Abstract
CzcD from Ralstonia metallidurans and ZitB from Escherichia coli are prototypes of bacterial members of the cation diffusion facilitator (CDF) protein family. Expression of the czcD gene in an E. coli mutant strain devoid of zitB and the gene for the zinc-transporting P-type ATPase zntA rendered this strain more zinc resistant and caused decreased accumulation of zinc. CzcD, purified as an amino-terminal streptavidin-tagged protein, bound Zn2+, Co2+, Cu2+, and Ni2+ but not Mg2+, Mn2+, or Cd2+, as shown by metal affinity chromatography. Histidine residues were involved in the binding of 2 to 3 mol of Zn2+ per mol of CzcD. ZitB transported 65Zn2+ in the presence of NADH into everted membrane vesicles with an apparent Km of 1.4 μM and a Vmax of 0.57 nmol of Zn2+ min−1 mg of protein−1. Conserved amino acyl residues that might be involved in binding and transport of zinc were mutated in CzcD and/or ZitB, and the influence on Zn2+ resistance was studied. Charged or polar amino acyl residues that were located within or adjacent to membrane-spanning regions of the proteins were essential for the full function of the proteins. Probably, these amino acyl residues constituted a pathway required for export of the heavy metal cations or for import of counter-flowing protons.
The cation diffusion facilitators (CDF) (T.C.2.A.4.1.1) (26) are a family of metal transport proteins found in a variety of organisms (16, 17, 19, 23). In contrast to other protein families, such as P-type ATPases or ABC transporters (26), all CDF proteins characterized to date transport only metals, and the majority are involved in Zn2+ transport (3, 16, 23). One of the first two CDF identified was the CzcD protein (15, 18) from the gram-negative bacterium Ralstonia metallidurans strain CH34 (previously Alcaligenes eutrophus [5]). This transporter is part of a cobalt-zinc-cadmium resistance system (Czc) and decreases the intracellular zinc concentration (1). The CzcD protein is composed of a hydrophobic, membrane-bound domain consisting of about 200 amino acid residues with probably six transmembrane α-helices and a 115-amino-acid hydrophilic domain located in the cytoplasm (1).
Escherichia coli detoxifies excess Zn2+ by using ZntA, a P-type ATPase, and the CDF protein ZitB (6), which is closely related to CzcD. Previously, site-directed mutagenesis was used to identify amino acyl residues H53, H159, D163, and D186 of ZitB as essential residues (12). These residues were located within predicted transmembrane domains (TMs) of this protein. In this study we compared a variety of additional mutations in ZitB and CzcD to better understand functional aspects of zinc binding and efflux. ZitB-dependent 65Zn2+ transport into everted membrane vesicles was driven by the proton motive force (PMF). These kinetics suggest that this protein is mainly responsible for zinc homeostasis under most physiological conditions.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and plasmid construction.
The media used to cultivate E. coli strains W3110 (wild type) (7), GG48 (ΔzitB::Cm zntA::Km) (6), and GR362 (ΔzntA::Km ΔzitB ΔzupT ΔznuABC ΔzntB::Cm) (8) were Tris-buffered mineral salts medium (13) containing 2 g of glucose liter−1 plus 1 g of yeast extract liter−1 (TGY), the same medium with 2 g of glycerol liter−1 plus 3 g of Casamino Acids liter−1 (TGC), and Luria-Bertani broth (LB) (27). Solid Tris-buffered media contained 20 g of agar liter−1. For protein purification, the expression strains were cultivated in Terrific broth (30).
The czcD gene was PCR amplified from megaplasmid pMOL30 (13) DNA by using the forward primer 5′-AAAGAATTCGGGCGCAGGTCAACTCACAC-3′ (EcoRI site underlined) and the reverse primer 5′-AAACCATGGTTTCCTCCTGCAGCAAGCGAC-3′ (NcoI site underlined). The amino-terminal membrane-bound part of czcD up to S203 was amplified with the same forward primer and reverse primer 5′-AAACCATGGCACGACTTCAGCAGGATC-3′ (NcoI site underlined). All fragments were cloned into the vector plasmid pASK5 (IBA GmbH, Göttingen, Germany) providing the ATG start codon, a Strep-TagII tag to the amino terminus, and the tetAp promoter. Correct cloning was verified by DNA sequence analysis.
Mutations and characterization of strains containing mutant proteins.
The Quick-Change site-directed mutagenesis system (Stratagene, La Jolla, Calif.) was used. Supercoiled pASK-IBA-derived plasmid DNA was used as a template in PCRs carried out with overlapping, antiparallel pairs of primers which contained the desired point mutations (16 PCR cycles plus one cycle per mutated base pair). The PCR products were treated with DpnI that exclusively degraded methylated template DNA. The resulting DNA was transformed into E. coli without prior ligation. Mutations were verified by DNA sequence analysis. Similarly, additional zitB mutants were generated as previously described (12). The metal resistance of E. coli cells containing plasmid pASK-IBA derivatives with the mutated genes, the wild type genes, and no insert was determined by using dose-response curves as described previously (7). To determine the 50% inhibitory concentration (IC50) (the metal concentration that led to a turbidity that was reduced by half) and the corresponding b value (slope of the sigmoidal dose-response curve), the data were adapted to the formula OD(c) = OD0/{1 + exp[(c − IC50)/b)}, which is a simplified version of a Hill-type equation introduced by Pace and Scholtz (21), where OD(c) is the turbidity at a given zinc concentration, OD0 is the turbidity with no added zinc, and c is the zinc concentration.
Uptake experiments with CzcD.
Cation uptake experiments in which the filtration method was used were performed as described previously (20), with some modifications. E. coli cells were incubated in TGY at 30°C. Cation uptake was started by addition of the radioactive cation 109Cd2+, 63Ni2+, 65Zn2+, or 57Co2+ (NEN, Cologne, Germany, or Amersham Pharmacia Biotech, Freiburg, Germany). Samples (400 μl) were filtered through membrane filters (pore size, 0.45 μm; Schleicher and Schuell, Dassel, Germany) and rinsed twice with 4 ml of 10 mM Tris-HCl (pH 7.0) buffer containing 10 mM MgCl2. The radioactivity remaining on the membrane filter was determined with a scintillation counter (LS6500; Beckman, Munich, Germany).
Uptake and transport experiments with ZitB.
Everted membrane vesicle preparation and uptake experiments were performed by using the filtration method described previously (14), with modifications. Buffer A (250 mM sucrose, 25 mM Tris-HCl, 150 mM KCl, 0.5 mM EDTA; pH 7) was used to prepare the vesicles from E. coli strain GR362 (6) bearing the pASK-IBA3 expression vector (IBA GmbH) either with or without the zitB gene inserted; for the final resuspension, EDTA was omitted from the buffer. Uptake experiments were performed in 1 ml of buffer B (same as buffer A except that the Tris-HCl concentration was 50 mM and 2 mM MgCl2 was added) containing approximately 0.1 mg of vesicle protein and various concentrations of 65ZnCl2. After 5 min of incubation at room temperature, uptake was initiated with 5 mM NADH in buffer B. At the intervals indicated below, 0.1 ml of the reaction mixture was filtered through a 0.2-μm-pore-size filter (Whatman Intl., Maidstone, England) and washed with 5 ml of buffer C (same as buffer B except that the concentration of MgCl2 was 10 mM and 10 mM ZnCl2 was added; pH 6.5). The filters were dried, and the radioactivity was determined by liquid scintillation analysis (Tri-Carb 2100T; Packard-Canberra, Meridian, Conn.). Identical assays without NADH were performed to determine nonspecific background binding.
Purification of CzcD.
Protein CzcD was purified by using Strep-TagII technology (IBA GmbH). Protein expression was performed with E. coli strain BL21 cells (Stratagene Europe, Amsterdam, The Netherlands) freshly transformed with the pASK expression plasmid. The transformants were cultivated overnight at 30°C in LB, diluted 1:100 into 1 liter of fresh Terrific broth, and cultivated with shaking at 30°C until the optical density at 600 nm was 1.5. Expression of CzcD was induced with 200 μg of anhydrotetracycline liter−1, and incubation was continued for 3 h. Cells were suspended in 30 ml of buffer W (100 mM Tris-HCl [pH 8.0], 1 mM EDTA) and lysed twice with a French press (SLM Aminco; SOPRA GmbH, Büttelborn, Germany) at 1,250 lb/in2 in the presence of a protease inhibitor (1 mM phenylmethylsulfonyl fluoride) and DNase I (10 mg · liter−1). Debris was removed by centrifugation (23,400 × g, 15 min, 4°C), and the membrane fraction was isolated by ultracentrifugation (100,000 × g, 2 h, 4°C). The membrane pellet was suspended in buffer W to a final protein concentration of 10 g · liter−1. CzcD was solubilized from 1 mg of membrane protein with 2 mg of n-dodecyl maltoside in the presence of 3.5 mg of phosphatidylcholine from soybean. The suspension was stirred on ice for 30 min, and residual membrane fragments were removed by ultracentrifugation (100,000 × g, 30 min, 4°C). The resulting solubilized protein fraction (100 mg of total protein) was applied to a streptactin affinity chromatography column (bed volume, 3 ml), which was washed with 30 ml of buffer W containing 0.05 g of n-dodecyl maltoside liter−1. Finally, CzcD was eluted with 100 mM Tris-HCl buffer (pH 8.0) containing 2.5 mM desthiobiotin and 0.05 g of dodecyl maltoside liter−1. The CzcD protein preparation was used to raise antibodies in rabbits (Seqlab, Göttingen, Germany).
E. coli cells containing mutant CzcD or ZitB proteins were cultivated overnight at 37°C in TGC, diluted 1:100 into 20 ml of fresh TGC, and cultivated with shaking at 37°C until the turbidity was 100 Klett units. Expression of the mutant proteins was induced by adding 200 μg of anhydrotetracycline liter−1, and incubation was continued for 3 h. Cells corresponding to 50 μg (dry weight) were lysed with sodium dodecyl sulfate (SDS) and subjected to SDS-polyacrylamide gel electrophoresis. The CzcD and ZitB proteins were visualized as indicated below.
Western blot experiments.
After polyacrylamide gel electrophoresis, the gel was blotted with a semidry transfer system (Biometra, Göttingen, Germany) for 30 min at a constant amperage (1 mA per cm2 of gel area) onto a polyvinylidene difluoride (PVDF) membrane (Roche, Mannheim, Germany). To avoid oligomerization, the CzcD protein was not heated before gel electrophoresis and the loading buffer contained β-mercaptoethanol. The membrane was blocked in a solution containing phosphate-buffered saline (PBS) (4 mM KH2PO4, 16 mM Na2HPO4, 115 mM NaCl), 5 ml of Tween 20 per liter, and 50 g of skim milk per liter with shaking at 4°C for 16 h. The PVDF membrane was washed three times (5 min each) with PBS-Tween (PBS, 1 ml of Tween 20 liter−1) and then for 5 min with PBS at 23°C. The membrane was incubated for 1 h at 23°C with shaking with the first antibody (polyclonal CzcD antibody, diluted in PBS-Tween [1:5,000]). The membrane was washed three times (15 min each) with PBS-Tween and for 5 min with PBS and incubated with the secondary antibody (a monoclonal anti-rabbit immunoglobulin G antibody conjugated with horseradish peroxidase [Sigma-Aldrich, Deisenhofen, Germany] diluted 1:50,000 in PBS-Tween) for 1 h at 23°C with shaking. Unbound antibody was washed off three times (15 min each) with PBS-Tween and once for 5 min with PBS. For detection, the PVDF membrane was incubated 1:1 with solution 1 (0.1 M Tris-HCl [pH 8.5], 0.4 mM p-coumaric acid, 2.5 mM 5-amino-2,3-dihydro-1,4-phthalazinedione [luminol]) and solution 2 (0.1 M Tris-HCl [pH 8.5], 5.4 M H2O2) for 1 min. Excess liquid was wiped off, and the membrane was exposed for 5 s to 5 min to ECL hyperfilm (Amersham Pharmacia Biotech, Uppsala, Sweden).
Covalent modification with DTNB or DEPC.
A 0.75-ml portion of CzcD (170 mg · liter−1) was modified with 0.5 mM dithionitrobenzoic acid (DTNB) at 23°C for 40 min as described previously (11). Formation of thionitrobenzoic acid was monitored over time at 412 nm by using an extinction coefficient of 14,000 cm−1 · M−1.
In a 600-μl (total volume) mixture, CzcD at a concentration of 3 μM or the carboxy-terminal part of CzcD at a concentration of 3 μM in 10 mM sodium phosphate buffer (pH 8.0) was treated with diethylpyrocarbonate (DEPC) (28) at 23°C until the reaction was complete (until there was no further increase in the adsorption at 242 nm). The concentration of DEPC used (30 μM) was equal to the molar concentrations of histidine residues of CzcD. To remove the covalent modification, CzcD was treated with 75 mM hydroxylamine (29) for 12 h at 0°C.
Metal affinity chromatography.
As described previously (14), 1 ml (bed volume) of fast-flow chelating Sepharose (Amersham Pharmacia Biotech, Freiburg, Germany) was loaded with 3-ml portions of 250 mM solutions of various divalent heavy metal cation chlorides (ZnCl2, CoCl2, CdCl2, NiCl2, CuCl2, MnCl2, and MgCl2). The column was washed with 2 ml of H2O and equilibrated with 2 ml of 10 mM sodium phosphate buffer (NaP buffer) (pH 7.2) containing 0.05 g of n-dodecyl maltoside liter−1. A sample containing 60 μg of CzcD in 300 μl of NaP buffer was applied to the column, which was washed with 10 ml of NaP buffer. Finally, all remaining protein was eluted with 3 ml of NaP buffer containing 10 mM EDTA. Fractions (300 μl) were collected and analyzed on a 15% polyacrylamide gel electrophoresis gel.
CD spectroscopy.
A circular dichroism (CD) spectrum of CzcD, dissolved in 100 mM Tris-HCl buffer (pH 8.0) containing 0.05 g of n-dodecyl maltoside liter−1, was obtained in the range from 203 to 250 nm with a CD spectrometer (62A DS; AVIV, Lakewood, N.J.).
Atomic absorption spectroscopy.
Purified CzcD protein was treated with 10 mM EDTA and dialyzed for 16 h against 100 mM Tris-HCl buffer (pH 8.0) containing 0.05 g of n-dodecyl maltoside liter−1 to remove excess EDTA. The protein was incubated in the presence of zinc cations at several concentrations and dialyzed again against 100 mM Tris-HCl buffer (pH 8.0) containing 0.05 g of n-dodecyl maltoside liter−1 to remove excess metal. The protein concentration was determined at 280 nm (32), and the protein was burned in an atomic absorption spectrophotometer (929 AA; ATI Unicam) in an acetylene-air mixture. The zinc content was determined at a wavelength of 232 nm by using a calibration curve.
RESULTS
CzcD rendered E. coli zinc resistant.
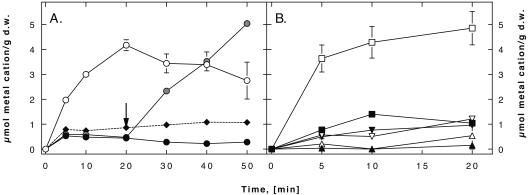
In E. coli strain GG48, the gene for the zinc-translocating P-type ATPase ZntA and the gene encoding the CDF protein ZitB were deleted, resulting in a highly zinc-sensitive strain (6, 25). Expression of R. metallidurans czcD in this strain led to increased zinc resistance, indicating that CzcD was expressed and active in E. coli (data not shown). E. coli strain GG48 carrying vector plasmid pASK5 accumulated about 3 μmol of Zn2+/g (dry weight) at an external concentration of 15 μM, whereas the strain expressing czcD from pASK5 accumulated 11% of this amount (Fig. 1A). CzcD caused a fivefold decrease in cadmium accumulation in GG48 cells but no decrease in accumulation of Ni2+ or Co2+ (Fig. 1B). Addition of the uncoupler carbonylcyanide (p-trifluoromethoxy)phenylhydrazone (FCCP) to czcD-expressing cells (Fig. 1A) led to an increase in zinc accumulation, and a level of 5 μmol/g (dry weight) was reached within 30 min. Thus, expression of CzcD decreased accumulation of Zn2+ and Cd2+ in E. coli strain GG48 cells. FCCP inhibition of this activity indicated that reduced accumulation might be the result of cation efflux driven by the proton motive force.
FIG. 1.
CzcD functionally complements a ΔzitB::Cm zntA::Km E. coli strain. (A) Accumulation of 65Zn2+ by E. coli GG48 (ΔzitB::Cm zntA::Km) (○) and strain GG48 containing the czcD gene (•) was determined by the filtration method with 15 μM zinc chloride. The arrow indicates when 10 μM FCCP was added to cells containing the czcD gene (gray circles). ♦, zinc uptake by W3110 wild-type cells. (B) Accumulation of 109Cd2+ (□ and ▪), 57Co2+ (▵ and ▴), or 63Ni2+ (▿ and ▾) by E. coli GG48 (ΔzitB::Cm zntA::Km) (open symbols) and strain GG48 containing the czcD gene (solid symbols), as determined at a metal cation concentration of 15 μM as described above. Mean values of duplicate determinations are shown; the error bars indicate standard deviations. d.w., cellular dry weight.
Spectrum of metals bound by CzcD.
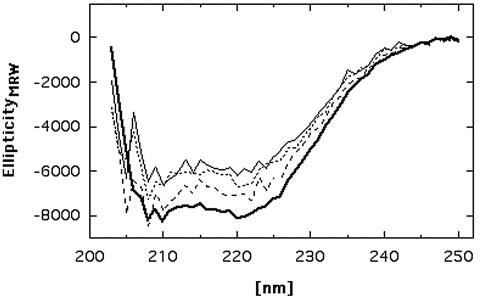
The CzcD protein was expressed in E. coli as an amino-terminal streptavidin-tagged fusion protein and purified to homogeneity (Fig. 2). CD spectroscopy revealed that purified CzcD contained significant α-helical portions (Fig. 3). Addition of 100 or 500 μM Zn2+ did not change the CD spectrum significantly, indicating that there was no conformational change (Fig. 3). After incubation with several Zn2+ concentrations, the metal ion contents of CzcD after dialysis were determined by atomic absorption spectroscopy to be 2.09 ± 0.48 mol of Zn2+ per mol of CzcD at 100 μM Zn2+ and 3.09 ± 0.56 mol of Zn2+ per mol of CzcD at 500 μM or 1 mM zinc. Thus, CzcD bound two or three Zn2+ cations per monomer.
FIG. 2.
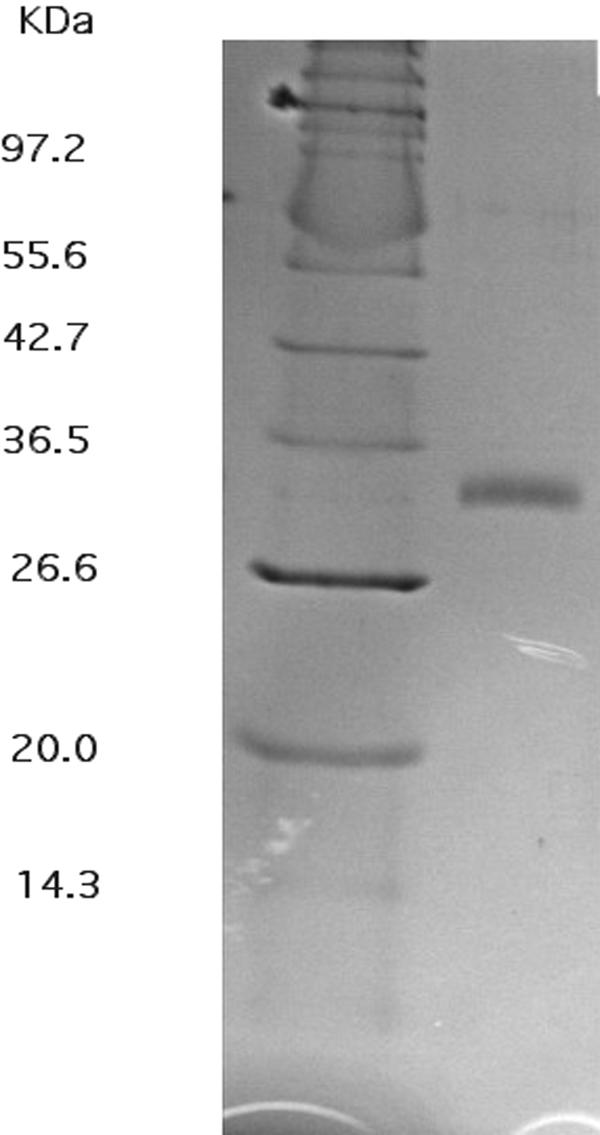
Coomassie blue-stained polyacrylamide gel containing purified CzcD. The gel contained purified CzcD in the right lane and a size marker in the left lane. The faint band corresponding to the twofold size of CzcD is indeed a dimer that starts to form during storage of the purified protein (data not shown).
FIG. 3.
CD spectrum of CzcD. The spectrum was generated with 0.24 g of CzcD per liter (thick solid line). Additionally, the CD spectrum of CzcD at a concentration of 0.1 g/liter was obtained in the presence of 100 μM Zn2+ (dotted line), in the presence of 500 μM Zn2+ (dashed line), or without added zinc (thin solid line). Due to strong light scattering at wavelengths below 203 nm, data points could not be collected at these wavelengths. EllipticityMRW, mean residue molar ellipticity.
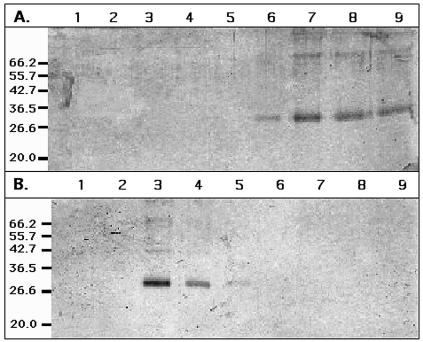
Binding of CzcD to heavy metal cations was also examined by metal affinity chromatography (Fig. 4 and Table 1). CzcD bound tightly to chelating affinity columns containing Zn2+ (Fig. 4A), Co2+, and Cu2+ and also bound significantly to an Ni2+ column (Table 1). In contrast, CzcD bound neither to Mn2+ or Cd2+ columns nor to a Mg2+ column that served as a negative control (Table 1 and Fig. 4B). In the case of Co2+, Ni2+, and Cd2+, the binding and transport data were not consistent, which is discussed below. Preincubation of CzcD with 1 mM Zn2+ led to decreased binding affinity of the zinc column for the zinc-loaded CzcD protein (Table 1).
FIG. 4.
CzcD retention by metal affinity chromatography. Chelating Sepharose fast-flow columns were prepared with Zn2+ (A) or Mg2+ (as a negative control) (B). Sixty micrograms of CzcD protein was applied to each column, and the columns were washed with 10 bed volumes of 10 mM sodium phosphate buffer (pH 7.2). Fractions (300 μl) were collected and applied to an SDS—15% polyacrylamide gel, which was silver stained. Lanes 1 to 5 contained the protein from the first five wash fractions, and lanes 6 to 9 contained the first four elution fractions. The positions of size markers are indicated on the left.
TABLE 1.
CzcD retention by metal affinity chromatographya
| Metal cation | Pretreatment of CzcD | % Retention |
|---|---|---|
| Zn2+ | None | 100 |
| Co2+ | None | 100 |
| Cu2+ | None | 100 |
| Ni2+ | None | 87 |
| Cd2+ | None | 0 |
| Mn2+ | None | 0 |
| Mg2+ | None | 0 |
| Zn2+ | Zincb | 75 |
| Zn2+ | DEPCc | 0 |
| Zn2+ | DEPC/hydroxylamined | 75 |
Chelating fast-flow Sepharose columns were loaded with several heavy metal cations and used for metal affinity chromatography with purified CzcD protein. After the protein was applied and washed with several bed volumes of 10 mM Na phosphate buffer (pH 7.2) containing 0.5 M NaCl, CzcD was eluted with the same buffer containing 10 mM EDTA. The fractions were collected and analyzed by polyacrylamide gel electrophoresis (original data are shown for Zn2+ and Mg2+ in Fig. 4). The gels were scanned, and the proportions of total CzcD in the wash and elution fractions were determined and used to calculate the percent retention.
The CzcD protein was incubated in the presence of 1 mM Zn2+ for 30 min at 0°C prior to chromatography.
The CzcD protein was incubated in the presence of 1 mM Zn2+ for 60 min at 0°C with DEPC at a concentration that was two times the concentration of histidine residues of CzcD.
The CzcD protein was incubated in the presence of 1 mM Zn2+ for 60 min at 0°C with DEPC and thin for 12 h at 0°C in the presence of 75 mM hydroxylamine.
Cysteine residues of CzcD.
CzcD contains a single Cys residue at position 290. This residue was titrated with DTNB in the presence or absence of 1 mM Zn2+ (data not shown). In the absence of zinc, 91.9% of the Cys residues were modified within 40 min, and 50% of the overall reaction was completed after 80 s. In the presence of 1 mM Zn2+, the corresponding values were 84.4% and 125.5 s. This indicates that access of the single Cys residue of CzcD was not significantly influenced by zinc.
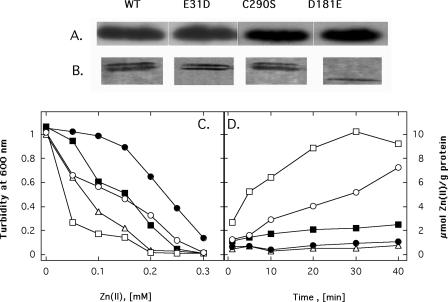
C290 in CzcD was changed to serine, and the mutant protein was expressed in E. coli strain GG48. As shown by Western blotting, the amounts of the mutant protein in the crude extract (Fig. 5A) and in membranes (Fig. 5B) of the cells were similar to the amounts of the wild-type protein. The cells were even more sensitive to zinc than cells containing only the vector plasmid (Fig. 5C). However, the CzcDC290S mutant protein mediated the same degree of diminished accumulation of zinc as the wild-type protein (Fig. 5D), indicating that there was a functional zinc transporter in vivo. This matched the results of the DTNB titration experiment with the purified CzcD protein, which indicated that the conserved Cys residue was not involved in zinc binding. The occurrence of the (reproducible) zinc-hypersensitive phenotype mediated by the CzcDC290S mutant protein is puzzling and cannot be explained at this time.
FIG. 5.
Effects of some amino acid changes on the function of CzcD. (A) Signals of wild-type CzcD (WT) and mutant proteins CzcDC290S, CzcDD181E, and CzcE31D in a Western blot with anti-CzcD antibodies. (B) Same protein signals in isolated cytoplasmic membranes. The bands were visualized by using the Strep-TagII detection system (IBA GmbH). The double band for the wild type and two mutant proteins is probably a gel migration artifact. (C and D) Effects of mutant proteins CzcDC290S (▵), CzcDD181E (□), and CzcE31D (▪) investigated by using dose-response curves (C) and by performing zinc accumulation experiments (D). (C) E. coli GG48 (ΔzitB::Cm zntA::Km) containing the pASK5 vector plasmid without an insert (○) and strain GG48 containing the wild-type czcD gene (•) or the mutated czcD genes, all cloned on plasmid pASK-IBA5 under control of the tetAp promoter, were cultivated in LB containing various concentrations of Zn2+ and 200 μg of the inducer anhydrotetracycline liter−1. The cells were cultivated with shaking for 14 h at 37°C, and the optical density at 600 nm was determined. The mean values for double determinations are shown. (D) Accumulation of 65Zn2+ by E. coli strain GG48 cells as determined by the filtration method with 15 μM 65Zn2+.
Histidine residues of CzcD.
CzcD contains nine His residues that are located in (H5, H7, and H9) or close to (H49) the amino terminus or in the hydrophilic carboxy-terminal domain (H234, H237, H251, H280, and H298) of the protein. CzcD was treated with DEPC to modify the accessible histidine residues of the complete protein. The molar amount of DEPC used was equivalent to twice the amount of histidine residues in the corresponding sample. When DEPC-treated CzcD protein was applied to a Zn2+ column, CzcD did not bind (Table 1). As a control, DEPC-treated CzcD protein was incubated in the presence of 75 mM hydroxylamine to remove the modification, and this protein was also applied to a Zn2+ affinity column. Twenty-five percent of the DEPC-hydroxylamine-treated CzcD protein was found in the wash fractions, and 75% was bound to the zinc-containing column (Table 1). Since DEPC modification of the His residues of CzcD prevented the protein from binding zinc, zinc binding probably involves some of the nine His residues of this protein.
To analyze the functional importance of these His residues, all of the residues except H234 were changed to Arg residues. The mutant proteins were expressed in E. coli strain GG48. The expression levels of the mutant proteins were verified by Western blotting of proteins in the crude extract by using anti-CzcD antibodies or by using streptactin conjugated to horseradish peroxidase to detect CzcD-Strep-TagII derivatives. Alteration of the amino-terminal His residues H5, H7, and H9 to Arg residues led to mutant proteins that could be expressed at levels similar to that of wild-type CzcD in membranes of E. coli (data not shown). The CzcDH5R and CzcDH9R mutant proteins exhibited one-half of the wild-type resistance to zinc (Table 2), indicating that these two residues alone were required for the function of CzcD. In contrast, the CzcDH7R protein did not confer zinc resistance to E. coli strain GG48. However, this mutant protein did not appear in the cytoplasmic membrane. Its production probably led to formation of inclusion bodies, because the protein was present in crude extracts of the cells (data not shown). Therefore, H7 may be required for correct membrane insertion of CzcD.
TABLE 2.
Effects of amino acid changes in CzcD and ZitB and corresponding positions in either protein
| CzcD | ZitB
|
||||||
|---|---|---|---|---|---|---|---|
| Change | Effect | IC50 of Zn2+ (μM)a | b (μM)b | Change | Effect | IC50 of Zn2+ (μM)a | b (μM)b |
| None | Wild-type CzcD | 204 ± 3 | 46 | None | Wild-type ZitB | 736 ± 114 | 146 |
| Control | No CzcD | 108 ± 9c | 55 | Control | No ZitB | 105 ± 48d | 114 |
| ΔC terminuse,f | ΔC terminus | No function | 122 ± 51 | 41 | |||
| H5R | Partially activeg | 163 ± 30 | 38 | H3h | |||
| H7R | No function | 105 ± 1 | 38 | H5h | |||
| H9R | Partially active | 154± 65 | 42 | H7h | |||
| H49R | Not expressed | NDi | H53R | No function | 241 ± 178 | 108 | |
| H49A | No function | 104 ± 4 | 30 | H53h | |||
| H237R | No function | 118 ± 18 | 36 | H243h | |||
| H251R | Not expressed | ND | H256h | ||||
| H251A | Partially active | 137 ± 12 | 60 | H256h | |||
| H280R | Not expressed | ND | H254h | ||||
| H280A | No function | 93 ± 15 | 40 | H284h | |||
| H298R | Not expressed | ND | N302h | ||||
| H298A | No function | 102 ± 7 | 45 | N302h | |||
| C290S | HyperSensitivej | 78 ± 0 | 45 | C294S | Wild type | 554 ± 341 | 117 |
| E31D | Partially active | 125 ± 7 | 36 | E35h | |||
| E31N | Not expressed | ND | E35h | ||||
| E31K | No function | 125 ± 7 | 31 | E35h | |||
| E154D | No function | 116 ± 12 | 34 | H159E | No function | 173 ± 34 | 73 |
| E154N | No function | 100 ± 13 | 31 | H159R | No function | 213 ± 69 | 156 |
| E209f | E214A | No function | 90 ± 48 | 183 | |||
| D46f | D50E | Partially active | 209 ± 19 | 72 | |||
| D46f | D50A | No function | 149 ± 17 | 77 | |||
| D53E | Hypersensitive | 60 ± 2 | 29 | D57h | |||
| D53A | Hypersensitive | 56 ± 6 | 47 | D57A | No function | 148 ± 17 | 77 |
| D53N | Hypersensitive | 48 ± 7 | 26 | D57h | |||
| D158A | No function | 85 ± 6 | 77 | D163A | No function | 192 ± 43 | 101 |
| D158E | No function | 112 ± 19 | 22 | D163E | No function | 122 ± 29 | 121 |
| D181E | Hypersensitive | 76 ± 61 | 56 | D186E | Partially active | 210 ± 33 | 75 |
| D181N | Hypersensitive | 59 ± 1 | 19 | D186h | |||
| D181A | Partially active | 136 ± 80 | 59 | D186A | No function | 102 ± 34 | 86 |
| M5OL | Hypersensitive | 63 ± 7 | 40 | M54L | Partially active | 286 ± 95 | 96 |
| R108f | R112A | Wild type | 744 ± 172 | 172 | |||
| N130f | N135A | No function | 168 ± 27 | 70 | |||
| S162f | S167A | No function | 127 ± 19 | 45 | |||
| F174f | W182L | Partially active | 201 ± 31 | 68 | |||
| W239f | W245L | Partially active | 461 ± 125 | 103 | |||
IC50, 50% inhibitory concentration (zinc concentration required to decrease the cell density of the growing culture to one-half the positive control value with no added zinc). Values are given with standard deviations.
b is the slope of the sigmoidal dose-response curve.
Value determined in Halle, Germany, for CzcD experiments.
Value determined in Tuczon, Ariz., for ZitB experiments.
ΔC terminus, deletion of the carboxy terminus.
Site that was not changed in CzcD but was changed in ZitB.
The phenotype was partially active if the IC50 of the wild type minus deviation was greater than the IC50 of the mutant plus deviation and the IC50 of the mutant minus deviation was less than the IC50 of the vector control plus deviation.
Site that was changed in CzcD but was not changed in ZitB.
ND, not determined.
The phenotype was hypersensitive if the IC50 of the mutant plus deviation was less than the IC50 of the vector control minus deviation.
The CzcDH237R mutant protein was stably expressed and inserted into the cytoplasmic membrane (data not shown), but it did not confer zinc resistance to E. coli (Table 2). Four mutant proteins (CzcDH49R, CzcDH251R, CzcDH280R, and CzcDH298R) were not expressed in vivo, indicating that they were unstable mutant proteins. However, when the His residues were changed to Ala residues, the mutant proteins (CzcDH49A, CzcDH251A, CzcDH280A, and CzcDH298A) were expressed. CzcDH251A, CzcDH280A, and CzcDH298A were inserted into the cytoplasmic membrane (data not shown), but they did not confer zinc resistance to E. coli (Table 2). CzcDH49A was present at lower concentrations in the cytoplasmic membranes than the wild-type protein (data not shown), which led to no increase in zinc resistance (Table 2). Thus, all His residues of CzcD tested were necessary for the full function of the protein or proper membrane insertion.
ZitB is driven by the proton motive force.
Further biochemical characterization of CzcD-mediated transport in the native host R. metallidurans failed, because this bacterium contains multiple zinc transport systems (31). Thus, ZitB was chosen for transport studies of a CDF protein, and the function of this protein was characterized in everted (inside-out) membrane vesicles that were isolated from E. coli mutant strain GR362 (8), which carries deletions in the genes for the zinc efflux pumps ZntA and ZitB and the zinc uptake proteins ZupT, ZntB, and ZnuABC.
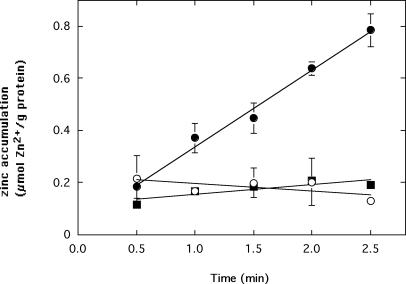
Figure 6 shows that everted membrane vesicles of strain GR362 accumulated Zn2+ only when ZitB and NADH were present. NADH was presumably needed to generate a PMF across the vesicle membrane by the respiratory chain. Addition of 5 mM Mg2+-ATP in place of NADH failed to stimulate zinc uptake (data not shown), probably because the F1F0-ATPase of E. coli functions much more slowly as a PMF-generating ATPase than as a PMF-driven ATP synthetase (10). As observed for CzcD, FCCP inhibited transport of zinc by ZitB (Fig. 6).
FIG. 6.
Zinc uptake into everted membrane vesicles is dependent upon ZitB: accumulation of 65Zn2+ with 5 μM ZnCl2 in everted membrane vesicles of E. coli strain GR362 carrying the pASK-IBA3 expression vector with zitB inserted (•), with zitB inserted in the presence of 10 μM FCCP (▪), or without zitB (○). Uptake was initiated at zero time by addition of 5 mM NADH, and vesicles were captured by filtration through a 0.2-μm-pore-size filter every 30 s for 2.5 min. 65Zn accumulation was determined by liquid scintillation analysis. Identical measurements obtained in the absence of NADH were subtracted from the values obtained at the corresponding time points as background.
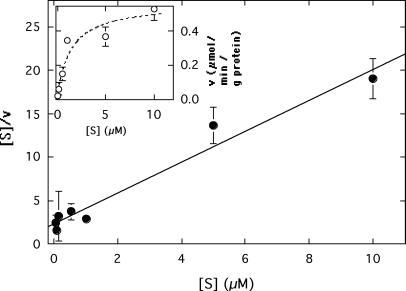
Nonradioactive Zn2+ at a concentration of 5 μM decreased the uptake of 5 μM 65Zn2+ to 40% of the control value without nonradioactive Zn2+, but the same concentration of nonradioactive Cd2+ decreased the zinc transport to 75% of the control value after 2.5 min (data not shown). Thus, ZitB was a better transport system for Zn2+ than for Cd2+. ZitB-dependent zinc uptake into the everted membrane vesicles showed substrate saturation (Fig. 7, inset) and occurred with an apparent Km of 1.4 ± 0.4 μM and a Vmax of 0.56 ± 0.17 μmol of Zn2+ · min−1 · g of protein−1 (Fig. 7).
FIG. 7.
Effect of substrate concentration ([S]) on the rate of NADH-dependent 65Zn accumulation (v). Zinc uptake was assayed as described in the legend to Fig. 6 at six different substrate concentrations. The initial uptake rate was plotted against the zinc concentration (inset). Km and Vmax were calculated from a Wolf-Hanes linear transformation of the data; Vmax was the reciprocal of the slope (0.56 ± 0.17 μmol/min/g of protein), and Km was the x intercept multiplied by Vmax (1.4 ± 0.4 μM). The regression coefficient of the linear Wolf-Hanes function was 96.4%, meaning that the error was 3.6%, and the mean deviation of the single data points was 27%, which resulted in a total error of 31%.
Carboxy-terminal parts of CzcD and ZitB were not essential for transport.
The histidine residues in the carboxy-terminal parts of both proteins were required for full function of CzcD and ZitB; however, in the first characterization of CzcD a truncated form of CzcD that contained only the first 200 amino acyl residues of this protein, which corresponded to only about two-thirds of the protein, was used (15). Thus, to investigate the relevance of the carboxy-terminal parts, mutant genes were constructed that expressed these proteins only up to CzcDL200 and ZitBL205. The latter construction conferred a small increase in zinc resistance to E. coli strain GG48 (Table 2), which was in the range of the experimental deviation. However, in carefully conducted dose-response experiments in which strain GG48 cells containing ZitB, ZitBL205, or neither protein were used, a low level of zinc resistance was reproducibly mediated by the mutated protein (data not shown).
When CzcDΔK201-N316 was expressed in E. coli strain GG48, cells accumulated 2.5 μmol of Zn2+/g (dry weight) after 15 min of incubation with 15 μM Zn2+ (data not shown). Cells containing an intact CzcD protein accumulated only 0.5 μmol/g (dry weight) under the same conditions, while cells without CzcD accumulated more than 4 μmol/g (dry weight) (Fig. 1). Thus, the truncated CzcD derivative diminished accumulation of zinc by the host cells, albeit to a lesser degree than the wild-type CzcD protein. These results indicated the importance of the carboxy-terminal parts of both proteins for full function, but they also indicated that a residual transport or detoxification activity was possible with the membrane-integrated parts alone.
Charged and polar amino acyl residues in predicted transmembrane α-helices.
Several charged or polar amino acyl residues in the predicted transmembrane α-helices (1) are conserved in CzcD, ZitB, and many closely related proteins belonging to group 2 of the CDF proteins (16). These amino acyl residues might be involved in cation export or proton import. Most of these amino acyl residues were mutated, and the effects of the mutations were investigated after expression of the corresponding proteins in E. coli strain GG48. In all cases, Western blot analysis verified regular expression levels of the mutant proteins and their proper insertion into the cytoplasmic membrane. The ZitBC294S mutation had no significant effect on the zinc resistance of E. coli strain GG48 (Table 2).
The most prominent conserved charged residue of TM 1 was a Glu residue that could be close to the periplasmic exit of this TM. While a CzcDE31N mutant protein was not expressed in E. coli strain GG48, mutant proteins CzcDE31D and CzcDE31K were expressed (data not shown), but they appeared in the cytoplasmic membrane in slightly smaller amounts than the corresponding wild-type proteins appeared (data for CzcDE31D are shown in Fig. 5; data for CzcDE31K are not shown). Both mutant proteins showed no significant degree of zinc resistance (Table 2).
TM 2 starts with a conserved Asp residue that was changed to a Glu or an Ala residue in ZitB. While the ZitBD50E mutant protein conferred an intermediate level of resistance, which placed this protein into the partially active category, the ZitBD50A protein mediated no zinc resistance (Table 2). The Asp residue was part of the highly conserved region of TM 2, DAXHMLTD. As stated above, the His residue was essential for the function of CzcD, and as described previously (12), it is also essential for the function of ZitB. Mutation of the following Met residue to Leu led to a partially active ZitB protein and to a CzcD mutant protein that increased zinc toxicity in the E. coli strain GG48 cells. Such a phenotype was categorized as hypersensitive (Table 2). Finally, the Asp residue at the end of the conserved motif was also essential; ZitBD57A, CzcDD53A, CzcDD53E, and CzcDD53N proteins were not able to confer metal resistance or led to hypersensitivity. Thus, the conserved motif DAXHMLTD in TM 2 was very important for the function of both CzcD and ZitB.
No conserved charged amino acyl residues are located in TMs 3 and 4, but surprisingly, N135 was essential for the function of ZitB (Table 2). The conserved motif DLLGSVG in TM 5 was also important. A ZitBS167A mutant protein did not confer zinc resistance, indicating the great importance of this region. The Asp residue at the beginning of the motif was also important, since CzcDD158E, CzcDD158A, ZitBD163A, and ZitBD163E mutant proteins conferred no zinc resistance (Table 2), although the amounts of these proteins in the cytoplasmic membranes were slightly smaller than the amounts of the wild-type proteins (data not shown). A glutamate (CzcD) or histidine (ZitB) residue four positions upstream of this Asp residue, although not conserved, was essential for the function of either protein, because CzcDE154D, CzcDE154N, ZitBH159R, and ZitBH159E mutant proteins did not confer any degree of zinc resistance. The amounts of these proteins in the cytoplasmic membrane were also smaller than the amounts of the corresponding wild-type proteins.
An Asp residue in TM 6 was reminiscent of the two essential Asp residues in TMs 2 and 5 and also proved to be critical. CzcDD181A and ZitBD186E mutant proteins displayed partial activity, ZitBD186A conferred no zinc resistance, and the mutant proteins CzcDD181E and CzcDD181N mediated zinc hypersensitivity (Table 2). In the case of CzcDD181E, production of this protein also led to increased accumulation of zinc by the E. coli cells, to a level that was more than the level observed in the complete absence of CzcD or ZitB (Fig. 5). However, the expression level of the mutant protein was about one-half that of the wild-type protein (Fig. 5A), and the membrane-inserted protein seemed to be unstable, as indicated by appearance of a smaller protein form (Fig. 5B). This may indicate that the mutant protein was not correctly inserted into the cytoplasmic membrane and consecutively degraded and thus functioned as a zinc importer. Alternatively, degradation of an unwanted zinc uptake system could also be the result of a cellular protection mechanism.
DISCUSSION
The bacterial CDF proteins that have been characterized to date are located in the cytoplasmic membrane and mediate diminished accumulation of divalent heavy metal cations (16). This is the result of cation efflux, as shown by active transport of zinc into inside-out membrane vesicles (Fig. 6). Cation efflux is driven by the PMF. Therefore, CDF proteins serving as cation efflux systems appear to be antiporters, as has been shown previously for a CDF protein from Bacillus subtilis (9) and for ZitB from E. coli (Fig. 6) (2).
The kinetic data for Zn2+ accumulation with ZitB in everted membrane vesicles (Km = 1.4 ± 0.4 μM) are inconsistent with recent data for the protein in reconstituted proteoliposomes, in which the Km was determined to be 104.9 μM (2). The physiological role of ZitB, however, predicts a low Km. Cells lacking ZitB but still carrying a functional copy of the gene for the P-type ATPase ZntA are not rendered any more sensitive to zinc than wild-type cells, while cells lacking both of these proteins are only slightly more sensitive to zinc than cells with a zntA deletion alone (6). This suggests that ZitB is a low-level detoxification-homeostasis protein, unlike ZntA, which is capable of rescuing cells from relatively high (millimolar) metal concentrations (6). This makes energetic sense, as ZntA is capable of catalyzing the transport of large quantities of metal but does so at the expense of direct ATP hydrolysis. Moreover, the Km of ZntA for Zn2+, 9 μM (6, 25), should be greater than that of ZitB, since the concentrations of substrate dealt with by ZntA are higher than the concentrations that ZitB can handle. While this does not validate either Km for ZitB, it does exclude any Km greater than that of ZntA. The assumption that there were factors that interacted with ZitB and were present in the everted membrane vesicles but not in the proteoliposomes may explain the observed differences in the Km values; however, these differences could also have been the result of experimental artifacts in either determination.
The membrane-spanning domains of CzcD and ZitB did not require the large carboxy-terminal domains of the corresponding proteins for cation transport per se, but these domains were essential for full function. Some conserved negatively charged amino acyl residues were identified in the membrane-spanning domains of both proteins. These residues probably form part of the transport pathway, which makes sense because positively charged ions or positive charges (protons) are transported. How the two processes, proton import and cation export, are coordinated is an interesting question. Mutations leading to hyperaccumulation of zinc and zinc hypersensitivity might be a key to this question. Hyperaccumulation of zinc might indicate additional zinc import by the mutant CDF protein, which seems to be unable to exchange cytoplasmic zinc for periplasmic protons. The residues in CzcD where mutations led to zinc hyperaccumulation were Asp residues in predicted transmembrane domains 2, 5, and 6 of the proteins. Asp residues have been shown to be involved in proton transfer pathways (4, 22), so two Asp residues, one of which is in the conserved DAXHMLTD motif of TM 2, might be part of an active site that exchanges periplasmic protons with cations coming from the cytoplasm. When a mutation prevents correct protonation of this site, cations are accepted from the periplasm and imported following the charge gradient portion ΔΨ of the PMF instead of being accepted from the cytosol and exchanged for periplasmic protons.
CzcD bound two or three zinc atoms per polypeptide, which requires a planar complex of 8 to 12 cation ligands. CzcD contains nine His residues, some of which are involved in binding of the protein to a zinc affinity column. This is no surprise, because the imidazole group of histidine has a higher affinity for borderline soft metals, such as zinc (24), while sulfhydryl groups bind more strongly to soft metals (e.g., Cd2+). Three His residues, all required for proper function or membrane insertion of CzcD, are located at the amino terminus of the protein. Four of five His residues in the hydrophilic carboxy-terminal part were also essential for full function of CzcD in E. coli. Yet another essential His residue is part of the conserved DAXHMLTD motif mentioned above. This is another piece of evidence suggesting that the conserved DAXHMLTD motif is a functionally important part of CzcD and ZitB.
CzcD mediates resistance to Co2+, Cd2+, and Zn2+ in R. metallidurans (1) and resistance to Zn2+ and Cd2+ in E. coli (Fig. 1). Cobalt resistance in E. coli was not observed, indicating that E. coli might harbor an unknown cobalt resistance system. CzcD showed binding to Zn2+, Co2+, Ni2+, and Cu2+ but not to its substrate Cd2+ or to Mn2+ (Table 1). This suggests that metal binding sites in the protein are different from the actual substrate exchange site for transport. At this point, the question of whether these bound metals perform a regulatory function or are themselves transported has not been answered. One possibility is that the binding sites could increase the local concentration of the CzcD substrates in the vicinity of the protein.
Acknowledgments
We thank Grit Schleuder for skillful technical assistance and Rainer Rudolph and Hauke Lilie for much help. We are grateful to Johanna Mansfeld for helping with the atomic absorption spectroscopy. Erik Fiedler and Stefan König are acknowledged for very fruitful discussions.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (grant Ni262/4-1, Graduiertenkollegs “Transport” and “Stress”) and Fonds der Chemischen Industrie to D.H.N. and by NIEHS grant ESO4940 with funds from EPA to C.R.
REFERENCES
- 1.Anton, A., C. Große, J. Reißman, T. Pribyl, and D. H. Nies. 1999. CzcD is a heavy metal ion transporter involved in regulation of heavy metal resistance in Ralstonia sp. strain CH34. J. Bacteriol. 181:6876-6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chao, Y., and D. Fu. 2004. Kinetic study of the antiport mechanism of an Escherichia coli zinc transporter, ZitB. J. Biol. Chem. 279:12043-12050. [DOI] [PubMed] [Google Scholar]
- 3.Gaither, L. A., and D. J. Eide. 2001. Eukaryotic zinc transporters and their regulation. Biometals 14:251-270. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg, M., T. Pribyl, S. Juhnke, and D. H. Nies. 1999. Energetics and topology of CzcA, a cation/proton antiporter of the RND protein family. J. Biol. Chem. 274:26065-26070. [DOI] [PubMed] [Google Scholar]
- 5.Goris, J., P. De Vos, T. Coenye, B. Hoste, D. Janssens, H. Brim, L. Diels, M. Mergeay, K. Kersters, and P. Vandamme. 2001. Classification of metal-resistant bacteria from industrial biotopes as Ralstonia campinensis sp. nov., Ralstonia metallidurans sp. nov. and Ralstonia basilensis Steinle et al. 1998 emend. Int. J. Syst. Evol. Microbiol. 51:1773-1782. [DOI] [PubMed] [Google Scholar]
- 6.Grass, G., B. Fan, B. P. Rosen, S. Franke, D. H. Nies, and C. Rensing. 2001. ZitB (YbgR), a member of the cation diffusion facilitator family, is an additional zinc transporter in Escherichia coli. J. Bacteriol. 183:4664-4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grass, G., and C. Rensing. 2001. Genes involved in copper homeostasis in Escherichia coli. J. Bacteriol. 183:2145-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grass, G., M. D. Wong, B. P. Rosen, R. L. Smith, and C. Rensing. 2002. ZupT is a Zn(II) uptake system in Escherichia coli. J. Bacteriol. 184:864-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guffanti, A. A., Y. Wei, S. V. Rood, and T. A. Krulwich. 2002. An antiport mechanism for a member of the cation diffusion facilitator family: divalent cations efflux in exchange for K+ and H+. Mol. Microbiol. 45:145-153. [DOI] [PubMed] [Google Scholar]
- 10.Hasan, S. M., T. Tsuchiya, and B. P. Rosen. 1978. Energy transduction in Escherichia coli: physiological and biochemical effects of mutation in the uncB locus. J. Bacteriol 133:108-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, S. W., S. Joo, G. Choi, H. S. Cho, B. H. Oh, and K. Y. Choi. 1997. Mutational analysis of the three cysteines and active-site aspartic acid 103 of ketosteroid isomerase from Pseudomonas putida biotype B. J. Bacteriol. 179:7742-7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee, S. M., G. Grass, C. J. Haney, B. Fan, B. P. Rosen, A. Anton, D. H. Nies, and C. Rensing. 2002. Functional analysis of the Escherichia coli zinc transporter ZitB. FEMS Microbiol. Lett. 215:273-278. [DOI] [PubMed] [Google Scholar]
- 13.Mergeay, M., D. Nies, H. G. Schlegel, J. Gerits, P. Charles, and F. van Gijsegem. 1985. Alcaligenes eutrophus CH34 is a facultative chemolithotroph with plasmid-bound resistance to heavy metals. J. Bacteriol. 162:328-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murgia, C., I. Vespignani, J. Cerase, F. Nobili, and G. Perozzi. 1999. Cloning, expression, and vesicular localization of zinc transporter Dri 27/ZnT4 in intestinal tissue and cells. Am. J. Physiol. Gastrointest. Liver Physiol. 277:G1231-G1239. [DOI] [PubMed] [Google Scholar]
- 15.Nies, D. H. 1992. CzcR and CzcD, gene products affecting regulation of resistance to cobalt, zinc, and cadmium (czc system) in Alcaligenes eutrophus. J. Bacteriol. 174:8102-8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nies, D. H. 2003. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol. Rev. 27:313-339. [DOI] [PubMed] [Google Scholar]
- 17.Nies, D. H., and N. Brown. 1998. Two-component systems in the regulation of heavy metal resistance, p. 77-103. In S. Silver and W. Walden (ed.), Metal ions in gene regulation. Chapman Hall, London, United Kingdom.
- 18.Nies, D. H., A. Nies, L. Chu, and S. Silver. 1989. Expression and nucleotide sequence of a plasmid-determined divalent cation efflux system from Alcaligenes eutrophus. Proc. Natl. Acad. Sci. USA 86:7351-7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nies, D. H., and S. Silver. 1995. Ion efflux systems involved in bacterial metal resistances. J. Ind. Microbiol. 14:186-199. [DOI] [PubMed] [Google Scholar]
- 20.Nies, D. H., and S. Silver. 1989. Plasmid-determined inducible efflux is responsible for resistance to cadmium, zinc, and cobalt in Alcaligenes eutrophus. J. Bacteriol. 171:896-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pace, C. N., and M. J. Scholtz. 1997. Measuring the conformational stability of a protein, p. 299-322. In T. Creighton (ed.), Protein structure: a practical approach. IRL Press, Oxford, United Kingdom.
- 22.Paddock, M. L., G. Feher, and M. Y. Okamura. 2003. Proton transfer pathways and mechanism in bacterial reaction centers. FEBS Lett. 555:45-50. [DOI] [PubMed] [Google Scholar]
- 23.Paulsen, I. T., and M. H. Saier, Jr. 1997. A novel family of ubiquitous heavy metal ion transport proteins. J. Membr. Biol. 156:99-103. [DOI] [PubMed] [Google Scholar]
- 24.Rensing, C., M. Ghosh, and B. P. Rosen. 1999. Families of soft-metal-ion-transporting ATPases. J. Bacteriol. 181:5891-5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rensing, C., B. Mitra, and B. P. Rosen. 1997. The zntA gene of Escherichia coli encodes a Zn(II)-translocating P-type ATPase. Proc. Natl. Acad. Sci. USA 94:14326-14331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saier, M. H., Jr. 2000. A functional-phylogenetic classification system for transmembrane solute transporters. Microbiol. Mol. Biol. Rev. 64:354-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd. ed. Cold Spring Harbor Laboratory., Cold Spring Harbor, N.Y.
- 28.Schiavo, G., G. Rossetto, A. Santucci, B. R. DasGupta, and C. Montecucco. 1992. Botulinum neurotoxins are zinc proteins. J. Biol. Chem. 267:23479-23483. [PubMed] [Google Scholar]
- 29.Shriver, Z., Y. Hu, and R. Sasisekharan. 1998. Heparinase II from Flavobacterium heparinum. Role of histidine residues in enzymatic activity as probed by chemical modification and site-directed mutagenesis. J. Biol. Chem. 273:10160-10167. [DOI] [PubMed] [Google Scholar]
- 30.Tartof, K. D., and C. A. Hobbs. 1987. Improved media for growing plasmid and cosmid clones. Bethesda Res. Lab. Focus 9:12. [Google Scholar]
- 31.von Rozycki, T., D. H. Nies, and M. H. J. Saier. Genomic analyses of transport proteins in Ralstonia metallidurans. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 32.Warburg, O., and W. Christian. 1942. Isolierung und Kristallisation des Gaerungsfermentes Enolase. Biochem. Z. 310:384-421. [Google Scholar]