ABSTRACT
BACKGROUND
Spinal cord atrophy occurs early in the multiple sclerosis (MS) disease course, is closely related to physical disability, and is a putative neuroprotective therapeutic outcome measure.
OBJECTIVE
This pilot study explored glatiramer acetate (GA)’s effect on spinal cord volume in patients with relapsing‐remitting MS (RRMS).
METHODS
Fifteen patients receiving daily subcutaneous GA were prospectively followed. At baseline, age was 43.6 ± 7.4 years, Expanded Disability Status Scale (EDSS) score was 1.4 ± 1.5, timed 25‐foot walk (T25FW) was 4.7 ± 1.1 seconds, and time on GA was 2.1 ± 3.1 years. Healthy controls (n = 10) with similar age and sex to the patients were also enrolled. The spinal cord was imaged at baseline and one year later with 3T magnetic resonance imaging. An active surface method measured the C1–C7 spinal cord volume from which we calculated the normalized area.
RESULTS
The spinal cord area showed no significant change in the MS group over one year (P = .19). Furthermore, the change in the spinal cord area did not differ significantly between the MS and control groups over one year (P = .26). In the MS group, the EDSS score (P = .44) and T25FW (P = .92) did not change significantly on‐study.
CONCLUSION
In this pilot study of RRMS, GA therapy was not associated with any ongoing spinal cord atrophy or any difference in the one‐year rate of spinal cord area change versus healthy controls. These results paralleled the lack of clinical worsening and may reflect a treatment effect of GA. Further studies are needed to confirm these preliminary findings.
Keywords: MRI, multiple sclerosis, glatiramer acetate, spinal cord atrophy
Introduction
Spinal cord atrophy is present in multiple sclerosis (MS) early in the disease course and is a major contributor to physical disability.1, 2, 3, 4 Improvements in magnetic resonance imaging (MRI) technology, both in scan acquisition and segmentation techniques, have led to a growing interest in the measurement of spinal cord atrophy to assess disease progression and treatment effects.1, 4, 5, 6, 7, 8 However, few studies to date have assessed the effects of MS disease‐modifying therapy (DMT) on spinal cord atrophy.1, 9, 10, 11, 12 Yet, there is increasing evidence to suggest that brain and spinal cord MS involvement are independent from each other1, 13, 14, 15, 16 and provide complementary information about disease severity and treatment effects.17
Glatiramer acetate (GA), given subcutaneously, is an approved DMT for MS that has been shown to reduce relapse rates, decrease cerebral MRI‐defined lesion load and activity, and limit increases in physical disability.18 While a few MS studies have demonstrated a reduction in brain atrophy in patients receiving GA,19, 20, 21, 22 its effect on spinal cord atrophy has not been studied. Our study explored the one‐year effect of GA treatment on cervical spinal cord volume.
Methods
Subjects
Our hospital's institutional review board approved this study. Participants gave written informed consent. The participant's medical record was examined, followed by a telephone interview to determine suitability to enter the study. We excluded those with a history of major medical, neurologic, or neuropsychiatric disorders; current or prior history of substance abuse; or any condition that precluded MRI. Subjects were also excluded if they had congenital or acquired spinal canal narrowing on MRI scans to avoid any confounding myelopathic effects on cord volume. All patients with MS had not experienced a relapse or corticosteroid use within the four weeks before study entry and had initiated GA therapy (20 mg/day subcutaneously) within the 10 years before study entry. Total enrollment was 25 subjects: 15 with relapsing‐remitting MS (RRMS) (13 women) and 10 healthy controls (8 women). Recruitment age was (mean ± SD) 43.6 ± 7.4 years for the MS group and 45.1 ± 5.7 years for healthy controls. There was no statistically significant difference in age and sex distribution between the two groups (P > .05, Table 1). An MS specialist neurologist performed clinical examinations every six months, and provided routine clinical care during the observation period. At baseline, patients had a disease duration (time since first symptoms) of 7.5 ± 7.2 years; the time on GA was 2.1 ± 3.1 years. Baseline Expanded Disability Status Scale (EDSS) scores and timed 25‐foot walk (T25FW) values are noted in Table 1.
Table 1.
Clinical and MRI Data in Patients with Multiple Sclerosis and Healthy Controls at Baseline and One‐Year Follow‐Up
| Patients | Controls | |
|---|---|---|
| Number of subjects | 15 | 10 |
| Age (years)† | 43.6 ± 7.4 | 45.1 ± 5.7 |
| Range | 27.4 ‐ 55.2 | 31.9 ‐ 49.4 |
| Women, n (%)†† | 13 (87%) | 8 (80%) |
| Expanded Disability Status Scale score* | ||
| Baseline | 1.4 ± 1.5 | – |
| Follow‐up | 1.2 ± 1.7 | – |
| Timed 25‐foot walk (seconds)** | ||
| Baseline | 4.7 ± 1.1 | – |
| Follow‐up | 4.9 ± 1.0 | – |
| Cervical spinal cord cross‐sectional area (mm2) | ||
| Baseline | 60.0 ± 9.9 | 61.3 ± 6.9 |
| Follow‐up | 61.6 ± 9.7 | 64.8 ± 8.6 |
| Change in cross‐sectional area (mm2)*** | 1.6 ± 4.5 | 3.5 ± 3.9 |
Note: Data are mean ± SD except as noted.
† P = .495; †† P = 1.0
* P = .44 (baseline vs. follow‐up); ** P = .92 (baseline vs. follow‐up); *** P = .26.
MRI Acquisition
All subjects underwent cervical spinal cord imaging on the same 3T scanner with a consistent acquisition protocol throughout the study. Spine MRI was performed with an eight‐channel phased array coil at 20‐mT/m maximal gradient strength at baseline and one year later. Spinal axial T2 fast spin‐echo images covered the whole spinal cord and had the following parameters: field‐of‐view (FOV), 24 × 19 cm; matrix size, 256 × 256; section thickness, 3 mm with no gap; repitition time (TR), 6,116.66 ms; echo time (TE), 110.24 ms; echo‐train length, 12; number of signal intensity averages, 2; flip angle, 90°; and voxel size, .937 × .937 × 3 mm.
MRI Analysis
MRI scans were transferred to our laboratory where analysis was performed using the Jim software package (Version 7.0, Xinapse Systems, Northants, United Kingdom; www.xinapse.com). Analysis was performed by two experienced observers who were unaware of clinical information. Spinal cord measurements were obtained from the C1–C7 vertebral levels, using a semiautomated pipeline. The cord contour was first determined using a highly reproducible active surface tool.5 Sagittal reconstructed images were cross‐referenced to the axial images to allow precise identification of vertebral levels. Manual adjustments were applied where necessary to assure accurate cord contours. The final cervical (C1–C7) spinal cord cross‐sectional area was obtained by dividing the total volume by the number of axial slices. This method of normalization is based on our previous work.23 The mean cross‐sectional area obtained in our laboratory by this T2‐derived technique shows high intrarater and interrater reliability, with coefficients of variation of .66% and .99%, respectively.24
In addition, the same trained observers assessed the number of spinal cord lesions in each subject. These data are provided for descriptive purposes only.
Statistical Analysis
On‐study one‐year change in spinal cord area was compared between the MS and NC groups using the exact Wilcoxon rank sum test. In the MS group, on‐study change in EDSS and T25W was tested using the Wilcoxon signed rank test. Between group age was compared with the exact Wilcoxon rank sum test and sex was compared with a Fisher's exact test. A P < .05 was considered statistically significant.
Results
There was no statistically significant difference in the on‐study change in spinal cord area between the MS and control groups over one year. The mean change in spinal cord area was 1.6 ± 4.5 mm2 in patients with MS and 3.5 ± 3.9 mm2 in the control group (P = .26) (Table 1, Fig 1).
Figure 1.

Spinal cord change in both groups over one year. Changes in cervical spinal cord cross‐sectional area (one‐year follow‐up minus baseline) in healthy controls (HC) and patients with multiple sclerosis (MS). Boxplots are presented with diamonds representing means, lines representing medians, areas extending from lower to upper quartiles, and whiskers extending to minima and maxima. The mean change in spinal cord area was 1.6 ± 4.5 mm2 with 95% confidence interval (−.9, 4.1) in MS, and 3.5 ± 3.9 mm2 with 95% confidence interval (.7, 6.3) in HC (P = .26).
Regarding disability measures, there was no statistically significant difference in EDSS scores between baseline and one‐year follow‐up in the MS group (1.4 ± 1.5 vs. 1.2 ± 1.7, P = .44). Similarly, there were no statistically significant on‐study changes in T25FW (4.7 ± 1.1 vs. 4.9 ± 1.0 seconds, P = .92) (Table 1).
The number of lesions per patient in the whole spinal cord was .40 ± .82 (range 0–2) at baseline, and .46 ± .82 (range 0–2) at follow‐up (Fig 2). Most of the MS subjects (73%) were free of lesions at both baseline and follow‐up scans. No spinal cord lesions were detected in the healthy controls at either the baseline or one‐year time point.
Figure 2.
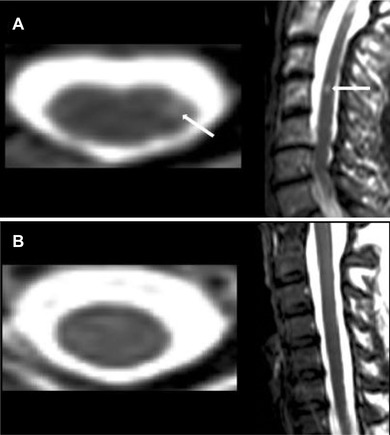
Sample images; MRI‐defined spinal cord lesion versus normal appearing spinal cord. Representative T2‐weighted 3T MRI scans are shown. (A) A hyperintense lesion (arrows) indicating a cervical spinal cord lesion (at the C4 vertebral level) is seen in the axial and sagittal planes of a 50‐year‐old woman with relapsing‐remitting MS (disease duration = 16 years, Expanded Disability Status Scale score = 0, timed 25‐foot walk = 4.6 seconds). (B) 31‐year‐old woman, healthy control, with no lesions (axial image shown from the C4 vertebral level).
Discussion
We report the results of a pilot study investigating the one‐year effect on cervical spinal cord volume of GA treatment in patients with RRMS. This was a “real‐world” study with no untreated comparison patient group available for comparison. This reflects the widespread usage and availability of numerous DMTs for RRMS. Atrophy was not detected in patients over one year and there was no difference in their spinal cord area change during the year as compared to healthy controls. These findings may represent a protective effect of GA in preventing spinal cord atrophy and neurodegeneration. Although this inference is not definitive because of the lack of a control untreated MS group, our study provides valuable information, as few studies have examined spinal cord atrophy in response to treatment in RRMS.
Previous studies have shown a partial but significant treatment effect on brain atrophy in MS patients treated with GA.19, 20, 21, 22 Such an effect can be demonstrated fairly consistently in placebo‐controlled phase III studies of a range of DMTs in patients with RRMS.22 In terms of the potential effect of GA on spinal cord atrophy, Shipova et al25 have shown significantly less cervical spinal cord atrophy in MS patients treated with GA versus those on interferon beta treatment. In their study, spinal cord atrophy measurement was based on the linear size of spinal cord on sagittal sections at the level of the inferior margin of the C2 vertebral body.
Similarly, in animal studies, GA treatment was associated with an increase in myelinated axons and decreased microglial/macrophage activation and T‐cell infiltration in an experimental allergic encephalomyelitis (EAE) model. Moreover, there were fewer amyloid precursor protein positive axons in the spinal cord of GA‐treated versus untreated EAE mice, suggesting reduced axonal degeneration.26 Other mechanistic studies have suggested that GA‐reactive Th2 lymphocytes may have anti‐inflammatory, neuroprotective, and bystander suppressive effects.18 GA treatment is hypothesized to exert its neuroprotective effect, in part, by upregulating brain‐derived neurotrophic factor (BDNF).27
A controversy exists regarding the time of appearance of spinal cord atrophy in the disease course of MS. While some studies have shown spinal cord atrophy early in the course of the disease including in patients with their first symptoms of demyelination or early stages of RRMS,3, 28, 29, 30, 31 others have not confirmed these findings and contend that spinal cord atrophy does not appear in MS until later stages of RRMS or in the secondary progressive phase of the disease.1, 5, 8, 23, 32 Furthermore, the presence of inflammation and edema, particularly in the early stages of MS, may mask spinal cord atrophy. This lack of sensitivity to early spinal cord atrophy in MS may be potentially overcome by focusing on gray matter (instead of whole spinal cord) volume loss.7 Hence, it is possible that our findings may have been limited by methodological issues. In addition, the patient characteristics of our sample presented a potential sensitivity bias, owing to the mild disability, and lack of spinal cord lesions in many of the patients, despite their advanced age and disease duration.
In terms of our technical approach, we obtained normalized spinal cord areas using a highly reproducible semiautomated segmentation tool. This segmentation approach was applied to 2‐dimensional images given their availability and our prior demonstration that these images were highly reproducible and sensitive to disease‐specific effects in assessing cord volume.24 Several groups have used high‐resolution 3‐dimensional images13, 33, 34, 35 and fully automated segmentation pipelines6, 8, 36 to measure spinal cord volume, which we did not employ in this study. Hence, it is possible that our MRI approach, both on the basis of scan acquisition and postprocessing technique, may have lacked the precision to detect ongoing spinal cord atrophy.
Conclusion
In this pilot study of patients with RRMS, GA therapy was not associated with any significant ongoing spinal cord atrophy over one year. There was also no significant difference in the one‐year rate of spinal cord area change compared to healthy controls. These results paralleled the lack of clinical worsening and may reflect a treatment effect of GA. However, further studies, addressing a range of methodologic issues and patient characteristics, and a larger sample size, are needed to confirm these preliminary findings.
Acknowledgement: None.
Funding: This work was supported by a research grant from Teva Neuroscience, who also provided a Medical Accuracy review of this manuscript.
Conflict of Interests: R. Bakshi has received consulting fees from AbbVie, Alkermes, Biogen, Novartis, and Questcor, and has received research support from Biogen, EMD‐Serono, Novartis, Sanofi‐Genzyme, and Teva. The other authors have nothing to disclose.
References
- 1. Lin X, Tench CR, Evangelou N, et al. Measurement of spinal cord atrophy in multiple sclerosis. J Neuroimaging 2004;14:20S‐6S. [DOI] [PubMed] [Google Scholar]
- 2. Minagar A, Toledo EG, Alexander JS, et al. Pathogenesis of brain and spinal cord atrophy in multiple sclerosis. J Neuroimaging 2004;14:5S‐10S. [DOI] [PubMed] [Google Scholar]
- 3. Bakshi R, Dandamudi VS, Neema M, et al. Measurement of brain and spinal cord atrophy by magnetic resonance imaging as a tool to monitor multiple sclerosis. J Neuroimaging 2005;15:30S‐45S. [DOI] [PubMed] [Google Scholar]
- 4. Gass A, Rocca MA, Agosta F, et al. MRI monitoring of pathological changes in the spinal cord in patients with multiple sclerosis. Lancet Neurol 2015;14:443‐54. [DOI] [PubMed] [Google Scholar]
- 5. Horsfield MA, Sala S, Neema M, et al. Rapid semi‐automatic segmentation of the spinal cord from magnetic resonance images: application in multiple sclerosis. Neuroimage 2010;50:446‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Leener B, Kadoury S, Cohen‐Adad J. Robust, accurate and fast automatic segmentation of the spinal cord. Neuroimage 2014;98:528‐36. [DOI] [PubMed] [Google Scholar]
- 7. Schlaeger R, Papinutto N, Panara V, et al. Spinal cord gray matter atrophy correlates with multiple sclerosis disability. Ann Neurol 2014;76:568‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yiannakas MC, Mustafa AM, De Leener B, et al. Fully automated segmentation of the cervical cord from T1‐weighted MRI using propseg: application to multiple sclerosis. Neuroimage Clin 2016;10:71‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kalkers NF, Barkhof F, Bergers E, et al. The effect of the neuroprotective agent riluzole on MRI parameters in primary progressive multiple sclerosis: a pilot study. Mult Scler 2002;8:532‐3. [DOI] [PubMed] [Google Scholar]
- 10. Lin X, Tench CR, Turner B, et al. Spinal cord atrophy and disability in multiple sclerosis over four years: application of a reproducible automated technique in monitoring disease progression in a cohort of the interferon beta‐1a (Rebif) treatment trial. J Neurol Neurosurg Psychiatry 2003;74:1090‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rudick RA. Impact of disease‐modifying therapies on brain and spinal cord atrophy in multiple sclerosis. J Neuroimaging 2004;14:54S‐64S. [DOI] [PubMed] [Google Scholar]
- 12. Filippi M, Preziosa P, Rocca MA. Magnetic resonance outcome measures in multiple sclerosis trials: time to rethink? Curr Opin Neurol 2014;27:290‐9. [DOI] [PubMed] [Google Scholar]
- 13. Filippi M, Bozzali M, Horsfield MA, et al. A conventional and magnetization transfer MRI study of the cervical cord in patients with MS. Neurology 2000;54:207‐13. [DOI] [PubMed] [Google Scholar]
- 14. Sombekke MH, Lukas C, Crusius JB, et al. Hla‐DRB1*1501 and spinal cord magnetic resonance imaging lesions in multiple sclerosis. Arch Neurol 2009;66:1531‐6. [DOI] [PubMed] [Google Scholar]
- 15. Cohen AB, Neema M, Arora A, et al. The relationships among MRI‐defined spinal cord involvement, brain involvement, and disability in multiple sclerosis. J Neuroimaging 2012;22:122‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kearney H, Rocca MA, Valsasina P, et al. Magnetic resonance imaging correlates of physical disability in relapse onset multiple sclerosis of long disease duration. Mult Scler 2014;20:72‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bakshi R, Neema M, Tauhid S, et al. An expanded composite scale of MRI‐defined disease severity in multiple sclerosis: MRDSS2. Neuroreport 2014;25:1156‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dhib‐Jalbut S. Glatiramer acetate (Copaxone) therapy for multiple sclerosis. Pharmacol Ther 2003;98:245‐55. [DOI] [PubMed] [Google Scholar]
- 19. Ge Y, Grossman RI, Udupa JK, et al. Glatiramer acetate (Copaxone) treatment in relapsing‐remitting ms: quantitative MR assessment. Neurology 2000;54:813‐7. [DOI] [PubMed] [Google Scholar]
- 20. Sormani MP, Rovaris M, Valsasina P, et al. Measurement error of two different techniques for brain atrophy assessment in multiple sclerosis. Neurology 2004;62:1432‐4. [DOI] [PubMed] [Google Scholar]
- 21. Bendfeldt K, Egger H, Nichols TE, et al. Effect of immunomodulatory medication on regional gray matter loss in relapsing‐remitting multiple sclerosis—a longitudinal MRI study. Brain Res 2010;1325:174‐82. [DOI] [PubMed] [Google Scholar]
- 22. Tsivgoulis G, Katsanos AH, Grigoriadis N, et al. The effect of disease modifying therapies on brain atrophy in patients with relapsing‐remitting multiple sclerosis: a systematic review and meta‐analysis. PLoS One 2015;10:e0116511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Healy BC, Arora A, Hayden DL, et al. Approaches to normalization of spinal cord volume: application to multiple sclerosis. J Neuroimaging 2012;22:e12‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim G, Khalid F, Oommen VV, et al. T1‐ vs. T2‐based MRI measures of spinal cord volume in healthy subjects and patients with multiple sclerosis. BMC Neurol 2015;15:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shipova EG, Spirin NN, Kasatkin DS, et al. State of the cervical section of the spinal cord in patients with remitting multiple sclerosis during immunomodulatory treatment. Neurosci Behav Physiol 2009;39:47‐51. [DOI] [PubMed] [Google Scholar]
- 26. Moore S, Khalaj AJ, Patel R, et al. Restoration of axon conduction and motor deficits by therapeutic treatment with glatiramer acetate. J Neurosci Res 2014;92:1621‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sarchielli P, Zaffaroni M, Floridi A, et al. Production of brain‐derived neurotrophic factor by mononuclear cells of patients with multiple sclerosis treated with glatiramer acetate, interferon‐beta 1a, and high doses of immunoglobulins. Mult Scler 2007;13:313‐31. [DOI] [PubMed] [Google Scholar]
- 28. Stevenson L, Leary SM, Losseff NA, et al. Spinal cord atrophy and disability in MS: a longitudinal study. Neurology 1998;51:234‐8. [DOI] [PubMed] [Google Scholar]
- 29. Brex PA, Leary SM, O'Riordan JI, et al. Measurement of spinal cord area in clinically isolated syndromes suggestive of multiple sclerosis. J Neurol Neurosurg Psychiatry 2001;70:544‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kearney H, Miller DH, Ciccarelli O. Spinal cord MRI in multiple sclerosis–diagnostic, prognostic and clinical value. Nat Rev Neurol 2015;11:327‐38. [DOI] [PubMed] [Google Scholar]
- 31. Schlaeger R, Papinutto N, Zhu AH, et al. Association between thoracic spinal cord gray matter atrophy and disability in multiple sclerosis. JAMA Neurol 2015;72:897‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rocca MA, Horsfield MA, Sala S, et al. A multicenter assessment of cervical cord atrophy among MS clinical phenotypes. Neurology 2011;76:2096‐102. [DOI] [PubMed] [Google Scholar]
- 33. Kearney H, Miszkiel KA, Yiannakas MC, et al. A pilot MRI study of white and grey matter involvement by multiple sclerosis spinal cord lesions. Mult Scler Relat Disord 2013;2:103‐8. [DOI] [PubMed] [Google Scholar]
- 34. Nair G, Absinta M, Reich DS. Optimized T1‐MPRAGE sequence for better visualization of spinal cord multiple sclerosis lesions at 3T. AJNR Am J Neuroradiol 2013;34:2215‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Papinutto N, Schlaeger R, Panara V, et al. Age, gender and normalization covariates for spinal cord gray matter and total cross‐sectional areas at cervical and thoracic levels: a 2D phase sensitive inversion recovery imaging study. PLoS One 2015;10:e0118576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen M, Carass A, Oh J, et al. Automatic magnetic resonance spinal cord segmentation with topology constraints for variable fields of view. Neuroimage 2013;83:1051‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]


