Abstract
Purpose
To analyse the prevalence of long‐term use of proton pump inhibitors (PPI) with respect to underlying diseases and drugs, and to find predictors for such treatment when an evident rationale for the PPI treatment is lacking.
Methods
The study cohort consisted of individuals, ≥65 years in 2010, residing in the Region Västra Götaland during 2005–2010. For individuals with and without long‐term use of PPI in 2010, we investigated the prevalence of an underlying diagnosis, that is, an acid‐related disease during the five preceding years, as well as concomitant long‐term use of antiplatelet agents or cyclooxygenase inhibitors.
Results
In all, 278 205 individuals (median age: 74 years; 55% female; median 3 drugs per person; 5% nursing home residents, 11% with multi‐dose drug dispensing) were included in the analyses, 32 421 (12%) of whom were on long‐term treatment with PPI in 2010. For 12 253 individuals (38%) with such treatment, no underlying rationale was found. In individuals without a disease‐ or a drug‐related reason for PPI use, nursing home residence, number of drugs, female sex, but not multi‐dose drug dispensing, were associated with long‐term use of PPI; adjusted odds ratios (95% confidence interval): 1.63 (1.49; 1.78), 1.27 (1.26; 1.28), 1.24 (1.19; 1.29), and 0.94 (0.88; 1.01), respectively.
Conclusions
Long‐term use of PPI occurs in one out of nine individuals in the older population. For four out of ten of these, no reason for PPI use can be identified. Nursing home residence, female sex, and greater number of drugs predict non‐rational long‐term use of PPI. © 2016 The Authors. Pharmacoepidemiology and Drug Safety published by John Wiley & Sons Ltd.
Keywords: pharmacoepidemiology, overuse, proton pump inhibitor
Introduction
In the last decades, the use of proton pump inhibitors (PPI) has increased considerably.1, 2 Indeed, between 2001 and 2010, the number of patients on PPI treatment more than doubled.1 PPIs are effective drugs for acid‐related diseases like gastroesophageal reflux disease, esophagitis, and peptic ulcer disease. They may also prevent gastrointestinal complications for patients treated with cyclooxygenase (COX) inhibitors or antiplatelet agents.3, 4
However, although PPIs have long been perceived relatively harmless, convincing evidence now exists that PPIs may cause serious adverse reactions. Indeed, meta‐analyses show that PPI treatment is associated with Clostridium difficile enteritis,5 fractures,6 pneumonia,7 and gastric premalignant lesions.8 Further, associations with dementia have been reported,9, 10 as well as cases of acute interstitial nephritis and vitamin/mineral deficiencies.11
The basic prerequisite for rational use of medicines, ascertaining that an acceptable benefit/risk balance is at least achievable, is that there is an underlying reason for the treatment for every treated individual. Thus, at initiation and maintenance of PPI treatment, an underlying acid‐related disease should be present, or concomitant treatment with a drug with known risk of gastrointestinal ulcerations/bleedings. As older people may be more susceptible to adverse reactions, it may be even more important in this age group that a clear rationale precedes long‐term use of PPI.12 This is illustrated by the inclusion of PPIs in recent indicator sets of prescribing quality, such as the EU(7)‐PIM list13 and the Screening Tool of Older Persons' Prescriptions (STOPP).14
In selected groups of patients in hospital care and nursing home facilities, overuse of PPIs has been shown, that is, treatment without a proper reason and a lack of follow‐up.15, 16, 17, 18, 19, 20 However, to the best of our knowledge, population‐based studies are lacking. Thus, the magnitude of the overuse of PPI in the older population is not known. To shed further light on this issue, we undertook this register‐based study with the aim to analyse the prevalence of long‐term use of PPI and underlying reasons, and to find predictors for such treatment when an evident rationale for the PPI treatment is lacking.
Methods
Data
We used four population‐based registers with individual‐level data:
the Total Population Register at Statistic Sweden including life events on all residents staying at least one year in Sweden21
the Swedish Prescribed Drug Register including prescription drugs dispensed in all pharmacies in Sweden22, 23
the Social Service Register at the National Board of Health and Welfare including social support approved for residents in Sweden at certain dates
the administrative healthcare register in the Region Västra Götaland (VEGA) containing diagnoses registered within medical records in this region
The study comprised data from 1 July 2005 to 31 December 2010, linked by the unique personal identity number.24 The Total Population Register contributed with dates of death, emigration/immigration, and moving into/out of Region Västra Götaland. From the Swedish Prescribed Drug Register, data on all dispensed prescription drugs were obtained. The Social Service Register, with acceptable data quality in 2007, 2008, and 2010, provided information on whether an individual lived in a nursing home or not. From the VEGA database, we extracted data on hospital as well as primary care diagnoses according to the International Statistical Classification of Diseases and Related Health Problems (ICD‐10).
Study population
The study population consisted of individuals, ≥65 years of age in 2010, residing in the Region Västra Götaland, the second largest region in Sweden encompassing 1.7 million inhabitants. Individuals with re‐used personal identity number were excluded to avoid mismatching between registers. Further, individuals who had moved into or out of the region during the study period were excluded, as were individuals deceased during this period.
Description of procedures
Long‐term use of PPI was defined as ≥3 filled PPI prescriptions in 2010 (A02BC in the Anatomical Therapeutic Chemical (ATC) classification system),25 or ≥20 dispensings within the multi‐dose drug dispensing system during this year. This definition was based on the facts that Swedish regulations allow drug use for a maximum of three months to be reimbursed at one purchase occasion and that multi‐dose drug dispensed drugs are filled every fortnight, respectively. Thus, the definition of long‐term users reflected filled prescriptions covering at least 75% of a year.
In order to detect potential disease‐related reasons for PPI use, we identified, for each individual, in primary as well as in hospital care during 2005–2009, the presence of ICD‐10 codes representing diagnoses where PPIs are commonly considered appropriate (K20, oesophagitis; K21, gastro‐oesophageal reflux disease; K22, other diseases of oesophagus; K25, gastric ulcer; K26, duodenal ulcer; K27, peptic ulcer, site unspecified; K28, gastrojejunal ulcer).
In order to evaluate the presence of potential drug‐related reasons for PPI use, that is, gastroprotection, we explored the long‐term use in 2010 of antiplatelet agents (B01AC) and COX inhibitors (M01A, antiinflammatory and antirheumatic products, non‐steroids, excluding M01AX05, glucosamine), using the same definition for long‐term use as for PPI.
In order to characterize individuals at the beginning of the year with potential long‐term use of PPI, we estimated the number of drugs in the medication list, recorded as a continuous variable, on 31 December 2009 as a proxy for burden of disease.26 For estimations of medication lists, we used the established method, that is also employed by the National Board of Health and Welfare.27 In short, a medication list, for individuals receiving their drugs via ordinary prescriptions, was constructed according to the filled prescriptions during the three month period preceding this date. Drugs were included in the medication list if the purchase covered treatment at the date in question according to (i) the date of filling the prescription; (ii) the amount of drug dispensed; and (iii) the prescribed dosage. Multi‐dose‐dispensed drugs were included in the medication list if filled within 14 days before 31 December 2009.
An individual was categorized as having multi‐dose drug dispensing if ≥1 drug was dispensed within this system in 2010. Further, the residence of each individual was determined according to the Social Service Register. If recorded in this register in 2007, 2008, or 2010, the individual was categorized as living in a nursing home. If not, the individual was categorized as community‐dwelling.
Sensitivity analyses
To investigate if the inclusion of more non‐specific diagnoses, where PPI may be considered, would have an impact on the results, additional diagnoses were included in the disease‐related reasons (K29, gastritis and duodenitis; K92.0, haematemesis; K92.1, melaena; K92.2, gastrointestinal haemorrhage, unspecified; B98.0, helicobacter pylori as the cause of diseases classified to other chapters; E16.4, increased secretion of gastrin; Q40.1, congenital hiatus hernia; R12.9, heartburn; R13.9, dysphagia). Further, additional drug groups associated with an increased risk of gastrointestinal complications were included in the drug‐related reasons: anticoagulants (B01AA, B01AE, and B01AF), selective serotonin reuptake inhibitors (SSRI, N06AB), and glucocorticoids (H02AB).28, 29, 30
As disease‐related reasons occurring in 2010 may justify long‐term PPI treatment this year, we also performed a sensitivity analysis including diagnoses up to 2010, that is, 2005–2010. To further explore the robustness of the results, a sensitivity analysis was performed where long‐term use of PPI, for individuals outside the multi‐dose drug dispensing system, was defined as ≥3 dispensings of PPI, each covering ≥90 days of treatment.
Statistics
Statistical analyses were performed using SPSS (IBM SPSS Statistics for Windows, Version 22.0, Armonk, NY). The Mann Whitney and Chi‐square tests were used for comparisons of characteristics between groups. Logistic regression was performed to obtain odds ratios and 95% confidence intervals (CI) for long‐term use of PPI according to disease‐ and/or drug‐related reasons for gastroprotection. Adjustments were made for age, sex, number of drugs (a proxy for burden of disease),26 residence (defined as nursing home or not), and multi‐dose drug dispensing (a system which has been associated with an extensive medication list and poor quality of drug treatment).31, 32, 33 To identify predictors of long‐term use of PPI in individuals without a reason for treatment, logistic regression with and without adjustments was performed in this subgroup. As non‐indications (K30.9, functional dyspepsia; R10, abdominal and pelvic pain; K52, other and unspecified noninfective gastroenteritis and colitis) may contribute to PPI prescribing, logistic regression analyses were also performed in the subgroup of individuals without a disease‐ or drug‐related reason, but with a non‐indication.
Results
In all, 278 205 individuals were included in the analyses (Figure 1). In 2010, the number of individuals who purchased ≥1 prescription of omeprazole, esomeprazole, pantoprazole, lansoprazole, and rabeprazole was 49 440 (17.8%), 4846 (1.7%), 3160 (1.4%), 1855 (0.7%), and 10 (0.004%), respectively. A total of 32 421 (11.7%) individuals were on long‐term treatment with PPI.
Figure 1.
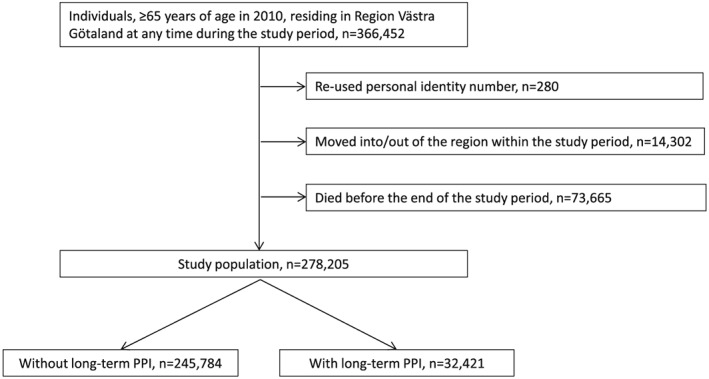
Flowchart of the studied population. The study period was 1 July 2005 to 31 December 2010
Characteristics of individuals, according to long‐term use of PPI in 2010, are presented in Table 1. Summarized, the median age was 74 years, ranging from 65 to 110 years, 153 142 (55.0%) individuals were women, 14 525 (5.2%) resided in a nursing home, and 29 376 (10.6%) had multi‐dose drug dispensing. The median (interquartile range) number of drugs at the onset of 2010 was 3 (0–5), ranging from 0 to 33.
Table 1.
Characteristics of individuals according to long‐term use of proton pump inhibitors (PPI). Values are presented as median (interquartile range) or number of individuals (percentage)
| All n = 278 205 | Disease‐ or drug‐related reason for PPI use n = 94 033 | No disease‐ or drug‐related reason for PPI use n = 184 172 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Without PPI n = 245 784 | With PPI n = 32 421 | P‐value | Without PPI n = 73 865 | With PPI n = 20 168 | P‐value | Without PPI n = 171 919 | With PPI n = 12 253 | P‐value | |
| Age, years | 74 (68–81) | 76 (70–83) | <0.0001 | 76 (70–83) | 76 (70–83) | 0.030 | 72 (68–79) | 75 (69–82) | <0.0001 |
| Female sex | 133 438 (54.3) | 19 704 (60.8) | <0.0001 | 37 799 (51.2) | 11 770 (58.4) | <0.0001 | 95 639 (55.6) | 7934 (64.8) | <0.0001 |
| Number of drugs (n) | 2 (0–5) | 6 (3–9) | <0.0001 | 4 (2–7) | 6 (3–10) | <0.0001 | 1 (0–4) | 5 (2–8) | <0.0001 |
| Nursing home resident | 11 085 (4.5) | 3440 (10.6) | <0.0001 | 4921 (6.7) | 2092 (10.4) | <0.0001 | 6164 (3.6) | 1348 (11.0) | <0.0001 |
| Multi‐dose drug dispensing | 22 713 (9.2) | 6663 (20.6) | <0.0001 | 10 053 (13.6) | 4246 (21.1) | <0.0001 | 12 660 (7.4) | 2417 (19.7) | <0.0001 |
For 20 168 (62.2%) individuals out of 32 421 with long‐term use of PPI, an underlying acid‐related disease or a drug‐related reason for PPI use could be identified. These individuals constituted 20.4% of all individuals with such a reason. For the remaining 12 253 (37.8%) individuals with long‐term use of PPI, no rationale for this treatment was found. These individuals constituted 6.7% of all individuals without a disease‐ or a drug‐related reason for PPI use.
The crude odds (95%CI) for long‐term use of PPI, compared to absence of such use, were 3.83 (3.74; 3.92) times greater for individuals with an underlying disease‐ or drug‐related reason (Table 2). After adjustments for age, sex, number of drugs, residence, and multi‐dose drug dispensing, the odds ratio was 2.55 (2.49; 2.62). Overall, disease‐related reasons predicted long‐term use of PPI to a greater extent than did drug‐related reasons; 8.30 (8.02; 8.59) versus 1.43 (1.39; 1.46).
Table 2.
Crude and adjusted odds ratios with 95% confidence intervals (CI) for long‐term use of proton pump inhibitors (PPI) according to potential disease‐ and/or drug‐related reasons for PPI use. Adjustments were made for age, sex, number of drugs, residence, and multi‐dose drug dispensing. Other values are given as number of individuals (percentage)
| Without PPI (reference) n = 245 784 | With PPI n = 32 421 | Crude OR (95%CI) | Adjusted OR (95%CI) | ||
|---|---|---|---|---|---|
| Any disease‐ or drug‐related reason below | 73 865 (30.1) | 20 168 (62.2) | 3.83 (3.74; 3.92) | 2.55 (2.49; 2.62) | |
| Disease‐related reason | Any diagnosis below | 9365 (3.8) | 8407 (25.9) | 8.84 (8.56; 9.13) | 8.30 (8.02; 8.59) |
| Oesophagitis | 539 (0.2) | 606 (1.9) | 8.67 (7.71; 9.74) | 7.89 (6.95; 8.95) | |
| Gastro‐oesophageal reflux disease | 5954 (2.4) | 5910 (18.2) | 8.98 (8.64; 9.33) | 8.97 (8.61; 9.35) | |
| Other diseases of oesophagus | 1167 (0.5) | 1182 (3.6) | 7.93 (7.31; 8.61) | 8.02 (7.35; 8.76) | |
| Gastric ulcer | 1606 (0.7) | 1450 (4.5) | 7.12 (6.62; 7.65) | 5.55 (5.13; 6.01) | |
| Duodenal ulcer | 1097 (0.4) | 770 (2.4) | 5.43 (4.95; 5.96) | 4.82 (4.36; 5.34) | |
| Peptic ulcer, site unspecified | 175 (0.07) | 148 (0.5) | 6.44 (5.17; 8.01) | 4.93 (3.88; 6.26) | |
| Gastrojejunal ulcer | 31 (0.01) | 16 (0.05) | 3.91 (2.14; 7.16) | 2.43 (1.23; 4.80) | |
| Drug‐related reason | Any drug below | 67 277 (27.4) | 15 220 (46.9) | 2.35 (2.29; 2.40) | 1.43 (1.39; 1.46) |
| Antiplateles agents | 69 048 (28.1) | 14 170 (43.7) | 1.99 (1.94; 2.04) | 1.14 (1.11; 1.17) | |
| COX inhibitors | 11 250 (4.6) | 3647 (11.2) | 2.64 (2.54; 2.75) | 2.26 (2.16; 2.36) | |
In the sensitivity analyses, including more non‐specific diagnoses in the disease‐related reasons as well as more non‐specific drugs in the drug‐related reasons, the adjusted odds ratio for long‐term use of PPI was 3.04 (2.95; 3.13). Further, the pattern regarding the odds ratios for disease‐ and drug‐related reasons was similar: 6.24 (6.06; 6.43) versus 1.64 (1.59; 1.68), respectively.
In the regression analyses in individuals without a disease‐ or a drug‐related reason for PPI use, nursing home residence, greater number of drugs, and female sex predicted long‐term use of PPI (Table 3). For individuals with multi‐dose dispensing, the crude but not the adjusted odds ratio predicted such treatment. For individuals with a non‐indication for PPI only, the results showed a similar pattern. However, in individuals with multi‐dose dispensing, the odds for PPI use were reduced. Number of drugs and nursing home residence predicted long‐term use of PPI to a smaller extent in individuals with an underlying disease‐ or drug‐related reason for PPI use, whereas the opposite was found for multi‐dose drug dispensing.
Table 3.
Crude and adjusted odds ratios (OR) with 95% confidence intervals (CI) for long‐term use of proton pump inhibitors (PPI) in individuals with and without a disease‐ or a drug‐related reason for such treatment, and in the subgroup of such individuals who in addition also had a non‐indication for PPI use
| With a disease‐ or drug‐related reason for PPI use (n = 94 033) | Without disease‐ or drug‐related reason for PPI use (n = 171 919) | Without disease‐ or drug‐related reason, but with a non‐indication, for PPI use (n = 17 231) | ||||
|---|---|---|---|---|---|---|
| Crude OR (95%CI) | Adjusted OR (95%CI)* | Crude OR (95%CI) | Adjusted OR (95%CI)* | Crude OR (95%CI) | Adjusted OR (95%CI)* | |
| Age (continuous variable) | 1.00 (1.00; 1.00) | 0.98 (0.98; 0.98) | 1.04 (1.03; 1.04) | 1.00 (0.99; 1.00) | 1.03 (1.02; 1.03) | 1.00 (0.99; 1.00) |
| Female sex (vs. male) | 1.34 (1.30; 1.38) | 1.25 (1.21; 1.30) | 1.47 (1.41; 1.52) | 1.24 (1.19; 1.29) | 1.34 (1.23; 1.45) | 1.17 (1.07; 1.28) |
| Number of drugs (continuous variable) | 1.14 (1.14; 1.15) | 1.14 (1.14; 1.15) | 1.28 (1.27; 1.28) | 1.27 (1.26; 1.28) | 1.21 (1.20; 1.22) | 1.21 (1.20; 1.22) |
| Nursing home resident (vs. community‐dwelling) | 1.62 (1.54; 1.71) | 1.19 (1.11; 1.28) | 3.32 (3.12; 3.54) | 1.63 (1.49; 1.78) | 3.14 (2.71; 3.63) | 2.09 (1.71; 2.54) |
| Multi‐dose drug dispensing (vs. ordinary prescription) | 1.69 (1.63; 1.76) | 1.24 (1.17; 1.31) | 3.09 (2.95; 3.24) | 0.94 (0.88; 1.01) | 2.31 (2.08; 2.57) | 0.78 (0.67; 0.91) |
All characteristics in the table included in the model.
In the sensitivity analysis where diagnoses up to 2010 were included, 21 493 (66.3%) individuals out of 32 421 with long‐term use of PPI had an underlying acid‐related disease or a drug‐related reason for PPI use. The adjusted odds ratio for long‐term use of PPI, compared to absence of such use, was 9.26 (8.97; 9.56) for individuals with disease‐related reasons, and 3.02 (2.94; 3.10) for individuals with disease‐ or drug‐related reasons.
In the sensitivity analysis excluding dispensed prescriptions of PPI covering less than 90 days of treatment, the number of patients on long‐term PPI was reduced to 23 692 (8.5%) individuals, 15 113 (63.8%) of whom had a disease‐ or drug‐related reason for PPI use. In the regression analysis, the adjusted odds for long‐term PPI use was 2.51 (2.44; 2.59) for individuals with a disease‐ or a drug‐related reason for such treatment.
Discussion
In this study, we show that long‐term use of PPI is common and occurs in one out of nine individuals in the older population. In six out of ten of these, an underlying rationale can be identified. Correspondingly, for four out of ten with long‐term use of PPI, no evident rationale can be identified. For these individuals, nursing home residence and female sex are predictors for long‐term use of PPI. Further, the odds of long‐term use of PPI, without an underlying reason, increase by the number of drugs in the medication list.
Regarding our finding that about 40% of older individuals on long‐term use of PPI lack an evident rationale for this treatment, other studies have reported similar15 as well as lower17, 18 and greater16, 19, 20 figures. Divergences in the results between the studies may be explained by characteristics of the populations studied, definitions used (including reasons considered appropriate for PPI treatment), and types of data used (including the extent of time period being covered). Indeed, with a shorter follow‐up and less extensive data, the chance of identifying relevant underlying diagnoses may be reduced. In our study, however, we had data, both from hospital and primary care, from the five year period preceding the year of long‐term use of PPI.
The absolute prevalence of PPI use is considerably lower in our study compared to others.15, 16, 18, 19 An explanation may be that our study reflects long‐term use of PPI, whereas previous studies focus on prevalence of PPI irrespective of length of treatment. Further, our study, as opposed to previous ones which were performed in selected inpatient groups, includes all older people irrespective of health status. Thus, our results illustrate that overtreatment with PPI is prevalent to a substantial extent in the society. Although medication reviews may not be the solution for this potential problem, because of health economics aspects36 as well as lack of effect on patient relevant outcomes,35, 36, 37 continuous education and feedback on prescribing patterns may help to improve the quality of drug treatment.38 Indeed, a simulation study reported an increased mortality among medical inpatients on PPI treatment, with net harm occurring in more than two in three patients,39 illustrating the need of further efforts within the field.
Disease‐related reasons predict long‐term PPI use to a greater extent than do drug‐related reasons. This may not be too surprising as, although the latter reasons have been reported to be the most frequent reasons for PPI use,15 they are more non‐specific. Nevertheless, as illustrated in previous studies,16, 17 inclusion of drug‐related reasons when determining the appropriateness of long‐term use of PPI is important, in order not to overestimate the prevalence of potentially inappropriate use.
For individuals without a disease‐ or a drug‐related reason for PPI treatment, the chance of long‐term use of PPI increases by almost 30% for each additional drug in the medication list. This finding needs to be emphasized, not only because of the risk of potential adverse reactions without a benefit to be expected, but also because of the risk of interactions associated with PPI.40 Thus, the effects of other drugs may be affected. A potential explanation for our finding of an increased risk of overuse of PPI in individuals treated with many drugs is that such treatment may reflect multiple comorbidities, and thus, a greater treatment complexity. Indeed, the number of drugs in the medication list is a poor indicator of prescribing quality,41 but a rather good proxy for burden of disease.26 Therefore, because of the general belief that PPIs are relatively safe, maintenance of initiated PPI treatment may occur without second thoughts because of other problems being more urgent to handle.
Encouragingly, long‐term use of PPI is about four times as common in individuals where an underlying reason can be identified, compared to individuals without such a reason. However, when relevant cofactors are considered in the analyses, these odds are reduced to a considerable extent. Consequently, other factors, not related to the medical need, may explain long‐term use of PPI. Indeed, nursing home residence was the most prominent predictor for non‐rationale long‐term use of PPI. These results are in concordance with a previous study reporting a large proportion of nursing home patients to have PPI treatment without an underlying diagnosis.17 Thus, the predictors for inappropriate use of PPI identified in the present study, having a long medication list and living in a nursing home, may help clinicians to focus their efforts.
Interestingly, for patients without a disease‐ or a drug‐related reason for PPI use but with a non‐indication, the odds for long‐term use of PPI, after relevant adjustments, are lower in individuals with multi‐dose drug dispensing. Indeed, drug treatment changes, including addition of drugs, have been reported to occur more seldom within this system,42 and this may contribute to this finding. Thus, when it comes to the use of PPIs, the system may be of benefit for the patient, as opposed to the results found regarding the quality of drug treatment in general.31, 32, 33
In this study, use of PPI is analysed in individuals 65 years or older, that is, the age group which has previously been identified to be more appropriately treated when it comes to these drugs.15 In future research, it may be of interest to investigate the use of PPIs in individuals below the age of 65.
The most important strength of this study is that it provides scientific knowledge on the prevalence of overuse of PPI in the elderly at the population level. The large number of individuals, representing all socioeconomic classes and both rural and urban areas, as well as the fact that few individuals were excluded, makes it reasonable to generalize from the results. In addition, linkage between registers allowed us to consider relevant cofactors in the regression analyses. Further, although the prevalence of long‐term use of PPI was somewhat reduced, the sensitivity analysis with a more restricted definition of such treatment revealed similar results concerning the extent of overtreatment.
Limitations of this study include, as always in register‐based studies, that the results depend on the quality and the content of the registers. However, all register data used in the present study are of good quality and have an almost complete coverage. Nevertheless, we cannot exclude that appropriate disease‐ or drug‐related indications may exist which are not captured by the register data. For example, COX inhibitors may be bought over‐the‐counter, and physicians may refrain from recording uncertain diagnoses. Further, an underlying diagnosis may have been recorded before 2005. Although we believe that these issues are of minor importance in the present study, we cannot exclude that the prevalence of overuse can be somewhat overestimated. On the other hand, as a diagnosis occurring during the five years preceding the long‐term use of PPI may not justify such treatment, our study may also underestimate the overuse of PPI. Interestingly, extending the capturing period of disease‐related reasons to the year in which the long‐term use of PPI occurred did not have a substantial impact on the results.
Summarized, our findings support the results of prior published studies showing that overuse of PPI in older people is not negligible. Indeed, we found that long‐term use of PPI occurs in 12% of the older population, and for 38% of these, no disease‐ or drug‐related reason can be identified. Further knowledge on the benefit/risk balance of such use is warranted.
Ethics Statement
The study complies with the Declaration of Helsinki. Ethics approval was obtained from the Regional Ethical Review Board in Gothenburg (Dnr 782‐11).
Conflict of Interest
The authors declare no conflicts of interest
Funding
The study was supported by the Swedish Research Council (521‐2013‐2639) and the Health and Medical Care Committee of the Region Västra Götaland (ALFGBG‐428711). The funding sources did not influence design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Key Points.
Use of proton pump inhibitors (PPI) is increasing and overuse has been shown in selected patient groups. For rational use of medicines and an acceptable benefit–risk balance for each patient, an underlying and appropriate reason for the treatment is a basic prerequisite.
In this register‐based study, comprising 278 205 individuals ≥65 years of age, we report that long‐term use of PPI occurs in 12% of the older population. For four in ten individuals on such treatment, no disease‐ or drug‐related reason can be identified.
For individuals without a disease‐ or a drug‐related reason for PPI use, nursing home residence, female sex, and greater number of drugs in the medication list are predictors for long‐term use of PPI.
Wallerstedt, S. M. , Fastbom, J. , Linke, J. , and Vitols, S. (2017) Long‐term use of proton pump inhibitors and prevalence of disease‐ and drug‐related reasons for gastroprotection—a cross‐sectional population‐based study. Pharmacoepidemiol Drug Saf, 26: 9–16. doi: 10.1002/pds.4135.
References
- 1. Mazer‐Amirshahi M, Mullins PM, van den Anker J, et al. Rising rates of proton pump inhibitor prescribing in US emergency departments. Am J Emerg Med 2014; 32: 618–622. doi:10.1016/j.ajem.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 2. Rotman SR, Bishop TF. Proton pump inhibitor use in the U.S. ambulatory setting, 2002–2009. PLoS One 2013; 8: e56060. doi:10.1371/journal.pone.0056060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mo C, Sun G, Lu ML, et al. Proton pump inhibitors in prevention of low‐dose aspirin‐associated upper gastrointestinal injuries. World J Gastroenterol 2015; 21: 5382–5392. doi:10.3748/wjg.v21.i17.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rostom A, Dube C, Wells G, et al. Prevention of NSAID‐induced gastroduodenal ulcers. Cochrane Database Syst Rev 2002; Cd002296. doi:10.1002/14651858.cd002296. [DOI] [PubMed] [Google Scholar]
- 5. Deshpande A, Pant C, Pasupuleti V, et al. Association between proton pump inhibitor therapy and Clostridium difficile infection in a meta‐analysis. Clin Gastroenterol Hepatol 2012; 10: 225–233. doi:10.1016/j.cgh.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 6. Ngamruengphong S, Leontiadis GI, Radhi S, et al. Proton pump inhibitors and risk of fracture: a systematic review and meta‐analysis of observational studies. Am J Gastroenterol 2011; 106: 1209–1218 quiz 19. doi:10.1038/ajg.2011.113. [DOI] [PubMed] [Google Scholar]
- 7. Sarkar M, Hennessy S, Yang YX. Proton‐pump inhibitor use and the risk for community‐acquired pneumonia. Ann Intern Med 2008; 149: 391–398. [DOI] [PubMed] [Google Scholar]
- 8. Song H, Zhu J, Lu D. Long‐term proton pump inhibitor (PPI) use and the development of gastric pre‐malignant lesions. Cochrane Database Syst Rev 2014; 12 Cd010623. doi:10.1002/14651858.CD010623.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci 2015; 265: 419–428. doi:10.1007/s00406-014-0554-0. [DOI] [PubMed] [Google Scholar]
- 10. Gomm W, von Holt K, Thome F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016; 73: 410–416. doi:10.1001/jamaneurol.2015.4791. [DOI] [PubMed] [Google Scholar]
- 11. Abraham NS. Proton pump inhibitors: potential adverse effects. Curr Opin Gastroenterol 2012; 28: 615–620. doi:10.1097/MOG.0b013e328358d5b9. [DOI] [PubMed] [Google Scholar]
- 12. Sheen E, Triadafilopoulos G. Adverse effects of long‐term proton pump inhibitor therapy. Dig Dis Sci 2011; 56: 931–950. doi:10.1007/s10620-010-1560-3. [DOI] [PubMed] [Google Scholar]
- 13. Renom‐Guiteras A, Meyer G, Thurmann PA. The EU(7)‐PIM list: a list of potentially inappropriate medications for older people consented by experts from seven European countries. Eur J Clin Pharmacol 2015; 71: 861–875. doi:10.1007/s00228-015-1860-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O'Mahony D, O'Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015; 44: 213–218. doi:10.1093/ageing/afu145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lodato F, Poluzzi E, Raschi E, et al. Appropriateness of PPI prescription in patients admitted to hospital: attitudes of general practitioners and hospital physicians in Italy. Eur J Intern Med 2016. doi:10.1016/j.ejim.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 16. Reid M, Keniston A, Heller JC, et al. Inappropriate prescribing of proton pump inhibitors in hospitalized patients. J Hosp Med 2012; 7: 421–425. doi:10.1002/jhm.1901. [DOI] [PubMed] [Google Scholar]
- 17. Patterson Burdsall D, Flores HC, Krueger J, et al. Use of proton pump inhibitors with lack of diagnostic indications in 22 Midwestern US skilled nursing facilities. J Am Med Dir Assoc 2013; 14: 429–432. doi:10.1016/j.jamda.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 18. Heidelbaugh JJ, Goldberg KL, Inadomi JM. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care 2010; 16: e228–e234. [PubMed] [Google Scholar]
- 19. Ramirez E, Lei SH, Borobia AM, et al. Overuse of PPIs in patients at admission, during treatment, and at discharge in a tertiary Spanish hospital. Curr Clin Pharmacol 2010; 5: 288–297. [DOI] [PubMed] [Google Scholar]
- 20. Zink DA, Pohlman M, Barnes M, et al. Long‐term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther 2005; 21: 1203–1209. doi:10.1111/j.1365-2036.2005.02454.x. [DOI] [PubMed] [Google Scholar]
- 21. Ludvigsson JF, Almqvist C, Bonamy AK, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol 2016; 31: 125–136. doi:10.1007/s10654-016-0117-y. [DOI] [PubMed] [Google Scholar]
- 22. Wallerstedt SM, Wettermark B, Hoffmann M. The first decade with the Swedish Prescribed Drug Register—a systematic review of the output in the scientific literature. Basic Clin Pharmacol Toxicol 2016; 119: 464–469. doi: 10.1111/bcpt.12613. [DOI] [PubMed] [Google Scholar]
- 23. Wettermark B, Hammar N, Fored CM, et al. The new Swedish Prescribed Drug Register—opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 2007; 16: 726–735. doi:10.1002/pds.1294. [DOI] [PubMed] [Google Scholar]
- 24. Ludvigsson JF, Otterblad‐Olausson P, Pettersson BU, et al. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol 2009; 24: 659–667. doi:10.1007/s10654-009-9350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC classification and DDD assignment 2013. [Available from: http://www.whocc.no/filearchive/publications/1_2013guidelines.pdf.
- 26. Brilleman SL, Salisbury C. Comparing measures of multimorbidity to predict outcomes in primary care: a cross sectional study. Fam Pract 2013; 30: 172–178. doi:10.1093/fampra/cms060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnell K, Fastbom J, Rosen M, et al. Inappropriate drug use in the elderly: a nationwide register‐based study. Ann Pharmacother 2007; 41: 1243–1248. doi:10.1345/aph.1K154. [DOI] [PubMed] [Google Scholar]
- 28. He Y, Wong IC, Li X, et al. The association between Non‐vitamin K antagonist oral anticoagulants and gastrointestinal bleeding: a meta‐analysis of observational studies. Br J Clin Pharmacol 2016. doi:10.1111/bcp.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dall M, Schaffalitzky de Muckadell OB, Lassen AT, et al. An association between selective serotonin reuptake inhibitor use and serious upper gastrointestinal bleeding. Clin Gastroenterol Hepatol 2009; 7: 1314–1321. doi:10.1016/j.cgh.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 30. Narum S, Westergren T, Klemp M. Corticosteroids and risk of gastrointestinal bleeding: a systematic review and meta‐analysis. BMJ Open 2014; 4: e004587. doi:10.1136/bmjopen-2013-004587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sjöberg C, Edward C, Fastbom J, et al. Association between multi‐dose drug dispensing and quality of drug treatment—a register‐based study. PLoS One 2011; 6: e26574. doi:10.1371/journal.pone.0026574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wallerstedt SM, Fastbom J, Johnell K, et al. Drug treatment in older people before and after the transition to a multi‐dose drug dispensing system—a longitudinal analysis. PLoS One 2013; 8: e67088. doi:10.1371/journal.pone.0067088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Belfrage B, Koldestam A, Sjöberg C, et al. Prevalence of suboptimal drug treatment in patients with and without multidose drug dispensing—a cross‐sectional study. Eur J Clin Pharmacol 2014; 70: 867–872. doi:10.1007/s00228-014-1683-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wallerstedt SM, Bladh L, Ramsberg J. A cost‐effectiveness analysis of an in‐hospital clinical pharmacist service. BMJ Open 2012; 2: e000329. doi:10.1136/bmjopen-2011-000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Christensen M, Lundh A. Medication review in hospitalised patients to reduce morbidity and mortality. Cochrane Database Syst Rev 2016; 2 Cd008986. doi:10.1002/14651858.CD008986.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hohl CM, McGrail K, Sobolev B. The effect of pharmacist‐led medication review in high‐risk patients in the emergency department: an evaluation protocol. CMAJ Open 2015; 3: E103–E110. doi:10.9778/cmajo.20140010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wallerstedt SM, Kindblom JM, Nylén K, et al. Medication reviews for nursing home residents to reduce mortality and hospitalization: systematic review and meta‐analysis. Br J Clin Pharmacol 2014; 78: 488–497. doi:10.1111/bcp.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O'Brien MA, Rogers S, Jamtvedt G, et al. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2007; Cd000409. doi:10.1002/14651858.CD000409.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pappas M, Jolly S, Vijan S. Defining appropriate use of proton‐pump inhibitors among medical inpatients. J Gen Intern Med 2016; 31: 364–371. doi:10.1007/s11606-015-3536-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yucel E, Sancar M, Yucel A, et al. Adverse drug reactions due to drug–drug interactions with proton pump inhibitors: assessment of systematic reviews with AMSTAR method. Expert Opin Drug Saf 2016; 15: 223–236. doi:10.1517/14740338.2016.1128413. [DOI] [PubMed] [Google Scholar]
- 41. Belfrage B, Koldestam A, Sjöberg C, et al. Number of drugs in the medication list as an indicator of prescribing quality: a validation study of polypharmacy indicators in older hip fracture patients. Eur J Clin Pharmacol 2015; 71: 363–368. doi:10.1007/s00228-014-1792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sjöberg C, Ohlsson H, Wallerstedt SM. Association between multi‐dose drug dispensing and drug treatment changes. Eur J Clin Pharmacol 2012; 68: 1095–1101. doi:10.1007/s00228-012-1230-9. [DOI] [PubMed] [Google Scholar]


