Abstract
During anaerobic growth of bacteria, organic intermediates of metabolism, such as pyruvate or its derivatives, serve as electron acceptors to maintain the overall redox balance. Under these conditions, the ATP needed for cell growth is derived from substrate-level phosphorylation. In Escherichia coli, conversion of glucose to pyruvate yields 2 net ATPs, while metabolism of a pentose, such as xylose, to pyruvate only yields 0.67 net ATP per xylose due to the need for one (each) ATP for xylose transport and xylulose phosphorylation. During fermentative growth, E. coli produces equimolar amounts of acetate and ethanol from two pyruvates, and these reactions generate one additional ATP from two pyruvates (one hexose equivalent) while still maintaining the overall redox balance. Conversion of xylose to acetate and ethanol increases the net ATP yield from 0.67 to 1.5 per xylose. An E. coli pfl mutant lacking pyruvate formate lyase cannot convert pyruvate to acetyl coenzyme A, the required precursor for acetate and ethanol production, and could not produce this additional ATP. E. coli pfl mutants failed to grow under anaerobic conditions in xylose minimal medium without any negative effect on their survival or aerobic growth. An ackA mutant, lacking the ability to generate ATP from acetyl phosphate, also failed to grow in xylose minimal medium under anaerobic conditions, confirming the need for the ATP produced by acetate kinase for anaerobic growth on xylose. Since arabinose transport by AraE, the low-affinity, high-capacity, arabinose/H+ symport, conserves the ATP expended in pentose transport by the ABC transporter, both pfl and ackA mutants grew anaerobically with arabinose. AraE-based xylose transport, achieved after constitutively expressing araE, also supported the growth of the pfl mutant in xylose minimal medium. These results suggest that a net ATP yield of 0.67 per pentose is only enough to provide for maintenance energy but not enough to support growth of E. coli in minimal medium. Thus, pyruvate formate lyase and acetate kinase are essential for anaerobic growth of E. coli on xylose due to energetic constraints.
All living systems generate the needed energy for cell maintenance and growth by catalyzing a set of coupled oxidation-reduction reactions. A critical component of these oxidation-reduction reactions is the redox balance of the sum of all metabolic reactions. With external input of an oxidant, such as dioxygen, the carbon and energy source can be completely oxidized to carbon dioxide with concomitant reduction of the terminal electron acceptor. However, under strict anaerobic conditions and in the absence of an external input of an oxidant, such as nitrate, metabolic intermediates serve the role of oxidant to maintain the overall redox balance. For many heterotrophic fermentative bacteria, the primary oxidant is pyruvate or derivatives of pyruvate, the terminal product of glycolysis. The redox state of total carbon in all final end products produced by the anaerobic cell should equal the redox state of the starting C compound, such as glucose carbon. Because of this constraint, the amount of energy available and ATP produced during anaerobic growth is significantly smaller than during complete oxidation of glucose to CO2 and H2O.
An anaerobic culture of a facultative bacterium like Escherichia coli is limited by ATP, and this is manifested by a lower growth rate and cell yield and higher glycolytic flux than those for an aerobic culture (9, 24, 31, 33). Under anaerobic conditions, the net yield of ATP per glucose is only 2 if pyruvate is reduced to lactate as in a homolactate-producing pfl mutant (37, 38). This yield can be increased by an additional ATP per glucose if two molecules of pyruvate are converted to one each of acetate and ethanol (24). The enzyme complex which starts this transformation of pyruvate to acetate and ethanol is pyruvate formate lyase, which metabolizes pyruvate to formate and acetyl coenzyme A (acetyl-CoA) (15). Thus, a bacterium that can convert glucose to acetate and ethanol will produce three net ATPs while still maintaining the overall redox balance of the carbon. In agreement with this possibility, both the ATP pool size and ATP/AMP ratio of a microaerobic (oxygen-limiting, less than 1 ppm) glucose minimal medium culture of a pfl mutant were reported to be about twofold lower than those for the wild type (38). Although this additional ATP produced at the acetate kinase step in fermentation of sugars is expected to provide a significant growth advantage (1), it has not been studied in detail. However, under severe ATP limitation, this difference in ATP yield may manifest itself more profoundly as a difference in growth rate and cell yield.
In E. coli, both transport and phosphorylation of glucose occur simultaneously at the expense of one ATP equivalent, phosphoenolpyruvate (26, 28). However, the cell expends two ATPs, one for transport (high-affinity ABC transporter) and the second for activation (phosphorylation) when xylose serves as the carbon source (19, 20). This need for two ATPs per internal production of one pentose phosphate reduces the net yield of ATP per xylose to 0.67 when lactate is the sole fermentation product (32). In glucose equivalents (6-carbon), the net ATP yield from xylose is 0.8 ATP, only 40% of the glucose-to-lactate fermentation value of 2.0 ATP. If the fermentation leads to acetate and ethanol, the net ATP yield from xylose will increase to 1.5 (1.8 ATP based on 6-carbon equivalent), a value close to the glucose-to-lactate yield of 2.0 but still less than the three ATPs for glucose to acetate and ethanol. This difference in the net ATP yield is expected to lower both the growth rate and the cell yield, as seen with ethanologenic E. coli (strain KO11 and E. coli B with plasmid pLOI297) growing in xylose medium compared to the values in glucose medium (specific growth rate of 0.19 h−1 for xylose and 0.3 h−1 for glucose [12]; Yglucose of 0.091 and Yxylose of 0.05 [g of dry-weight cells · g of sugar−1] [17]).
During the construction of E. coli K-12 derivatives for the production of lactic acid, acetic acid, and pyruvic acid, the gene coding for pyruvate formate lyase was deleted (4, 5, 37). These and other pflB mutants required acetate for anaerobic growth, in agreement with previous observations (35). However, even with acetate supplementation, the pflB mutants failed to grow with xylose as a carbon source under anaerobic conditions. We have investigated the physiological basis for this anaerobic xylose-negative phenotype of the pflB mutant. Results presented here provide evidence that the inability of the pflB mutant to grow with xylose anaerobically is a consequence of insufficient net ATP yield from this pentose.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All bacterial cultures are derivatives of E. coli K-12 unless indicated otherwise and are listed in Table 1. Strain W3110 (ATCC 27325) was used as the wild type. Cultures were grown in either rich medium (Luria-Bertani [LB]) or minimal salts medium described previously (18). For routine aerobic cultures the sugar concentration in minimal medium was 0.3%, and for anaerobic growth conditions the sugar concentration was increased to 1%. Minimal medium was supplemented with sodium acetate (0.1%) during anaerobic growth. Aerobic cultures were grown in 125-ml Erlenmeyer flasks with 10 ml of medium at 200 RPM in a shaker. Anaerobic cultures were grown in 13- by 100-mm screw-cap tubes filled to the top with medium. For pH-controlled anaerobic fermentations, 250-ml cultures were grown at 37°C in a custom-made pH-stat at a constant pH of 7.0 (adjusted with 2N KOH), as described previously (2). After inoculation, the medium was sparged with argon for 10 min at a flow rate of about 100 ml per min. Argon was maintained in the gas phase by continuous addition throughout the experiment. Inoculum for fermentation experiments was grown aerobically in the same medium until the culture reached the mid- to late-exponential phase of growth. Immediately after inoculation, the optical density (OD) of the culture at 420 nm was about 0.05 (Beckman DU640 spectrophotometer).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain | Relevant genotype | Source or reference |
|---|---|---|
| W3110 | Wild type | ATCC 27325 |
| BW545 | Δ(lacU)169, rpsL | Lab. collection |
| AH218 | Δ(focA-pflB)-FRT-Km-FRT | 37 |
| AH243 | W3110 Δ(focA-pflB)-FRT, Km | This study |
| AH245 | Δ(araFGH)-Km | W3110 × P1(BW27269) |
| AH247 | W3110 Δ(araE)-Km | This study |
| AH248 | Δ(focA-pflB) Δ(araFGH)-Km | AH243 × P1(BW27269) |
| AH249 | Δ(focA-pflB) ΦPCP13-araE+ | AH243 × P1 (BW27752) |
| AH250 | Δ(focA-pflB) Δ(araE)-Km | AH243 × P1(AH247) |
| AH257 | W3110 Δ(xylE)-FRT-Km-FRT | This study |
| AH280 | W3110 Δ(xylG)-FRT-Km-FRT | This study |
| BW18499 | Δ(codB-lacI)3 zfb-223::Tn10 ackA202 rpoS396(Am)? rph-1 ΔglnL2001 | CGSC; 10 |
| BW25113 | lacIqrrnBT14 ΔlacZW116 hsdR514 araBADAH33ΔrhaBADLD78 | B. Wanner |
| BW27269 | BW25113 Δ(araFGH)-Km903 | CGSC |
| BW27752 | BW25113 Φ(ΔaraEp PCP13-araE) | CGSC; 16 |
| BW25141(pKD4) | p(FRT-Km-FRT) | B. Wanner |
| BW25113(pKD46) | BW25113 p(λ-γβexo under ParaB)-repA101ts | B. Wanner |
| DH5α(pCP20) | p(FLPase) | B. Wanner |
| LCB898 | pflB1 | CGSC; 35 |
| SE1265 | LCB898, zca::Tn10 | This study |
| SE1900 | BW545, pflB-1 zca::Tn10 | BW545 × P1(SE1265) |
| SE2358 | W3110 Δ(focA-pflB)-FRT-Km-FRT | W3110 × P1(AH218) |
| YK19 | ackA202 zfb-223::Tn10 | W3110 × P1(BW18499) |
| E. coli B | Wild type | ATCC 11303 |
| SE2273 | E. coli B, pflB1 | This study |
| Plasmids | ||
| pAH286 | pBR322-(PCP6-xylE+) Apr | This study |
| pAH291 | pTOPO-(xylE+) Apr | This study |
Construction of mutants.
Mutants with gene deletions were constructed using the methods described by Datsenko and Wanner (8) with appropriate gene-specific primers flanking the region of the gene to be deleted. Construction of a Δ(focA-pflB) mutation with kanamycin resistance genes flanked by FRT sequences was described previously (37). The kanamycin resistance gene and one of the FRT sequences from the deletion strains constructed by this method were removed as described by Datsenko and Wanner, using the yeast FLP recombinase (8). Mutations were transduced to appropriate backgrounds using phage P1 as before (27).
Construction of xylE plasmids.
Two xylE plasmids with different copy numbers and promoters were constructed. Plasmid pAH291 contains the xylE gene and 455 bp of upstream DNA from the translation start site in a high-copy-number plasmid, PCRII-TOPO (Invitrogen). In this plasmid, the xylE gene is expected to be expressed from the native promoter. The xylE gene with the upstream sequence was amplified by PCR using two primers, XylE3 (TCTGCATTACCGATCACCATCGT) and XylE2 (AATCCCGGGTGGACAGGAAGATTACAGCGT), and the product was cloned into plasmid vector PCRII-TOPO (Invitrogen), appropriately linearized. In plasmid pAH286, the xylE gene was expressed from a synthetic promoter, PCP6 (14). The xylE gene without the promoter was amplified by PCR using the primers XylE1 (TTAAAGAATTCTCTAGAGGAGAGGTCTTGAATGAATACCCAGT) and XylE2. The XylE1 primer at the 5′ end contains in series restriction sites for EcoRI and XbaI and the sequence GGAG nine bases upstream of the “A” in the ATG for effective translation initiation. The XylE2 primer has an AvaI site at the 5′ end. The PCR-amplified product was hydrolyzed by EcoRI and AvaI and cloned into the EcoRI-AvaI fragment from plasmid pBR322, resulting in plasmid pAH285. The PCP6 promoter DNA along with an erythromycin resistance gene was removed from plasmid pAH281 as an XbaI fragment and cloned into the XbaI site immediately upstream of the xylE gene in plasmid pAH285. In this plasmid, pAH286, the xylE gene is expressed from the synthetic promoter PCP6 (14). Both plasmids complemented an E. coli mutant with deletions in both xylE and xylFGH for growth in xylose minimal medium under aerobic conditions.
Analytical methods.
Cell density was determined as the OD at 420 nm after appropriate dilutions (Beckman DU640). Fermentation products and sugars were determined by high-pressure liquid chromatography, using HP 1090 (Hewlett-Packard) fitted with an Aminex HPX-87H column (Bio-Rad Laboratories). The mobile phase was 4 mM H2SO4 at 0.4 ml per min. Organic acids, sugars, and ethanol were detected by a filter photometric detector (210 nm) and/or a refractive index monitor, in series, as described previously (34).
For determination of xylulose kinase activity, cultures were grown in a pH-stat at pH 7.0 in minimal medium with both glucose and xylose (0.5% each) and sodium acetate (0.1%). Cells were harvested when the wild type utilized about 50% of the xylose from the medium as seen by base consumption and were collected by centrifugation at 4°C (5,000 × g; 10 min) and washed with 50 mM Tris-HCl buffer, pH 7.5. Cells were broken by passage through a French pressure cell (20,000 lb/in2). Broken-cell suspension was centrifuged at 30,000 × g for 30 min, and the supernatant was centrifuged again at 100,000 × g for 1 h. The clear supernatant was used in the assay. Xylulose kinase activity was determined as described previously (32) but with [γ-32P]ATP. The concentration of cold ATP in the 20-μl reaction was 10 mM, and that of [γ-32P]ATP was 10 μCi (3,000 Ci per mmol) (Perkin-Elmer, NEN). After the reaction was stopped with 20 mM EDTA, xylulose-dependent ATP hydrolysis was determined as described previously (29).
Quantitative real-time PCR experiments.
For determination of xylose-specific mRNA levels in the cell, cells were grown in minimal medium with glucose (0.5%) and xylose (0.5%) as carbon sources and sodium acetate (0.1%) at a constant pH of 7.0 in a pH-stat (adjusted with 2 N KOH) under an argon gas phase. Fresh aerobic cultures in mid- to late-exponential phase of growth were used to inoculate at an initial cell density of 0.1 OD at 420 nm (Beckman DU640 spectrophotometer). Cultures were maintained at 37°C until all the glucose was exhausted and the wild-type culture utilized at least 50% of the xylose in the medium. Total RNAs from the cells were isolated by using a modified hot-phenol procedure described previously (32). xylA- and xylF-specific mRNAs were reverse transcribed from 5 μg of total RNA by Superscript II reverse transcriptase (Invitrogen), using primers XylA2 (AGGCTGCTGCCACGGACGATT) and XylF2 (CCGAGAGATTCTGCCTTTTTCAC), respectively. The xylA cDNA was amplified using the primers XylA1 (CAAACCCGTTAGCATTCCGTCA) and XylA2. The expected product was 161 bp long, extending from +68 to +228 from the translation start site (A in the ATG) for the xylA gene. The primers used for amplifying the xylF gene were XylF1 (GAACATTCTACTCACCCTTTGC) and XylF2. These two primers amplified a fragment of 153 bp between +12 and +164 from the translation start site of xylF. The xylA or xylF cDNA was amplified by using the primers in a master mix containing SYBR green (Bio-Rad Laboratories), using iCycler (Bio-Rad Laboratories). DNA containing xylA or xylF served as the standard for quantitation of the amount of xylA- or xylF-specific cDNA/mRNA in the RNA sample.
RESULTS AND DISCUSSION
The pflB mutant is defective for anaerobic growth in xylose minimal medium.
E. coli wild-type strain W3110 grew in minimal medium with either glucose or xylose as the sole C source under anaerobic conditions (with pH control) with growth rates of 0.35 and 0.2 h−1, respectively. An isogenic Δ(focA-pflB) derivative, strain SE2358, grew in glucose minimal medium supplemented with acetate under anaerobic conditions with a growth rate of 0.3 h−1 but failed to grow with xylose as the C source, although xylose supported growth of the mutant under aerobic conditions. This inability of the pfl mutant to grow with xylose under anaerobic conditions was independent of the growth temperature and control of the medium at pH 7.0. Strain SE1900, an E. coli K-12 derivative with a point mutation in the pflB gene, also did not grow with xylose as a sole C source under anaerobic conditions. Strain SE2273, a pflB derivative of E. coli B, also failed to grow in xylose minimal medium under anaerobic conditions. These results show that the pflB gene is essential for growth of E. coli with xylose in the absence of oxygen.
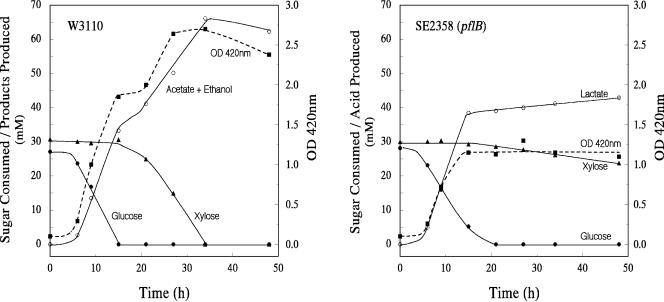
Since the pflB mutant did not grow in xylose minimal medium, it was difficult to differentiate between the ability of the mutant to transport and ferment xylose from the ability to support growth. To overcome this limitation, the wild-type strain, W3110, and the pflB mutant (strain SE2358) were grown in minimal medium containing limiting amounts of both glucose and xylose in a pH-stat at pH 7.0 (Fig. 1). Strain W3110 exhibited an expected diauxie and utilized glucose preferentially over xylose, since xylose utilization is additionally controlled by CRP-cAMP (23, 30). Main fermentation products produced by the wild type were acetate, ethanol, and formate. Only small amounts of lactate (2 mM) and succinate (11 mM) were detected in the spent medium. Under the same conditions, the cell yield of strain SE2358 (pflB) was only half of that of the wild-type parent. Growth of the pfl mutant ceased after the glucose in the medium was exhausted. Xylose was utilized at a very low rate (0.19 mmol h−1 compared to the rate of 1.9 mmol h−1 for the wild type), and the increase in lactate during this phase (after about 20 h; Fig. 1) was directly proportional to the xylose consumed. During the glucose utilization phase, strain SE2358 produced about 6 mM succinate, and this was not appreciably increased during the xylose phase. With these low sugar concentrations, the efficiency of xylose fermentation to products was about 70% for both the wild type and the mutant, indicating that the pfl mutant is apparently growing but only at about 1/10 the growth rate of the wild type on xylose. This would represent a generation time of about 35 h for the pflB mutant in xylose minimal medium under anaerobic conditions. During the xylose phase, the cells did not lose their viability, which is in agreement with the very low growth rate of the culture.
FIG. 1.
Anaerobic growth and fermentation profile of E. coli wild type, strain W3110, and an isogenic Δ(focA-pflB) mutant, strain SE2358. Minimal salts medium contained glucose (0.5%), xylose (0.5%), and sodium acetate (0.1%). Cultures were grown at 37°C at pH 7.0 in a pH-stat under argon gas phase.
Total RNA from both the wild type and the pfl mutant was isolated when the amount of xylose consumed by the wild type reached about the midpoint. Using two sets of appropriate DNA primers, the amounts of xylA and xylF mRNA in the total RNA were determined by quantitative real-time PCR. The relative mRNA levels of xylA, representing the xylAB operon, encoding xylose isomerase and xylulose kinase, respectively, and xylF, part of the ABC transporter for xylose (XylFGH), in the pflB mutant were about 70% of the parent levels. The xylulose kinase activity was about 85% of the parent level in the mutant. These results show that the xyl operons are transcribed and translated in the pflB mutant even when the organism is not growing with xylose as the C source under anaerobic conditions.
Acetate kinase-minus mutant is also xylose negative under anaerobic conditions.
The results presented above suggest that the ATP derived from the pyruvate-to-acetyl-CoA-to-acetate pathway is a requirement for supporting anaerobic growth of E. coli on xylose. Since acetate kinase is the enzyme that converts acetyl phosphate to acetate and ATP, an ackA mutant is also expected to be defective in anaerobic growth on xylose. The results presented in Table 2 show that strain YK19, with the ackA202 mutation, also failed to grow with xylose under anaerobic conditions. The rate of anaerobic growth on glucose of strain YK19 was slightly lower than that of the wild-type parent but not significantly different from that of the pflB mutant in the same medium. Aerobic growth of the ackA or pflB mutant on either glucose or xylose was unaffected. These results are in agreement that the ATP produced during the interconversion of acetyl-CoA to acetate is essential for growth of E. coli on xylose in minimal medium under anaerobic conditions. This additional ATP is probably responsible also for the observed increase in the cell yield of glucose-grown cultures by 1.5-fold (Fig. 1).
TABLE 2.
Growth rates of E. coli pfl and ackA mutants on glucose and xylose in minimal mediumb
| Strain | Relevant genotype | Specific growth rate (h−1)
|
|||
|---|---|---|---|---|---|
| Aerobic
|
Anaerobic
|
||||
| Glucose | Xylose | Glucose | Xylose | ||
| W3110 | Wild type | 0.69 | 0.59 | 0.26 | 0.19 |
| AH243 | Δ(focA-pflB) | 0.70 | 0.60 | 0.21 | NGa |
| YK19 | ackA202 | 0.64 | 0.57 | 0.22 | NG |
NG, no detectable growth.
Cultures were grown in minimal medium with the indicated sugars and sodium acetate without pH control. See Methods for details.
These results further confirm that the net ATP yield from xylose (0.67) is only slightly higher than the need for maintenance energy of an anaerobic culture of a pflB (ackA) mutant of E. coli. The maintenance energy requirement for an E. coli culture growing in glucose minimal medium was calculated to be 18.9 mmol of ATP per g of cell dry weight per h (25). Apparently, due to the need for two ATPs per xylulose-5-phosphate production, the specific rate of net ATP production from xylose is close to the calculated value for maintenance energy for these pH-controlled, anaerobic batch cultures on xylose minimal medium in the absence of acetyl-CoA production. The net anaerobic ATP yield from xylose may even be lower if the ATP consumed during the production of acetyl-CoA from external acetate by the acetyl-CoA synthetase for biosynthesis is also included in these calculations.
Growth of pflB mutant in LB-xylose medium.
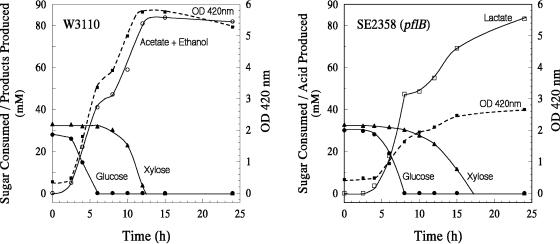
The cell yield (YATP) for E. coli is expected to be higher in rich medium than in minimal medium, as seen with Enterobacter, due to the availability of small molecule building blocks in the rich medium (31). If the ATP yield from xylose of the pflB mutant is the same when grown in rich or minimal medium, the availability of most of the building blocks in LB medium could spare the ATP used for small molecule biosynthesis, which in turn can be used for growth of the pfl mutant in complex medium with xylose as the fermentable sugar. Since both pflB and ackA mutants have the same anaerobic xylose-minus phenotype, all subsequent experiments were carried out with the pflB deletion mutant. With the same amount of total sugar (0.5% each of glucose and xylose), the cell yield of the wild type doubled in the complex medium compared to that in the minimal medium (Fig. 2 and 1, respectively). A similar increase was observed with the pflB mutant, which also included limited growth during the xylose phase at a lower rate. In contrast to the inability of the pflB mutant to ferment xylose when cultured in minimal medium (Fig. 1), xylose was completely fermented by the LB-xylose culture (Fig. 2). Although the pflB mutant utilized all the added sugars, the cell yield from xylose was only about 40% of that for the wild type, suggesting poor efficiency in converting xylose carbon to cell material due to ATP limitation during anaerobic growth.
FIG. 2.
Anaerobic growth and fermentation profile of E. coli strain W3110 and a Δ(focA-pflB) mutant, strain SE2358, in rich medium. LB medium was supplemented with glucose (0.5%) and xylose (0.5%). Cultures were grown at 37°C at pH 7.0 in a pH-stat under argon gas phase.
Anaerobic growth of pflB mutant in minimal medium with other pentoses.
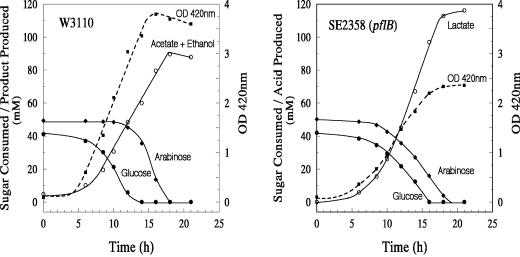
In order to test whether the observed xylose-negative growth phenotype of pfl mutants is specific for xylose or for all pentoses, strain SE2358 was cultured in minimal medium with either arabinose or ribose as the C source. Even the wild-type strain used in this experiment, W3110, failed to grow on ribose anaerobically, and this pentose was not used in further experiments. E. coli strain W3110 grew in minimal medium with both glucose and arabinose with an expected diauxie in sugar utilization (Fig. 3) (23). This diauxie was not apparent in the growth or production of fermentation products, probably due to a combination of very short transition time from the end of glucose to the beginning of arabinose and the high rate of arabinose utilization (compared to that for xylose [Fig. 1]). Strain SE2358, the pfl mutant, grew in minimal medium containing both glucose and arabinose, and both sugars were utilized simultaneously. The rate of glucose utilization by the pfl mutant was lower than that by the parent strain, probably accounting for the co-utilization of both sugars. The cell yield of strain SE2358 was also significantly lower than that of the wild-type parent strain in the glucose-arabinose medium (Fig. 3). Lower cell yield combined with higher fermentation product yield (115 mM lactate for strain SE2358 compared to 90 mM concentration of acetate plus ethanol for the wild type) (Fig. 3) suggests that arabinose is not as efficient as glucose in supporting growth of the pfl mutant (Fig. 1). However, the pflB mutant did grow in arabinose minimal medium, in contrast to results in xylose minimal medium. Similar results were also obtained with the ackA mutant, strain YK19 (data not presented).
FIG. 3.
Anaerobic growth and fermentation profile of E. coli strain W3110 and a Δ(focA-pflB) mutant, strain SE2358. Minimal salts medium was supplemented with glucose (0.75%), arabinose (0.75%), and sodium acetate (0.1%). Cultures were grown at 37°C at pH 7.0 in a pH-stat under argon gas phase.
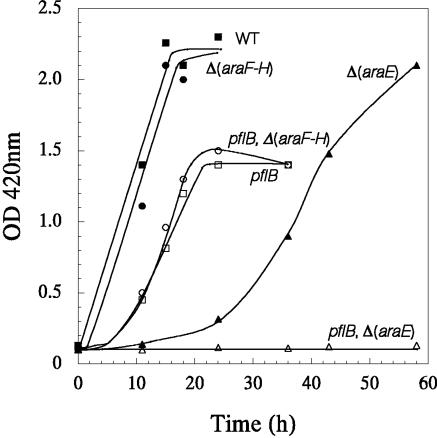
Arabinose is transported by two arabinose-inducible transport systems; a low-affinity, high-capacity arabinose/H+ symport (AraE) and a high-affinity ABC-type transporter (AraFGH) (7). The two transport systems are analogous to the two xylose transporters (13, 20). The observed growth of the pfl mutant in arabinose minimal medium (Fig. 3) suggests that the pfl mutant is utilizing ATP-independent AraE for transport of arabinose and thus conserving ATP for growth. It has been previously reported that araE expression is enhanced by arabinose and that the H+/arabinose symport is the main arabinose transporter in wild-type E. coli (16). In agreement with these published results, an araFGH mutant lacking the ABC transporter (strain AH245) grew in arabinose minimal medium anaerobically at a rate comparable to that of the parent (μ of 0.19 h−1 and 0.23 h−1, respectively) (Fig. 4). In contrast, an araE deletion mutant (strain AH247) had a longer lag and a significantly lower growth rate (μ of 0.07 h−1) than the wild-type parent. A pflB araE double mutant (AH250) did not grow in arabinose minimal medium, while the growth characteristics of strain AH248, a pfl Δ(araFGH) double mutant, and a pfl mutant were similar (μ of 0.15 h−1 for strain AH248 and 0.13 h−1 for the isogenic pfl mutant, strain AH243). These results show that in E. coli, ATP-independent arabinose/H+ symport is conserving ATP during sugar transport, leading to the observed growth of the pflB mutant on arabinose under anaerobic conditions. Although ATP can be utilized to generate an H+ gradient, the energy needed for arabinose symport is apparently independent of ATP-dependent energization of the membrane. Since the amount of succinate produced by the various mutants was comparable, the electron flow to fumarate through the membrane-bound fumarate reductase may provide the needed energy for arabinose/H+ symport (3).
FIG. 4.
Anaerobic growth of various arabinose transport mutants of E. coli in arabinose (1%) minimal medium with sodium acetate (0.1%). Wild type, W3110; Δ(araFGH), AH245; Δ(araE), AH247; pflB ΔfocA-pflB, AH243; pflB Δ(araF-H), AH248; pflB Δ(araE), AH250. Cultures were grown at 37°C at pH 7.0 in a pH-stat under argon gas phase.
Xylose transport mutants.
Based on the results of the xylose and arabinose utilization patterns, the XylE, xylose/H+ symport is apparently not the main contributor to xylose transport in E. coli, and xylose is preferentially transported by the high-affinity ABC-type XylFGH transport system. This was corroborated by the growth characteristics of xylG and xylE deletion derivatives, especially under anaerobic conditions (Table 3). Specific growth rate of a xylG mutant, AH280, utilizing only the XylE (H+/symport) for transport, was reduced by about 50% even under aerobic conditions in xylose minimal medium. Growth of this mutant in xylose minimal medium under anaerobic conditions was minimal, reaching a specific growth rate of only 0.03 h−1 compared to 0.19 h−1 for the parent. Increasing the copy number of the xylE gene with the native promoter (plasmid pAH291) did not increase the growth rate of the xylG deletion mutant (pfl+) either aerobically or anaerobically. When xylE was expressed from a synthetic promoter (plasmid pAH286), the growth rate of the xylG mutant (pfl+) on xylose only increased by about twofold under anaerobic growth conditions, although aerobic growth rates of both the wild type and the xylG mutant with the plasmid pAH286 were comparable. This anaerobic growth rate of the xylG mutant with the plasmid pAH286 expressing xylE was still only about 30% of that of the wild type. Neither plasmid supported growth of the pfl mutant on xylose anaerobically. These results show that the xylose/H+ symport is not an effective xylose transporter in E. coli, and under anaerobic conditions, xylose is primarily transported by the ATP-dependent ABC transporter. As expected, deleting the xylE gene had only a minimal effect (about 20%) on the growth rate of the mutant, strain AH257, in xylose minimal medium (Table 3).
TABLE 3.
Growth rates of E. coli mutants on glucose and xylose in minimal medium
| Strain | Genotype | Transport systema | Specific growth rate (h−1)
|
||
|---|---|---|---|---|---|
| Glucose (−O2) | Xylose (−O2) | Xylose (+O2) | |||
| W3110 | Wild type | Both | 0.31 | 0.19 | 0.47 |
| AH280 | ΔxylG | XylE | 0.30 | 0.03 | 0.25 |
| AH280 (pAH291) | ΔxylG, p-xylE+ | XylE | 0.31 | 0.03 | 0.27 |
| AH280 (pAH286) | ΔxylG, PCP6-xylE+ | XylE | 0.29 | 0.06 | 0.50 |
| AH243 | Δ(focA-pflB) | Both | 0.23 | NGb | 0.47 |
| AH243 (pAH286) | Δ(focA-pflB), PCP6-xylE+ | Both | 0.25 | NG | 0.46 |
| AH243 (pAH291) | Δ(focA-pflB), p-xylE+ | Both | 0.25 | NG | 0.35 |
| AH257 | ΔxylE | XylFGH | 0.31 | 0.15 | 0.43 |
Transport system represents either the XylFGH-ATP-dependent ABC transporter, XylE-xylose/H+ symport, or both.
NG, no detectable growth.
Xylose transport by AraE.
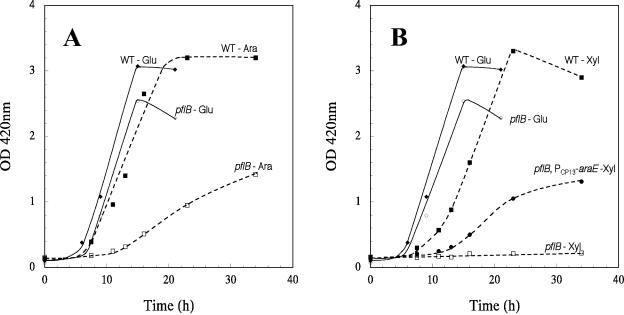
It is well established that sugar symport systems have limited specificity and transport heterologous sugars (7). It is possible that the AraE protein can also transport xylose, but under normal physiological conditions araE would be repressed by the AraC protein and could not contribute to xylose transport. In the presence of both arabinose and xylose, arabinose would be preferentially transported and utilized over xylose, and when the arabinose concentration decreased below the required critical level, the araE again would be repressed by the free AraC protein. In order to overcome this physiological limitation, the araE gene was expressed from a synthetic promoter (16), and the mutation was transduced into the pflB deletion strain AH243. The transductant, strain AH249, grew at about the same rate in xylose minimal medium (μ of 0.12 h−1) as the corresponding pfl mutant (strain AH243) grew on arabinose (μ of 0.13 h−1) (Fig. 5). These results show that an ATP-independent xylose transport is essential for growth of the pfl mutant in xylose minimal medium.
FIG. 5.
Expression of araE from a synthetic promoter supports growth of a pfl mutant of E. coli in xylose (1%) minimal medium with sodium acetate. WT, wild type, strain W3110; pflB, AH243; pflB PCP13-araE, AH249. Cultures were grown at 37°C at pH 7.0 in a pH-stat under argon gas phase.
In E. coli, xylose is primarily transported by the high-affinity XylFGH, with a stoichiometric requirement for ATP. This higher energy demand for xylose transport and further phosphorylation, coupled with the inability to produce energy from pyruvate, as in the case of a pflB mutant, does not allow sufficient net ATP to support anaerobic growth. The pyruvate formate lyase, phosphotransacetylase, acetate kinase pathway is required not only for growth of E. coli on xylose but also for growth of other bacteria on limiting sugars (11, 36). Lactococcus lactis and Lactobacillus pentosus produce lactate as the sole product during glucose or lactose fermentation. In the presence of galactose or limiting concentration of glucose, these bacteria additionally produce acetate and ethanol as fermentation products (6, 21). It has been established that the pyruvate formate lyase is induced under these carbon-limiting conditions (22). It is apparent from these studies and the present study on the E. coli pfl mutant that one of the physiological roles of pyruvate formate lyase in bacteria is to provide the precursor acetyl phosphate for production of an additional ATP at the level of acetate kinase to support a higher growth rate of the bacterium under fermentative conditions.
Acknowledgments
We thank Jensen for providing plasmids containing synthetic promoters and M. Berlyn, CGSC, for providing strains used in this study.
This work was supported in part by grants from the Department of Energy (DE-FC36-01GO11073 and FG02-96ER20222) and U.S. Department of Agriculture (00-52104-9704 and 01-35504-10669).
Footnotes
Florida Agricultural Experiment Station Journal Series no. R-10339.
REFERENCES
- 1.Bauchop, T., and S. R. Elsden. 1960. The growth of microorganisms in relation to their energy supply. J. Gen. Microbiol. 23:457-469. [DOI] [PubMed] [Google Scholar]
- 2.Beall, D. S., K. Ohta, and L. O. Ingram. 1991. Parametric studies of ethanol production from xylose and other sugars by recombinant Escherichia coli. Biotechnol. Bioeng. 38:296-303. [DOI] [PubMed] [Google Scholar]
- 3.Bernhard, T. H., and G. Gottschalk. 1978. Cell yields of Escherichia coli during anaerobic growth on fumarate and molecular hydrogen. Arch. Microbiol. 116:235-238. [DOI] [PubMed] [Google Scholar]
- 4.Causey, T. B., K. T. Shanmugam, L. P. Yomano, and L. O. Ingram. 2004. Engineering Escherichia coli for efficient conversion of glucose to pyruvate. Proc. Natl. Acad. Sci. USA 101:2235-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Causey, T. B., S. Zhou, K. T. Shanmugam, and L. O. Ingram. 2003. Engineering the metabolism of Escherichia coli W3110 for the conversion of sugar to redox-neutral and oxidized products: homoacetate production. Proc. Natl. Acad. Sci. USA 100:825-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cselovszky, J., G. Wolf, and W. P. Hammes. 1992. Production of formate, acetate, and succinate by anaerobic fermentation of Lactobacillus pentosus in the presence of citrate. Appl. Microbiol. Biotechnol. 37:94-97. [Google Scholar]
- 7.Daruwalla, K. R., A. T. Paxton, and P. J. Henderson. 1981. Energization of the transport systems for arabinose and comparison with galactose transport in Escherichia coli. Biochem. J. 200:611-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Graef, M. R., S. Alexeeva, J. L. Snoep, and M. J. T. De Mattos. 1999. The steady-state internal redox state (NADH/NAD) reflects the external redox state and is correlated with catabolite adaptation in Escherichia coli. J. Bacteriol. 181:2351-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng, J., M. R. Atkinson, W. R. McCleary, J. B. Stock, B. L. Wanner, and A. J. Ninfa. 1992. Role of phosphorylated metabolic intermediates in the regulation of glutamine synthetase synthesis in Escherichia coli. J. Bacteriol. 174:6061-6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garrigues, C., P. Loubiere, N. D. Lindley, and M. Cocaign-Bousquet. 1997. Control of the shift from homolactic acid to mixed-acid fermentation in Lactococcus lactis: predominant role of the NADH/NAD+ ratio. J. Bacteriol. 179:5282-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez, R., H. Tao, K. T. Shanmugam, S. W. York, and L. O. Ingram. 2002. Global gene expression differences associated with changes in glycolytic flux and growth rate in Escherichia coli during the fermentation of glucose and xylose. Biotechnol. Prog. 18:6-20. [DOI] [PubMed] [Google Scholar]
- 13.Henderson, P. J. F., and M. C. J. Maiden. 1990. Homologous sugar transport proteins in Escherichia coli and their relatives in both prokaryotes and eukaryotes. Philos. Trans. R. Soc. Lond. B 326:391-410. [DOI] [PubMed] [Google Scholar]
- 14.Jensen, P. R., and K. Hammer. 1998. The sequence of spacers between the consensus sequences modulates the strength of prokaryotic promoters. Appl. Environ. Microbiol. 64:82-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kessler, D., and J. Knappe. 1996. Anaerobic dissimilation of pyruvate, p. 199-205. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 16.Khlebnikov, A., K. A. Datsenko, T. Skaug, B. L. Wanner, and J. D. Keasling. 2001. Homogeneous expression of the PBAD promoter in Escherichia coli by constitutive expression of the low-affinity high-capacity AraE transporter. Microbiology 147:3241-3247. [DOI] [PubMed] [Google Scholar]
- 17.Lawford, H. G., and J. D. Rousseau. 1995. Comparative energetics of glucose and xylose metabolism in ethanologenic recombinant Escherichia coli B. Appl. Biochem. Biotechnol. 51-52:179-195. [DOI] [PubMed] [Google Scholar]
- 18.Lee, J. H., P. Patel, P. Sankar, and K. T. Shanmugam. 1985. Isolation and characterization of mutant strains of Escherichia coli altered in H2 metabolism. J. Bacteriol. 162:344-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin, E. C. C. 1996. Dissimilatory pathways for sugars, polyols, and carboxylates, p. 307-342. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 20.Linton, K. J., and C. F. Higgins. 1998. The Escherichia coli ATP-binding cassette (ABC) proteins. Mol. Microbiol. 28:5-13. [DOI] [PubMed] [Google Scholar]
- 21.Melchiorsen, C. R., N. B. Jensen, B. Christensen, K. Vaever Jokumsen, and J. Villadsen. 2001. Dynamics of pyruvate metabolism in Lactococcus lactis. Biotechnol. Bioeng. 74:271-279. [DOI] [PubMed] [Google Scholar]
- 22.Melchiorsen, C. R., K. V. Jokumsen, J. Villadsen, H. Israelsen, and J. Arnau. 2002. The level of pyruvate-formate lyase controls the shift from homolactic to mixed-acid product formation in Lactococcus lactis. Appl. Microbiol. Biotechnol. 58:338-344. [DOI] [PubMed] [Google Scholar]
- 23.Montalvo, V. H., F. Valle, F. Bolivar, and G. Gosset. 2001. Characterization of sugar mixtures utilization by an Escherichia coli mutant devoid of the phosphotransferase system. Appl. Microbiol. Biotechnol. 57:186-191. [DOI] [PubMed] [Google Scholar]
- 24.Neijssel, O. M., M. J. T. de Mattos, and D. W. Tempest. 1996. Growth yield and energy distribution, p. 1683-1692. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 25.Pirt, S. J. 1965. The maintenance energy of bacteria in growing cultures. Proc. R. Soc. Lond. B 163:224-231. [DOI] [PubMed] [Google Scholar]
- 26.Postma, P. W., J. W. Lengeler, and G. R. Jacobson. 1993. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev. 57:543-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosentel, J. K., F. Healy, J. A. Maupin-Furlow, J. H. Lee, and K. T. Shanmugam. 1995. Molybdate and regulation of mod (molybdate transport), fdhF, and hyc (formate hydrogenlyase) operons in Escherichia coli. J. Bacteriol. 177:4857-4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saier, M. H., and J. Reizer. 1994. The bacterial phosphotransferase system: new frontiers 30 years later. Mol. Microbiol. 13:755-764. [DOI] [PubMed] [Google Scholar]
- 29.Self, W. T., A. Hasona, and K. T. Shanmugam. 2001. N-terminal truncations in the FhlA protein result in formate- and MoeA-independent expression of the hyc (formate hydrogenlyase) operon of Escherichia coli. Microbiology 147:3093-3104. [DOI] [PubMed] [Google Scholar]
- 30.Song, S., and C. Park. 1997. Organization and regulation of the d-xylose operons in Escherichia coli K-12: XylR acts as a transcriptional activator. J. Bacteriol. 179:7025-7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stouthamer, A. H. 1977. Energetic aspects of the growth of micro-organisms. Symp. Soc. Gen. Microbiol. 27:285-315. [Google Scholar]
- 32.Tao, H., R. Gonzalez, A. Martinez, M. Rodriguez, L. O. Ingram, J. F. Preston, and K. T. Shanmugam. 2001. Engineering a homo-ethanol pathway in Escherichia coli: increased glycolytic flux and levels of expression of glycolytic genes during xylose fermentation. J. Bacteriol. 183:2979-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tempest, D. W., and O. M. Neijssel. 1984. The status of YATP and maintenance energy as biologically interpretable phenomena. Annu. Rev. Microbiol. 38:459-486. [DOI] [PubMed] [Google Scholar]
- 34.Underwood, S. A., S. Zhou, T. B. Causey, L. P. Yomano, K. T. Shanmugam, and L. O. Ingram. 2002. Genetic changes to optimize carbon partitioning between ethanol and biosynthesis in ethanologenic Escherichia coli. Appl. Environ. Microbiol. 68:6263-6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varenne, S., F. Casse, M. Chippaux, and M. C. Pascal. 1975. A mutant of Escherichia coli deficient in pyruvate formate lyase. Mol. Gen. Genet. 141:181-184. [DOI] [PubMed] [Google Scholar]
- 36.Yamada, T., S. Takahashi-Abbe, and K. Abbe. 1985. Effects of oxygen on pyruvate formate-lyase in situ and sugar metabolism of Streptococcus mutans and Streptococcus sanguis. Infect. Immun. 47:129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou, S., T. B. Causey, A. Hasona, K. T. Shanmugam, and L. O. Ingram. 2003. Production of optically pure d-lactic acid in mineral salts medium by metabolically engineered Escherichia coli W3110. Appl. Environ. Microbiol. 69:399-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu, J., and K. Shimizu. 2004. The effect of pfl gene knockout on the metabolism for optically pure D-lactate production by Escherichia coli. Appl. Microbiol. Biotechnol. 64:367-375. [DOI] [PubMed] [Google Scholar]