Abstract
Neuroleptic malignant syndrome is an unpredictable iatrogenic neurologic emergency condition, mainly arising as an idiosyncratic reaction to antipsychotic agent use. It is characterized by distinctive clinical features including a change in mental status, generalized rigidity, hyperpyrexia, and dysautonomia. It can be lethal if not diagnosed and treated properly. Mortality and morbidity attributed to this syndrome have recently declined markedly due to greater awareness, earlier diagnosis, and intensive care intervention. In most cases, the syndrome occurs as a result of a rapid increase in a dose of neuroleptic, especially one of the long-acting ones. Pathophysiology behind this syndrome is attributed to a dopamine receptor blockade inside the neurons rendered by the offending drug and excessive calcium release from the sarcoplasmic reticulum of skeletal myocytes. Laboratory tests, although not diagnostic, may assist in assessing the severity of the syndrome and also the consequent complications. The syndrome has been described in all age groups and occurs more in males than in females. Genetics appears to be central regarding the etiology of the syndrome. Stopping the use of the offending agent, cold intravenous fluids, and removal of the causative agent and its possible active metabolites is the cornerstone of treatment. Periodic observation of psychotic patients recently started on antipsychotic medications, especially those being treated with depot preparations, may aid to an early diagnosis of the syndrome and lead to early treatment.
Keywords: neuroleptic malignant syndrome, dopamine receptors, rhabdomyolysis, renal shutdown, hyperpyrexia, sarcoplasmic reticulum
Introduction
Prelude and history
Neuroleptic malignant syndrome (NMS) is a neurologic emergency condition that may arise as a result of administration of potent psychotropic agents. Its pathophysiology is still unclear. It includes the following symptoms: muscle rigidity, hyperpyrexia, autonomic nervous system imbalance, mental disturbances (manifested mostly as delirium), and abnormal metabolic changes. In atypical cases, which are considered to be associated with the use of lower potency agents, rigidity may be milder or even absent.1 Importantly, hyperpyrexia, an essential diagnostic criterion for NMS, can be absent in such cases.2,3 The complexity of making diagnosis is due to the isolated occurrence of autonomic malfunction, hyperpyrexia, Parkinsonian-like muscle rigidity, and creatine kinase (CK) elevation. Individually, these symptoms are not necessarily considered as a precursor of NMS.4,5 It appears that the incidence of this iatrogenic ailment has decreased partly due to the discovery of the so-called second-generation (nonconventional or atypical) antipsychotics and partly due to the changes in prescription guidelines that the psychiatrist follows when treating psychoses and other neurologic conditions that might necessitate the use of these major tranquilizers. If NMS is not diagnosed early and vigorously treated in a fully equipped intensive care unit,6,7 then the condition can be fatal or give rise to permanent morbid sequelae.8,9 A danger is that NMS can be easily overlooked, especially after the prescription of nonconventional antipsychotic agents as first-line treatment to combat psychoses, especially schizophrenia. The major tranquilizers were discovered in 1956 and clinically used in 1960 by a French group, who studied the effects of the potent conventional antipsychotic, haloperidol. They described the syndrome that was given the French name “syndrome malin des neuroleptiques”, later translated into English and termed NMS.10,11
Contributing risk factors
Strong evidence shows that a genetic defect is a major risk factor. Identical twins and a mother with her two daughters were presented with NMS.12 Senile dementia of Lewy-type (another potential contributing factor) is characterized by the presence of protein aggregates (α-synuclein and ubiquitin) in neurons of these individuals, usually detected as a postmortem finding in the brain. In the USA alone, 1.3 million individuals are affected by this type of dementia.13 It is unclear why males <40 years of age are at a greater risk of developing NMS (male:female ratio is 2:1).14,15 This could be attributed to 1) the higher use of antipsychotics in men of this age group, 2) the adherence to medication, 3) differences in pharmacokinetics toward the drug and the pharmacodynamics of the drugs in the patients of different sexes, and 4) other factors not yet fully elucidated.16–19 Point 3 is not totally accepted, and this is indeed of controversial nature.20 Postpartum women are liable to develop psychiatric diseases, such as psychosis and depression, and thus they are likely to use psychotropics, therefore are at risk of suffering from NMS.21 The most obvious risk factor related to the development of NMS is neuroleptic therapy itself. Thus, the time-course of administering the drug, the type of neuroleptic used (high-potency drugs endanger most), rapid titration of the desired therapeutic serum levels, and the oily long-acting depot forms of neuroleptics (such as haloperidol decanoate, risperdal consta, fluphenazine decanoate, and fluphenazine enanthate) are well-established risk factors regarding the development of NMS.22–24 Previous episodes of NMS, a recent episode of catatonia, and extreme agitation are documented risk factors. This is because these conditions are over represented in this patient group, and thus NMS might be overlooked,25–28 or at least not diagnosed early enough. The incidence of NMS is ~0.2%–3.23%.14 The reported numbers are probably low because physicians and psychiatrists have become aware of the serious side effects that neuroleptics can cause, even the most recently designed ones.23,29
Origin and proposed theories
The molecular basis of NMS is attributed to a sudden pronounced reduction of dopamine activity. This might be due to the withdrawal of dopaminergic agents or to the reduction in dose, or from the antagonism of dopamine receptors with antipsychotics or other dopamine-antagonizing preparations. In schizophrenic patients, it has been proposed that proinflammatory cytokines such as interleukin 1 (IL-1)-α, IL-6, and tumor necrosis factor, are involved in mechanisms that lead to development of neuroleptic intolerance. These proinflammatory cytokines activate the key kynurenine enzymes: indoleamine 2,3-dioxygenase and kynurenine-3-monooxygenase that catalyzes the production of 3-hydroxykynurenine (3-HK). In NMS, the existence of this harmful biomaterial (3-HK) initiates the activation of the scavenging process carried out by the microglia (macrophages of central nervous system [CNS]) to produce the neurotoxin quinolinic acid instead of neuroprotective kynurenic acid (KYNA).30,31 A potential decrease in the KYNA pool will diminish firing rates and burst firing activities of dopamine neurons of the midbrain with decreased intake and uptake of the amino acid tyrosine, which is attributed to dysphagia and physical hypoactivity because of muscle rigidity and altered consciousness.32 In schizophrenia a partial decrease in BH4 will occur. BH4 is an essential cofactor of the aromatic amino acid hydroxylase enzymes used in degradation of phenylalanine and the biosynthesis of certain neurotransmitters including dopamine, and it is a cofactor in production of NO. This decrease in the BH4 pool will affect nigrostriatal dopamine neurons, causing a deficiency in dopamine biosynthesis.33
BH4 is related to the free radical NO. NO dilates blood vessels, inhibits thrombocyte activity,34 and reduces the sympathetic vasoconstriction responsiveness, both in resting and contracting skeletal myocytes.35,36
Failure in the NO system results in the accumulation of a large amount of free radicals that impairs the mitochondrial electron transport chain, thus decreasing the cellular production of adenosine triphosphate (ATP).37 As a result, muscle cells are damaged, causing hyperpyrexia, and cellular constituents, for example, electrolytes (potassium, phosphate) and proteins (myoglobin, creatine, and CK) leak into the blood stream.
One of the motives behind design of second-generation antipsychotics was for treatment of NMS. Unfortunately, it was found that these new agents were also capable of causing this syndrome. For example, clozapine, olanzapine, risperidone, quetiapine, and ziprasidone have been criticized for being causative agents.23 Actually, the potent nonselective dopamine receptor (D) antagonist neuroleptics, such as haloperidol (a butyrophenone), promethazine, and chlorpromazine (phenothiazines) are well-known causative factors behind this secondary drug effect.38 Dopaminergic drugs, for example, levodopa, used in Parkinson’s disease, especially when abruptly discontinued25 may induce NMS as a side effect. It is known that the antiemetic metoclopramide (a chlorbenzamide derivative), expressing antidopaminergic action, can cause NMS.39 Importantly, the hematologic and biochemical changes seen in NMS, such as leukocyosis and elevated serum CK levels, are a result of the use of an antipsychotic. These agents enhance calcium release from the sarcoplasmic reticulum of skeletal myocytes, ultimately causing muscle rigidity and eventually rhabdomyolysis.40 NMS as a side effect has also been reported following the use of other drugs that are not known to have antidopaminergic potential. It is evident that many drugs used in treatment of psychiatric diseases are not antipsychotic agents (Table 1).11,41–43
Table 1.
A list of some known drugs (of different categories) that can cause neuroleptic malignant syndrome
| Drug | Pharmacologic category |
|---|---|
| Amoxapine | Antidepressant, tetracyclic |
| Amisulpride | Nonconventional antipsychotic |
| Aripiprazole | Nonconventional antipsychotic |
| Chlorpromazine | Conventional antipsychotic, anticholinergic, and antihistaminic |
| Citalopram | SSRI antidepressant |
| Clozapine | Potent nonconventional antipsychotic |
| Desipramine | Antidepressant, tricyclic |
| Domperidone | Dopamine antagonist, antiemetic |
| Dosulepine | Antidepressant, tricyclic |
| Droperidol | Antipsychotic, antiemetic, and neuroleptanalgesic |
| Fluphenazine | Conventional antipsychotic |
| Haloperidol | Potent conventional antipsychotic |
| Lithium salts | Mood stabilizer |
| Metoclopramide | Antiemetic |
| Olanzapine | Nonconventional antipsychotic |
| Paliperidone | Nonconventional antipsychotic |
| Perphenazine | Potent conventional antipsychotic |
| Phenelzine | Antidepressant, anxiolytic, and irreversible nonselective MAOI |
| Prochlorperazine | Antiemetic, conventional antipsychotic |
| Promethazine | Antihistaminic, conventional antipsychotic |
| Quetiapine | Nonconventional antipsychotic |
| Reserpine | Antihypertensive, antipsychotic |
| Risperidone | Nonconventional antipsychotic |
| Tetrabenazine | VMA2 inhibitor, used in movement disorders (hyperkinesia) |
| Thioridazine | Nonconventional antipsychotic |
| Valproate | Anticonvulsant |
| Ziprasidone | Nonconventional antipsychotic |
Abbreviations: MAOI, mono amine oxidase inhibitor; SSRI, selective serotonin reuptake inhibitors; VMA2, vesicular monoamine transporter 2.
Landmarks of recent directions in research
The most important of these are the following
First, regarding dopamine receptor blockade and the consequence of reduced dopamine activity, it was in the past belief that psychosis was a result of high neurotransmitter levels in dopaminergic neurons and its action on dopamine receptors, especially those of dopamine subtype 2 receptors (D2). One reason for designing atypical antipsychotics was to have at hand agents with lower D2 receptor affinity (the proposed target receptors of the vast majority of conventional psychotropics). Unfortunately, these new agents were also capable of causing NMS.
Second, recent studies indicate a genetic defect as a component in NMS,44 and this actually supports sympathoadrenal hyperactivity as another etiologic model in NMS. This would imply that a defect in calcium regulatory enzymes within the autonomic sympathetic neurons probably has an initiating role in the pathophysiology of this syndrome.45 This would in fact link the syndrome to what is known as malignant hyperthermia, where NMS is considered as the neurogenic variant of this condition. Furthermore, both conditions are closely linked to a defect in synthesis of calcium-related proteins.
Clinical features of the neuroleptic syndrome
It is evident that the clinical presentation of NMS is not homogeneous. Indeed, the clinical signs/symptoms are rather heterogeneous, making diagnosis difficult, especially in the early phase.46 NMS usually starts as an unexplainable collection of several symptoms: tremor and muscle cramps, unstable blood pressure, and disturbance of mental status, for example, anxiety, agitation, delirium, and fulminant coma (terminal stage).
Muscle rigidity in NMS is most likely caused by blockade of dopamine D2 receptors and can occur in many forms. Choreiform movements can also be seen in some cases of NMS. Caution should be taken here, because drugs other than antipsychotics can cause this unwanted effect such as anticonvulsants and levodopa.47,48 Oculogyric crisis, can be manifested in NMS especially when the intoxication is induced by haloperidol, chlorpromazine, fluphenazine, or olanzapine.49 Clinicians have to take into consideration that oculogyric crisis can also be seen in certain forms of epileptic fits (versive seizures).
According to an analytic study of a population of NMS cases, 70% demonstrated the following sequence of events ending in coma at the terminal phase: mental status changes appear first, followed by rigidity, then hyperpyrexia, and finally autonomic dysfunction (dysautonomia).50 Dysautonomia may include diaphoresis, nausea, vomiting, unstable blood pressure, and cardiac arrhythmias.
Importantly, hyperpyrexia can be delayed for >24 hours (after the appearance of the first symptoms). This might lead to diagnostic confusion even among expert clinicians.51 The substantial variations that occur in clinical presentation have to be taken into consideration, and the signs and symptoms need to be evaluated twice before a final diagnosis of NMS can be made. Clinicians have to be aware that signs and symptoms presented in NMS, related to atypical antipsychotic agents, differ from those seen with older (typical) remedies.52 Once the above-mentioned symptoms have appeared, then progress occurs very rapidly and the classical clinical picture appears within 2–3 days. In certain cases of NMS, one can observe sialorrhea (ptyalism). This is hypersalivation, which can cause drooling if dysphagia coexists. Sialorrhea in NMS can also cause aspiration pneumonia, as stated elsewhere. This reflects the significance that NMS patients are best admitted to intensive care unit (ICU), where nasogastric and endotracheal tubes can be instituted easily by expert personnel, when necessary. Hypersalivation probably will be more prominent when NMS is caused by clozapine and/or amisulpride, as these two agents can manifest this unwanted effect. Hematologic and biochemical changes are then inevitable: leukocytosis and elevated serum CK levels, which are attributed to hyperkinesia and subsequent rhabdomyolysis.53 The patient may suffer hypertensive crisis (resultant of dysautonomia), hyperpyrexia, and finally metabolic acidosis (coma). Almost 50% of patients show an unexplained slow pattern of electroencephalographic (EEG) changes.54 It has been hypothesized that these EEG findings can be attributed to changes in neuronal pathways resulting from the blockade of dopamine receptors.55 The exact pathophysiology behind hyperpyrexia is also unclear but is believed to be associated with the blockade of the hypothalamic dopamine receptor.56
Differential diagnosis
The dilemma of differential diagnosis in NMS is sometimes further complicated by the paradox of coexisting pathologies giving a similar clinical picture, such as myxedema crisis resultant of hypothyroidism and other pathologies, for example, catatonia of different underlying reasons.57
Another situation is where skin burns coexist with NMS (although a rare coincidence),58–60 giving rise to diagnostic difficulties, because these two situations have many similar symptoms in common, although differing in etiology. The problem with the above-mentioned disorder (NMS concomitant with hypothyroid crisis) is that both conditions can be precipitated by antipsychotic use (ie, occur as side effects). In fact, these two pathologies are the opposite of one another, NMS being a hypermetabolic state whereas hypothyroid crisis is a hypometabolic one. This is the reason why the latter (hypothyroid crisis) can curtail the classic clinical manifestations of NMS.61
In all suspected NMS cases, a careful review of drug intake by the patient is mandatory, moreover, a broad list of differential diagnoses in all cases of hyperpyrexia and rigidity, should be drawn-up and evaluated.62
Clinical findings in NMS can sometimes be easily misinterpreted by a nonexpert as being symptoms of a nontreated mental illness.63 The likelihood of developing NMS is very small in the following patient groups: 1) those already admitted to a psychiatric institution, being stabilized during a therapeutic period with constant maintenance doses of psychotropics and 2) those who neither have a history of medicament incompliance nor the intake of psychedelic substances that could worsen their psychosis, and were thus subjected to an increase in their doses of antipsychotics.
On a clinical basis NMS should be carefully distinguished from the following clinical categories: encephalitis, toxic encephalopathy, malignant hyperthermia, heat stroke, serotonin syndrome (selective serotonin reuptake inhibitors [SSRIs] toxicity), malignant catatonia (neurogenic motor immobility),64 and status epilepticus.42,65
As one can see, some of these above-mentioned categories are related to NMS, such as 1) serotonin syndrome, 2) malignant hyperpyrexia, 3) malignant catatonia, and 4) clozapine-induced hyperpyrexia. Here, clinical expertise is the most important tool used to distinguish these from true cases of NMS.
Serotonin syndrome
This is the most common diagnosis related to NMS. It is one of the serious side effects of SSRI. Patients who have a similar presentation makes it difficult to be distinguished from NMS.66 Milestones that characterize serotonin syndrome are shivering, hyperreflexia, myoclonus, and ataxia.67,68 Other minor features include gastrointestinal symptoms such as diarrhea, nausea, and vomiting, which are rarely seen in NMS. If present, rigidity and hyperpyrexia are milder here than anticipated in NMS.
Malignant hyperpyrexia
This is a genetic disorder, usually triggered by the inhalation of potent halogenated anesthetics (eg, halothane) or depolarizing neuromuscular junction blockers such as succinylcholine and during induction of general anesthesia. Symptoms are hyperthermia, muscle rigidity, and dysautonomia, identical to that in NMS, though in a more abrupt manner.
Malignant catatonia
Poses a dilemma when to be differentiated from NMS. It shares hyperpyrexia and rigidity with NMS, but it differs in the existence of prodromal behavioral symptoms (of some weeks), such as psychosis, agitation, and catatonic excitement. The motor symptoms of malignant catatonia are dystonia, waxy flexibility, and repetitive stereotypic movements. These form a category distinct from that usually seen in NMS.69,70 These two below-mentioned disorders can thus give rise to diagnostic challenges and thereby produce controversy among clinicians because they are very difficult to distinguish from one another on a clinical basis alone.28,44,71
Clozapine-induced hyperpyrexia
Clozapine is a major atypical neuroleptic agent recommended in the treatment of resistant schizophrenia.72 It has proved its effectiveness in treating both negative and positive symptoms in patients with refractory (resistant) schizophrenia, a reason that accounts for its frequent use. The drug has a spectrum of side effects (not discussed in this review), importantly including fever which is mentioned here.73 Clozapine-induced hyperpyrexia is a known common side effect following the use of this antipsychotic,74 usually being encountered within the first 4 weeks of treatment and has a prevalence that varies from 0.5% to 55%.75,76 It lasts for 2.5 days on average and if treatment is not discontinued then fever abates between 8 and 16 days.77 This condition can be considered as a variant of NMS.78 There is also a miscellaneous group of unrelated neurologic and medical disorders that should be taken into consideration with regard to differential diagnosis in NMS patients. The clinical symptoms of these disorders can overlap and mimic those of NMS. This is especially true for patients with extrapyramidal side effects (EPS) resulting from concomitant antipsychotic use. These disorders are listed in Table 2 and should not be overlooked because they have serious implications for both prognosis and treatment.1,42
Table 2.
Unrelated disorders as elements necessary to be differentially diagnosed from neuroleptic malignant syndrome
| Category | Description | Detail |
|---|---|---|
| CNS | Infections | Meningitis, encephalitis, tetanus |
| Diseases | Autoimmune encephalitis, seizures, CNS vasculitis | |
| Trauma | Acute hydrocephalus | |
| Acute spinal cord injuries | ||
| Systemic infections | Pneumonia, sepsis | |
| Endocrine diseases | Thyrotoxicosis | |
| Pheochromocytoma | ||
| Drugs | Intoxication | Phencyclidine, ecstasy, cocaine, amphetamine, lithium salts |
| Impaired thermoregulation | Neuroleptics can predispose heat stroke | |
| Withdrawal | Intrathecal baclofen | |
| Neuromuscular | Acute dystonia | Muscle rigidity |
| Porphyria | CNS and dermatologic types | Accumulation of porphyrin in the body |
Abbreviation: CNS, central nervous system.
Is NMS avoidable or inevitable?
This is a perplexing question to answer. In recent years, there has been an increase in the use of antipsychotics in cases other than psychoses such as in treatment of trauma patients, for example, burns58 and other situations where pain is the chief complaint (eg, neuritis and migraine).79 It is true that nowadays one is more aware of NMS, and there are clear general rules concerning the therapeutic doses of many neuroleptics. New remedies are constantly coming into the drug market for therapeutic use. It can be difficult to predict what side effects a new drug are likely to initiate, even if this drug has passed phase 2 or even phase 3 stages. These new agents could be neuroleptics or agents belonging to other drug groups. On an accumulative basis many drugs can produce side effects, that is, after prolonged use with successive doses (not as an idiosyncratic side effect of a single dose). As mentioned elsewhere in this review, drugs of very different categories can precipitate NMS, thus it is not exclusively induced by neuroleptics.
How to approach a suspected case of NMS
A history of rapid dose escalation of the psychotropic agent being used, or a switch from one agent to a more potent one, especially a depot parenteral preparation, or even sometimes a switch from one agent to another of the same potency, should raise an alarm suggesting NMS, especially whenever hyperpyrexia and rigidity are prodromal features.22,26,27,80 As mentioned, there is no diagnostic laboratory test for NMS. Laboratory data only strengthen diagnosis or at least reduce the number of other possibilities. The parameters shown in Table 3, as suggested by intensive care units, are usually requested. Importantly, the results of blood tests in cases of NMS are usually the following: leukocytosis, thrombocytosis (very rarely thrombocytopenia81), thick serum (because of rhabdomyolysis), and dehydration (because of hyperpyrexia). The rigidity and hyperpyrexia in NMS contribute to muscle damage (necrosis of skeletal myocytes) that explains the elevated levels of the following biochemical parameters: CK, liver function tests (aspartate aminotransferase and alanine aminotransferase), and lactate dehydrogenase. A CK level that exceeds 1,000 IU/L is a useful indicator for NMS,82–85 moreover, the degree of elevation reflects the severity of the condition, giving a clue regarding prognosis.51 The increase can be as high as 10,000 IU/L.4,44,86–88 Basically, the increase is not pathognomonic in diagnosis89 nor is any other positive laboratory parameter alone. This is because CK levels can be within the normal physiologic values especially when the muscle rigidity is not clearly developed, particularly in the early onset phase of NMS. Some clinicians attribute mild increases in CK levels to be a result of intramuscular injections and physical restraints (especially in catatonic psychotic patients) and sometimes idiopathic, without having any logical specific explanation.45,86
Table 3.
Suggested workup blood tests required when neuroleptic malignant syndrome is suspected
| Requested test | Why requested |
|---|---|
| Complete blood picture | To exclude leukocytosis and all kinds of hemolysis and its consequences |
| Blood cultures | To exclude possibilities of septic shock (coma) |
| LFTs | To exclude hepatic failure for one reason or another |
| BUN and creatinine levels | To exclude renal failure |
| Calcium, phosphate, potassium, and sodium levels | To exclude electrolyte imbalances and hemolysis |
| CK level | To exclude or prove rhabdomyolysis or other myocytes type necrosis |
| Serum iron level | Because of rhabdomyolysis and other hemolytic pathologies |
| Urine myoglobin level | To exclude myoglobinuria |
| Arterial blood gas analysis | To exclude respiratory failure and metabolic acidosis |
| Coagulation studies | To exclude hepatic failure and DIC |
| Serum and urine toxicologic screening | To exclude ASA, cocaine and amphetamines poisoning |
Abbreviations: ASA, acetyl salicylic acid; BUN, blood urea nitrogen; CK, creatine kinase; DIC, disseminated intravascular coagulopathy; LFTs, liver function tests.
Necrosis of skeletal myocytes progresses quickly to rhabdomyolysis, in turn leading to myoglobinemia, hyperkalemia, hyperphosphatemia, hypercalcemia, and hyperuricemia. In a study on 1,346 NMS patients, rhabdomyolysis was reported as the most prevalent complication (30.1%) compared to other associated complications.90 Electrolyte imbalances can cause serious cardiac arrhythmias that may end up in cardiac arrest if not treated promptly. Both classes of antipsychotics, which are the main causal actors of NMS, can cause serious cardiac side effects regardless of electrolyte imbalance; orthostatic hypotension is one of these. This undesired autonomic side effect can also be encountered with atypical antipsychotics, for example, clozapine. Among the typical antipsychotics, the low potency agents (phenothiazines) are most commonly known to cause this undesired effect.91 The other serious side effect psychotropic agents can cause is the prolongation of the QT interval. This electrocardiographic finding has a pivotal clinical relevance, as this can cause life-threatening cardiac arrhythmias, the worst of which is torsades de pontes and sudden death.92 This serious cardiac side effect has been seen in patients who have been treated with amisulpride, pimozide, sertindole, thioridazine, and ziprasidone.93 Clinicians should be aware of these side effects when regarding a case of NMS. Another problem is myoglobinemia that leads to myoglobinuria which can ultimately promote renal failure.51,94 A low serum iron concentration of 5.71 μmol/L (normal value: 11–32 μmol/L) is frequent in NMS. Although sensitive in 92%–100% of cases it is not a pathognomonic biochemical marker for NMS.88,95 Clinicians need to investigate other possibilities behind hyperpyrexia, depending on the dimensions of the manifested clinical picture. Other reasons causing fever have to be ruled out, for example, infections of the urinary tract, respiratory system, and CNS (encephalitis and meningitis). Blood, urine, and cerebrospinal fluid (CSF) culture and sensitivity tests should be requested. Examination of CSF can exclude meningitis as being causative of fever (pyrexia of an unknown origin) and disturbed mental status.
Concerning imaging (computerized axial tomography [CAT] or magnetic resonance imaging [MRI]), this investigation is not strongly recommended since it does not yield urgent diagnostic aid for NMS. Imaging will only rule out structural lesions in the CNS, such as tumors, intracranial hemorrhage, or head injuries that can possibly lead to coma. Certain cerebral hemorrhages such as pontine hemorrhage can initiate the onset of pyrexia.96,97 Thus, cerebral imaging techniques might afford a useful tool to rule this out or other above-mentioned possible diagnostic categories. Some neurologists believe that MRI should be performed very urgently whenever NMS is suspected in order to rule out autoimmune encephalitis.98,99 If this is diagnosed then one can start treatment with immunosuppressive agents. In this sense, MRI is preferred over CAT scanning because it will be more informative.100,101 To exclude aspiration pneumonia (Mendelson’s syndrome) and consequent respiratory failure, chest X-ray is usually requested. The values of laboratory parameters found in a typical case of NMS are shown in Table 4.
Table 4.
Laboratory data expected to be seen in a typical case of neuroleptic malignant syndrome
| Parameters | Changes |
|---|---|
| Enzymes and abnormal protein in plasma | |
| LDH | Increased |
| CK | Increased in (50%–100% of cases) |
| ALP | Increased |
| ASAT and ALAT | Increased |
| Myoglobin | Myoglobinemia |
| Serum electrolytes and proteolysis remnants | |
| Phosphate | Hyperphosphatemia |
| Potassium | Hyperkalemia |
| Calcium | Hypocalcemia |
| Magnesium | Hypomanesemia |
| Sodium | Hypo or hypernatremia |
| Uric acid | Hyperuricemia |
| Urea | Uremia (increased BUN) |
| Serum iron | Decreased |
| Elements of blood | |
| Leukocytes | Leukocytosis (70%–80% of cases) |
| Blood platelets (thrombocytes) | Thrombocytosis (thrombocytopenia*) |
| Urine | |
| Protein | Proteinuria (increased protein) |
| Myoglobin | Myoglobinuria (increased myoglobin) |
| pH (blood gas analysis) | Decreased (metabolic acidosis) |
Note:
Very rarely, for more information see the study by Ghani et al.81
Abbreviations: ALP, alkaline phosphatase; ALAT, alanine aminotransferase; ASAT, aspartate aminotransferase; BUN, blood urea nitrogen; CK, creatine kinase; LDH, lactate dehydrogenase.
Management and lines of treatment
Management should be started actively once the syndrome is suspected, that is, when the individual has the criteria mentioned above. He/she should be admitted to a well-equipped ICU with circulatory and ventilator support. Active treatment should commence immediately, generally including, first, the removal of the offending agent and effects of its active metabolites (whenever possible) and second, cooling down the patient using cooling blankets and applying ice-packs to the groin and axillae.
The optimal pharmacologic treatment is still to be elucidated, and there is no general agreement among clinicians (especially between internalists and doctors in an ICU) concerning the therapeutic significance of currently used drugs to treat NMS. In fact all these drugs are administered for symptomatic treatment of the complications, not the syndrome itself.
Being a rare condition, most of the published work regarding the lines of treatment of NMS are based on case reports, meta-analysis, or opinions of experts in the field.46 In general, one can say that there are two basic lines of treatment, one being a biological approach and the other parallel supportive therapy.62
Biological treatment
Dantrolene has been recommended as a muscle relaxant for treating muscular rigidity, and dopamine-agonist drugs such as bromocriptine have proved to be beneficial.102 Due to its dopaminergic and anticholinergic pharmacologic effects, amantadine is another alternative. Apomorphine may be used, but still it is not an optimal choice because it is not supported by major evidence.103 Minor tranquilizers such as benzodiazepines are a good choice for treating agitation and catatonia. The benefits achieved by the above-mentioned drugs are claimed to be uncertain, at least by some research groups.104 The evidence that supports the use of the above-mentioned agents actually is limited because 1) these agents are frequently used anecdotally, since they lack scientific evidence regarding efficiency, 2) absence of evidence-based optimal pharmacologic treatment, and 3) high morbidity and mortality rates in this syndrome. Electroconvulsive therapy (ECT), which has been suggested by certain groups, has no proven empirical benefits. The reason for its use in NMS though is attributed to its efficacy in treating malignant catatonia and Parkinsonism.105,106 One further issue can be mentioned in this context: the drive for the use of ECT comes from the frequent need for psychotropic agents in a setting where these cannot be used.107 The mainstay of the treatment is, thus, supportive once having ceased the use of the offending drug(s).
Complementary supportive treatment
Comatose NMS patients should have an open intravenous (IV) channel for administration of fluid and, if necessary, drugs. The purpose of IV fluids is to avoid dehydration resulting from hyperpyrexia and to avoid acute renal failure because of highly elevated blood myoglobin that may induce renal damage. This can be avoided by aggressive (contentious) intravenous hydration with cooled IV fluids to induce alkalization of urine and diuresis. These measures can prevent renal failure and enhance excretion of the by-products of muscle breakdown. One should also consider prophylactic endotracheal intubation in patients with excessive salivation (sialorrhea) to avoid aspiration pneumonia, dysphagia, hypoxemia (in case use of a ventilator is evaluated), metabolic acidosis (successive performance of blood gas analysis, with a display of blood pH), severe rigidity with hyperthermia and coma. A nasogastric tube might be necessary to feed comatosed patients, particularly if the coma situation prevails longer than expected, or when necessary according to a clinical evaluation. With these supportive techniques, symptoms will resolve within a couple of weeks or so, or longer (~4 weeks) if the syndrome was initially brought about by long-acting antipsychotics (ie, depot injections).
Prognosis
Concerning the prognosis of this syndrome, this is mainly dependent on early diagnosis and active intervention without delay in a well-equipped ICU. Although the majority of cases can be successfully managed, we still have to be aware that ~10% of cases can be fatal, regardless of early diagnosis and treatment. Hyperpyrexia, rhabdomyolysis, and neuronal damage can lead to amnesia (memory impairment), which could be temporary or persistent in certain cases.108 Among the elderly, acute respiratory failure, acute renal shutdown, infections (septic shock), and coexisting congestive heart failure are significant predictors of mortality in this rare syndrome. The acute respiratory insufficiency is the strongest independent mortality prognosticator.90
What if the patient needs an antipsychotic after recovery from this syndrome: should an antipsychotic be prescribed or not? A question difficult to answer. The majority of psychiatric centers recommend a drug from the atypical group (second-generation or nonconventional) of a low-potency type.42
Discussion and future perspectives
NMS is a life-threatening iatrogenic neurologic emergency, usually but not always associated with the administration of psychotropic drugs and manifested as a characteristic clinical syndrome. One should expect the occurrence of NMS in Parkinson’s patients, especially those treated with levodopa preparations, where these suddenly have been reduced or withdrawn as an iatrogenic mishap, or because of the complications initiated by the drug.44,80,86,109 Regardless of clinical alertness, it might not be easy to differentiate NMS from a case of, for example, adrenocorticotropic hormone (ACTH) deficiency. Patients with ACTH deficiency can manifest a clinical picture very similar to that of NMS.110,111
In such cases hydrocortisone therapy should be evaluated. The incidence of NMS varies widely, ranging from 0.02% to 3% among patients on psychotropic drug therapy.44,51 A wide range variation of this type may reflect the following: 1) differences among sampled populations, for example, an inpatient versus outpatient, 2) differences in methods of surveillance, and 3) definition of the disease itself. All of these criteria have a lot to say regarding the wide spectrum of statistical values.
The dilemma of how to diagnose this syndrome actually originates from the fact that one cannot predict its occurrence, in other words, it can occur after an orphan dose of a drug (usually antipsychotic) or after continuing therapy with the same remedy at the same dose for many years.112 It is thus an idiosyncratic condition. On a clinical basis, the diagnosis is settled when four features of the syndrome are fulfilled: change of mental status, rigidity, hyperpyrexia, vegetative dysregulation of the autonomic nervous system (including blood pressure fluctuations). When any two of these above-mentioned features appear, diagnosis should be suspected within the setting of psychotropic drug therapy (when treating psychosis) or dopamine withdrawal (in cases of Parkinsonism). Withdrawal of therapy with intrathecal baclofen has, also in several cases, been associated with an NMS-like syndrome.113 The difference here is that increased muscular tone is of a rebound spastic-type rather than being a rigid one, otherwise the spectrum of symptoms appears quite identical to those of NMS. Clinicians have to take into consideration that the importance of differential diagnosis, including meningitis, encephalitis, septic shock (systemic infections), heat stroke, and other iatrogenic dysautonomias. Concerning the results from laboratory investigations, these should assist in ruling out the possibility of the above-mentioned tentative diagnoses, especially the elevated CK values. An elevated level of this enzyme is a common observation in NMS although it is not a pathognomonic finding in the syndrome. The approach to a case of NMS, and its general management, should be based on a hierarchy of the severity of clinical features and thus enable the correct diagnosis to be made.44,71
Taken together, when there is any possible suggestion of NMS, the administration of psychotropics should be stopped, and the patient should be admitted for close observation to evaluate the clinical signs and to perform the relevant laboratory investigations. This should be done in an ICU, especially for patients who have significant hyperpyrexia and rigidity. This is because these individuals need aggressive supportive care (see above). One should evaluate biological treatment with dantrolene, bromocriptine, and/or amantadine in patients who have significantly elevated CK values or hyperpyrexia on the first presentation, and in those who are irresponsive to withdrawal of a psychotropic drug (or the offending drug) within the first 48 hours of admission. Patients who do not respond to medical therapy during the first 7 days, especially those with persistent catatonia after the resolution of other symptoms, lethal catatonia should be regarded as an alternative diagnosis or as a concomitant sequel, then ECT should be seriously considered.114 Should the patient need antipsychotic therapy after NMS has subsided, risk of the syndrome will still be there although it may be minimized if the following guidelines are followed:
Therapy should be postponed for at least 14 days or more, until all the residual symptoms have subsided (especially those of EPS).
An agent of lower potency should be chosen where the possibility of NMS relapse is minimal (or less likely).
The initial start dose should be the lowest possible (ie, lowest recommended), where the clinician can increase the dose gradually by titration in order to establish the lowest possible therapeutic level that clinically controls psychosis.
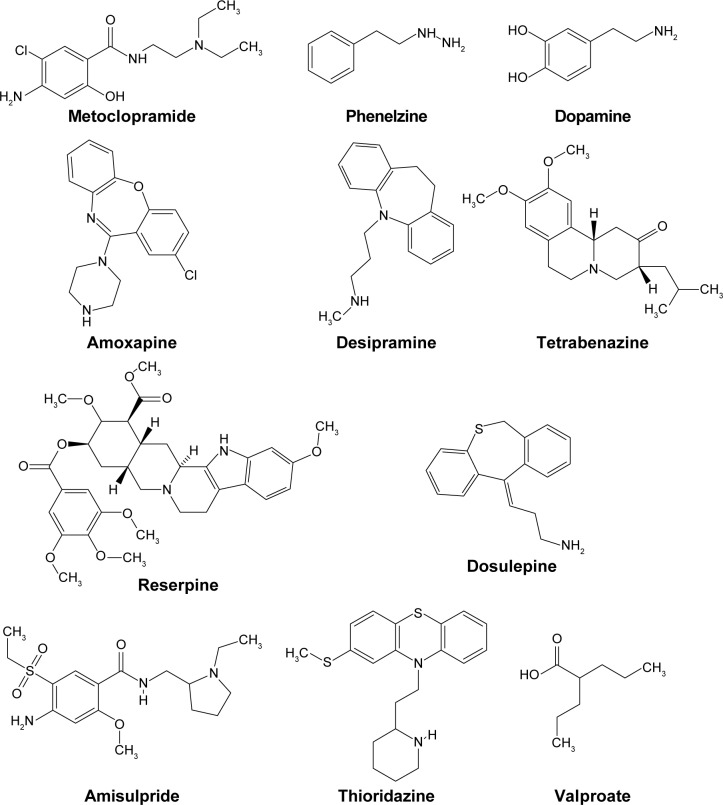
In cases where lithium is needed as an adjuvant medication, in addition to an antipsychotic agent (ie, when such a combination is indicated clinically), then the clinician should find another combination as an alternative treatment in order to avoid the use of lithium. This is simply because lithium, even as monotherapy, can potentially result in a relapse.115 The patient has to be advised to drink sufficient amounts of liquids (water and alcohol-free beverages) to keep the body hydrated, to control body temperature, and to ensure proper kidney function. Environmental therapy has a very important role here, since patients should be under observation with respect to their fluid intake even when the patient is on an atypical antipsychotic therapy regime.23 Overhydration (water intoxication) has also been blamed for causing NMS,116 so a balanced water intake is a pivotal factor for both optimal metabolism and the pharmacology of the antipsychotic agent(s) the patient is using, and to maintain normal renal function. The patients themselves should be orientated and alerted about the early symptoms of possible relapse, and they should be called in to a psychiatric outpatient institution for a periodic checkup and clinical observation. As pointed out here, and also stated elsewhere, the real pathophysiology of psychosis is unknown and the dopamine hypothesis of psychosis alone can neither explain the biochemical basis of psychosis nor the pathophysiology of NMS. Indeed the psychotropic agents currently in use are not optimal for treatment of psychosis, simply because they are not selective enough in their actions. They can thus induce a huge number of known and unknown idiosyncratic devastating side effects, including the syndrome currently being discussed, namely NMS.117 The fight against psychosis with the design of better agents that possess more selective actions, such as monotherapy, will almost certainly continue for decades. Time and economic resources will be needed in addition to ambitious and devoted scientists to achieve this important goal. This will require that the scientists (biochemists, pharmaceutical chemists, and psychopharmacologists) will need to channel resources in the direction toward the area of psychosis, a currently unexplained and poorly investigated medical issue. Another aspect is, can we explain the pathophysiology of psychosis dependent only on the basis of receptor and ligand (neurotransmitter) interaction alone and treatment based on the receptor–drug theory of pharmacology? Furthermore, what about the intercalation mechanism of psychotropics into the membranal phospholipids of neurons and myocytes118 and the biomembranes of internal cellular organelles of these excitable cells, such as endoplasmic reticulum that releases intracellular calcium? The majority of agents that can initiate the onset of NMS, listed in Table 1 have amphiphilic chemical structures,119,120 that is, they have lipophilic and hydrophilic moieties (Figure 1). This property makes these agents intercalate in-between the lipid tails of biomembrane phospholipids. This may plausibly explain why none of the psychotropics currently in use are selective for dopamine subtype D2 receptors (the proposed receptors for psychotropic drugs). We consider them as being nonselective because these agents often produce a spectrum of side effects that cannot be explained by solely being dependent on the receptor–drug theory. Indeed, most scientific research concerning NMS does not encompass the issue of neuronal biomembrane integrity related to sound and normal neuronal function. The question that imposes itself in this context is the following: is there any significance, or therapeutic, or biological advantage for the use of micronutrients such as antioxidants, for example, selenium-containing compounds, the water soluble vitamins (C and B), the fat soluble vitamins (E and A), and the polyunsaturated fatty acids as adjuvants to psychotropics in order to minimize the possible occurrence of side effects including NMS? New psychotropics will have to be more selective and thus more potent against the proposed receptors that they are designed for. Evidence exists that some of the neuroleptics currently in use can release free radicals and these can precipitate NMS.121,122 It would thus be logical to consider treating the syndrome with antioxidants, at least as adjuvant remedies.123 One interesting issue concerning valproate is that it can precipitate NMS,124 but can also be used in treatment of this syndrome.125 This illustrates the fact that we still know very little about the pathophysiology of this morbidity, and the exact cause of NMS is still unknown. More research must be done in order to elucidate these issues. It is highly likely that once we have understood the pathophysiology behind NMS, we will plausibly be able to answer the following question: how do psychotropics exert their pharmacologic function at the cellular (neuronal) level? Indeed these two issues (NMS and pharmacologic actions of antipsychotics) are two sides of the same coin, and each may aid in explaining the other. Currently available theories (or hypotheses) have limitations in being able to explain all clinical manifestations of this syndrome and other complications that are precipitated, especially by antipsychotics in general.
Figure 1.
Chemical structures of dopamine and some of the agents referred to in this work.
One of the issues causing a great dilemma in NMS is the hyperpyrexia, where there is no convincing explanation for its pathophysiology. Animal model studies have shown that orexin-A (an excitatory neuropeptide) can cause thermogenesis in a manner unrelated to muscle activity. This action is controlled centrally via the sympathetic nervous system. This neuropeptide and its other isoforms are involved in controlling body temperature in a complex reaction with the dopaminergic pathways of the brain.126 In one experiment, an orexin-1 receptor antagonist (SB-334867) increased the number of naturally active dopamine neurons A9 and A10 while the number was decreased by the action of acutely administered haloperidol or olanzapine. The same antagonist spontaneously decreased the number of active dopamine cells A9 and A10, that is, it caused the reverse. This animal model experiment indicates that the orexin-1 receptor plays a role in the effects of psychotropics on dopamine pathways, and probably the clinical effects of these agents (therapeutic and side effects).127 In another animal experiment, it was also demonstrated that the administration of quetiapine delays the sympathetic firing rate (muscle and colonic temperature and heart rate) caused by orexin-A injected into the lateral cerebral ventricle.128 In a similar experiment, this time with olanzapine, it was observed that this antipsychotic blocks the sympathetic firing rate, thus affecting muscular and colonic temperatures, and also heart rate.129 These experiments might give a clue regarding how certain psychotropics exert their pharmacologic effects, these could be therapeutic and/or undesired effects.
A final issue of importance that requires mention is the animal model of this syndrome, developed by methods of bioassay. This, however, does not correspond to the human variant of this syndrome,71,130 and is thus of no practical use.
Finally, it is crucial to gain an understanding of the pathophysiology of psychiatric diseases at the biomolecular level in neurons in order to be in a position to design more selective agents. These should be potent as well as being devoid of grave side effects, such as the induction of NMS.
Conclusion
As the majority of NMS cases have been attributed to the use of antipsychotic agents, especially first-generation (conventional or typical) drugs, one should be careful when prescribing such agents to psychotic patients. The current trend in many psychiatric centers of the developed world is the use of second-generation (atypical) agents. NMS, however, has not been totally abolished.131 In other words, the syndrome can still be encountered even with the availability of the second-generation of newly designed agents,132 although the clinical picture might be milder than what is encountered in NMS with typical antipsychotics.10,11 Amisulpride, a relatively newly designed atypical antipsychotic, has also been shown to be associated with the occurrence of NMS.133 This has to be attributed to the fact that we do not know the nature of the exact biological changes that occur in the neurons of psychotic patients. Once we have understood this dilemma, we can then prepare better agents with least possible side effects. It is also necessary to mention here that the use of drugs other than antipsychotic agents can lead to NMS, for example, drugs such as metoclopramide (antiemetic), amoxapine (tetracyclic antidepressant), and lithium (mood stabilizer) have been recognized as being perpetrators of NMS.134 In other words, it is not only antipsychotics alone that should be blamed for being behind the occurrence of this syndrome. A retrospective survey on patients in a study showed that citalopram (an SSRI antidepressant) can trigger acute dystonia which could be the prodromal stage of NMS.135 The same thing is also true for metoclopramide (Table 1), and as stated before, this indeed makes the prediction of NMS almost impossible. It is likely that the number of agents that can precipitate NMS will most probably increase year by year. Because there is no pathognomonic laboratory test to pinpoint the diagnosis of this idiosyncratic syndrome, careful periodic clinical observation of psychotic patients is warranted, especially those who have recently started taking antipsychotics (particularly those being treated with oily depot injections of long-acting potent first-generation drugs in outpatient clinics of psychiatric centers). This action may help the early diagnosis of NMS and thus ensure an early start of treatment intervention with the hope of minimum negative consequences. It is clear that the majority of drugs that are associated with the induction of NMS are either antipsychotics or antidepressants. These groups of drugs are cornerstones as biological treatment tools in contemporary clinical psychiatry. It is evident that these drugs have no selective actions when prescribed as monotherapy and the optimal therapy may require polypharmacy, a fact that increases the spectrum of anticipated side effects, including NMS.136,137
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Carbone JR. The neuroleptic malignant and serotonin syndromes. Emerg Med Clin North Am. 2000;18(2):317–325. doi: 10.1016/s0733-8627(05)70127-9. [DOI] [PubMed] [Google Scholar]
- 2.Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am. 1993;77(1):185–202. doi: 10.1016/s0025-7125(16)30278-4. [DOI] [PubMed] [Google Scholar]
- 3.Caroff SN, Mann SC. Neuroleptic malignant syndrome and malignant hyperthermia. Anaesth Intensive Care. 1993;21(4):477–478. [PubMed] [Google Scholar]
- 4.Adnet P, Lestavel P, Krivosic-Horber R. Neuroleptic malignant syndrome. Br J Anaesth. 2000;85(1):129–135. doi: 10.1093/bja/85.1.129. [DOI] [PubMed] [Google Scholar]
- 5.Hasan S, Buckley P. Novel antipsychotics and the neuroleptic malignant syndrome: a review and critique. Am J Psychiatry. 1998;155(8):1113–1116. doi: 10.1176/ajp.155.8.1113. [DOI] [PubMed] [Google Scholar]
- 6.Kuchibatla SS, Cheema SA, Chakravarthy KS, Sayeh HG. A case report of neuroleptic malignant syndrome. BMJ Case Rep. 2009;2009 doi: 10.1136/bcr.07.2008.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel U, Agrawal M, Krishnan P, Niranjan S. Neuroleptic malignant syndrome presenting as pulmonary edema and severe bronchorrhea. J Natl Med Assoc. 2002;94(4):279–282. [PMC free article] [PubMed] [Google Scholar]
- 8.Adityanjee, Sajatovic M, Munshi KR. Neuropsychiatric sequelae of neuroleptic malignant syndrome. Clin Neuropharmacol. 2005;28(4):197–204. doi: 10.1097/01.wnf.0000172079.80795.5f. [DOI] [PubMed] [Google Scholar]
- 9.Belvederi Murri M, Guaglianone A, Bugliani M, et al. Second-generation antipsychotics and neuroleptic malignant syndrome: systematic review and case report analysis. Drugs R D. 2015;15(1):45–62. doi: 10.1007/s40268-014-0078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delay J, Pichot P, Lemperiere T, Elissalde B, Peigne F. Un neuroleptiques majeur non phénothiazine et non réserpine, l’halopéridol dans le traitement des psychoses. [A non-phenothiazine and non-reserpine major neuroleptic, haloperidol, in the treatment of psychoses] Ann Med Psychol (Paris) 1960;118(1):145–152. French. [PubMed] [Google Scholar]
- 11.Buckley PF, Hutchinson M. Neuroleptic malignant syndrome. J Neurol Neurosurg Psychiatry. 1995;58(3):271–273. doi: 10.1136/jnnp.58.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otani K, Horiuchi M, Kondo T, Kaneko S, Fukushima Y. Is the predisposition to neuroleptic malignant syndrome genetically transmitted? Br J Psychiatry. 1991;158:850–853. doi: 10.1192/bjp.158.6.850. [DOI] [PubMed] [Google Scholar]
- 13.Zweig YR, Galvin JE. Lewy body dementia: the impact on patients and caregivers. Alzheimers Res Ther. 2014;6(2):21. doi: 10.1186/alzrt251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelonero AL, Levenson JL, Pandurangi AK. Neuroleptic malignant syndrome: a review. Psychiatr Serv. 1998;49(9):1163–1172. doi: 10.1176/ps.49.9.1163. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez JL, Palacios-Araus L, Echevarria S, Herran A, Campo JF, Riancho JA. Neuroleptic malignant syndrome in the acquired immunodeficiency syndrome. Postgrad Med J. 1997;73(866):779–784. doi: 10.1136/pgmj.73.866.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aleman A, Kahn RS, Selten JP. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry. 2003;60(6):565–571. doi: 10.1001/archpsyc.60.6.565. [DOI] [PubMed] [Google Scholar]
- 17.Haack S, Seeringer A, Thurmann PA, Becker T, Kirchheiner J. Sex-specific differences in side effects of psychotropic drugs: genes or gender? Pharmacogenomics. 2009;10(9):1511–1526. doi: 10.2217/pgs.09.102. [DOI] [PubMed] [Google Scholar]
- 18.Leung A, Chue P. Sex differences in schizophrenia, a review of the literature. Acta Psychiatr Scand Suppl. 2000;401:3–38. doi: 10.1111/j.0065-1591.2000.0ap25.x. [DOI] [PubMed] [Google Scholar]
- 19.Pollock BG. Gender differences in psychotropic drug metabolism. Psychopharmacol Bull. 1997;33(2):235–241. [PubMed] [Google Scholar]
- 20.Chaves AC, Seeman MV. Sex selection bias in schizophrenia antipsychotic trials. J Clin Psychopharmacol. 2006;26(5):489–494. doi: 10.1097/01.jcp.0000236652.78168.ee. [DOI] [PubMed] [Google Scholar]
- 21.Alexander PJ, Thomas RM, Das A. Is risk of neuroleptic malignant syndrome increased in the postpartum period? J Clin Psychiatry. 1998;59(5):254–255. doi: 10.4088/jcp.v59n0509a. [DOI] [PubMed] [Google Scholar]
- 22.Keck PE, Jr, Pope HG, Jr, Cohen BM, McElroy SL, Nierenberg AA. Risk factors for neuroleptic malignant syndrome. A case-control study. Arch Gen Psychiatry. 1989;46(10):914–918. doi: 10.1001/archpsyc.1989.01810100056011. [DOI] [PubMed] [Google Scholar]
- 23.Khaldi S, Kornreich C, Choubani Z, Gourevitch R. Antipsychotiques atypiques et syndrome malin des neuroleptiques: bréve revue de la littérature. [Neuroleptic malignant syndrome and atypical antipsychotics: a brief review] Encephale. 2008;34(6):618–624. doi: 10.1016/j.encep.2007.11.007. French. [DOI] [PubMed] [Google Scholar]
- 24.Gupta V, Magon R, Mishra BP, Sidhu GB, Mahajan R. Risk factors in neuroleptic malignant syndrome. Indian J Psychiatry. 2003;45(1):30–35. [PMC free article] [PubMed] [Google Scholar]
- 25.Keyser DL, Rodnitzky RL. Neuroleptic malignant syndrome in Parkinson’s disease after withdrawal or alteration of dopaminergic therapy. Arch Intern Med. 1991;151(4):794–796. [PubMed] [Google Scholar]
- 26.Hermesh H, Aizenberg D, Weizman A, Lapidot M, Mayor C, Munitz H. Risk for definite neuroleptic malignant syndrome. A prospective study in 223 consecutive in-patients. Br J Psychiatry. 1992;161:254–257. doi: 10.1192/bjp.161.2.254. [DOI] [PubMed] [Google Scholar]
- 27.Berardi D, Amore M, Keck PE, Jr, Troia M, Dell’Atti M. Clinical and pharmacologic risk factors for neuroleptic malignant syndrome: a case-control study. Biol Psychiatry. 1998;44(8):748–754. doi: 10.1016/s0006-3223(97)00530-1. [DOI] [PubMed] [Google Scholar]
- 28.Koch M, Chandragiri S, Rizvi S, Petrides G, Francis A. Catatonic signs in neuroleptic malignant syndrome. Compr Psychiatry. 2000;41(1):73–75. doi: 10.1016/s0010-440x(00)90135-4. [DOI] [PubMed] [Google Scholar]
- 29.Sawant NS, Kate NS, Bhatankar SS, Kulkarni PS. Neuroleptic malignant syndrome in cycloserine-induced psychosis. Indian J Pharmacol. 2015;47(3):328–329. doi: 10.4103/0253-7613.157134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guillemin GJ, Smith DG, Kerr SJ, et al. Characterisation of kynurenine pathway metabolism in human astrocytes and implications in neuropathogenesis. Redox Rep. 2000;5(2–3):108–111. doi: 10.1179/135100000101535375. [DOI] [PubMed] [Google Scholar]
- 31.Guillemin GJ, Smythe G, Takikawa O, Brew BJ. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia. 2005;49(1):15–23. doi: 10.1002/glia.20090. [DOI] [PubMed] [Google Scholar]
- 32.Fukuda K. Integrated theory to unify status among schizophrenia and manic depressive illness. Med Hypotheses. 2015;85(4):506–511. doi: 10.1016/j.mehy.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Nagatsu T, Ichinose H. Regulation of pteridine-requiring enzymes by the cofactor tetrahydrobiopterin. Mol Neurobiol. 1999;19(1):79–96. doi: 10.1007/BF02741379. [DOI] [PubMed] [Google Scholar]
- 34.Riddell DR, Owen JS. Nitric oxide and platelet aggregation. Vitam Horm. 1999;57:25–48. doi: 10.1016/s0083-6729(08)60639-1. [DOI] [PubMed] [Google Scholar]
- 35.Jendzjowsky NG, Just TP, Jones KE, DeLorey DS. Acute tetrahydrobiopterin supplementation attenuates sympathetic vasoconstrictor responsiveness in resting and contracting skeletal muscle of healthy rats. Physiol Rep. 2014;2(10) doi: 10.14814/phy2.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vasquez-Vivar J, Kalyanaraman B, Martasek P. The role of tetrahydrobiopterin in superoxide generation from eNOS: enzymology and physiological implications. Free Radic Res. 2003;37(2):121–127. doi: 10.1080/1071576021000040655. [DOI] [PubMed] [Google Scholar]
- 37.Mary K, Ian J. Tetrahydrobiopterin deficiency. In: Natan G, Hans HG, editors. Oxidative Stress and Free Radical Damage in Neurology. Oxidative Stress in Applied Basic Research and Clinical Practice. New York, NY: Springer; 2011. pp. 225–234. [Google Scholar]
- 38.Berman BD. Neuroleptic malignant syndrome: a review for neurohospitalists. Neurohospitalist. 2011;1(1):41–47. doi: 10.1177/1941875210386491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wittmann O, Sadot E, Bisker-Kassif O, Scolnik D, Tavor O, Glatstein MM. Neuroleptic malignant syndrome associated with metoclopramide use in a boy: case report and review of the literature. Am J Ther. 2016 doi: 10.1097/MJT.0000000000000320. [DOI] [PubMed] [Google Scholar]
- 40.Musselman ME, Saely S. Diagnosis and treatment of drug-induced hyperthermia. Am J Health Syst Pharm. 2013;70(1):34–42. doi: 10.2146/ajhp110543. [DOI] [PubMed] [Google Scholar]
- 41.Chandran GJ, Mikler JR, Keegan DL. Neuroleptic malignant syndrome: case report and discussion. CMAJ. 2003;169(5):439–442. [PMC free article] [PubMed] [Google Scholar]
- 42.Strawn JR, Keck PE, Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870–876. doi: 10.1176/ajp.2007.164.6.870. [DOI] [PubMed] [Google Scholar]
- 43.Seitz DP, Gill SS. Neuroleptic malignant syndrome complicating antipsychotic treatment of delirium or agitation in medical and surgical patients: case reports and a review of the literature. Psychosomatics. 2009;50(1):8–15. doi: 10.1176/appi.psy.50.1.8. [DOI] [PubMed] [Google Scholar]
- 44.Velamoor VR. Neuroleptic malignant syndrome. Recognition, prevention and management. Drug Saf. 1998;19(1):73–82. doi: 10.2165/00002018-199819010-00006. [DOI] [PubMed] [Google Scholar]
- 45.Gurrera RJ. Is neuroleptic malignant syndrome a neurogenic form of malignant hyperthermia? Clin Neuropharmacol. 2002;25(4):183–193. doi: 10.1097/00002826-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Nagel M, Freisberg S, Junghanns K, Moll CK, Willenborg B. Das maligne neuroleptische syndrome (NMS) – Eine systematische ϋbersicht. [The neuroleptic malignant syndrome] Fortschr Neurol Psychiatr. 2015;83(7):373–380. doi: 10.1055/s-0035-1553246. German. [DOI] [PubMed] [Google Scholar]
- 47.Gerlach M, Riederer P, Scheller D. Mechanisms underlying and medical management of L-Dopa-associated motor complications. J Neural Transm. 2011;118(12):1659–1660. doi: 10.1007/s00702-011-0728-0. [DOI] [PubMed] [Google Scholar]
- 48.Wild EJ, Tabrizi SJ. The differential diagnosis of chorea. Pract Neurol. 2007;7(6):360–373. doi: 10.1136/pn.2007.134585. [DOI] [PubMed] [Google Scholar]
- 49.Praharaj SK, Jana AK, Sarkar S, Sinha VK. Olanzapine-induced tardive oculogyric crisis. J Clin Psychopharmacol. 2009;29(6):604–606. doi: 10.1097/JCP.0b013e3181c00b08. [DOI] [PubMed] [Google Scholar]
- 50.Velamoor VR, Norman RM, Caroff SN, Mann SC, Sullivan KA, Antelo RE. Progression of symptoms in neuroleptic malignant syndrome. J Nerv Ment Dis. 1994;182(3):168–173. doi: 10.1097/00005053-199403000-00007. [DOI] [PubMed] [Google Scholar]
- 51.Levenson JL. Neuroleptic malignant syndrome. Am J Psychiatry. 1985;142(10):1137–1145. doi: 10.1176/ajp.142.10.1137. [DOI] [PubMed] [Google Scholar]
- 52.Neuhut R, Lindenmayer JP, Silva R. Neuroleptic malignant syndrome in children and adolescents on atypical antipsychotic medication: a review. J Child Adolesc Psychopharmacol. 2009;19(4):415–422. doi: 10.1089/cap.2008.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Latham J, Campbell D, Nichols W, Mott T. Clinical inquiries. How much can exercise raise creatine kinase level – and does it matter? J Fam Pract. 2008;57(8):545–547. [PubMed] [Google Scholar]
- 54.Arslankoylu AE, Kutuk MO, Okuyaz C, Toros F. Neuroleptic malignant syndrome due to risperidone misdiagnosed as status epilepticus. Pediatr Rep. 2011;3(3):e19. doi: 10.4081/pr.2011.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henderson VW, Wooten GF. Neuroleptic malignant syndrome: a pathogenetic role for dopamine receptor blockade? Neurology. 1981;31(2):132–137. doi: 10.1212/wnl.31.2.132. [DOI] [PubMed] [Google Scholar]
- 56.Figa-Talamanca L, Gualandi C, Di Meo L, Di Battista G, Neri G, Lo Russo F. Hyperthermia after discontinuance of levodopa and bromocriptine therapy: impaired dopamine receptors a possible cause. Neurology. 1985;35(2):258–261. doi: 10.1212/wnl.35.2.258. [DOI] [PubMed] [Google Scholar]
- 57.Sahoo MK, Agarwal S, Biswas H. Catatonia versus neuroleptic malignant syndrome: the diagnostic dilemma and treatment. Ind Psychiatry J. 2014;23(2):163–165. doi: 10.4103/0972-6748.151703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gragnani A, Cezillo MV, Oliveira AF, Ferreira LM. Neuroleptic malignant syndrome in trauma patient. Burns. 2015;41(6):1147–1151. doi: 10.1016/j.burns.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 59.Nachreiner R, Balledux J, Zieger M, Viegas O, Sood R. Neuroleptic malignant syndrome associated with metoclopramide in a burn patient. J Burn Care Res. 2006;27(2):237–241. doi: 10.1097/01.BCR.0000202644.17987.3F. [DOI] [PubMed] [Google Scholar]
- 60.Still J, Friedman B, Law E, Deppe S, Epperly N, Orlet H. Neuroleptic malignant syndrome in a burn patient. Burns. 1998;24(6):573–575. doi: 10.1016/s0305-4179(98)00056-4. [DOI] [PubMed] [Google Scholar]
- 61.Dixit S, Dutta MK, Namdeo M. A rare case of myxedema coma with neuroleptic malignant syndrome (NMS) J Clin Diagn Res. 2015;9(5):VD01–VD03. doi: 10.7860/JCDR/2015/13008.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Al Danaf J, Madara J, Dietsche C. Neuroleptic malignant syndrome: a case aimed at raising clinical awareness. Case Rep Med. 2015;2015:769576. doi: 10.1155/2015/769576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Margetic B, Aukst-Margetic B. Neuroleptic malignant syndrome and its controversies. Pharmacoepidemiol Drug Saf. 2010;19(5):429–435. doi: 10.1002/pds.1937. [DOI] [PubMed] [Google Scholar]
- 64.Northoff G. Catatonia and neuroleptic malignant syndrome: psychopathology and pathophysiology. J Neural Transm. 2002;109(12):1453–1467. doi: 10.1007/s00702-002-0762-z. [DOI] [PubMed] [Google Scholar]
- 65.Sachdev PS. A rating scale for neuroleptic malignant syndrome. Psychiatry Res. 2005;135(3):249–256. doi: 10.1016/j.psychres.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 66.Haddow AM, Harris D, Wilson M, Logie H. Clomipramine induced neuroleptic malignant syndrome and pyrexia of unknown origin. BMJ. 2004;329(7478):1333–1335. doi: 10.1136/bmj.329.7478.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lejoyeux M, Fineyre F, Ades J. The serotonin syndrome. Am J Psychiatry. 1992;149(10):1410–1411. doi: 10.1176/ajp.149.10.1410b. [DOI] [PubMed] [Google Scholar]
- 68.Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):705–713. doi: 10.1176/ajp.148.6.705. [DOI] [PubMed] [Google Scholar]
- 69.Castillo E, Rubin RT, Holsboer-Trachsler E. Clinical differentiation between lethal catatonia and neuroleptic malignant syndrome. Am J Psychiatry. 1989;146(3):324–328. doi: 10.1176/ajp.146.3.324. [DOI] [PubMed] [Google Scholar]
- 70.Fleischhacker WW, Unterweger B, Kane JM, Hinterhuber H. The neuroleptic malignant syndrome and its differentiation from lethal catatonia. Acta Psychiatr Scand. 1990;81(1):3–5. doi: 10.1111/j.1600-0447.1990.tb06439.x. [DOI] [PubMed] [Google Scholar]
- 71.Caroff SN, Mann SC, Keck PE., Jr Specific treatment of the neuroleptic malignant syndrome. Biol Psychiatry. 1998;44(6):378–381. doi: 10.1016/s0006-3223(97)00529-5. [DOI] [PubMed] [Google Scholar]
- 72.Patel SS, Allin MP. Physical complications in early clozapine treatment: a case report and implications for safe monitoring. Ther Adv Psychopharmacol. 2011;1(1):25–29. doi: 10.1177/2045125311398284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bruijnzeel D, Suryadevara U, Tandon R. Antipsychotic treatment of schizophrenia: an update. Asian J Psychiatr. 2014;11:3–7. doi: 10.1016/j.ajp.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 74.Lowe CM, Grube RR, Scates AC. Characterization and clinical management of clozapine-induced fever. Ann Pharmacother. 2007;41(10):1700–1704. doi: 10.1345/aph.1K126. [DOI] [PubMed] [Google Scholar]
- 75.Jeong SH, Ahn YM, Koo YJ, Kang UG, Kim YS. The characteristics of clozapine-induced fever. Schizophr Res. 2002;56(1–2):191–193. doi: 10.1016/s0920-9964(01)00262-6. [DOI] [PubMed] [Google Scholar]
- 76.Young CR, Bowers MB, Jr, Mazure CM. Management of the adverse effects of clozapine. Schizophr Bull. 1998;24(3):381–390. doi: 10.1093/oxfordjournals.schbul.a033333. [DOI] [PubMed] [Google Scholar]
- 77.Kohen I, Afzal N, Hussain S, Manu P. Increases in C-reactive protein may predict recurrence of clozapine-induced fever. Ann Pharmacother. 2009;43(1):143–146. doi: 10.1345/aph.1L467. [DOI] [PubMed] [Google Scholar]
- 78.Bruno V, Valiente-Gomez A, Alcoverro O. Clozapine and fever: a case of continued therapy with clozapine. Clin Neuropharmacol. 2015;38(4):151–153. doi: 10.1097/WNF.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 79.Marmura MJ. Use of dopamine antagonists in treatment of migraine. Curr Treat Options Neurol. 2012;14(1):27–35. doi: 10.1007/s11940-011-0150-9. [DOI] [PubMed] [Google Scholar]
- 80.Shalev A, Hermesh H, Munitz H. Mortality from neuroleptic malignant syndrome. J Clin Psychiatry. 1989;50(1):18–25. [PubMed] [Google Scholar]
- 81.Ghani SO, Ahmed W, Marco LA. Neuroleptic malignant syndrome and severe thrombocytopenia: case report and literature review. Ann Clin Psychiatry. 2000;12(1):51–54. doi: 10.1023/a:1009079127829. [DOI] [PubMed] [Google Scholar]
- 82.Butwicka A, Krystyna S, Retka W, Wolanczyk T. Neuroleptic malignant syndrome in an adolescent with CYP2D6 deficiency. Eur J Pediatr. 2014;173(12):1639–1642. doi: 10.1007/s00431-013-2208-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gupta S, Racaniello AA. Neuroleptic malignant syndrome associated with amoxapine and lithium in an older adult. Ann Clin Psychiatry. 2000;12(2):107–109. doi: 10.1023/a:1009028315246. [DOI] [PubMed] [Google Scholar]
- 84.Maniam T, Rahman MA. All elevated creatine kinase is not neuroleptic malignant syndrome. Med J Malaysia. 1994;49(3):252–254. [PubMed] [Google Scholar]
- 85.Venkatasubramanian G, Yogananda BH, Gangadhar BN. Risperidone-induced neuroleptic malignant syndrome: a case report. Indian J Psychiatry. 2000;42(1):101–103. [PMC free article] [PubMed] [Google Scholar]
- 86.Adityanjee The myth of elevated serum creatine phosphokinase level and neuroleptic malignant syndrome. Br J Psychiatry. 1991;158:706–707. doi: 10.1192/bjp.158.5.706. [DOI] [PubMed] [Google Scholar]
- 87.Hermesh H, Manor I, Shiloh R, et al. High serum creatinine kinase level: possible risk factor for neuroleptic malignant syndrome. J Clin Psychopharmacol. 2002;22(3):252–256. doi: 10.1097/00004714-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 88.Rosebush P, Stewart T. A prospective analysis of 24 episodes of neuroleptic malignant syndrome. Am J Psychiatry. 1989;146(6):717–725. doi: 10.1176/ajp.146.6.717. [DOI] [PubMed] [Google Scholar]
- 89.Nisijima K, Shioda K. A rare case of neuroleptic malignant syndrome without elevated serum creatine kinase. Neuropsychiatr Dis Treat. 2014;10:403–407. doi: 10.2147/NDT.S58677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Modi S, Dharaiya D, Schultz L, Varelas P. Neuroleptic malignant syndrome: complications, outcomes, and mortality. Neurocrit Care. 2016;24(1):97–103. doi: 10.1007/s12028-015-0162-5. [DOI] [PubMed] [Google Scholar]
- 91.Mackin P. Cardiac side effects of psychiatric drugs. Hum Psychopharmacol. 2008;23(Suppl 1):3–14. doi: 10.1002/hup.915. [DOI] [PubMed] [Google Scholar]
- 92.Beach SR, Celano CM, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics. 2013;54(1):1–13. doi: 10.1016/j.psym.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 93.Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–962. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- 94.Eiser AR, Neff MS, Slifkin RF. Acute myoglobinuric renal failure. A consequence of the neuroleptic malignant syndrome. Arch Intern Med. 1982;142(3):601–603. [PubMed] [Google Scholar]
- 95.Lee JW. Serum iron in catatonia and neuroleptic malignant syndrome. Biol Psychiatry. 1998;44(6):499–507. doi: 10.1016/s0006-3223(98)00109-7. [DOI] [PubMed] [Google Scholar]
- 96.Lee HC, Kim JM, Lim JK, Jo YS, Kim SK. Central hyperthermia treated with baclofen for patient with pontine hemorrhage. Ann Rehabil Med. 2014;38(2):269–272. doi: 10.5535/arm.2014.38.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sung CY, Lee TH, Chu NS. Central hyperthermia in acute stroke. Eur Neurol. 2009;62(2):86–92. doi: 10.1159/000222778. [DOI] [PubMed] [Google Scholar]
- 98.Takahashi Y, Mori H, Mishina M, et al. Autoantibodies and cell-mediated autoimmunity to NMDA-type GluRepsilon2 in patients with Rasmussen’s encephalitis and chronic progressive epilepsia partialis continua. Epilepsia. 2005;46(Suppl 5):152–158. doi: 10.1111/j.1528-1167.2005.01024.x. [DOI] [PubMed] [Google Scholar]
- 99.Lancaster E. The diagnosis and treatment of autoimmune encephalitis. J Clin Neurol. 2016;12(1):1–13. doi: 10.3988/jcn.2016.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Splendiani A, Felli V, Di Sibio A, et al. Magnetic resonance imaging and magnetic resonance spectroscopy in a young male patient with anti-N-methyl-D-aspartate receptor encephalitis and uncommon cerebellar involvement: a case report with review of the literature. Neuroradiol J. 2016;29(1):30–35. doi: 10.1177/1971400915609333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Heine J, Pruss H, Bartsch T, Ploner CJ, Paul F, Finke C. Imaging of autoimmune encephalitis – relevance for clinical practice and hippocampal function. Neuroscience. 2015;309:68–83. doi: 10.1016/j.neuroscience.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 102.Dhib-Jalbut S, Hesselbrock R, Mouradian MM, Means ED. Bromocriptine treatment of neuroleptic malignant syndrome. J Clin Psychiatry. 1987;48(2):69–73. [PubMed] [Google Scholar]
- 103.Bonuccelli U, Piccini P, Corsini GU, Muratorio A. Apomorphine in malignant syndrome due to levodopa withdrawal. Ital J Neurol Sci. 1992;13(2):169–170. doi: 10.1007/BF02226968. [DOI] [PubMed] [Google Scholar]
- 104.Rosenberg MR, Green M. Neuroleptic malignant syndrome. Review of response to therapy. Arch Intern Med. 1989;149(9):1927–1931. doi: 10.1001/archinte.149.9.1927. [DOI] [PubMed] [Google Scholar]
- 105.Hermesh H, Aizenberg D, Weizman A. A successful electroconvulsive treatment of neuroleptic malignant syndrome. Acta Psychiatr Scand. 1987;75(3):237–239. doi: 10.1111/j.1600-0447.1987.tb02782.x. [DOI] [PubMed] [Google Scholar]
- 106.Trollor JN, Sachdev PS. Electroconvulsive treatment of neuroleptic malignant syndrome: a review and report of cases. Aust N Z J Psychiatry. 1999;33(5):650–659. doi: 10.1080/j.1440-1614.1999.00630.x. [DOI] [PubMed] [Google Scholar]
- 107.Davis JM, Janicak PG, Sakkas P, Gilmore C, Wang Z. Electroconvulsive therapy in the treatment of the neuroleptic malignant syndrome. Convuls Ther. 1991;7(2):111–120. [PubMed] [Google Scholar]
- 108.Mendhekar DN, Duggal HS. Persistent amnesia as a sequel of olanzapine-induced neuroleptic malignant syndrome. J Neuropsychiatry Clin Neurosci. 2006;18(4):552–553. doi: 10.1176/jnp.2006.18.4.552. [DOI] [PubMed] [Google Scholar]
- 109.Wu YF, Kan YS, Yang CH. Neuroleptic malignant syndrome associated with bromocriptine withdrawal in Parkinson’s disease – a case report. Gen Hosp Psychiatry. 2011;33(3):301.e7–e8. doi: 10.1016/j.genhosppsych.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 110.Sekijima Y, Hoshi KI, Kasai H, et al. Three patients with isolated adrenocorticotropin deficiency presenting with neuroleptic malignant syndrome-like symptoms. Intern Med. 2001;40(6):510–514. doi: 10.2169/internalmedicine.40.510. [DOI] [PubMed] [Google Scholar]
- 111.Morigaki Y, Iga J, Kameoka N, Sumitani S, Ohmori T. Psychiatric symptoms in a patient with isolated adrenocorticotropin deficiency: case report and literature review. Gen Hosp Psychiatry. 2014;36(4):449.e443–e445. doi: 10.1016/j.genhosppsych.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 112.Pope HG, Jr, Aizley HG, Keck PE, Jr, McElroy SL. Neuroleptic malignant syndrome: long-term follow-up of 20 cases. J Clin Psychiatry. 1991;52(5):208–212. [PubMed] [Google Scholar]
- 113.Coffey RJ, Edgar TS, Francisco GE, et al. Abrupt withdrawal from intrathecal baclofen: recognition and management of a potentially life-threatening syndrome. Arch Phys Med Rehabil. 2002;83(6):735–741. doi: 10.1053/apmr.2002.32820. [DOI] [PubMed] [Google Scholar]
- 114.Luchini F, Medda P, Mariani MG, Mauri M, Toni C, Perugi G. Electroconvulsive therapy in catatonic patients: efficacy and predictors of response. World J Psychiatry. 2015;5(2):182–192. doi: 10.5498/wjp.v5.i2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oruch R, Elderbi MA, Khattab HA, Pryme IF, Lund A. Lithium: a review of pharmacology, clinical uses, and toxicity. Eur J Pharmacol. 2014;740:464–473. doi: 10.1016/j.ejphar.2014.06.042. [DOI] [PubMed] [Google Scholar]
- 116.Suzuki T, Uchida H, Watanabe K, Kashima H. A potentially aborted neuroleptic malignant syndrome following seclusion against uncontrollable water intoxication. Psychopharmacol Bull. 2008;41(1):164–170. [PubMed] [Google Scholar]
- 117.Oruch R, Pryme IF, Lund A. The ideal antipsychotic: hybrid between typical “haloperidol” and the atypical “colzapine” antipsychotic. J Bioanal Biomed. 2015;7(4):124–135. [Google Scholar]
- 118.Oruch R, Lund A, Pryme IF, Holmsen H. An intercalation mechanism as a mode of action exerted by psychotropic drugs: results of altered phospholipid substrate availabilities in membranes? J Chem Biol. 2010;3(2):67–88. doi: 10.1007/s12154-009-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Oruch R, Pryme IF, Holmsen H. Effects of psychotropic drugs on the thrombin-induced liberation of arachidonate in human platelets. Saudi Med J. 2008;29(10):1397–1407. [PubMed] [Google Scholar]
- 120.Oruch R, Hodneland E, Pryme IF, Holmsen H. Psychotropic drugs interfere with the tight coupling of polyphosphoinositide cycle metabolites in human platelets: a result of receptor-independent drug intercalation in the plasma membrane? Biochim Biophys Acta. 2008;1778(10):2165–2176. doi: 10.1016/j.bbamem.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 121.Kulkarni SK, Naidu PS. Pathophysiology and drug therapy of tardive dyskinesia: current concepts and future perspectives. Drugs Today (Barc) 2003;39(1):19–49. doi: 10.1358/dot.2003.39.1.799430. [DOI] [PubMed] [Google Scholar]
- 122.Kaminska T, Szuster-Ciesielska A, Wysocka A, Marmurowska-Michalowska H, Dubas-Slemp H, Kandefer-Szerszen M. Serum cytokine level and production of reactive oxygen species (ROS) by blood neutrophils from a schizophrenic patient with hypersensitivity to neuroleptics. Med Sci Monit. 2003;9(7):CS71–CS75. [PubMed] [Google Scholar]
- 123.Dursun SM, Oluboka OJ, Devarajan S, Kutcher SP. High-dose vitamin E plus vitamin B6 treatment of risperidone-related neuroleptic malignant syndrome. J Psychopharmacol. 1998;12(2):220–221. doi: 10.1177/026988119801200214. [DOI] [PubMed] [Google Scholar]
- 124.Verma R, Junewar V, Rathaur BP. An atypical case of neuroleptic malignant syndrome precipitated by valproate. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2013-202578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tadke RR, Suryavanshi P. Use of intravenous valproate in a patient with neuroleptic malignant syndrome. J Neuropsychiatry Clin Neurosci. 2006;18(1):131–132. doi: 10.1176/jnp.18.1.131. [DOI] [PubMed] [Google Scholar]
- 126.Monda M, Viggiano A, Viggiano E, Messina G, Tafuri D, De Luca V. Sympathetic and hyperthermic reactions by orexin A: role of cerebral catecholaminergic neurons. Regul Pept. 2007;139(1–3):39–44. doi: 10.1016/j.regpep.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 127.Rasmussen K, Hsu MA, Yang Y. The orexin-1 receptor antagonist SB-334867 blocks the effects of antipsychotics on the activity of A9 and A10 dopamine neurons: implications for antipsychotic therapy. Neuropsychopharmacology. 2007;32(4):786–792. doi: 10.1038/sj.npp.1301239. [DOI] [PubMed] [Google Scholar]
- 128.Monda M, Viggiano A, Viggiano E, Messina G, Tafuri D, De Luca V. Quetiapine lowers sympathetic and hyperthermic reactions due to cerebral injection of orexin A. Neuropeptides. 2006;40(5):357–363. doi: 10.1016/j.npep.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 129.Monda M, Viggiano A, Mondola R, et al. Olanzapine blocks the sympathetic and hyperthermic reactions due to cerebral injection of orexin A. Peptides. 2008;29(1):120–126. doi: 10.1016/j.peptides.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 130.Tanii H, Taniguchi N, Niigawa H, et al. Development of an animal model for neuroleptic malignant syndrome: heat-exposed rabbits with haloperidol and atropine administration exhibit increased muscle activity, hyperthermia, and high serum creatine phosphokinase level. Brain Res. 1996;743(1–2):263–270. doi: 10.1016/s0006-8993(96)01059-1. [DOI] [PubMed] [Google Scholar]
- 131.Schattner A, Kitroser E, Cohen JD. Fatal neuroleptic malignant syndrome associated with quetiapine. Am J Ther. 2016;23(5):e1209–e1210. doi: 10.1097/MJT.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 132.Lertxundi U, Ruiz AI, Aspiazu MA, et al. Adverse reactions to antipsychotics in Parkinson disease: an analysis of the Spanish pharmacovigilance database. Clin Neuropharmacol. 2015;38(3):69–84. doi: 10.1097/WNF.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 133.Harter C, Obier C, Druschky KF, Eikelmann B. Malignes neuroleptisches syndrom durch amisulprid. [Malignant neuroleptic syndrome associated with amisulpride] Nervenarzt. 2008;79(1):86–89. doi: 10.1007/s00115-007-2343-8. German. [DOI] [PubMed] [Google Scholar]
- 134.Yaman A, Kendirli T, Odek C, et al. Neuroleptic malignant syndrome associated with metoclopramide in a child. Turk J Pediatr. 2014;56(5):535–537. [PubMed] [Google Scholar]
- 135.Moosavi SM, Ahmadi M, Monajemi MB. Acute dystonia due to citalopram treatment: a case series. Glob J Health Sci. 2014;6(6):295–299. doi: 10.5539/gjhs.v6n6p295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sharon N, Cullen C, Martinez K. A complex presentation of pediatric neuroleptic malignant syndrome related to polypharmacy in a 12-year-old male. J Child Adolesc Psychopharmacol. 2016;26(6):571–573. doi: 10.1089/cap.2014.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang Y, Guo Y, Zhang A. Neuroleptic malignant syndrome in a patient treated with lithium carbonate and haloperidol. Shanghai Arch Psychiatry. 2014;26(6):368–370. doi: 10.11919/j.issn.1002-0829.214099. [DOI] [PMC free article] [PubMed] [Google Scholar]