Abstract
Pulmonary hypertension (PH), a serious complication of sickle cell disease (SCD), causes significant morbidity and mortality. Although a recent study determined that hemin release during hemolysis triggers endothelial dysfunction in SCD, the pathogenesis of SCD-PH remains incompletely defined. This study examines peroxisome proliferator-activated receptor γ (PPARγ) regulation in SCD-PH and endothelial dysfunction. PH and right ventricular hypertrophy were studied in Townes humanized sickle cell (SS) and littermate control (AA) mice. In parallel studies, SS or AA mice were gavaged with the PPARγ agonist, rosiglitazone (RSG), 10 mg/kg/day, or vehicle for 10 days. In vitro, human pulmonary artery endothelial cells (HPAECs) were treated with vehicle or hemin for 72 hours, and selected HPAECs were treated with RSG. SS mice developed PH and right ventricular hypertrophy associated with reduced lung levels of PPARγ and increased levels of microRNA-27a (miR-27a), v-ets avian erythroblastosis virus E26 oncogene homolog 1 (ETS1), endothelin-1 (ET-1), and markers of endothelial dysfunction (platelet/endothelial cell adhesion molecule 1 and E selectin). HPAECs treated with hemin had increased ETS1, miR-27a, ET-1, and endothelial dysfunction and decreased PPARγ levels. These derangements were attenuated by ETS1 knockdown, inhibition of miR-27a, or PPARγ overexpression. In SS mouse lung or in hemin-treated HPAECs, activation of PPARγ with RSG attenuated reductions in PPARγ and increases in miR-27a, ET-1, and markers of endothelial dysfunction. In SCD-PH pathogenesis, ETS1 stimulates increases in miR-27a levels that reduce PPARγ and increase ET-1 and endothelial dysfunction. PPARγ activation attenuated SCD-associated signaling derangements, suggesting a novel therapeutic approach to attenuate SCD-PH pathogenesis.
Keywords: peroxisome proliferator-activated receptor γ, sickle cell disease, microRNA-27a, v-ets avian erythroblastosis virus E26 oncogene homolog 1, pulmonary hypertension
Clinical Relevance
The current study provides novel evidence that microRNA-27a (miR-27a) is up-regulated in mice with sickle cell disease and in hemin-treated human pulmonary artery endothelial cells through v-ets avian erythroblastosis virus E26 oncogene homolog 1 activation. Increases in miR-27a down-regulate peroxisome proliferator-activated receptor γ (PPARγ) and contribute to endothelial dysfunction. Activation of PPARγ stimulates reciprocal inhibition of v-ets avian erythroblastosis virus E26 oncogene homolog 1, miR-27a and pulmonary endothelial dysfunction. We believe these findings provide the first indication that targeting PPARγ activation may represent a novel therapeutic approach to attenuate sickle cell disease–pulmonary hypertension pathogenesis through modulation of microRNA levels.
Sickle cell disease (SCD), an autosomal recessive disorder caused by a mutant β-hemoglobin (HbS), is associated with a large spectrum of pathologic complications caused by the aggregation and polymerization of HbS within red blood cells (RBCs) (1, 2). The sickling of abnormal RBCs results in anemia and vasoocclusion, causing acute chest syndrome (3, 4) and multiorgan dysfunction. SCD affects millions of people worldwide. In the United States, one of every 365 African Americans and one of every 36,000 Hispanic Americans are born with SCD, and ∼100,000 people are living with SCD (5).
Pulmonary hypertension (PH), defined as an elevation of the mean pulmonary artery pressure to >25 mm Hg (6, 7) is an increasingly recognized complication of SCD that is associated with high morbidity and mortality (8–10). Two-year mortality rates among patients with SCD and PH have been reported to be as high as 40–50% (10, 11), and ∼6–10.4% of patients with SCD ultimately develop PH (12–14). Despite the magnitude of this problem, the pathogenesis of SCD-PH remains incompletely defined. Current evidence suggests that SCD-PH, similar to other forms of PH, involves endothelial dysfunction with increased production of vasoconstrictors (e.g., endothelin-1 [ET-1]) (15), and reduced production of vasodilators (e.g., prostacyclin [16] and nitric oxide [17, 18]). Interactions between RBCs and endothelium may contribute to the pathogenesis of vasoocclusive crisis in SCD (19). The release of hemoglobin and hemin during hemolysis triggers endothelial dysfunction and contributes to PH pathogenesis (20). Recent studies in sickle cell (SS) patients demonstrate that EC adhesion molecules, including selectins and vascular endothelial cadherin, play an important role in the recruitment and binding of inflammatory cells to vascular endothelium (21). Because SCD-PH morbidity and mortality remain unacceptably high, there is an urgent need for novel therapeutic strategies.
Mouse models of hemolysis, including the Berkeley SS mouse (18, 22), the spherocytosis mouse (23), and the alloimmune hemolysis mouse, develop spontaneous PH and right heart failure (18). To further examine the pathogenesis of SCD-PH and explore novel therapeutic targets, the current study capitalized on an existing mouse model of SCD (24–26). In this study, we used a humanized knockin mouse model of SCD (27) (the Townes model) in which mouse HbS genes are replaced with human γ-and βS-hemoglobin genes and mouse α-hemoglobin genes are replaced with human α-hemoglobin genes. Because our laboratory and others have shown that stimulating peroxisome proliferator-activated receptor γ (PPARγ) with thiazolidinedione (TZD) ligands attenuates PH in experimental models (28–32), whereas reduced PPARγ expression is associated with PH pathogenesis (29, 33, 34), we compared SS mice with littermate control (AA) mice to determine if PPARγ plays an important role in SCD-associated pulmonary vascular dysfunction and PH pathogenesis.
PPARγ is a ligand-activated transcription factor that belongs to the nuclear hormone receptor superfamily. The PPARγ receptor is activated by a diverse spectrum of ligands that includes naturally occurring fatty acids and their metabolites, as well as synthetic TZD medications, which are currently used in this country to treat patients with type 2 diabetes. Once activated, PPARγ forms a heterodimer with the retinoid X receptor and binds to PPAR response elements in the promoter region of responsive genes to increase their expression. PPARγ activation can also reduce the expression of selected genes though transrepression mechanisms (35). For example, PPARγ inhibits transcription factors, such as early growth response 1 (36, 37), Sp1 transcription factor (37, 38), and v-ets avian erythroblastosis virus E26 oncogene homolog (ETS)1 (ETS1) (39, 40). ETS1, a member of the ETS family of transcription factors (41), plays an important role in proliferation, migration, vascular remodeling, apoptosis, and senescence (42). Recent studies demonstrate that PPARγ reduction increases ETS1 expression and vascular smooth muscle cell migration and proliferation (39), whereas PPARγ activation inhibits ETS1 expression and attenuates migration by inhibiting ETS1 expression (40, 43). Furthermore, PPARγ attenuates hypoxic increases in proliferative mediators (e.g., ET-1 and nicotinamide adenine dinucleotide phosphate oxidase 4), in part through inhibition of nuclear factor-κB p65 (22, 32, 44, 45). Interestingly, mounting evidence demonstrates that pharmacological activation of PPARγ attenuates pulmonary vascular cell proliferation and PH (46), whereas loss of PPARγ expression or function is associated with PH (47). Taken together, these findings led us to postulate in the current study that: (1) chronic hemolysis in SCD stimulates loss of PPARγ and increased expression of the proliferative mediator, ET-1, which contributes to PH pathogenesis; and (2) targeting PPARγ with existing pharmacological ligands may represent a novel therapeutic approach in SCD to attenuate increases in proliferative mediators and SCD-PH pathogenesis.
Pathways leading to reductions in PPARγ in PH continue to be defined (29, 33, 34). We reported recently that microRNA-27a (miR-27a) plays an important role in hypoxic reductions in PPARγ (37). Evolving evidence indicates that dysregulation of microRNAs (miRNA or miR) contribute to PH pathogenesis (48–52). miRNAs are endogenous, noncoding, single-stranded RNAs of ∼22 nucleotides that constitute a novel class of post-transcriptional gene regulators (53, 54). miRNAs negatively regulate the expression of their target genes through translational repression or mRNA degradation (54). Recent studies have provided compelling evidence that miRNAs regulate pulmonary vascular remodeling by controlling endothelial cell (EC) and smooth muscle cell differentiation and proliferation (48, 49, 51, 52, 55–57). The potential role of miRNAs as master regulators of cell differentiation in physiological and pathological processes in the lung has been reviewed recently (58–60). However, the precise post-transcriptional mechanisms by which PPARγ activation regulates SCD-PH have not been defined. In the current study, we explored the hypothesis that hemolysis increases the levels of miR-27a to reduce PPARγ expression through post-transcriptional mechanisms, leading to increased ET-1 expression, endothelial dysfunction, and PH. The current study demonstrates that SCD-related hemolysis stimulates miR-27a expression, reduces PPARγ expression, and increases ET-1 in human pulmonary artery endothelial cells (HPAECs) in vitro and in the SS mouse lung in vivo. Pharmacological activation of PPARγ attenuates these derangements, suggesting that therapeutic targeting of this receptor provides a novel strategy for reducing SCD-associated pulmonary endothelial dysfunction and PH.
Materials and Methods
Reagents
HPAECs were obtained from Invitrogen (Carlsbad, CA). ET-1, PPARγ, ETS1, and glyceraldehyde phosphate dehydrogenase antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The following reagents were obtained from Sigma-Aldrich (St. Louis, MO): hemin, fetal bovine serum (FBS), dimethyl sulfoxide (DMSO), and methyl cellulose. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from ATCC (Manassas, VA). The PPARγ ligand, rosiglitazone (RSG), was obtained from Cayman Chemical (Ann Arbor, MI).
Measurement of PH in SS Mice
The recently described Townes humanized mouse model of SCD was used in the current study (27), and male and female SS and AA mice were examined at age 15–17 weeks, as reported (32). All animals were given unrestricted access to water and standard mouse chow. To assess PH, SS and AA mice were subjected to measurements of right ventricular systolic pressure (RVSP) and right ventricular hypertrophy (RVH), as reported (32). Briefly, after being anesthetized with isoflurane, RVSP was measured by introducing a 1.2-F microtip pressure transducer catheter (Transonic, Ithaca, NY) into the right internal jugular vein and advancing it to the right ventricle (RV), and RV pressures were monitored continuously for 10 minutes. RVH was assessed by dissecting the heart and calculating the ratio of the weight of the RV to the weight of the left ventricle (LV) and septum (S) [Fulton’s Index, RV:(LV + S) weight ratio]. Selected animals were gavaged daily with 100 μl RSG (10 mg/kg/d in 100 μl 0.5% methyl cellulose) or with an equivalent volume of vehicle for 10 days, as we reported previously (32). All experiments using mice were approved by the Institutional Animal Care and Use Committee of the Atlanta Veterans Affairs Medical Center.
Hemin-Treated HPAECs and Cell Proliferation Assay
To examine the effect of RSG on hemin-induced cell proliferation, HPAECs were treated with DMSO as a control, or hemin (HEM, 5 μM), for 72 hours, as reported (61). Hemin concentrations in this range have been measured in patients with SCD (62). Selected HPAECs were treated with RSG (10 μM) or an equivalent volume of vehicle added directly to the culture media during the last 24 hours of hemin treatment, and cell proliferation was measured using MTT assays, as we have reported (46).
miR-27a Down-Regulation
To confirm the role of miR-27a in alterations in PPARγ expression, HPAECs (passages 3–6) were transfected with anti–miR-27a (50 nM) or an equivalent amount of scrambled anti-miR negative control using lipofectamine RNAiMax (QIAGEN, Valencia, CA). After transfection for 6 hours, media were replaced with endothelial growth medium (EGM) containing 10% FBS. HPAECs were treated with DMSO or HEM (5 μM) and then cultured for 72 hours. Alterations in miR-27a, ET-1, PPARγ, platelet/endothelial cell adhesion molecule 1 (PECAM1), and E-selectin or endothelial adhesion molecule 1 (E-SEL) levels were examined using real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR).
PPARγ Overexpression in HPAECs In Vitro
To overexpress PPARγ, HPAECs were transfected with adenovirus containing a PPARγ plasmid (AdPPARγ, 25 multiplicity of infection) or control green fluorescent protein (GFP) plasmid, as we reported previously (63). Six hours after transfection, adenovirus-containing medium was replaced with fresh 10% FBS EGM, and HPAECs were then treated with DMSO or HEM (5 μM) for 72 hours. Levels of miR-27a, ETS1, ET-1, PPARγ, PECAM-1, and E-SEL were determined by qRT-PCR.
HPAEC Transfection and RNA Interference Studies
HPAECs were transfected with scrambled or ETS1 (5′-UCAGUAGAGAGAAGUGUACGGCU TT-3′, 3′-UAAGUCAUCUCUCUUCACAUGCCGAA A-5′) RNA interference (RNAi) duplex (10 nM) using RNAiMax transfection reagent (Invitrogen) according to the manufacturer’s instructions. After transfection for 6 hours, the transfection media were replaced with EGM containing 10% FBS. HPAECs were then treated with DMSO or HEM (5 μM) for 72 hours. HPAEC lysates were then harvested and examined for ETS1, miR-27a, ET-1, and PPARγ levels using qRT-PCR and Western blot assays.
miRNA and mRNA qRT-PCR Analysis
To measure miR-27a, PPARγ, ETS1, ET-1, E-SEL, and PECAM1 levels in HPAECs or SS mouse lungs, small RNAs (<200 nt) and large RNAs (>200 nt) were isolated using the mirVana kit (Invitrogen). The levels of miR-27a expression were analyzed by qRT-PCR using the QIAGEN miRNA primer assay according to the manufacturer’s instructions. RNU6B (miRNA) was used as an endogenous control. PPARγ, ETS1, ET-1, PECAM1, and E-SEL mRNA levels in the same sample were determined and quantified using specific mRNA primers, as described previously (46). Levels of 9S mRNA were used as an endogenous control.
Western Blot Analysis
After treatment with RSG or vehicle, protein homogenates from mouse lungs or hemin-treated HPAECs were subjected to Western blot analysis, as reported (46). Primary antibodies were purchased from Santa Cruz Biotechnology and included ET-1 rabbit polyclonal antibody (1:250 dilution, Cat. No. SC-98727, 24 kD), PPARγ rabbit polyclonal antibody (1:500 dilution, Cat. No. SC-7196, 54 kD), ETS1 antibody (1:500 dilution, Cat. No. SC-350, 55 kD), PECAM1 (1:500 dilution, Cat. No. SC-8306, 130 kD), E-SEL (1:500 dilution, Cat. No. SC-14011, 115 kD), and CDK4 antibody (1:500 dilution, Cat. No. SC-260, 32 kD). Proteins were visualized using a peroxidase-coupled antigoat, antirabbit, or antimouse IgG in the presence of LumiGlo reagent (Thermo Scientific, Waltham, MA). Bands were identified by chemiluminescence, quantified by laser densitometry (Bio-Rad, Hercules, CA), and normalized to CDK4 levels within the same lane.
Statistical Analysis
For all measurements, data are presented as mean ± SE. All data were analyzed using analysis of variance. Post hoc analysis used the Student-Newman-Keuls test to detect differences between specific groups. In studies comparing only two experimental groups, data were analyzed with Student’s t test to determine the significance of treatment effects. The level of statistical significance was taken as P < 0.05. Statistical analyses were performed using GraphPad Prism, Version 5.0 software (LaJolla, CA).
Results
Townes SS Mice Develop PH and RVH Spontaneously
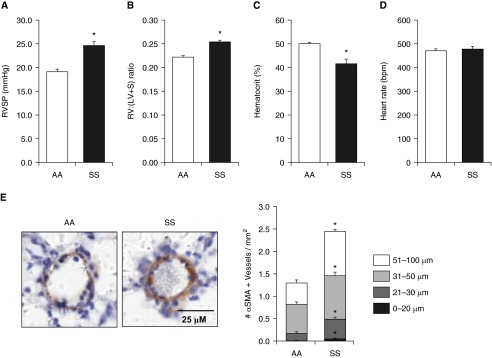
Because PH is an important complication of SCD (64, 65) and is associated with an increased mortality risk (12–14), we measured RVSP and RVH in SS and AA mice. As illustrated in Figure 1, Townes SS mice of either sex at 15–17 weeks of age spontaneously developed PH (Figure 1A) and RVH (Figure 1B). As reported previously (27), Townes SS mice showed decreased hematocrit values compared with AA mice (Figure 1C), whereas no differences in heart rate were observed between AA and SS mice (Figure 1D). To evaluate pulmonary vascular remodeling in SS and AA mice, α-smooth muscle actin–stained lung sections were used, and the number of muscularized vessels per square millimeter was counted. As illustrated in Figure 1E, pulmonary vascular remodeling was increased in SS compared with AA lungs.
Figure 1.
Compared with littermate control (AA) mice, sickle cell (SS) mice had increases in right ventricular systolic pressure (RVSP) and right ventricular hypertrophy and reduced hematocrit. (A and D) AA and SS mice were anesthetized, and the right internal jugular vein of each mouse was surgically exposed and cannulated with a pressure transducer that was advanced to record RVSP and heart rate. Each bar represents the mean RVSP in mm Hg ± SE. *P < 0.05 versus AA (n = 6–13). (B) The right ventricular (RV) to left ventricular (LV) + septum (S) weight ratio [RV:(LV + S)] is presented as an index of right ventricular hypertrophy. *P < 0.05 versus AA (n = 6). (C) Hematocrits were measured as the volume of red blood cells in AA and SS mice whole blood. *P < 0.05 versus AA (n = 6–13). (E) The number of α smooth muscle actin (αSMA)–positive vessels (0–20, 21–30, 31–50, and 51–100 µm in luminal diameter) per square millimeter was determined. Each bar represents the mean ± SE αSMA-positive vessels per square millimeter (n = 6). *P < 0.05 versus AA. Scale bar, 25 μM.
Alterations in PPARγ and ET-1 in SS Mice and Hemin-Treated HPAECs
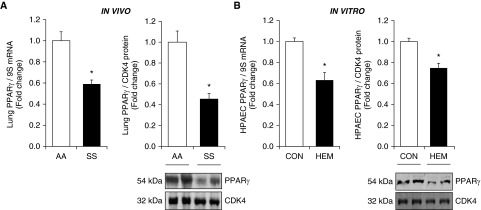
Because mounting evidence demonstrates that PPARγ activation attenuates PH (46), whereas loss of PPARγ expression is associated with PH (47), we examined PPARγ levels in SCD-PH. As illustrated in Figure 2A, PPARγ mRNA and protein levels were reduced in SS mouse lungs. Because ET-1 levels are increased in the plasma of patients with SCD (66–68) and because PPARγ negatively regulates ET-1 (46), we measured ET-1 mRNA and protein levels in these same models. As expected, ET-1 expression was increased in SS compared with AA lungs (see Figure E1A in the online supplement). Circulating hemin levels are increased in SCD and they contribute to vascular endothelial dysfunction (69, 70), and plasma hemin concentrations in patients with SCD were ∼4.2 μM compared with 0.2 μM in healthy control subjects (62). Hemin, released into the circulation during hemolysis, causes inflammation, oxidative damage, and endothelial dysfunction and contributes to SCD-PH pathogenesis (71). Furthermore, plasma cell free hemin levels are elevated in Townes SS mice (72) and they contribute to endothelial barrier dysfunction (73). Thus, we examined the dose-dependent (0–10 μM) effects of hemin on HPAECs (Figure E2). Treatment with hemin (5 μM) was sufficient to reduce PPARγ mRNA and protein levels (Figure 2B) and increase HPAEC ET-1 mRNA and protein levels (Figure E1B). Hemin treatment was therefore selected to further model SCD-induced endothelial signaling pathways in vitro.
Figure 2.
Levels of peroxisome proliferator-activated receptor γ (PPARγ) are reduced in SS mice and in hemin-treated human pulmonary artery endothelial cells (HPAECs). Whole lung homogenates were collected from SS and AA mice. (A) Lung PPARγ levels were measured with quantitative RT-PCR (qRT-PCR) and/or Western blotting and are expressed relative to lung mRNA (9S mRNA) or CDK4. *P < 0.05 versus AA (n = 6). HPAECs were treated with dimethyl sulfoxide vehicle (CON) or hemin (HEM, 5 µM) for 72 hours. (B) Mean HPAEC PPARγ levels were measured with qRT-PCR and/or Western blotting. Each bar represents the mean PPARγ level ± SE expressed as fold change versus CON. *P < 0.05 versus CON (n = 6).
To examine the role of endothelial dysfunction in SCD-PH pathogenesis, we examined levels of markers of endothelial dysfunction, including E-SEL (Figure E3A) and PECAM1 (Figure E3B). Each of these markers of endothelial dysfunction was increased in the lungs of SS mice compared with those of AA mice. In parallel, hemin treatment was sufficient to increase HPAEC mRNA and protein levels of these same markers of endothelial dysfunction in vitro (Figures E3C and E3D).
miR-27a Is Increased in SS Mice and Hemin-Treated HPAECs
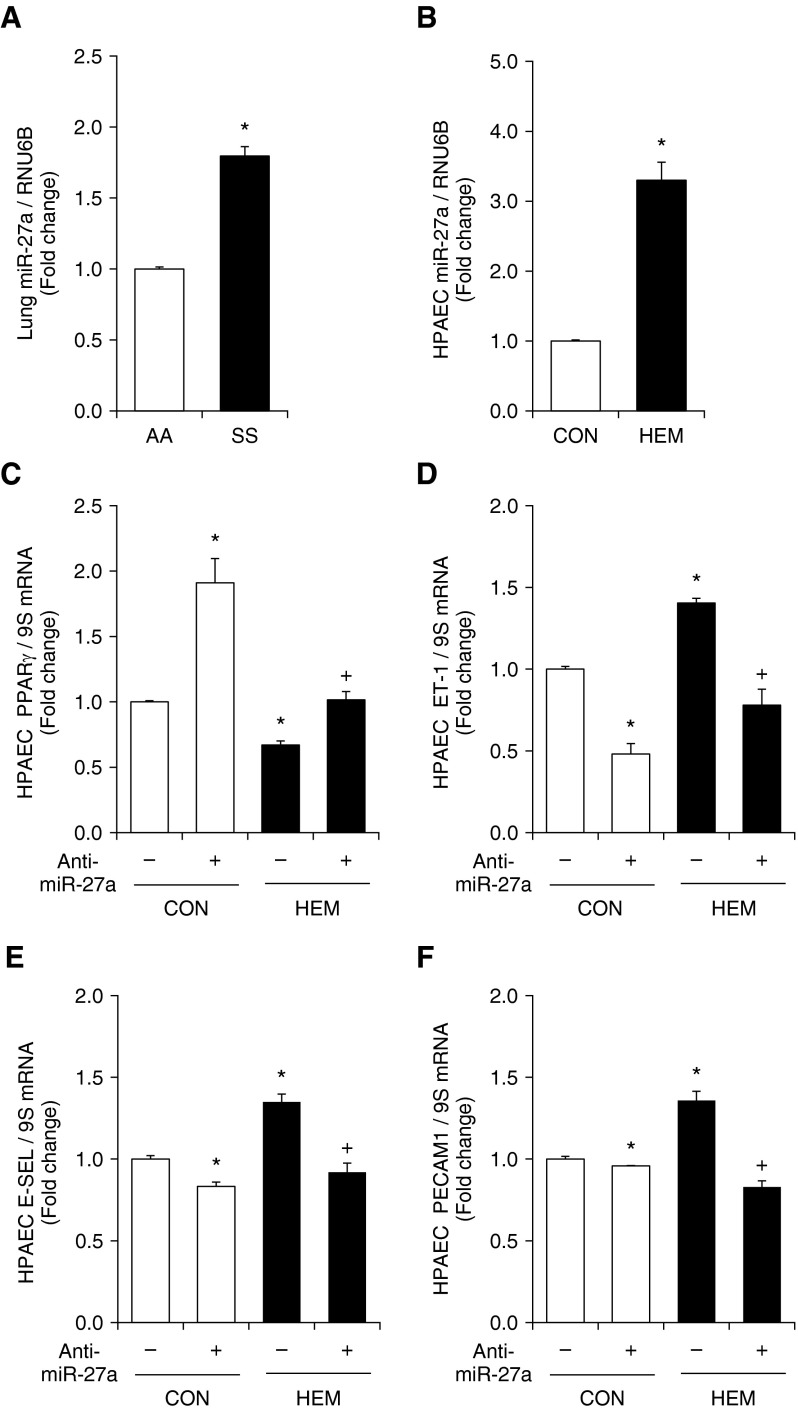
To examine potential miRNAs that negatively regulate PPARγ, a bioinformatics approach using multiple prediction algorithms (miRBase, PicTar, and TargetScan v6.1) was used to identify binding sites for miRNAs in the 3′UTR of PPARγ. This analysis indicated miR-27a/b, miR-130a/b, miR-301a/b, and miR-454 as potential regulators of PPARγ. Samples from SS and AA mouse lungs or hemin-treated HPAECs were used to screen for alterations in these miRNAs. As illustrated in Figure 3A, of the miRNAs that were examined and predicted to regulate PPARγ, miR-27a was increased in SS mouse lung and miR-130b, miR-301a/b, and miR-454 were selectively increased in SS mouse lung (Figure E4A). As illustrated in Figure 3B, compared with control conditions, hemin increased the levels of miR-27a and miR-27b only in HPAECs in vitro (Figure E4B). Because increased levels of miR-27a are predicted to reduce PPARγ, and because miR-27a reduced lung PPARγ levels in a hypoxia-induced model of PH (37), subsequent studies focused on miR-27a in SS mouse lung and hemin-treated HPAECs.
Figure 3.
MicroRNA-27a (miR-27a) regulates PPARγ in SS mice and in hemin-treated HPAECs. Anti–miR-27a reduces endothelin-1 (ET-1) and markers of endothelial dysfunction and increases PPARγ expression in hemin-treated HPAECs. (A) Whole lung homogenates were collected from SS and AA mice. qRT-PCR was performed on lung tissue for miR-27a predicted to regulate PPARγ expression. Each bar represents the mean miR-27a level ± SE relative to RNA, U6B small nuclear (RNU6B) and normalized to control values. *P < 0.05 versus AA (n = 3–6). (B) HPAECs were treated with dimethyl sulfoxide vehicle (CON) or hemin (HEM, 5 µM) for 72 hours. *P < 0.05 versus CON (n = 3–6). HPAEC miR-27a was isolated and subjected to qRT-PCR analysis together with RNU6B. Each bar represents the mean miR-27a level ± SE relative to RNU6B and normalized to control values. *P < 0.05 versus CON (n = 3–6). (C–F) HPAECs were treated with either scrambled or 50 nM anti–miR-27a for 6 hours and then treated with dimethyl sulfoxide vehicle (CON) or hemin (HEM, 5 µM) for 72 hours. HPAECs were then collected, and mRNAs were isolated. qRT-PCR was performed for (C) PPARγ, (D) ET-1, (E) endothelial adhesion molecule 1 (E-SEL), and (F) platelet/endothelial cell adhesion molecule 1 (PECAM1) levels. Each bar represents the mean mRNA level ± SE relative to ribosomal S9 (9S RNA) expressed as fold change versus CON. *P < 0.05 versus CON; +P < 0.05 versus HEM (n = 6–9).
miR-27a Inhibition Reduces ET-1 Expression and Endothelial Dysfunction through PPARγ Up-Regulation in Hemin-Treated HPAECs
We have reported previously that 50 nM anti–miR-27a reduced miR-27a levels by 50% and increased PPARγ levels in HPAECs (37). To confirm the role of miR-27a in hemin-induced reductions in PPARγ and increases in ET-1 and endothelial dysfunction, loss-of-miR-27a function was accomplished by transfecting HPAECs with an miR-27a inhibitor. Anti–miR-27a increased PPARγ levels in control HPAECs and attenuated reductions in PPARγ levels in hemin-treated HPAECs (Figure 3C). Anti–miR-27a also lowered HPAEC ET-1 mRNA levels in control cells and attenuated hemin-stimulated increases in ET-1 mRNA (Figure 3D). In addition, inhibition of miR-27a attenuated hemin-induced increases in E-SEL and PECAM1 mRNA levels (Figures 3E and 3F).
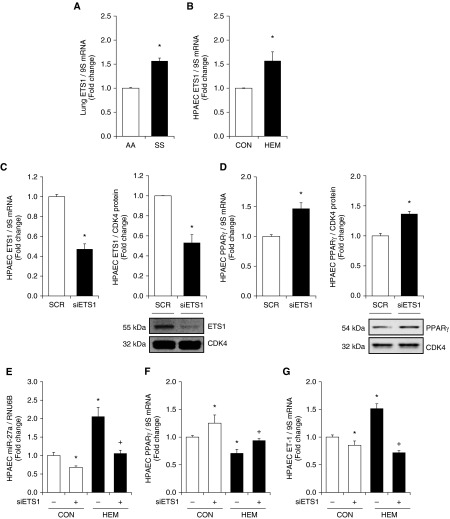
ETS1 Knockdown Attenuates Increases in miR-27a and ET-1
To further examine the mechanisms underlying increases in miR-27a in SS mouse lungs and hemin-treated HPAECs, in silico analysis of the miR-27a promoter using PuTmiR software was performed (74). The upstream promoter region of miR-27a (chromosome X: 13,808,373–13,809,373 bp) contains a putative ETS binding site (5′-ACTTCCT-3′), suggesting that ETS may directly regulate transcriptional expression of miR-27. As shown in Figures 4A and 4B, ETS1 mRNA levels were increased in SS mouse lungs and hemin-treated HPAECs. In preliminary studies, we screened two different targeted regions, and subsequent studies focused on small interfering RNA targeting ETS1 transcript 1 (V1) that significantly decreased ETS1 levels (Figure E5). To further examine the role of ETS1 in hemin-induced miR-27a expression, RNA interference duplexes to ETS1 (10 nM) were used to directly reduce HPAEC ETS1 levels. Figures 4C and 4D illustrate that knockdown of HPAEC ETS1 decreased ETS1 mRNA and protein levels, but increased HPAEC PPARγ mRNA and protein levels. The findings in Figures 4E–4G illustrate that depletion of ETS1 was sufficient to attenuate hemin-induced (1) increases in HPAEC miR-27a, (2) decreases in PPARγ expression, and (3) increases in ET-1.
Figure 4.
ETS1 knockdown attenuates increases in miR-27a and ET-1 levels and decreases in PPARγ expression in hemin-treated HPAECs. (A) Whole lung homogenates were collected from SS and AA mice. Lung ETS1 levels were measured with qRT-PCR and expressed relative to lung 9S mRNA. *P < 0.05 versus AA (n = 6). (B) HPAECs were treated with dimethyl sulfoxide vehicle (CON) or hemin (HEM, 5 µM) for 72 hours. Mean HPAEC ETS1 levels were measured with qRT-PCR. Each bar represents the mean ETS1 level ± SE relative to 9S expressed as fold change versus CON. *P < 0.05 versus CON (n = 5). (C–E) HPAECs were treated with 10 nM scrambled (CON) or small interfering RNA duplexes to ETS1 for 72 hours. HPAEC mRNA or protein was then isolated. Each bar represents the mean ± SE (C) ETS1 or (D) PPARγ relative to 9S in the same sample expressed as fold change versus CON. *P < 0.05 versus CON (n = 3). Western blotting was used to detect (C) ETS1 and (D) PPARγ protein levels. Representative blots depicting ETS1 or PPARγ protein in siETS1-treated HPAECs are shown. (E–G) HPAECs were treated with 10 nM scrambled (CON) or small interfering RNA duplexes to ETS1 for 6 hours and then treated with dimethyl sulfoxide vehicle (CON) or hemin (HEM, 5 µM) for 72 hours. MicroRNAs (miRNA) or mRNA were isolated and subjected to qRT-PCR analysis for miR-27a, PPARγ, ET-1, and RNU6B or 9S. Each bar represents the mean ± SE (E) miR-27a, (F) PPARγ, or (G) ET-1 relative to RNU6B or 9S expressed as fold change versus CON. *P < 0.05 versus CON; +P < 0.05 versus HEM (n = 3–6). ETS1, v-ets avian erythroblastosis virus E26 oncogene homolog 1; SCR, scrambled small interfering RNA; siETS1, ETS1 small interfering RNA.
PPARγ Regulates miR-27a and ET-1 Expression and Endothelial Dysfunction
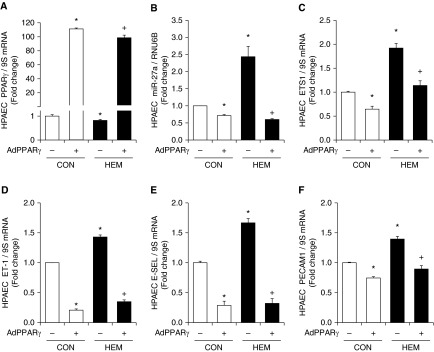
We demonstrated previously that miR-27a and PPARγ were mutually repressive in the hypoxic pulmonary vasculature (37). To further determine if alterations in PPARγ expression are sufficient to regulate miR-27a, ET-1, and endothelial dysfunction, AdPPARγ expression construct was used to examine PPARγ gain of function in hemin-induced signaling. As shown in Figure E6, AdPPARγ caused large and dose-dependent increases in HPAEC PPARγ protein levels. As shown in Figure 5, AdPPARγ at 25 multiplicity of infection increased PPARγ (Figure 5A) and decreased miR-27a (Figure 5B) and ETS1 (Figure 5C) mRNA levels in control cells. PPARγ overexpression also prevented hemin-induced reductions in HPAEC PPARγ and increases in miR-27a and ETS1 levels. Similarly, PPARγ overexpression reduced ET-1, E-SEL, and PECAM1 mRNA levels in control HPAECs and attenuated hemin-induced increases in mRNA levels of these markers of endothelial dysfunction (Figures 5D–5F).
Figure 5.
PPARγ gain-of-function attenuates miR-27a, ETS1, and ET-1 expression and endothelial dysfunction in hemin-treated HPAECs. HPAECs were transfected with adenovirus containing a PPARγ plasmid (AdPPARγ, 25 multiplicity of infection) or green fluorescent protein (AdPPARγ−) constructs for 6 hours and were then treated with dimethyl sulfoxide vehicle (CON) or hemin (HEM, 5 µM) for 72 hours. miRNA or mRNA were isolated and subjected to qRT-PCR analysis (n = 3–9). (A) HPAEC PPARγ mRNA levels are expressed relative to 9S in the same sample as fold change versus CON. (B) HPAEC miR-27a levels are expressed relative to RNU6B as fold change versus CON. HPAEC (C) ETS1, (D) ET-1, (E) E-SEL, and (F) PECAM1 mRNA levels are expressed relative to 9S as fold change versus CON/AdPPARγ−. All bars represent mean ± SE. *P < 0.05 versus CON; +P < 0.05 versus HEM.
PPARγ Activation Attenuates Increases in miR-27a and ETS1 Levels and Restores PPARγ in SS Mouse Lungs and Hemin-Treated HPAECs
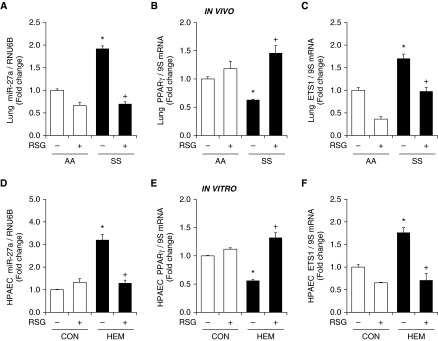
Because PPARγ levels were reduced in the lungs of SS mice, and because, as we have reported previously, pharmacological PPARγ activation attenuates increases in miR-27a levels in the lung and attenuates hypoxia-induced PH (37), we sought to examine the impact of PPARγ activation on miR-27a levels in SS mouse lungs and hemin-treated HPAECs. As shown in Figure 6, consistent with Figures 2 and 3, miR-27a was significantly increased in SS mouse lungs, whereas PPARγ levels were significantly reduced. Treating mice with the PPARγ ligand, RSG, using a regimen that attenuated hypoxia-induced PH (32), attenuated increases in miR-27a and ETS1 mRNA levels and restored PPARγ levels in SS mouse lungs (Figures 6A–6C). Similarly, in HPAECs treated with hemin for 72 hours, treatment with RSG during the last 24 hours attenuated hemin-induced increases in miR-27a and ETS1 and decreases in PPARγ (Figures 6D–6F). To further examine phenotypic changes in hemin-treated HPAECs and the effect of RSG on hemin-induced cell proliferation, HPAEC proliferation was measured with MTT assays. Figure E7 shows that hemin significantly increased HPAEC proliferation and that RSG treatment attenuated hemin-induced HPAEC proliferation. Taken together, the findings in Figures 3–5 provide compelling evidence that miR-27a down-regulates PPARγ to stimulate ET-1 expression and endothelial dysfunction, and that activation of PPARγ can negatively regulate miR-27a in these conditions.
Figure 6.
The PPARγ ligand rosiglitazone (RSG) attenuates increases in miR-27a and ETS1 levels and reductions in PPARγ in SS mouse lung and hemin-treated HPAECs. (A) Whole lung homogenates were collected from AA and SS mice. Selected AA and SS mice were gavaged with RSG (10 mg/kg/d) or vehicle for 10 days. qRT-PCR was performed on AA and SS lung tissues. Lung miR-27a levels are expressed relative to RNU6B and normalized to control values. Each bar represents the mean ± SE. *P < 0.05 versus AA/−RSG; +P < 0.05 versus SS/−RSG, (n = 3–6). Lung (B) PPARγ and (C) ETS1 mRNA levels were measured with qRT-PCR and expressed relative to lung 9S mRNA levels. Each bar represents the mean PPARγ and ETS1 mRNA level ± SE expressed as fold change versus AA/−RSG. *P < 0.05 versus AA/−RSG; +P < 0.05 versus SS/−RSG (n = 3–6). HPAECs were treated with dimethyl sulfoxide vehicle (CON) or hemin (HEM, 5 μM) for 72 hours. During the final 24 hours of hemin exposure, selected HPAECs were treated with or without RSG (10 μM). HPAECs were then collected, and miRNAs or total RNAs were isolated. qRT-PCR was performed for (D) miR-27a, (E) PPARγ, and (F) ETS1 levels. Each bar represents the mean ± SE miR-27a, PPARγ, or ET-1 level relative to RNU6B or 9S as indicated. *P < 0.05 versus CON/−RSG; +P < 0.05 versus HEM/−RSG (n = 3–6).
Discussion
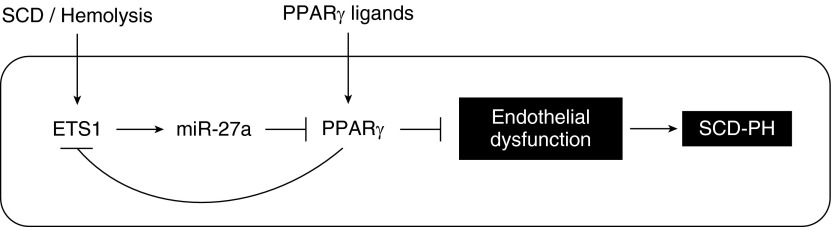
In the current study, we demonstrate, we believe for the first time, that (1) the Townes humanized SS mouse model spontaneously develops PH, RVH, and pulmonary vascular remodeling, (2) PPARγ levels are reduced in lungs from SS mice and in hemin-treated HPAECs, and (3) miR-27a levels are increased in SS mouse lung and in hemin-treated HPAECs through pathways involving the ETS1 transcription factor. Furthermore, reductions in PPARγ promote increased expression of ET-1 and markers of endothelial dysfunction in vivo and in vitro. Activation of PPARγ with the pharmacological ligand RSG or with adenoviral-mediated PPARγ overexpression attenuates these derangements, suggesting that therapeutically targeting PPARγ provides a novel strategy for reducing SCD-associated pulmonary endothelial dysfunction and PH (Figure 7).
Figure 7.
Schema of putative pathways increasing miR-27a and reducing PPARγ that increase ET-1 and endothelial dysfunction in sickle cell disease (SCD). The current findings suggest that products of hemolysis in SCD, such as hemin, activate the transcription factor ETS1 to stimulate the expression of miR-27a. Increases in miR-27a reduce PPARγ, leading to increased ET-1, endothelial dysfunction, and pulmonary hypertension (PH). Stimulation of PPARγ with pharmacological ligands such as RSG attenuates increases in miR-27a and downstream ET-1 levels and endothelial dysfunction, suggesting that this pathway may provide an opportunity for new therapeutic targeting and intervention in SCD-PH.
ET-1 is elevated in both patients with SCD and those with PH and plays a key role in the pathophysiology of SCD and PH. Although several potential therapeutic strategies have been investigated in SCD-PH, none have significantly affected SCD-PH outcomes (11, 75–79). The importance of ET-1 in the pathogenesis of SCD-PH is well established (80–83). ET-1 causes endothelial dysfunction and stimulates vasoconstriction and migration and proliferation of pulmonary vascular wall cells in PH (81, 84). However, in clinical trials in SCD-PH, the ET-1 receptor antagonist, bosentan, failed to significantly reduce pulmonary vascular resistance (85). Thus, despite existing evidence that ET-1 plays an important pathophysiological role in SCD-PH pathogenesis, new therapeutic strategies that regulate ET-1 as well as other pathways involved in SCD-PH may lead to more effective and widely applicable therapeutic approaches for SCD-PH. We demonstrated previously that PPARγ ligands favorably modulate several components of the ET-1 signaling pathway (46) and also attenuate other proliferative signaling pathways including nicotinamide adenine dinucleotide phosphate oxidase 4 (32), platelet-derived growth factor (32), and thrombospondin-1 (86) in hypoxia-induced PH pathogenesis. These findings indicate that the therapeutic potential of targeting PPARγ in SCD-PH merits additional evaluation and led us to the current study.
Chronic hemolysis in SCD stimulates steady-state inflammation (87, 88) through subclinical vascular endothelial injury and transient vasoocclusive events (70, 89). The vascular endothelium in the lung and other organs senses these SCD-related stimuli including hypoxia, ischemia-reperfusion, hemoglobin, and hemin. Studies in SS mice demonstrate a role for adhesion molecules in the interaction of erythrocytes with the endothelium (22, 90–93). Furthermore, adhesion molecules are increased in SCD and are associated with PH and mortality (21). Current evidence suggests that SCD-related hemolysis and its byproducts, hemoglobin and hemin, induce endothelial dysfunction and PH in SCD. We postulated that these hemolysis-induced derangements in endothelial function play an important role in overall pulmonary vascular dysfunction, transducing signals from the blood to other cellular compartments of the vascular wall (72, 73). Our findings are consistent with these concepts and demonstrate that endothelial adhesion molecules, including E-SEL and PECAM1, are increased significantly in the lungs of SS mice and in hemin-treated HPAECs (Figure E3). Hemin triggers vasoocclusion through increases in and activation of these adhesion molecules (90). The current studies demonstrate that PPARγ activation attenuates endothelial dysfunction in the SS mouse lung and in hemin-treated HPAECs (Figures 5 and 6), supporting the concept that therapeutic targeting of this receptor provides a novel strategy for reducing SCD-PH. In addition, unpublished studies by our group indicate that PPARγ activation may also exert direct and beneficial effects on the myocardium in experimental models of PH.
We reported recently that hypoxia-induced PH was associated with reductions in PPARγ, and that activating the PPARγ receptor attenuates hypoxia-induced reductions in PPARγ in mouse lungs and HPAECs (32, 46). The current study extends these observations by providing novel evidence that PPARγ is reduced in the lungs of SS but not AA mice. Because treatment with pathobiologically relevant concentrations of hemin was sufficient to reduce PPARγ in HPAECs in vitro (Figure 1), these findings suggest that products of intravascular hemolysis trigger these reductions in PPARγ. Furthermore, our findings indicate that PPARγ activation restores PPARγ in SS mouse lung and in hemin-treated HPAECs (Figures 5 and 6), supporting the concept that stimulating PPARγ may provide a novel therapeutic strategy in SCD-PH.
We demonstrated previously that hypoxic exposure sufficient to induce PH in vivo and pulmonary artery EC proliferation in vitro increased miR-27a and reduced PPARγ (37). Furthermore, PPARγ activation attenuated hypoxia-induced reductions in PPARγ by attenuating hypoxia-induced increases in miR-27a levels. Emerging evidence supports the role of miRNAs in PH pathogenesis (94). Both miR-648 (95) and miR-199a2 (96) were reported to contribute to SCD pathogenesis in the Berkeley SS mouse. Our findings extend these observations to suggest that miR-27a also contributes to SCD-PH. Levels of miR-27a were increased in the lungs of SS mice and hemin-treated HPAECs (Figure 3). Anti–miR-mediated inhibition of miR-27a (Figure 3), activation of PPARγ with RSG (Figure 6), or overexpression of PPARγ (Figure 5) attenuated increases in miR-27a levels in SS mouse lung and HPAECs. These results provide novel evidence that PPARγ activation suppresses increases in miR-27a in SCD, which is consistent with previous reports of the repressive effect of PPARγ activation on miR-27a under hypoxic conditions (37).
To further explore the mechanisms for increases in miR-27a levels in SCD, we performed an in silico analysis of the upstream promoter region of miR-27a and identified putative ETS1 binding sites. Recent studies reported that hemin increases ETS1 mRNA levels in human erythroleukemic cell lines (97), that dominant-negative loss of PPARγ function increases ETS1 expression (39), and that PPARγ activation attenuates ETS1 activity (36). Therefore, to further investigate whether ETS1 activates the miR-27a promoter in SCD and whether it might also be regulated by PPARγ, we examined the effects of PPARγ activation on ETS1 in SS mouse lung and in hemin-treated HPAECs. The importance of ETS1 was supported by evidence that small interfering RNA-mediated ETS1 depletion attenuated hemin-induced (1) increases in HPAEC miR-27a and ET-1 and (2) decreases in PPARγ expression (Figure 4). Taken together, these novel findings indicate that hemin and perhaps other stimuli in SCD can activate ETS transcription factors to increase miR-27a levels. As reported previously (37), miR-27a reduces PPARγ, which attenuates ET-1 levels and prevents endothelial dysfunction. Because of the mutually repressive relationship between miR-27a and PPARγ (37), our findings suggest that stimulating the PPARγ receptor with exogenous PPARγ ligands can reverse pathogenic programs of gene expression in SCD. These findings highlight a previously unrecognized pathobiological cascade in SCD that may be amenable to therapeutic intervention.
This study has several important limitations. First, although the current study demonstrates that hemin increases ETS1 and miR-27a levels, it does not directly address how hemin increases ETS1 levels. ETS1 knockdown attenuated hemin-induced increases in miR-27a levels and increased PPARγ mRNA and protein levels in HPAECs. These findings indicate that ETS1 stimulates miR-27a expression in HPAECs and suggest that PPARγ activation could attenuate ETS1 and miR-27a promoter activity through transrepression. Therefore, additional studies will be required to further define how hemin directly stimulates ETS1. Second, although discordant patterns of miRNA expression have been reported in experimental rodent models and in human PH (98), reductions in PPARγ have been reported in several experimental models of PH (28, 30, 32, 33, 46, 99), in human PAH lung tissue (33), and in pulmonary artery ECs isolated from patients with idiopathic pulmonary arterial hypertension (37). These findings suggest that common mechanisms that regulate PPARγ during PH pathogenesis in multiple models and species merit additional investigation and may provide new insights into the pathobiology and therapy of PH. Finally, the current study used RSG as a pharmacological agonist to stimulate PPARγ activity. RSG is a TZD PPARγ ligand currently used in the clinical management of patients with type 2 diabetes. However, RSG, as well as other drugs in this class, has been recognized to have significant adverse effects in patients with diabetes (100). Cardiovascular responses elicited by different TZD PPARγ ligands in human subjects are distinct. Furthermore, it is difficult to extrapolate the therapeutic effects of drugs across different groups of PH (101). Thus, although the current study, together with other reports, suggests therapeutic benefits of pharmacological PPARγ activation in PH, the potential of this therapeutic strategy will require additional study and more refined pharmacological tools that activate PPARγ without causing adverse effects.
Conclusions
To our knowledge, the current study of SCD-PH pathogenesis provides the first evidence that miR-27a reduces PPARγ expression and increases ET-1 expression and endothelial dysfunction through post-transcriptional mechanisms in vivo and in vitro. Furthermore, this study provides novel evidence that ETS1 mRNA levels were increased in SS mouse lungs and in hemin-treated HPAECs and that ETS1 loss of function was sufficient to attenuate hemin-induced increases in HPAEC miR-27a and ET-1 and decreases in PPARγ expression. These results suggest that targeting PPARγ activation may represent a novel therapeutic approach in SCD-PH pathogenesis.
Acknowledgments
Acknowledgments
The authors thank Dr. Claudia R. Morris for her careful review and valuable editorial comments in preparation of the manuscript. They also thank Dr. Solomon F. Ofori-Acquah, Dr. Samit Ghosh, and Dr. Fang Tan for their assistance with and critical input into the initial concepts and design of this study.
Footnotes
This work was supported by Veterans Affairs Basic Laboratory Research and Development Merit Review Award 1I01BX001910 (C.M.H.), National Institutes of Health National Heart, Lung, and Blood Institute R01 Grant HL102167 (C.M.H. and R.L.S.), Emory/Children’s Healthcare of Atlanta Grant CEB F16788-00 (B.-Y.K.), and American Heart Association National Scientist Development Grant 13SDG14150004 (B.-Y.K.).
The contents of this report represent the views of the authors and do not represent the views of the Department of Veterans Affairs or the U.S. Government.
Author Contributions: Conception, hypothesis delineation, and design: B.-Y.K. and C.M.H.; acquisition of data, analysis, and interpretation: B.-Y.K., K.P., J.M.K., T.C.M., R.L.S., D.A., and C.M.H.; and writing of the article: B.-Y.K. and C.M.H.
This article has an online data supplement, which is accessible from this issue's table of contents online at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0166OC on September 9, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ingram VM. A specific chemical difference between the globins of normal human and sickle-cell anaemia haemoglobin. Nature. 1956;178:792–794. doi: 10.1038/178792a0. [DOI] [PubMed] [Google Scholar]
- 2.Ingram VM. Gene mutations in human haemoglobin: the chemical difference between normal and sickle cell haemoglobin. Nature. 1957;180:326–328. doi: 10.1038/180326a0. [DOI] [PubMed] [Google Scholar]
- 3.Lopez BL, Davis-Moon L, Ballas SK, Ma XL. Sequential nitric oxide measurements during the emergency department treatment of acute vasoocclusive sickle cell crisis. Am J Hematol. 2000;64:15–19. doi: 10.1002/(sici)1096-8652(200005)64:1<15::aid-ajh3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 4.Morris CR, Kuypers FA, Larkin S, Vichinsky EP, Styles LA. Patterns of arginine and nitric oxide in patients with sickle cell disease with vaso-occlusive crisis and acute chest syndrome. J Pediatr Hematol Oncol. 2000;22:515–520. doi: 10.1097/00043426-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med. 2010;38(4) Suppl:S512–S521. doi: 10.1016/j.amepre.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 6.Barst RJ, McGoon M, Torbicki A, Sitbon O, Krowka MJ, Olschewski H, Gaine S. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(12) Suppl S:40S–47S. doi: 10.1016/j.jacc.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 7.Galiè N, Manes A, Negro L, Palazzini M, Bacchi-Reggiani ML, Branzi A. A meta-analysis of randomized controlled trials in pulmonary arterial hypertension. Eur Heart J. 2009;30:394–403. doi: 10.1093/eurheartj/ehp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lilienfeld DE, Rubin LJ. Mortality from primary pulmonary hypertension in the United States, 1979-1996. Chest. 2000;117:796–800. doi: 10.1378/chest.117.3.796. [DOI] [PubMed] [Google Scholar]
- 9.Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, Klug PP. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 10.Powars D, Weidman JA, Odom-Maryon T, Niland JC, Johnson C. Sickle cell chronic lung disease: prior morbidity and the risk of pulmonary failure. Medicine (Baltimore) 1988;67:66–76. [PubMed] [Google Scholar]
- 11.Castro O, Hoque M, Brown BD. Pulmonary hypertension in sickle cell disease: cardiac catheterization results and survival. Blood. 2003;101:1257–1261. doi: 10.1182/blood-2002-03-0948. [DOI] [PubMed] [Google Scholar]
- 12.Fonseca GH, Souza R, Salemi VM, Jardim CV, Gualandro SF. Pulmonary hypertension diagnosed by right heart catheterisation in sickle cell disease. Eur Respir J. 2012;39:112–118. doi: 10.1183/09031936.00134410. [DOI] [PubMed] [Google Scholar]
- 13.Parent F, Bachir D, Inamo J, Lionnet F, Driss F, Loko G, Habibi A, Bennani S, Savale L, Adnot S, et al. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med. 2011;365:44–53. doi: 10.1056/NEJMoa1005565. [DOI] [PubMed] [Google Scholar]
- 14.Mehari A, Gladwin MT, Tian X, Machado RF, Kato GJ. Mortality in adults with sickle cell disease and pulmonary hypertension. JAMA. 2012;307:1254–1256. doi: 10.1001/jama.2012.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ergul S, Brunson CY, Hutchinson J, Tawfik A, Kutlar A, Webb RC, Ergul A. Vasoactive factors in sickle cell disease: in vitro evidence for endothelin-1-mediated vasoconstriction. Am J Hematol. 2004;76:245–251. doi: 10.1002/ajh.20107. [DOI] [PubMed] [Google Scholar]
- 16.Graido-Gonzalez E, Doherty JC, Bergreen EW, Organ G, Telfer M, McMillen MA. Plasma endothelin-1, cytokine, and prostaglandin E2 levels in sickle cell disease and acute vaso-occlusive sickle crisis. Blood. 1998;92:2551–2555. [PubMed] [Google Scholar]
- 17.Bunn HF, Nathan DG, Dover GJ, Hebbel RP, Platt OS, Rosse WF, Ware RE. Pulmonary hypertension and nitric oxide depletion in sickle cell disease. Blood. 2010;116:687–692. doi: 10.1182/blood-2010-02-268193. [DOI] [PubMed] [Google Scholar]
- 18.Hsu LL, Champion HC, Campbell-Lee SA, Bivalacqua TJ, Manci EA, Diwan BA, Schimel DM, Cochard AE, Wang X, Schechter AN, et al. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood. 2007;109:3088–3098. doi: 10.1182/blood-2006-08-039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stuart MJ, Setty BN. Sickle cell acute chest syndrome: pathogenesis and rationale for treatment. Blood. 1999;94:1555–1560. [PubMed] [Google Scholar]
- 20.Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, Hazen SL, Vichinsky EP, Morris SM, Jr, Gladwin MT. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato GJ, Martyr S, Blackwelder WC, Nichols JS, Coles WA, Hunter LA, Brennan ML, Hazen SL, Gladwin MT. Levels of soluble endothelium-derived adhesion molecules in patients with sickle cell disease are associated with pulmonary hypertension, organ dysfunction, and mortality. Br J Haematol. 2005;130:943–953. doi: 10.1111/j.1365-2141.2005.05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaul DK, Liu XD, Chang HY, Nagel RL, Fabry ME. Effect of fetal hemoglobin on microvascular regulation in sickle transgenic-knockout mice. J Clin Invest. 2004;114:1136–1145. doi: 10.1172/JCI21633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frei AC, Guo Y, Jones DW, Pritchard KA, Jr, Fagan KA, Hogg N, Wandersee NJ. Vascular dysfunction in a murine model of severe hemolysis. Blood. 2008;112:398–405. doi: 10.1182/blood-2007-12-126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Townes TM. Gene replacement therapy for sickle cell disease and other blood disorders. Hematology (Am Soc Hematol Educ Program) 2008:193–196. doi: 10.1182/asheducation-2008.1.193. [DOI] [PubMed] [Google Scholar]
- 25.De Franceschi L, Platt OS, Malpeli G, Janin A, Scarpa A, Leboeuf C, Beuzard Y, Payen E, Brugnara C. Protective effects of phosphodiesterase-4 (PDE-4) inhibition in the early phase of pulmonary arterial hypertension in transgenic sickle cell mice. FASEB J. 2008;22:1849–1860. doi: 10.1096/fj.07-098921. [DOI] [PubMed] [Google Scholar]
- 26.Sabaa N, de Franceschi L, Bonnin P, Castier Y, Malpeli G, Debbabi H, Galaup A, Maier-Redelsperger M, Vandermeersch S, Scarpa A, et al. Endothelin receptor antagonism prevents hypoxia-induced mortality and morbidity in a mouse model of sickle-cell disease. J Clin Invest. 2008;118:1924–1933. doi: 10.1172/JCI33308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu LC, Sun CW, Ryan TM, Pawlik KM, Ren J, Townes TM. Correction of sickle cell disease by homologous recombination in embryonic stem cells. Blood. 2006;108:1183–1188. doi: 10.1182/blood-2006-02-004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crossno JT, Jr, Garat CV, Reusch JE, Morris KG, Dempsey EC, McMurtry IF, Stenmark KR, Klemm DJ. Rosiglitazone attenuates hypoxia-induced pulmonary arterial remodeling. Am J Physiol Lung Cell Mol Physiol. 2007;292:L885–L897. doi: 10.1152/ajplung.00258.2006. [DOI] [PubMed] [Google Scholar]
- 29.Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW, et al. An antiproliferative BMP-2/PPARγ/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest. 2008;118:1846–1857. doi: 10.1172/JCI32503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim EK, Lee JH, Oh YM, Lee YS, Lee SD. Rosiglitazone attenuates hypoxia-induced pulmonary arterial hypertension in rats. Respirology. 2010;15:659–668. doi: 10.1111/j.1440-1843.2010.01756.x. [DOI] [PubMed] [Google Scholar]
- 31.Matsuda Y, Hoshikawa Y, Ameshima S, Suzuki S, Okada Y, Tabata T, Sugawara T, Matsumura Y, Kondo T.[Effects of peroxisome proliferator-activated receptor gamma ligands on monocrotaline-induced pulmonary hypertension in rats[in Japanese]. Nihon Kokyuki Gakkai Zasshi 200543283–288. [PubMed] [Google Scholar]
- 32.Nisbet RE, Bland JM, Kleinhenz DJ, Mitchell PO, Walp ER, Sutliff RL, Hart CM. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. Am J Respir Cell Mol Biol. 2010;42:482–490. doi: 10.1165/rcmb.2008-0132OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ameshima S, Golpon H, Cool CD, Chan D, Vandivier RW, Gardai SJ, Wick M, Nemenoff RA, Geraci MW, Voelkel NF. Peroxisome proliferator-activated receptor gamma (PPARγ) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ Res. 2003;92:1162–1169. doi: 10.1161/01.RES.0000073585.50092.14. [DOI] [PubMed] [Google Scholar]
- 34.Guignabert C, Alvira CM, Alastalo TP, Sawada H, Hansmann G, Zhao M, Wang L, El-Bizri N, Rabinovitch M. Tie2-mediated loss of peroxisome proliferator-activated receptor-γ in mice causes PDGF receptor-β-dependent pulmonary arterial muscularization. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1082–L1090. doi: 10.1152/ajplung.00199.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ricote M, Glass CK. PPARs and molecular mechanisms of transrepression. Biochim Biophys Acta. 2007;1771:926–935. doi: 10.1016/j.bbalip.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okada M, Yan SF, Pinsky DJ. Peroxisome proliferator-activated receptor-γ (PPAR-γ) activation suppresses ischemic induction of Egr-1 and its inflammatory gene targets. FASEB J. 2002;16:1861–1868. doi: 10.1096/fj.02-0503com. [DOI] [PubMed] [Google Scholar]
- 37.Kang BY, Park KK, Green DE, Bijli KM, Searles CD, Sutliff RL, Hart CM. Hypoxia mediates mutual repression between microRNA-27a and PPARγ in the pulmonary vasculature. PLoS One. 2013;8:e79503. doi: 10.1371/journal.pone.0079503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han S, Ritzenthaler JD, Rivera HN, Roman J. Peroxisome proliferator-activated receptor-γ ligands suppress fibronectin gene expression in human lung carcinoma cells: involvement of both CRE and Sp1. Am J Physiol Lung Cell Mol Physiol. 2005;289:L419–L428. doi: 10.1152/ajplung.00002.2005. [DOI] [PubMed] [Google Scholar]
- 39.Meredith D, Panchatcharam M, Miriyala S, Tsai YS, Morris AJ, Maeda N, Stouffer GA, Smyth SS. Dominant-negative loss of PPARγ function enhances smooth muscle cell proliferation, migration, and vascular remodeling. Arterioscler Thromb Vasc Biol. 2009;29:465–471. doi: 10.1161/ATVBAHA.109.184234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogawa D, Nomiyama T, Nakamachi T, Heywood EB, Stone JF, Berger JP, Law RE, Bruemmer D. Activation of peroxisome proliferator-activated receptor γ suppresses telomerase activity in vascular smooth muscle cells. Circ Res. 2006;98:e50–e59. doi: 10.1161/01.RES.0000218271.93076.c3. [DOI] [PubMed] [Google Scholar]
- 41.Watson DK, McWilliams MJ, Lapis P, Lautenberger JA, Schweinfest CW, Papas TS. Mammalian ETS-1 and ETS-2 genes encode highly conserved proteins. Proc Natl Acad Sci USA. 1988;85:7862–7866. doi: 10.1073/pnas.85.21.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Findlay VJ, LaRue AC, Turner DP, Watson PM, Watson DK. Understanding the role of ETS-mediated gene regulation in complex biological processes. Adv Cancer Res. 2013;119:1–61. doi: 10.1016/B978-0-12-407190-2.00001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goetze S, Kintscher U, Kim S, Meehan WP, Kaneshiro K, Collins AR, Fleck E, Hsueh WA, Law RE. Peroxisome proliferator-activated receptor-γ ligands inhibit nuclear but not cytosolic extracellular signal-regulated kinase/mitogen-activated protein kinase-regulated steps in vascular smooth muscle cell migration. J Cardiovasc Pharmacol. 2001;38:909–921. doi: 10.1097/00005344-200112000-00013. [DOI] [PubMed] [Google Scholar]
- 44.Lu X, Bijli KM, Ramirez A, Murphy TC, Kleinhenz J, Michael Hart C. Hypoxia downregulates PPARγ via an ERK 1/2-NF-κB-Nox4-dependent mechanism in human pulmonary artery smooth muscle cells. Free Radic Biol Med. 2013;63:151–160. doi: 10.1016/j.freeradbiomed.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu X, Murphy TC, Nanes MS, Hart CM. PPARγ regulates hypoxia-induced Nox4 expression in human pulmonary artery smooth muscle cells through NF-κB. Am J Physiol Lung Cell Mol Physiol. 2010;299:L559–L566. doi: 10.1152/ajplung.00090.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang BY, Kleinhenz JM, Murphy TC, Hart CM. The PPARγ ligand rosiglitazone attenuates hypoxia-induced endothelin signaling in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 2011;301:L881–L891. doi: 10.1152/ajplung.00195.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kleinhenz JM, Kleinhenz DJ, You S, Ritzenthaler JD, Hansen JM, Archer DR, Sutliff RL, Hart CM. Disruption of endothelial peroxisome proliferator-activated receptor-gamma reduces vascular nitric oxide production. Am J Physiol Heart Circ Physiol. 2009;297:H1647–H1654. doi: 10.1152/ajpheart.00148.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caruso P, MacLean MR, Khanin R, McClure J, Soon E, Southgate M, MacDonald RA, Greig JA, Robertson KE, Masson R, et al. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol. 2010;30:716–723. doi: 10.1161/ATVBAHA.109.202028. [DOI] [PubMed] [Google Scholar]
- 49.Drake KM, Zygmunt D, Mavrakis L, Harbor P, Wang L, Comhair SA, Erzurum SC, Aldred MA. Altered microRNA processing in heritable pulmonary arterial hypertension: an important role for Smad-8. Am J Respir Crit Care Med. 2011;184:1400–1408. doi: 10.1164/rccm.201106-1130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo L, Qiu Z, Wei L, Yu X, Gao X, Jiang S, Tian H, Jiang C, Zhu D. The microRNA-328 regulates hypoxic pulmonary hypertension by targeting at insulin growth factor 1 receptor and L-type calcium channel-α1C. Hypertension. 2012;59:1006–1013. doi: 10.1161/HYPERTENSIONAHA.111.185413. [DOI] [PubMed] [Google Scholar]
- 51.Pullamsetti SS, Doebele C, Fischer A, Savai R, Kojonazarov B, Dahal BK, Ghofrani HA, Weissmann N, Grimminger F, Bonauer A, et al. Inhibition of microRNA-17 improves lung and heart function in experimental pulmonary hypertension. Am J Respir Crit Care Med. 2012;185:409–419. doi: 10.1164/rccm.201106-1093OC. [DOI] [PubMed] [Google Scholar]
- 52.Yang S, Banerjee S, Freitas Ad, Cui H, Xie N, Abraham E, Liu G. miR-21 regulates chronic hypoxia-induced pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol. 2012;302:L521–L529. doi: 10.1152/ajplung.00316.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 54.Di Guardo A, Profeta G, Crisafulli C, Sidoti G, Zammataro M, Paolini I, Filippi A. Obstructive sleep apnoea in patients with obesity and hypertension. Br J Gen Pract. 2010;60:325–328. doi: 10.3399/bjgp10X484174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brock M, Samillan VJ, Trenkmann M, Schwarzwald C, Ulrich S, Gay RE, Gassmann M, Ostergaard L, Gay S, Speich R, et al. AntagomiR directed against miR-20a restores functional BMPR2 signalling and prevents vascular remodelling in hypoxia-induced pulmonary hypertension. Eur Heart J. 2014;35:3203–3211. doi: 10.1093/eurheartj/ehs060. [DOI] [PubMed] [Google Scholar]
- 56.Courboulin A, Paulin R, Giguère NJ, Saksouk N, Perreault T, Meloche J, Paquet ER, Biardel S, Provencher S, Côté J, et al. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med. 2011;208:535–548. doi: 10.1084/jem.20101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bockmeyer CL, Maegel L, Janciauskiene S, Rische J, Lehmann U, Maus UA, Nickel N, Haverich A, Hoeper MM, Golpon HA, et al. Plexiform vasculopathy of severe pulmonary arterial hypertension and microRNA expression. J Heart Lung Transplant. 2012;31:764–772. doi: 10.1016/j.healun.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 58.Hassan T, McKiernan PJ, McElvaney NG, Cryan SA, Greene CM. Therapeutic modulation of miRNA for the treatment of proinflammatory lung diseases. Expert Rev Anti Infect Ther. 2012;10:359–368. doi: 10.1586/eri.11.175. [DOI] [PubMed] [Google Scholar]
- 59.Joshi SR, McLendon JM, Comer BS, Gerthoffer WT. MicroRNAs-control of essential genes: implications for pulmonary vascular disease. Pulm Circ. 2011;1:357–364. doi: 10.4103/2045-8932.87301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.White K, Loscalzo J, Chan SY. Holding our breath: the emerging and anticipated roles of microRNA in pulmonary hypertension. Pulm Circ. 2012;2:278–290. doi: 10.4103/2045-8932.101395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ghosh S, Tan F, Yu T, Li Y, Adisa O, Mosunjac M, Ofori-Acquah SF. Global gene expression profiling of endothelium exposed to heme reveals an organ-specific induction of cytoprotective enzymes in sickle cell disease. PLoS One. 2011;6:e18399. doi: 10.1371/journal.pone.0018399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, III, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 63.Kang BY, Park KK, Kleinhenz JM, Murphy TC, Green DE, Bijli KM, Yeligar SM, Carthan KA, Searles CD, Sutliff RL, et al. Peroxisome proliferator-activated receptor γ and microRNA 98 in hypoxia-induced endothelin-1 signaling. Am J Respir Cell Mol Biol. 2016;54:136–146. doi: 10.1165/rcmb.2014-0337OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 65.Sutton LL, Castro O, Cross DJ, Spencer JE, Lewis JF. Pulmonary hypertension in sickle cell disease. Am J Cardiol. 1994;74:626–628. doi: 10.1016/0002-9149(94)90760-9. [DOI] [PubMed] [Google Scholar]
- 66.Hammerman SI, Kourembanas S, Conca TJ, Tucci M, Brauer M, Farber HW. Endothelin-1 production during the acute chest syndrome in sickle cell disease. Am J Respir Crit Care Med. 1997;156:280–285. doi: 10.1164/ajrccm.156.1.9611085. [DOI] [PubMed] [Google Scholar]
- 67.Rybicki AC, Benjamin LJ. Increased levels of endothelin-1 in plasma of sickle cell anemia patients. Blood. 1998;92:2594–2596. [PubMed] [Google Scholar]
- 68.Werdehoff SG, Moore RB, Hoff CJ, Fillingim E, Hackman AM. Elevated plasma endothelin-1 levels in sickle cell anemia: relationships to oxygen saturation and left ventricular hypertrophy. Am J Hematol. 1998;58:195–199. doi: 10.1002/(sici)1096-8652(199807)58:3<195::aid-ajh6>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 69.Belhassen L, Pelle G, Sediame S, Bachir D, Carville C, Bucherer C, Lacombe C, Galacteros F, Adnot S. Endothelial dysfunction in patients with sickle cell disease is related to selective impairment of shear stress-mediated vasodilation. Blood. 2001;97:1584–1589. doi: 10.1182/blood.v97.6.1584. [DOI] [PubMed] [Google Scholar]
- 70.Minneci PC, Deans KJ, Zhi H, Yuen PS, Star RA, Banks SM, Schechter AN, Natanson C, Gladwin MT, Solomon SB. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115:3409–3417. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Potoka KP, Gladwin MT. Vasculopathy and pulmonary hypertension in sickle cell disease. Am J Physiol Lung Cell Mol Physiol. 2015;308:L314–L324. doi: 10.1152/ajplung.00252.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghosh S, Adisa OA, Chappa P, Tan F, Jackson KA, Archer DR, Ofori-Acquah SF. Extracellular hemin crisis triggers acute chest syndrome in sickle mice. J Clin Invest. 2013;123:4809–4820. doi: 10.1172/JCI64578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ghosh S, Tan F, Ofori-Acquah SF. Spatiotemporal dysfunction of the vascular permeability barrier in transgenic mice with sickle cell disease. Anemia. 2012;2012:582018. doi: 10.1155/2012/582018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bandyopadhyay S, Bhattacharyya M. PuTmiR: a database for extracting neighboring transcription factors of human microRNAs. BMC Bioinformatics. 2010;11:190. doi: 10.1186/1471-2105-11-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gladwin MT, Schechter AN. Nitric oxide therapy in sickle cell disease. Semin Hematol. 2001;38:333–342. doi: 10.1016/s0037-1963(01)90027-7. [DOI] [PubMed] [Google Scholar]
- 76.Little JA, Hauser KP, Martyr SE, Harris A, Maric I, Morris CR, Suh JH, Taylor J, Castro O, Machado R, et al. Hematologic, biochemical, and cardiopulmonary effects of L-arginine supplementation or phosphodiesterase 5 inhibition in patients with sickle cell disease who are on hydroxyurea therapy. Eur J Haematol. 2009;82:315–321. doi: 10.1111/j.1600-0609.2009.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Machado RF, Martyr S, Kato GJ, Barst RJ, Anthi A, Robinson MR, Hunter L, Coles W, Nichols J, Hunter C, et al. Sildenafil therapy in patients with sickle cell disease and pulmonary hypertension. Br J Haematol. 2005;130:445–453. doi: 10.1111/j.1365-2141.2005.05625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morris CR, Morris SM, Jr, Hagar W, Van Warmerdam J, Claster S, Kepka-Lenhart D, Machado L, Kuypers FA, Vichinsky EP. Arginine therapy: a new treatment for pulmonary hypertension in sickle cell disease? Am J Respir Crit Care Med. 2003;168:63–69. doi: 10.1164/rccm.200208-967OC. [DOI] [PubMed] [Google Scholar]
- 79.Sullivan KJ, Goodwin SR, Evangelist J, Moore RD, Mehta P. Nitric oxide successfully used to treat acute chest syndrome of sickle cell disease in a young adolescent. Crit Care Med. 1999;27:2563–2568. doi: 10.1097/00003246-199911000-00039. [DOI] [PubMed] [Google Scholar]
- 80.Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, Kimura S, Masaki T, Duguid WP, Stewart DJ. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 81.McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006;114:1417–1431. doi: 10.1161/CIRCULATIONAHA.104.503540. [DOI] [PubMed] [Google Scholar]
- 82.Stewart DJ, Levy RD, Cernacek P, Langleben D. Increased plasma endothelin-1 in pulmonary hypertension: marker or mediator of disease? Ann Intern Med. 1991;114:464–469. doi: 10.7326/0003-4819-114-6-464. [DOI] [PubMed] [Google Scholar]
- 83.Yoshibayashi M, Nishioka K, Nakao K, Saito Y, Matsumura M, Ueda T, Temma S, Shirakami G, Imura H, Mikawa H. Plasma endothelin concentrations in patients with pulmonary hypertension associated with congenital heart defects. Evidence for increased production of endothelin in pulmonary circulation. Circulation. 1991;84:2280–2285. doi: 10.1161/01.cir.84.6.2280. [DOI] [PubMed] [Google Scholar]
- 84.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 85.Barst RJ, Mubarak KK, Machado RF, Ataga KI, Benza RL, Castro O, Naeije R, Sood N, Swerdlow PS, Hildesheim M, et al. ASSET study group. Exercise capacity and haemodynamics in patients with sickle cell disease with pulmonary hypertension treated with bosentan: results of the ASSET studies. Br J Haematol. 2010;149:426–435. doi: 10.1111/j.1365-2141.2010.08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Green DE, Kang BY, Murphy TC, Hart CM. Peroxisome proliferator-activated receptor gamma (PPARγ) regulates thrombospondin-1 and Nox4 expression in hypoxia-induced human pulmonary artery smooth muscle cell proliferation. Pulm Circ. 2012;2:483–491. doi: 10.4103/2045-8932.105037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 88.Liang X, Lin T, Sun G, Beasley-Topliffe L, Cavaillon JM, Warren HS. Hemopexin down-regulates LPS-induced proinflammatory cytokines from macrophages. J Leukoc Biol. 2009;86:229–235. doi: 10.1189/jlb.1208742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ofori-Acquah SF, King J, Voelkel N, Schaphorst KL, Stevens T. Heterogeneity of barrier function in the lung reflects diversity in endothelial cell junctions. Microvasc Res. 2008;75:391–402. doi: 10.1016/j.mvr.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Belcher JD, Chen C, Nguyen J, Milbauer L, Abdulla F, Alayash AI, Smith A, Nath KA, Hebbel RP, Vercellotti GM. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 2014;123:377–390. doi: 10.1182/blood-2013-04-495887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Embury SH, Matsui NM, Ramanujam S, Mayadas TN, Noguchi CT, Diwan BA, Mohandas N, Cheung AT. The contribution of endothelial cell P-selectin to the microvascular flow of mouse sickle erythrocytes in vivo. Blood. 2004;104:3378–3385. doi: 10.1182/blood-2004-02-0713. [DOI] [PubMed] [Google Scholar]
- 92.Mahaseth H, Vercellotti GM, Welch TE, Bowlin PR, Sonbol KM, Hsia CJ, Li M, Bischof JC, Hebbel RP, Belcher JD. Polynitroxyl albumin inhibits inflammation and vasoocclusion in transgenic sickle mice. J Lab Clin Med. 2005;145:204–211. doi: 10.1016/j.lab.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 93.Setty BN, Stuart MJ. Vascular cell adhesion molecule-1 is involved in mediating hypoxia-induced sickle red blood cell adherence to endothelium: potential role in sickle cell disease. Blood. 1996;88:2311–2320. [PubMed] [Google Scholar]
- 94.Zhou G, Chen T, Raj JU. MicroRNAs in pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2015;52:139–151. doi: 10.1165/rcmb.2014-0166TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li C, Gonsalves CS, Eiymo Mwa Mpollo MS, Malik P, Tahara SM, Kalra VK. MicroRNA 648 targets ET-1 mRNA and is cotranscriptionally regulated with MICAL3 by PAX5. Mol Cell Biol. 2015;35:514–528. doi: 10.1128/MCB.01199-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li C, Mpollo MS, Gonsalves CS, Tahara SM, Malik P, Kalra VK. Peroxisome proliferator-activated receptor-α-mediated transcription of miR-199a2 attenuates endothelin-1 expression via hypoxia-inducible factor-1α. J Biol Chem. 2014;289:36031–36047. doi: 10.1074/jbc.M114.600775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Clausen PA, Athanasiou M, Chen Z, Dunn KJ, Zhang Q, Lautenberger JA, Mavrothalassitis G, Blair DG. ETS-1 induces increased expression of erythroid markers in the pluripotent erythroleukemic cell lines K562 and HEL. Leukemia. 1997;11:1224–1233. doi: 10.1038/sj.leu.2400735. [DOI] [PubMed] [Google Scholar]
- 98.Schlosser K, Taha M, Deng Y, Jiang B, Stewart DJ. Discordant regulation of microRNA between multiple experimental models and human pulmonary hypertension. Chest. 2015;148:481–490. doi: 10.1378/chest.14-2169. [DOI] [PubMed] [Google Scholar]
- 99.Hansmann G, Wagner RA, Schellong S, Perez VA, Urashima T, Wang L, Sheikh AY, Suen RS, Stewart DJ, Rabinovitch M. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-gamma activation. Circulation. 2007;115:1275–1284. doi: 10.1161/CIRCULATIONAHA.106.663120. [DOI] [PubMed] [Google Scholar]
- 100.Rizos CV, Elisaf MS, Mikhailidis DP, Liberopoulos EN. How safe is the use of thiazolidinediones in clinical practice? Expert Opin Drug Saf. 2009;8:15–32. doi: 10.1517/14740330802597821. [DOI] [PubMed] [Google Scholar]
- 101.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]