Abstract
Aim
To analyse the regenerative potential of leucocyte‐ and platelet‐rich fibrin (L‐PRF) during periodontal surgery.
Materials and Methods
An electronic and hand search were conducted in three databases. Only randomized clinical trials were selected and no follow‐up limitation was applied. Pocket depth (PD), clinical attachment level (CAL), bone fill, keratinized tissue width (KTW), recession reduction and root coverage (%) were considered as outcome. When possible, meta‐analysis was performed.
Results
Twenty‐four articles fulfilled the inclusion and exclusion criteria. Three subgroups were created: intra‐bony defects (IBDs), furcation defects and periodontal plastic surgery. Meta‐analysis was performed in all the subgroups. Significant PD reduction (1.1 ± 0.5 mm, p < 0.001), CAL gain (1.2 ± 0.6 mm, p < 0.001) and bone fill (1.7 ± 0.7 mm, p < 0.001) were found when comparing L‐PRF to open flap debridement (OFD) in IBDs. For furcation defects, significant PD reduction (1.9 ± 1.5 mm, p = 0.01), CAL gain (1.3 ± 0.4 mm, p < 0.001) and bone fill (1.5 ± 0.3 mm, p < 0.001) were reported when comparing L‐PRF to OFD. When L‐PRF was compared to a connective tissue graft, similar outcomes were recorded for PD reduction (0.2 ± 0.3 mm, p > 0.05), CAL gain (0.2 ± 0.5 mm, p > 0.05), KTW (0.3 ± 0.4 mm, p > 0.05) and recession reduction (0.2 ± 0.3 mm, p > 0.05).
Conclusions
L‐PRF enhances periodontal wound healing.
Keywords: bone regeneration, gingival recession, intra‐bony defects, leucocyte–platelet‐rich fibrin, open flap debridement, platelet‐rich fibrin, tissue regeneration
In the last 20 years, platelet concentrates (PCs) have emerged as a potential regenerative material, used alone or as scaffold for other graft materials. PCs are blood extracts, obtained after processing a whole blood sample, mostly through centrifugation (Dohan et al. 2014a). In 1970, Matras (1970) published the first article on PCs using fibrin glue to improve skin wound healing. But it was not until Marx's studies (Marx et al. 1998, Marx 2001) that the use of PCs also gained interest in oral and maxillofacial surgery. Since then, different techniques have been developed and with them, a variety of preparations. The first PCs generation (Fig. 1) include platelet‐rich plasma (PRP) and plasma rich in growth factors (PRGF). Their preparation requires anticoagulants at the moment of blood collection to avoid coagulation. Consequently, the fibrin polymerization occurs rapidly, resulting in a weak fibrin network (Dohan et al. 2006a). They are used as liquid solution or in gel form after adding bovine thrombin and calcium chloride. Due to the difficulties in the preparation and the inconsistent outcome of PRP and PRGF formulations, a second PCs generation was introduced in 2001 by Choukroun and co‐workers (Choukroun 2001, Dohan et al. 2006a, 2014a). The use of platelet‐rich fibrin (PRF) is simple and requires neither anticoagulant, bovine thrombin nor calcium chloride. It is nothing more than centrifuged blood without any additives (Table S1). Whole blood is centrifuged without anticoagulants at high spin so that three layers are obtained: red blood corpuscles (RBCs) at the bottom of the tube, platelet‐poor plasma (PPP) on the top and an intermediate layer called “buffy coat” where most leucocytes and platelets are concentrated.
Figure 1.
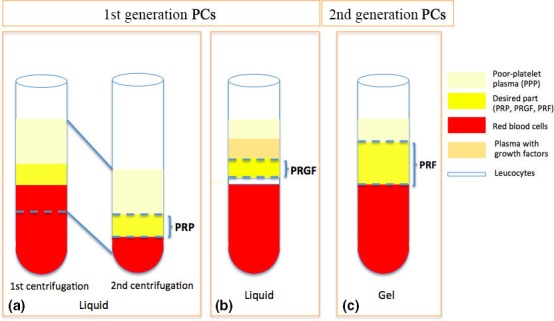
Differences among PCs preparation. (a) platelet‐rich plasma (PRP): after the first centrifugation, the platelet‐poor plasma, the “yellow” part called buffy coat and a few red blood cells are carefully collected (pipetting) and centrifuged again in order to obtain the PRP (Dohan et al. 2006a,b,c); (b) PRGF: after centrifugation, the blood is divided in five layers; by pipetting, the undesired parts are discarded; the most concentrated part with growth factors (PRGF) is collected (Anitua, 2001); (c) PRF: after centrifugation, a fibrin clot is obtained in the middle of the tube, which is ready to be used (Dohan et al. 2006a).
This buffy coat or L‐PRF is a bioactive construct that stimulates the local environment for differentiation and proliferation of stem and progenitor cells (Dohan et al. 2006b). It acts as an immune regulation node with inflammation control abilities, including a slow continuous release of growth factors over a period of 7–14 days (Dohan et al. 2006c). Rich in fibrin, platelets (±95% of initial blood), leucocytes (±50% of initial blood), monocytes and stem cells, L‐PRF can be further transformed into a membrane, circa 1 mm in thickness, by careful compression (Dohan et al. 2010) (Appendix S1). Its strong fibrin architecture and its superior mechanical properties distinguish it from other kinds of PCs (Khorshidi et al. 2016). PRP, for example, has a thin and non‐condensed fibrin network with a low tensile strength so that it is less useful as a space maintainer (Burnouf et al. 2013). The strong fibrin network in L‐PRF is explained by the physiological concentrations of thrombin during its preparation. Rowe et al. (2007) concluded that a high thrombin concentration resulted in a high‐interconnected fibre mesh with a fine fibre structure. However, as thrombin concentration decreased, fibre size increased as well as the mechanical properties. Apart from the biological and mechanical properties, antimicrobial effects have also been described (Yang et al. 2015).
The main aim of this systematic review was to study the beneficial effect of L‐PRF used as sole filling material and as adjunct to conventional techniques in periodontal surgery.
Materials and Methods
The protocol of this systematic review was based on the guidelines of the Belgian Centre for Evidence‐Based Medicine (CEBAM), Belgian Branch of the Dutch Cochrane Centre. It was conducted in accordance with the Transparent Reporting of Systematic Reviews and Meta‐analyses (PRISMA statement, Moher et al. 2009).
Focused PICO question
The following statements were used to conduct the systematic search:
Population (P) = systemically healthy humans (ASA I) with loss of periodontal tissues.
Intervention (I) = use of L‐PRF (protocol 2700 r.p.m./12 min. or 3000 r.p.m./10 min.) as sole biomaterial or in combination to other biomaterials in periodontal surgery.
Comparison (C) = traditional techniques: open flap debridement with or without grafting, periodontal plastic surgery via coronally advanced flap, with or without connective tissue graft.
Outcome (O) = alveolar bone and/or periodontal wound healing.
A PICO question was created to define the search strategy: Does L‐PRF promote periodontal wound healing in systemically healthy patients (ASA I) during periodontal surgery compared to traditional techniques?
Search strategy
An electronic search was performed in three Internet databases: the National Library of Medicine, Washington, DC (MEDLINE‐PubMed), EMBASE (Excerpta Medical Database by Elsevier), and Cochrane Central Register of Controlled Trials (CENTRAL). The search terms were defined by combining (Mesh Terms OR Key Words) from “Population” AND (Mesh Terms OR Key Words) from “Intervention”, as shown in Table S2.
The search was limited to studies involving humans. No language or time restrictions were applied in the first search. However, only studies in English were included for selection. No follow‐up limitations were used. The last electronic search was performed on the 31st of July 2015.
This search was enriched by hand searches, citation screening and expert recommendations. All reference lists of selected papers as well as related reviews were scanned for possible additional studies.
Screening and selection
The titles and abstracts obtained from the first search were screened independently by two reviewers (A.C., N.M.). When publications did not meet the inclusion criteria, they were excluded upon reviewer's agreement. Any disagreement between the two reviewers was resolved by discussion. All full texts of the eligible articles were obtained and examined by both reviewers. The articles that fulfilled all selection criteria were processed for data extraction. Given some variability in the preparation of L‐PRF, two different protocols (2700 r.p.m./12 min. or 3000 r.p.m./10 min.) were included. The inclusion and exclusion criteria are summarized in Table S3.
Assessment of heterogeneity
The heterogeneity of the included studies was judged based on following factors: (1) study design and evaluation period, (2) subject characteristics and smoking habits, and (3) surgical protocol used: (a) centrifugation protocol (2700 r.p.m./12 min. or 3000 r.p.m./10 min.), (b) mL blood used to prepare L‐PRF and (c) number of clots/membranes (if used).
Quality assessment
The quality assessment, performed by both reviewers (A.C., N.M.), was based on the Cochrane Collaboration's tool for assessing risk of bias. Six quality criteria were verified: (1) sequence generation or randomization component, (2) allocation concealment, (3) blinding of participants, personnel and outcome assessors, (4) incomplete/missing outcome data, (5) selective outcome reporting and (6) other sources of bias. In case of any doubt, the authors were contacted for clarification or to provide missing information. Low risk of bias was indicated if all quality criteria were “present”, moderate risk of bias if one or more key domains were “unclear” and high risk of bias if one or more key domains were “absent”.
Data analysis
The analysed variables were as follows: pocket depth (PD) reduction, clinical attachment level (CAL) gain, bone fill (mm and %), keratinized tissue width (KTW) gain, tissue thickness gain, recession reduction and root coverage (%) at 6 months. For all variables in each group, mean values and standard deviation (SD) were extracted. All data were arranged in groups for the inter‐group comparison (L‐PRF versus control group). When possible, a meta‐analysis was performed. The mean difference was calculated and a 95% confidence interval (CI) was computed. Forest plots were created to display the analysis.
Results
Search and selection
As a result of the electronic and hand search, 205 articles were obtained, of which 23 were duplicate and consequently removed (Fig. 2). A total of 182 articles was included for title and abstract screening. From those, 25 articles were included for full text review. One article was excluded after full text screening, which was conducted independently by two reviewers (A.C., N.M.) (Table S4). Twenty‐four randomized control trials (RCTs) fulfilled the inclusion criteria and were included for analysis.
Figure 2.
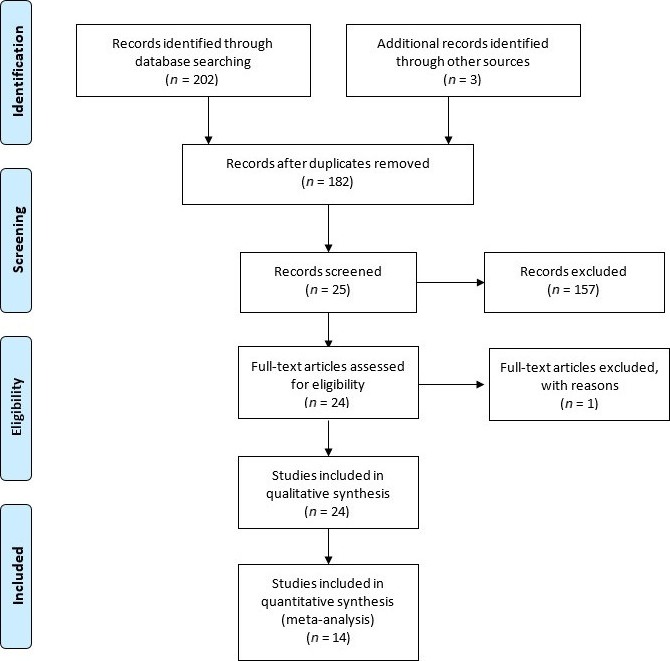
PRISMA flow diagram.
The included articles were classified into three subgroups, depending on the indication for the use of L‐PRF (Tables 1, 2, 3):
Table 1.
L‐PRF for intra‐bony defects. Papers have been arranged by subapplications (L‐PRF + OFD versus OFD, L‐PRF versus PRP, L‐PRF versus L‐PRF + BPBM, L‐PRF + DFDBA versus DFDBA, L‐PRF versus Emdogain®, L‐PRF versus nano‐bone®, L‐PRF versus ABG, L‐PRF in furcation lesions: L‐PRF + OFD versus OFD)
| Authors (year) | Study design, Duration | No. of participants baseline (end), gender, age (mean/range), Smoking (?, No, Yes) | Groups C: control T: test | L‐PRF preparation | Surgical protocol | Results |
|---|---|---|---|---|---|---|
| L‐PRF + OFD versus OFD | ||||||
| Thorat et al. (2011) |
RCT Parallel Single‐blind 9 months |
40 – (32) 18 ♀, 22 ♂ Mean age: 31 ± 2 Range: ? Smoking: ? |
2 and 3 walls IBDs C: n = 16, OFD T: n = 16, OFD + L‐PRF |
Hardware:a
Setting: 400 g/12 min. |
1 L‐PRF clot 1 L‐PRF membrane 10 ml blood/clot |
L‐PRF + OFD
versus
OFD
SS more PD reduction (4.5 versus 3.5 mm), CAL gain (3.7 versus 2.1 mm) and bone fill (47% versus 29%) in favour of L‐PRF group (p < 0.05). |
| Sharma & Pradeep (2011b) |
RCT Parallel Double‐blind 9 months |
42 – (35) 18 ♀, 24 ♂ Mean age: 35 ± 6 Range: 30–50 Smoking: No |
3 walls IBDs C: n = 17 (28 sites), OFD T: n = 18 (28 sites), L‐PRF |
Hardware: ? Setting: 3000 r.p.m./10 min. |
1 L‐PRF clot 2 L‐PRF membrane 10 ml blood/clot |
L‐PRF + OFD
versus
OFD
SS more PD reduction (4.5 versus 3.2 mm) and bone fill (48.2% versus 1.8%) in L‐PRF group (p < 0.001). NSS CAL gain between groups (3.1 versus 2.7 mm) (p > 0.05). |
| Rosamma et al. (2012) |
RCT Split‐mouth Not blind 12 months |
15 – (15) 9 ♀, 6 ♂ Mean age: 29 ± 7 Range: 17–44 Smoking: No |
3 walls IBDs C: n = 15, OFD T: n = 15, OFD + L‐PRF |
Hardware: b
Setting: 3000 r.p.m./10 min. |
1 L‐PRF clot 0 L‐PRF membrane 10 ml blood/clot |
L‐PRF + OFD
versus
OFD
SS PD reduction (4.6 versus 2.4 mm), CAL gain (4.7 versus 1.4 mm) and radiographic intra‐bony defect depth (1.9 versus 0.6 mm) in favour of L‐PRF sites (p < 0.00). |
| Ajwani et al. (2015) |
RCT Split‐mouth Single‐blind 9 months |
20 – (20) 10 ♀, 10 ♂ Mean age: 30.5 Range: ? Smoking: No |
2 and 3 walls IBDs C: n = 20, OFD T: n = 20, OFD + L‐PRF |
Hardware:c
Setting: 3000 r.p.m./10 min. |
1 L‐PRF clot 0 L‐PRF membrane 10 ml blood/clot |
L‐PRF + OFD
versus
OFD
NSS improvement in PD (1.9 versus 1.6 mm) and CAL (1.8 versus 1.3 mm) (p > 0.05). SS more bone fill (2.6 versus 1.3 mm) in favour of L‐PRF group (p < 0.05). |
| Pradeep et al. (2015) |
RCT Parallel Triple‐blind 9 months |
126 – (120) 60 ♀, 60 ♂ Mean age: 41 ± 6 Range: 30–50 Smoking: No |
3 walls IBDs C: n = 30, OFD T1: n = 30, OFD + L‐PRF T2: n = 30, OFD + 1% MF T3: n = 30, OFD + L‐PRF + 1% MF |
Hardware: ? Setting: 3000 r.p.m./10 min. |
2 L‐PRF clot 0 L‐PRF membrane 10 ml blood/clot |
L‐PRF + OFD
versus
OFD
SS PD reduction (4.0 versus 3.0 mm), CAL gain (4.0 versus 2.9 mm) in favour or T1 compared to C (p < 0.05). |
| L‐PRF versus PRP | ||||||
| Pradeep et al. (2012) |
RCT Parallel Double‐blind 9 months |
54 – (50) 27 ♀, 27 ♂ Mean age: 36.8 Range: ? Smoking: No |
3 wall IBDs C: n = 17 (30 sites), OFD T1: n = 16 (30 sites), L‐PRF T2: n = 17 (30 sites), PRP |
Hardware: ? Setting: 3000 r.p.m./10 min. |
1 L‐PRF clot 2 L‐PRF membrane 10 ml blood/clot |
L‐PRF
versus
PRP
SS PD reduction (T1: 3.7 versus T2: 3.7 versus C: 2.7 mm) and bone fill (T1 55% versus C: 2.9 mm) and bone fill (T1 55% versus T2: 56% versus C: 1.5%) in favour of L‐PRF and PRP groups (p < 0.05). NSS CAL gain (T1: 3.17 versus T2: 2.9 versus C: 2.9 mm) (p > 0.05). |
| L‐PRF versus L‐PRF + BPBM | ||||||
| Lekovic et al. (2012) |
RCT Split‐mouth Double‐blind 6 months |
17 – (17) 11 ♀, 6 ♂ Mean age: 44 ± 9 Range: ? Smoking: 12 non smokers/5 smokers |
2 and 3 walls IBDs C: n = 17, L‐PRF T: n = 17, L‐PRF + BPBM |
Hardware:d
Setting: 1000 g/10 min. |
1 L‐PRF clot 1 L‐PRF membrane 10 ml blood/clot |
L‐PRF
versus
L‐PRF + BPBM
SS PD reduction (4.4 versus 3.3 mm), CAL gain (2.4 versus 3.8 mm), and bone fill (2.1 versus 4.6 mm) in favour of L‐PRF‐BPBM group (p < 0.001). |
| L‐PRF + DFDBA versus DFDBA | ||||||
| Bansal & Bharti (2013) |
RCT Split‐mouth Not blind 6 months |
10 – (10) Gender: ? Mean age: ? Range: ? Smoking: ? |
Walls IBDs not mentioned C: n = 10, DFDBA T: n = 10, L‐PRF + DFDBA |
Hardware: ? Setting: 3000 r.p.m./10 min. |
1 L‐PRF clot 0 L‐PRF membrane 10 ml blood/clot |
L‐PRF + DFDBA
versus
DFDBA
SS PD reduction (4.0 versus 3.1 mm) and CAL gain (3.4 versus 2.3 mm) in favour of L‐PRF group (p < 0.05). NNSD for bone fill (2.3 versus 1.9 mm) and alveolar crest resorption (0.02 versus 0.04 mm) (p > 0.01). |
| Shah et al. (2015) |
RCT Split‐mouth Not blind 6 months |
20 – (20) Gender: ? Mean age: ? Range: 20–55 Smoking: No |
2 and 3 walls IBDs C: n = 20, OFD + DFDBA T: n = 20, OFD + L‐PRF |
Hardware: ? Setting: 3000 r.p.m./10 min. |
? L‐PRF clot 0 L‐PRF membrane 10 ml blood/clot |
L‐PRF + DFDBA
versus
DFDBA
NSS PD reduction (3.6 versus 3.7 mm), CAL (2.9 versus 2.9 mm) and GML (−0.4 versus −0.3 mm) |
| Agarwal et al. (2016) |
RCT Split‐mouth Double‐blind 12 months |
32 – (30) 14 ♀, 18 ♂ Mean age: 52 ± 7 Range: ? Smoking: No |
2 and 3 walls IBDs C: n = 32, DFDBA + saline T: n = 32, DFDBA + L‐PRF |
Hardware: ? Setting: 400 g/12 min. |
1? L‐PRF clot >1 L‐PRF membrane 10 ml blood/clot |
L‐PRF + DFDBA
versus
DFDBA
SS PD reduction (4.2 versus 3.6 mm), CAL gain (3.7 versus 2.6 mm), REC (0.5 versus 1.0 mm), bone fill (3.5 versus 2.5 mm) and defect resolution (3.7 versus 2.7 mm) in favour of DFDBA + L‐PRF group (p < 0.05). |
| L‐PRF versus Emdogain® | ||||||
| Gupta et al. (2014) |
RCT 6 months |
30 – (30) 15 ♀, 15 ♂ Mean age: ? Range: 30–65 Smoking: No |
3 walls IBDs C: n = 22, OFD + Emdogain® T: n = 22, OFD + L‐PRF |
Hardware:c
Setting: 3000 r.p.m./12 min. |
? L‐PRF clot 0 L‐PRF membrane 10 ml blood/clot |
L‐PRF
versus
Emdogain
®
NSS PD reduction (1.8 versus 1.8 mm) and CAL gain (2.0 versus 1.8 mm) (p > 0.05). SS more defect resolution in Emdogain® group (43% versus 32%) (p < 0.05). |
| L‐PRF versus nano‐bone® | ||||||
| Elgendy & Abo Shady (2015) |
RCT Split‐mouth Not blind 6 months |
20 – (20) Gender: ? Mean age: C: 40 ± 6, T: 44 ± 8 Range: ? Smoking: No or light smokers (<10 cig/day) |
Walls IBDs not mentioned C: n = 20, OFD + nano‐bone® T: n = 20, L‐PRF + nano‐bone® |
Hardware: ? Setting: 3000 r.p.m./10 min. |
? L‐PRF clot 0 L‐PRF membrane 10 ml blood/clot |
L‐PRF
versus
nano‐bone
®
SS PD reduction (7.1 versus 6.7 mm) and CAL gain (7.4 versus 7.1 mm) in favour of L‐PRF group (p < 0.01). |
| L‐PRF versus ABG | ||||||
| Mathur et al. (2015) |
RCT Parallel Not blind 6 months |
25 – (25) 11 ♀, 14 ♂ Mean age: 40 ± 5 Range: ? Smoking: ? |
3 walls IBDs C: n = 19, OFD + ABG T: n = 19, OFD + L‐PRF |
Hardware:c
Setting: 3000 r.p.m./10 min. |
? L‐PRF clot 0 L‐PRF membrane 10 ml blood/clot |
L‐PRF
versus
ABG
NSS PD reduction (2.6 versus 2.4 mm), and CAL gain (2.5 versus 2.6 mm) (p > 0.05). |
ABG, autologous bone graft; BPBM, Bovine porous bone mineral; C, control group; CAL, clinical attachment level; DFDBA, demineralized freeze‐dried bone allograft; IBDs, intra‐bony defects; NSS, no statistically significant; OFD, open flap debridement; PD, pocket depth; PRP, platelet‐rich plasma; REC, gingival recession; SS, statistically significant; T, test group.
Process protocol, Nice, France.
KW‐70,AlmicroTM Instruments, Ambala Cantt., Haryana, India.
R‐4C, REMI, Mumbai, India.
Labofuge 300, Kendro Laboratory Products GmbH, Osterrode, Germany.
Table 2.
L‐PRF for furcation defects. Papers have been arranged by subapplications (L‐RF + OFD versus OFD)
| Authors (year) | Study design, Duration | No. of participants baseline (end), gender, age (mean/range), Smoking (?, No, Yes) | Groups C: control T: test | L‐PRF preparation | Surgical protocol | Results |
|---|---|---|---|---|---|---|
| L‐PRF + OFD versus OFD | ||||||
| Sharma & Pradeep (2011a) |
RCT Split‐mouth Double‐blind 9 months |
18 – (18) 8 ♀, 10 ♂ Mean age: 34.2 Range: ? Smoking: No |
Furcation degree II C: n = 18, OFD T: n = 18, L‐PRF |
Hardware: ? Setting: 3000 r.p.m./10 min. |
1 L‐PRF clot 2 L‐PRF membrane 10 ml blood/clot |
L‐PRF + OFD
versus
OFD
SS PD reduction (4.1 versus 2.9 mm), CAL gain (2.3 versus 1.2 mm) and bone fill (50% versus 16.7%) in favour of L‐PRF group (p < 0.001). |
| Bajaj et al. (2013) |
RCT Parallel Double‐blind 9 months |
42 – (37) 20 ♀, 22 ♂ Mean age: 39.4 Range: ? Smoking: ? |
Furcation degree II C: n = 12 (23), OFD T1: n = 12 (24), L‐PRF T2: n = 13 (25), PRP |
Hardware:a
Setting: 400 g/10 min. |
1 L‐PRF clot 2 L‐PRF membrane 10 ml blood/clot |
L‐PRF + OFD
versus
OFD
SS PD reduction (T1: 4.2 versus T2: 3.9 versus C: 1.5 mm), CAL gain (T1: 2.8 versus T2: 2.7 versus C: 1.3 mm) and bone fill (T1: 44% versus T2: 42% versus C: 2.8%) (p < 0.001). |
C, control group; CAL, clinical attachment level; OFD, open flap debridement; PD, pocket depth; PRP, platelet‐rich plasma; SS, statistically significant; T, test group.
R‐4C, REMI, Mumbai, India.
Table 3.
L‐PRF for periodontal plastic surgery. Papers have been arranged by subapplications (CAF + L‐PRF versus CAF, CAF + L‐PRF versus CAF + CTG, L‐PRF versus EMD)
| Authors (year) | Study design, Duration | No. of participants baseline (end), gender, age (mean/range), Smoking (?, No, Yes) | Groups C: control T: test | L‐PRF preparation | Surgical protocol | Results |
|---|---|---|---|---|---|---|
| CAF + L‐PRF versus CAF | ||||||
| Aroca et al. (2009) |
RCT Split‐mouth Not blind 6 months |
20 – (20) 15 ♀, 5 ♂ Mean age: 31.7 Range: 22–47 Smoking: No or ≤20 cig/day |
C: n = 21, CAF T: n = 21, CAF + L‐PRF |
Hardware:a
Setting: 3000 r.p.m./10 min. |
4? L‐PRF membrane Modified CAF 10 ml blood/clot |
CAF + L‐PRF
versus
CAF
SS more root coverage at 3 months (91.5% versus 80%) and 6 months (88% versus 81%) in favour of control group (p < 0.01). NSSD for PD reduction in both groups. more CAL gain (2.6 versus 2.5 mm) and GTH (0.0 versus 0.3 mm) in favour of control group (p > 0.05). |
| Padma et al. (2013) |
RCT Split‐mouth Not blind 6 months |
15 – (15) Gender: ? Mean age: ? Range: 18–35 Smoking: No |
C: n = 15, CAF T: n = 15, CAF + L‐PRF |
Hardware: ? Setting: 3000 r.p.m./10 min. |
1 L‐PRF membrane CAF 10 ml blood/clot |
CAF + L‐PRF
versus
CAF
SS more root coverage (100% versus 68%) in favour of L‐PRF group (p < 0.05). SS more WKG (2.4 versus 2.2 mm) in favour of L‐PRF group (p < 0.05). |
| Gupta et al. (2015) |
RCT Parallel Not blind 6 months |
26 – (26) 10 ♀, 16 ♂ Mean age: 37 ± 9 Range: ? Smoking: No |
C: n = 15, CAF T: n = 15, CAF + L‐PRF |
Hardware:b
Setting: 2700 r.p.m./12 min. |
1 L‐PRF membrane Modified CAF 10 ml blood/clot |
CAF + L‐PRF
versus
CAF
NSSD for outcomes in both groups for any parameter (p > 0.05). |
| Thamaraiselvan et al. (2015) |
RCT Parallel Single‐blind 6 months |
20 – (20) 2 ♀, 18 ♂ Mean age: ? Range: 21–47 Smoking: No |
C: n = 10, CAF T: n = 10, CAF + L‐PRF |
Hardware: ? Setting: 3000 r.p.m./10 min. |
1 L‐PRF membrane + surgical site rinsed with L‐PRF exudate CAF 10 ml blood/clot |
CAF + L‐PRF
versus
CAF
NSSD for outcomes in both groups for any parameter (p > 0.05). |
| CAF + L‐PRF versus CAF + CTG | ||||||
| Jankovic et al. (2012) |
RCT Split‐mouth Single‐blind 6 months |
15 – (15) 10 ♀, 5 ♂ Mean age: ? Range: 19–47 Smoking: No |
C: n = 15, CAF + CTG T: n = 15, CAF + L‐PRF |
Hardware: ? Setting: 3000 r.p.m./10 min. |
1 L‐PRF membrane CAF 10 ml blood/clot |
CAF + L‐PRF
versus
CAF + CTG
NSSD for PD, CAL and root coverage for L‐PRF and CTG group (p > 0.05). SS more gain of keratinized tissue width (0.8 versus 1.4 mm) for CTG group (p < 0.05). SS enhanced healing in L‐PRF group (p < 0.05). |
| Eren & Atilla (2014) |
RCT Split‐mouth Single‐blind 6 months |
27 – (22) 13 ♀, 9 ♂ Mean age: 34 ± 13 Range 18.5 Smoking: No |
C: n = 22, CAF + SCTG T: n = 22, CAF + L‐PRF |
Hardware:c
Setting: 400 g/12 min |
1 L‐PRF membrane CAF 10 ml blood/clot |
CAF + L‐PRF
versus
CAF + CTG
NSSD for root coverage in L‐PRF group (92.7%) and control group (94.2%) (p > 0.05). NSSD for complete root coverage in L‐PRF group (72.7%) and control group (77.3%) (p > 0.05). |
| Keceli et al. (2015) |
RCT Split‐mouth Single‐blind 6 months |
40 – (40) 27 ♀, 13 ♂ Mean age: 40 ± 7 Range: ? Smoking: No |
C: n = 20, CAF + CTG T: n = 20, CAF + CTG + L‐PRF |
Hardware: ? Setting: 3000 r.p.m./10 min. |
1 L‐PRF membrane CAF 10 ml blood/clot |
CAF + L‐PRF
versus
CAF + CTG
NSSD for outcomes in both groups for any parameter (p > 0.05). |
| Tunali et al. (2015) |
RCT Split‐mouth Single‐blind 12 months |
10 – (10) 6 ♀, 4 ♂ Mean age: 34.2 Range: 25–52 Smoking: No |
C: n = 10, CAF + CTG T: n = 10, CAF + L‐PRF |
Hardware:a
Setting: 2700 r.p.m./12 min. |
1 L‐PRF membrane CAF 10 ml blood/clot |
CAF + L‐PRF
versus
CAF + CTG
Similar outcomes in both groups for any parameter. |
| L‐PRF versus EMD | ||||||
| Jankovic et al. (2010) |
RCT Split‐mouth Not blind 12 months |
20 – (20) 12 ♀, 8 ♂ Mean age: ? Range: 21–48 Smoking: No |
C: n = 20, CAF + EMD T: n = 20, CAF + L‐PRF |
Hardware: ? Setting: 3000 r.p.m./10 min. |
1 L‐PRF membrane Modified CAF 10 ml blood/clot |
L‐PRF versus EMD More complete root coverage (65% versus 60%) in L‐PRF group. Similar WKG between groups. |
C, control group; CAF, coronally advanced flap; CAL, clinical attachment level; CTG, connective tissue graft; EMD, Emdogain®; GTH, gingival thickness; PD, pocket depth; SCTG, subepithelial connective tissue graft; T, test group; WKG, width of keratinized gingiva.
EBA 20, Hettich GmbH & Co KG, Tuttlingen, Germany.
RC‐4, REMI, Mumbai, India.
Nüve Laboratory Equipments, NF200, Ankara, Turkey.
- Intra‐bony defect fill: n = 13
- 1
-
2L‐PRF versus PRP versus OFD: n = 1, Pradeep et al. (2012).
-
3L‐PRF versus bovine porous bone mineral (BPBM): n = 1, Lekovic et al. (2012).
- 4
-
5L‐PRF versus Emdogain®: n = 1, Gupta et al. (2014).
-
6L‐PRF versus nano‐bone®: n = 1, Elgendy & Abo Shady (2015).
-
7L‐PRF versus autologous bone graft (ABG): n = 1, Mathur et al. (2015).
Furcation defects: n = 2, Sharma & Pradeep (2011a), and Bajaj et al. (2013).
- Periodontal plastic surgery: n = 9
- 1
- 2
-
3CAF + L‐PRF versus CAF +Emdogain® (EMD): n = 1, Jankovic et al. (2010).
Assessment of heterogeneity
Study design and evaluation period
All studies were RCTs and frequently presented a split‐mouth design. The articles with these characteristics are the following: intra‐bony defects (IBDs) 7/13 (Lekovic et al. 2012, Rosamma et al. 2012, Bansal & Bharti 2013, Ajwani et al. 2015, Elgendy & Abo Shady 2015, Shah et al. 2015, Agarwal et al. 2016), furcation defects 1/2 (Sharma & Pradeep 2011a), plastic surgery 7/9 (Aroca et al. 2009, Jankovic et al. 2010, 2012, Padma et al. 2013, Eren & Atilla 2014, Keceli et al. 2015, Tunali et al. 2015). The follow‐up ranged slightly (IBDs 6–12 months, furcation defects 9 months and plastic surgery 6–12 months).
Subject characteristics and smoking habits
Healthy subjects with no active periodontal disease were included in all the studies. The studies that did not include smokers are the following: IBDs 9/13 (Sharma & Pradeep 2011a,b, Lekovic et al. 2012, Rosamma et al. 2012, Pradeep et al. 2012, 2015, Gupta et al. 2014, Ajwani et al. 2015, Shah et al. 2015, Agarwal et al. 2016), furcation defects 1/2 (Sharma & Pradeep 2011a), plastic surgery 8/9 (Jankovic et al. 2010, 2012, Padma et al. 2013, Eren & Atilla 2014, Gupta et al. 2015, Keceli et al. 2015, Thamaraiselvan et al. 2015, Tunali et al. 2015).
Surgical protocol
A wide variety of surgical protocols was used. This heterogeneity can be derived from Tables 1, 2, 3.
Quality assessment
Appendix S2–S4 shows the quality assessment for the included studies. All articles on furcation defects and periodontal plastic surgery showed a moderate risk of bias. Similarly, 12 articles using L‐PRF in IBD had a moderate risk, and one had a low risk of bias.
Quantitative assessment
The extracted data were continuous. The articles with split‐mouth design and parallel design were not analysed separately. The control group and test group from the articles with split‐moth design were considered as independent. As shown in the Figs 3 and 4, the studies with split‐mouth design do not differ from those with parallel design. Random effects were used due to the heterogeneity of the data.
Figure 3.
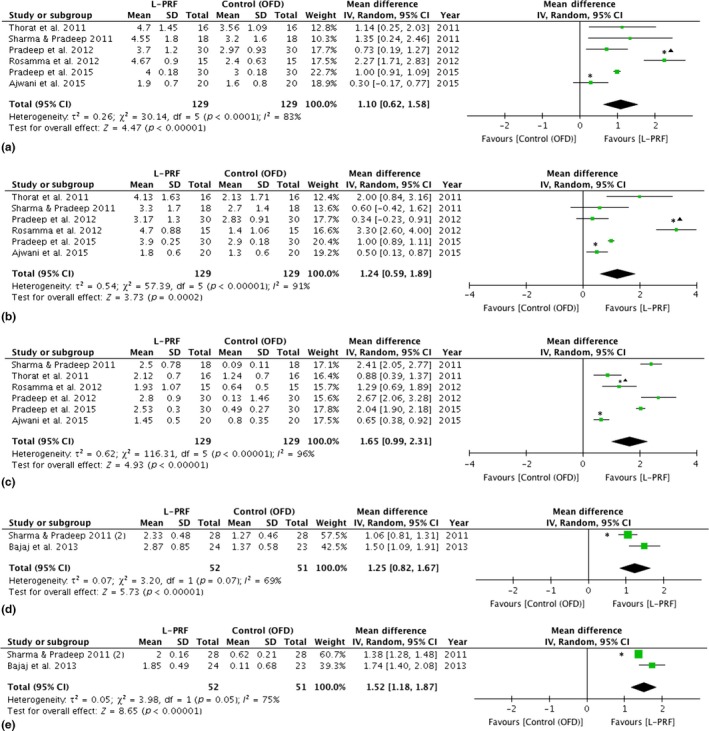
Forest plot comparing OFD versus OFD + L‐PRF in the treatment of intra‐bony defects (IBDs) and furcation defects.: ▲ different follow‐up from the rest of the studies included. *: study with split‐mouth design. (a) Forest plot comparing OFD versus OFD + L‐PRF in the treatment of IBDs, PD reduction (mm). (b) Forest plot comparing OFD versus OFD + L‐PRF in the treatment of IBDs, CAL gain (mm). (c) Forest plot comparing OFD versus OFD + L‐PRF in the treatment of IBDs, bone fill (mm). (d) Forest plot comparing OFD versus OFD + L‐PRF in the treatment of furcation defects, CAL gain (mm). (e) Forest plot comparing OFD versus OFD + L‐PRF in the treatment of furcation defects, bone fill (mm). CAL, clinical attachment level; OFD, open flap debridement; PD, Pocket depth.
Figure 4.
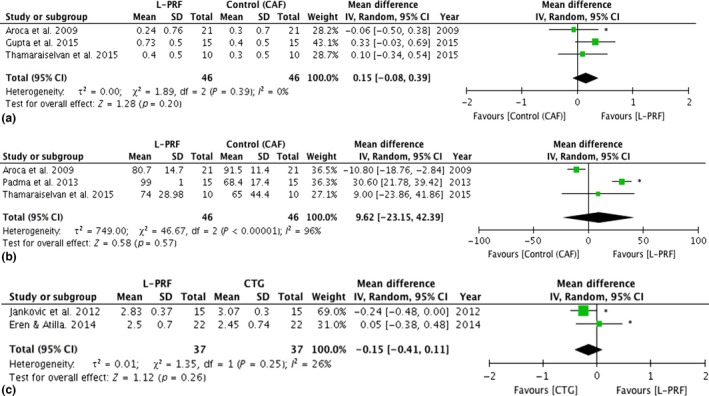
Forest plot comparing CAF + L‐PRF versus CAF and CAF + L‐PRF versus CAF + CTG in periodontal plastic surgery. *: study with split‐mouth design. (a) Forest plot comparing CAF versus CAF + L‐PRF in periodontal plastic surgery, recession reduction (mm). (b) Forest plot comparing CAF versus CAF + L‐PRF in periodontal plastic surgery, root coverage 6 months (%). (c) Forest plot comparing CAF + CTG versus CAF + L‐PRF in periodontal plastic surgery, recession reduction (mm). CAF, Coronally advanced flap; CTG, connective tissue graft.
Intra‐bony defects
In the articles on IBDs, benefits in terms of PD reduction, CAL gain and bone fill were shown when L‐PRF was used alone or in combination with other biomaterials (Table 1). Six out of 13 articles (Sharma & Pradeep 2011b, Thorat et al. 2011, Pradeep et al. 2012, 2015, Rosamma et al. 2012, Ajwani et al. 2015) could be used for a meta‐analysis since they reported on similar outcome measures comparing OFD to OFD + L‐PRF (Fig. 3a‐c). The meta‐analysis of IBDs showed a statistical significant difference for PD reduction (mean difference: 1.1 mm, p < 0.001, CI: 0.6–1.6), CAL gain (mean difference: 1.2 mm, p < 0.001, CI: 0.5–1.9), amount of bone fill in mm (mean difference: 1.7 mm, p < 0.001, CI: 1.0–2.3) and bone fill when scored as % (mean difference: 46.0%, p < 0.001, CI: 33.2–58.7), all in favour of L‐PRF.
Furcation defects
Two articles were included for furcation defects (Sharma & Pradeep 2011a, Bajaj et al. 2013). A meta‐analysis could be performed for both articles, comparing OFD to OFD + L‐PRF (Fig. 3d,e). Statistical significant differences could be found for PD reduction (mean difference: 1.9 mm, p = 0.01, CI: 0.4–3.5), CAL gain (mean difference: 1.3 mm, p < 0.001, CI: 0.8–1.7), amount of bone fill in mm (mean difference: 1.5 mm, p < 0.001, CI:1.2–1.9), bone fill when scored as % (37.6%, p < 0.001, CI: 30.6–44.5), again in favour of L‐PRF (Table 2).
Periodontal plastic surgery
In case of a CAF, some studies reported some benefits when L‐PRF membranes were added, but others failed to show this advantage (Table 3). When the use of a CTG in a CAF procedure was compared to the use of L‐PRF membranes, similar results were obtained. Two meta‐analyses could be performed, one comparing a CAF alone versus a CAF with L‐PRF, and another comparing a CAF with L‐PRF versus a CAF with a CTG. The following variables were considered: PD reduction, CAL gain, KTW gain, tissue thickness gain, recession reduction and root coverage at 6 months.
For the first comparison (CAF +L‐PRF versus CAF, Fig. 4a,b), three articles could be included for a meta‐analysis (Aroca et al. 2009, Gupta et al. 2015, Thamaraiselvan et al. 2015). The analysis showed no statistical significant difference in PD reduction (mean difference: 0.2 mm, p = 0.2, CI: −0.08 to 0.4), CAL gain (mean difference: 0.4 mm, p = 0.09, CI: −0.06 to 0.8), KTW gain (mean difference: 0.3 mm, p = 0.1, CI: −0.06 to 0.6), tissue thickness (mean difference: 0.2 mm, p = 0.09, CI: −0.03 to 0.4) and root coverage at 6 months (mean difference: 9.6%, p = 0.6, CI: −23.2 to 42.4), although the results showed a trend that L‐PRF was superior for all of these variables. However, statistically significant difference could be found for recession depth reduction (mean difference: 0.6 mm, p < 0.01, CI: 0.2–1.1), in favour of the for the L‐PRF treatment.
For the second comparison (CAF + L‐PRF versus CAF + CTG, Fig. 4c) also three articles could be used for a meta‐analysis (Jankovic et al. 2012, Eren & Atilla 2014, Tunali et al. 2015). No statistical significant differences could be found for all of the variables: PD reduction (mean difference: 0.2 mm, p = 0.4, CI: −0.5 to 0.2), CAL gain (mean difference: 0.2 mm, p = 0.3, CI: −0.3 to 0.7), KTW gain (mean difference: 0.3 mm, p = 0.2, CI: −0.7 to 0.2) and recession reduction (mean difference: 0.2 mm, p = 0.2, CI: −0.4 to 0.1). Root coverage could not be included in this meta‐analysis since only one article (Jankovic et al. 2012) fully analysed this variable; Eren & Atilla (2014), and Tunali et al. (2015) did not include the standard deviations.
The adverse events were only registered in some articles within the group of periodontal plastic surgery (Aroca et al. 2009, Jankovic et al. 2010, 2012, Eren & Atilla 2014, Gupta et al. 2015). Each article analysed the adverse events with a different scale, so no meta‐analysis could be performed. Five out of the nine articles on periodontal plastic surgery reported on pain, swelling and hypersensitivity. All of them observed less side effects in L‐PRF sites.
Discussion
L‐PRF has often shown a positive effect when applied during periodontal surgery. Although it has been classified as a platelet concentrate (Dohan et al. 2014a), it can also be considered as a living tissue graft due to its cellular content and its constant release of growth factors for more than 7 days (Dohan et al. 2006b).
This review demonstrates that L‐PRF has many applications but there is no clear standard protocol per surgical procedure. For example, the number of clots used varies enormously, as well as the amount of blood drawn to prepare L‐PRF. The type of centrifuge and setting also differed from one study to another. More standardized protocols are necessary in order to better compare and standardize outcomes.
The effectiveness of L‐PRF in the treatment of intra‐bony defects has been studied by different research groups (Rock 2013, Shah et al. 2014). In these studies, L‐PRF was placed in the defect and L‐PRF membranes were used to cover the defect similar to a guided tissue regeneration (GTR) membrane. Clinical and radiographic evaluations showed statistically significant greater PD reduction, CAL gain and radiographic intra‐bony defect fill in the L‐PRF group (Table 3). Different graft materials were also compared to L‐PRF during GTR. The outcomes showed a favourable effect of L‐PRF in all clinical parameters measured, or an improvement of the outcomes in studies where L‐PRF was combined with other biomaterials (Table 3). Although very limited data exist, the use of L‐PRF in furcation defects has also shown favourable results.
For periodontal plastic surgery, the comparison of CAF + L‐PRF versus CAF led to controversial results. Although most articles did not show statistically significant differences, L‐PRF was superior for all of the parameters recorded. Comparing CAF + L‐PRF versus CAF + CTG, L‐PRF might be an alternative to a connective tissue graft. The latter is supported by some case reports (Anilkumar et al. 2009, Agarwal et al. 2013, Singh & Bharti 2013). In this systematic review, a mean root coverage of 86.5% at 6 months has been recorded for CAF + L‐PRF treatment. For CAF + EMD and CAF + CTG, a mean root coverage of 91.2% and 90.3% was, respectively, reported in a recent systematic review at 6 months (Cairo et al. 2008).
Some limitations have to be taken into consideration while processing this systematic review. Most of the included articles showed a moderate risk of bias. In those articles, the power analysis was often performed after the recruitment of the participants, where for a RCT it should be done prior to the recruitment in order to determine the sample size. Working in the opposite way, a selective outcome reporting bias can be introduced. Additionally, the allocation concealment and blinding methods were frequently not applied which increased the risk of bias.
Meta‐analysis could be performed in the three indications. However, also here the results of certain studies have to be considered very cautiously. For instance, for the IBDs subgroup, Ajwani et al. (2015) obtained the worst results compared to the rest of the selected articles. The reason could be that two‐ and three‐wall IBDs were included but not analysed separately. Moreover, only one L‐PRF clot without membrane was used, so the stability of one L‐PRF clot in a two‐wall defect without the use of a membrane might not have been ideal. Given the importance of stability in GTR, the use of L‐PRF clots in two‐ or one‐wall defects should be accompanied by a L‐PRF membrane. In periodontal plastic surgery, Aroca et al. (2009) published the only article that reported better outcome for the control group (CAF). However, smokers (<20 cig/day) were also included, though smoking negatively influences the healing process and affects complete root coverage (Chambrone et al. 2009, De Sanctis & Clementini 2014). Tobacco smoke might directly affect the peripheral blood cells within the L‐PRF (Armilli et al. 2012), yielding to uncertain outcomes.
Regardless the limitations of the included studies, it is worth pointing out some strengths of this systematic review. A total number of 722 participants was enrolled in the selected studies (479 in intra‐bony defects, 55 in furcation defects, 188 in periodontal plastic surgery). Taking into consideration the rather short history of L‐PRF, this review comprehends a quite large sample of patients. Moreover, the follow‐up varied slightly in the articles included for meta‐analysis. The duration in the follow‐up ranged from 9 to 12 months in the studies selected for quantitative assessment for the IBDs group. Considering furcation defects and periodontal plastic surgery, all of them had a follow‐up of 9 and 6 months, respectively. Moreover, only the two most accepted protocols of centrifugation (3000 r.p.m./10 min. or 2,700 r.p.m./12 min.) were included. All other protocols that were not explained in detail or with a non‐standardized procedure were excluded. A correct handling of L‐PRF is of the outmost importance. It should be clearly distinguished what L‐PRF is and what not. For example, L‐PRF and PRP contain different cell concentrations, release different amount of growth factors, and have different mechanical properties although both come from a blood sample (Dohan et al. 2006a).
Conclusion
Favourable effects on hard and soft tissue healing and postoperative discomfort reduction were often reported when L‐PRF was used. Nevertheless, standardization of the protocol is needed to obtain an optimal effect of L‐PRF in regenerative procedures. Correct handling of L‐PRF as well as the use of enough clots/membranes per surgical site might be crucial to obtain benefits from this technique. This biomaterial can be taken into consideration due to its reported good biological effects, low costs and ease of preparation.
Clinical Relevance.
Scientific rationale for the study: The aim of this systematic review and meta‐analysis is to extensively analyse the additional regenerative potential of L‐PRF during periodontal surgery.
Principal findings: The meta‐analysis showed significant clinical benefits of L‐PRF for the treatment of IBDs and for furcation defects, and similar outcomes when a connective tissue graft (CTG) was replaced by L‐PRF membranes during periodontal plastic surgery.
Practical implications: These results indicate that L‐PRF has favourable effects periodontal wound healing, and postoperative discomfort reduction. Nevertheless, standardization of the protocol is needed to obtain an optimal effect.
Supporting information
Table S1. Description of platelet concentrates (PCs) characteristics. Although PRP and PRF can be prepared with or without leucocytes (Dohan et al. 2010, Dohan et al. 2014a), this table presents the most common formulations.
Table S2. Search terms used for PUBMED, EMBASE and CENTRAL.
Table S3. Inclusion and exclusion criteria.
Table S4. Excluded articles and reason for exclusion.
Appendix S1. L‐PRF preparation. A. Blood is withdrawn from the patient. B. Tubes are centrifuged within 60 s after blood collection without any additives. C. After 12 min. of centrifugation, a clear separation between the platelet‐ poor plasma, the buffy coat and the red blood cells is obtained. D. L‐PRF is presented in the middle of the tube. E. Different L‐PRF forms can be produced: liquid, clots or membranes.
Appendix S2. Quality assessment for IBDs. Cochrane tool's for assessment of risk of bias for RCTs.
Appendix S3. Quality assessment for furcation defects. Cochrane tool's for assessment of risk of bias for RCTs.
Appendix S4. Quality assessment for IBDs for periodontal plastic surgery. Cochrane tool's for assessment of risk of bias for RCTs.
Acknowledgements
We acknowledge GC Europe N.V. for the chair in bio‐regeneration and Intra Lock International Inc. for the chair in optimized osseointegration. The authors also acknowledge Wim Coucke for his support in the statistical analysis and Mrs. Trudy Bekkering from the Belgian Centre for Evidence‐Based Medicine (CEBAM).
Castro AB, Meschi N, Temmerman A, Pinto N, Lambrechts P, Teughels W, Quirynen M. Regenerative potential of leucocyte‐ and platelet‐rich fibrin. Part A: intra‐bony defects, furcation defects and periodontal plastic surgery. A systematic review and meta‐analysis. J Clin Periodontol 2017; 44: 67–82. doi: 10.1111/jcpe.12643
Conflict of interest and source of funding statement
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
The study was self‐funded by the authors and their institution.
References
- Agarwal, A. , Gupta, N. D. & Jain, A. (2016) Platelet rich fibrin combined with decalcified freeze‐dried bone allograft for the treatment of human intrabony periodontal defects: a randomized split mouth clinical trail. Acta Odontologica Scandinavica 74, 36–43. [DOI] [PubMed] [Google Scholar]
- Agarwal, K. , Chandra, C. , Agarwal, K. & Kumar, N. (2013) Lateral sliding bridge flap technique along with platelet rich fibrin and guided tissue regeneration for root coverage. Journal of Indian Society of Periodontology 17, 801–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajwani, H. , Shetty, S. , Gopalakrishnan, D. , Katharira, R. , Kulloli, A. , Dolas, R. S. & Pradeep, A. R. (2015) Comparative evaluation of platelet‐rich fibrin biomaterial and open flap debridement in the treatment of two and three wall intrabony defects. Journal of International Oral Health 7, 32–37. [PMC free article] [PubMed] [Google Scholar]
- Anilkumar, K. , Geetha, A. , Umasudhakar, Ramakrishnan, T. , Vijayalakshmi, R. & Pameela, E. (2009) Platelet‐rich‐fibrin: a novel root coverage approach. Journal of Indian Society of Periodontology, 13, 50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anitua, E. (2001) The use of plasma‐rich growth factors (PRGF) in oral surgery. Practical procedures & aesthetic dentistry 16, 487–493. [PubMed] [Google Scholar]
- Armilli, S. , Damratoski, B. E. , Bombick, B. , Borgerding, M. F. & Prasad, G. L. (2012) Evaluation of cytotoxicity of different tobacco product preparation. Regulatory Toxicology and Pharmacology 64, 350–360. [DOI] [PubMed] [Google Scholar]
- Aroca, S. , Keglevich, T. , Barbieri, B. , Gera, I. & Etienne, D. (2009) Clinical evaluation of a modified coronally advanced flap alone or in combination with a platelet‐rich fibrin membrane for the treatment of adjacent multiple gingival recessions: a 6‐month study. Journal of Periodontology 80, 244–252. [DOI] [PubMed] [Google Scholar]
- Bajaj, P. , Pradeep, A. R. , Agarwal, E. , Rao, N. S. , Naik, S. B. , Priyanka, N. & Kalra, N. (2013) Comparative evaluation of autologous platelet‐rich fibrin and platelet‐rich plasma in the treatment of mandibular degree II furcation defects: a randomized controlled clinical trial. Journal of Periodontal Research 48, 573–581. [DOI] [PubMed] [Google Scholar]
- Bansal, C. & Bharti, V. (2013) Evaluation of efficacy of autologous platelet‐rich fibrin with demineralized‐freeze dried bone allograft in the treatment of periodontal intrabony defects. Journal of Indian Society of Periodontology 17, 361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnouf, T. , Goubran, H. A. , Chen, T. & Ou, K. (2013) Blood‐derived biomaterials and platelet growth factors in regenerative medicine. Blood Reviews 27, 77–89. [DOI] [PubMed] [Google Scholar]
- Cairo, F. , Pagliaro, U. & Nieri, M. (2008) Treatment of gingival recession with coronally advanced flap procedures: a systematic review. Journal of Clinical Periodontology 35 (Suppl. 8), 136–162. [DOI] [PubMed] [Google Scholar]
- Chambrone, L. , Chambrone, D. , Pustiglioni, F. E. , Chambrone, L. A. & Lima, L. A. (2009) The influence of tobacco smoking to the outcome achieved by root‐coverage procedures: a systematic review. Journal of the American Dental Association 140, 294–306. [DOI] [PubMed] [Google Scholar]
- Choukroun, J. (2001) Une opportunité en paro‐implantologie: le PRF. Implantodontie, 42, 55–62. French. [Google Scholar]
- De Sanctis, M. & Clementini, M. (2014) Flap approaches in plastic periodontal and implant surgery: critical elements in design and execution. Journal of Clinical Periodontology 41 (Suppl. 15), S108–S122. [DOI] [PubMed] [Google Scholar]
- Dohan, D. M. , Andia, I. , Zumstein, M. A. , Zhang, C. Q. , Pinto, N. R. & Bielecki, T. (2014a) Classification of platelet concentrates (Platelet‐Rich Plasma‐PRP, Platelet‐Rich Fibrin‐PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons Journal 4, 3–9. [PMC free article] [PubMed] [Google Scholar]
- Dohan, D. M. , Choukroun, J. , Diss, A. , Dohan, S. L. , Dohan, A. J. , Mouhyi, J. & Gigly, B. (2006a) Platelet‐rich fibrin (PRF): a second‐generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 101, E37–E44. [DOI] [PubMed] [Google Scholar]
- Dohan, D. M. , Choukroun, J. , Diss, A. , Dohan, S. L. , Dohan, A. J. , Mouhyi, J. & Gogly, B. (2006b) Platelet‐rich fibrin (PRF): a second‐generation platelet concentrate. Part II: platelet‐related biologic features. Oral Surgery Oral Medicine Oral Pathology Oral Radiology and Endodontics 101, e45–e50. [DOI] [PubMed] [Google Scholar]
- Dohan, D. M. , Choukroun, J. , Diss, A. , Dohan, S. L. , Dohan, A. J. , Mouhyi, J. & Gogly, B. (2006c) Platelet‐rich fibrin (PRF): a second‐generation platelet concentrate. Part III: Leucocyte activation: a new feature for platelet concentrates? Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 101, E51–E55. [DOI] [PubMed] [Google Scholar]
- Dohan, D. M. , Del Corso, M. , Diss, A. , Mouhyi, J. & Charrier, J. B. (2010) Three‐dimensional architecture and cell composition of a Choukroun's platelet‐rich fibrin clot and membrane. Journal of Periodontology 81, 546–555. [DOI] [PubMed] [Google Scholar]
- Elgendy, E. A. & Abo Shady, T. E. (2015) Clinical and radiographic evaluation of nanocrystalline hydroxyapatite with or without platelet‐rich fibrin membrane in the treatment of periodontal intrabony defects. Journal of Indian Society of Periodontology 19, 61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren, G. & Atilla, G. (2014) Platelet‐rich fibrin in the treatment of localized gingival recessions: a split‐mouth randomized clinical trial. Clinical Oral Investigations 18, 1941–1948. [DOI] [PubMed] [Google Scholar]
- Gupta, S. , Banthia, R. , Singh, P. , Banthia, P. , Raje, S. & Aggarwal, N. (2015) Clinical evaluation and comparison of the efficacy of coronally advanced flap alone and in combination with platelet rich fibrin membrane in the treatment of Miller Class I and II gingival recessions. Contemporary Clinical Dentistry 6, 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, S. J. , Jhingran, R. , Gupta, V. , Bains, V. K. , Madan, R. & Rizvi, I. (2014) Efficacy of platelet‐rich fibrin vs. enamel matrix derivative in the treatment of periodontal intrabony defects: a clinical and cone beam computed tomography study. Journal of International Academy of Periodontology 16, 86–96. [PubMed] [Google Scholar]
- Jankovic, S. , Aleksic, Z. , Klokkevold, P. , Lekovic, V. , Dimitrijevic, B. , Kenney, E. B. & Camargo, P. (2012) Use of platelet‐rich fibrin membrane following treatment of gingival recession: a randomized clinical trial. International Journal of Periodontics and Restorative Dentistry 32, e41–e50. [PubMed] [Google Scholar]
- Jankovic, S. , Aleksic, Z. , Milinkovic, I. & Dimitrijevic, B. (2010) The coronally advanced flap in combination with platelet‐rich fibrin (PRF) and enamel matrix derivative in the treatment of gingival recession: a comparative study. European Journal of Esthetic Dentistry 5, 260–273. [PubMed] [Google Scholar]
- Keceli, H. G. , Kamak, G. , Erdemir, E. O. , Evqiner, M. S. & Dolgun, A. (2015) The adjunctive effect of Platelet‐Rich Fibrin to Connective Tissue Graft in the treatment of buccal recession defects: results of a randomized, parallel‐group controlled trial. Journal of Periodontology 86, 1221‐1230. [DOI] [PubMed] [Google Scholar]
- Khorshidi, H. , Raoofi, S. , Bagheri, R. & Banihashemi, H. (2016) Comparison of the mechanical properties of early leukocyte‐ and platelet‐rich fibrin versus PRGF/endoret membranes. International Journal of Dentistry, 1849207. Published online 2016 Jan 6. doi: 10.1155/2016/1849207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekovic, V. , Milinkovic, I. , Aleksic, Z. , Jankovic, S. , Stankovic, P. , Kenney, E. B. & Camargo, P. M. (2012) Platelet‐rich fibrin and bovine porous bone mineral vs. platelet‐rich fibrin in the treatment of intrabony periodontal defects. Journal of Periodontal Research 47, 409–417. [DOI] [PubMed] [Google Scholar]
- Marx, R. E. (2001) Platelet‐rich plasma (PRP): what is PRP and what is not PRP? Implant Dentistry 10, 225–228. [DOI] [PubMed] [Google Scholar]
- Marx, R. E. , Carlson, E. R. , Eichstaedt, R. M. , Schimmele, S. R. , Strauss, J. E. & Georgeff, K. R. (1998) Platelet‐rich plasma: growth factor enhancement for bone grafts. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics, 85, 638–646. [DOI] [PubMed] [Google Scholar]
- Mathur, A. , Bains, V. K. , Gupta, V. , Jhingran, R. & Singh, G. P. (2015) Evaluation of intrabony defects treated with platelet‐rich fibrin or autogenous bone graft: a comparative analysis. European Journal of Dentistry 9, 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matras, H. (1970) Effect of various fibrin preparations on reimplantations in the rat skin. Österreichische Zeitschrift für Stomatologie 67, 338–359. [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. & Altman, D. G. (2009) Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 6, 1–6. [PMC free article] [PubMed] [Google Scholar]
- Padma, R. , Shilpa, A. , Kumar, P. A. , Nagasri, M. , Kumar, C. & Sreedhar, A. (2013) A split mouth randomized controlled study to evaluate the adjunctive effect of platelet‐rich fibrin to coronally advanced flap in Miller's class‐I and II recession defects. Journal of Indian Society of Periodontology 17, 631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradeep, A. R. , Nagpal, K. , Karvekar, S. , Patnaik, K. , Naik, S. B. & Guruprasad, C. N. (2015) Platelet rich fibrin with 1% metformin for the treatment of intrabony defects in chronic periodontitis: a randomized controlled clinical trial. Journal of Periodontology 86, 632–641. [DOI] [PubMed] [Google Scholar]
- Pradeep, A. R. , Rao, N. S. , Agarwal, E. , Bajaj, P. , Kumari, M. & Naik, S. B. (2012) Comparative evaluation of autologous platelet‐rich fibrin and platelet‐rich plasma in the treatment of 3‐wall intrabony defects in chronic periodontitis: a randomized controlled clinical trial. Journal of Periodontology 83, 1499–1507. [DOI] [PubMed] [Google Scholar]
- PRISMA statement . Preferred Reporting Items for Systematic Reviews and Meta‐Analyses. Available at: http://www.prisma-statement.org/ (accessed 04 augustus 2015).
- Rock, L. (2013) Potential of platelet rich fibrin in regenerative periodontal therapy: literature review. The Canadian Journal of Dental Hygiene 47, 33–37. [Google Scholar]
- Rosamma, V. , Raghunath, A. & Sharma, N. (2012) Clinical effectiveness of autologous platelet rich fibrin in the management of infrabony periodontal defects. Singapore Dental Journal 33, 5–12. [DOI] [PubMed] [Google Scholar]
- Rowe, S. L. , Lee, S. Y. & Stegemann, J. P. (2007) Influence of thrombin concentration on the mechanical and morphological properties of cell‐seeded fibrin hydrogels. Acta Biomaterialia 3, 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, M. , Deshpande, N. , Bharwani, A. , Nadig, P. , Doshi, V. & Dave, D. (2014) Effectiveness of autologous platelet‐rich fibrin in the treatment of intra‐bony defects: a systematic review and meta‐analysis. Journal of Indian Society of Periodontology 18, 698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, M. , Patel, J. , Dave, D. & Shah, S. (2015) Comparative evaluation of platelet‐rich fibrin with demineralized freeze‐dried bone allograft in periodontal infrabony defects: a randomized controlled clinical study. Journal of Indian Society of Periodontology 19, 56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, A. & Pradeep, A. R. (2011a) Autologous platelet‐rich fibrin in the treatment of mandibular degree II furcation defects: a randomized clinical trial. Journal of Periodontology 82, 1396–1403. [DOI] [PubMed] [Google Scholar]
- Sharma, A. & Pradeep, A. R. (2011b) Treatment of 3‐wall intrabony defects in patients with chronic periodontitis with autologous platelet‐rich fibrin: a randomized controlled clinical trial. Journal of Periodontology 82, 1705–1712. [DOI] [PubMed] [Google Scholar]
- Singh, J. & Bharti, V. (2013) Laterally positioned flap‐revised technique along with platelet rich fibrin in the management of Miller class II gingival recession. Dental Research Journal 10, 268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thamaraiselvan, M. , Elavarasu, S. , Thangakumaran, S. , Gadagi, J. S. & Arthie, T. (2015) Comparative clinical evaluation of coronally advanced flap with or without platelet rich fibrin membrane in the treatment of isolated gingival recession. Journal of Indian Society of Periodontology 19, 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorat, M. , Pradeep, A. R. & Pallavi, B. (2011) Clinical effect of autologous platelet‐rich fibrin in the treatment of intra‐bony defects: a controlled clinical trial. Journal of Clinical Periodontology 38, 925–932. [DOI] [PubMed] [Google Scholar]
- Tunali, M. , Ozdemir, H. , Arabaciota, T. , Gürbüzer, B. , Pikdoken, L. & Firatli, E. (2015) Clinical evaluation of autologous platelet‐rich fibrin in the treatment of multiple adjacent gingival recession defects: a 12‐month study. International Journal of Periodontics and Restorative Dentistry 35, 105–114. [DOI] [PubMed] [Google Scholar]
- Yang, L. C. , Hu, S. W. , Yan, M. , Yang, J. J. , Tsou, S. H. & Lin, Y. Y. (2015) Antimicrobial activity of plasma‐rich plasma and other plasma preparations against periodontal pathogens. Journal of Periodontology 86, 310–318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Description of platelet concentrates (PCs) characteristics. Although PRP and PRF can be prepared with or without leucocytes (Dohan et al. 2010, Dohan et al. 2014a), this table presents the most common formulations.
Table S2. Search terms used for PUBMED, EMBASE and CENTRAL.
Table S3. Inclusion and exclusion criteria.
Table S4. Excluded articles and reason for exclusion.
Appendix S1. L‐PRF preparation. A. Blood is withdrawn from the patient. B. Tubes are centrifuged within 60 s after blood collection without any additives. C. After 12 min. of centrifugation, a clear separation between the platelet‐ poor plasma, the buffy coat and the red blood cells is obtained. D. L‐PRF is presented in the middle of the tube. E. Different L‐PRF forms can be produced: liquid, clots or membranes.
Appendix S2. Quality assessment for IBDs. Cochrane tool's for assessment of risk of bias for RCTs.
Appendix S3. Quality assessment for furcation defects. Cochrane tool's for assessment of risk of bias for RCTs.
Appendix S4. Quality assessment for IBDs for periodontal plastic surgery. Cochrane tool's for assessment of risk of bias for RCTs.


