Abstract
The nonmuscle (nm) myosin light-chain kinase isoform (MLCK), encoded by the MYLK gene, is a vital participant in regulating vascular barrier responses to mechanical and inflammatory stimuli. We determined that MYLK is alternatively spliced, yielding functionally distinct nmMLCK splice variants including nmMLCK2, a splice variant highly expressed in vascular endothelial cells (EC) and associated with reduced EC barrier integrity. We demonstrated previously that the nmMLCK2 variant lacks exon 11, which encodes a key regulatory region containing two differentially phosphorylated tyrosine residues (Y464 and Y471) that influence vascular barrier function during inflammation. In this study, we used minigene constructs and RT-PCR to interrogate biophysical factors (mechanical stress) and genetic variants (MYLK single-nucleotide polymorphisms [SNPs]) that are potentially involved in regulating MYLK alternative splicing and nmMLCK2 generation. Human lung EC exposed to pathologic mechanical stress (18% cyclic stretch) produced increased nmMLCK2 expression relative to levels of nmMLCK1 with alternative splicing significantly influenced by MYLK SNPs rs77323602 and rs147245669. In silico analyses predicted that these variants would alter exon 11 donor and acceptor sites for alternative splicing, computational predictions that were confirmed by minigene studies. The introduction of rs77323602 favored wild-type nmMLCK expression, whereas rs147245669 favored alternative splicing and deletion of exon 11, yielding increased nmMLCK2 expression. Finally, lymphoblastoid cell lines selectively harboring these MYLK SNPs (rs77323602 and rs147245669) directly validated SNP-specific effects on MYLK alternative splicing and nmMLCK2 generation. Together, these studies demonstrate that mechanical stress and MYLK SNPs regulate MYLK alternative splicing and generation of a splice variant, nmMLCK2, that contributes to the severity of inflammatory injury.
Keywords: ventilator-induced lung injury, cyclic stretch, splicing, single nucleotide polymorphism, bioinformatics
Clinical Relevance
Ventilator-induced lung injury is induced in critically ill patients undergoing high-tidal mechanical ventilation. The myosin light-chain kinase, encoded by the MYLK gene, is a critical regulator of lung vascular endothelial barrier disruption and recovery during ventilator-induced lung injury. This study demonstrates that single-nucleotide polymorphisms regulate MYLK gene splicing, thereby influencing endothelial barrier integrity.
Acute respiratory distress syndrome (ARDS) is a critical care illness characterized by lung vascular leakage of fluid, cells, and protein into the alveolar space, a perturbation evoked by circulating inflammatory mediators such as bacterial endotoxin, tumor necrosis factor-α (TNF-α), and IL-1 (1). The lung vascular barrier is also compromised by excessive mechanical stress as a consequence of exposure to high shear stress (2), or by exposure to cyclic stretch (CS)–induced mechanical stress (3) produced by high tidal volume mechanical ventilation, a syndrome termed ventilator-induced lung injury (4). The lung endothelial cell (EC) cytoskeleton, a dynamic network of proteins involved in cell shape maintenance and cell–cell interactions, is highly responsive to changes in mechanical stress and is centrally involved in lung vascular barrier regulation and maintenance of vascular integrity (2, 5–8). The balance between actomyosin cytoskeleton contractile forces and cell–cell and cell–matrix interactions is dependent on the activities of a central bioregulatory cytoskeletal effector protein, myosin light-chain kinase (MLCK, gene code MYLK) (6) that phosphorylates myosin light chains on Ser18 and Thr19 to produce localized contraction. We have shown previously that lung vascular endothelium express the nonmuscle (nm) isoform of MLCK (nmMLCK) and that nmMLCK enzymatic activity is increased by permeability-enhancing agents such as thrombin (7) and TNF-α (9), resulting in EC stress fiber formation, cellular contraction, paracellular gap formation, and loss of vascular integrity (10). As a result, nmMLCK has proved to be an attractive therapeutic target in preclinical models of lung inflammation and injury produced by mechanical ventilation (8, 11), endotoxin (8, 12), and allergen challenge (13). For example, mice overexpressing nmMLCK in the endothelium exhibit markedly enhanced neutrophil diapedesis and vascular permeability in response to inflammatory stimuli, compared with wild-type mice (13). The complexity of nmMLCK involvement in vascular barrier regulation is highlighted by its dual role as a critical participant in barrier-restoration responses elicited by barrier-promoting agonists such as sphingosine-1-phosphate (S1P) (14) and hepatocyte growth factor (15). EC activation in response to S1P and hepatocyte growth factor drives nmMLCK translocation to the cell periphery and into lamellipodia to accelerate paracellular gap closure during the recovery phase of acute lung injury (14, 15). Consistent with MYLK as a candidate gene in inflammatory lung processes, we have identified coding and promoter MYLK polymorphisms that confer increased risk of sepsis, ARDS (16–19), and asthma (19–21) and that influence the severity of disease, particularly in patients of African descent (16–21).We have determined that these single nucleotide polymorphisms (SNPs) functionally alter MYLK promoter activity, alter secondary messenger RNA (mRNA) structure, and influence nmMLCK translocation to lamellipodia to restore vascular barrier integrity (21–23).
In contrast to the smooth-muscle MLCK isoform (1,147 amino acids; 135 kD), the nmMLCK isoform (1,914 amino acids; 210 kD), exhibits a unique N-terminus that contains sites for post-translational modification that regulate nmMLCK interactions with actin (24) and other actin-binding proteins (25) and alters Ca++ requirements for optimal enzymatic activity (26–28). Another distinction between smooth-muscle MLCK and nmMLCK is that MYLK undergoes alternative splicing to yield only nmMLCK variants, including nmMLCK2, the major MYLK alternatively spliced variant expressed in human lung endothelium (27). The nmMLCK transcript contains 34 exons, including the regulatory exon 11, which encodes two key tyrosine residues (Y464 and Y471). Exon 11 is deleted in the alternatively spliced nmMLCK2 variant, thereby altering regulation via post-translational modifications such as phosphorylation catalyzed by pp60 src (10) and c-Abl kinases (29). Consistent with the involvement of Y464 and Y471 phosphorylation (residing in exon 11) in regulating nmMLCK trafficking to subcellular locations to promote paracellular gap closure (10, 22, 25, 29), mice overexpressing nmMLCK2 (lacking exon 11) exhibit increased susceptibility to lung injury (30).
Given these unique contributions of wild-type nmMLCK and the alternatively spliced nmMLCK2 variant to the regulation of inflammatory lung vascular permeability, we analyzed the participation of CS-mediated mechanical stress and SNPs in the regulation of MYLK alternative splicing. Using minigene constructs and RT-PCR to estimate the relative levels of nmMLCK1/nmMLCK2, we now demonstrate that excessive mechanical stress promotes MYLK alternative splicing and nmMLCK2 generation in human lung EC. In silico approaches led to the identification of MYLK SNPs that directly influence splicing patterns, results validated in lymphoblastoid cell lines harboring these SNPs. Together, these results indicate that MYLK alternative splicing is a highly regulated process with important ramifications in defining the magnitude of inflammatory injury.
Materials and Methods
CS and RT-PCR Analysis of MYLK in Human EC
CS experiments were performed using the FX-5000 Flexcell Tension Plus System (Flexcell International, McKeesport, PA) equipped with a 25-mm loading station, as we have described previously (31). EC were plated in BioFlex 6-well culture plates coated with collagen-1 (Flexcell International) in complete medium containing 10% fetal bovine serum. Plates were loaded onto the Flexcell system and exposed to 5% or 18% high-magnitude CS for 8 hours. Control plates were placed in the same incubator during the stretch period. RNA was isolated using Trizol according to the manufacturer’s instructions. First-strand complementary DNA synthesis was performed using oligo (dT) primers and the Protoscript AMV First-Strand cDNA Synthesis System (New England Biolabs, Ipswich, MA). Semiquantitative RT-PCR was performed using primers MLCK-for GCCAGGAGGTCAAGGAAAAT and MLCK-rev CCAAGGCATTCTCAGCTAGG. Human TATA binding protein (TBP) was used as a control for normalization of gene expression using primers (TBP-for TGACCCAGGGTGCCATGA and TBP-rev TGAATAGGCTGTGGGGTCAG). Band intensities were quantitated using Imagelab (Bio-Rad, Hercules, CA) and were normalized to wild-type nmMLCK1 values under static conditions.
Analysis of SNPs
Analysis of the SNP variants was performed using Splicing-based Analysis of Variants (SPANR) (32) and SpliceScan (33).
Minigene Analyses of MYLK Splice Variants
A portion of the MYLK comprising exon 10 to exon 12 was cloned into pCMV6-Entry (Origene, Rockville, MD). RNA was isolated from transfected HEK-293 cells and controls using Trizol, purified using the Purelink Mini RNA kit (Life Technologies, Carlsbad, CA) and was treated with Turbo DNase free (Life Technologies) to eliminate any contamination from genomic DNA and transfected DNA. Five hundred nanograms of RNA was reverse transcribed using the primer CMV6R (2 pmol per reaction of 20 μl), dNTPs, and 10U Transcriptor (Roche, Mannheim, Germany). To avoid detection of the endogenous MYLK gene, CMV6R was designed to be specific to pCMV6-Entry and carried no significant homology to the MYLK gene. Conditions were set at 55°C for 30 minutes, followed by 85°C for 5 minutes to inactivate the reverse transcriptase. PCR was performed using MLCK exon10F (GGCCAGAGGGATTCAGCATT) and MLCK exon12R (ACCTCCATCACGGCAAGC) located on exon 10 and exon 12, respectively, using GoTaq DNA polymerase (Promega, Madison, WI) to yield products with expected sizes of 317 and 110 bp, corresponding to nmMLCK1 and nmMLCK2. PCR products were visualized using a Gel doc, band intensities were quantified using Imagelab (Bio-Rad), and ratios of exon inclusion (nmMLCK1/nmMLCK1 + nmMLCK2) and exon skipping (nmMLCK2/nmMLCK1 + nmMLCK2) were calculated.
RT-PCR Analysis of Lymphoblastoid Cell Lines
A total of 1 μg of RNA isolated from lymphoblast cell lines was reverse transcribed using anchored Oligo-dt (20) (Integrated DNA Technologies, Coralville, IA) and Superscript III (Life Technologies) in a 20-μl reaction at 50°C for 60 minutes, followed by inactivation of the reverse transcriptase at 70°C for 15 minutes. PCR and quantitation of products were performed as described previously.
Statistical Analysis
Data were analyzed using Student’s t test, and the statistical threshold was set at P < 0.05.
Results
CS-Induced Alterations in MYLK Alternative Splicing
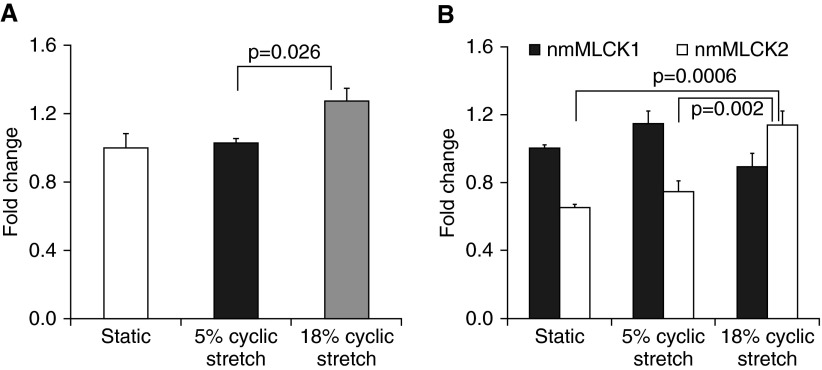
To analyze the effect of CS-mediated mechanical stress on MYLK splicing, we subjected human lung EC to 5% CS, mirroring the physiological levels of mechanical stress that correspond to normal breathing, and to 18% CS, reflecting the pathological levels of mechanical stress that mimic high tidal volume ventilation. EC exposed to 18% CS for 8 hours exhibited higher levels of total nmMLCK mRNA (RT-PCR) when compared with EC exposed to 5% CS or EC exposed to static conditions only (Figure 1A). We next determined if higher levels of total nmMLCK reflected alterations in the relative balance of wild-type (nmMLCK1) and nmMLCK2 levels in response to mechanical stress. When EC nmMLCK expression levels were normalized to static conditions, the quantitation of mRNA levels of wild-type nmMLCK1 and alternatively spliced nmMLCK2 revealed selective increases in nmMLCK2 mRNA levels in response to exposure to 18% CS (Figure 1B), whereas nmMLCK1 and nmMLCK2 levels were unaltered by exposure to 5% CS. These studies indicate that excessive mechanical stress, but not physiologic mechanical stress, produces selective increases in the expression of the vascular barrier-disrupting nmMLCK splice variant, nmMLCK2.
Figure 1.
Effects of cyclic stretch (CS) on expression of nonmuscle myosin light-chain kinase (nmMLCK) and splice variants. (A) Analysis of total nmMLCK mRNA. Levels of total nmMLCK were increased in response to 18% CS. Cells were exposed to CS for 8 hours, and control cells under static conditions were maintained in the same incubator. (B) Levels of nmMLCK1 versus nmMLCK2 in endothelial cells. Levels were normalized to nmMLCK1 under static conditions and demonstrate increased nmMLCK2 expression under 18% CS. Data are presented as mean ± SE of three independent experiments.
In Silico Analysis to Identify MYLK SNPs That Influence Alternative Splicing
As noted above, we have previously identified MYLK SNPs that increase the risk and severity of inflammatory lung diseases such as asthma, sepsis, and ARDS (13, 16–21). We next used in silico bioinformatic approaches to identify 19 SNPs within exon 11 and 10 SNPs within exon-flanking nucleotide sequences that potentially regulate MYLK alternative splicing. These SNPs were then analyzed for their potential influence on alternative splicing, using SPANR (32) (see Table E1 in the online supplement), with two SNPs, rs147245669 and rs77323602, selected as top-ranking SNPs on the basis of dPSI of −58.9 and 5.44, respectively, a metric that indicates the percentage of transcripts with the exon spliced in (32). Complementing this SPANR analysis, we also used SpliceScan software (33) and demonstrated that the strong donor motif AAGGTGAGT (strength of 0.92) in rs77323602 would favor inclusion of exon 11 and therefore would be predicted to yield greater levels of wild-type nmMLCK1 (Table 1). In contrast, the donor motif AAAGTGAAT in rs147245669 was classified as a weak donor site, thereby promoting exon 11 skipping, and would conversely be predicted to favor greater nmMLCK2 expression. In summary, bioinformatics analyses indicated that the MYLK SNP rs77323602 would favor wild-type nmMLCK1 expression and that rs147245669 would contribute to nmMLCK2 expression.
Table 1.
Summary of Bioinformatics Analyses of Top-Ranking SNPs on the Human MYLK Gene
| Chromosome | Position | SNP ID | Reference Allele | Alternate Allele | Frequency | dPSI | SpliceScan Change | Predicted Outcome |
|---|---|---|---|---|---|---|---|---|
| 3 | 123451738 | rs147245669 | C | T | 0.0005 | −58.9 | Donor = 0.01 Exon 11 score = 0 |
Increased MLCK 2; more skipping |
| 3 | 123451743 | rs77323602 | T | C | 0.01 | 5.44 | Donor = 0.92 Exon 11 score = 0.15 |
Increased MLCK1; more inclusion |
Definition of abbreviations: C, cytosine; dPSI, change in percent of transcripts with the exon spliced in; MLCK, myosin light-chain kinase; SNP, single-nucleotide polymorphism; T, thymine.
Verification of Bioinformatic Analyses Using a MYLK Minigene
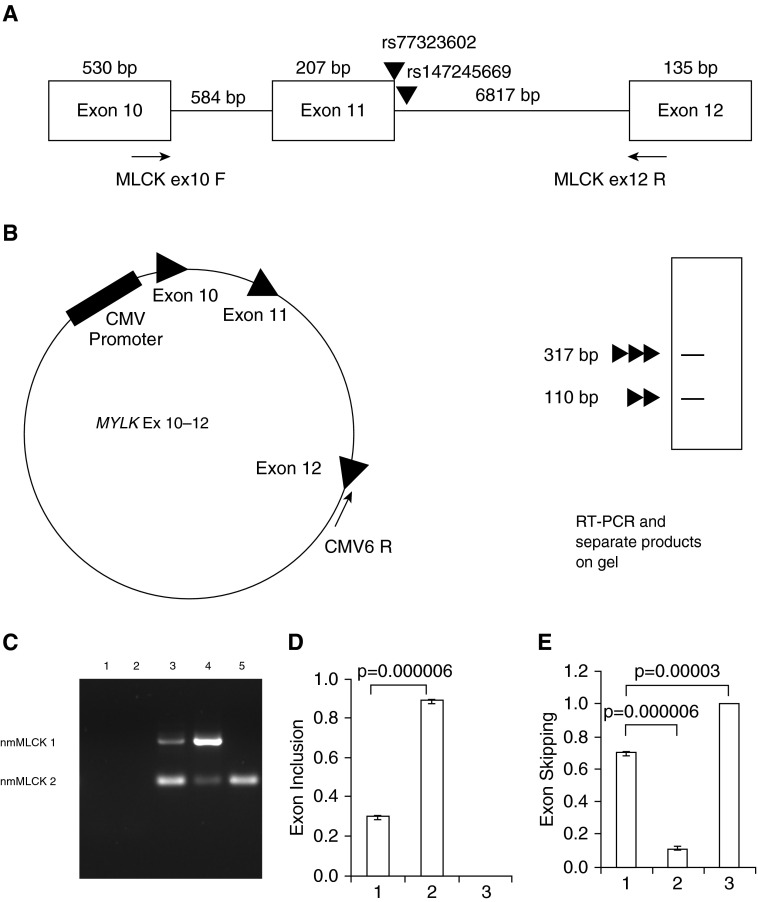
To test the validity of these in silico predictions, a minigene system was developed consisting of a MYLK exon 10–12 plasmid containing MYLK exons 10–12 and the introns in between 10–12 that were cloned into the vector pCMV6-Entry (Origene) (Figures 2A and 2B). The MYLK Ex 10–12 plasmid was transfected into HEK293 cells and RNA was extracted and reverse transcribed using a primer specific to the pCMV6-Entry vector backbone (Figure 2B), yielding two major PCR products corresponding to the expected size of wild-type nmMLCK1 (exons 10, 11, and 12) and nmMLCK2 (exons 10 and 12 only) (Figure 2C, lane 3). PCR product bands were not present in untransfected cells or in cells transfected with the vector pCMV6-Entry, indicating the specificity of the assay (Figure 2C, lanes 1 and 2). We next introduced MYLKs SNPs into the MYLK Ex 10–12 to generate minigene-rs77323602 and minigene-rs147245669. Transfection of minigene-rs77323602 into HEK cells resulted in significantly greater nmMLCK 1 product and less nmMLCK2 product, compared with the wild-type minigene (Figure 2C, lane 4). Consistent with in silico predictions, cells transfected with minigene-rs147245669 (Figure 2C, lane 5) exhibited nmMLCK2 expression only, results confirmed by statistical analysis of multiple PCR experiments (Figures 2D and 2E).
Figure 2.
Schematic of exons 10–12 of the MYLK gene and minigene analysis. (A) Genomic organization of exons 10–12 of the MYLK gene and two single-nucleotide polymorphisms (SNPs) under investigation, rs77323602 on exon 11 and rs147245669 on intron 11–12. Primers MLCK ex10 F and MLCK ex12 R were used to distinguish the two splice variants nmMLCK1 and nmMLCK2 in polymerase chain reaction (PCR) reactions. (B) Entire region corresponding to exons 10–12 was cloned into pCMV6-Entry to generate the minigene MLCK Ex10–12. Shown also is the primer CMV6 R, used for generation of minigene-specific reverse transcript (RT) products. (C) Representative gel showing RT-PCR analysis of the minigene; 293 cells were transfected with constructs as indicated, and RT-PCR was performed. Lane 1: untransfected; lane 2: vector transfected cells; lane 3: minigene; lane 4: minigene-rs77323602; lane 5: minigene-rs147245669. SNPs on the minigene altered splicing of nmMYLK (compare lanes 3, 4, and 5). The data were obtained from three independent experiments and are shown as mean ± SE. (D and E) Densitometry analysis of the RT-PCR products shown in C showing levels of exon inclusion (B) and exon skipping (C). Bars correspond to (1) minigene, (2) minigene-rs77323602, and (3) minigene-rs147245669. Exon inclusion was defined by nmMLCK1/(nmMLCK1 + nmMLCK2), and exon skipping by nmMLCK2/(nmMLCK1 + nmMLCK2) band intensities. bp, base pair; CMV, cytomegalovirus.
Analysis of MYLK Splicing and nmMLCK2 Generation in Human Lymphoblastoid Cell Lines
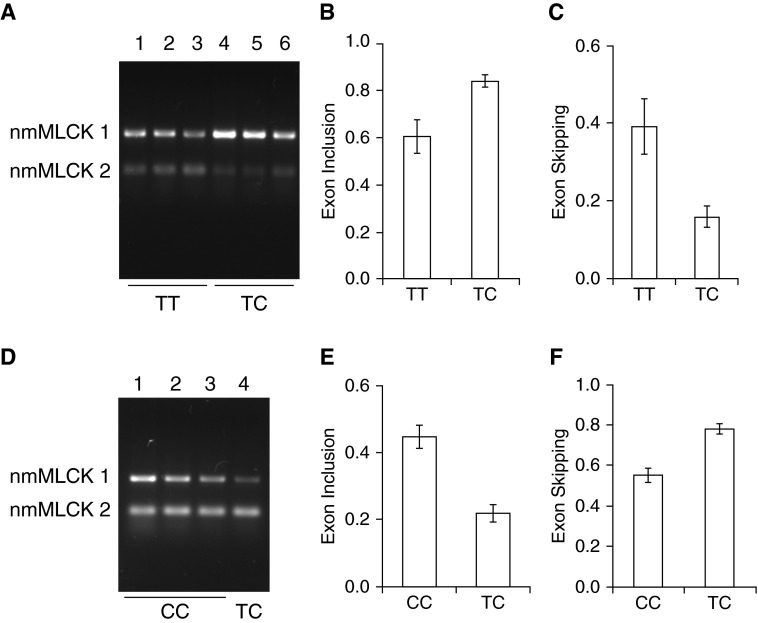
Further studies of the effects of the SNPs were performed in human lymphoblastoid cells because lymphoblast cell lines incorporating these SNPs are readily available and it was technically challenging to engineer these SNPs in primary human EC. To further validate the influence of MYLK SNPs rs147245669 and rs77323602 on MYLK alternative splicing, we surveyed the 1,000 genome database and identified individuals harboring these SNPs (Table 2). We next obtained specific lymphoblastoid cell lines (Coriell Institute, Camden, NJ) that were generated from three individuals (GM 20334, GM 20322, and GM19900) with a TT genotype for SNP rs77323602, and lymphoblastoid cell lines from three individuals (GM20332, GM19908, and GM19916) with a TC genotype for SNP rs77323602. RNA was isolated next, and semiquantitative RT-PCR was performed to determine exon inclusion and exon exclusion ratios and nmMLCK1 and 2 levels (Figure 3A). Densitometric quantification confirmed that individuals with a TC genotype exhibited higher exon inclusion ratios, implying that nmMLCK1 expression was favored (Figure 3B), reflecting reduced exon skipping (Figure 3C).
Table 2.
List of Lymphoblast Cell Lines Used in this Study and Genotypes at Two SNP (rs147245669 and rs77323602) Loci
| Cell Line | Population | Genotype at rs147245669 | Genotype at rs77323602 |
|---|---|---|---|
| GM 19900 | ASW | C/C | T/T |
| GM 19908 | ASW | C/C | T/C |
| GM 19916 | ASW | C/C | T/C |
| GM 20322 | ASW | C/C | T/T |
| GM 20332 | ASW | C/C | T/C |
| GM 20334 | ASW | C/C | T/T |
| GM 20756 | TSI | C/C | T/T |
| GM 20757 | TSI | T/C | T/T |
| GM 20760 | TSI | C/C | T/T |
| GM 20761 | TSI | C/C | T/T |
Definition of abbreviations: ASW, African ancestry in Southwestern United States; C/C, cytosine/cytosine; GM, Genome (prefix in the Coriell Institute catalog); SNP, single-nucleotide polymorphism; T/C, thymine/cytosine; TSI, Toscani in Italia; T/T, thymine/thymine.
Figure 3.
Effect of rs77323602 and rs147245669 SNPs on MYLK splicing in lymphoblastoid cells. (A) Analysis of SNP rs77323602. Representative gel showing analysis of nmMLCK1 and nmMLCK2 splice variants in lymphoblastoid cells showing that nmMLCK1 levels were increased in lymphoblastoid lines carrying the “TC” genotype. Lymphoblastoid lines GM20334, GM20322, and GM19900 in lanes 1, 2, and 3, respectively, represent the parental alleles (TT) and GM20332, GM19908, and GM19916 in lanes 4, 5, and 6, respectively, represent the “TC” variant. (B and C) Densitometry of RT-PCR analysis shown in A indicating exon inclusion (B) and exon skipping (C). (D) Analysis of SNP rs147245669. Representative gel showing analysis of nmMLCK1 and nmMLCK2 splice variants in lymphoma cells showing that nmMLCK2 levels were increased in the individual carrying the SNP rs147245669. Lymphoma lines GM20760, GM20761, and GM20757 represent the “CC” genotype in lanes 1, 2, and 3, respectively, whereas GM20757 represents the “TC” variant in lane 4. Levels of nmMLCK2 were increased as a result of the SNP rs147245669. (E and F) Densitometry of RT-PCR analysis showing exon inclusion (E) and exon skipping (F). Exon inclusion was defined by nmMLCK1/(nmMLCK1 + nmMLCK2), and exon skipping by nmMLCK2/(nmMLCK1 + nmMLCK2) band intensities. All data were obtained from three independent experiments and are presented as mean ± SE. C/C, cytosine/cytosine; GM, Genome (prefix in the Coriell Institute catalog); T/C, thymine/cytosine; T/T thymine/thymine.
To investigate the influence of rs147245669, lymphoblastoid cell lines were obtained from three individuals (GM20760, GM 20761, and GM20756) carrying a CC genotype and from a single individual with a TC genotype (GM 20757, harboring SNP rs147245669). RT-PCR analysis showed that GM 20757 (with the TC genotype) exhibited reduced nmMLCK1 levels (Figure 3D, lane 4), as indicated by a decrease in exon 11 inclusion (Figure 3E) and an increase in exon skipping (Figure 3F). These findings are again consistent with in silico predictions and generated minigene data that SNP rs147245669 favors an increase in exon skipping and nmMLCK2 generation.
Discussion
In the current study, we report that exposure to pathological mechanical stress (lung injury-producing mechanical stress) as well as MYLK genetic variants both significantly influence the magnitude of alternative splicing to yield greater levels of nmMLCK2, an alternatively spliced nmMLCK variant with reduced enzymatic regulation and cellular spatial regulation caused by loss of key post-translational modifications (Y464 and Y471 phosphorylation) residing within the skipped exon. Consequently, altering the ratio of the two isoforms using small interfering RNA against nmMLCK2 or against both isoforms (nmMLCK1/2) causes significant changes in transendothelial electrical resistance (TER) responses on thrombin stimulation (Figures E1A and E2A) and the existence of dose-dependent effects, because nmMLCK1/2 knockdown cells show further reduction (Figures E2A and E2B) in resistance, in comparison to nmMLCK2 knockdown cells (Figures E1A and E1B) and loss of TER responses over baseline. We have reported previously that the barrier agonist S1P protected cells from the thrombin-induced resistance decrease and caused an increase in TER over baseline (34), supporting distinct roles of the two isoforms in regulating the vascular barrier in response to thrombin. These results provide for a fine-tuned post-transcriptional control mechanism of nmMLCK participation in lung injury and repair that is influenced by genetic variation and environmental perturbation in the form of excessive mechanical stress.
In severe acute lung injury such as ARDS, increased vascular hyperpermeability produces persistent life-threatening alveolar flooding contributing to the observed mortality rates of 30–40% (1, 35, 36). This lung endothelial hyperpermeability reflects loss of EC barrier integrity caused by actin cytoskeletal remodeling and an imbalance between barrier-disruptive stress fibers and protective peripheral cortical actin (6), processes tightly regulated by nmMLCK activity and spatial-specific myosin light-chain phosphorylation (Ser18 and Thr19). We have shown that mice deficient in nmMLCK, the primary isoform present in human endothelium (27), are protected against the injurious effects of excessive mechanical stress produced in vitro or by mechanical ventilation in preclinical models of ventilator-induced lung injury (8) and have provided substantial insights into the mechanisms underlying nmMLCK-mediated barrier regulation (7, 14, 25, 26, 29–31, 37–44). The major nmMLCK alternatively spliced variant, nmMLCK2, is highly expressed in lung endothelium (27) and is distinguished from the wild-type nmMLCK1 by the absence of a 69 amino acid stretch encoded by exon 11 that is contained in nmMLCK1. Exon 11 in wild-type nmMLCK1 includes two key tyrosines (Tyr464 and Tyr471) that are phosphorylation sites for c-Abl and p60 src kinases (6, 10) and are critical to lung vascular barrier regulation (10, 29). For example, cAbl is activated by barrier-protective agents such as sphingosine1-phosphate, resulting in nmMLCK1 binding to the actin-binding protein, cortactin, and thereby promoting nmMLCK1 translocation to the lamellipodia (25, 45), paracellular gap resolution, and reduced vascular leak (29). The selective deletion of exon 11 is highly proinflammatory because this precludes the regulatory effects present on exon 11 that influence nmMLCK’s participation in lung vascular barrier restoration. The alternatively spliced nmMLCK variant, nmMLCK2, promotes vascular hyperpermeability (30) and retards the recovery of vascular barrier integrity (46). Significantly, mice overexpressing nmMLCK2 show increased lung injury in response to LPS and mechanical ventilation (30). These data suggest that nmMLCK2 is uniquely generated by alternative splicing and is functionally distinct from wild-type nmMLCK1.
MYLK, the gene encoding both wild-type nmMLCK1 and the variant, nmMLCK2, has been characterized as a candidate gene whose SNPs influence the susceptibility to and the severity of inflammatory lung disorders such as sepsis- and trauma-induced ARDS and asthma (13, 16–21). We confirmed the role of MYLK SNPs in alternative splicing and generation of mMLCK2 by designing a minigene comprising exons 10–12 of the MYLK gene. Minigenes have been used to analyze splicing and exhibit the advantage of engineering mutations and analyzing the effects of other splicing factors by cotransfection (47). Using our minigene system, we validated the in silico predictions for SNPs rs77323602 and rs147245669 in alternative splicing and observed greater nmMLCK1 expression in the presence of the SNP rs77323602 and only nmMLCK2 in the presence of rs147245669. These results were in strong synchrony with the Bayesian splice sensor (33) and SPANR (identification of cis splicing elements) (32) software predictions of greater exon skipping in the presence of rs147245669 and reduced exon skipping by rs77323602. In addition, these predictions and results with the minigene assays were strongly validated in our studies using lymphoblastoid cells harboring these SNPs. The absence of wild-type nmMLCK1 expression in the presence of the SNP rs147245669 suggests that either the site is essential for recognition of the splicing signals by the spliceosome or that other regions besides exons 10–12 present on the minigene are critical for alternative splicing.
It has been estimated that 22–25% of human disease mutations will cause alterations in protein splicing patterns (48). We have previously identified race-specific SNPs in MYLK that affect susceptibility to ARDS and asthma (13, 16–21). For example, SNPs P21H, S147P, and V261A increase the susceptibility of African American patients to sepsis- and trauma-induced ARDS and asthma (16, 20). Other MYLK SNPs were shown to affect transcription factor binding (rs820336 can regulate binding of FOXN1) and MYLK expression (patients with asthma with rs936170 show reduced expression of MYLK) (17). We now demonstrate a role for rs77323602 and rs147245669 in the regulation of MYLK splicing. The location of these SNPs at the splicing boundary suggests that these SNPs affect the binding of splicing machinery components. However, additional SNP effects cannot be ruled out because SNP rs77323602 (on exon 11) may alter mRNA stability, as we have shown for SNP rs9840993 (23), and increased MYLK mRNA half-life, leading to better translation efficiency. Consistent with our data, the presence of an SNP at the splice donor site in the MER tyrosine kinase gene causes exon skipping, resulting in a frame shift and premature termination, affecting the MER tyrosine kinase activity in retinal pigment epithelial cells (49).
Although the low frequency of the two SNPs under investigation (rs147245669 and rs77323602 with frequencies of 0.0005 and 0.01, respectively) has precluded analyses of the clinical significance in our ARDS cohort, the knowledge of the functional contributions of these SNPs to MYLK splicing is invaluable. Functional validation of genetic variants provides future possibilities for personalized medicine, especially for diseases with high mortality rates and those lacking specific cures, such as ARDS. In addition, rare SNPs, including rs77323602 (MAF, 0.07 in YRI and absent in EUR) (data from single nucleotide polymorphism database), exist in various ethnic groups with differential frequency, and functional interrogation of these SNPs can provide the foundation for understanding the genetic contribution to health disparities and disease heterogeneity among various ethnic groups.
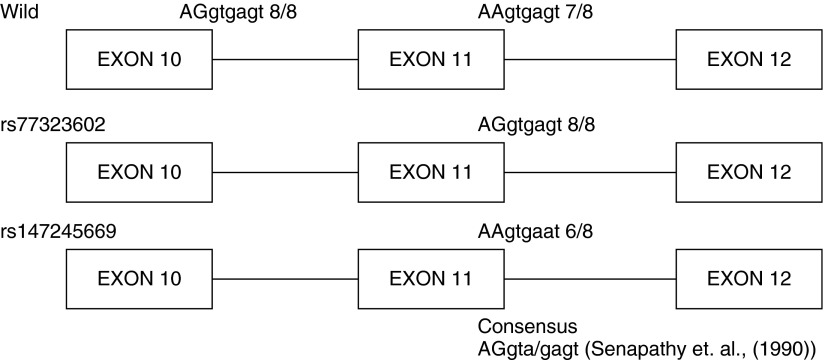
The simplistic model of the minigene provides an attractive tool to analyze alternative splicing. A drawback of the minigene system is the absence of other elements, including other introns and exons that potentially regulate splicing. Therefore, to validate our findings using the minigene, we identified lymphoblast cell lines (available from the Coriell Institute from the 1,000 genome project) that harbor these SNPs in complete genomes and analyzed nmMLCK1/nmMLCK2 mRNA ratios by RT-PCR. We replicated our initial minigene findings and demonstrated that rs77323602 reduced exon skipping and produced greater exon inclusion (resulting in increased expression of wild-type nmMLCK1) and that rs147245669 produced increased exon skipping (resulting in increased nmMLCK2 expression). In light of these findings, we analyzed the splice donor sites at exon 10 and exon 11 of the nmMLCK transcript on the basis of the splice donor site consensus sequence, AGgta/gagt (50) (Figure 4), and observed a strong conservation of the splice donor sites at exon 10. However, the splice donor site at exon 11 was weaker, with only seven residues matching the consensus. The presence of the SNP rs77323602 at the splice donor site of exon 11 restores the donor site to the consensus, strengthening the splice site, and would be predicted to improve the recognition of the splice donor site by the splicing machinery and inclusion of the exon 11 to generate wild-type nmMLCK1. In contrast, the presence of the SNP rs147245669 causes the substitution of an “a” at residue 7 of the consensus AAgtgagt, thereby weakening the splice site and lowering recognition of the splice donor signals, followed by exon skipping and greater levels of nmMLCK2 expression. In this context, the exon definition model in which the splicing machinery marks the exon and is the predominant form in higher organisms with longer introns is favored (51). On the basis of our data, we cannot rule out the possibility that the SNPs we have studied may affect other determinants of splicing, such as exonic splice silencers, exonic splice enhancers, intronic splice silencers, and/or intronic splice enhancers.
Figure 4.
Analysis of splice donor consensus sequence sites in the MYLK gene. The splice donor sites within the exons 10–12 on nmMLCK were analyzed with reference to the splice donor consensus as determined by Senapathy and colleagues (50). The sequence at the splice donor site is indicated over the exons; exonic sequences are capitalized and introns are in lower case. The number of residues conforming to the splice donor consensus is also shown.
It is plausible that SNPs and mechanical stress regulate alternate splicing by independent mechanisms. The occurrence of the two SNPs (rs147245669 and rs77323602) is at a region defined as a splicing junction that is critical for splice site identification and assembly of the spliceosome (discussed above). Consequently, alteration in the sequence by the presence of the SNPs affects splicing, which we have demonstrated using a minigene and lymphoblast lines, reflecting a direct effect on spliceosome assembly or the splicing machinery. Analysis of point mutations at the splice site at the splice junction has been shown to directly affect mRNA splicing (52). On the other hand, mechanical stretch has been shown to directly affect alternate splicing of tenascin, collagen XII, and versican, which are attributed to alteration of calcium signaling and to mechanical signals transmitted from the exterior of the cell to the interior through the Integrin, FAK, and ERK pathways (53).
Conclusions
In summary, using minigene constructs and RT-PCR, we have demonstrated that human lung endothelium exposed to excessive mechanical stress (18% CS) produced increased nmMLCK2 expression, with alternative splicing significantly influenced by MYLK SNPs rs77323602 and rs147245669. In addition, lymphoblastoid cell lines harboring these MYLK SNPs (rs77323602, rs147245669) directly validated SNP-specific effects on MYLK alternative splicing and nmMLCK2 generation. These studies demonstrate that mechanical stress and MYLK SNPs regulate MYLK alternative splicing and nmMLCK2 generation, a splice variant that contributes to the susceptibility and severity of ventilator-induced lung injury and inflammatory lung injury.
Footnotes
Author Contributions: J.B.M. was the primary investigator, involved in the design, performance, and analysis of the experiments, and was the primary author of the manuscript; A.Y.T. contributed bioinformatic analysis and to the writing of the manuscript; H.F. was involved in the design, performance, and analysis of the experiments; S.M.D. and T.W. helped design the experiments and contributed to the writing and analysis; J.G.N.G. was the leader of this team and contributed to and oversaw the design, experiments, and writing.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0053OC on August 16, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Birukov KG, Birukova AA, Dudek SM, Verin AD, Crow MT, Zhan X, DePaola N, Garcia JG. Shear stress-mediated cytoskeletal remodeling and cortactin translocation in pulmonary endothelial cells. Am J Respir Cell Mol Biol. 2002;26:453–464. doi: 10.1165/ajrcmb.26.4.4725. [DOI] [PubMed] [Google Scholar]
- 3.Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD, Garcia JG. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol. 2003;285:L785–L797. doi: 10.1152/ajplung.00336.2002. [DOI] [PubMed] [Google Scholar]
- 4.Dos Santos CC, Slutsky AS. Invited review: mechanisms of ventilator-induced lung injury: a perspective. J Appl Physiol (1985) 2000;89:1645–1655. doi: 10.1152/jappl.2000.89.4.1645. [DOI] [PubMed] [Google Scholar]
- 5.Davis HW, Crimmins DL, Thoma RS, Garcia JG. Phosphorylation of calmodulin in the first calcium-binding pocket by myosin light chain kinase. Arch Biochem Biophys. 1996;332:101–109. doi: 10.1006/abbi.1996.0321. [DOI] [PubMed] [Google Scholar]
- 6.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol (1985) 2001;91:1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- 7.Garcia JG, Davis HW, Patterson CE. Regulation of endothelial cell gap formation and barrier dysfunction: role of myosin light chain phosphorylation. J Cell Physiol. 1995;163:510–522. doi: 10.1002/jcp.1041630311. [DOI] [PubMed] [Google Scholar]
- 8.Mirzapoiazova T, Moitra J, Moreno-Vinasco L, Sammani S, Turner JR, Chiang ET, Evenoski C, Wang T, Singleton PA, Huang Y, et al. Non-muscle myosin light chain kinase isoform is a viable molecular target in acute inflammatory lung injury. Am J Respir Cell Mol Biol. 2011;44:40–52. doi: 10.1165/rcmb.2009-0197OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrache I, Birukov K, Zaiman AL, Crow MT, Deng H, Wadgaonkar R, Romer LH, Garcia JG. Caspase-dependent cleavage of myosin light chain kinase (MLCK) is involved in TNF-α-mediated bovine pulmonary endothelial cell apoptosis. FASEB J. 2003;17:407–416. doi: 10.1096/fj.02-0672com. [DOI] [PubMed] [Google Scholar]
- 10.Birukov KG, Csortos C, Marzilli L, Dudek S, Ma SF, Bresnick AR, Verin AD, Cotter RJ, Garcia JG. Differential regulation of alternatively spliced endothelial cell myosin light chain kinase isoforms by p60(Src) J Biol Chem. 2001;276:8567–8573. doi: 10.1074/jbc.M005270200. [DOI] [PubMed] [Google Scholar]
- 11.Parker JC. Inhibitors of myosin light chain kinase and phosphodiesterase reduce ventilator-induced lung injury. J Appl Physiol (1985) 2000;89:2241–2248. doi: 10.1152/jappl.2000.89.6.2241. [DOI] [PubMed] [Google Scholar]
- 12.Wainwright MS, Rossi J, Schavocky J, Crawford S, Steinhorn D, Velentza AV, Zasadzki M, Shirinsky V, Jia Y, Haiech J, et al. Protein kinase involved in lung injury susceptibility: evidence from enzyme isoform genetic knockout and in vivo inhibitor treatment. Proc Natl Acad Sci USA. 2003;100:6233–6238. doi: 10.1073/pnas.1031595100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang T, Moreno-Vinasco L, Ma SF, Zhou T, Shimizu Y, Sammani S, Epshtein Y, Watterson DM, Dudek SM, Garcia JG. Nonmuscle myosin light chain kinase regulates murine asthmatic inflammation. Am J Respir Cell Mol Biol. 2014;50:1129–1135. doi: 10.1165/rcmb.2013-0434OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudek SM, Jacobson JR, Chiang ET, Birukov KG, Wang P, Zhan X, Garcia JG. Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J Biol Chem. 2004;279:24692–24700. doi: 10.1074/jbc.M313969200. [DOI] [PubMed] [Google Scholar]
- 15.Liu F, Schaphorst KL, Verin AD, Jacobs K, Birukova A, Day RM, Bogatcheva N, Bottaro DP, Garcia JG. Hepatocyte growth factor enhances endothelial cell barrier function and cortical cytoskeletal rearrangement: potential role of glycogen synthase kinase-3β. FASEB J. 2002;16:950–962. doi: 10.1096/fj.01-0870com. [DOI] [PubMed] [Google Scholar]
- 16.Gao L, Grant A, Halder I, Brower R, Sevransky J, Maloney JP, Moss M, Shanholtz C, Yates CR, Meduri GU, et al. Novel polymorphisms in the myosin light chain kinase gene confer risk for acute lung injury. Am J Respir Cell Mol Biol. 2006;34:487–495. doi: 10.1165/rcmb.2005-0404OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao L, Grant AV, Rafaels N, Stockton-Porter M, Watkins T, Gao P, Chi P, Muñoz M, Watson H, Dunston G, et al. Polymorphisms in the myosin light chain kinase gene that confer risk of severe sepsis are associated with a lower risk of asthma. J Allergy Clin Immunol. 2007;119:1111–1118. doi: 10.1016/j.jaci.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Christie JD, Ma SF, Aplenc R, Li M, Lanken PN, Shah CV, Fuchs B, Albelda SM, Flores C, Garcia JG. Variation in the myosin light chain kinase gene is associated with development of acute lung injury after major trauma. Crit Care Med. 2008;36:2794–2800. doi: 10.1097/ccm.0b013e318186b843. [DOI] [PubMed] [Google Scholar]
- 19.Acosta-Herrera M, Pino-Yanes M, Ma SF, Barreto-Luis A, Corrales A, Cumplido J, Pérez-Rodríguez E, Campo P, Eng C, García-Robaina JC, et al. Fine mapping of the myosin light chain kinase (MYLK) gene replicates the association with asthma in populations of Spanish descent. J Allergy Clin Immunol. 2015;136:1116–1118.e9. doi: 10.1016/j.jaci.2015.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flores C, Ma SF, Maresso K, Ober C, Garcia JG. A variant of the myosin light chain kinase gene is associated with severe asthma in African Americans. Genet Epidemiol. 2007;31:296–305. doi: 10.1002/gepi.20210. [DOI] [PubMed] [Google Scholar]
- 21.Han YJ, Ma SF, Wade MS, Flores C, Garcia JG. An intronic MYLK variant associated with inflammatory lung disease regulates promoter activity of the smooth muscle myosin light chain kinase isoform. J Mol Med (Berl) 2012;90:299–308. doi: 10.1007/s00109-011-0820-9. [DOI] [PubMed] [Google Scholar]
- 22.Shen K, Ramirez B, Mapes B, Shen GR, Gokhale V, Brown ME, Santarsiero B, Ishii Y, Dudek SM, Wang T, et al. Structure-function analysis of the non-muscle myosin light chain kinase (nmMLCK) isoform by NMR spectroscopy and molecular modeling: Influence of MYLK variants. PLoS One. 2015;10:e0130515. doi: 10.1371/journal.pone.0130515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang T, Zhou T, Saadat L, Garcia JG. A MYLK variant regulates asthmatic inflammation via alterations in mRNA secondary structure. Eur J Hum Genet. 2015;23:874–876. doi: 10.1038/ejhg.2014.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vilitkevich EL, Khapchaev AY, Kudryashov DS, Nikashin AV, Schavocky JP, Lukas TJ, Watterson DM, Shirinsky VP. Phosphorylation regulates interaction of 210-kDa myosin light chain kinase n-terminal domain with actin cytoskeleton. Biochemistry (Mosc) 2015;80:1288–1297. doi: 10.1134/S0006297915100090. [DOI] [PubMed] [Google Scholar]
- 25.Dudek SM, Birukov KG, Zhan X, Garcia JG. Novel interaction of cortactin with endothelial cell myosin light chain kinase. Biochem Biophys Res Commun. 2002;298:511–519. doi: 10.1016/s0006-291x(02)02492-0. [DOI] [PubMed] [Google Scholar]
- 26.Clayburgh DR, Rosen S, Witkowski ED, Wang F, Blair S, Dudek S, Garcia JG, Alverdy JC, Turner JR. A differentiation-dependent splice variant of myosin light chain kinase, MLCK1, regulates epithelial tight junction permeability. J Biol Chem. 2004;279:55506–55513. doi: 10.1074/jbc.M408822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazar V, Garcia JG. A single human myosin light chain kinase gene (MLCK; MYLK) Genomics. 1999;57:256–267. doi: 10.1006/geno.1999.5774. [DOI] [PubMed] [Google Scholar]
- 28.Verin AD, Lazar V, Torry RJ, Labarrere CA, Patterson CE, Garcia JG. Expression of a novel high molecular-weight myosin light chain kinase in endothelium. Am J Respir Cell Mol Biol. 1998;19:758–766. doi: 10.1165/ajrcmb.19.5.3125. [DOI] [PubMed] [Google Scholar]
- 29.Dudek SM, Chiang ET, Camp SM, Guo Y, Zhao J, Brown ME, Singleton PA, Wang L, Desai A, Arce FT, et al. Abl tyrosine kinase phosphorylates nonmuscle myosin light chain kinase to regulate endothelial barrier function. Mol Biol Cell. 2010;21:4042–4056. doi: 10.1091/mbc.E09-10-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moitra J, Evenoski C, Sammani S, Wadgaonkar R, Turner JR, Ma SF, Garcia JG. A transgenic mouse with vascular endothelial over-expression of the non-muscle myosin light chain kinase-2 isoform is susceptible to inflammatory lung injury: role of sexual dimorphism and age. Transl Res. 2008;151:141–153. doi: 10.1016/j.trsl.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adyshev DM, Moldobaeva N, Mapes B, Elangovan V, Garcia JG. MicroRNA regulation of nonmuscle myosin light chain kinase expression in human lung endothelium. Am J Respir Cell Mol Biol. 2013;49:58–66. doi: 10.1165/rcmb.2012-0397OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiong HY, Alipanahi B, Lee LJ, Bretschneider H, Merico D, Yuen RK, Hua Y, Gueroussov S, Najafabadi HS, Hughes TR, et al. RNA splicing. The human splicing code reveals new insights into the genetic determinants of disease. Science. 2015;347:1254806. doi: 10.1126/science.1254806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Churbanov A, Rogozin IB, Deogun JS, Ali H. Method of predicting splice sites based on signal interactions. Biol Direct. 2006;1:10. doi: 10.1186/1745-6150-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaphorst KL, Chiang E, Jacobs KN, Zaiman A, Natarajan V, Wigley F, Garcia JG. Role of sphingosine-1 phosphate in the enhancement of endothelial barrier integrity by platelet-released products. Am J Physiol Lung Cell Mol Physiol. 2003;285:L258–L267. doi: 10.1152/ajplung.00311.2002. [DOI] [PubMed] [Google Scholar]
- 35.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS, Slutsky AS ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 36.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 37.Borbiev T, Birukova A, Liu F, Nurmukhambetova S, Gerthoffer WT, Garcia JG, Verin AD. p38 MAP kinase-dependent regulation of endothelial cell permeability. Am J Physiol Lung Cell Mol Physiol. 2004;287:L911–L918. doi: 10.1152/ajplung.00372.2003. [DOI] [PubMed] [Google Scholar]
- 38.Garcia JG, Pavalko FM, Patterson CE. Vascular endothelial cell activation and permeability responses to thrombin. Blood Coagul Fibrinolysis. 1995;6:609–626. doi: 10.1097/00001721-199510000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Garcia JG, Schaphorst KL, Shi S, Verin AD, Hart CM, Callahan KS, Patterson CE. Mechanisms of ionomycin-induced endothelial cell barrier dysfunction. Am J Physiol. 1997;273:L172–L184. doi: 10.1152/ajplung.1997.273.1.L172. [DOI] [PubMed] [Google Scholar]
- 40.Garcia JG, Verin AD, Schaphorst K, Siddiqui R, Patterson CE, Csortos C, Natarajan V. Regulation of endothelial cell myosin light chain kinase by Rho, cortactin, and p60(src) Am J Physiol. 1999;276:L989–L998. doi: 10.1152/ajplung.1999.276.6.L989. [DOI] [PubMed] [Google Scholar]
- 41.Gilbert-McClain LI, Verin AD, Shi S, Irwin RP, Garcia JG. Regulation of endothelial cell myosin light chain phosphorylation and permeability by vanadate. J Cell Biochem. 1998;70:141–155. doi: 10.1002/(sici)1097-4644(19980701)70:1<141::aid-jcb14>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 42.Verin AD, Gilbert-McClain LI, Patterson CE, Garcia JG. Biochemical regulation of the nonmuscle myosin light chain kinase isoform in bovine endothelium. Am J Respir Cell Mol Biol. 1998;19:767–776. doi: 10.1165/ajrcmb.19.5.3126. [DOI] [PubMed] [Google Scholar]
- 43.Wadgaonkar R, Linz-McGillem L, Zaiman AL, Garcia JG. Endothelial cell myosin light chain kinase (MLCK) regulates TNFα-induced NFκB activity. J Cell Biochem. 2005;94:351–364. doi: 10.1002/jcb.20250. [DOI] [PubMed] [Google Scholar]
- 44.Wang P, Verin AD, Birukova A, Gilbert-McClain LI, Jacobs K, Garcia JG. Mechanisms of sodium fluoride-induced endothelial cell barrier dysfunction: role of MLC phosphorylation. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1472–L1483. doi: 10.1152/ajplung.2001.281.6.L1472. [DOI] [PubMed] [Google Scholar]
- 45.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown M, Adyshev D, Bindokas V, Moitra J, Garcia JG, Dudek SM. Quantitative distribution and colocalization of non-muscle myosin light chain kinase isoforms and cortactin in human lung endothelium. Microvasc Res. 2010;80:75–88. doi: 10.1016/j.mvr.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodriguez-Martin T, Anthony K, Garcia-Blanco MA, Mansfield SG, Anderton BH, Gallo JM. Correction of tau mis-splicing caused by FTDP-17 MAPT mutations by spliceosome-mediated RNA trans-splicing. Hum Mol Genet. 2009;18:3266–3273. doi: 10.1093/hmg/ddp264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sterne-Weiler T, Sanford JR. Exon identity crisis: disease-causing mutations that disrupt the splicing code. Genome Biol. 2014;15:201. doi: 10.1186/gb4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brea-Fernández AJ, Pomares E, Brión MJ, Marfany G, Blanco MJ, Sánchez-Salorio M, González-Duarte R, Carracedo A. Novel splice donor site mutation in MERTK gene associated with retinitis pigmentosa. Br J Ophthalmol. 2008;92:1419–1423. doi: 10.1136/bjo.2008.139204. [DOI] [PubMed] [Google Scholar]
- 50.Senapathy P, Shapiro MB, Harris NL. Splice junctions, branch point sites, and exons: sequence statistics, identification, and applications to genome project. Methods Enzymol. 1990;183:252–278. doi: 10.1016/0076-6879(90)83018-5. [DOI] [PubMed] [Google Scholar]
- 51.Amit M, Donyo M, Hollander D, Goren A, Kim E, Gelfman S, Lev-Maor G, Burstein D, Schwartz S, Postolsky B, et al. Differential GC content between exons and introns establishes distinct strategies of splice-site recognition. Cell Reports. 2012;1:543–556. doi: 10.1016/j.celrep.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 52.Krawczak M, Cooper DN. Gene deletions causing human genetic disease: mechanisms of mutagenesis and the role of the local DNA sequence environment. Hum Genet. 1991;86:425–441. doi: 10.1007/BF00194629. [DOI] [PubMed] [Google Scholar]
- 53.Liu H, Tang L. Mechano-regulation of alternative splicing. Curr Genomics. 2013;14:49–55. doi: 10.2174/138920213804999156. [DOI] [PMC free article] [PubMed] [Google Scholar]