Abstract
Lung cellular senescence and inflammatory response are the key events in the pathogenesis of chronic obstructive pulmonary disease (COPD) when cigarette smoke (CS) is the main etiological factor. Telomere dysfunction is induced by either critical shortening or disruption of the shelterin complex, leading to cellular senescence. However, it remains unknown whether disruption of the shelterin complex is responsible for CS-induced lung cellular senescence. Here we show that telomere protection protein 1 (TPP1) levels are reduced on telomeres in lungs from mice with emphysema, as well as in lungs from smokers and from patients with COPD. This is associated with persistent telomeric DNA damage, leading to cellular senescence. CS disrupts the interaction of TPP1 with the Sirtuin 1 (Sirt1) complex, leading to increased TPP1 acetylation and degradation. Lung fibroblasts deficient in Sirt1 or treated with a selective Sirt1 inhibitor exhibit increased cellular senescence and decreased TPP1 levels, whereas Sirt1 overexpression and pharmacological activation protect against CS-induced TPP1 reduction and telomeric DNA damage. Our findings support an essential role of TPP1 in protecting CS-induced telomeric DNA damage and cellular senescence, and therefore provide a rationale for a potential therapy for COPD, on the basis of the shelterin complex, in attenuating cellular senescence.
Keywords: shelterin complex, telomeric DNA damage, Sirtuin1, cellular senescence, emphysema
Clinical Relevance
Telomere dysfunction is induced by either critical shortening or disruption of the shelterin complex, leading to cellular senescence. This study reveals that shelterin complex telomere protection protein 1 (TPP1) levels were reduced on telomeres associated with persistent telomeric DNA damage, leading to cellular senescence in various models of chronic obstructive pulmonary disease. Sirtuin 1 deacetylase regulates TPP1 levels and pharmacological activation protect against cigarette smoke–induced TPP1 reduction and telomeric DNA damage. This study provides a rationale for a potential therapy of chronic obstructive pulmonary disease on the basis of shelterin complex in attenuating cellular senescence.
Telomeres are the protective caps at the ends of each DNA with a TTAGGG repeat sequence of hundreds to thousands of base pairs. Telomere capping protects the chromosomes against deterioration or end-to-end fusion and prevents recognition by DNA damage pathways in response to genotoxic stress (1–4), thus maintaining the genomic stability. A six-subunit protein assembly called the shelterin/telosome complex, which consists of telomere protection protein 1 (TPP1), i.e., ACD (adrenocortical dysplasia homolog)/shelterin component TPP1, telomere repeat factor (TRF) 1, TRF2, TRF1 interacting nuclear factor 2 (TIN2), repressor activator protein 1, and protection of telomere protein 1 (POT1), maintains telomere capping and integrity (3, 5, 6). Telomere shortening is the hallmark of aging. Current studies suggest a critical role of telomere structure and organization, as well as telomere capping proteins in aging and in various age-associated disorders (7–10). Genetic knockout studies have uncovered an intriguing role played by individual shelterin complex proteins that regulate telomere stability and hence determine cell fate (11, 12).
Cigarette smoke (CS) is the main cause of chronic obstructive pulmonary disease (COPD). CS elicits an inflammatory response which results in destruction of the alveolar wall, and premature lung aging or cellular senescence (13–16). Recent evidence has explicitly shown a critical role of cellular senescence during the development of COPD/emphysema (15, 17, 18), but the precise molecular mechanism is not yet known. Human studies have observed telomere shortening in circulating cells isolated from smokers and patients with COPD (19, 20). However, it is not known whether telomere capping proteins/shelterin complex have any role in CS-induced telomere shortening and/or telomeric DNA damage during the development of COPD. In this study, we hypothesize that CS induces telomere DNA damage caused by disrupted shelterin complex, leading to cellular senescence in the pathogenesis of COPD. We show that shelterin complex protein TPP1 levels were reduced in CS-exposed mouse lungs with emphysema and in lungs from smokers and patients with COPD. CS caused telomeric DNA damage because of TPP1 reduction, which exaggerated the CS-induced cellular senescence. We also show that Sirtuin1 (Sirt1) reduction was responsible for reduced levels of TPP1 by enhancing its acetylation followed by degradation. To the best of our knowledge, this is the first study to show the role of telomere capping protein TPP1 in CS-induced cellular senescence during the development of COPD/emphysema.
Materials and Methods
All the experimental details for cells and treatments, constructs and transfections, CS exposure to mice, measurement of senescence-associated β-galactosidase (SA-β-gal) activity, immunofluorescence staining, telomere chromatin immunoprecipitation (ChIP), measurement of telomere dysfunction–induced foci (TIFs), telomere length assay, immunoprecipitation, TPP1 and Sirt1 flow cytometry, and Western blot analysis are presented in the online data supplement. Brief information is provided below.
Cells and Treatments
Primary mouse lung fibroblasts, human lung fibroblast (HFL1, ATCC-CCL153) and human small airway epithelial cells (SAECs) from normal subjects and patients with COPD (Lonza, Allendale, NJ) were used and were treated with cigarette smoke extract (CSE) in concentrations of 0.5%, 0.25%, and 0.2% for different experiments.
Constructs and Transfections
Myc-tagged TPP1 vectors were obtained from Origene (Rockville, MD). HFL1 cells were transfected using various carriers (see online supplement).
Telomere ChIP
ChIP was performed as per the protocol (ChIP assay kit; Millipore, Danvers, MA).
Measurement of TIFs
TIFs were measured using the telomere-specific fluorescent FISH PNA probe (Roche Diagnostics, Indianapolis, IN).
Telomere Length Assay
Telomere length was measured in cells using a telomere length assay kit (Roche Diagnostics).
Mice Exposure to CS
Eight-week-old male C57BL/6J mice were exposed to smoke generated using 3R4F cigarettes, by a Teague smoking machine (Model TE-10, Teague Enterprises, Woodland, CA) at a concentration of ∼100 mg/m3 total particulate matter (see online supplement).
Human Samples
The lung tissue specimens from normal, lifelong nonsmokers, smokers, and patients with COPD were collected by the Department of Medicine and Pathology, Helsinki University Central Hospital, with clinical characteristics as described previously (14).
Statistical Analysis
Statistical analysis of significance was calculated using one-way analysis of variance for multigroup comparisons using GraphPad Prism 6 (La Jolla, CA). The results are shown as the mean ± SEM. P < 0.05, P < 0.01, and P < 0.001 were considered statistically significant.
Results
TPP1 Is Reduced in Human SAECs and in Lung Tissues from Patients with COPD
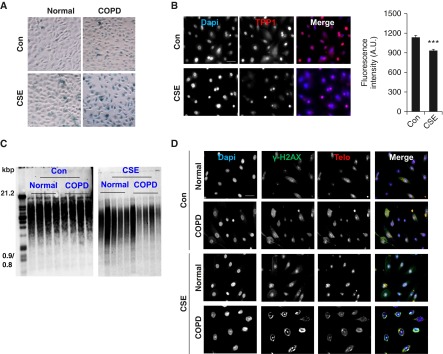
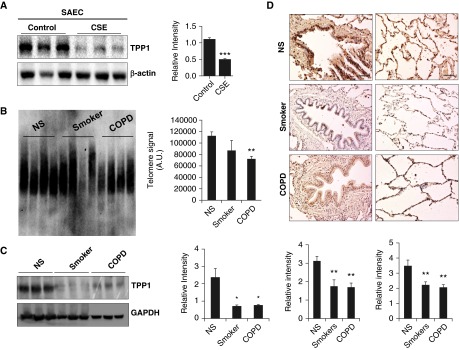
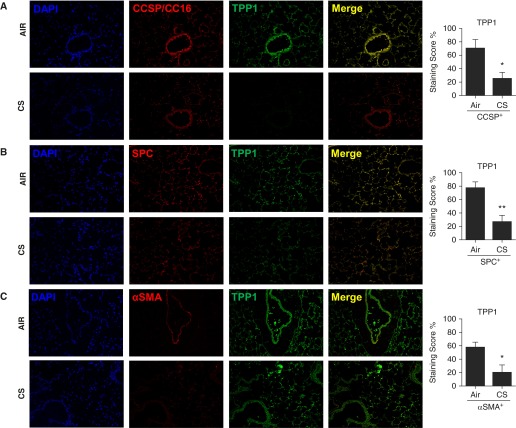
We hypothesized that TPP1 would be required for protection of the shelterin/telomere complex and that TPP1 would be disrupted by CS in lung cells. We determined TPP1-mediated protection against telomere dysfunction and cellular senescence in COPD by using SAECs from normal subjects and patients with COPD. CSE treatment (0.2% for 15 d) induced cellular senescence in human SAECs, which was augmented in patients with COPD (Figure 1A). This is consistent with our previous results (13). CSE treatment led to a greater degree of reduction in nuclear TPP1 levels as measured by immunofluorescence (Figures 1B and Figures E1A and E1B in the online supplement). In contrast to the effect seen in mouse lungs, there was a significant reduction in telomere length with CSE treatment in human SAECs (normal, 100.9 ± 9.6% versus COPD, 64.9 ± 6.2%, expressed as a percentage reduction calculated on the basis of arbitrary densitometry readings; P < 0.01), and this effect was not regulated by COPD (Figure 1C). Furthermore, CSE (0.2% for 15 d) caused telomeric DNA damage in normal human SAECs, which was further augmented in cells from patients with COPD (Figure 1D). We next determined the levels of TPP1 and the telomere length in CSE-treated (0.2% for 15 d) human SAECs and in lungs from nonsmokers, smokers, and patients with COPD. CSE treatment in SAECs caused significant reduction in TPP1 levels as measured by immunoblot analysis (Figure 2A). We found that telomere length was significantly reduced in the lungs of patients with COPD (Figure 2B), and that there was a greater reduction in TPP1 levels in the lungs of patients with COPD compared with those of nonsmokers (Figures 2C and 2D). We performed immunofluorescence double/triple staining for lung fibroblasts in emphysematous mice using α smooth muscle actin to show the abundance of TPP1 and its reduction after 6 months of chronic CS exposure (Figures 3A–3C). Similar observations were found in airway (Club/Clara Cell Secretory Protein+) and alveolar (SPC+) epithelial cells (Figures 3A–3C). This justified the use of lung fibroblasts and airway epithelial cells for mechanistic cell culture studies. The findings were reproduced in human SAEC and HFL1 cells. Thus, our results indicate that TPP1 may be a critical regulator of telomere dysfunction and cellular senescence during the development of COPD.
Figure 1.
Small airway epithelial cells (SAECs) from patients with chronic obstructive pulmonary disease (COPD) show increased senescence and telomere dysfunction, together with telomere protection protein 1 (TPP1) reduction, which is augmented by cigarette smoke extract (CSE) treatment. SAECs from normal subjects and from patients with COPD were treated with CSE (0.2%) for 10 days. (A) Representative images showing senescence-associated β-galactosidase activity in human SAECs. (B) Representative images of cells stained with TPP1 (red) and Dapi (4′6-diamidino-2-phenylindole, blue), showing TPP1 expression in SAECs from normal subjects. Scale bar: 100 μm. The average fluorescent intensity of TPP1 was calculated in SAECs treated with CSE using MetaMorph software. (C) Southern blot–based telomere length assay was performed in SAECs treated with CSE. (D) Representative images of CSE-treated SAECs from normal subjects and from patients with COPD were stained with γH2AX (green) and teloprobe (Telo, red), together with Dapi (blue) for telomere dysfunction–induced foci. Scale bar: 100 µm. Data are shown as mean ± SEM (n = 3–5). ***P < 0.001 versus control (Con). A.U., arbitrary units. Con, untreated control.
Figure 2.
TPP1 reduction is observed in primary airway epithelial cells, mouse lung cells, and lungs of patients with COPD. (A) SAECs showing reduced TPP1 levels after being treated with CSE (0.2%) for 15 days. Densitometry of the corresponding bands was normalized to β-actin loading control. (B) Southern blot–based telomere length assay was performed in lungs from nonsmokers (NS), smokers, and patients with COPD. Densitometry of bands for telomere length is presented. (C) Western blot of TPP1 in lung homogenates from NS, smokers, and patients with COPD was performed, and representative glyceraldehyde phosphate dehydrogenase (GAPDH) was used as a loading control. Densitometry of the corresponding bands was normalized to GADPH. (D) Representative images of bronchial staining of TPP1 in lungs from NS, smokers, and patients with COPD are presented; histogram shows the average intensity of TPP1 staining using MetaMorph software. Representative images of the alveolar region of TPP1 staining in lungs from NS, smokers, and patients with COPD are presented. Average intensity of TPP1 staining was calculated using MetaMorph software. Scale bars: 100 μm. Data are shown as mean ± SEM (n = 3–4). *P < 0.05, **P < 0.01 versus NS; ***P < 0.001 versus control.
Figure 3.
TPP1 reduction in airway epithelium, alveolar type II cells, and lung fibroblasts by chronic cigarette smoke (CS) exposure in emphysematous mice. (A) TPP1 expression in CCSP+ (Club/Clara Cell Secretory Protein, CC16 in the small airways [CC16/CC10]) cells, (B) proSPC+ (pro-surfactant protein C) alveolar type II cells, and (C) α smooth muscle actin (αSMA)+ lung fibroblasts was reduced by chronic CS exposure in mice. Immunofluorescence staining scores for TPP1 expression in CCSP+ CC10 airway, SPC+ alveolar epithelial cells, and αSMA+ lung fibroblasts were determined semiquantitatively in a blinded fashion and are presented as staining score percentage (%). Data are shown as mean ± SEM (n = 3–4/group). *P < 0.05, **P < 0.01 versus air group.
CS Causes Cellular Senescence and Telomeric DNA Damage in Lung Fibroblasts
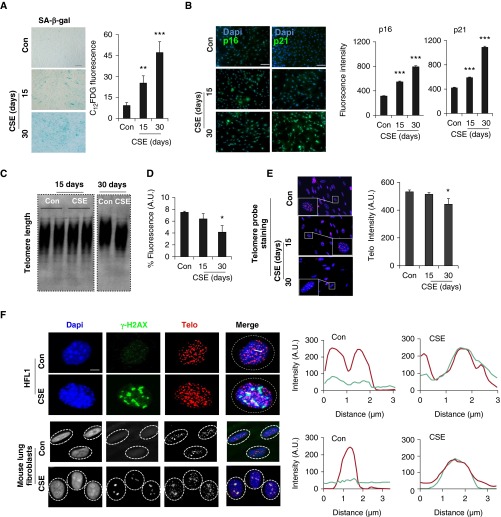
Cellular senescence is one of the major contributing factors to the pathogenesis of COPD (14, 18). However, it remains unknown whether CS-induced cellular senescence involves the telomere dysfunction and shelterin complex pathway. To study this, we first treated HFL1 cells with CSE to induce cellular senescence, as described earlier (13). As expected, we found that CSE (0.5%) treatment for both 15 days and 30 days induced cellular senescence, which is characterized by increased SA-β-gal activity (Figure 4A), p16 (CDKN2A or INK4A) and p21 (CDKN1A) protein abundance (Figure 4B). This is the standard in vitro model of CS-induced cellular senescence (13, 14).
Figure 4.
CSE-induced cellular senescence and telomere dysfunction–induced foci (TIF) formation in lung fibroblasts. (A) Human lung fibroblast (HFL1) cells were treated with CSE (0.5%) for 15 days and 30 days, and senescence-associated β-galactosidase (SA-β-gal) staining was performed for cellular senescence. Scale bar: 100 µm. Degree of cellular senescence measured by C12FDG fluorescence using FACS was shown as fluorescent intensity. (B) Immunofluorescence was performed in HFL1 cells treated with CSE (0.5%) for 15 days and 30 days to determine the expression of p16 and p21. Scale bars: 100 µm. Fluorescence intensity for p16 and p21 expression was measured quantitatively in HFL1 cells using MetaMorph software. (C) Telomere length assay was performed on the basis of the Southern blot–based technique in HFL1 cells treated with CSE (0.25%) for 15 days and 30 days. (D) Telomere length measurement by FACS, which is shown as % intensity of the telomere fluorescence. (E) Representative images of HFL1 cells showing telomere staining. Cells treated with CSE were stained with Telo (red) and Dapi (blue). Quantitative measurement of telomere length by fluorescence intensity in HFL1 cells treated with CSE at indicated time points. Insets showing magnified telomere staining. (F) Representative images of TIFs in CSE-treated HFL1 cells (0.5%) and mouse lung fibroblasts (0.25%) for 15 days. Cells were stained with γ-H2AX (blue) and Telo (red), together with Dapi (blue). Scale bar: 100 µm. Line scan data of corresponding images show the degree of colocalization between Telo (red) and γ-H2AX (green). Data are shown as mean ± SEM (n = 3–4). *P < 0.05, **P < 0.01, ***P < 0.001 versus control. FACS, fluorescence-activated cell sorting; C12FDG, 5-dodecanoylaminofluorescein di-β-d-galactopyranoside.
Next, we determined telomere length and telomeric DNA damage in HFL1 cells treated with CSE. We could not find any obvious changes in telomere length after 15 days of CSE treatment, whereas a significant decrease in telomere length was observed after 30 days of CSE. This was associated with an increase in cellular senescence (Figures 4C and 4D). These results suggest that telomere length shortening is preceded by cellular senescence with CSE treatment. One of the apparent changes caused by telomere dysfunction is the formation of telomere dysfunction-induced foci (TIFs). We wondered whether CSE treatment induces TIFs during cellular senescence. TIFs were measured as a colocalization between telomere probe by the fluorescence in situ hybridization and γ-H2AX (a DNA damage response factor). As shown in Figures 4E and 4F, increased TIFs were observed with 15 days of CSE treatment in both HFL1 and mouse lung fibroblasts, as represented by a line scan showing the degree of colocalization between γ-H2AX and telomere. These results suggest that CS-induced cellular senescence is associated with TIF formation and hence telomere dysfunction.
CS Reduces TPP1 Levels and Its Association with Telomeres in HFL1 Cells
Shelterin is a protein complex that protects chromosomes from DNA repair mechanisms during stress (1). Oxidative stress may lead to telomere DNA damage by engaging the shelterin complex. TPP1 is an integral part of the shelterin complex, and altered TPP1, TIN2, and TRF2 levels destabilize the shelterin complex and may contribute to premature aging or senescence (1, 21). Here, we determined the levels of telomere capping proteins (shelterin complex) and their interaction with telomeres in HFL1 cells treated with CSE. We first performed quantitative immunofluorescence to measure the levels of nuclear TPP1 and TIN2 in HFL1 cells treated with CSE (0.5%) for 15 days. Nuclear abundance of TPP1 and TIN2 was reduced with CSE (Figures E1A–E1C in the online supplement). Similarly, CSE (0.5%) treatment for 15 days reduced the levels of TPP1, but no changes were detected in TIN2 or TRF2, in whole cell lysates from HFL1 cells (Figures E1D and E1E). Telomere ChIP was performed to measure the association of both TPP1 and TIN2 with telomere. As shown in Figure E1F, CSE treatment decreased the association of both TPP1 and TIN2 with the telomeres. This decreased association may be a result of altered levels of TPP1 and TIN2 in the nucleus or may have been caused by CS-induced post-translational changes in these protein complexes. We further used the small interfering RNA for TPP1 in fibroblasts treated with CSE. Silencing of TPP1 augmented the proinflammatory mediator release, suggesting that TPP1 has a role in regulating the cellular senescence secretory phenotype, together with cellular senescence (data not shown).
CS-Mediated TPP1 Reduction and Telomeric DNA Damage Are Regulated by Sirt1 in Lung Fibroblasts
Sirt1 deficiency aggravated, whereas Sirt1 overexpression protected against CS-induced TPP1 reduction in bronchial and alveolar epithelial cells. These results indicate that although telomere length is not significantly altered by chronic CS exposure in mouse lungs, reduced TPP1 levels may play an important role in inducing cellular senescence and hence emphysema.
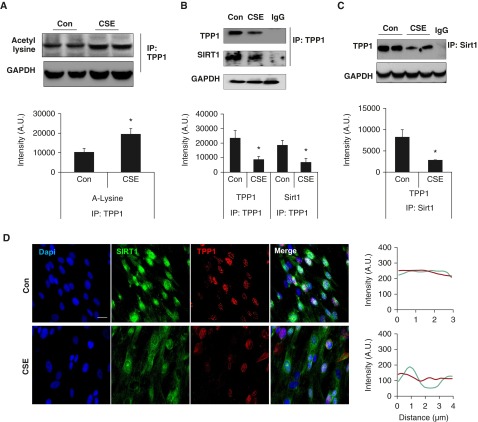
The post-translational modifications of TPP1 may either enhance shelterin complex stability or lead to TPP1 degradation (21–23). On the basis of our previous finding that Sirt1, a protein deacetylase, protects against CS-induced lung cellular senescence (14), we investigated whether Sirt1 interacts with TPP1 during CSE-induced cellular senescence. We performed coimmunoprecipitation of TPP1 with or without CSE (0.5% for 15 d) in HFL1 cells and probed with acetyl lysine (Figure 5A) and the Sirt1 antibody (Figure 5B). Comparatively, Sirt1 pull-down was performed and the lysate was probed for the TPP1 antibody (Figure 5C). This was confirmed by coimmunofluorescence for Sirt1 and TPP1 in HFL1 cells (Figure 5D). We next investigated whether TPP1 is acetylated during CSE treatment in HFL1 cells. TPP1 was immunoprecipated and probed for acetyl lysine. CSE treatment increased TPP1 acetylation in as early as 24 hours. At this time point, the total TPP1 levels were not significantly changed (data not shown) between the control and CSE-treated HFL1 cells. These results suggest that CS-induced disruption of TPP1 and Sirt1 interaction leads to TPP1 acetylation, followed by its reduction.
Figure 5.
CSE causes TPP1 acetylation and disrupts its interaction with Sirtuin 1 (Sirt1) in HFL1 cells. HFL1 cells were treated with CSE (0.5%) for (A) 24 hours or (B–D) 15 days. (A) Cell lysates were immunoprecipitated by TPP1 antibody, followed by probing with anti–acetyl lysine antibody. (B) Immunoprecipitated TPP1 immunocomplex was used for Western blot, which was probed with Sirt1 and TPP1 antibodies. (C) Western blot images of immunoprecipitation of Sirt1, followed by its probing with TPP1. Representative GAPDH was used as a loading control, which was run separately from the same samples without IP. Densitometry of the corresponding blots is presented. (D) Representative images of TPP1 (green) and Sirt1 (red) showing the colocalization in the nucleus, which was stained with Dapi (blue). Scale bar: 100 µm. Line scan data of corresponding images show the degree of colocalization between TPP1 and Sirt1. Data are shown as mean ± SEM (n = 3–4). *P < 0.05 versus control. IP, immunoprecipitation.
TPP1 Is Reduced in Mouse Lungs with CS-Induced Emphysema
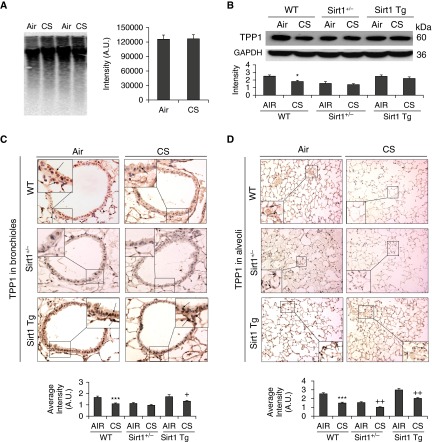
We next investigated whether there is an association between telomere length or TPP1 and CS-induced emphysema. We measured the telomere length using a well-established Southern blot assay. Chronic CS exposure did not have any effect on telomere length in mouse lungs (Figure 6A). We next determined the TPP1 levels and found a significant reduction in its levels in lungs exposed to CS (Figure 6B). Immunohistochemistry showed that a decrease in TPP1 abundance was restricted mostly to lung structural cells, which was confirmed by the semiquantitative analysis (Figures 6C–6D).
Figure 6.
TPP1 reduction is observed in mouse lungs with emphysema. Sirt1+/−, Sirt1 Tg, and WT mice were exposed to CS for 6 months. (A) Telomere length assay was performed in mouse lungs on the basis of a Southern blot–based technique. (B) Western blot of TPP1 in lung homogenates was performed, and representative GAPDH was used as a loading control. Densitometry of the corresponding bands was normalized to GADPH. Representative images of TPP1 expression in (C) the bronchial airways (insets showing magnified image of stained bronchial epithelial cells) and (D) the alveolar region in mouse lungs and histogram show the average intensity of TPP1 staining in the bronchial and alveolar regions calculated using MetaMorph software. Arrows indicate dark brown staining for TPP1 expression in bronchial airway and alveolar epithelial cells shown in (C) and (D). Data are shown as mean ± SEM (n = 3–4). *P < 0.05, ***P < 0.001 versus WT-Air; +P < 0.05, ++P < 0.01 versus WT-CS. Sirt1+/−, Sirt1 heterozygous knockout; Sirt1 Tg, Sirt1 transgenic; WT, wild type.
Sirt1 Regulation of TPP1 in Mouse Lungs with CS-Induced Emphysema
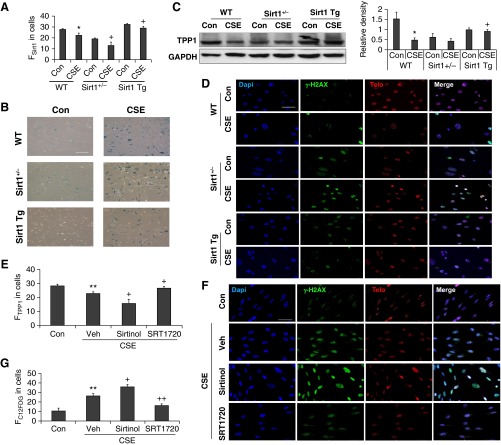
To further determine whether Sirt1 protects against CS-induced TPP1 reduction and telomere DNA damage, we isolated lung fibroblasts from Sirt1-deficient and transgenic mice and treated them with CSE (0.25% for 15 d) (Figure 7A). Consistent with our previous findings (14), CSE treatment increased SA-β-gal activity in mouse lung fibroblasts, which was augmented by Sirt1 deficiency. This effect was attenuated in CSE-treated lung fibroblasts from Sirt1 transgenic mice (Figure 7B). We next determined the effect of Sirt1 expression on TPP1 levels. Sirt1 overexpression attenuated CS-induced TPP1 reduction in lung fibroblasts (Figure 7C), whereas Sirt1 deficiency augmented CSE (0.25% for 15 d)–induced telomeric DNA as measured by TIF formation (Figure 7D). Furthermore, HFL1 cells were treated with a selective Sirt1 activator (SRT1720, 10 μM) and inhibitor (sirtinol, 1 μM). As shown in Figures 7E–7G, sirtinol treatment further aggravated CSE-induced TPP1 reduction, telomeric DNA damage, and cellular senescence, which were reduced by SRT1720. These results suggest that Sirt1 reduction reduced the levels of TPP1 and hence led to telomeric DNA damage during CS-induced cellular senescence.
Figure 7.
Sirt1 regulates CSE-induced TPP1 reduction and TIF formation in lung fibroblasts. (A–D) Mouse lung fibroblasts isolated from Sirt1+/−, Sirt1 Tg, and WT mice were treated with CSE (0.25% for 15 d). (A) Sirt1 levels determined by FACS in CSE-treated lung fibroblasts from WT, Sirt1+/−, and Sirt1 Tg mice. (B) Representative images of SA-β-gal staining in mouse lung fibroblasts treated with CSE. Scale bar: 100 µm. (C) Western blot of TPP1 in whole cell extracts of lung fibroblasts obtained from WT, Sirt1+/−, and Sirt1 Tg mice. Representative GAPDH was used as a loading control. Densitometry of the corresponding TPP1 bands normalized to GAPDH. (D) Lung fibroblasts were stained with Dapi (blue), γH2AX (green), and Telo (red) for TIF formation. Scale bar: 100 µm. (E–G) HFL1 cells were treated with CSE (0.5%) for 15 days in the presence of SIRT1 inhibitor (sirtinol, 1 μM) or activator (SRT1720, 10 μM). (E) TPP1 levels were determined by the FACS in CSE-treated HFL1 cells in the presence of sirtinol or SRT1720. (F) HFL1 cells were stained with γ-H2AX (green) and Telo (red) for TIF formation, together with Dapi (blue). Scale bar: 100 µm. (G) C12FDG fluorescence in CSE-treated HFL1 cells measured by the FACS was plotted as FC12FDG in cells, which showed that the degree of cellular senescence was arbitrary. Data are shown as mean ± SEM (n = 3–4). *P < 0.05, **P < 0.01 versus Con; +P < 0.05, ++P < 0.01 versus CSE group. FC12FDG, fluorescent intensity of 5-dodecanoylaminofluorescein di-β-d-galactopyranoside; FSirt1, fluorescent intensity of Sirt1; FTPP1, fluorescent intensity of TPP1; Veh, vehicle.
TPP1 Overexpression Protects against CS-Induced Cellular Senescence and Telomere DNA Damage
To explicitly determine that TPP1 is a critical component in CS-induced cellular senescence and telomere DNA damage, we used a genetic approach to increase TPP1 expression. HFL1 cells were transfected with TPP1 plasmid in the presence of CSE treatment (0.5% for 15 d). TPP1 overexpression restored its loss caused by CSE treatment, as measured by fluorescence-activated cell sorting analysis (Figure E2A). Nuclear levels of TPP1 and TIN2 proteins were also increased in TPP1-overexpressed HFL1 cells after CSE treatment (Figure E2B). Furthermore, CSE-induced telomeric DNA damage (a colocalization of γ-H2AX and telomere probe) and cellular senescence (SA-β-gal activity) were reduced by TPP1 overexpression (Figures E2C and E2D). These results suggest that TPP1 reduction is a critical determinant of CS-induced telomere DNA damage and cellular senescence.
Discussion
Telomeres are the DNA repeats that protect the ends of chromosomes from recognition by DNA repair machinery and hence maintain the genome stability. Telomere repeat length is shortened with every cell division and once reaching a threshold, induces replicative senescence and organismal aging. Telomere ends are considered the favored genomic regions for oxidative and genotoxic stress because of the guanine triplet, which is susceptible to oxidative modifications, and because of the inefficient DNA repair on an extended single strand of telomeres. Telomere structure and stability are maintained by shelterin protein complex, the aberrant expression of which may have deleterious consequences. Several studies have shown telomere length attrition in smokers and patients with COPD (19, 20, 24). However, the role of telomeres and shelterin complex in CS-induced cellular senescence during COPD is not known. In this study, we showed that CS causes a reduction of telomere capping protein TPP1, and hence augments cellular senescence and the development of COPD/emphysema. However, further study is required to determine the role of telomere degradation, if any, by DNases and telomerase in CS-induced telomere shortening. Nevertheless, TPP1 is modified post-translationally by acetylation with CS treatment, resulting in its degradation which is reversed by Sirt1.
CS accelerates lung aging by inducing cellular senescence in lung structural cells (14, 15, 18), including epithelial and mesenchymal cells such as smooth muscle cells or fibroblasts. Senescent cells initiate a myriad of damaging stress responses, including release of senescence-associated secretory phenotype (25, 26) and oxidative stress (13). We show that CSE treatment for 15 days induced cellular senescence in lung fibroblasts and epithelial cells, whereas telomere length did not reveal any significant differences between the CSE and the control group. These findings suggest a telomere length–independent mechanism for cellular senescence. Although replicative senescence, associated with telomere shortening during cell division, was not observed because CSE treatment did not increase cell division, there was growth restriction by CSE (data not shown). We next investigated whether CSE induces any changes in shelterin proteins, since 30 days of CSE led to reduced telomere length. Our results show that the level of shelterin protein TTP1 was significantly reduced in CSE-treated lung fibroblasts and in human SAECs in emphysematous mouse lungs as well as in the lungs of patients with COPD. Decreased abundance of TPP1 expression in lung fibroblasts was observed in emphysematous mice after 6 months of chronic CS exposure. Similar observations of TPP1 reduction were found in airway (Club/Clara Cell Secretory Protein+) and alveolar (SPC+) cells in the lungs of mice (emphysematous) after chronic CS exposure.
Importantly, TPP1 overexpression attenuated CS-induced telomeric DNA damage and cellular senescence in lung fibroblasts. Therefore, CSE-induced telomere uncapping may initiate a cellular senescence response, irrespective of telomere shortening, whereas persistent telomere deprotection and DNA degradation may induce its shortening. This is in agreement with the finding that telomere uncapping induces telomere attrition and TIF formation (27). Telomere deprotection induced by changes in its capping proteins is a critical phenomenon in regulating cellular senescence (12). Specifically, TPP1 is an integral regulator of telomere maintenance through governing of TIN2’s interactions with POT1 or telomerase recruitment (1, 28, 29), and mouse embryonic fibroblasts from the TPP1 heterozygous mice show increased cellular senescence (30). We have shown that TPP1 overexpression reduced CS-induced telomeric DNA damage and cellular senescence. These results further support the notion that TPP1 protects against CS-induced telomeric DNA damage by the telomere stability mechanisms (e.g., via interactions with TIN2 and POT1) (31), rather than via telomerase-dependent extension. It has been shown that uncapped dysfunctional telomeres, through TRF2 mutation, lead to progerin production, which collaborates with telomere dysfunction to trigger cellular senescence (11, 32). It is unclear whether CS exposure produces progerin or whether TPP1 reduction has any effect on it. Altogether, CS causes TIF formation via TPP1 reduction, leading to cellular senescence, although studies in TPP1 knockout mice are required to determine whether these mice are susceptible to CS-induced lung cellular senescence and hence COPD/emphysema.
It has been shown that TPP1 undergoes post-translational modifications that affect the shelterin complex stability (21, 33). Our results indicate that under conditions of oxidative stress induced by CSE, TPP1 was acetylated and possibly degraded. Previous studies have shown that Sirt1 interacts with telomeres and maintains telomere length (34, 35). Hence, we surmise that CS-induced TPP1 acetylation and reduction are attributable to Sirt1 reduction. This is supported by our finding that Sirt1 activation by a pharmacological activator, SRT1720, prevented CS-induced TPP1 degradation, whereas a selective Sirt1 inhibitor, sirtinol, caused further TPP1 reduction in human lung fibroblasts. Similarly, TPP1 degradation was increased in lung fibroblasts from Sirt1-deficient mice, whereas Sirt1 overexpression protected against CSE-induced TPP1 reduction in lung fibroblasts. This is consistent with the finding that SIRT1 improves healthy aging and ameliorates age-related senescence of mesenchymal stem cells via modulating telomere shelterin TPP1 (36). Importantly, we showed that TPP1 interacts with Sirt1 under normal conditions, and that this was disrupted by CS. Further study is ongoing to determine whether Sirt1 protects against CS-induced TPP1 acetylation and to identify the corresponding residues. In addition to TPP1, Sirt1 may regulate other molecules including telomerase, poly ADP ribose polymerase, and histones, thereby protecting against CS-induced telomere dysfunction (34, 35); this needs further investigation. CS treatment exaggerated the alveolar damage and led to reduced TPP1 levels in the Sirt1 knockout mice. Although Sirt1 activator SRT1720 or transgenic overexpression (Sirt1 Tg) attenuated CS-induced telomere DNA damage and cellular senescence, it remains unknown whether the Sirt1 activator reduced TIF formation and cellular senescence in TPP1 knockout cells. There is a vicious feedback between telomere dysfunction and chronic low-grade inflammation, which accelerates aging and the development of COPD (11, 24, 37). This is corroborated by our study showing Sirt1’s protection against CS-induced telomere dysfunction and lung inflammation (14). Overall, this offers proof that Sirt1 is upstream of TPP1 in regulating telomere function and cellular senescence during CS-induced development of COPD.
It is interesting to note that the telomere length was not changed in lungs of mice with CS-induced emphysema. This may be because of the nonhomogenous changes in telomere length among lung structure and inflammatory/immune cells, and due to the ultralong telomeres in mice as compared with human (38, 39), which affects the difference between control and CS-exposed lungs. From another viewpoint, telomere shortening may not be necessary for CS-induced lung cellular senescence; this needs further investigation. We observed telomere shortening in the lungs of patients with COPD. Further study is required to differentiate the role of telomeric DNA damage and telomere shortening in lung cellular senescence during the development of COPD, using lung samples from patients with different stages of COPD.
Conclusions
In conclusion, CS-induced cellular senescence is mediated by telomere attrition, in which TPP1 plays a critical role. We have also shown that the shelterin complex is highly destabilized in the lungs of a mouse model of CS-induced emphysema, as well as in human smokers and in patients with COPD. These findings provide new insights into telomere dysfunction and shelterin complex disruption during CS-induced lung cellular senescence, which can be explored further in an attempt to develop a potential therapeutic intervention for respiratory disorders, in particular, COPD.
Acknowledgments
Acknowledgments
The authors thank Dr. Vuokko L. Kinnula (late) and Dr. Witold Mazur (University of Helsinki, Finland) for providing the human tissue samples from nonsmokers, smokers, and patients with chronic obstructive pulmonary disease, which were used previously in our published studies. They also thank Dr. Vera Gorbunova at the University of Rochester for her advice on telomere biology and related assays.
Footnotes
This work was supported by National Institutes of Health Grant 1R01HL085613 (I.R.), American Lung Association Grant RG-266456-N (H.Y.), and National Institute of Environmental Health Sciences Center Grant P30-ES01247.
Author Contributions: T.A., H.Y., and I.R. conceived and designed the experiments; T.A., I.K.S., A.M.T., C.A.L., and J.G. performed the experiments; T.A. and A.M.T. analyzed the data; and T.A., I.K.S., H.Y., and I.R. wrote and revised/edited the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0198OC on August 25, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Nandakumar J, Bell CF, Weidenfeld I, Zaug AJ, Leinwand LA, Cech TR. The TEL patch of telomere protein TPP1 mediates telomerase recruitment and processivity. Nature. 2012;492:285–289. doi: 10.1038/nature11648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cesare AJ, Kaul Z, Cohen SB, Napier CE, Pickett HA, Neumann AA, Reddel RR. Spontaneous occurrence of telomeric DNA damage response in the absence of chromosome fusions. Nat Struct Mol Biol. 2009;16:1244–1251. doi: 10.1038/nsmb.1725. [DOI] [PubMed] [Google Scholar]
- 3.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 4.Suram A, Herbig U. The replicometer is broken: telomeres activate cellular senescence in response to genotoxic stresses. Aging Cell. 2014;13:780–786. doi: 10.1111/acel.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood AM, Laster K, Rice EL, Kosak ST. A beginning of the end: new insights into the functional organization of telomeres. Nucleus. 2015;6:172–178. doi: 10.1080/19491034.2015.1048407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sfeir A, Kabir S, van Overbeek M, Celli GB, de Lange T. Loss of Rap1 induces telomere recombination in the absence of NHEJ or a DNA damage signal. Science. 2010;327:1657–1661. doi: 10.1126/science.1185100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15:482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 8.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 9.Holohan B, De Meyer T, Batten K, Mangino M, Hunt SC, Bekaert S, De Buyzere ML, Rietzschel ER, Spector TD, Wright WE, et al. Decreasing initial telomere length in humans intergenerationally understates age-associated telomere shortening. Aging Cell. 2015;14:669–677. doi: 10.1111/acel.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alder JK, Guo N, Kembou F, Parry EM, Anderson CJ, Gorgy AI, Walsh MF, Sussan T, Biswal S, Mitzner W, et al. Telomere length is a determinant of emphysema susceptibility. Am J Respir Crit Care Med. 2011;184:904–912. doi: 10.1164/rccm.201103-0520OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alder JK, Barkauskas CE, Limjunyawong N, Stanley SE, Kembou F, Tuder RM, Hogan BL, Mitzner W, Armanios M. Telomere dysfunction causes alveolar stem cell failure. Proc Natl Acad Sci USA. 2015;112:5099–5104. doi: 10.1073/pnas.1504780112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sfeir A, de Lange T. Removal of shelterin reveals the telomere end-protection problem. Science. 2012;336:593–597. doi: 10.1126/science.1218498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad T, Sundar IK, Lerner CA, Gerloff J, Tormos AM, Yao H, Rahman I. Impaired mitophagy leads to cigarette smoke stress-induced cellular senescence: implications for chronic obstructive pulmonary disease. FASEB J. 2015;29:2912–2929. doi: 10.1096/fj.14-268276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao H, Chung S, Hwang JW, Rajendrasozhan S, Sundar IK, Dean DA, McBurney MW, Guarente L, Gu W, Rönty M, et al. SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J Clin Invest. 2012;122:2032–2045. doi: 10.1172/JCI60132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nyunoya T, Monick MM, Klingelhutz A, Yarovinsky TO, Cagley JR, Hunninghake GW. Cigarette smoke induces cellular senescence. Am J Respir Cell Mol Biol. 2006;35:681–688. doi: 10.1165/rcmb.2006-0169OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moriyama C, Betsuyaku T, Ito Y, Hamamura I, Hata J, Takahashi H, Nasuhara Y, Nishimura M. Aging enhances susceptibility to cigarette smoke-induced inflammation through bronchiolar chemokines. Am J Respir Cell Mol Biol. 2010;42:304–311. doi: 10.1165/rcmb.2009-0025OC. [DOI] [PubMed] [Google Scholar]
- 17.Zhou F, Onizawa S, Nagai A, Aoshiba K. Epithelial cell senescence impairs repair process and exacerbates inflammation after airway injury. Respir Res. 2011;12:78. doi: 10.1186/1465-9921-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence in patients with pulmonary emphysema. Am J Respir Crit Care Med. 2006;174:886–893. doi: 10.1164/rccm.200509-1374OC. [DOI] [PubMed] [Google Scholar]
- 19.Savale L, Chaouat A, Bastuji-Garin S, Marcos E, Boyer L, Maitre B, Sarni M, Housset B, Weitzenblum E, Matrat M, et al. Shortened telomeres in circulating leukocytes of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179:566–571. doi: 10.1164/rccm.200809-1398OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rode L, Bojesen SE, Weischer M, Vestbo J, Nordestgaard BG. Short telomere length, lung function and chronic obstructive pulmonary disease in 46,396 individuals. Thorax. 2013;68:429–435. doi: 10.1136/thoraxjnl-2012-202544. [DOI] [PubMed] [Google Scholar]
- 21.Zemp I, Lingner J. The shelterin component TPP1 is a binding partner and substrate for the deubiquitinating enzyme USP7. J Biol Chem. 2014;289:28595–28606. doi: 10.1074/jbc.M114.596056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rai R, Li JM, Zheng H, Lok GT, Deng Y, Huen MS, Chen J, Jin J, Chang S. The E3 ubiquitin ligase Rnf8 stabilizes Tpp1 to promote telomere end protection. Nat Struct Mol Biol. 2011;18:1400–1407. doi: 10.1038/nsmb.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han X, Liu D, Zhang Y, Li Y, Lu W, Chen J, Songyang Z. Akt regulates TPP1 homodimerization and telomere protection. Aging Cell. 2013;12:1091–1099. doi: 10.1111/acel.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amsellem V, Gary-Bobo G, Marcos E, Maitre B, Chaar V, Validire P, Stern JB, Noureddine H, Sapin E, Rideau D, et al. Telomere dysfunction causes sustained inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;184:1358–1366. doi: 10.1164/rccm.201105-0802OC. [DOI] [PubMed] [Google Scholar]
- 25.Kumar M, Seeger W, Voswinckel R. Senescence-associated secretory phenotype and its possible role in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2014;51:323–333. doi: 10.1165/rcmb.2013-0382PS. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Wu L, Qu JM, Bai CX, Merrilees MJ, Black PN. Pro-inflammatory phenotype of COPD fibroblasts not compatible with repair in COPD lung. J Cell Mol Med. 2012;16:1522–1532. doi: 10.1111/j.1582-4934.2011.01492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eller MS, Liao X, Liu S, Hanna K, Bäckvall H, Opresko PL, Bohr VA, Gilchrest BA. A role for WRN in telomere-based DNA damage responses. Proc Natl Acad Sci USA. 2006;103:15073–15078. doi: 10.1073/pnas.0607332103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frescas D, de Lange T. TRF2-tethered TIN2 can mediate telomere protection by TPP1/POT1. Mol Cell Biol. 2014;34:1349–1362. doi: 10.1128/MCB.01052-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frescas D, de Lange T. Binding of TPP1 protein to TIN2 protein is required for POT1a,b protein-mediated telomere protection. J Biol Chem. 2014;289:24180–24187. doi: 10.1074/jbc.M114.592592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takai KK, Kibe T, Donigian JR, Frescas D, de Lange T. Telomere protection by TPP1/POT1 requires tethering to TIN2. Mol Cell. 2011;44:647–659. doi: 10.1016/j.molcel.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajavel M, Mullins MR, Taylor DJ. Multiple facets of TPP1 in telomere maintenance. Biochim Biophys Acta. 2014;1844:1550–1559. doi: 10.1016/j.bbapap.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao K, Blair CD, Faddah DA, Kieckhaefer JE, Olive M, Erdos MR, Nabel EG, Collins FS. Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts. J Clin Invest. 2011;121:2833–2844. doi: 10.1172/JCI43578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Chen LY, Han X, Xie W, Kim H, Yang D, Liu D, Songyang Z. Phosphorylation of TPP1 regulates cell cycle-dependent telomerase recruitment. Proc Natl Acad Sci USA. 2013;110:5457–5462. doi: 10.1073/pnas.1217733110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Bonis ML, Ortega S, Blasco MA. SIRT1 is necessary for proficient telomere elongation and genomic stability of induced pluripotent stem cells. Stem Cell Rep. 2014;2:690–706. doi: 10.1016/j.stemcr.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palacios JA, Herranz D, De Bonis ML, Velasco S, Serrano M, Blasco MA. SIRT1 contributes to telomere maintenance and augments global homologous recombination. J Cell Biol. 2010;191:1299–1313. doi: 10.1083/jcb.201005160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herranz D, Muñoz-Martin M, Cañamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, Serrano M. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun. 2010;1:3. doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jurk D, Wilson C, Passos JF, Oakley F, Correia-Melo C, Greaves L, Saretzki G, Fox C, Lawless C, Anderson R, et al. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat Commun. 2014;2:4172. doi: 10.1038/ncomms5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kipling D, Cooke HJ. Hypervariable ultra-long telomeres in mice. Nature. 1990;347:400–402. doi: 10.1038/347400a0. [DOI] [PubMed] [Google Scholar]
- 39.Hemann MT, Greider CW. Wild-derived inbred mouse strains have short telomeres. Nucleic Acids Res. 2000;28:4474–4478. doi: 10.1093/nar/28.22.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]