Abstract
Escherichia coli heat shock transcription factor σ32 is rapidly degraded in vivo, with a half-life of about 1 min. A set of proteins that includes the DnaK chaperone team (DnaK, DnaJ, GrpE) and ATP-dependent proteases (FtsH, HslUV, etc.) are involved in degradation of σ32. To gain further insight into the regulation of σ32 stability, we isolated σ32 mutants that were markedly stabilized. Many of the mutants had amino acid substitutions in the N-terminal half (residues 47 to 55) of region 2.1, a region highly conserved among bacterial σ factors. The half-lives ranged from about 2-fold to more than 10-fold longer than that of the wild-type protein. Besides greater stability, the levels of heat shock proteins, such as DnaK and GroEL, increased in cells producing stable σ32. Detailed analysis showed that some stable σ32 mutants have higher transcriptional activity than the wild type. These results indicate that the N-terminal half of region 2.1 is required for modulating both metabolic stability and the activity of σ32. The evidence suggests that σ32 stabilization does not result from an elevated affinity for core RNA polymerase. Region 2.1 may, therefore, be involved in interactions with the proteolytic machinery, including molecular chaperones.
When cells or organisms are exposed to high temperature, synthesis of a set of heat shock proteins (HSPs) is rapidly induced. This induction generally occurs at the transcriptional level and is mediated by specific transcription factors. In Escherichia coli, the level of heat shock transcription factor σ32 (encoded by the rpoH gene) increases rapidly and transiently upon a temperature upshift, directing RNA polymerase to transcribe heat shock genes that encode HSPs, such as molecular chaperones and ATP-dependent proteases (9, 10, 56). The increase in the σ32 level depends on both increased synthesis and stabilization of the normally unstable σ32 (half-life, ∼1 min). The stabilization and enhanced synthesis of σ32 observed upon temperature upshift represent two distinct events that presumably involve different signaling pathways (15, 52).
Upon a temperature upshift from 30 to 42°C, the rate of σ32 synthesis increases 10-fold within 3 to 4 min at the translational level (41), as mediated by the secondary structure of the 5′ portion of rpoH mRNA (26, 31, 57). A high temperature directly disrupts the mRNA secondary structure, perhaps without involvement of other cellular factors, leading to enhanced ribosome entry and initiation of translation (27). Thus, the rate of synthesis of σ32 is primarily determined by the ambient temperature. Besides translational induction, marked stabilization of σ32 (eightfold stabilization) occurs for the first 4 to 5 min upon a heat shock, and this is followed by rapid destabilization (41). Transient stabilization of σ32 has been thought to occur by titration of DnaK and/or DnaJ chaperones away from σ32 by unfolded proteins accumulating at a high temperature, since these chaperones are involved in dealing with both σ32 and unfolded proteins (5, 7).
The DnaK chaperone team (DnaK, DnaJ, and GrpE) is required for rapid σ32 degradation because σ32 is markedly stabilized in dnaK, dnaJ, or grpE mutants (40, 47). The DnaK chaperone team is also involved in degradation of short-lived proteins, such as SulA and RcsA, and abnormal proteins, including puromycyl fragments, canavanine-containing proteins, and a nonsecreted form of alkaline phosphatase (12, 14, 18, 42). Two possible roles of the DnaK chaperone team in proteolysis have been proposed; one of these roles assumes that chaperones act in protein turnover only as accessory factors that help maintain substrates in a soluble form (14), and the other assumes that chaperones promote formation of protease-substrate complexes (12). Since σ32 stabilized in chaperone-deficient cells remains soluble and exhibits high transcriptional activity, the DnaK chaperone team may well play an active role, although the exact roles of chaperones in σ32 turnover, including their binding sites, remain unknown.
FtsH (HflB), a member of the AAA family of proteins (23), is the first protease that has been shown to degrade σ32 (11, 49). It is a membrane-bound ATP-dependent metalloprotease with an active site facing the cytoplasm, and it forms a ring structure consisting of six protomers (34). It degrades membrane proteins that are not assembled into functional complexes (1, 19) and cytoplasmic proteins (33, 38). Cytoplasmic ATP-dependent proteases, including HslUV (ClpYQ), also participate in σ32 degradation appreciably, although the relative contributions of FtsH and other proteases remain unknown (16, 17, 55).
To gain further insight into the mechanisms of σ32 degradation, we isolated a number of σ32 mutants that were stabilized in vivo by screening for hyperactive molecules. Some of these stable mutants had amino acid changes in the N-terminal half of region 2.1, one of the regions highly conserved among bacterial σ factors (22).
MATERIALS AND METHODS
Bacteria, plasmids, and phage.
E. coli K-12 strain MC4100 [F− araD Δ(argF-lac)U169 rpsL relA flbB deoC ptsF rbsR] and derivatives of this strain were used in most experiments. KY1612 [MC4100 ΔrpoH30::kan zhf50::Tn10 (λpF13-groEp-lacZ)] lacks σ32 and cannot grow at temperatures above 20°C (58). Prophage λpF13-groEp-lacZ carried lacZ under control of the groE promoter (groEp) (53). All rpoH mutant alleles were cloned under the isopropyl-β-d-thiogalactopyranoside (IPTG)-dependent trc promoter (5′-TGTTGACAATTAATCATCCGGCTCGTATAATGTG-3′; −35 and −10 hexamer sequences are underlined) on derivatives of the multicopy pTrc99A vector (Amersham Biosciences). pTrc99A was cut with NcoI, blunt ended with ExoVII, and self-ligated to obtain pKV1142 lacking four nucleotides of the NcoI site. A derivative of pKV1142 containing a weaker trc promoter (5′-TGTTGACAATTAAATCCATCCGGCTCGTATAATGTG-3′) was obtained by inserting two nucleotides (indicated by boldface type) into the spacer between the −35 and −10 sequences by PCR (pKV1585). A promoterless intact rpoH gene was amplified by PCR by using pKV7 (29) as the template, an upstream primer (5′-CCGGAATTCCGATTGAGAGGATTTGAATGACTGACAAAATGC-3′) containing an EcoRI site (GAATTC; underlined) and the initiation codon (ATG; underlined), and a downstream primer (5′-CGCGGATCCACAATCTGCGTGGGGATTGGCGTTTTGCCGG-3′) containing a downstream region of rpoH and a BamHI site (underlined). Amplified DNA fragments were cut with EcoRI and BamHI and cloned into pKV1142 and pKV1585 cut with the same enzymes; the resulting plasmids carrying rpoH were designated pKV1425 and pKV1637, respectively. The mutation from GCG to GAG at the 76th codon of rpoH was introduced into pKV1425 and pKV1637 by site-directed mutagenesis, yielding pKV1693 and pKV1845, respectively. All rpoH mutant alleles were constructed with pKV1637 and pKV1845 by site-directed mutagenesis. The sequence of each rpoH allele was confirmed by DNA sequence analysis.
Media, chemicals, and reagents.
L broth (48) was generally used for growing cells; ampicillin (50 μg/ml) was added when necessary. The synthetic medium used was medium E (48) supplemented with 0.5% glucose, 2 μg of thiamine per ml, and all of the amino acids (20 μg/ml each) except methionine and tryptophan; ampicillin was used at a concentration of 10 μg/ml. IPTG was added when necessary. l-[35S]methionine (29.6 TBq/mmol) was obtained from American Radiolabeled Chemicals and Amersham Biosciences. Other chemicals were purchased from Nacalai Tesque (Kyoto, Japan) or Wako Pure Chemicals (Osaka, Japan).
Radioactive labeling of proteins.
The assay for radioactive labeling of proteins was carried out essentially as described elsewhere (54). Cells were grown in synthetic medium at 30°C. Samples were taken and pulse-labeled with [35S]methionine (100 μCi/ml) at the mid-log phase. To determine protein stability, pulse-labeled cells were incubated with excess unlabeled methionine (200 μg/ml), and samples taken at intervals were examined for radioactivities associated with σ32 by immunoprecipitation and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Quantitation of σ32 bands was done with a BAS1800 BioImaging analyzer (Fuji Film, Tokyo, Japan).
Other methods.
Nucleic acid manipulation (36), mutagenic PCR (6), SDS-PAGE (48), immunoblotting (16), and site-directed mutagenesis (37) were performed as described previously.
RESULTS
Initial screening of σ32 mutants with increased stability.
The cellular level of σ32 is adjusted by regulation of its stability, as well as by regulation of its translational efficiency. To isolate σ32 mutants that specifically had increased stability, we used parental rpoH containing several undefined mutations that released the rpoH mRNA from translational inhibition. We expected that stabilization of σ32 would result in an increased level of protein, which in turn would increase transcription from heat shock promoters. The rpoH coding region amplified by PCR under error-prone conditions was ligated with a vector, placing rpoH under control of the IPTG-dependent trc promoter. The resulting plasmids were transformed into an rpoH deletion strain lacking σ32 (KY1612) and carrying λpF13-groEp-lacZ prophage to monitor σ32 activity, and cells were plated on L agar without IPTG and incubated at 30°C. Since KY1612 cells cannot grow at temperatures above 20°C (58), selection of transformants at 30°C guaranteed that potential σ32 mutants had transcriptional activity at least capable of supporting growth at 30°C, thus excluding mutants that had undergone drastic structural changes. β-Galactosidase activity expressed from the groE heat shock promoter served as an indicator of the transcriptional activity of σ32. In the absence of IPTG, the rate of synthesis of σ32 expressed in cells harboring wild-type rpoH on plasmid (pKV1425) was about fourfold higher than the rate of synthesis in the wild-type strain carrying only the chromosomal rpoH gene (data not shown). Darker blue colonies on L agar plates containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) were picked at 30°C, because transformants that produced stable σ32 mutants were expected to exhibit higher β-galactosidase activity.
Screening of over 20,000 transformants resulted in 55 initial candidates with apparently higher σ32 activity. When the σ32 level was examined by immunoblotting, it was significantly elevated in 27 of the transformants tested. Many of these transformants showed slower growth even in rich media, possibly due to increased σ32 levels. To minimize deleterious effects on cell growth, some rpoH mutants were recloned into a plasmid carrying a weaker trc promoter that contained two additional nucleotides between the −35 and −10 sequences (pKV1585). In spite of these efforts, however, growth of the ΔrpoH strains carrying mutant rpoH on the plasmid was slow in synthetic medium, which prevented pulse-labeling experiments.
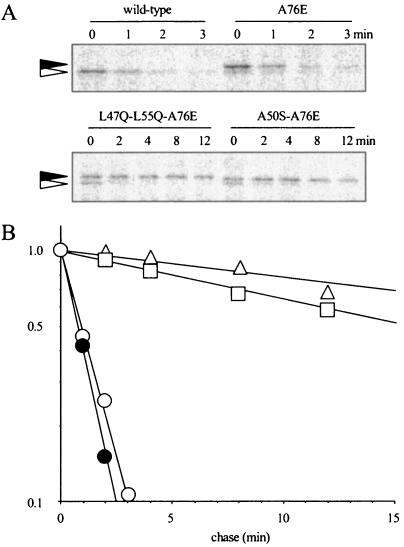
The resulting plasmids were, therefore, transformed into a wild-type rpoH+ strain (MC4100), and the stability of each mutant σ32 was examined by performing pulse-chase and immunoprecipitation experiments. Two rpoH mutant alleles encoding σ32 with particularly longer half-lives had several mutations that caused amino acid changes. Mutations responsible for σ32 stabilization were identified by swapping the restriction fragments between wild-type rpoH and each of the mutant alleles, and the mutation identified in this way was individually introduced into wild-type rpoH on plasmid pKV1637 by site-directed mutagenesis. One of the mutants containing two adjacent mutations that changed leucine residues to glutamine residues at positions 47 and 55 (designated L47Q-L55Q) produced σ32 with a half-life that was more than 10-fold longer than that of the wild type (Fig. 1). The half-life of σ32 containing the L47Q mutation alone and the half-life of σ32 containing the L55Q mutation alone were about three- and twofold longer than the half-life of the wild-type σ32, respectively (Fig. 2). Another mutant had an A50S substitution (alanine replaced by serine) at position 50 and produced about fourfold more stable σ32 than the wild type produced (Fig. 1).
FIG. 1.
Stability of σ32 in some mutants obtained by initial screening. MC4100 cells expressing wild-type σ32 from the trc promoter (pKV1425) or mutant σ32 from the weak trc promoter (pKV1637 derivatives) were grown in synthetic medium containing IPTG (100 μM for the wild type and 10 μM for the mutants) at 30°C to the mid-log phase and then were pulse-labeled with [35S]methionine (100 μCi/ml) for 1 min and chased with excess unlabeled methionine (200 μg/ml). To exclude the possibility that the mutant σ32 was stabilized as the result of an increased level, wild-type σ32 (control) was expressed from the intact trc promoter with a higher inducer concentration. Samples were taken at intervals starting at 1 min (wild type) or 3 min (mutants) (defined as time zero), and radiolabeled σ32 was analyzed by immunoprecipitation, followed by SDS-PAGE (10% polyacrylamide gel). Under these conditions, small amounts of wild-type σ32 expressed from the chromosomal rpoH were not detectable due to both a low synthesis rate and rapid degradation. (A) Typical results of SDS-PAGE. (B) Plot of average values calculated from three experiments. Symbols: ○, wild-type σ32; ▵, L47Q-L55Q; □, A50S.
FIG. 2.
Summary of stabilities (half-lives) of σ32 mutants localized in the N-terminal half of region 2.1. The stabilities of various σ32 mutants were determined by pulse-chase experiments essentially as described in the legend to Fig. 1. Synthetic medium with 500 μM IPTG (E48K-A76E, A49S-A76E, T52A-A76E, L53A-A76E) or without IPTG (the rest of the mutants) was used to grow cells. All σ32 mutants were expressed from pKV1637 or pKV1845 derivatives in rpoH+ MC4100 cells. The averages of three experiments are shown, and the wild-type value was defined as 1. Solid bars, σ32 mutants without the A76E mutation; shaded bars, σ32 mutants with the A76E mutation. Conserved regions of σ32 and amino acid changes in the N-terminal half of region 2.1 are shown at the top.
Further screening of stable σ32 mutants.
Since the parental rpoH used in the experiments described above contained several uncharacterized mutations which caused amino acid changes that made it difficult to identify and characterize mutations responsible for σ32 stabilization, further screening was carried out with parental rpoH containing three well-defined mutations expected to disrupt the rpoH mRNA secondary structure appreciably. These mutations changed the G residues at positions 123 and 177 (A in the initiation codon was defined as position 1) to A and the T at position 180 to C; none of these changes altered the amino acids encoded. Indeed, the rate of synthesis of σ32 encoded by this rpoH allele was about 2.5-fold higher than rate of synthesis of the wild-type σ32 at 30°C, and the synthesis was induced only slightly (∼twofold) upon a shift to 42°C (synthesis was induced about fivefold in the case of wild-type σ32) (data not shown).
Error-prone random mutagenesis was carried out by using the parental rpoH gene described above as the template in PCR. To minimize deleterious effects of excessive σ32 on cell growth, PCR-amplified fragments were cloned under control of the weak trc promoter on pKV1585, and the resulting plasmids were transformed into ΔrpoH strain KY1612. Through analyses similar to the initial screening, several σ32 mutants that contained the A50T, K51E, or I54T mutation and exhibited three- to fivefold-higher stability than the wild-type σ32 were obtained (Fig. 2). Remarkably, these amino acid residues, as well as those found earlier, were all localized in the N-terminal half of region 2.1, a region highly conserved among bacterial σ factors, indicating that this region is directly or indirectly involved in modulating degradation of σ32.
In the course of the initial screening, we found that the mobility of the σ32 mutant containing the A76E mutation on SDS-polyacrylamide gels was distinctly lower. To distinguish the mutant σ32 proteins from wild-type σ32 on the gel and to examine possible effects of mutant σ32 on the stability of wild-type σ32, the A76E mutation was introduced into each of the σ32 mutants. The half-life of wild-type σ32 expressed from the chromosomal DNA was found to be 1 to 2 min in cells producing any of the σ32 mutants tested, indicating that production of σ32 mutants did not affect the stability of wild-type σ32. Although the A76E mutation alone hardly affected the stability (Fig. 3), some stable σ32 mutants, such as the A50S mutant, were further stabilized by having both the original and A76E mutations simultaneously (Fig. 2 and 3). The reasons why only some of the specific mutants were further stabilized by the A76E mutation are unknown.
FIG. 3.
Stability of σ32 containing the A76E mutation. The stabilities of wild-type σ32 and mutant σ32 proteins containing the A76E mutation were determined in wild-type (rpoH+) MC4100 cells by pulse-chase experiments. Synthetic medium without IPTG was used to facilitate comparison of the stabilities of mutant σ32 protein expressed from rpoH on the plasmid and wild-type σ32 protein expressed from the chromosomal rpoH gene. (A) Results of a typical experiment. Since the rate of synthesis of mutant σ32 from plasmids was low in the absence of IPTG, wild-type σ32 expressed from the chromosomal rpoH gene could be detected simultaneously. The open arrowheads indicate the position of wild-type σ32 expressed both from the chromosomal rpoH gene and from pKV1425; the solid arrowheads indicate the position of mutant σ32 containing the A76E mutation expressed from plasmids (pKV1693 or pKV1845 derivatives). (B) Plot of average values calculated from three experiments. Symbols: ○, wild type; •, A76E; ▵, L47Q-L55Q-A76E; □, A50S-A76E.
Directed mutagenesis in the N-terminal half of region 2.1.
The results described above indicated that at least some amino acid residues in the N-terminal half of region 2.1 are critically involved in degradation of σ32. To examine the specificity of the mutation in this region, additional σ32 mutants with mutations affecting adjacent amino acid residues (E48K, A49S, T52A, and L53A) were constructed, and their stabilities were examined by performing pulse-chase and immunoprecipitation experiments. However, none of these mutants appeared to produce σ32 that was significantly more stable than the wild-type σ32, although precise half-lives could not be determined due to bands that overlapped wild-type σ32 bands resulting from the chromosomal rpoH gene on SDS-polyacrylamide gels. When these mutant σ32 proteins containing the A76E mutation were constructed and their half-lives were examined, they were all unstable, like the wild-type protein (Fig. 2). These results, combined with those presented above, indicate that only some specific amino acid residues in the N-terminal half of region 2.1 are required for rapid degradation of σ32.
Since in the stable σ32 mutants obtained up to this point hydrophobic amino acids were replaced by polar amino acids (L47Q, A50S, A50T, and I54T) or a basic amino acid was replaced by an acidic amino acid (K51E), these changes might potentially alter the σ32 structure. We therefore constructed mutants with L47A and I54A mutations which still exhibited hydrophobicity at the corresponding amino acid residues and examined the protein stability. Surprisingly, both of these mutant proteins were found to be even more stable than the proteins isolated by screening for higher σ32 activity (Fig. 2), suggesting that the bulky hydrophobic amino acid side chains at positions 47 and 54 are important for the rapid turnover characteristic of σ32.
Stable mutant σ32 proteins exhibited higher transcriptional activity.
When HSP synthesis was examined with the mutants producing stable σ32, the level of HSPs (DnaK and GroEL) was much greater in most mutants than in wild-type cells. To quantitatively assess σ32 activity, the intracellular level of σ32 was varied by varying the IPTG concentration (0 to 500 μM), and the levels of GroEL and σ32 in the mutant and wild type were compared by immunoblotting. As shown for a typical mutant in Fig. 4, the levels of DnaK and GroEL increased in parallel with an increased σ32 level for both wild-type and mutant σ32, but the levels were much higher in the mutant (I54A) than in the wild-type at every IPTG concentration tested. In the absence of IPTG, the I54A level was fourfold higher than the wild-type σ32 level due to stabilization, which led to increased levels of the heat shock proteins. However, when the concentration of wild-type σ32 was increased to a similar level with 10 μM IPTG, the levels of the heat shock proteins hardly changed, indicating that the activity of wild-type σ32 was repressed by an unknown mechanism (see below) and that I54A was partially liberated from repression. Repression of σ32 activity clearly occurred even in the case of I54A, because while the I54A level with 500 μM IPTG was 7.5-fold higher than the level without IPTG, the levels of the heat shock proteins were only 3.3-fold higher with 500 μM IPTG. While the levels of heat shock proteins produced in cells expressing I54A with 10 μM IPTG were comparable to the levels produced in cells expressing wild-type σ32 with 500 μM IPTG, the levels of the I54A mutant were about 10-fold less than the level of the wild-type σ32. Similar results were obtained for the L47Q-L55Q mutant (data not shown). These data strongly suggest that some amino acid residues (such as Ile54) in the N-terminal half of region 2.1 participate in regulation of both σ32 activity and stability. Under the conditions used here, no significant amount of wild-type σ32 expressed from the chromosomal DNA was detected.
FIG. 4.
Higher activity of stable mutant σ32. MC4100 cells harboring plasmids were grown at 30°C in synthetic medium without IPTG (lanes 1 and 6) or with IPTG at a concentration of 10 μM (lanes 2 and 7), 50 μM (lanes 3 and 8), 100 μM (lanes 4 and 9), or 500 μM (lanes 5 and 10). Whole-cell proteins were analyzed by SDS-PAGE (10% polyacrylamide gel), followed by staining with Coomassie blue (upper panel) or immunoblotting (lower panel). The values shown below the lane numbers indicate the levels of GroEL (upper panel) or σ32 (lower panel) relative to the level in lane 1 and were determined by immunoblotting serial dilutions of samples on separate gels. Lanes 1 to 5, MC4100 expressing wild-type σ32 from the trc promoter (pKV1425); lanes 6 to 10, MC4100 expressing I54A from the weak trc promoter (pKV1637 derivative).
σ32 stabilization does not result from elevated affinity for core RNA polymerase.
The increased levels of HSPs observed with the I54A and L47Q-L55Q mutants imply that the number of σ32 molecules associated with core RNA polymerase during steady-state growth increases in cells containing these stable mutant σ32 proteins. Stabilization of σ32 might therefore result from elevated affinity for core RNA polymerase, since region 2.1 has been thought to be one of the sites involved in binding to core RNA polymerase (21, 28, 39, 51) and σ32 bound to polymerase is hardly degraded by proteases in vitro (4, 17). To exclude this possibility, we constructed and examined the stability of stable σ32 mutants containing the Q80R mutation, which is known to reduce the affinity for core RNA polymerase (13) and not to affect the σ32 stability (44). When the Q80R mutation was introduced into two stable σ32 mutants, L47Q-L55Q and I54A, the resulting σ32 mutants (L47Q-L55Q-Q80R and I54A-Q80R) showed reduced DnaK and GroEL levels compared to wild-type σ32 (Fig. 5A and data not shown), unlike I54A (Fig. 4), presumably as a result of reduced affinity for core RNA polymerase. Nonetheless, both of these mutants remained stable and seemed to be more stable than the corresponding mutants without Q80R (Fig. 2, 3, and 5B), although the reason for further stabilization is unclear (see below). These results are consistent with the notion that these mutations in region 2.1 stabilize σ32 by reducing the affinity for some component(s) of the proteolytic machinery, leading to a higher σ32 level, which in turn promotes binding to RNA polymerase and increases HSP synthesis.
FIG. 5.
Stabilization of σ32 mutants with reduced affinity for core RNA polymerase. (A) MC4100 cells harboring plasmids were grown at 30°C in synthetic medium without IPTG (lanes 1 and 6) or with IPTG at a concentration of 10 μM (lanes 2 and 7), 50 μM (lanes 3 and 8), 100 μM (lanes 4 and 9), or 500 μM (lanes 5 and 10). Analyses of whole-cell proteins (upper panel) and immunoblotting (lower panel) were performed as described in the legend to Fig. 4. Lanes 1 to 5, MC4100 expressing wild-type σ32 from the trc promoter (pKV1425); lanes 6 to 10, MC4100 expressing I54A-Q80R from the weak trc promoter (pKV1637 derivative). (B) Stability of L47Q-L55Q-Q80R and I54A-Q80R as determined by pulse-chase experiments essentially as described in the legend to Fig. 1, except that in the case of the wild type, the time-zero sample was taken 3 min after addition of unlabeled methionine, like mutant σ32. Cells were grown in synthetic medium with IPTG at a concentration of 500 μM (wild type) or 10 μM (mutants). Results for a typical experiment are shown. (Upper panel) Lanes 1 to 5, wild type; lanes 6 to 10, L47Q-L55Q-Q80R; lanes 11 to 15, I54A-Q80R. (Lower panel) Symbols: ○, wild type; ▵, L47Q-L55Q-Q80R; □, I54A-Q80R.
DISCUSSION
The E. coli heat shock transcription factor, σ32, is extremely unstable in vivo, with a half-life of about 1 min during steady-state growth. We isolated a number of σ32 mutants that are highly or moderately stabilized (two- to ninefold), and in the present study we scrutinized the effects of amino acid changes exclusively in the N-terminal half of conserved region 2.1. The amino acid residues that were specifically shown to be involved in modulating σ32 stability are Leu47, Ala50, Lys51, Ile54, and Leu55. The results are consistent with previous data which indicated that an internal region (residues 36 to 122) of σ32 is critical for rapid degradation (3). Although some of the amino acid changes at these positions, such as L47Q and I54T, might alter the local protein structure with changing hydrophobicity of side chains, the σ32 mutant containing the L47A or I54A mutation, perhaps not leading to an appreciable structural alteration, was also found to be quite stable (Fig. 2). It appears likely that the side chains of Leu47 and Ile54 are particularly important for controlling the in vivo stability of σ32. On the other hand, our data suggest that several adjacent residues (Glu48, Ala49, Thr52, and Leu53) are not critically involved in determining σ32 stability. Stable mutants similar to those reported here were isolated by Gross and colleagues (C. A. Gross, personal communication).
Although we focused on amino acid residues in the N-terminal half of region 2.1, we did obtain other stable σ32 mutants, each of which had several amino acid changes outside this region. These mutants are currently being analyzed to identify residues crucial for controlling stability. Previous studies revealed that region C of σ32 (amino acids 122 to 144) is involved in the control of σ32 stability (30). However, we did not obtain any mutants containing mutations in region C in the present screening analysis. The latter region overlaps one of the regions involved in the binding to DnaK (25) and to core RNA polymerase (2). The potential role of region C in σ32 stability remains unresolved. In any event, it appears likely that other regions of σ32 also participate in modulating σ32 stability. This is consistent with the fact that the stabilization of mutant σ32 observed in the present work was at most 15-fold (in the case of L47Q-L55Q) (Fig. 1), and the most stable mutants were less stable than most other proteins in E. coli. On the other hand, we should not underestimate the role of region 2.1, particularly because some stable σ32 mutants with mutations in region 2.1, when combined with the Q80R mutation, became more stable than the corresponding original mutants. It is worth noting that while production of any of the stable mutant σ32 proteins resulted in higher levels of HSPs, production of stable mutant σ32 proteins also carrying the Q80R mutation did not result in higher levels of HSPs. The results imply that the increased synthesis of HSPs, including proteases, in these mutants (without Q80R) should in turn accelerate degradation of σ32 by changing the equilibrium of the association between stable mutant σ32 and the proteolytic machinery.
The finding that stable σ32 mutants containing the additional Q80R mutation that reduces the affinity for core RNA polymerase are more stable than the original mutants suggests that stabilization caused by mutations in region 2.1 results from reduced affinity of σ32 for components of the proteolytic machinery, although much work is required to determine the nature of the involvement of this region in modulating σ32 stability. In addition, this region might also be a contact site for factors regulating the transcriptional activity of σ32, as suggested by the fact that cells expressing a mutant σ32 protein, such as I54A, exhibit higher levels of HSPs than cells expressing wild-type σ32 (Fig. 4). This is not unexpected, since the DnaK chaperone team has been thought to modulate the transcriptional activity as well as the stability of σ32 (8, 43, 45, 46, 50). Specifically, region 2.1 might be a recognition and/or contact site for the chaperones. This idea is supported by the fact that a 13-mer synthetic oligopeptide corresponding to amino acids 47 to 59 shows high affinity for DnaK (25). According to the results presented by Rüdiger et al. (35), DnaK has a tendency to associate with oligopeptides containing a hydrophobic core surrounded by positive charged amino acids. The N-terminal half of region 2.1 of σ32 contains Leu53, Ile54, Leu55, and Lys51, which seems to be an ideal sequence for DnaK binding (Fig. 2). It has been reported that substrate polypeptides accommodated by DnaK are in an extended conformation (20, 59). In line with the structure of σ70-type σ factors, however, it is likely that region 2.1 of σ32 assumes an α-helix structure (24, 28, 32, 51). It is therefore tempting to speculate that the α-helix structure of region 2.1 undergoes alteration upon binding with DnaK, which in turn inhibits σ32 transcriptional activity and promotes σ32 degradation. In vitro experiments focusing on the structure of region 2.1 are necessary to explore this possibility further.
Acknowledgments
We are grateful to C. A. Gross (University of California) for communicating results prior to publication. T.Y. thanks C. Wada (Institute for Virus Research, Kyoto University), whose kind hospitality made his contribution possible. We also thank K. Yamaguchi (Kanazawa University) for help with the DNA sequence analysis.
This work was supported by grants from Yamada Science Foundation and from the Japan Society for the Promotion of Science.
REFERENCES
- 1.Akiyama, Y., A. Kihara, and K. Ito. 1996. Subunit a of proton ATPase Fo sector is a substrate of the FtsH protease in Escherichia coli. FEBS Lett. 399:26-28. [DOI] [PubMed] [Google Scholar]
- 2.Arsène, F., T. Tomoyasu, A. Mogk, C. Schirra, A. Schulze-Specking, and B. Bukau. 1999. Role of region C in regulation of the heat shock gene-specific sigma factor of Escherichia coli, σ32. J. Bacteriol. 181:3552-3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertani, D., A. B. Oppenheim, and F. Narberhaus. 2001. An internal region of the RpoH heat shock transcription factor is critical for rapid degradation by the FtsH protease. FEBS Lett. 493:17-20. [DOI] [PubMed] [Google Scholar]
- 4.Blaszczak, A., C. Georgopoulos, and K. Liberek. 1999. On the mechanism of FtsH-dependent degradation of the σ32 transcriptional regulator of Escherichia coli and the role of the DnaK chaperone machine. Mol. Microbiol. 31:157-166. [DOI] [PubMed] [Google Scholar]
- 5.Bukau, B. 1993. Regulation of the Escherichia coli heat shock response. Mol. Microbiol. 9:671-680. [DOI] [PubMed] [Google Scholar]
- 6.Cadwell, R. C., and G. F. Joyce. 1995. Mutagenic PCR, p. 583-589. In C. W. Dieffenbach and G. S. Dveksler (ed.), PCR primer: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 7.Craig, E. A., and C. A. Gross. 1991. Is Hsp70 the cellular thermometer? Trends Biochem. Sci. 16:135-140. [DOI] [PubMed] [Google Scholar]
- 8.Gamer, J., G. Multhaup, T. Tomoyasu, J. S. McCarty, S. Rüdiger, H.-J. Schönfeld, C. Schirra, H. Bujard, and B. Bukau. 1996. A cycle of binding and release of the DnaK, DnaJ and GrpE chaperones regulates activity of the Escherichia coli heat shock transcription factor σ32. EMBO J. 15:607-617. [PMC free article] [PubMed] [Google Scholar]
- 9.Georgopoulos, C., K. Liberek, M. Zylicz, and D. Ang. 1994. Properties of the heat shock proteins of Escherichia coli and the autoregulation of the heat shock response, p. 209-249. In R. I. Morimoto, A. Tissières, and C. Georgopoulos (ed.), The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 10.Gross, C. A. 1996. Function and regulation of the heat shock proteins, p. 1382-1399. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 11.Herman, C., D. Thévenet, R. D'Ari, and P. Bouloc. 1995. Degradation of σ32, the heat shock regulator in Escherichia coli, is governed by HflB. Proc. Natl. Acad. Sci. USA 92:3516-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang, H.-C., M. Y. Sherman, O. Kandror, and A. L. Goldberg. 2001. The molecular chaperone DnaJ is required for the degradation of a soluble abnormal protein in Escherichia coli. J. Biol. Chem. 276:3920-3928. [DOI] [PubMed] [Google Scholar]
- 13.Joo, D. M., N. Ng, and R. Calendar. 1997. A σ32 mutant with a single amino acid change in the highly conserved region 2.2 exhibits reduced core RNA polymerase affinity. Proc. Natl. Acad. Sci. USA 94:4907-4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jubete, Y., M. R. Maurizi, and S. Gottesman. 1996. Role of the heat shock protein DnaJ in the Lon-dependent degradation of naturally unstable proteins. J. Biol. Chem. 271:30798-30803. [DOI] [PubMed] [Google Scholar]
- 15.Kanemori, M., H. Mori, and T. Yura. 1994. Induction of heat shock proteins by abnormal proteins results from stabilization and not increased synthesis of σ32 in Escherichia coli. J. Bacteriol. 176:5648-5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanemori, M., K. Nishihara, H. Yanagi, and T. Yura. 1997. Synergistic roles of HslVU and other ATP-dependent proteases in controlling in vivo turnover of σ32 and abnormal proteins in Escherichia coli. J. Bacteriol. 179:7219-7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanemori, M., H. Yanagi, and T. Yura. 1999. Marked instability of the σ32 heat shock transcription factor at high temperature: implications for heat shock regulation. J. Biol. Chem. 274:22002-22007. [DOI] [PubMed] [Google Scholar]
- 18.Keller, J. A., and L. D. Simon. 1988. Divergent effects of a dnaK mutation on abnormal protein degradation in Escherichia coli. Mol. Microbiol. 2:31-41. [DOI] [PubMed] [Google Scholar]
- 19.Kihara, A., Y. Akiyama, and K. Ito. 1995. FtsH is required for proteolytic elimination of uncomplexed forms of SecY, an essential protein translocase subunit. Proc. Natl. Acad. Sci. USA 92:4532-4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landry, S. J., R. Jordan, R. McMacken, and L. M. Gierasch. 1992. Different conformations for the same polypeptide bound to chaperones DnaK and GroEL. Nature 355:455-457. [DOI] [PubMed] [Google Scholar]
- 21.Lesley, S. A., and R. R. Burgess. 1989. Characterization of the Escherichia coli transcription factor σ70: localization of a region involved in the interaction with core RNA polymerase. Biochemistry 28:7728-7734. [DOI] [PubMed] [Google Scholar]
- 22.Lonetto, M., M. Gribskov, and C. A. Gross. 1992. The σ70 family: sequence conservation and evolutionary relationships. J. Bacteriol. 174:3843-3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lupas, A. N., and J. Martin. 2002. AAA proteins. Curr. Opin. Struct. Biol. 12:746-753. [DOI] [PubMed] [Google Scholar]
- 24.Malhotra, A., E. Severinova, and S. A. Darst. 1996. Crystal structure of a σ70 subunit fragment from E. coli RNA polymerase. Cell 87:127-136. [DOI] [PubMed] [Google Scholar]
- 25.McCarty, J. S., S. Rüdiger, H.-J. Schönfeld, J. Schneider-Mergener, K. Nakahigashi, T. Yura, and B. Bukau. 1996. Regulatory region C of the E. coli heat shock transcription factor, σ32, constitutes a DnaK binding site and is conserved among eubacteria. J. Mol. Biol. 256:829-837. [DOI] [PubMed] [Google Scholar]
- 26.Morita, M., M. Kanemori, H. Yanagi, and T. Yura. 1999. Heat-induced synthesis of σ32 in Escherichia coli: structural and functional dissection of rpoH mRNA secondary structure. J. Bacteriol. 181:401-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morita, T. M., Y. Tanaka, T. S. Kodama, Y. Kyogoku, H. Yanagi, and T. Yura. 1999. Translational induction of heat shock transcription factor σ32: evidence for a built-in RNA thermosensor. Genes Dev. 13:655-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murakami, K. S., S. Masuda, and S. A. Darst. 2002. Structural basis of transcription initiation: RNA polymerase holoenzyme at 4 Å resolution. Science 296:1280-1284. [DOI] [PubMed] [Google Scholar]
- 29.Nagai, H., R. Yano, J. W. Erickson, and T. Yura. 1990. Transcriptional regulation of the heat shock regulatory gene rpoH in Escherichia coli: involvement of a novel catabolite-sensitive promoter. J. Bacteriol. 172:2710-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagai, H., H. Yuzawa, M. Kanemori, and T. Yura. 1994. A distinct segment of the σ32 polypeptide is involved in DnaK-mediated negative control of the heat shock response in Escherichia coli. Proc. Natl. Acad. Sci. USA 91:10280-10284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagai, H., H. Yuzawa, and T. Yura. 1991. Interplay of two cis-acting mRNA regions in translational control of σ32 synthesis during the heat shock response of Escherichia coli. Proc. Natl. Acad. Sci. USA 88:10515-10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narberhaus, F., and S. Balsiger. 2003. Structure-function studies of Escherichia coli RpoH (σ32) by in vitro linker insertion mutagenesis. J. Bacteriol. 185:2731-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogura, T., K. Inoue, T. Tatsuta, T. Suzaki, K. Karata, K. Young, L.-H. Su, C. A. Fierke, J. E. Jackman, C. R. H. Raetz, J. Coleman, T. Tomoyasu, and H. Matsuzawa. 1999. Balanced biosynthesis of major membrane components through regulated degradation of the committed enzyme of lipid A biosynthesis by the AAA protease FtsH (HflB) in Escherichia coli. Mol. Microbiol. 31:833-844. [DOI] [PubMed] [Google Scholar]
- 34.Ogura, T., and A. J. Wilkinson. 2001. AAA+ superfamily ATPases: common structure-diverse function. Genes Cells 6:575-597. [DOI] [PubMed] [Google Scholar]
- 35.Rüdiger, S., L. Germeroth, J. Schneider-Mergener, and B. Bukau. 1997. Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J. 16:1501-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 37.Sawano, A., and A. Miyawaki. 2000. Directed evolution of green fluorescent protein by a new versatile PCR strategy for site-directed and semi-random mutagenesis. Nucleic Acids Res. 28:e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shotland, Y., S. Koby, D. Teff, N. Mansur, D. A. Oren, K. Tatematsu, T. Tomoyasu, M. Kessel, B. Bukau, T. Ogura, and A. B. Oppenheim. 1997. Proteolysis of the phage λ CII regulatory protein by FtsH (HflB) of Escherichia coli. Mol. Microbiol. 24:1303-1310. [DOI] [PubMed] [Google Scholar]
- 39.Shuler, M. F., K. M. Tatti, K. H. Wade, and C. P. Moran, Jr. 1995. A single amino acid substitution in σE affects its ability to bind core RNA polymerase. J. Bacteriol. 177:3687-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Straus, D., W. Walter, and C. A. Gross. 1990. DnaK, DnaJ, and GrpE heat shock proteins negatively regulate heat shock gene expression by controlling the synthesis and stability of σ32. Genes Dev. 4:2202-2209. [DOI] [PubMed] [Google Scholar]
- 41.Straus, D. B., W. A. Walter, and C. A. Gross. 1987. The heat shock response of E. coli is regulated by changes in the concentration of σ32. Nature 329:348-351. [DOI] [PubMed] [Google Scholar]
- 42.Straus, D. B., W. A. Walter, and C. A. Gross. 1988. Escherichia coli heat shock gene mutants are defective in proteolysis. Genes Dev. 2:1851-1858. [DOI] [PubMed] [Google Scholar]
- 43.Straus, D. B., W. A. Walter, and C. A. Gross. 1989. The activity of σ32 is reduced under conditions of excess heat shock protein production in Escherichia coli. Genes Dev. 3:2003-2010. [DOI] [PubMed] [Google Scholar]
- 44.Tatsuta, T., D. M. Joo, R. Calendar, Y. Akiyama, and T. Ogura. 2000. Evidence for an active role of the DnaK chaperone system in the degradation of σ32. FEBS Lett. 478:271-275. [DOI] [PubMed] [Google Scholar]
- 45.Tatsuta, T., T. Tomoyasu, B. Bukau, M. Kitagawa, H. Mori, K. Karata, and T. Ogura. 1998. Heat shock regulation in the ftsH null mutant of Escherichia coli: dissection of stability and activity control mechanisms of σ32 in vivo. Mol. Microbiol. 30:583-593. [DOI] [PubMed] [Google Scholar]
- 46.Taura, T., N. Kusukawa, T. Yura, and K. Ito. 1989. Transient shut off of Escherichia coli heat shock protein synthesis upon temperature shift down. Biochem. Biophys. Res. Commun. 163:438-443. [DOI] [PubMed] [Google Scholar]
- 47.Tilly, K., J. Spence, and C. Georgopoulos. 1989. Modulation of stability of the Escherichia coli heat shock regulatory factor σ32. J. Bacteriol. 171:1585-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tobe, T., K. Ito, and T. Yura. 1984. Isolation and physical mapping of temperature-sensitive mutants defective in heat-shock induction of proteins in Escherichia coli. Mol. Gen. Genet. 195:10-16. [DOI] [PubMed] [Google Scholar]
- 49.Tomoyasu, T., J. Gamer, B. Bukau, M. Kanemori, H. Mori, A. J. Rutman, A. B. Oppenheim, T. Yura, K. Yamanaka, H. Niki, S. Hiraga, and T. Ogura. 1995. Escherichia coli FtsH is a membrane-bound, ATP-dependent protease which degrades the heat-shock transcription factor σ32. EMBO J. 14:2551-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomoyasu, T., T. Ogura, T. Tatsuta, and B. Bukau. 1998. Levels of DnaK and DnaJ provide tight control of heat shock gene expression and protein repair in Escherichia coli. Mol. Microbiol. 30:567-581. [DOI] [PubMed] [Google Scholar]
- 51.Vassylyev, D. G., S. Sekine, O. Laptenko, J. Lee, M. N. Vassylyeva, S. Borukhov, and S. Yokoyama. 2002. Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6 Å resolution. Nature 417:712-719. [DOI] [PubMed] [Google Scholar]
- 52.Wild, J., W. A. Walter, C. A. Gross, and E. Altman. 1993. Accumulation of secretory protein precursors in Escherichia coli induces the heat shock response. J. Bacteriol. 175:3992-3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yano, R., M. Imai, and T. Yura. 1987. The use of operon fusions in studies of the heat-shock response: effects of altered sigma 32 on heat-shock promoter function in Escherichia coli. Mol. Gen. Genet. 207:24-28. [DOI] [PubMed] [Google Scholar]
- 54.Yano, R., H. Nagai, K. Shiba, and T. Yura. 1990. A mutation that enhances synthesis of σ32 and suppresses temperature-sensitive growth of the rpoH15 mutant of Escherichia coli. J. Bacteriol. 172:2124-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yura, T., M. Kanemori, and M. T. Morita. 2000. The heat shock response: regulation and function, p. 3-18. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, D.C.
- 56.Yura, T., H. Nagai, and H. Mori. 1993. Regulation of heat shock response in bacteria. Annu. Rev. Microbiol. 47:321-350. [DOI] [PubMed] [Google Scholar]
- 57.Yuzawa, H., H. Nagai, H. Mori, and T. Yura. 1993. Heat induction of σ32 synthesis mediated by mRNA secondary structure: a primary step of the heat shock response in Escherichia coli. Nucleic Acids Res. 21:5449-5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou, Y.-N., N. Kusukawa, J. W. Erickson, C. A. Gross, and T. Yura. 1988. Isolation and characterization of Escherichia coli mutants that lack the heat shock sigma factor σ32. J. Bacteriol. 170:3640-3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu, X., X. Zhao, W. F. Burkholder, A. Gragerov, C. M. Ogata, M. E. Gottesman, and W. A. Hendrickson. 1996. Structural analysis of substrate binding by the molecular chaperone DnaK. Science 272:1606-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]