Abstract
The flhD operon is the master operon of the flagellar regulon and a global regulator of metabolism. The genome sequence of the Escherichia coli K-12 strain MG1655 contained an IS1 insertion sequence element in the regulatory region of the flhD promoter. Another stock of MG1655 was obtained from the E. coli Genetic Stock Center. This stock contained isolates which were poorly motile and had no IS1 element upstream of the flhD promoter. From these isolates, motile subpopulations were identified after extended incubation in motility agar. Purified motile derivatives contained an IS5 element insertion upstream of the flhD promoter, and swarm rates were sevenfold higher than that of the original isolate. For a motile derivative, levels of flhD transcript had increased 2.7-fold, leading to a 32-fold increase in fliA transcript and a 65-fold increase in flhB::luxCDABE expression from a promoter probe vector. A collection of commonly used lab strains was screened for IS element insertion and motility. Five strains (RP437, YK410, MC1000, W3110, and W2637) contained IS5 elements upstream of the flhD promoter at either of two locations. This correlated with high swarm rates. Four other strains (W1485, FB8, MM294, and RB791) did not contain IS elements in the flhD regulatory region and were poorly motile. Primer extension determined that the transcriptional start site of flhD was unaltered by the IS element insertions. We suggest that IS element insertion may activate transcription of the flhD operon by reducing transcriptional repression.
An important source of genome plasticity is derived from transpositional events of insertion sequence (IS) elements (34, 35). They generally encode no functions other than those involved in their mobility (for a review, see reference 30) and display a nonrandom distribution in the chromosome of Escherichia coli (9, 16). Many IS elements have been shown to activate the expression of neighboring genes, for example, through the formation of hybrid promoters or disruption of transcriptional repression. This has also been seen with cryptic operons, which depend upon mutations for activation. Two examples in E. coli are the bgl and ade operons, which can be activated by IS element insertion upstream or downstream of the promoter (18, 42, 49, 50, 53). The chitobiose operon, chb (formerly cel), was thought to be cryptic but can be induced by chitobiose, as well as being activated by IS element insertion upstream of the structural genes under noninducing conditions (40, 44).
Flagellar motility enables bacteria to escape from detrimental conditions and to reach more favorable environments. In E. coli, the flagellar regulon involves the expression of at least 14 operons in a regulated cascade to produce functional flagellar and chemotaxis machinery (for a review, see reference 14). The flhD operon at the apex of the flagellar regulon has been identified as the primary target of regulation by many environmental factors (for a review, see reference 61). It consists of two genes, flhD and flhC, whose products form a heterotetrameric transcriptional regulatory complex, FlhD/FlhC (29). The flhD operon is a global regulator, having pleiotropic effects on gene regulation. Through microarray studies, an additional 29 putative operon targets of known function have been identified. These are involved in nitrogen and carbon metabolism and adaptation to anaerobiosis, mediated by the methyl-accepting chemotaxis protein Aer (47, 48).
The flhD operon regulatory region is a direct target for several regulatory proteins: These are the cyclic AMP-catabolite gene activator protein complex, which is an activator in response to an alleviation of catabolite repression (60); OmpR∼P, which represses at high osmolarity (57); histone-like nucleoid-structuring (H-NS) protein, an activator (5, 60); LrhA, a repressor (27); RcsAB, a repressor (17); and HdfR, a repressor (23). In two E. coli K-12 genome sequencing projects, IS elements were present in the flhD operon regulatory region: first, a 768-bp IS1 element was present for strain MG1655 (6); second, an 1,195-bp IS5 element was present in strain W3110, from sequencing of the Kohara library clone 339 (20). In contrast, no IS elements were present in the flhD operon of other lab strains such as CS520 (4, 57), FB8 (60), and, intriguingly, MG1655 (27).
In this study, we show that IS5 and IS1 elements can spontaneously insert into the regulatory region of the flhD operon and increase expression. We obtained an MG1655 stock from the E. coli Genetic Stock Center (stock CGSC 8003) and found that it contained cells which were poorly motile and had no IS1 element upstream of the flhD promoter. Extended incubation in motility agar identified motile subpopulations containing IS element insertions in the flhD promoter regulatory region. Furthermore, an IS element was present upstream of the flhD operon promoter for a number of different lab strains, and this was consistent with increased motility.
MATERIALS AND METHODS
Bacterial strains, plasmids, and primers.
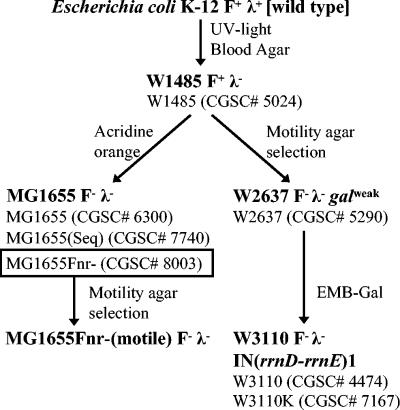
All strains used in this study are derivatives of E. coli K-12 and are listed with plasmids in Table 1. All oligonucleotide primers used in this study are available upon request. The E. coli Genetic Stock Center has at least three stocks classified as MG1655; these include MG1655 (CGSC 6300), MG1655Fnr− (CGSC 8003), and MG1655(Seq) (CGSC 7740). We have kept the designations assigned by the stock center to avoid confusion. We used stock MG1655Fnr− (CGSC 8003) predominately in our experiments. It was previously designated MG1655 (CGSC 6300) but was found to have an fnr-267 mutation (59). These three stocks of MG1655 all originated from a single sample of MG1655 sent to the stock center.
TABLE 1.
List of strains and plasmids
| Strain or plasmid | Genotype or description | Reference or source |
|---|---|---|
| Strains | ||
| MG1655Fnr−a | F− λ−fnr-267 rph-1 | CGSC 8003 |
| MG1655Fnr−(motile) | MG1655Fnr− selected for motility | This study |
| MG1655(Seq)a | F− λ−rph-1 | CGSC 7740 |
| MG1655a | F− λ−rph-1 | CGSC 6300 |
| W3110b | F− λ− IN(rrnD-rrnE)1 rph-1 | CGSC 4474 |
| W3110Kb | F− λ− IN(rrnD-rrnE)1 rph-1 | CGSC 7167 |
| W1485 | F+ λ−rph-1 | CGSC 5024 |
| W2637 | F− λ− IN (rrnD-rrnE)1 rph-1 | CGSC 5290 |
| RP437 | F−thi thr leu his rpsL eda | 41 |
| YK410 | F−araD139 ΔlacU169 rpsL thi nalA thyA pyrC46 his | 24 |
| MC1000 | F− λ−araD139 Δ(ara-leu)7697 Δ(lacIY)74 galU galK rpsL | 13 |
| FB8 | UTH1038 | 10 |
| MM294 | F− λ−glnV44(AS) endA1 thi-1 hsdR17 creC510 | CGSC 6315 |
| RB791 | λ− IN(rrnD-rrnE)1 lacIp4000(lacIq) | CGSC 6564 |
| MC4100 | F− λ−araD139 Δ(argF-lac)U169 rspL150 relA1 flhD5301 fruA25 deoC1 | 12 |
| Plasmids | ||
| pT7-7 | T7 promoter; Apr | 64 |
| pXL27 | pT7-7-flhD-flhC; Apr | 29 |
| pDEW201 | luxCDABE promoter probe vector; Apr | T. K. Van Dyk; 67 |
| pDEW534 | pDEW201-serA::luxCDABE | T. K. Van Dyk; 47 |
| pDEWflhB | pDEW201-flhB::luxCDABE | This study |
| pDEWgltB | pDEW201-gltB::luxCDABE | This study |
| pDEWgcvT | pDEW201-gcvT::luxCDABE | This study |
| pDEWompT | pDEW201-ompT::luxCDABE | This study |
Stock of strain MG1655 at the E. coli Genetic Stock Center.
Stock of strain W3110 at the E. coli Genetic Stock Center.
Growth conditions.
Strains were maintained on Luria-Bertani broth agar plates (tryptone, 10 g liter−1; yeast extract, 5 g liter−1; sodium chloride, 10 g liter−1 [solidified with 15 g of agar liter−1]) at +4°C for a maximum of 2 weeks. Strains were inoculated into the indicated volume of tryptone broth (TB) (tryptone, 10 g liter−1; sodium chloride, 5 g liter−1) as the edge of an individual colony and grown overnight with shaking (250 rpm). Thymine (20 μg ml−1) was used to supplement the growth medium for all cultures when strain YK410 (thyA; Table 1) was grown. Penicillin (100 μg ml−1) was added to media as required to maintain plasmids. All experiments were performed at 33°C, except where indicated.
PCR.
PCR Supermix (High Fidelity) (Invitrogen) was used in the reaction mixtures according to the manufacturer's instructions; all primers were added at a concentration of 1 μM. The reaction mix contained either 1 μl of genomic DNA, 1 μl from an overnight culture, or a picked (single) colony in a total volume of 50 μl. The reactions were amplified with the following cycling conditions: initial denaturation at 94°C for 2 min followed by 27 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for between 1 and 4 min 30 s, depending on the expected product size; a final extension step of 72°C for 10 min was then applied. PCR products were analyzed by agarose gel electrophoresis (1.5% [wt/vol] agarose). PCR products were sequenced by the University of Illinois at Chicago Research Resources Center DNA sequencing facility.
Motility and growth rate assays.
To investigate swimming motility, aliquots of 2 μl from overnight cultures were inoculated into semisolid motility agar (TB solidified with 0.3% [wt/vol] agar). Swarm diameters were measured hourly, and swarm rates were calculated from the linear phase of a graph of swarm diameter against time. To determine the growth rate, overnight cultures were diluted 1:200 into 40 ml of TB in a 50-ml Falcon tube and incubated. The optical density at 600 nm (OD600) was measured at 30-min intervals and used to calculate generations per hour. To examine the motility of cells by eye, an aliquot was taken from cultures at an OD600 of 0.4 to 0.6 and visualized with the aid of a Zeiss light microscope and phase-contrast optics.
RNA isolation and cDNA synthesis.
Overnight cultures were diluted 1:200 into 28 ml of TB in a 50-ml Falcon tube and grown to an OD600 of 0.5. Growth of the cultures and RNA degradation were inhibited by adding 25 ml of culture to 2.5 ml of stop solution (5% phenol in ethanol). Bacterial pellets were stored at −70°C. RNA was isolated by a hot phenol-sodium dodecyl sulfate method (15), with one phenol, one phenol-chloroform, and one chloroform extraction, followed by isopropanol precipitation. Final cleanup of the RNA was performed with an RNeasy minicolumn (QIAGEN). Synthesis of cDNA was performed using SuperScript II reverse transcriptase (Invitrogen) according to the manufacturer's instructions, modified to produce cDNA from 20 μg of RNA with incubation at 42°C for 2.5 h. At the end of the incubation period, the residual RNA was removed by alkaline hydrolysis. The reaction mixtures were cleaned up by using Microcon YM-30 concentrators (Amicon), with four washings, each wash with 450 μl of H2O, prior to elution.
Real-time PCR.
PCR was performed with the SYBR green kit (PE Biosystems). The reaction mixture contained 100 ng of cDNA, 1× SYBR green buffer, 2.5 mM MgCl2, a 0.25 mM concentration of each deoxynucleoside triphosphate, a 0.05 μM concentration of each primer, 0.01 U of AmpErase UNG, and 1 U of Taq Gold polymerase. The reaction was performed with 50 cycles of 30 s at 94°C, 30 s at 55°C, and 1 min at 72°C and monitored in an iCycler iQ real-time PCR detection system (Bio-Rad). A standard curve was derived from plasmids at known concentrations and used to convert threshold crossings to log copy numbers. All PCR fragments yielded a single band on an agarose gel.
Plasmid constructions.
DNA manipulations, transformations, and restriction analyses were performed according to standard procedures (51). Promoter region DNA was generated by PCR for the following operons: flhB, gltB, ompT, and gcvT. The fragments were first cloned into the pCR2.1-TOPO vector (Invitrogen) and were then directionally cloned into the EcoRI and SacI sites of the Photorhabdus luminescens luxCDABE-based promoter probe vector pDEW201 to generate translational fusions (67).
Luminescence assay.
Cells containing luxCDABE promoter fusions were diluted 1:200 into 30 ml of TB, with penicillin selection in a 50-ml Falcon tube, and grown with shaking. At 1-h intervals, OD600 and light production over 2 s in a MiniLumat 9506 luminometer (EG & G Berthold) were measured.
Primer extension.
Primer extension was done by a modification of the method of Sambrook and Russell (51). Briefly, 30 pmol of primer was end labeled with [γ-32P]ATP (6,000 Ci/mmol, 10 μCi/μl), using 30 U of polynucleotide kinase (Invitrogen) at 37°C for 30 min, followed by inactivation at 80°C for 10 min. To the end-labeled primer (26 ng), 50 μg of RNA was added and ethanol precipitated using cold sodium acetate (pH 5.2), and the precipitate was washed with 70% ethanol. The primer was annealed to the RNA in reverse transcriptase buffer, and reverse transcription was then performed in 20 μl with 200 U of Superscript III Reverse Transcriptase (Invitrogen) at 50°C for 90 min, followed by inactivation at 70°C for 15 min. Formamide-based stop solution (MBI Fermentas) (10 μl) was added. A sequencing ladder was also prepared using the same end-labeled primer (2 ng) and 10 fmol of the flhD operon regulatory region DNA per reaction (A, C, G, T), using a CycleReader DNA sequencing kit (MBI Fermentas) according to the manufacturer's instructions. The samples were denatured and analyzed by denaturing polyacrylamide gel electrophoresis at 1,500 V for approximately 2 h using an 8% SequaGel (National Diagnostics). The image was visualized with a PhosphorImager (Molecular Dynamics).
RESULTS
Observation of motile subpopulations of MG1655 in motility agar.
There are a number of versions of MG1655 (including CGSC 6300, 7740, and 8003) in the E. coli Stock Center. The sequenced version (CGSC 7740) is highly motile and has an IS1 element inserted into the promoter region. Another MG1655 stock (MG1655Fnr−, CGSC 8003) is poorly motile and does not have an IS element in the promoter region. We have shown that from a population of poorly motile MG1655 cells a motile variant can be screened for by extended incubation on motility agar.
In the next experiment, to preserve each isolate for further analysis, a portion of the overnight culture (1 ml) was also frozen at −70°C as a 33% glycerol stock and genomic DNA was prepared (43). An isolate of MG1655Fnr− was inoculated into 25 ml of TB and grown overnight. Cells were inoculated across the center of a motility agar plate and incubated. Prior to 10 h, a dense slow outgrowth was seen for MG1655Fnr− around the point of the inoculum (Fig. 1A). However, after 10 h there was an outgrowth of motile subpopulations from this, represented by the formation of motility halos (Fig. 1B). Motile subpopulation isolates were purified from the motility agar. A comparison of motility in motility agar at 33°C demonstrated that the swarm rate had increased sevenfold for the motile derivatives in comparison to the original poorly motile isolate (Fig. 1C). The motility of the cultures was confirmed by microscopy, during late-exponential growth (OD600, 0.4 to 0.6). The motile derivatives were highly motile, whereas the original isolate showed motility only slightly above that attributed to Brownian motion (data not shown). There was no difference in growth rate for the motile derivatives in comparison to the original isolate of MG1655Fnr− in tryptone broth at 33°C; both divided at 1.9 generations h−1. Complementation of an original poorly motile isolate of MG1655Fnr− by FlhD/FlhC expressed from a plasmid conferred increased motility (Fig. 1D). This suggested that MG1655Fnr− was poorly motile due to reduced expression of the flhD operon. No outgrowth was seen in motility agar for MC4100 (flhD), which is nonmotile (Fig. 1E).
FIG. 1.
Outgrowth of motile subpopulations of MG1655Fnr− from an inoculum of poorly motile MG1655Fnr− cells in motility agar. Overnight cultures in TB were inoculated into semisolid TB medium containing 0.3% (wt/vol) agar as a streak of 30 μl (A, B, and E) or as 2-μl stabs (C and D) and incubated at 33°C. (A) A motility agar plate after 8 h of incubation, inoculated with MG1655Fnr−; (B) the same plate after 10.5 h of incubation. At 10.5 h outgrowth of motile subpopulations is visible. (C) A motility agar plate inoculated with MG1655Fnr− (left) and a motile isolate of MG1655Fnr− (right). The motile isolate was obtained by purification from the outgrowth of a motile subpopulation of MG1655Fnr− from a previous motility agar plate. (D) Swarming at 8 h of MG1655Fnr− transformed with the pT7-7 expression plasmid without an insert (left) and with the pXL27 plasmid expressing FlhD/FlhC (pT7-7 with the flhD and flhC genes inserted) (right). (E) A motility agar plate inoculated with nonmotile MC4100 (flhD) after 10.5 h of incubation. The scale bar is 1 cm.
Presence of IS elements in the flhD operon regulatory region of laboratory strains.
The sequenced MG1655 isolate obtained from the E. coli stock center [MG1655(Seq), CGSC 7740] was motile and contained an IS1 element in the regulatory region of the flhD operon (6). In the study by Lehnen et al. (27), MG1655 was poorly motile and the flhD sequence was without an IS element insert. This suggested that an IS element insertion in the flhD operon regulatory region might increase expression. A PCR screen showed that an approximately 1,200-bp insertion had occurred in the regulatory region of the flhD operon of six motile isolates of MG1655Fnr− purified from motility agar (Fig. 2). Sequencing demonstrated that an 1,195-bp IS5 element insertion had occurred in the flhD operon regulatory region.
FIG. 2.
PCR screen for IS element insertion into the regulatory region of the flhD operon for MG1655Fnr− and a motile derivative of MG1655Fnr− purified from motility agar. MG1655(Seq) and W3110K were included as controls for strains with IS elements in the flhD regulatory region. The positions of the primers are shown (A). Two separate reactions were used; the reverse primer was the same in each case (5′-GGAATGTTGCGCCTCACCG-3′), but two different forward primers were used either downstream (PCR 1; 5′-CCCCCTCCGTTGTATGTGCG-3′) or upstream (PCR 2; 5′-CCTGTTTCATTTTTGCTTGCTAGC-3′) of an IS element insertion hot spot. (B) PCR products. PCR 1 was used to generate product in lanes 1 to 4. PCR 2 was used to generate product in lanes 5 to 8. The genomic DNA templates in the reaction mixtures were MG1655Fnr− (lanes 1 and 5), motile MG1655Fnr− purified from outgrowth of motile subpopulations of MG1655Fnr− after incubation in motility agar (lanes 2 and 6), MG1655(Seq) (lanes 3 and 7), and W3110K (lanes 4 and 8). The sizes of the molecular standards in lane M are noted to the right. Sequencing of the DNA confirmed an IS5 element had inserted into the regulatory region of the flhD operon of the motile derivative of MG1655Fnr− (lane 6).
Several laboratory strains derived from K-12 were investigated for the presence or absence of IS elements in the flhD operon regulatory region and their relative motilities (Table 2). The lineage of strains MG1655 and W3110 is well documented (Fig. 3), so strains W1485 and W2637 were examined. Four strains were examined which have been used in studies of flhD regulation or chemotaxis, as they are motile and the motility might be expected to be due to an IS element insertion in the flhD operon regulatory region: FB8, RP437, YK410, and MC1000 (41, 47, 48, 60). Two additional strains examined were MM294 and RB791 (51). Motility was assayed by measuring swarm rate in semisolid TB medium at 33°C. The presence or absence of an IS element in the flhD operon regulatory region was confirmed by PCR for every overnight culture. The presence of an IS element in the flhD operon regulatory region correlated with elevated motility. The swarm rates of strains or stocks containing isolates with an IS element in the flhD operon regulatory region [RP437, W3110K, W3110, MC1000, W2637, YK410, MG1655Fnr−(motile), and MG1655(Seq)] were 4.4 to 7.5 mm h−1, while the swarm rates of strains or stocks containing isolates with a regulatory region without an IS element insert (MG1655Fnr−, W1485, RB791, MG1655, FB8, and MM294) were 1.0 to 2.4 mm h−1.
TABLE 2.
Swarm rates of E. coli K-12 strain derivatives with or without an IS element in the regulatory region of the flhD operon
| Strain | IS elementa | Swarm rate (mm h−1)b |
|---|---|---|
| Without IS element | ||
| MG1655Fnr− | 1.0 | |
| W1485 | 1.1 | |
| RB791 | 1.1 | |
| MG1655 | 1.2 | |
| FB8 | 2.3 | |
| MM294 | 2.4 | |
| With IS element | ||
| RP437 | IS5 (−166 to −169) | 4.4 |
| W3110K | IS5 (−96 to −99) | 5.1 |
| W3110 | IS5 (−96 to −99) | 5.7 |
| MC1000 | IS5 (−96 to −99) | 5.9 |
| W2637c | IS5 (−96 to −99) | 6.1 |
| YK410 | IS5 (−166 to −169) | 6.8 |
| MG1655Fnr−(motile) | IS5 (−96 to −99) | 7.0 |
| MG1655(Seq) | IS1 (−100 to −107) | 7.5 |
Coordinates of the target sequences of the IS elements relative to the transcription start site described by Soutourina et al. (60) are indicated in parentheses. All IS5 elements were oriented in the upstream direction, according to their defined left and right ends (25). The IS1 in MG1655(Seq) was oriented in the downstream direction (38).
Swarm diameters were measured every hour for overnight cultures inoculated into motility agar with incubation at 33°C. Mean swarm rates are shown for at least three replicates; standard deviations were less than 20% of the mean in each case.
The W2637 stock (CGSC 5290) also contained a high proportion of nonmotile cells. However, colonies that were composed of motile cells were used, as W2637 was reportedly derived by motility agar selection (3) and therefore should have a motile phenotype.
FIG. 3.
Derivation of MG1655 and W3110. This information has been taken from Bachmann (3) and Jensen (21). Below the strains (bold type) are the strain stocks from the E. coli Genetic Stock Center used in this study. The MG1655 stocks were generated from lyophilized cultures originating from a single sample sent to the stock center. The W3110 stocks were generated from different samples sent to the stock center. MG1655Fnr−(motile) was obtained in this study by purification of isolates from the outgrowth of motile subpopulations of MG1655Fnr− formed in motility agar.
Increased expression of the flhD operon by an IS5 element insertion in the regulatory region and the effect on FlhD/FlhC- and FlhD-regulated operons.
Gene expression characteristics for one of six motile subpopulation derivatives of MG1655Fnr− purified from motility agar which had an IS5 element insertion in the flhD operon regulatory region were compared to those of the original poorly motile isolate (Table 3). The derivative, named MG1655Fnr−(motile), may not be isogenic with its parent, as it is possible that a secondary event also occurred in the genome in addition to the IS5 insertion during incubation of MG1655Fnr− in motility agar. However, since five other motile candidates were also obtained which contained IS element insertions in the flhD operon regulatory region, this phenomenon appears specific. Real-time PCR was used to compare flhD and fliA mRNA levels of MG1655Fnr−(motile) to its poorly motile parent. A modest increase in flhD mRNA levels of 2.7-fold was seen for the motile derivative, while levels of mRNA for fliA (a direct target of FlhD/FlhC) were 32-fold higher. Expression of various promoter fusions to a promoterless luxCDABE cassette from a moderate-copy-number plasmid in MG1655Fnr−(motile) was compared that for its poorly motile parent. The gltB, ompT, and gcvT operons have been identified as candidates for FlhD regulation in the absence of FlhC, while the serA operon has been proposed as a target for regulation by FlhD/FlhC (47). The flhB operon was also examined as a flagellar control. In MG1655Fnr−(motile), expression of flhB::luxCDABE was 65-fold higher and expression of gltB::luxCDABE was 3.1-fold higher. There was only a small increase in expression for serA::luxCDABE, gcvT::luxCDABE, and ompT::luxCDABE fusions.
TABLE 3.
Expression of flhD and FlhD/FlhC- and FlhD-regulated genes in poorly motile MG1655Fnr− and a motile derivativea after selection for motility
| mRNA or fusion | Expression ratio (motile/poorly motile) |
|---|---|
| mRNAsb | |
| flhD | 2.7 ± 0.4 |
| fliA | 31.6 ± 15.5 |
| luxCDABE fusionsc | |
| flhB | 64.5 ± 25.1 |
| gltB | 3.1 ± 0.9 |
| gcvT | 1.5 ± 0.6 |
| serA | 1.5 ± 0.3 |
| ompT | 1.4 ± 0.1 |
The derivative, designated MG1655Fnr−(motile) in this study, was purified from motility agar inoculated with MG1655Fnr− after selection for motility. It contained an IS5 element insertion in the regulatory region of the flhD operon.
Real-time PCR was used to determine copy numbers of flhD and fliA mRNA per nanogram of cDNA prepared from late-exponential-phase cultures (at an OD600 of 0.5). Cultures were prepared in duplicate, and levels of transcript from each culture were measured four times.
Batch cultures of cells containing luxCDABE promoter fusions were grown in triplicate, and light units and OD600 were measured at seven points through the growth curve. Expression was calculated as specific light units per milliliter per OD600 unit, and at each time point the ratio of expression for motile cells to that for poorly motile cells was derived. The mean ratio of expression for all time points is shown: Standard deviations are indicated.
Additional genetic variation in MG1655 stocks.
Recently, Soupene et al. (59) have shown that MG1655 stocks from different sources vary in several respects including the presence of a large deletion around the fnr locus (fnr-267) in some isolates. Stock MG1655Fnr− (CGSC 8003) is now known to be a mixture of cells with (fnr-267) and without (fnr+) the deletion; hence, it has been renamed from MG1655 (CGSC 6300) to MG1655Fnr− (CGSC 8003) (http://cgsc.biology.yale.edu/) (59). We always used individual colonies in our experiments, so the fact that this stock is a mixture is not important to our results. We screened the flhD operon from 20 colonies generated from stock CGSC 8003: 19 had an flhD operon regulatory region without an insert and were poorly motile, and one was comprised of motile cells with an insert which we determined to be an IS1 element, in the same position and orientation as in MG1655(Seq) (CGSC 7740). Using the PCR screen described by Soupene et al. (59) with primers fnr-5′ and fnr-3′, we found that the motile isolate of CGSC 8003 was fnr+, while the poorly motile isolates we used in this study did not produce PCR products, suggesting that they carried the fnr-267 deletion (data not shown). Isolates of a replacement stock MG1655 (CGSC 6300) were fnr+ and had an flhD operon regulatory region without inserts. These isolates were poorly motile and also formed motile subpopulations in motility agar, with purified isolates having IS element insertions upstream of the flhD operon (data not shown). Therefore, both fnr+ and fnr-267 isolates are capable of generating IS element insertions in the flhD operon regulatory region, so the variation at the fnr locus appears unrelated to the variation in strains at the flhD operon regulatory region, which we report here.
Transcriptional control of the flhD operon and hot spots for IS element insertion in the regulatory region.
Three insertion points for IS elements in the regulatory region were identified (Fig. 4). In each case the target sequence becomes duplicated upon insertion to flank the element. Soutourina et al. (60) previously described the (+1) transcription start site of the flhD operon regulatory region. A 4-bp target site (5′-TTAA-3′) exists at −96 to −99 bp for IS5 elements. An IS5 element was identified in strains W3110, MC1000, W2637, and MG1655Fnr−(motile). In each case the orientation of the 1,195-bp IS5 element was in the upstream direction, according to its defined left and right ends (25). MC1000 has a C→T substitution at position 1134 of the IS5 element. Another 4-bp target site (5′-CTAG-3′) for IS5 insertion exists at −166 to −169 bp relative to the transcription start site. An IS5 element was identified here for strains RP437 and YK410, and in both cases the orientation was in the upstream direction. The IS1 insertion target site was identified in strain MG1655 previously (6). An 8-bp sequence (5′-CATTTATG-3′) at −100 to −107 bp was a target for a 768-bp IS1 element, isoform IS1A (also known as IS1E) (66).
FIG. 4.
The regulatory region of the flhD operon showing hot spots of IS element insertion. IS1 (768 bp) and IS5 (1,195 bp) elements have been determined to specifically insert into regions of the flhD operon regulatory region. The nature of the IS element is shown above the target sequence (boxes with broken lines). The target sequence becomes duplicated and flanks each end of the IS element upon insertion. Horizontal arrows indicate the orientation of the IS element inserts, according to their defined left and right ends (25, 38). The positions of the binding sites are indicated as follows: H-NS, black lines below the sequence (60); OmpR, black bars above the sequence (57); LrhA, grey bar above the sequence (27); catabolite gene activator protein (CAP), grey bar above the sequence (60); and RcsAB, grey bar above the sequence (17). The transcriptional start site (+1) of the flhD operon is indicated by a solid bent arrow.
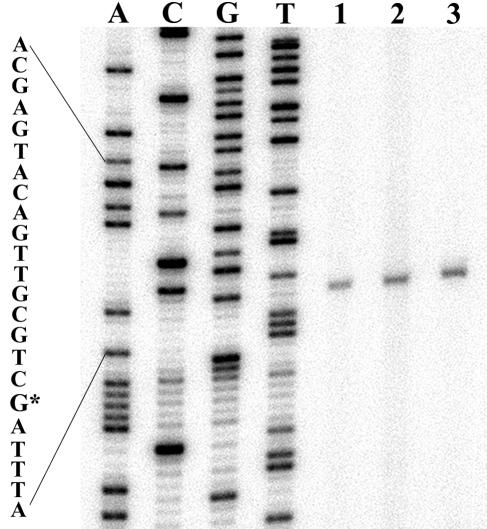
The transcription start site was unaltered by IS1 or IS5 element insertion in the flhD operon regulatory region, based on primer extension analysis (Fig. 5). RNA was isolated from RP437 (IS5), MG1655(Seq) (IS1), and MG1655Fnr−(motile) (IS5) for primer extension. A sequencing ladder showed that the transcriptional start site was the same as that determined by Soutourina et al. (60). In that study strain FB8 was used, which is without an IS element insertion in the flhD operon regulatory region (60). The transcriptional start site from poorly motile MG1655Fnr−, which is also without an IS element insertion in the flhD operon regulatory region, was examined, but the signal was very weak (data not shown). This was consistent with this strain having reduced expression of the flhD operon, since the same quantity of RNA (50 μg) was used in each cDNA synthesis reaction.
FIG. 5.
Determination of the transcriptional start site of the flhD operon for strains having an IS element inserted into the regulatory region. As a reference, a DNA sequencing ladder is shown (lanes ACGT). This was obtained with the same primer as that used for primer extension. The start site is indicated (G*) and is the same as that determined by Soutourina et al. (60). For primer extension, 50 μg of RNA was used in each reaction mixture, prepared from cultures grown to an OD600 of 0.5. Lane 1, RP437 (1,195-bp IS5 element inserted at a target site at position −166 to −169); lane 2, MG1655(Seq) (768-bp IS1 element inserted at a target site at position −100 to −107); lane 3, MG1655Fnr−(motile) (1,195-bp IS5 element inserted at a target site at position −96 to −99).
DISCUSSION
We have shown that IS5 and IS1 elements can spontaneously insert into the regulatory region of the flhD operon, increase expression, and improve motility. We have found that derivatives of E. coli K-12 can be separated into two groups on the basis of the absence or presence of an IS element in the regulatory region of the flhD operon, and we have been able to generate cells with an IS element insert there from cells without one. A modest increase in flhD expression of 2.7-fold by IS5 element insertion was observed and is significant because the signal becomes amplified in the regulatory cascade of the flagellar regulon. This modulation of flhD expression significantly increased fliA (32-fold) and flhB::luxCDABE (65-fold) expression. All the flagellar operons depend upon FlhD/FlhC for expression, and FlhD/FlhC has been shown to bind the flhB and fliA operon promoters directly and to activate transcription in vitro (29). A gltB::luxCDABE fusion was moderately regulated by the increase in flhD expression, while serA::luxCDABE, gcvT::luxCDABE, and ompT::luxCDABE fusions showed slight regulation. These targets for regulation have been identified by microarray analysis by comparing expression ratios of the wild-type to those of flhD::kan, flhD, and flhC mutants and are less well understood in terms of regulation by FlhD/FlhC (in the case of serA) and FlhD alone (in the case of gltB, gcvT, and ompT) (47). Since these operons are regulated by other factors in addition to control through the flhD operon, a 2.7-fold modulation in flhD expression would be expected to have a less marked effect than for a flagellar target. The flhD operon is not cryptic like the bgl and ade operons, since cells without an IS element insertion in the regulatory region were poorly motile, not nonmotile, flhD expression was 2.7-fold lower, and light production from flhB::luxCDABE was still significant in MG1655Fnr− (data not shown).
The mechanism of increased expression of the flhD operon by IS element insertion into the regulatory region is not by the formation of hybrid promoters, since the transcriptional start site was unaltered in primer extension analysis. This implies that activation has occurred through a decrease in transcriptional repression. Examples of disruption of transcriptional repression in E. coli include ompC, where an IS1 element insertion perturbed regulation by OmpR (39); sodA, where an IS5 element was suggested to abolish repression by Fur (8), and fnr, where the autoregulation was postulated to be disrupted by IS5 element insertion (7). The binding sites of the repressors LrhA and OmpR appear to be affected by the IS1 insertion point and the downstream IS5 insertion point (Fig. 4). For the bgl and ade operons, gene silencing is abolished by IS element insertion through a mechanism which has been suggested to involve disruption of a repressing nucleoprotein complex of which H-NS is a component (11, 42). In a study of the bgl operon, IS5 activation was abolished by internal deletions but was restored by providing an IS5-encoded gene product, Ins5A, necessary for transposition, in trans (53). The implication is that interaction of Ins5A with the ends of IS5 leads to changes in the topology of the bgl promoter region. Analogous to the bgl and ade operons, the natural topology of the flhD operon regulatory region has been suggested to be partly provided by H-NS regulation, implying that an alteration of chromosome topology by IS element insertion may enhance flhD expression. Although, unlike the bgl and ade operons, where H-NS is a repressor, H-NS has been proposed as a positive regulator of the flhD operon (5, 60, 62). H-NS regulation of the flhD operon is complex, however; although an hns mutant was nonmotile, in vitro transcription of the flhD operon by H-NS was negative (60). Furthermore, part of the regulation by H-NS is indirect and occurs via HdfR (23).
The two insertion points of IS5 fit the known preferred target sequence, YTAR (30). It is known that IS1 elements can generate direct target duplications of 8 to 10 bp (and even 14 bp) and have a preference for AT-rich target regions, which is consistent with the target site identified in MG1655 (6, 30). It will be interesting to determine the insertion frequency at each point and whether or not this is affected by the transcription factors which bind at that region. Although subpopulations having an IS element insertion upstream of the flhD operon promoter were more motile, and therefore have a selective advantage in motility agar, we are not suggesting that this is a case of directed evolution. The directed mutation hypothesis, which states that mutations occur more frequently when they are advantageous, has been rejected for activation of the bgl operon (31).
The flhD operon is under complex regulation to respond to the heterogeneous and transient environments E. coli encounters naturally. It will be important to determine how the IS element insertions affect regulation of the flhD operon and whether IS element regulation of the flhD operon is important for E. coli in its natural environment. Flagellar synthesis is known to inhibited by the following conditions: catabolite repression (1, 58, 68), high temperature (1), high osmolarity (57), low pH (62), high concentrations of salts, carbohydrates, and low-molecular-weight alcohols (28, 55), salicylate (26), and acetate (46). In contrast, expression of flhD increased after long-term adaptation to acetate and propionate (45). Synthesis of flagella is also regulated by growth phase (2), transition from surface to liquid (19), quorum sensing by QseBC (63), phosphatidylethanolamine and phosphatidylglycerol synthesis (22, 33, 54, 65), heat shock proteins DnaK, DnaJ, and GrpE (56), and cell cycle control (32, 36).
The level of motility of E. coli isolates in comparison to one another is obviously determined by more complex factors than the presence or absence of an IS element insertion in the flhD regulatory region. For example, the genome content of strains can be highly variable. Even in a comparison of W3110 and MG1655, which are closely related and have the same chromosome size, W3110 lacks ∼80 of the ORFs (65 kb) present in MG1655 (37). Furthermore, other cis-acting mutations such as point mutations in the regulatory region of flhD or trans-acting mutations in the genes for repressors of flhD could activate expression, or mutations elsewhere in the flagellar regulon may alter motility. If a larger sample of motile subpopulation isolates of MG1655Fnr− had been purified from the motility agar, it is probable that other types of mutation would have been seen. Moreover, different strains may activate flhD regulation differently because of differences in IS element compositions of the genome (16, 52). Also, some strains that are sufficiently motile may not form motile subpopulations in semisolid media. In conclusion, motility of E. coli is under complex regulation, of which IS element integration into the regulatory region of the flhD operon is part, and this can lead to inter- as well as intrapopulation diversity.
Acknowledgments
We thank William Hendrickson (University of Illinois at Chicago) for helpful advice, Philippe N. Bertin (Université Louis Pasteur, Strasbourg, France) for supplying strains, Mary Berlyn (E. coli Genetic Stock Center) for supplying strains and for help in tracing the lineage of MG1655, Tapan K. Misra (University of Illinois at Chicago) for questions regarding strains, Rhonda T. Fleming and Wael Refaat Abdel-Fattah (University of Illinois at Chicago) for technical assistance, Andrés Campos (University of Illinois at Chicago) for help with preparation of the figures, and Christopher O'Connor (University of Illinois at Chicago) for critical reading of the manuscript.
This work was supported by grant GM59484 from the National Institutes of Health.
REFERENCES
- 1.Adler, J., and B. Templeton. 1967. The effect of environmental conditions on Escherichia coli. J. Gen. Microbiol. 46:175-184. [DOI] [PubMed] [Google Scholar]
- 2.Amsler, C. D., M. Cho, and P. Matsumura. 1993. Multiple factors underlying the maximum motility of Escherichia coli as cultures enter postexponential growth. J. Bacteriol. 175:6238-6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmann, B. J. 1996. Derivations and genotypes of some mutant derivatives of Escherichia coli K-12, p. 2460-2488. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 4.Bartlett, D. H., B. B. Frantz, and P. Matsumura. 1988. Flagellar transcriptional activators FlbB and FlaI: gene sequences and 5′ consensus sequences of operons under FlbB and FlaI control. J. Bacteriol. 170:1575-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertin, P., E. Terao, E. Hee Lee, P. Lejeune, C. Colson, A. Danchin, and E. Collatz. 1994. The H-NS protein is involved in the biogenesis of flagella in Escherichia coli. J. Bacteriol. 176:5537-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 7.Bonnefoy, V., M. Fons, J. Ratouchniak, M.-C. Pascal, and M. Chippaux. 1988. Aerobic expression of the nar operon of Escherichia coli in a fnr mutant. Mol. Microbiol. 2:419-425. [DOI] [PubMed] [Google Scholar]
- 8.Bowen, S. W., and H. M. Hassan. 1993. Characterization of cis-acting regulatory mutations causing anaerobic expression of the sodA gene in Escherichia coli. Arch. Biochem. Biophys. 302:372-379. [DOI] [PubMed] [Google Scholar]
- 9.Boyd, E. F., and D. L. Hartl. 1997. Nonrandom location of IS1 elements in the genomes of natural isolates of Escherichia coli. Mol. Biol. Evol. 14:725-732. [DOI] [PubMed] [Google Scholar]
- 10.Bruni, C. B., V. Colantuoni, L. Sbordone, R. Cortese, and F. Blasi. 1977. Biochemical and regulatory properties of Escherichia coli K-12 hisT mutants. J. Bacteriol. 130:4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caramel, A., and K. Schnetz. 1998. Lac and λ repressors relieve silencing of the Escherichia coli bgl promoter. Activation by alteration of a repressing nucleoprotein complex. J. Mol. Biol. 284:875-883. [DOI] [PubMed] [Google Scholar]
- 12.Casadaban, M. J. 1976. Transposition and fusion of the lac genes to selected promoters using bacteriophage Lambda and Mu. J. Mol. Biol. 104:541-555. [DOI] [PubMed] [Google Scholar]
- 13.Casadaban, M. J., and S. N. Cohen. 1980. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J. Mol. Biol. 138:179-207. [DOI] [PubMed] [Google Scholar]
- 14.Chilcott, G. S., and K. T. Hughes. 2000. Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar Typhimurium and Escherichia coli. Microbiol. Mol. Biol. Rev. 64:694-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chuang, S. E., D. L. Daniels, and F. R. Blattner. 1993. Global regulation of gene expression Escherichia coli. J. Bacteriol. 175:2026-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deonier, R. C. 1996. Native insertion sequence elements: locations, distributions, and sequence relationships, p. 2000-2011. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 17.Francez-Charlot, A., B. Laugel, A. Van Gemert, N. Dubarry, F. Wiorowski, M.-P. Castanié-Cornet, C. Gutierrez, and K. Cam. 2003. RcsCDB His-Asp phosphorelay system negatively regulates the flhDC operon in Escherichia coli. Mol. Microbiol. 49:823-832. [DOI] [PubMed] [Google Scholar]
- 18.Hall, B. G. 1998. Activation of the bgl operon by adaptive mutation. Mol. Biol. Evol. 15:1-5. [DOI] [PubMed] [Google Scholar]
- 19.Harshey, R. M., and T. Matsuyama. 1994. Dimorphic transition in Escherichia coli and Salmonella typhimurium: surface-induced differentiation into hyperflagellate swarmer cells. Proc. Natl. Acad. Sci. USA 91:8631-8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itoh, T., H. Aiba, T. Baba, K. Hayashi, T. Inada, K. Isono, H. Kasai, S. Kimura, M. Kitakawa, M. Kitagawa, K. Makino, T. Miki, K. Mizobuchi, H. Mori, T. Mori, K. Motomura, S. Nakade, Y. Nakamura, H. Nashimoto, Y. Nishio, T. Oshima, N. Saito, G. Sampei, Y. Seki, S. Sivasundaram, H. Tagami, J. Takeda, K. Takemoto, C. Wada, Y. Yamamoto, and T. Horiuchi. 1996. A 460-kb DNA sequence of the Escherichia coli K-12 genome corresponding to the 40.1-50.0 min region on the linkage map. DNA Res. 3:379-392. [DOI] [PubMed] [Google Scholar]
- 21.Jensen, K. F. 1993. The Escherichia coli K-12 “wild types” W3110 and MG1655 have an rph frameshift mutation that leads to pyrimidine starvation due to low pyrE expression levels. J. Bacteriol. 175:3401-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitamura, E., Y. Nakayama, H. Matsuzaki, K. Matsumoto, and I. Shibuya. 1994. Acidic-phospholipid deficiency represses the flagella master operon through a novel regulatory region in Escherichia coli. Biosci. Biotech. Biochem. 58:2305-2307. [DOI] [PubMed] [Google Scholar]
- 23.Ko, M., and C. Park. 2000. H-NS-dependent regulation of flagellar synthesis is mediated by a LysR family protein. J. Bacteriol. 182:4670-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komeda, Y., and T. Iino. 1979. Regulation of expression of the flagellin gene (hag) in Escherichia coli K-12: analysis of hag-lac gene fusions. J. Bacteriol. 139:721-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kröger, M., and G. Hobom. 1982. Structural analysis of insertion sequence IS5. Nature 297:159-162. [DOI] [PubMed] [Google Scholar]
- 26.Kunin, C. M., T. Hua Hua, and L. O. Bakaletz. 1995. Effect of salicylate on expression of flagella by Escherichia coli and Proteus, Providencia, and Pseudomonas spp. Infect. Immun. 63:1796-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehnen, D., C. Blumer, T. Polen, B. Wackwitz, V. F. Wendisch, and G. Unden. 2002. LrhA as a new transcriptional key regulator of flagella, motility and chemotaxis genes in Escherichia coli. Mol. Microbiol. 45:521-532. [DOI] [PubMed] [Google Scholar]
- 28.Li, C., C. J. Louise, W. Shi, and J. Adler. 1993. Adverse conditions which cause lack of flagella in Escherichia coli. J. Bacteriol. 175:2229-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, X., and P. Matsumura. 1994. The FlhD/FlhC complex, a transcriptional activator of the Escherichia coli flagellar class II operons. J. Bacteriol. 176:7345-7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahillon, J., and M. Chandler. 1998. Insertion sequences. Microbiol. Mol. Biol. Rev. 62:725-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mittler, J. E., and R. E. Lenski. 1992. Experimental evidence for an alternative to directed mutation in the bgl operon. Nature 356:446-448. [DOI] [PubMed] [Google Scholar]
- 32.Mizushima, T., R. Koyanagi, T. Katayama, T. Miki, and K. Sekimizu. 1997. Decrease in expression of the master operon of flagellin synthesis in a dnaA46 mutant of Escherichia coli. Biol. Pharm. Bull. 20:327-331. [DOI] [PubMed] [Google Scholar]
- 33.Mizushima, T., R. Koyanagi, E. Suzuki, A. Tomura, K. Kutsukake, T. Miki, and K. Sekimizu. 1995. Control by phosphatidylglycerol of expression of the flhD gene in Escherichia coli. Biochim. Biophys. Acta 1245:397-401. [DOI] [PubMed] [Google Scholar]
- 34.Naas, T., M. Blot, W. M. Fitch, and W. Arber. 1994. Insertion sequence-related genetic variation in resting Escherichia coli K-12. Genetics 136:721-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naas, T., M. Blot, W. M. Fitch, and W. Arber. 1995. Dynamics of IS-related genetic rearrangements in resting Escherichia coli K-12. Mol. Biol. Evol. 12:198-207. [DOI] [PubMed] [Google Scholar]
- 36.Nishimura, A., and Y. Hirota. 1989. A cell division regulatory mechanism controls the flagellar regulon in Escherichia coli. Mol. Gen. Genet. 216:340-346. [DOI] [PubMed] [Google Scholar]
- 37.Ochman, H., and I. B. Jones. 2000. Evolutionary dynamics of full genome content in Escherichia coli. EMBO J. 19:6637-6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohtsubo, H., and E. Ohtsubo. 1978. Nucleotide sequence of an insertion element, IS1. Proc. Natl. Acad. Sci. USA 75:615-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozawa, Y., S. Mizushima, and T. Mizuno. 1990. Osmoregulatory expression of the ompC gene in Escherichia coli K-12; IS1 insertion in the upstream regulatory region results in constitutive activation of the promoter. FEMS Microbiol. Lett. 68:295-300. [DOI] [PubMed] [Google Scholar]
- 40.Parker, L. L., and B. G. Hall. 1990. Mechanisms of activation of the cyptic cel operon of Escherichia coli K12. Genetics 124:473-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parkinson, J. S. 1978. Complementation analysis and deletion mapping of Escherichia coli mutants defective in chemotaxis. J. Bacteriol. 135:45-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petersen, C., L. Birk Møller, and P. Valentin-Hansen. 2002. The cryptic adenine deaminase gene of Escherichia coli. Silencing by the nucleoid-associated DNA-binding protein, H-NS, and activation by insertion elements. J. Biol. Chem. 277:31373-31380. [DOI] [PubMed] [Google Scholar]
- 43.Pitcher, D. G., N. A. Saunders, and R. J. Owen. 1989. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett. Appl. Microbiol. 8:151-156. [Google Scholar]
- 44.Plumbridge, J., and O. Pellegrini. 2004. Expression of the chitobiose operon of Escherichia coli is regulated by three transcription factors: NagC, ChbR and CAP. Mol. Microbiol. 52:437-449. [DOI] [PubMed] [Google Scholar]
- 45.Polen, T., D. Rittmann, V. F. Wendisch, and H. Sahm. 2003. DNA microarray analyses of the long-term adaptive response of Escherichia coli to acetate and propionate. Appl. Environ. Microbiol. 69:1759-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prüβ, B. M. 1998. Acetyl phosphate and the phosphorylation of OmpR are involved in the regulation of cell division rate in Escherichia coli. Arch. Microbiol. 170:141-146. [DOI] [PubMed] [Google Scholar]
- 47.Prüβ, B. M., J. W. Campbell, T. K. Van Dyk, C. Zhu, Y. Kogan, and P. Matsumura. 2003. FlhD/FlhC is a regulator of anaerobic respiration and the Entner-Doudoroff pathway through induction of the methyl-accepting chemotaxis protein Aer. J. Bacteriol. 185:534-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prüβ, B. M., X. Liu, W. Hendrickson, and P. Matsumura. 2001. FlhD/FlhC-regulated promoters analyzed by gene array and lacZ gene fusions. FEMS Microbiol. Lett. 197:91-97. [DOI] [PubMed] [Google Scholar]
- 49.Reynolds, A. E., J. Felton, and A. Wright. 1981. Insertion of DNA activates the cryptic bgl operon in E. coli K12. Nature 293:625-629. [DOI] [PubMed] [Google Scholar]
- 50.Reynolds, A. E., S. Mahadevan, S. F. J. LeGrice, and A. Wright. 1984. Enhancement of bacterial gene expression by insertion elements or by mutation in a CAP-cAMP binding site. J. Mol. Biol. 191:85-89. [DOI] [PubMed] [Google Scholar]
- 51.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 52.Sawyer, S. A., D. E. Dykhuizen, R. F. DuBose, L. Green, T. Mutangadura-Mhlanga, D. F. Wolczyk, and D. L. Hartl. 1987. Distribution and abundance of insertion sequences among natural isolates of Escherichia coli. Genetics 115:51-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schnetz, K., and B. Rak. 1992. IS5: a mobile enhancer of transcription in Escherichia coli. Proc. Natl. Acad. Sci. USA 89:1244-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi, W., M. Bogdanov, W. Dowhan, and D. R. Zusman. 1993. The pss and psd genes are required for motility and chemotaxis in Escherichia coli. J. Bacteriol. 175:7711-7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi, W., C. Li, C. J. Louise, and J. Adler. 1993. Mechanism of adverse conditions causing lack of flagella in Escherichia coli. J. Bacteriol. 175:2236-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi, W., Y. Zhou, J. Wild, J. Adler, and C. A. Gross. 1992. DnaK, DnaJ, and GrpE are required for flagellum synthesis in Escherichia coli. J. Bacteriol. 174:6256-6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shin, S., and C. Park. 1995. Modulation of flagellar expression in Escherichia coli by acetyl phosphate and the osmoregulator OmpR. J. Bacteriol. 177:4696-4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silverman, M., and M. Simon. 1974. Characterization of Escherichia coli flagellar mutants that are insensitive to catabolite repression. J. Bacteriol. 120:1196-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soupene, E., W. C. van Heeswijk, J. Plumbridge, V. Stewart, D. Bertenthal, H. Lee, G. Prasad, O. Paliy, P. Charernnoppakul, and S. Kustu. 2003. Physiological studies of Escherichia coli strain MG1655: growth defects and apparent cross-regulation of gene expression. J. Bacteriol. 185:5611-5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soutourina, O., A. Kolb, E. Krin, C. Laurent-Winter, S. Rimsky, A. Danchin, and P. Bertin. 1999. Multiple control of flagellum biosynthesis in Escherichia coli: role of H-NS protein and the cyclic AMP-catabolite activator protein complex in transcription of the flhDC master operon. J. Bacteriol. 181:7500-7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soutourina, O. A., and P. N. Bertin. 2003. Regulation cascade of flagellar expression in Gram-negative bacteria. FEMS Microbiol. Rev. 27:505-523. [DOI] [PubMed] [Google Scholar]
- 62.Soutourina, O. A., E. Krin, C. Laurent-Winter, F. Hommais, A. Danchin, and P. N. Bertin. 2002. Regulation of bacterial motility in response to low pH in Escherichia coli: the role of H-NS protein. Microbiology 148:1543-1551. [DOI] [PubMed] [Google Scholar]
- 63.Sperandio, V., A. G. Torres, and J. B. Kaper. 2002. Quorum sensing Escherichia coli regulators B and C (QseBC): a novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol. Microbiol. 43:809-821. [DOI] [PubMed] [Google Scholar]
- 64.Tabor, S. 1990. Expression using the T7 RNA polymerase/promoter system, p. 16.2.1-16.2.11. In F. A. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J.A. Smith, and K. Struhl (ed.), Current protocols in molecular biology. Greene Publishing and Wiley-Interscience, New York, N.Y.
- 65.Tomura, A., T. Ishikawa, Y. Sagara, T. Miki, and K. Sekimizu. 1993. Requirement of phosphatidylglycerol for flagellation of Escherichia coli. FEBS Lett. 329:287-290. [DOI] [PubMed] [Google Scholar]
- 66.Umeda, M., and E. Ohtsubo. 1991. Four types of IS1 with differences in nucleotide sequence reside in the Escherichia coli K-12 chromosome. Gene 98:1-5. [DOI] [PubMed] [Google Scholar]
- 67.Van Dyk, T. K., and R. A. Rosson. 1998. Photorhabdus luminescens luxCDABE promoter probe vectors. Methods Mol. Biol. 102:85-95. [DOI] [PubMed] [Google Scholar]
- 68.Yokota, T., and J. S. Gots. 1970. Requirement of adenosine 3′,5′-cyclic phosphate for flagella formation in Escherichia coli and Salmonella typhimurium. J. Bacteriol. 103:513-516. [DOI] [PMC free article] [PubMed] [Google Scholar]