Abstract
Sarcoidosis is characterized by noncaseating granulomas with an unknown cause that present primarily in the lung. Propionibacterium acnes, an immunogenic commensal skin bacterium involved in acne vulgaris, has been implicated as a possible causative agent of sarcoidosis. Here, we demonstrate that a viable strain of P. acnes isolated from a patient with sarcoidosis and instilled intratracheally into wild-type mice can generate pulmonary granulomas similar to those observed in patients with sarcoidosis. The formation of these granulomas is dependent on the administration of viable P. acnes. We also found that mice deficient in the innate immunity adapter protein MyD88 had a greater number and a larger area of granuloma lesions compared with wild-type mice administered P. acnes. Early after P. acnes administration, wild-type mice produced proinflammatory mediators and recruited neutrophils into the lung, a response that is dependent on MyD88. In addition, there was an increase in granuloma number and size after instillation with P. acnes in mice deficient in CybB, a critical component of nicotinamide adenine dinucleotide phosphate oxidase required for the production of reactive oxygen species in the phagosome. Myd88−/− or Cybb−/− mice both had increased persistence of P. acnes in the lung, together with enhanced granuloma formation. In conclusion, we have generated a mouse model of early granuloma formation induced by a clinically relevant strain of P. acnes isolated from a patient with sarcoidosis, and, using this model, we have shown that a deficiency in MyD88 or CybB is associated with impaired bacterial clearance and increased granuloma formation in the lung.
Keywords: granulomas, Propionibacterium acnes, sarcoidosis
Clinical Relevance
The cause of sarcoidosis is unknown; however, Propionibacterium acnes has been identified in the granulomas of patients with sarcoidosis. Demonstrating a novel model of granuloma formation in mice using a clinically relevant strain of P. acnes supports the idea of a bacterial component of sarcoidosis disease pathology.
Sarcoidosis is a systemic disease with an unknown cause that is characterized by noncaseating granulomas found primarily in the lung; however, these granulomas are also commonly found in the liver, skin, lymph nodes, eyes, and heart. These granulomas are composed of multinucleated giant cells, macrophages, and lymphoid cells, and although the exact cause of sarcoidosis is unknown, it is thought to result from the combination of genetic susceptibility and exposure to a specific antigen, either environmental or infectious (1). Propionibacterium acnes has been implicated in the development of sarcoidosis because frequently it can be cultured out of the lymph nodes of patients with sarcoidosis (2) and it has been localized within sarcoidosis granulomas (3). In addition, P. acnes has been indicated as driving differential cytokine responses in the peripheral blood mononuclear cells of patients with sarcoidosis (4). However, the role of P. acnes in sarcoidosis is contentious because it is viewed primarily as commensal skin bacteria (5) and it is also cultured occasionally out of the lymph nodes of healthy control patients (2).
Some granuloma-producing animal models of sarcoidosis using different bacteria and bacterial products have been proposed; however, there is yet to be a consensus on the ideal animal model (6–9). The anaerobic gram-positive P. acnes is the strongest bacterial candidate for a causative agent of sarcoidosis (10) because it is the only bacterium to be cultured from sarcoidosis lesions (2) and it has been localized within sarcoidosis granulomas (3). Previous models of sarcoid-like granuloma formation have been developed using heat-killed P. acnes; however, the use of heat to kill bacteria may denature bacterial surface proteins and runs the risk of generating an artificial immune response (11). In addition to relying on heat-killed P. acnes, these models require adjuvants and multiple administrations of heat-killed P. acnes in a 2- to 8-week sensitization period before challenge with heat-killed P. acnes (6, 7, 12). Although the requirement of multiple sensitizations may represent the inherent intricacies of developing a mouse model of a chronic disease within the dynamic pulmonary environment, it may also complicate the model unnecessarily through the addition of multiple time points. Furthermore, the granuloma response in these models is not robust or well quantified, and the mechanisms of this granuloma formation are not addressed.
For these reasons, it is imperative to design a mouse model of the initial stages of granuloma formation using a single dose of live P. acnes to retain the original structure of bacterial surface antigens. We show that viable P. acnes isolated from the lymph node of a patient with sarcoidosis and administered intratracheally to mice results in the formation of pulmonary granulomas in wild-type mice. Using this model, we show that MyD88, an adapter for Toll-like receptor (TLR)/IL-1–like receptor signaling, and CybB (also known as Nox2), a major component of nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase), are critical in granuloma development. Some of the results of these studies have been reported previously in the form of abstracts (13, 14).
Materials and Methods
Animals
Wild-type (Wt) (C57BL/6), Myd88−/− and Cybb−/− mice (both on a C57BL/6 background) were bred and maintained in a specific pathogen-free environment at the Animal Care Facility of the University of Michigan. Mice between 6 and16 weeks of age were used for experiments. All animal studies were reviewed and approved by the University of Michigan Committee on the Use and Care of Animals.
P. acnes Culture
A clinical isolate of P. acnes was cultured from a patient with sarcoidosis lymph node as reported previously (15). P. acnes was grown anaerobically in Schaedler broth (Sigma, St. Louis, MO) at 37°C and frozen at −80 °C in 1:10 Glycerol (Fisher, Waltham, MA) in phosphate-buffered saline (Gibco, Carlsbad, CA) until administration.
In Vivo P. acnes Administration
P. acnes culture aliquots were thawed, washed, and suspended in 10 ml of Schaedler broth (Sigma) anaerobically for 3 hours at 37 °C. The mice were then anesthetized with isoflurane and intratracheally administered 109 CFU in phosphate-buffered saline (Gibco) of either viable or heat-killed (60–80°C for 30 min) P. acnes in 50 μl. After 9 days, the mice were killed, and the size and number of granulomas in the lung were quantified with pattern recognition software (see online supplement).
P. acnes Immunohistochemistry
An antibody specific to P. acnes (PAB) was developed and produced as described previously (3). Both naive mice and mice 9 days after P. acnes administration were killed, and the lungs were removed. Individual sections of the whole lung were fixed and imbedded flat in paraffin in preparation for sectioning. Serial sections of the lung tissue were cut and were stained with PAB and hematoxylin and eosin as described previously (3).
Flow Cytometry
The mice were killed at 0, 3, and 9 days after P. acnes administration, and the lungs were removed and digested with collagenase (Sigma) as described previously (16). A single-cell suspension was prepared, and Fc receptors were blocked with CD16/32 (eBioscience, San Diego, CA), and viable cells were stained with Live/Dead (Invitrogen, Carlsbad, CA). The cells were then stained with surface markers (see online supplement). Cells were then fixed using the Cytofix/Cytoperm kit from Becton, Dickinson and Co. (Franklin Lakes, NJ).
P. acnes Viability Studies
The mice were killed at 0 and 3 days after P. acnes administration, and lungs were removed, homogenized, plated on Schaedler agar (Sigma), and then grown anaerobically at 37°C.
Data Analysis
All analysis was performed using Prism 7 (GraphPad Software, Inc., San Diego, CA). Normal Gaussian distribution was determined by the D'Agostino-Pearson omnibus test. Normal data were analyzed using unpaired two-tailed t tests, one-way analysis of variance, or two-way analysis of variance, as appropriate. Welch’s correction was used in data sets with unequal SDs. Nonparametric data were analyzed using the Mann–Whitney U test and the Kruskal–Wallis test, as appropriate.
Results
Intratracheal Administration of Viable P. acnes Produces Sarcoid-Like Pulmonary Granulomas in Wild-Type Mice
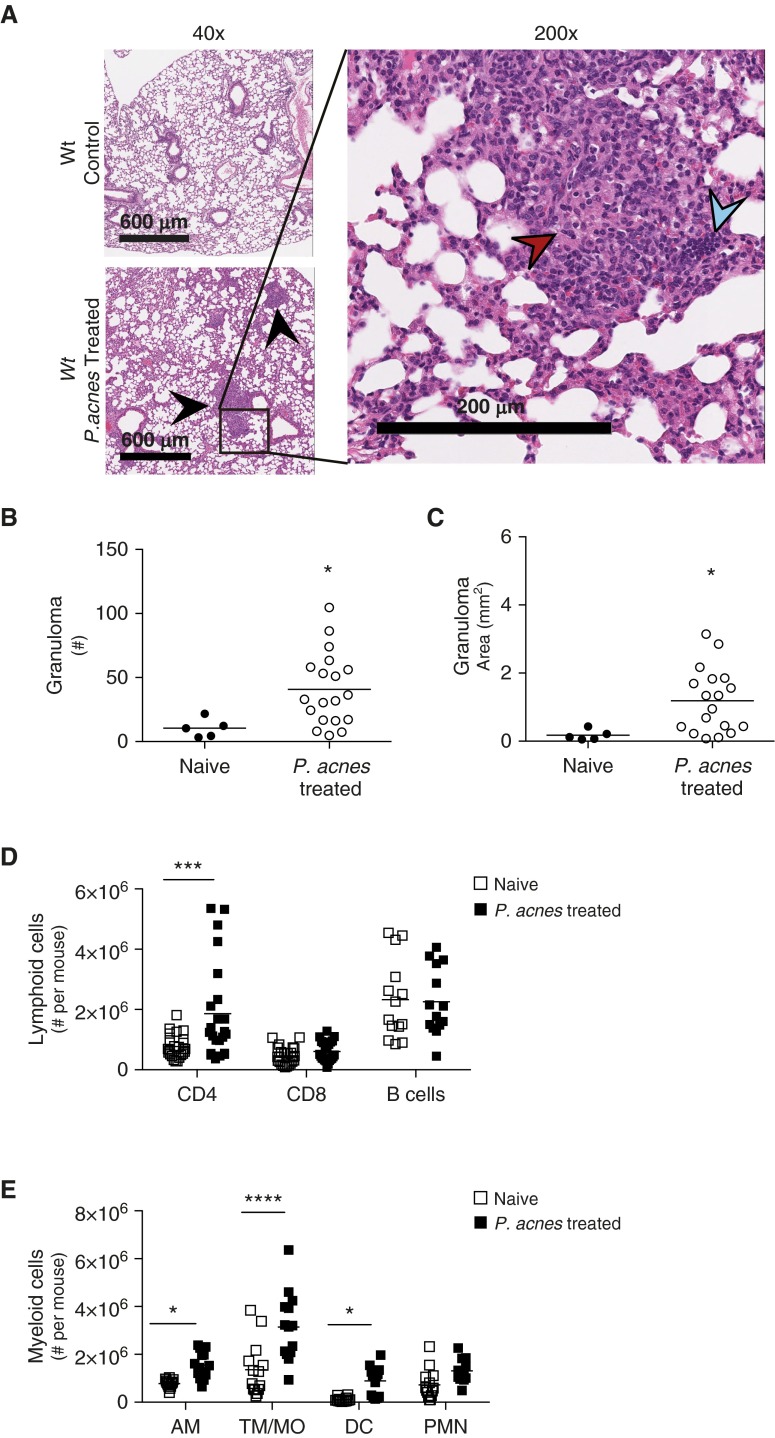
Wild-type (Wt) mice were administered viable P. acnes intratracheally, and granulomas were observed in their lungs (Figure 1A, bottom left, and right) but not in the naive Wt control mice (Figure 1A, top left). The compact organization of epithelioid macrophages and lymphoid cells can be observed at a higher magnification (Figure 1A, right). To quantify the size and number of granulomas in the lungs after P. acnes inoculation, we used the pattern-matching software Spatially Invariant Vector Quantization (SIVQ). This program allows for novel identification of user-generated patterns irrespective of spatial organization, by allowing for rotational sampling (17). In addition, SIVQ also automatically calculates the area of these patterns (18), allowing for objective and automated granuloma quantification by number (Figure 1B) as well as area (Figure 1C). Administration of P. acnes to Wt mice resulted in an average of 41 granulomas and 1 mm2 of granulomas in a 600-μm section of lung analyzed every 200 μm, compared with uninfected control lungs (Figures 1B and 1C). Moreover, after enzymatic dispersion of naive and P. acnes–administered lungs, there was an increase in CD4+ T cells (Figure 1D), and both CD4+ and CD8+ T cells isolated from the lungs of mice administered P. acnes produced more IFN-γ (see Figure E1 in the online supplement). There was no change in the number of T cells producing either IL-2 or IL-4 after P. acnes administration (data not shown). In addition, there was an increase in alveolar macrophages, tissue macrophages/monocytes, and dendritic cells after treatment with P. acnes (Figure 1E). The increase in myeloid cells and CD4+ T cells, as well as the increase in IFN-γ–producing CD4+ cells in the lung, mirrors the cellular composition of granulomas and the localized Th1 cytokine production observed in patients with sarcoidosis (19, 20). Furthermore, the administration of an additional clinical isolate of P. acnes to Wt mice also drove granuloma formation (Figure E2). These data indicate that a single dose of viable P. acnes can initiate a granuloma response in the lung.
Figure 1.
Generation of early pulmonary granuloma model. Wild-type (Wt) mice were administered 109 viable Propionibacterium acnes intratracheally and were killed after 9 days to generate a mouse model of sarcoid-like granuloma formation. Naive Wt mice were used as control mice. (A) Naive (top left) and P. acnes–administered Wt mouse lungs (bottom left) were removed, fixed, embedded, and examined for granulomas (black arrowheads) via hematoxylin and eosin staining at low magnification. Scale bars: 600 μm. At higher magnification in P. acnes–administered mice (right), epithelioid macrophages (red arrowhead) and lymphoid cells (blue arrowhead) can be observed. Scale bar: 200 μm. Granuloma (B) numbers and (C) area were quantified using pattern recognition software Spatially Invariant Vector Quantization (SIVQ) from three tissue sections 200 μm apart; each individual data point represents the average of those measurements per mouse. (D and E) Naive and P. acnes–administered lungs were also perfused with phosphate-buffered saline, digested into single-cell suspensions, analyzed for cell type composition via flow cytometry, and gated as described in Materials and Methods. The number of total viable (D) lymphoid cells and (E) myeloid cells is shown. Lymphoid cells are CD4+, CD8+ T cells, and B cells; myeloid cells are AM, TM/MO, DC, and PMN. Statistical significance was determined using unpaired t tests (B and C) and multiple t tests (D and E). *P < 0.05, ***P < 0.001, and ****P < 0.0001. AM, alveolar macrophages; DC, dendritic cells; PMN, neutrophils; TM/MO, tissue macrophages/monocytes.
Viability of P. acnes Is Key to Granuloma Formation
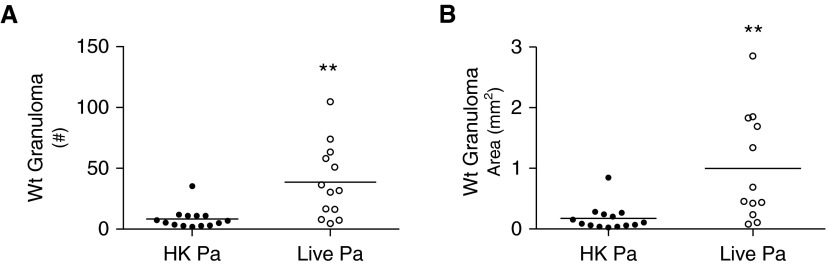
Wt mice were administered either viable P. acnes or an identical dose of heat-killed P. acnes, and the mice receiving nonviable heat-killed P. acnes had smaller and fewer granulomas through SIVQ analysis compared with Wt mice administered viable P. acnes (Figures 2A and 2B). The number and area of granulomas detected in mice administered a single dose of heat-killed P. acnes were similar to those detected in naive Wt mice (Figures 1B and 1C) and are indicative of background levels of SIVQ. These data indicate that P. acnes viability is crucial to single-challenge granuloma development in Wt mice.
Figure 2.
Viable P. acnes drives granuloma formation in Wt mice. Wt mice were administered 109 viable or heat-killed P. acnes intratracheally and were killed after 9 days. Granuloma (A) number and (B) area from Wt mice were quantified using pattern recognition software SIVQ from three tissue sections 200 μm apart; each individual data point represents the average of those measurements per mouse. Statistical significance was determined using unpaired t tests. **P < 0.01. HK, heat killed; Pa, P. acnes.
Mice Deficient in MyD88 Have Enhanced P. acnes–Driven Granuloma Development
Because granuloma formation was shown to rely on P. acnes viability, we wished to explore the role of innate immunity in P. acnes–driven granuloma formation, because previous reports have linked TLR gene polymorphisms to the development of granulomatous diseases (21, 22). To determine whether multiple TLR deficiencies potentially play a role, we administered viable P. acnes to Myd88−/− mice intratracheally. MyD88 is a signaling adaptor protein for multiple members of the TLR/IL1R superfamily pathways and is critically important for antimicrobial host defense (23). The lungs from Myd88−/− mice administered P. acnes demonstrated an increase in pulmonary granulomas (Figure 3A, bottom left) compared with the lungs of either naive Myd88−/− control mice (Figure 3A, top left) or P. acnes–administered Wt mice (Figure 1A, bottom left). Under higher magnification, epithelioid macrophages and lymphoid cells could also be seen in Myd88−/− mice (Figure 3A, right). In addition, Myd88−/− mice administered P. acnes had three times the number (Figure 3B) and five times the area (Figure 3C) of granulomas of Wt mice administered P. acnes. Similar to P. acnes–treated Wt mice, after enzymatic dispersion, Myd88−/− mouse lungs had increased amounts of CD4+ T cells (Figure 3D), as well as alveolar macrophages, tissue macrophage/monocytes, and dendritic cells (Figure 3E). These data suggest that the innate host defense adapter protein MyD88 is key to controlling granuloma development, and in its absence, granulomas increase in both number and size.
Figure 3.
Increased granulomas in Myd88−/− mice. Myd88−/− mice were administered 109 viable P. acnes intratracheally and were killed after 9 days to test the role of MyD88 in granuloma formation. Naive Myd88−/− mice were used as control mice. (A) Naive (top left) and P. acnes–administered (bottom left) Myd88−/− mouse lungs were removed, fixed, embedded, and examined for granulomas (black arrowheads) via hematoxylin and eosin staining at low magnification (left). Scale bars: 600 μm. At higher magnification in P. acnes–administered mice (right), epithelioid macrophages (red arrowhead) and lymphoid cells (blue arrowhead) can be observed. Scale bar: 200 μm. Granuloma (B) numbers and (C) area were quantified using pattern recognition software SIVQ from three tissue sections 200 μm apart; each individual data point represents the average of those measurements per mouse. (D and E) Naive and P. acnes–administered lungs from Myd88−/− mice were perfused with phosphate-buffered saline, digested into single-cell suspensions, analyzed for cell type composition via flow cytometry, and gated as described in Materials and Methods. The number of total viable (D) lymphoid and (E) myeloid cells is shown. Lymphoid cells are CD4+, CD8+ T cells, and B cells; myeloid cells are AM, TM/MO, DC, and PMN. Statistical significance was determined using unpaired t tests (B), Mann–Whitney tests (C), and multiple t tests (D and E). **P < 0.01, ***P < 0.005, and ****P < 0.0001.
Administration of P. acnes Drives Early MyD88-Dependent Proinflammatory Mediator Production in the Lungs
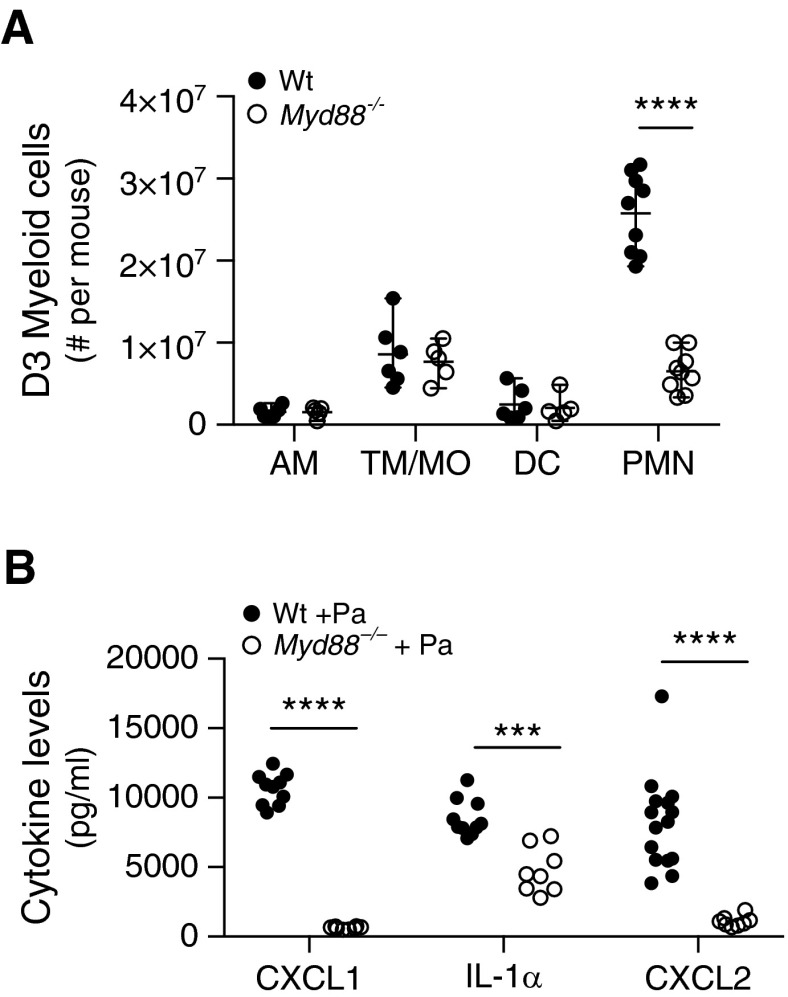
On the basis of the increased granuloma formation observed in Myd88−/− mice after P. acnes treatment, we hypothesized that innate myeloid cell types may play a role early in the development of granulomas. Single-cell suspensions were prepared from enzymatically digested mouse lungs at several time points after P. acnes administration. Three days after P. acnes administration, there was a significant increase in neutrophils in the lungs of Wt mice; however, this response was abrogated in Myd88−/− mice, whereas the number of other myeloid cells remained similar (Figure 4A). We also observed that neutrophil chemoattractants CXCL1 and CXCL2 (24, 25), as well as proinflammatory IL-1α (26), were produced rapidly after P. acnes administration in Wt mice lung homogenates, and all were reduced significantly in Myd88−/− lung homogenates (Figure 4B). Tumor necrosis factor-α (TNF-α), CC chemokine ligand 2, and IL-17A were not detected in response to P. acnes treatment in the lung (data not shown). These data suggest that a deficiency in Myd88-dependent proinflammatory pathways results in increased granuloma formation in the lung through the reduction of bacterial-driven neutrophil recruitment.
Figure 4.
P. acnes drives MyD88-dependent proinflammatory mediator production. Wt and Myd88−/− mice were administered 109 viable P. acnes intratracheally and were killed after (A) 3 days or (B) 6 hours to look at early cellular recruitment and proinflammatory mediator production. Single-cell suspension of lung digest was stained for myeloid cell types: AM, TM/MO, DC, and PMN. Six hours after P. acnes administration, the lungs were homogenized and analyzed for the level of CXCL1, CXCL2, and IL-1α in lung homogenate. (B) Background levels of naive lung homogenate were subtracted from each mouse genotype. Statistical significance was determined using multiple t tests (A) and two-way analysis of variance with Sidak’s multiple comparisons (B). ***P < 0.0005, ****P < 0.0001. CXCL, CXC chemokine ligand.
Neutrophil Depletion Had No Effect on Granuloma Formation
Because Wt mice had a large influx of neutrophils after P. acnes treatment but Myd88−/− mice did not, we hypothesized that a lack of neutrophils may drive granuloma formation. To test this, we depleted neutrophils in Wt mice using an anti-Ly6G depleting antibody (1A8) the day before P. acnes treatment and then every other day until the mice were killed. The mice treated with 1A8 had no increase in granuloma number or size, although they had significant depletion of neutrophils (Figure E3). These data indicate either that neutrophils do not play a central role in the prevention of granuloma formation or that other cell types are able to compensate for neutrophil functions in neutrophil-depleted mice.
Inability To Produce Reactive Oxygen Species Promotes Increased Granuloma Formation
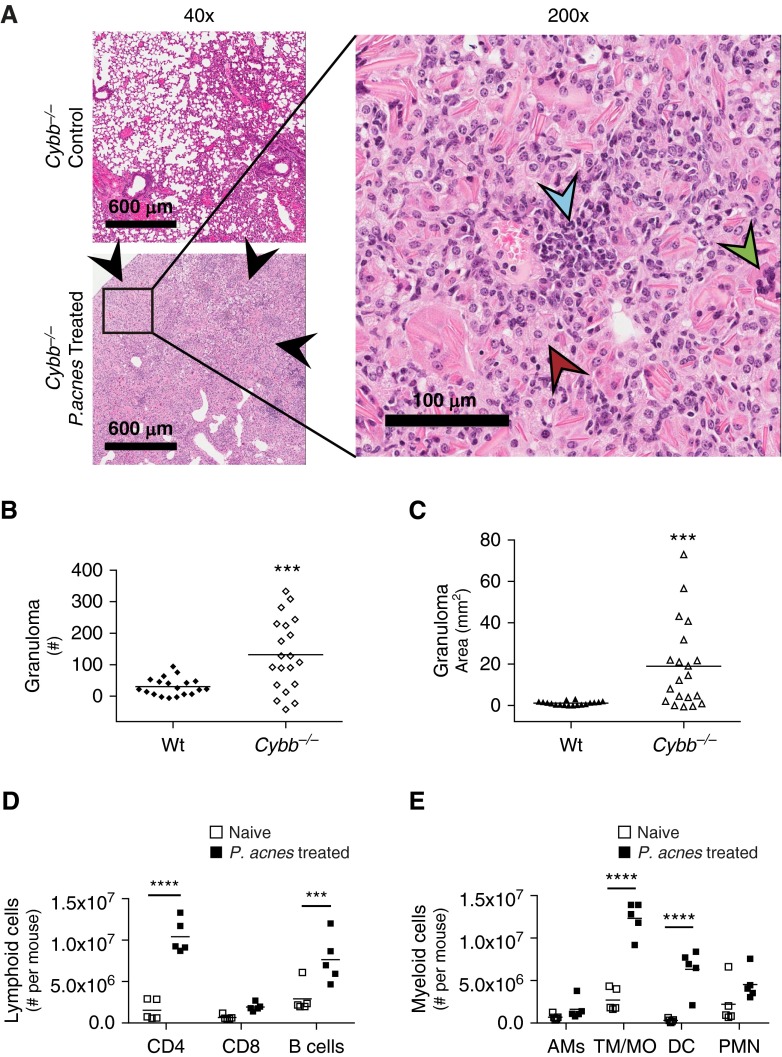
On the basis of the defective proinflammatory signaling in Myd88−/− mice and the requirement of P. acnes viability in generating a granulomatous response, we hypothesized that bacterial killing and clearance were key to granuloma formation. To test this, we used Cybb−/− mice that lack CybB/Nox2, a critical component of phagosome NADPH oxidase, crucial for bacterial killing and clearance in the lung through the production of reactive oxygen species (ROS) (27, 28). The Cybb−/− mice generated enhanced granulomas in response to P. acnes (Figure 5A, bottom left) compared with untreated Cybb−/− mice (Figure 5A, top left) or naive Wt mice (Figure 1A, top left). Consistent with those of Wt and Myd88−/− mice, Cybb−/− granulomas were composed of epithelioid macrophages and lymphoid cells (Figure 5A, right). Giant cells, characteristic of granulomas (29), can also be identified in Cybb−/− mice administered P. acnes (Figure 5A, right). Similar to that which we observed in Myd88−/− mice, the administration of P. acnes to Cybb−/− mice resulted in a fourfold increase in granuloma number (Figure 5B) and an 18-fold increase in granuloma area in Cybb−/− mice compared with Wt mice (Figure 5C). Similar to Wt and Myd88−/− mice, Cybb−/− mice had an increase in CD4+ T cells (Figure 5D), as well as in tissue macrophages/monocytes and dendritic cells (Figure 5E). However, unlike Myd88−/− mice, P. acnes-treated Cybb−/− mice had increases compared with naive Cybb−/− mice of 5.7-fold, 1.9-fold, and 1.6-fold, respectively, for CD4+, CD8+, and B cells (Figure E4A). Cybb−/− mice and Myd88−/− mice administered P. acnes had a 19.4-fold and a 17.4-fold increase, respectively, in dendritic cells (Figure E4B), suggesting similar cell recruitment into the lungs of Wt, Myd88−/−, and Cybb−/− mice. Furthermore, the robust increase in granuloma formation in Cybb−/− mice demonstrates that the loss of NADPH oxidase function enhances granuloma formation.
Figure 5.
Increased granulomas in Cybb−/− mice. Cybb−/− mice were administered 109 viable P. acnes intratracheally and were killed after 9 days to test the role of CybB/Nox2 in granuloma formation. Naive Cybb−/− mice were used as control mice. (A) Naive (top left) and P. acnes–administered (bottom left) Cybb−/− mouse lungs were removed, fixed, embedded, and examined for granulomas (black arrowheads) via hematoxylin and eosin staining at low magnification (left). Scale bars: 600 μm. At higher magnification in P. acnes–administered mice (right), epithelioid macrophages (red arrowhead) and lymphoid cells (blue arrowhead), together with giant cells (green arrowhead), can be observed. Scale bar: 100 μm. Granuloma (B) numbers and (C) area were quantified using pattern recognition software SIVQ from three tissue sections 200 μm apart; each individual data point represents the average of those measurements per mouse. (D and E) Naive and P. acnes–administered lungs from Cybb−/− mice were perfused with phosphate-buffered saline, digested into single-cell suspensions, analyzed for cell type composition via flow cytometry, and gated as described in Materials and Methods. The number of total viable (D) lymphoid and (E) myeloid cells is shown. Lymphoid cells are CD4+, CD8+ T cells, and B cells; myeloid cells are AM, TM/MO, DC, and PMN. Statistical significance was determined using unpaired t tests (B), Mann–Whitney tests (C), and multiple t tests (D and E). ***P < 0.005, and ****P < 0.0001.
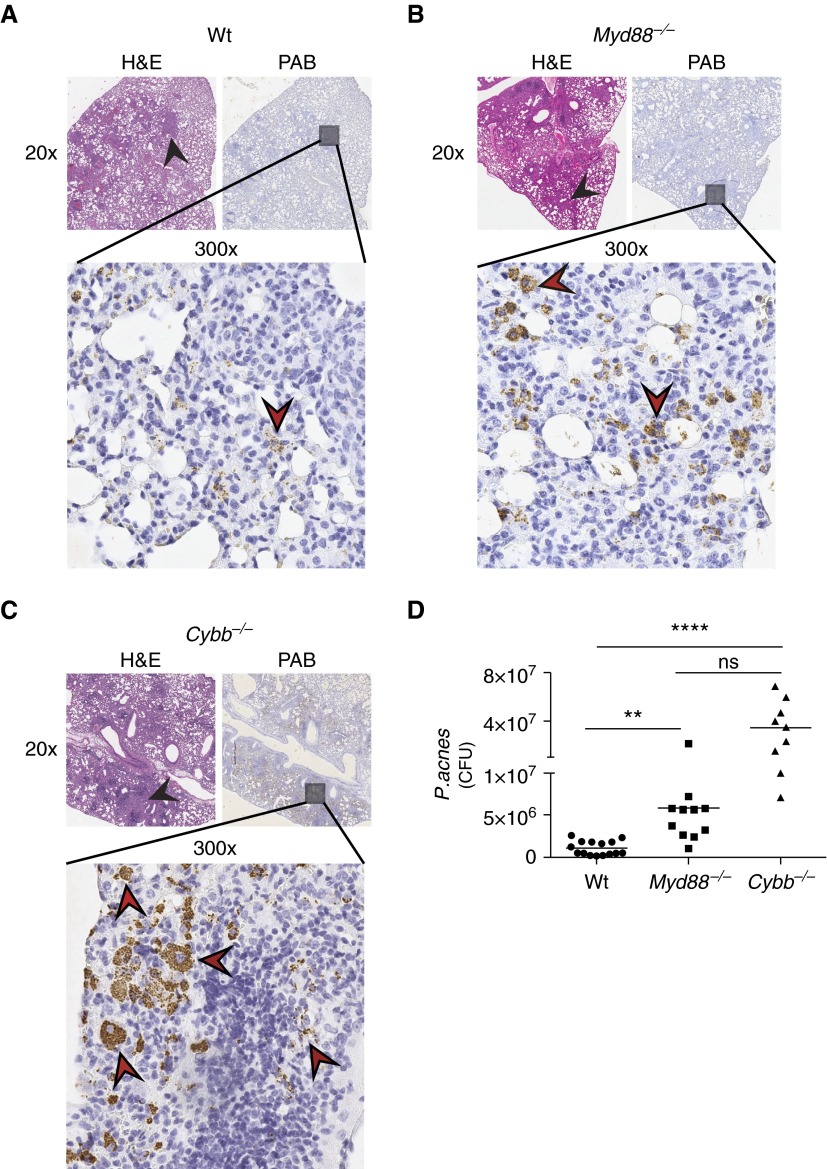
Persistence of P. acnes in the Lung Stimulates Granuloma Formation
Because of the increased number and size of granulomas in Myd88−/− (Figure 3) and Cybb−/− mice (Figure 5), we hypothesized that bacterial persistence either through lack of killing or clearance of P. acnes antigen was driving granuloma formation. Serial sections of P. acnes–treated lung tissue from Wt, Myd88−/−, and Cybb−/− mice were stained with hematoxylin and eosin (Figures 6A–6C, left) to identify granulomas and a P. acnes-specific stain (3) (Figures 6A–C, right, and bottom). Despite the presence of granulomas, almost no P. acnes bacteria were detected in the lungs of Wt administered P. acnes (Figure 6A), and all these were localized within granulomas. In contrast, numerous P. acnes bacteria were observed in Myd88−/− mice (Figure 6B) and, to a much greater extent, in Cybb−/− mice (Figure 6C), compared with Wt mice. Interestingly, P. acnes–specific staining can be observed intracellularly in Cybb−/− mice (Figure 6C). No P. acnes–specific staining was observed in any strain (Wt, Myd88−/−, Cybb−/−) of uninfected mice (data not shown). These data indicate that P. acnes antigen, from either live or dead bacteria, continues to persist in the lungs of Myd88−/− and Cybb−/− mice after Wt mice have almost completely cleared P. acnes from their lungs. To quantify the number of viable bacteria in the lungs of mice treated with P. acnes, we homogenized the lungs of Wt, Myd88−/−, and Cybb−/− mice early after P. acnes administration and plated the lung homogenate; colony-forming units were enumerated after 2 weeks of anaerobic growth. We observed that both knockout strains had significantly higher levels of viable P. acnes in their lungs after P. acnes treatment compared with the levels in Wt mice (Figure 6D). Moreover, there was a trend toward increased bacterial burden in the lungs of Cybb−/− compared with Myd88−/− mice, although the differences that were observed did not reach statistical significance. Collectively, these data suggest that defects in bacterial killing or clearance in the lung drive the initial steps of granuloma formation.
Figure 6.
Increased P. acnes burden in Myd88−/− and Cybb−/− mice. (A) Wt, (B) Myd88−/−, and (C) Cybb−/− mice were administered 109 viable P. acnes intratracheally and were killed after 9 days. Serial sections of P. acnes–administered mouse lungs were removed, fixed, embedded, and examined for granuloma formation (black arrowheads) via hematoxylin and eosin staining at low magnification (left upper panels) and PAB (right upper panels). PAB staining (brown) can be observed (red arrowheads, bottom panels). Three days after P. acnes administration, Wt, Myd88−/−, and Cybb−/− mouse lungs were homogenized, plated, and grown on Schaedler agar. (D) CFU were enumerated. Statistical significance was determined using the Kruskal–Wallis test with multiple comparisons. **P < 0.01, ****P < 0.0001. H&E, hematoxylin and eosin; ns, not significant; PAB, P. acnes-specific stain.
Discussion
P. acnes has been linked clinically with the granuloma-forming disease sarcoidosis (3). Previous mouse models of sarcoid-like granuloma formation have required heat-killed bacteria and multiple sensitization procedures to produce granulomas (6–8, 12). However, without the use of adjuvants, immune priming, or bacterial manipulation, we were able to produce a model of the initial stages of pulmonary granuloma formation using a single challenge of viable P. acnes. By placing living, whole, and unmodified P. acnes directly into the lungs of Wt mice, we mimicked the most likely route of exposure of P. acnes to the pulmonary microenvironment. Wt mice administered one dose of heat-killed bacteria in our model did not form granulomas (Figures 2A and 2B). This lack of multiple doses or use of adjuvants in our model may explain why other models using multiple doses of heat-killed bacteria were able to observe granuloma formation (6, 7). Viable bacteria appear to be critical for the formation of granulomas in Wt mice in a single-dose challenge model.
Intratracheal administration of viable P. acnes into the lungs of Wt mice produces pulmonary granulomas with a compact organization of epithelioid macrophages surrounded by lymphoid cells (Figure 1A), and these granulomas can be quantified in both number and size by using SIVQ software (Figures 1B and 1C). The macrophages in granulomas are often said to be epithelioid when their morphology resembles epithelial cells with elongated nuclei and diffuse cell borders (Figure 1A), which often fuse into multinucleated giant cells (Figure 5A) and are generated from infiltrating monocytes/tissue macrophages (30), which are clearly present in our mouse model of granuloma formation (Figure 1E). We also observed an increase in dendritic cells in the lung (Figure 1E) after P. acnes administration, and, interestingly, some patients with the granulomatous disease sarcoidosis also have increased dendritic cells in their bronchoalveolar lavage fluid (31). The mice administered P. acnes also had, together with granuloma formation, an increase in T cells, dendritic cells, and tissue macrophages/monocytes in their lungs (Figures 1D and 1E), similar to the lungs of patients with sarcoidosis (32).
The development of this rapid and reproducible mouse model of early pulmonary granuloma formation allowed us to investigate the innate mechanisms of granuloma formation. Because of conflicting reports on the role of various TLR gene polymorphisms in human populations regarding susceptibility to sarcoidosis (21, 33–36), we attempted to investigate the role of multiple TLRs simultaneously by using MyD88, a downstream adapter molecule in proinflammatory TLR/IL1R superfamily signaling (23). At least one targeted gene study has linked polymorphisms in MyD88 with susceptibility to sarcoidosis in a human population (37). We observed a significant increase in granuloma number and granuloma size after P. acnes treatment in the absence of MyD88 signaling, compared with in wild-type mice (Figure 3). This suggests that MyD88 signaling plays a role in the prevention of granulomas and that its absence results in an increase in granuloma size and number. This may explain, at least in part, the increased sarcoidosis susceptibility observed in humans with MyD88 polymorphisms (37).
To investigate the differences between Wt and Myd88−/− mouse granuloma formation, the lungs of Wt mice and Myd88−/− mice were compared for differences in cellular recruitment after P. acnes administration. Early after P. acnes administration, Wt mice experienced a large influx of neutrophils representing ∼65% of the viable lung cells, whereas this response was significantly impaired in Myd88−/− mice (Figure 4A). Wt mice also had increased levels of neutrophil chemoattractants CXCL1 and CXCL2, and proinflammatory IL-1α (Figure 4B), early after P. acnes administration. It is interesting to note that together with many other proinflammatory roles, IL-1α is also involved in neutrophil recruitment and priming (38). These data suggest that neutrophils are recruited preferentially in response to P. acnes treatment and that recruitment is a result of MyD88-dependent production of cytokines and chemokines.
The cause of sarcoidosis is a complex immune paradox consisting of a localized hyperinflammatory environment coupled with peripheral anergy (39). Despite the evidence that granulomas form in response to persistent antigen stimulation (40), and that this antigen is likely bacterial in origin (10), most work on sarcoidosis has centered on T cells and, to a lesser extent, tissue macrophages and dendritic cells (41). A few reports suggest that the presence of neutrophils in the lung may be diagnostic of progressive sarcoid disease and poor patient outcome (42, 43), which correlates with our data showing an increase of neutrophils in Wt mice (Figure 4A) and a rapid increase in neutrophil recruitment chemokines in the lung (Figure 4C) after P. acnes treatment. The heightened presence of neutrophils in the lungs of both patients with sarcoidosis and Wt mice early after P. acnes administration may be an indicator of bacterial load.
The alveoli of the lung are occupied by alveolar macrophages, which are highly phagocytic, representing the first line of defense in pulmonary infections, as well as in the clearance of debris. In contrast to their counterparts in other organs, alveolar macrophages dampen inflammatory signals to prevent the damage to lung architecture that would occur with an unnecessary inflammatory immune response. However, when the alveolar macrophages are unable to handle the antigenic burden, the alveolar macrophages play a prominent role in coordinating an immune response by recruiting neutrophils, together with other cells, to the lung (44). Alveolar macrophages in Myd88−/− mice are known to be deficient in the production of innate early proinflammatory signals (45). Stimulation of ex vivo alveolar macrophages from Wt mice with P. acnes resulted in a robust dose-dependent production of TNF-α and, consistent with other findings (46), this TNF-α production was completely abolished in Myd88−/− mice (Figure E5). This shows that, together with the lack of CXCL1, CXCL2, and IL-1α, Myd88−/− mice have widespread defects in P. acnes–driven proinflammatory signaling that is mediated at least in part by alveolar macrophages. This proinflammatory signaling defect in Myd88−/− mice results in defective neutrophil recruitment (Figure 4), implicating neutrophils in the development of granulomas. However, when Wt mice were treated with neutrophil-depleting antibody 1A8 and were administered P. acnes, there was no difference in the number or area of granulomas despite a significant reduction in neutrophils (Figure E3). These data may be explained by one of three scenarios: (1) neutrophils were not required for granuloma formation, (2) the low levels of neutrophils remaining after depletion were sufficient for granuloma formation, or (3) other cells were able to compensate for the neutrophil deficiency.
The enhanced granuloma phenotype we observed in Myd88−/− mice, together with defective early proinflammatory cytokine production, led us to the hypothesis that bacterial killing and clearance was key to granuloma formation. To test this hypothesis, we used Cybb−/− mice that are unable to produce ROS, a major component of both alveolar macrophage and neutrophil-mediated bacterial killing (28, 47). In our P. acnes–driven model of granuloma formation, Cybb−/− mice had dramatically increased granuloma formation (Figure 5A), with no defect in neutrophil recruitment (Figure E6), demonstrating that a lack of phagosome NADPH oxidase–mediated bacterial clearance increased granuloma formation. Interestingly, others have observed an increase in Nox2 (CybB) messenger RNA in tissue biopsy specimens from sarcoidosis-specific granulomas (48). These data are also consistent with the results of a recent unbiased genome-wide association study identifying Rab23, a component of bacterial killing mediated through phagolysosome fusion, as a risk factor for sarcoidosis (49). Furthermore, we observed an increase in the persistence of P. acnes in the lungs of Myd88−/− and Cybb−/− mice compared with Wt mice after bacterial administration, through both P. acnes colocalization in the granulomas (Figures 6A–6C) and P. acnes bacterial burden in the lung (Figure 6D). This defect in P. acnes clearance can be observed dramatically in P. acnes–stained lung sections from Cybb−/− mice, where macrophages are laden with P. acnes (Figure 6C). This additional bacterial burden in Myd88−/− mice most likely drives the enhanced inflammatory cell recruitment through MyD88-independent signaling, which results in the increased granuloma formation observed in these mice (Figure 3). These data are consistent with recent reports that a broad-spectrum antimycobacterial therapy can yield clinical improvement in patients with sarcoidosis (50) and suggest that the increased presence of bacteria contributes to the development of granuloma formation. Either way, it is clear that the consequence of defective bacterial clearance via production of ROS by CybB or defective proinflammatory signaling via MyD88 can promote granuloma development in mice administered P. acnes.
Conclusions
In summary, we have generated a mouse model of early granuloma formation through intratracheal administration of live P. acnes directly into the lungs of mice. These mice generate pulmonary granulomas with no experimental manipulation of P. acnes itself or of the immune system, such as adjuvants or multiple sensitizations. Furthermore, after P. acnes administration, these mice have increased numbers of T cells, tissue macrophages/monocytes, dendritic cells, and neutrophils in their lungs, which correspond to the composition of human sarcoid granulomas. In addition, soon after P. acnes administration, neutrophils and neutrophil-recruiting chemokines are produced in the lung in a MyD88-dependent manner. Mice deficient in innate proinflammatory signaling (Myd88−/−) and ROS-dependent bacterial killing (Cybb−/−) produce significantly more granulomas than do Wt mice, which is most likely because of an increased presence of bacterial antigen in their lungs. This study therefore provides a sorely needed animal model of early granuloma formation and lays a foundation for future investigations into the role of innate immunity in the development of granulomas.
Acknowledgments
Acknowledgments
The authors thank Benjamin Murdock (Department of Neurology, University of Michigan) and Eric White (Department of Internal Medicine, University of Michigan) for careful review and discussion of the manuscript. They also thank Ulysses Balis and Jason Hipp (Department of Pathology, University of Michigan) for the generous gift of SIVQ software as well as for SIVQ software training.
Footnotes
This work was supported by Public Health Service Grant T32 HL007517, National Center for Advancing Translational Science Grant UL1TR000433, a Mallinckrodt Research Fellowship (J.L.W), and National Institutes of Health Grant R01 DK61707 (G.N.).
Author Contributions: Conception and design: J.L.W. and G.N.; data collection: J.L.W., S.G.E., J.T.H., T.N.M., B.P.W., and Y.E.; analysis and interpretation: J.L.W. and G.N.; and drafting and review of the manuscript for important intellectual content: J.L.W., Y.E., and G.N.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0035OC on September 8, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 2.Abe C, Iwai K, Mikami R, Hosoda Y. Frequent isolation of Propionibacterium acnes from sarcoidosis lymph nodes. Zentralbl Bakteriol Mikrobiol Hyg A. 1984;256:541–547. doi: 10.1016/s0174-3031(84)80032-3. [DOI] [PubMed] [Google Scholar]
- 3.Negi M, Takemura T, Guzman J, Uchida K, Furukawa A, Suzuki Y, Iida T, Ishige I, Minami J, Yamada T, et al. Localization of Propionibacterium acnes in granulomas supports a possible etiologic link between sarcoidosis and the bacterium. Mod Pathol. 2012;25:1284–1297. doi: 10.1038/modpathol.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furusawa H, Suzuki Y, Miyazaki Y, Inase N, Eishi Y. Th1 and Th17 immune responses to viable Propionibacterium acnes in patients with sarcoidosis. Respir Investig. 2012;50:104–109. doi: 10.1016/j.resinv.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Belkaid Y, Segre JA. Dialogue between skin microbiota and immunity. Science. 2014;346:954–959. doi: 10.1126/science.1260144. [DOI] [PubMed] [Google Scholar]
- 6.Iio K, Iio TU, Okui Y, Ichikawa H, Tanimoto Y, Miyahara N, Kanehiro A, Tanimoto M, Nakata Y, Kataoka M. Experimental pulmonary granuloma mimicking sarcoidosis induced by Propionibacterium acnes in mice. Acta Med Okayama. 2010;64:75–83. doi: 10.18926/AMO/32852. [DOI] [PubMed] [Google Scholar]
- 7.Kishi J, Nishioka Y, Kuwahara T, Kakiuchi S, Azuma M, Aono Y, Makino H, Kinoshita K, Kishi M, Batmunkh R, et al. Blockade of Th1 chemokine receptors ameliorates pulmonary granulomatosis in mice. Eur Respir J. 2011;38:415–424. doi: 10.1183/09031936.00070610. [DOI] [PubMed] [Google Scholar]
- 8.Nishiwaki T, Yoneyama H, Eishi Y, Matsuo N, Tatsumi K, Kimura H, Kuriyama T, Matsushima K. Indigenous pulmonary Propionibacterium acnes primes the host in the development of sarcoid-like pulmonary granulomatosis in mice. Am J Pathol. 2004;165:631–639. doi: 10.1016/S0002-9440(10)63327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swaisgood CM, Oswald-Richter K, Moeller SD, Klemenc JM, Ruple LM, Farver CF, Drake JM, Culver DA, Drake WP. Development of a sarcoidosis murine lung granuloma model using Mycobacterium superoxide dismutase A peptide. Am J Respir Cell Mol Biol. 2011;44:166–174. doi: 10.1165/rcmb.2009-0350OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eishi Y. Etiologic aspect of sarcoidosis as an allergic endogenous infection caused by Propionibacterium acnes. BioMed Res Int. 2013;2013:935289. doi: 10.1155/2013/935289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan FH. The Elements of Immunology. Chennai, India: Pearson Education India; 2009. [Google Scholar]
- 12.McCaskill JG, Chason KD, Hua X, Neuringer IP, Ghio AJ, Funkhouser WK, Tilley SL. Pulmonary immune responses to Propionibacterium acnes in C57BL/6 and BALB/c mice. Am J Respir Cell Mol Biol. 2006;35:347–356. doi: 10.1165/rcmb.2005-0285OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Werner J, Escolero S, Hewitt J, Mak T, Yoshinobu E, Núñez G. Regulation of granuloma formation via Myd88 and Nox2 in a model of pulmonary sarcoidosis (MPF5P.741) J Immunol. 2015;194:137.4. [Google Scholar]
- 14.Werner JL, Escolero S, Zeng M, Mak T, Núñez G. Regulation of granuloma formation via Myd88 and Nox2 in a model of pulmonary sarcoidosis [abstract] Am J Respir Crit Care Med. 2014;189:A110. [Google Scholar]
- 15.Furukawa A, Uchida K, Ishige Y, Ishige I, Kobayashi I, Takemura T, Yokoyama T, Iwai K, Watanabe K, Shimizu S, et al. Characterization of Propionibacterium acnes isolates from sarcoid and non-sarcoid tissues with special reference to cell invasiveness, serotype, and trigger factor gene polymorphism. Microb Pathog. 2009;46:80–87. doi: 10.1016/j.micpath.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Werner JL, Gessner MA, Lilly LM, Nelson MP, Metz AE, Horn D, Dunaway CW, Deshane J, Chaplin DD, Weaver CT, et al. Neutrophils produce interleukin 17A (IL-17A) in a dectin-1- and IL-23-dependent manner during invasive fungal infection. Infect Immun. 2011;79:3966–3977. doi: 10.1128/IAI.05493-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hipp JD, Cheng JY, Toner M, Tompkins RG, Balis UJ. Spatially Invariant Vector Quantization: a pattern matching algorithm for multiple classes of image subject matter including pathology. J Pathol Inform. 2011;2:13. doi: 10.4103/2153-3539.77175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hipp J, Cheng J, Daignault S, Sica J, Dugan MC, Lucas D, Yagi Y, Hewitt S, Balis UJ. Automated area calculation of histopathologic features using SIVQ. Anal Cell Pathol (Amst) 2011;34:265–275. doi: 10.3233/ACP-2011-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prasse A, Georges CG, Biller H, Hamm H, Matthys H, Luttmann W, Virchow JC., Jr Th1 cytokine pattern in sarcoidosis is expressed by bronchoalveolar CD4+ and CD8+ T cells. Clin Exp Immunol. 2000;122:241–248. doi: 10.1046/j.1365-2249.2000.01365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moller DR. Cells and cytokines involved in the pathogenesis of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:24–31. [PubMed] [Google Scholar]
- 21.Veltkamp M, van Moorsel CHM, Rijkers GT, Ruven HJT, Grutters JC. Genetic variation in the Toll-like receptor gene cluster (TLR10-TLR1-TLR6) influences disease course in sarcoidosis. Tissue Antigens. 2012;79:25–32. doi: 10.1111/j.1399-0039.2011.01808.x. [DOI] [PubMed] [Google Scholar]
- 22.Pabst S, Baumgarten G, Stremmel A, Lennarz M, Knüfermann P, Gillissen A, Vetter H, Grohé C. Toll-like receptor (TLR) 4 polymorphisms are associated with a chronic course of sarcoidosis. Clin Exp Immunol. 2006;143:420–426. doi: 10.1111/j.1365-2249.2006.03008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 24.Rovai LE, Herschman HR, Smith JB. The murine neutrophil-chemoattractant chemokines LIX, KC, and MIP-2 have distinct induction kinetics, tissue distributions, and tissue-specific sensitivities to glucocorticoid regulation in endotoxemia. J Leukoc Biol. 1998;64:494–502. doi: 10.1002/jlb.64.4.494. [DOI] [PubMed] [Google Scholar]
- 25.Day RB, Link DC. Regulation of neutrophil trafficking from the bone marrow. Cell Mol Life Sci. 2012;69:1415–1423. doi: 10.1007/s00018-011-0870-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dinarello CA. The interleukin-1 family: 10 years of discovery. FASEB J. 1994;8:1314–1325. [PubMed] [Google Scholar]
- 27.Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 28.Piotrowski WJ, Kurmanowska Z, Antczak A, Marczak J, Majewski S, Górski P. Superoxide anion production by bronchoalveolar lavage cells in relation to cellular composition and lung function in sarcoidosis and chronic bronchitis. Pol Arch Med Wewn. 2009;119:777–784. [PubMed] [Google Scholar]
- 29.Kumar SN, Prasad TS, Narayan PA, Muruganandhan J. Granuloma with langhans giant cells: an overview. J Oral Maxillofac Pathol. 2013;17:420–423. doi: 10.4103/0973-029X.125211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Postlethwaite AE, Jackson BK, Beachey EH, Kang AH. Formation of multinucleated giant cells from human monocyte precursors. Mediation by a soluble protein from antigen-and mitogen-stimulated lymphocytes. J Exp Med. 1982;155:168–178. doi: 10.1084/jem.155.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ten Berge B, Kleinjan A, Muskens F, Hammad H, Hoogsteden HC, Hendriks RW, Lambrecht BN, Van den Blink B. Evidence for local dendritic cell activation in pulmonary sarcoidosis. Respir Res. 2012;13:33. doi: 10.1186/1465-9921-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agostini C, Facco M, Chilosi M, Semenzato G. Alveolar macrophage-T cell interactions during Th1-type sarcoid inflammation. Microsc Res Tech. 2001;53:278–287. doi: 10.1002/jemt.1094. [DOI] [PubMed] [Google Scholar]
- 33.Gabrilovich MI, Walrath J, van Lunteren J, Nethery D, Seifu M, Kern JA, Harding CV, Tuscano L, Lee H, Williams SD, et al. Disordered Toll-like receptor 2 responses in the pathogenesis of pulmonary sarcoidosis. Clin Exp Immunol. 2013;173:512–522. doi: 10.1111/cei.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asukata Y, Ota M, Meguro A, Katsuyama Y, Ishihara M, Namba K, Kitaichi N, Morimoto S, Kaburaki T, Ando Y, et al. Lack of association between Toll-like receptor 4 gene polymorphisms and sarcoidosis-related uveitis in Japan. Mol Vis. 2009;15:2673–2682. [PMC free article] [PubMed] [Google Scholar]
- 35.Schürmann M, Kwiatkowski R, Albrecht M, Fischer A, Hampe J, Müller-Quernheim J, Schwinger E, Schreiber S. Study of Toll-like receptor gene loci in sarcoidosis. Clin Exp Immunol. 2008;152:423–431. doi: 10.1111/j.1365-2249.2008.03621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gazouli M, Koundourakis A, Ikonomopoulos J, Gialafos EJ, Rapti A, Gorgoulis VG, Kittas C. CARD15/NOD2, CD14, and Toll-like receptor 4 gene polymorphisms in Greek patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2006;23:23–29. [PubMed] [Google Scholar]
- 37.Daniil Z, Mollaki V, Malli F, Koutsokera A, Antoniou KM, Rodopoulou P, Gourgoulianis K, Zintzaras E, Vassilopoulos G. Polymorphisms and haplotypes in MyD88 are associated with the development of sarcoidosis: a candidate-gene association study. Mol Biol Rep. 2013;40:4281–4286. doi: 10.1007/s11033-013-2513-7. [DOI] [PubMed] [Google Scholar]
- 38.Rider P, Carmi Y, Guttman O, Braiman A, Cohen I, Voronov E, White MR, Dinarello CA, Apte RN. IL-1α and IL-1β recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol. 2011;187:4835–4843. doi: 10.4049/jimmunol.1102048. [DOI] [PubMed] [Google Scholar]
- 39.Loke WS, Herbert C, Thomas PS. Sarcoidosis: Immunopathogenesis and immunological markers. Int J Chronic Dis. 2013;2013:928601. doi: 10.1155/2013/928601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.du Bois RM, Goh N, McGrath D, Cullinan P. Is there a role for microorganisms in the pathogenesis of sarcoidosis? J Intern Med. 2003;253:4–17. doi: 10.1046/j.1365-2796.2003.01073.x. [DOI] [PubMed] [Google Scholar]
- 41.Broos CE, van Nimwegen M, Hoogsteden HC, Hendriks RW, Kool M, van den Blink B. Granuloma formation in pulmonary sarcoidosis. Front Immunol. 2013;4:437. doi: 10.3389/fimmu.2013.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tutor-Ureta P, Citores MJ, Castejón R, Mellor-Pita S, Yebra-Bango M, Romero Y, Vargas JA. Prognostic value of neutrophils and NK cells in bronchoalveolar lavage of sarcoidosis. Cytometry B Clin Cytom. 2006;70:416–422. doi: 10.1002/cyto.b.20120. [DOI] [PubMed] [Google Scholar]
- 43.Ziegenhagen MW, Rothe ME, Schlaak M, Müller-Quernheim J. Bronchoalveolar and serological parameters reflecting the severity of sarcoidosis. Eur Respir J. 2003;21:407–413. doi: 10.1183/09031936.03.00010403. [DOI] [PubMed] [Google Scholar]
- 44.Aberdein JD, Cole J, Bewley MA, Marriott HM, Dockrell DH. Alveolar macrophages in pulmonary host defence the unrecognized role of apoptosis as a mechanism of intracellular bacterial killing. Clin Exp Immunol. 2013;174:193–202. doi: 10.1111/cei.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coady A, Sil A. MyD88-dependent signaling drives host survival and early cytokine production during Histoplasma capsulatum infection. Infect Immun. 2015;83:1265–1275. doi: 10.1128/IAI.02619-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhoj VG, Sun Q, Bhoj EJ, Somers C, Chen X, Torres JP, Mejias A, Gomez AM, Jafri H, Ramilo O, et al. MAVS and MyD88 are essential for innate immunity but not cytotoxic T lymphocyte response against respiratory syncytial virus. Proc Natl Acad Sci USA. 2008;105:14046–14051. doi: 10.1073/pnas.0804717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Christophi GP, Caza T, Curtiss C, Gumber D, Massa PT, Landas SK. Gene expression profiles in granuloma tissue reveal novel diagnostic markers in sarcoidosis. Exp Mol Pathol. 2014;96:393–399. doi: 10.1016/j.yexmp.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hofmann S, Fischer A, Till A, Müller-Quernheim J, Häsler R, Franke A, Gäde KI, Schaarschmidt H, Rosenstiel P, Nebel A, et al. GenPhenReSa Consortium. A genome-wide association study reveals evidence of association with sarcoidosis at 6p12.1. Eur Respir J. 2011;38:1127–1135. doi: 10.1183/09031936.00001711. [DOI] [PubMed] [Google Scholar]
- 50.Drake WP, Oswald-Richter K, Richmond BW, Isom J, Burke VE, Algood H, Braun N, Taylor T, Pandit KV, Aboud C, et al. Oral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled study. JAMA Dermatol. 2013;149:1040–1049. doi: 10.1001/jamadermatol.2013.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]