyroid parameters, TSH and triiodothyronine (T3), were assayed with an electrochemiluminescence immunoassay and the E2010 (Roche Diagnostics, Mannheim, Germany). Thyroxine (T4) was measured by the CEDIA T4 test (homogeneous enzyme immunotest) on a Hitachi 917.
Diagnostic criteria
The diagnosis of hepatic steatosis was based on the criteria defined by Saverymuttu et al. and the severity divided into ‘mild’ (grade 1), ‘moderate’ (grade 2) and ‘severe’ (grade 3) depending on the degree of fatty infiltration 22. Subclinical hypothyroidism was defined as a raised TSH (>4 μU mL−1) and peripheral euthyroidism (T4 normal).
Statistical analysis
All statistical analyses were carried out with the statistic software sas 9.2 (SAS Institute Inc., Cary, North Carolina, USA). First of all, a descriptive evaluation was performed. The mean and standard deviation were calculated for continuous variables and the absolute and relative frequencies for categorical data. To determine differences between the groups with and without NAFLD, the Wilcoxon rank‐sum test was applied for continuous variables and the chi‐squared test for categorical variables, unless the numbers were too small, when Fischer's exact test was used. TSH values were divided into four equal groups (quartiles) for the univariate and multivariate analyses. The quartiles were as follows: Q1: TSH ≤ 1.72 μU mL−1, Q2: TSH 1.73–2.30 μU mL−1, Q3: TSH 2.31–3.22 μU mL−1, Q4: TSH ≥ 3.23 μU mL−1; Q1: T3 ≤ 1.42 nmol L−1, Q2: T3 1.43–1.61 nmol L−1, Q3: T3 1.62–1.84 nmol L−1, Q4: T3 ≥ 1.85 nmol L−1; Q1: T4 ≤ 7.07 nmol L−1, Q2: T4 7.08–8.00 nmol L−1, Q3: T4 8.01–9.01 nmol L−1, Q4: T4 ≥ 9.02 nmol L−1. The TSH quartiles were first of all checked by univariate analysis for a relationship with hepatic steatosis. Then, in a multivariate logistic regression analysis, the association between hepatic steatosis and TSH (quartiles) adjusted for age, BMI‐SDS and stage of puberty was examined. In further regression models, the liver function tests (ALT, AST and GGT) and the homeostatic model assessment (HOMA) index were additionally introduced separately into the model, and the relationship between hepatic steatosis and the TSH quartiles investigated after inclusion of the liver function tests and adjustment for age, BMI‐SDS and stage of puberty. All tests were two‐tailed. The level of significance was set at α = 5%. The p‐value was given to four decimal places, and the odds ratio (OR) and 95% confidence interval were corrected to three decimal places.
Results
Descriptive data analysis
Data from 332 children and adolescents (48.8% male and 51.2% female) were evaluated. The mean age of the population was 14.0 ± 1.8 years, whereby the youngest subject was 10 and the oldest 19. Tanner's scores for the stage of puberty revealed 56 prepubertal subjects, 155 subjects in puberty and 121 who were postpubertal. The prepubertal and postpubertal groups showed an unequal gender distribution with more prepubertal boys (m = 83.9%; f = 16.1%) and postpubertal girls (m = 24.0%; f = 76.0%). A diagnosis of subclinical hypothyroidism was made in 35 subjects (10.5%). Overt hypothyroidism or hyperthyroidism was not found in any of the subjects.
Hepatic steatosis was demonstrated in 99 (29.8%) subjects (66.7% male and 33.3% female). Depending on the diagnosis, the subjects were divided into two groups, and a comparison was made between those with NAFLD and those without (Table 1). The NAFLD group had significantly higher values of both BMI‐SDS (p < 0.0001) and waist‐to‐hip ratio (p < 0.0001). Liver function tests results for ALT, AST and GGT (p < 0.0001) were also significantly higher in the NAFLD group than in the subjects without fatty liver changes. The HOMA‐IR (insulin resistance) was also significantly higher (p < 0.0001), as was the systolic blood pressure (p = 0.0012) (Table 1). Taking the thyroid function tests into account, the only significant difference was found for TSH: the mean of 2.8 ± 1.1 mIU L−1 in the NAFLD group was significantly higher than the level measured in the control group (p = 0.0007). On the other hand, T3 and T4 levels were not significantly different in the two groups. Considering the thyroid function tests in relation to the degree of hepatic steatosis diagnosed on ultrasound, there was a significant rise in TSH with an increasing degree of steatosis (p = 0.0027). Such a difference could not be demonstrated for either T3 or T4 (p = 0.3475, p = 0.6203).
Table 1.
Characteristics of the study population
| Parameter | Subjects without NAFLD (n = 233) mean ± SD (range) | Subjects with NAFLD (n = 99) mean ± SD (range) | Total (n = 332) | p‐value |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Male | 96 (41.2%) | 66 (66.7%) | 162 (48.8%) | <0.0001 |
| Female | 137 (58.8%) | 33 (33.3%) | 170 (51.2%) | |
| Age (years) | 13.9 ± 1.8 (10.1–19.5) | 14.1 ± 1.9 (10.3–18.3) | 14.0 ± 1.8 (10.1–19.5) | 0.4997 |
| BMI‐SDS | 2.5 ± 0.5 (1.2–4.6) | 2.8 ± 0.4 (1.8–3.8) | 2.6 ± 0.5 (1.2–4.6) | <0.0001 |
| WHR (m) | 0.9 ± 0.1 (0.7–2.1) | 1.0 ± 0.1 (0.8–1.2) | 0.9 ± 0.1 (0.7–2.1) | <0.0001 |
| AST (U L−1) | 12.7 ± 3.2 (5.1–28.1) | 18.3 ± 8.6 (8.2–64.0) | 14.4 ± 6.0 (5.1–64.0) | <0.0001 |
| ALT (U L−1) | 11.4 ± 4.2 (6.1–37.5) | 21.3 ± 15.4 (5.4–112.6) | 14.3 ± 10.1 (5.4–112.6) | <0.0001 |
| GGT (U L−1) | 12.3 ± 3.8 (2.2–31.9) | 18.8 ± 11.9 (6.8–86.6) | 14.2 ± 7.8 (2.2–86.6) | <0.0001 |
| LDL (mg dL−1) | 118.7 ± 32.8 (38.0–225.0) | 125.8 ± 33.0 (47.0–217.0) | 120.8 ± 33.0 (38.0–225.0) | 0.0751 |
| HDL (mg dL−1) | 46.1 ± 8.9 (26.7–79.7) | 45.0 ± 8.7 (29.1–69.5) | 45.8 ± 8.8 (26.7–79.7) | 0.3444 |
| Cholesterol (mg dL−1) | 185.1 ± 32.3 (106.0–289.0) | 195.1 ± 34.8 (115.0–277.0) | 188.1 ± 33.3 (106.0–289.0) | 0.0179 |
| Triglycerides (mg dL−1) | 98.0 ± 46.2 (20.8–338.0) | 130.1 ± 67.8 (32.1–432.0) | 107.6 ± 55.5 (20.8–432.0) | <0.0001 |
| Insulin | 15.1 ± 8.1 (3.2–59.5) | 23.0 ± 14.2 (5.4–64.2) | 17.5 ± 10.9 (3.2–64.2) | <0.0001 |
| Leptin | 31.1 ± 18.9 (1.5–117.4) | 33.7 ± 17.5 (6.3–92.8) | 31.9 ± 18.5 (1.5–117.4) | 0.0887 |
| HOMA | 3.2 ± 1.9 (0.5–13.2) | 5.1 ± 3.6 (1.1–21.2) | 3.8 ± 2.7 (0.5–21.2) | <0.0001 |
| Blood pressure | ||||
| Systolic | 125.5 ± 12.0 (100.0–160.0) | 129.3 ± 10.9 (105.0–160.0) | 126.6 ± 11.8 (100.0–160.0) | 0.0012 |
| Diastolic | 76.4 ± 8.9 (60.0–115.0) | 78.7 ± 10.6 (60.0–120.0) | 77.1 ± 9.5 (60.0–120.0) | 0.0577 |
| T3 (nmol L−1) | 1.6 ± 0.3 (0.9–2.8) | 1.7 ± 0.4 (0.9–2.7) | 1.6 ± 0.3 (0.9–2.8) | 0.2380 |
| T4 (µg dL−1) | 8.0 ± 1.4 (4.1–13.3) | 8.0 ± 1.4 (3.7–11.2) | 8.0 ± 1.4 (3.7–13.3) | 0.8709 |
| TSH (μU mL−1) | 2.5 ± 1.4 (0.5–11.5) | 2.8 ± 1.1 (0.7–6.7) | 2.6 ± 1.3 (0.5–11.5) | 0.0007 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; GGT, gamma‐glutamyltransferase; HDL, high density lipoprotein; HOMA, homeostasis model assessment; LDL, low density lipoprotein; NAFLD, non‐alcoholic fatty liver disease; SD, standard deviation; SDS, standard deviation scores; T3, triiodothyronine; T4, thyroxine; TSH, thyroid‐stimulating hormone; WHR, waist‐to‐hip ratio.
In the in‐depth analysis, the results of the thyroid function tests were subdivided into four equal groups (quartiles), and the corresponding prevalence of NAFLD determined. Here, an increasing prevalence of NAFLD could be seen with increasing TSH values in the first three quartiles (Fig. 1). Univariate analysis showed a significant association of TSH with hepatic steatosis (p = 0.0023). After adjustment for age, BMI‐SDS and stage of puberty (prepubertal, intrapubertal and postpubertal), TSH also showed a significant association with hepatic steatosis (p = 0.0020). The association of rising TSH levels with hepatic steatosis also remained significant when ALT (p = 0.0193) or GGT (p = 0.0431) was included in the regression model, but the p‐value was just above the level of significance when other lab tests (AST, HOMA index) were included (Table 2).
Figure 1.
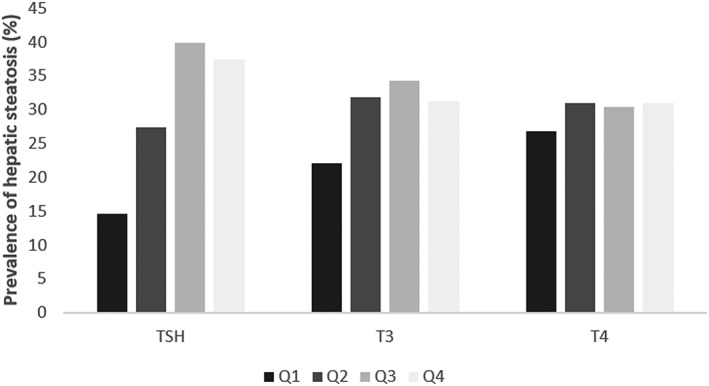
Prevalence of hepatic steatosis in relation to thyroid function in the present study. Q = quartile.
Table 2.
Logistic regression analysis of the relationship between hepatic steatosis and the TSH quartiles in various models
| Total population (n = 332) | ||
|---|---|---|
| Variable | OR (95% CI) | p‐value |
| TSH* | ||
| Q 1 | Reference | |
| Q 2 | 2.199 (1.010–4.787) | |
| Q 3 | 3.850 (1.812–8.181) | 0.0023 |
| Q 4 | 3.477 (1.631–7.412) | |
| TSH** | ||
| Q 1 | Reference | |
| Q 2 | 2.125 (0.956–4.724) | |
| Q 3 | 3.431 (1.576–7.470) | 0.0020 |
| Q 4 | 3.219 (1.474–7.028) | |
| TSH** | ||
| Q 1 | Reference | |
| Q 2 | 2.203 (0.907–5.355) | |
| Q 3 | 3.372 (1.414–8.041) | 0.0472 |
| Q 4 | 2.700 (1.140–6.392) | |
| AST | ||
| Q 1 | Reference | |
| Q 2 | 1.266 (0.493–3.255) | |
| Q 3 | 2.177 (0.899–5.273) | <0.0001 |
| Q 4 | 10.674 (4.544–25.075) | |
| TSH** | ||
| Q 1 | Reference | |
| Q 2 | 2.542 (1.034–6.251) | |
| Q 3 | 3.681 (1.532–8.845) | 0.0193 |
| Q 4 | 3.527 (1.462–8.505) | |
| ALT | ||
| Q 1 | Reference | |
| Q 2 | 1.455 (0.528–4.007) | |
| Q 3 | 3.626 (1.406–9.353) | <0.0001 |
| Q 4 | 16.392 (6.428–41.800) | |
| TSH** | ||
| Q 1 | Reference | |
| Q 2 | 2.167 (0.930–5.051) | |
| Q 3 | 3.137 (1.375–7.158) | 0.0431 |
| Q 4 | 2.796 (1.212–6.452) | |
| GGT | ||
| Q 1 | Reference | |
| Q 2 | 1.489 (0.637–3.484) | <0.0001 |
| Q 3 | 1.535 (0.660–3.568) | |
| Q 4 | 7.053 (3.104–16.027) | |
| TSH** | ||
| Q 1 | Reference | |
| Q 2 | 1.829 (0.805–4.157) | |
| Q 3 | 2.828 (1.270–6.300) | 0.0731 |
| Q 4 | 2.349 (1.041–5.303) | |
| HOMA index | ||
| Q 1 | Reference | |
| Q 2 | 1.393 (0.617–3.142) | |
| Q 3 | 1.417 (0.619–3.244) | 0.0002 |
| Q 4 | 4.788 (2.107–10.884) | |
Univariate.
Regression model adjusted for age, BMI‐SDS and stage of puberty.
AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index; CI, confidence interval; GGT, gamma‐glutamyltransferase; HOMA, homeostasis model assessment; OR, odds ratio; WHR, waist‐to‐hip ratio.
Significant values are highlighted in bold.
Discussion
The study presented here shows a significant relationship between increased TSH levels in euthyroid severely obese children and adolescents and the occurrence of hepatic steatosis, independent of age, gender, stage of puberty, BMI‐SDS and other risk markers for NAFLD. Higher TSH levels are associated not only with a higher risk of NAFLD, but also with a greater degree of fatty infiltration found on ultrasound scanning. The results of our study emphasize the possible significance of subclinical hypothyroidism as a predictor of metabolic comorbidity in obese children and adolescents.
Our study has certain limitations. The cross‐sectional design allows the investigation of association, but not causality. Furthermore, the children in our study were not a population‐based paediatric group, but hospital patients. It is therefore not possible to extrapolate our results to the general paediatric population. In addition, the diagnosis of NAFLD was made on the basis of ultrasound scans and not confirmed by histology. However, various studies have shown a high sensitivity (85%) and specificity (94%) for ultrasound in the diagnostic investigation of hepatic steatosis 23. A good correlation between the steatosis score determined on ultrasound and the histological grade of steatosis is also found in children 24.
Thyroid hormone status has a considerable effect on energy metabolism as well as on the cardiovascular system. Patients with overt hypothyroidism have unfavourable changes in the classical cardiovascular risk factors, in particular, clearly elevated total cholesterol and low‐density lipoprotein cholesterol levels 25. The possible relationship between subclinical hypothyroidism and increased cardiovascular morbidity and mortality is still a controversial subject of debate. However, recent meta‐analyses of epidemiological studies provide evidence that subclinical hypothyroidism in adult patients is associated with an increased risk of coronary heart disease and increased mortality 9. In contrast, there is little data on a possible relationship between subclinical hypothyroidism and NAFLD, although it would seem likely, because patients with overt hypothyroidism often have fatty infiltration of the liver 15. Metabolic changes in hypothyroidism have clear similarities with key metabolic features of NAFLD or NASH, namely, reduced beta‐oxidation of fatty acids, increased peroxidation of lipids, which is an important source of oxidative stress in liver tissue and a cause of cellular injury 13 and, in particular, insulin resistance 14. Furthermore, alterations in leptin‐signalling or FGF‐21‐signalling pathways may provide pathophysiological linkage of hypothyroidism and NAFLD (reviewed in 26). For example, increased levels of leptin have been observed in hypothyroidism, which may promote hepatic insulin resistance and also hepatic collagen synthesis 27.
In a large‐scale study on more than 10 000 adult Caucasian patients, Targher et al. showed a progressive increase in AST, ALT and GGT with rising TSH levels, independent of age, gender, lipid status and fasting glucose 28. A Chinese study on similarly large population of adult patients came to contradictory conclusions 16. In their cross‐sectional study, however, Chung et al. investigated 4648 subjects, of whom 2324 were euthyroid and 2324 had hypothyroidism. They showed here an association between hepatic steatosis and hypothyroidism or TSH levels 16.
To our knowledge, there are currently only three studies that have looked at a possible relationship between thyroid dysfunction and NAFLD in children and adolescents 17, 18, 29. A cross‐sectional study by Torun et al. with 109 obese subjects aged between 9 and 15 years showed a significant correlation of rising TSH levels with an increasing degree of hepatic steatosis, as determined on ultrasound scanning 18.
The present study confirms the findings of Torun et al. 18 in a large‐scale, well‐characterized population of central European children and adolescents and also shows the association between rising TSH levels and the ultrasound grade of fatty liver to be independent of possible confounders. It must be emphasized that the association of TSH with the severity of fatty liver described in our study can be demonstrated in children and adolescents who are euthyroid with respect to T3 and T4 as well as in those who are euthyroid to TSH. In addition, there was no change in the T3/T4 ratio in our study, unlike that found in other smaller studies on obese children and adolescents with NAFLD 29.
In a study population of 402 overweight or obese children and adolescents aged between 6 and 16 years, Pacifico et al. showed that an elevated TSH >4 mIU L−1 with euthyroid metabolism is associated with hepatic steatosis, giving significantly higher ORs of 2.10 without raised ALT levels and of 2.42 when ALT was also raised 17. Using the same arbitrary cut‐off for TSH (>4 mIU L−1) as in Pacifico's study and adjusting for the same possible confounders, the OR for the presence of steatosis was not statistically significant in our study population (data not shown). One possible reason for this finding may be the different distribution of the TSH levels and the different number of subjects with TSH > 4 mIU L−1 in the two study populations. Only 10.2% of the children and adolescents in our study had TSH levels above 4 mIU L−1, while twice as many had TSH levels > 4 mIU L−1 in the study by Pacifico et al. As our results clearly show, the relationship between rising TSH levels and the probability/odds of having hepatic steatosis seems to be effective not only on the threshold or outside the normal age‐related range, but even more so already within the normal range for TSH (and T3 and T4). Obese children and adolescents with a TSH level of 1.73–2.30 μU mL−1 (in Q2) have twice the probability of hepatic steatosis than children with peripheral euthyroidism and a TSH level ≤ 1.72 μU mL−1 (Q1). Adjusted for age, stage of puberty and BMI‐SDS, the probability of hepatic steatosis again increases when the TSH level reaches the next quartile (TSH 2.31–3.22 μU mL−1) but does not increase any further. This finding suggests that discreet elevations of TSH could be a previously overlooked sign of NAFLD and supports the idea that subclinical disorders of thyroid function are also associated with an unfavourable metabolic risk profile in children and adolescents 11.
As the pathogenesis of NAFLD is not yet completely understood 1 and the disease is associated with increased morbidity and mortality in children as well as adults 30, the identification of other relevant factors is extremely important to allow causal treatment and prevent sequelae. Given the current database, the lack of comparability between studies and the differences in methodology, further large‐scale studies are needed to evaluate TSH as an independent risk factor for NAFLD.
Conflict of Interest Statement
All authors have no conflicts of interest to declare.
Author contributions
T. E.‐M. K., T. G. and C. D. wrote the manuscript. D. H., W. K., M. W., C. D. were involved in the design and conduction of the study. S. O. and D. H. collected and analysed the data. S. O., D. H., W. K., M. W. and C. D. were involved in data interpretation and manuscript writing. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by grants from the German Federal Ministry for Education and Research (BMBF, project funding reference number 01GI1120A).
Kaltenbach, T. E.‐M. , Graeter, T. , Oeztuerk, S. , Holzner, D. , Kratzer, W. , Wabitsch, M. , and Denzer, C. (2017) Thyroid dysfunction and hepatic steatosis in overweight children and adolescents. Pediatric Obesity, 12: 67–74. doi: 10.1111/ijpo.12110.
References
- 1. Nobili V, Alkhouri N, Alisi A, et al. Nonalcoholic fatty liver disease: a challenge for pediatricians. JAMA Pediatr 2015; 169: 170–176. [DOI] [PubMed] [Google Scholar]
- 2. Wiegand S, Keller KM, Robl M, et al. Obese boys at increased risk for nonalcoholic liver disease: evaluation of 16,390 overweight or obese children and adolescents. Int J Obes (Lond) 2010; 34: 1468–1474. [DOI] [PubMed] [Google Scholar]
- 3. Strauss RS, Barlow SE, Dietz WH. Prevalence of abnormal serum aminotransferase values in overweight and obese adolescents. J Pediatr 2000; 136: 727–733. [PubMed] [Google Scholar]
- 4. Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics 2006; 118: 1388–1393. [DOI] [PubMed] [Google Scholar]
- 5. Burgert TS, Taksali SE, Dziura J, et al. Alanine aminotransferase levels and fatty liver in childhood obesity: associations with insulin resistance, adiponectin, and visceral fat. J Clin Endocrinol Metab 2006; 91: 4287–4294. [DOI] [PubMed] [Google Scholar]
- 6. Denzer C, Thiere D, Muche R, et al. Gender‐specific prevalences of fatty liver in obese children and adolescents: roles of body fat distribution, sex steroids, and insulin resistance. J Clin Endocrinol Metab 2009; 94: 3872–3881. [DOI] [PubMed] [Google Scholar]
- 7. Nobili V, Alisi A, Grimaldi C, et al. Non‐alcoholic fatty liver disease and hepatocellular carcinoma in a 7‐year‐old obese boy: coincidence or comorbidity? Pediatr Obes 2014; 9: e99–e102. [DOI] [PubMed] [Google Scholar]
- 8. Zhang HX, Xu XQ, Fu JF, Lai C, Chen XF. Predicting hepatic steatosis and liver fat content in obese children based on biochemical parameters and anthropometry. Pediatr Obes 2015; 10: 112–117. [DOI] [PubMed] [Google Scholar]
- 9. Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 2010; 304: 1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reinehr T. Thyroid function in the nutritionally obese child and adolescent. Curr Opin Pediatr 2011; 23: 415–420. [DOI] [PubMed] [Google Scholar]
- 11. Denzer C, Karges B, Nake A, et al. Subclinical hypothyroidism and dyslipidemia in children and adolescents with type 1 diabetes mellitus. Eur J Endocrinol 2013; 168: 601–608. [DOI] [PubMed] [Google Scholar]
- 12. Brufani C, Manco M, Nobili V, Fintini D, Barbetti F, Cappa M. Thyroid function tests in obese prepubertal children: correlations with insulin sensitivity and body fat distribution. Horm Res Paediatr 2012; 78: 100–105. [DOI] [PubMed] [Google Scholar]
- 13. Pucci E, Chiovato L, Pinchera A. Thyroid and lipid metabolism. Int J Obes Relat Metab Disord 2000; 24(Suppl. 2): S109–S112. [DOI] [PubMed] [Google Scholar]
- 14. Bakker SJ, ter Maaten JC, Popp‐Snijders C, Slaets JP, Heine RJ, Gans RO. The relationship between thyrotropin and low density lipoprotein cholesterol is modified by insulin sensitivity in healthy euthyroid subjects. J Clin Endocrinol Metab 2001; 86: 1206–1211. [DOI] [PubMed] [Google Scholar]
- 15. Liangpunsakul S, Chalasani N. Is hypothyroidism a risk factor for non‐alcoholic steatohepatitis? J Clin Gastroenterol 2003; 37: 340–343. [DOI] [PubMed] [Google Scholar]
- 16. Chung GE, Kim D, Kim W, et al. Non‐alcoholic fatty liver disease across the spectrum of hypothyroidism. J Hepatol 2012; 57: 150–156. [DOI] [PubMed] [Google Scholar]
- 17. Pacifico L, Bonci E, Ferraro F, Andreoli G, Bascetta S, Chiesa C. Hepatic steatosis and thyroid function tests in overweight and obese children. Int J Endocrinol 2013; 381014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Torun E, Ozgen IT, Gokce S, Aydin S, Cesur Y. Thyroid hormone levels in obese children and adolescents with non‐alcoholic fatty liver disease. J Clin Res Pediatr Endocrinol 2014; 6: 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kromeyer‐Hauschild K, Wabitsch M, Geller F, et al. Perzentilen für den Body Mass‐Index für das Kindes‐ und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Monatschr Kinderheilkd 2001; 149: 807–818. [Google Scholar]
- 20. Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr 1990; 44: 45–60. [PubMed] [Google Scholar]
- 21. Raivio T, Dunkel L. Inhibins in childhood and puberty. Best Pract Res Clin Endocrinol Metab 2002; 16: 43–52. [DOI] [PubMed] [Google Scholar]
- 22. Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J (Clin Res Ed) 1986; 292: 13–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hernaez R, Lazo M, Bonekamp S, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta‐analysis. Hepatology 2011; 54: 1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shannon A, Alkhouri N, Carter‐Kent C, et al. Ultrasonographic quantitative estimation of hepatic steatosis in children With NAFLD. J Pediatr Gastroenterol Nutr 2011; 53: 190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cappola AR, Ladenson PW. Hypothyroidism and atherosclerosis. J Clin Endocrinol Metab 2003; 88: 2438–2444. [DOI] [PubMed] [Google Scholar]
- 26. Eshraghian A, Hamidian Jahromi A. Non‐alcoholic fatty liver disease and thyroid dysfunction: a systematic review. World J Gastroenterol 2014; 20: 8102–8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saxena NK, Ikeda K, Rockey DC, Friedman SL, Anania FA. Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology 2002; 35: 762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Targher G, Montagnana M, Salvagno G, et al. Association between serum TSH, free T4 and serum liver enzyme activities in a large cohort of unselected outpatients. Clin Endocrinol (Oxf) 2008; 68: 481–484. [DOI] [PubMed] [Google Scholar]
- 29. Bilgin H, Pirgon O. Thyroid function in obese children with non‐alcoholic fatty liver disease. J Clin Res Pediatr Endocrinol 2014; 6: 152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of non‐alcoholic fatty liver disease in children: a follow‐up study for up to 20 years. Gut 2009; 58: 1538–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]


