Abstract
Purpose
To describe the geographic variation in anti-osteoporosis drug therapy prescriptions before and after a hip fracture during 1999-2013 in the UK.
Methods
We used primary care data (Clinical Practice Research Datalink) to identify patients with a hip fracture and primary care prescriptions of any anti-osteoporosis drugs prior to the index hip fracture and up to five years after. Geographic variations in prescribing before and after availability of generic oral bisphosphonates were analysed. Multivariable logistic regression models were adjusted for gender, age and body mass index (BMI).
Results
13,069 patients (76% female) diagnosed with a hip fracture during 1999-2013 were identified. 11% had any anti-osteoporosis drug prescription in the six months prior to the index hip fracture. In the 0-4 months following a hip fracture 5% of patients were prescribed anti-osteoporosis drugs in 1999, increasing to 51% in 2011 to then decrease to 39% in 2013.
The independent predictors (OR (95%CI)) of treatment initiation included gender (male:0.42 (0.36-0.49)), BMI (0.98 per kg/m2 increase (0.97-1.00)) and geographic region (1.29 (0.89-1.87) North East vs. 0.56(0.43-0.73) South Central region). Geographic differences in prescribing persisted over the 5-year follow-up. If all patients were treated at the rate of the highest performing region, then nationally an additional 3,214 hip fracture patients would be initiated on therapy every year.
Conclusions
Significant geographic differences exist in prescribing of anti-osteoporosis drugs after hip fracture despite adjustment for potential confounders. Further work examining differences in health care provision may inform strategies to improve secondary fracture prevention after hip fracture.
Mini Abstract.
Fragility fractures of the hip have a major impact on the lives of patients and their families. This study highlights significant geographical variation in secondary fracture prevention with even the highest performing regions failing the majority of patients despite robust evidence supporting the benefits of diagnosis and treatment.
Keywords: hip fracture, secondary fracture prevention, geographic variation, osteoporosis, epidemiology, primary care data
Introduction
Fragility fractures of the hip are associated with significant morbidity, increased risk of subsequent falls and other fractures as well as higher mortality [1–3]. About 87,000 hip fractures occur annually in the UK, mostly in elderly individuals with underlying bone fragility as a result of osteoporosis. Almost half of all hip fracture patients have had a prior fracture [2]. The estimated risk of a second hip fracture ranges from 2.3% to 10.6%, with the majority of second hip fractures occurring within a few years after the first [4]. One-year mortality estimates following fracture range from 8.4% to 36% [2].
Anti-osteoporosis drugs (e.g. bisphosphonates) and interventions to help patients to avoid falls can potentially halve the risk of further hip fractures[5]. Persistence with anti-osteoporotic drug therapy is important for reducing the number of secondary fractures, and discontinuing therapy is associated with a 32% increase in fracture risk [6]. Despite cost effective medicines that reduce re-fracture [7], there has been a failure to translate research evidence and guidance into routine clinical care with reported low rates of prescribing for patients surviving a hip fracture [8]. We have previously demonstrated a significant increase in treatment initiation following the availability of generic bisphosphonates and publication of national guidance for secondary fracture prevention (Hawley 2016 submitted).
Geographic variations in health care delivery have been used to inform health care policy [9]. Geographical variation that remains after adjustment for demographic factors is unlikely to be due to differences in disease prevalence or patient preferences. UK health care policy places duties on health services to reduce variations in access to, and outcomes from, health care services for patients, and to assess and report on how well they have fulfilled this duty . The aim of the study was to describe geographic variation in prescription of anti-osteoporosis drug therapy before and after a hip fracture during 1999-2013 within the UK.
Methods
Data sources
Primary care data from the Clinical Practice Research Datalink (CPRD) were used to identify patients with a hip fracture. The CPRD covers 11.3 million people from 674 UK practices, with a current coverage of approximately 6.9% of the UK population who are broadly representative of the UK population in terms of age, sex and ethnicity [10]. The Office for National Statistics database on mortality was linked and validated with the data within CPRD. While the data is anonymised at the participant level, geographical information is recorded by dividing the UK into 13 geographic regions.
Study Population
Hip fractures occurring between 1 January 1999 and 30 September 2013 among patients over the age of 60 years were identified using READ codes as defined a priori after consensus by two clinicians experienced in clinical practice and epidemiological research [11]. To ensure that primary not secondary hip fractures had been captured, patients had to have no record of a hip fracture in the three years preceding the identified hip fracture.
The treatment outcomes were defined as the proportion of patients who were treated with anti-osteoporosis medications 6 months prior to hip fracture, within 4 months of primary hip fracture and up to five years after were calculated for each geographical region. Patients who died or who were lost to follow-up prior to the relevant time periods were not included. Medications classified as ‘anti-osteoporosis’ included oral bisphosphonates, hormone replacement therapy (HRT), selective oestrogen receptor modulators (SERMS), strontium ranelate, denosumab and teriparatide. Outcomes were also stratified by gender and calendar period of primary hip fracture (1999-2004 vs. 2005-2013), reflecting the availability of generic bisphosphonates and national guidelines for secondary fracture prevention
The main predictor was geographical region, which is pre-defined in CPRD, and extracted at the patient level. A priori, we use the region with the largest number of cases as the referent region, the North West. The following potential confounders were also extracted: gender and calendar period of primary hip fracture, body mass index (BMI, kg/m2), socio-economic status (Index of Multiple Deprivation 2004), Charlson Index of Comorbidity, other specific comorbid conditions, smoking status (current, Ex, and non-smoker) and drinking status (current, Ex, and non-drinker) and other previous non-hip fractures.
Statistical analysis
Descriptive statistical techniques were used to present variations in prevalence of prescribing by geographical region combining both incident and prevalent users in pre-defined time periods before and after the index fracture. Multiple imputation using chained equations was used to account for missing data on body mass index, smoking and drinking [12]. Twenty imputed datasets were generated using all potential factors (including the outcome) and estimated parameters were combined using Rubin’s rules. Many patients had missing data on Index of Multiple Deprivation (41%), which was defined as ‘missing not at random’ because the score can only be calculated for patients in England. This factor was included in a complete case model as a confounder in a sensitivity analysis.
Independent risk factors for prescription with an anti-osteoporosis medicine were identified using multivariable logistic regression models. All potential predictors were assessed in univariate models, and then in multivariable models using backward-stepwise selection. A parsimonious multivariable model was identified from the full model using cut-offs of p- entry 0.049 and p- exit 0.10. Univariate and multivariate models were applied to patients with complete data (N=6,019) and the imputed data for all patients (N=13,069). A sensitivity analysis was conducted on the imputed datasets for patients who had data on Index of Multiple Deprivation (N=7,676), which resulted in similar odds ratios as those for the imputed data for all patients.
An additional sensitivity analysis was conducted using Fine and Gray survival regression models to take into account the competing risk of mortality [13]. Patients were censored at the date of death or at the end of follow-up. All analyses were performed using STATA v14.1.
Results
A cohort of 13,069 patients diagnosed with a primary hip fracture during 1999-2013 was identified in CPRD; their descriptive factors are described in Table 1. Following the index hip fracture, mortality was high with 14% of patients dying within 4 months and an additional 8% dying within a year.
Table 1.
Baseline characteristics among primary hip fracture patients within the Clinical Practice Research Datalink during 1999-2013, UK
| Characteristic | Number | % | |
|---|---|---|---|
| Calendar period of hip fracture | 1999-2004 | 5,738 | 44 |
| 2005-2013 | 7,331 | 56 | |
| Gender | Female | 9,995 | 76 |
| Male | 3,074 | 24 | |
| Age at hip fracture | 60-69 years | 1,199 | 9 |
| 70-79 years | 3,291 | 25 | |
| 80-89 years | 6,095 | 47 | |
| ≥90 years | 2,484 | 19 | |
| Body Mass Index (kg/m2) | <18.5 | 864 | 7 |
| 18.5-24.9 | 5,352 | 41 | |
| 25.0-29.9 | 3,169 | 24 | |
| 30-34.9 | 944 | 7 | |
| ≥35 | 236 | 2 | |
| Missing | 2,504 | 19 | |
| Index of Multiple Deprivation (quintile of deprivation) | Affluent | 1,694 | 13 |
| 2 | 1,812 | 14 | |
| 3 | 1,491 | 11 | |
| 4 | 1,550 | 12 | |
| Deprived | 1,129 | 9 | |
| Missing | 5,393 | 41 | |
| Smoking | No | 7,343 | 56 |
| Yes | 1,697 | 13 | |
| Ex | 3,021 | 23 | |
| Missing | 1,008 | 8 | |
| Drinking | Yes | 7,261 | 56 |
| No | 3,391 | 26 | |
| Ex | 349 | 3 | |
| Missing | 2,068 | 16 | |
| Charlson co-morbidity index | 0 | 6,737 | 52 |
| 1 | 2,250 | 17 | |
| 2 | 1,979 | 15 | |
| ≥3 | 2,103 | 16 | |
| Region | East Midlands | 783 | 6 |
| East of England | 1,363 | 10 | |
| London | 977 | 7 | |
| North East | 338 | 3 | |
| North West | 1,921 | 15 | |
| Northern Ireland | 678 | 5 | |
| Scotland | 681 | 5 | |
| South Central | 1,031 | 8 | |
| South East Coast | 1,277 | 10 | |
| South West | 1,323 | 10 | |
| Wales | 922 | 7 | |
| West Midlands | 1,104 | 8 | |
| Yorkshire & the Humber | 671 | 5 | |
| Co-morbid conditions | Asthma | 1,796 | 14 |
| Malabsorption Syndromes | 21 | 0 | |
| Inflammatory Bowel Disease | 189 | 1 | |
| Hypertension | 6,568 | 50 | |
| Hyperlipidaemia | 1,884 | 14 | |
| Ischemic heart disease | 2,816 | 22 | |
| Cerebro-Vascular Disease | 1,453 | 11 | |
| Chronic Obstructive Pulmonary Disorder | 1,214 | 9 | |
| Chronic Renal Failure | 712 | 5 | |
| Cancer | 2,674 | 20 | |
| Previous fractures | Previous Spinal fracture | 178 | 1 |
| Previous Wrist fracture | 1,270 | 10 | |
| Previous Humerus fracture | 67 | 1 | |
| Previous Pelvis fracture | 187 | 1 | |
| Previous Rib fracture | 321 | 2 | |
| Previous Other non-hip fracture | 699 | 5 | |
| Previous joint replacement | 1,143 | 9 | |
| Mortality | 0-4 months | 1,854 | 14 |
| 5-12 months | 1,061 | 8 | |
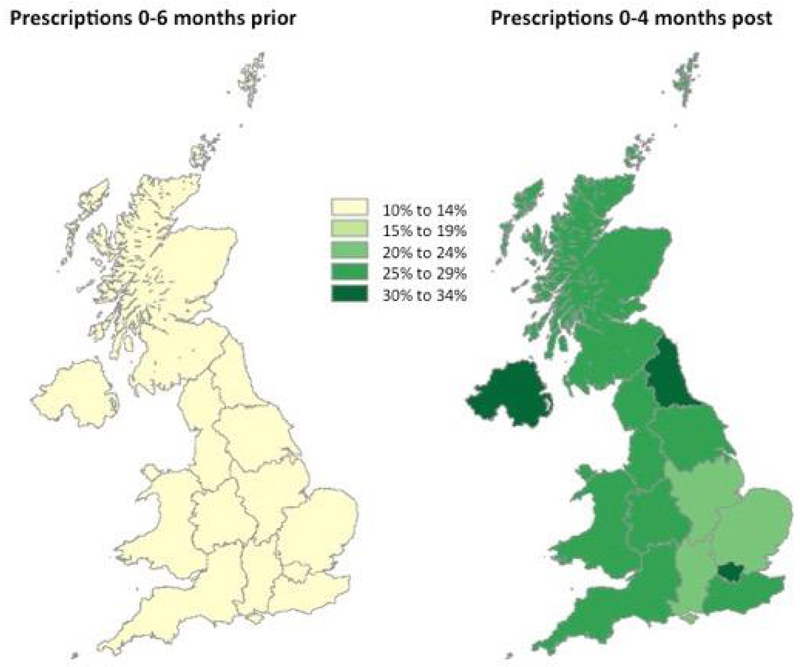
Overall, rates of prescribing of anti-osteoporosis drugs in the 6 months prior to index hip fracture were very low (11%) with no significant geographical variation (Figure 1). Geographic differences in prior prescription did not predict prescribing patterns following hip fracture.
Figure 1.
Percentage of patients on any anti-osteoporosis medicine 6 months prior to primary hip fracture and 0-4 months following primary hip fracture by geographical region within the Clinical Practice Research Datalink, 1999-2013, UK
Legend: Bars show the percentage of patients prescribed any anti-osteoporosis medication in the 6 months prior to and within 4 months post index hip fracture.
Nationally, in the 0-4 months following a hip fracture 5% of patients were prescribed an anti-osteoporosis drug in 1999, which increased to 51% in 2011 (p<0.001) and significantly decreased to 39% in 2013 (p<0.001).
During the entire study period (1999-2013) independent predictors of treatment initiation included men (OR=0.43 95% CI: 0.39-0.49, p=<0.001), increasing BMI (OR=0.98 95%CI: 0.97-0.99, p=0.002) and region (OR=1.44 95% CI: 1.10-1.88, p=0.008 North East vs. OR=0.77 95%CI: 0.64-0.93, p=0.006 South Central, with North West region as reference category) (Table 2). If all patients were treated at the rate of the highest performing region, then nationally 3,214 additional hip fracture patients would be initiated on therapy every year.
Table 2.
Estimated odds ratios (OR) of patients receiving a prescription within 4 months of primary hip fracture within the Clinical Practice Research Datalink, 1999-2013, UK
| Complete case analysis N = 6,019 | Multiple Imputed data on BMI, smoking and drinking N=13,069 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | P value | Univariate N=13,069 | Multivariate | P value | |||||||||
| Characteristic | Number | % | OR | 95% CI | OR | 95% CI | % | OR | 95% CI | OR | 95% CI | |||
| Region (ref: North West) | East Midlands | 256 | 4 | 0.94 | 0.70-1.26 | 1.00 | 0.73-1.37 | 0.990 | 6 | 0.76 | 0.62-0.92 | 0.81 | 0.66-1.00 | 0.053 |
| East of England | 854 | 14 | 0.93 | 0.77-1.13 | 0.86 | 0.70-1.07 | 0.179 | 10 | 0.83 | 0.71-0.97 | 0.89 | 0.75-1.06 | 0.203 | |
| London | 476 | 8 | 0.95 | 0.75-1.19 | 0.92 | 0.71-1.18 | 0.496 | 7 | 1.08 | 0.91-1.28 | 1.08 | 0.90-1.30 | 0.426 | |
| North East | 153 | 3 | 1.33 | 0.94-1.89 | 1.26 | 0.87-1.84 | 0.224 | 3 | 1.34 | 1.04-1.71 | 1.44 | 1.10-1.88 | 0.008 | |
| North West | 1,183 | 20 | 1.00 | 1.00 | 15 | 1.00 | 1.00 | |||||||
| Northern Ireland | 5 | 1.19 | 0.98-1.44 | 1.18 | 0.96-1.45 | 0.125 | ||||||||
| Scotland | 5 | 0.94 | 0.77-1.14 | 0.91 | 0.74-1.12 | 0.381 | ||||||||
| South Central | 463 | 8 | 0.69 | 0.54-0.88 | 0.56 | 0.42-0.73 | <0.001 | 8 | 0.82 | 0.69-0.97 | 0.77 | 0.64-0.93 | 0.006 | |
| South East Coast | 598 | 10 | 0.87 | 0.70-1.07 | 0.82 | 0.64-1.04 | 0.098 | 10 | 0.98 | 0.84-1.15 | 0.99 | 0.84-1.18 | 0.926 | |
| South West | 916 | 15 | 0.93 | 0.77-1.12 | 0.91 | 0.74-1.11 | 0.353 | 10 | 0.90 | 0.76-1.05 | 0.93 | 0.78-1.10 | 0.395 | |
| Wales | 7 | 0.96 | 0.81-1.15 | 0.96 | 0.79-1.16 | 0.665 | ||||||||
| West Midlands | 746 | 12 | 0.90 | 0.74-1.10 | 0.88 | 0.70-1.09 | 0.228 | 8 | 0.90 | 0.76-1.07 | 0.91 | 0.76-1.09 | 0.298 | |
| Yorkshire & the Humber | 374 | 6 | 1.07 | 0.83-1.37 | 1.07 | 0.82-1.40 | 0.628 | 5 | 0.93 | 0.76-1.13 | 1.06 | 0.86-1.32 | 0.580 | |
| Calendar period of hip fracture | 1999-2004 | 2,170 | 36 | 1.00 | 1.00 | 44 | 1.00 | 1.00 | ||||||
| 2005-2013 | 3,849 | 64 | 5.52 | 4.76-6.41 | 6.00 | 5.15-7.00 | <0.001 | 56 | 5.67 | 5.14-6.25 | 6.14 | 5.55-6.79 | <0.001 | |
| Gender | Female | 4,547 | 76 | 1.00 | 1.00 | 76 | 1.00 | 1.00 | ||||||
| Male | 1,472 | 24 | 0.46 | 0.40-0.53 | 0.42 | 0.36-0.50 | <0.001 | 24 | 0.50 | 0.45-0.55 | 0.43 | 0.39-0.49 | <0.001 | |
| Age at hip fracture (years) | 60-69 years | 537 | 9 | 0.76 | 0.62-0.93 | 0.86 | 0.69-1.08 | 0.206 | 9 | 0.85 | 0.74-0.98 | 0.95 | 0.81-1.11 | 0.520 |
| 70-79 years | 1,567 | 26 | 0.89 | 0.78-1.01 | 1.01 | 0.88-1.17 | 0.859 | 25 | 0.97 | 0.89-1.07 | 1.11 | 1.00-1.24 | 0.043 | |
| 80-89 years | 2,858 | 47 | 1.00 | 1.00 | 47 | 1.00 | 1.00 | |||||||
| >90 years | 1,057 | 18 | 0.71 | 0.60-0.83 | 0.59 | 0.50-0.70 | <0.001 | 19 | 0.70 | 0.63-0.78 | 0.61 | 0.54-0.69 | <0.001 | |
| Body Mass Index (kg/m2) prior to hip fracture | 0.98 | 0.97-0.99 | 0.98 | 0.97-0.99 | 0.005 | 100 | 0.99 | 0.98-1.00 | 0.98 | 0.97-0.99 | 0.002 | |||
| Index of Multiple Deprivation quintile (ref=affluent) | 0.96 | 0.92-0.99 | 0.93 | 0.89-0.97 | 0.003 | |||||||||
| Smoking | Non-smoker | 3,614 | 60 | 1.00 | 1.00 | 61 | 1.00 | 1.00 | ||||||
| Current | 762 | 13 | 0.77 | 0.65-0.93 | 0.89 | 0.73-1.09 | 0.260 | 14 | 0.83 | 0.74-0.94 | 0.92 | 0.80-1.06 | 0.236 | |
| Ex-smoker | 1,643 | 27 | 1.00 | 0.88-1.13 | 1.00 | 0.87-1.16 | 0.970 | 25 | 1.14 | 1.04-1.25 | 1.03 | 0.93-1.14 | 0.573 | |
| Drinking | Current | 4,135 | 69 | 1.00 | 1.00 | 66 | 1.00 | 1.00 | ||||||
| Non-drinker | 1,683 | 28 | 1.04 | 0.92-1.18 | 0.95 | 0.83-1.09 | 0.494 | 31 | 1.01 | 0.92-1.10 | 0.92 | 0.83-1.02 | 0.121 | |
| Ex-drinker | 201 | 3 | 0.93 | 0.68-1.28 | 0.81 | 0.58-1.14 | 0.224 | 3 | 0.99 | 0.78-1.27 | 0.77 | 0.60-1.00 | 0.048 | |
| Charlson co-morbidity index (ref=0) | 0.99 | 0.96-1.02 | 0.95 | 0.92-0.99 | 0.004 | 100 | 1.01 | 0.98-1.03 | 0.95 | 0.93-0.98 | <0.001 | |||
| Co-morbid conditions | Hypertension | 3337 | 55 | 1.38 | 1.23-1.54 | 1.17 | 1.03-1.32 | 0.016 | 50 | 1.49 | 1.37-1.61 | 1.20 | 1.10-1.31 | <0.001 |
| Previous fractures | Spine fracture | 106 | 2 | 1.89 | 1.28-2.78 | 1.83 | 1.20-2.79 | 0.005 | 1 | 2.32 | 1.72-3.13 | 2.15 | 1.55-2.98 | <0.001 |
| Humerus fracture | 29 | <1 | 2.19 | 1.06-4.55 | 2.90 | 1.32-6.37 | 0.008 | 1 | 1.98 | 1.22-3.22 | 2.15 | 1.28-3.64 | 0.004 | |
| Other non-hip fracture | 350 | 6 | 1.38 | 1.10-1.72 | 1.41 | 1.10-1.80 | 0.006 | 5 | 1.44 | 1.22-1.69 | 1.36 | 1.14-1.62 | 0.001 | |
Legend: Odds Ratios (OR) shown for complete case and multiply imputed univariate and mutually adjusted Cox models.
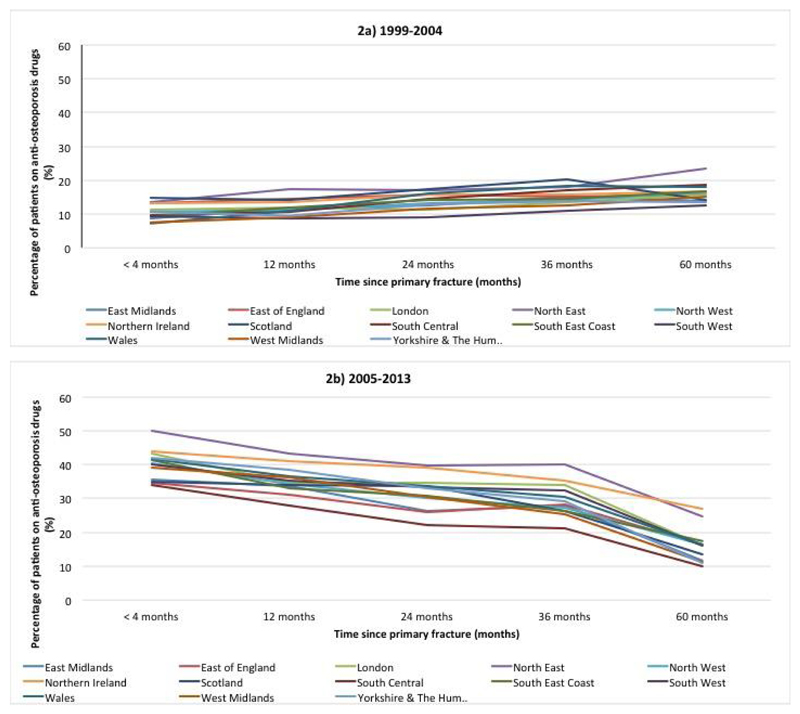
There was a significant interaction between geographic region and calendar period of hip fracture (p=0.0047) (Table 3). During 1999-2004, overall 10% of patients with a primary hip fracture were prescribed anti-osteoporosis drugs within 4 months with little variation by geographic region (Figure 2a). However during 2005-2013, this increased to 40% with marked variations between regions (50% of the patients in the North East compared with 34% of patients in the South Central region) (Figure 2b). The percentage of patients on anti-osteoporosis drugs both in the 0-6 months pre and 0-4 post hip fracture increased from 59% to 75% from 1999-2004 to 2005-2013. Further, the percentage of patients who were on anti-osteoporosis drugs before their hip fracture and then stopped immediately aftewarrds, reduced from 41% to 25% between these time periods.
Table 3.
Estimated odds ratios (OR) of patients receiving a prescription within 4 months of primary hip fracture by region and calendar period of diagnosis within the Clinical Practice Research Datalink, 1999-2013, UK
| 1999-2004 | 2005-2013 | |||||
|---|---|---|---|---|---|---|
| Region | OR | 95% CI | P value | OR | 95% CI | P value |
| East Midlands | 0.87 | 0.56-1.33 | 0.513 | 0.79 | 0.62-1.01 | 0.057 |
| East of England | 1.42 | 1.02-1.97 | 0.037 | 0.76 | 0.62-0.92 | 0.006 |
| London | 1.09 | 0.74-1.60 | 0.665 | 1.07 | 0.87-1.33 | 0.51 |
| North East | 1.40 | 0.82-2.38 | 0.221 | 1.48 | 1.08-2.03 | 0.015 |
| North West (Referent) | 1.00 | 1.00 | ||||
| Northern Ireland | 1.37 | 0.90-2.11 | 0.144 | 1.14 | 0.90-1.44 | 0.284 |
| Scotland | 1.47 | 0.98-2.22 | 0.062 | 0.77 | 0.60-0.98 | 0.033 |
| South Central | 0.92 | 0.62-1.38 | 0.694 | 0.73 | 0.59-0.90 | 0.004 |
| South East Coast | 0.94 | 0.65-1.37 | 0.748 | 1.01 | 0.83-1.23 | 0.922 |
| South West | 0.93 | 0.65-1.34 | 0.707 | 0.93 | 0.77-1.14 | 0.493 |
| Wales | 0.65 | 0.42-1.02 | 0.062 | 1.04 | 0.84-1.29 | 0.700 |
| West Midlands | 0.74 | 0.49-1.13 | 0.158 | 0.95 | 0.78-1.17 | 0.634 |
| Yorkshire & the Humber | 1.06 | 0.69-1.62 | 0.790 | 1.07 | 0.83-1.38 | 0.599 |
Legend: Univariate comparison of prescription of anti-osteoporosis drugs in 1999-2004 vs. 2005-2013 by region.
Figure 2.
Prescription rates of any anti-osteoporosis medication after primary hip fracture by geographical region during 1999-2004 (2a) and during 2005-13 (2b) within the Clinical Practice Research Datalink, UK
Legend: This figure shows for each time period, the proportion of patients alive and followed up within CPRD receiving a prescription of anti-osteoporosis medication.
Following an index hip fracture, there was a significant decline in prescription rates such that by 5 years only 15% were on any anti-osteoporosis therapy (Figures 2a and 2b). Regional differences in prescribing persisted over the 5-year follow-up; 10% of patients were on therapy at five years in the South Central region in contrast with those in the North East (25%) and in Northern Ireland (27%) during 2005-13. While there was a significant difference in medication initiation by calendar year, the overall prescribing rates at 5 years were similar between 1999-2004 and 2005 – 2013 (15.9% vs. 15.8%, respectively). Models adjusting for the competing risk of death produced similar findings (see supplementary table).
Discussion
Summary of key findings
This study has confirmed significant geographic variation in initiation of anti-osteoporosis medication after hip fracture in the UK. However, even the best performing geographical region had lower initiation rates than anticipated and longer-term prescribing of therapy remained low.
Geographical variation of care
Geographical variation in health care use has been used for many decades to highlight areas for further investigation and potentially significant change in routine clinical care. Historically, the description of an upto eightfold difference in tonsillectomy between comparable towns within England and USA [14] led to dis-investment in tonsillectomy and subsequent clinical trials to identify the subgroups who do benefit. More recent reviews of studies of geographical variation have confirmed its value to highlight a potential priorities for evidence synthesis and/or dissemination to inform local commissioning with the aims of standardising current practice around current best evidence [9]. While typically applied to high volume procedures, geographical variation in care has been shown to also apply where there is underuse of healthcare [15]. Variation in care has been identified in a number of disease areas including radiotherapy for cancer [16], ischaemic heart disease[17] but not childhood asthma[18].-,
The major drivers of geographical variation in health care delivery are a) difference in physicians’ ability to diagnose patients b) difference in physicians’ belief in the benefits of the intervention [19]. These are underpinned by variation in technology diffusion [20] and gaps in the clinical knowledge of clinicians or how they apply their knowledge [21]. In case of the tonsillectomy in the early 20th Century, the geographical variation in physicians’ diagnosis and treatment was due to the lack of trial evidence for the intervention. In contrast, the observed variation in secondary fracture prevention is not due to a lack of robust evidence for how to diagnose high-risk patients or the lack of evidence for the treatment benefit and likely reflects inadequate technology diffusion.
A number of tools have been validated to identify and diagnose patients at high risk that would benefit from pharmacotherapy such as FRAX [22] and Qfracture [23]. The National Osteoporosis Guidance Group recommendations deliver treatment thresholds within FRAX as a decision aid to support physicians in the diagnosis of patients who would benefit from therapy [24]. The FRAX tool has now been tested in a large scale randomized controlled trial and demonstrated an impressive 24% reduction in hip fracture associated with an 80% prescription rate in those identified at high risk vs. 12% in the control group [25]. The lower treatment rates and lack of difference in the pre-fracture period suggests a) selection and maintainence of anti-osteoporosis prescriptions in high risk patients who go on to fracture is poor in primary care b) post-fracture variation in secondary fracture prevention most likely reflects the variable presence of fracture liaison services within the UK [26].
Another issue is the belief of benefit of secondary fracture prevention amongst the wider health care community and policy makers. Despite a number of trials demonstrating fracture reduction from 20 to 70% using anti-osteoporosis medications [5] and the cost-effectiveness of these interventions [27], secondary fracture prevention in the UK remains poor with less than a third of the expected number of patients treated for secondary fracture prevention included in the UK Quality Outcome Framework in 2013/14, a national re-imbursement scheme for primary care [28]. Further, recently published perspectives in general medical journals, based on opinion and not the balance of published literature [29], have questioned the effectiveness of pharmacotherapy for secondary fracture prevention causing confusion over the benefits of secondary prevention denying high risk patients therapy and leading to avoidable fragility fractures to the detriment of patients, their family, carers and the society as a whole.
Medication persistence
While in the randomized controlled trials used to register agents, persistence with therapy was over 90% [30], real world data has consistently described poor persistence in the real world setting with rates of primary non-adherence of up to 30% [31] and secondary non-adherence between 30 and 50% at one year [32]. Persistance has been reported as higher in the one year after fragility fracture but still low in those aged 80 and over[33]. Non-adherence to anti-osteoporosis medication is associated with a 30- 40% increase in the risk of fracture [6]. In this study, the geographic variation in medication prescriptions persisted from initiation to 5 years after the index fracture. However the higher rates of medication initiation after 2005 did not translate to higher rates of prescriptions at 5 years across geographical regions. The poor adherence to anti-osteoporosis therapies is well known and monitoring has been recognized as one of the essential components for a secondary fracture prevention care pathway to be effective [34]. The most efficient methods for monitoring have yet to be determined. The major reasons for lack of persistence with oral bisphosphonates, the first line anti-osteoporosis therapy used in most cases, include patient characteristics (experiencing side effects, insufficient motivation, lack of perceived benefit and the complex administration [35]) and physician characteristics (overestimation of patient adherence [36] and physician misinformation [35]),
Improving medication persistence to anti-osteoporosis therapy is challenging. A number of strategies such as providing reminders [37], motivational telephone interventions [38], personal training using telephone calls and group meetings [39] have been tested and do not improve treatment persistence to anti-osteoporosis. However, the longer treatment interval and simpler administration regimes with denosumab have been shown to increase 12-month persistence rates to over 80% in routine clinical practice [40, 41].
Secondary fracture prevention care gap and Fracture Liaison Services
International bodies such as the ASBMR and IOF [42, 43] have published guidelines on service models to improve secondary fracture prevention to reduce subsequent fractures [44]. These recommendations recommend that Fracture Liaison Services be created to close the care gap in secondary fracture prevention. However, in the UK, less than 40% of hospitals in England had established such a service by 2010 and there is marked variability in service delivery with more than 50% of services identifying less than 50% of their expected fragility fracture caseload annually[26]. Published criteria and standards are now available as part of an improvement programme to improve the quality of Fracture Liaison Services and ensure they are both effective and efficient[45].
Strengths and Limitations
This is a large study using real world data that used validated methods to ascertain the primary fracture. Two statistical methods were used to analyse the data given we did not know how mortality may differ in the data. As competing risk methods such as Fine & Grey need to be used if the mortality rates differ between exposure groups, and mortality after hip fracture did not vary by region, both Cox and Fine & Grey gave similar findings. Regional denominators were not available and so the proportion of patients presenting with a hip fracture per region is not known. While primary care data in the UK captures prescribing of oral anti-osteoporosis medication, the prescribing of parenteral therapies such as teriparatide, zoledronate and denosumab is likely underestimated, as a proportion will be prescribed in the secondary care setting with inconsistent recording in the primary care record. This may account for some of the differences in prescribing rates between 2011 and 2013. While we did not have access to dispensing data and, from other sources, the rates of primary non-adherence is up to 30%[31]. in the UK if a patient does not pick up their prescription then the pharmacy feeds this back to the primary care physician and future scripts are not issued and these patients would be identified as non-adherent.
Conclusions
Fragility fractures of the hip have a major impact on the lives of patients and their families as well as to health care and society. This study highlights significant geographical variation in secondary fracture prevention with even the highest performing regions failing the majority of patients despite robust evidence supporting the benefits of diagnosis and treatment. Further health services research including the use of guidelines and decision aids are needed to close this care gap and prevent avoidable fragility fractures and their clinical and economic sequalae.
Supplementary Material
Acknowledgements
The ReFRESH study group consists of Dr Andrew David Judge, Dr Muhammad Kassim Javaid, Professor Nigel Arden, Professor Cyrus Cooper, Professor Andrew Farmer, Dr Daniel Prieto-Alhambra, Dr Jose Leal, Professor Michael Goldacre, Professor Alastair Gray, Dr Janet Lippett, Dr Rachael Gooberman-Hill and Laura Graham.
Role of the funding source
This work was supported by the National Institutes of Health and Research (NIHR) Health Services and Delivery Research programme HS&DR) (project number 11/1023/01); and from the Oxford NIHR Musculoskeletal Biomedical Research Unit, Nuffield Orthopaedic Centre, University of Oxford. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the HS&DR programme, NIHR, NHS or the Department of Health. This study is based in part on data from the Clinical Practice Research Datalink obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. The funding source had no role in the design and conduct of the study, in the collection, analysis and interpretation of the data, or in the preparation, review or approval of the manuscript.
Footnotes
Author contributions
AS analysed the data and drafted the paper with MKJ. DPA and SH assisted with the analysis and commented on the paper. AD cleaned the data and commented on the paper. JL and CC commented on the paper. AJ and MKJ designed the study, oversaw the analysis and revised the paper.
Competing interests
AS, SH and AD have no competing financial interests relevant to the submitted work. DPA, JL, CC, MKJ and AJ received grants from NIHR HS&DR during the conduct of the study. Outside the submitted work, MKJ reports personal fees from Lilly UK, Amgen, Sevier, Merck, Medtronic, Internis, Consilient Health, Stirling Anglia, Mereo Biopharma and Optasia. He serves on the Scientific Committee of the National Osteoporosis Society and International Osteoporosis Foundation; DPA received grants from Bioiberica S.A. and Amgen Spain S.A.; CC received personal fees from Servier, Amgen, Eli Lilly, Merck, Medtronic and Novartis. AJ has received consultancy, lecture fees and honoraria from Servier, UK Renal Registry, Oxford Craniofacial Unit, IDIAP Jordi Gol, Freshfields Bruckhaus Deringer, has held advisory board positions (which involved receipt of fees) from Anthera Pharmaceuticals, INC., and received research sponsorship from ROCHE.
References
- 1.Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int. 2009;20:1633–1650. doi: 10.1007/s00198-009-0920-3. [DOI] [PubMed] [Google Scholar]
- 2.Cooper C, Mitchell P, Kanis JA. Breaking the fragility fracture cycle. Osteoporos Int. 2011;22:2049–2050. doi: 10.1007/s00198-011-1643-9. [DOI] [PubMed] [Google Scholar]
- 3.Johnell O, Kanis JA, Oden A, Sernbo I, Redlund-Johnell I, Petterson C, De Laet C, Jonsson B. Fracture risk following an osteoporotic fracture. Osteoporos Int. 2004;15:175–179. doi: 10.1007/s00198-003-1514-0. [DOI] [PubMed] [Google Scholar]
- 4.Melton LJ, 3rd, Kearns AE, Atkinson EJ, Bolander ME, Achenbach SJ, Huddleston JM, Therneau TM, Leibson CL. Secular trends in hip fracture incidence and recurrence. Osteoporos Int. 2009;20:687–694. doi: 10.1007/s00198-008-0742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freemantle N, Cooper C, Diez-Perez A, Gitlin M, Radcliffe H, Shepherd S, Roux C. Results of indirect and mixed treatment comparison of fracture efficacy for osteoporosis treatments: a meta-analysis. Osteoporos Int. 2013;24:209–217. doi: 10.1007/s00198-012-2068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross S, Samuels E, Gairy K, Iqbal S, Badamgarav E, Siris E. A meta-analysis of osteoporotic fracture risk with medication nonadherence. Value Health. 2011;14:571–581. doi: 10.1016/j.jval.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 7.NICE. Alendronate, etidronate, risedronate, raloxifene, strontium ranelate and teriparatide for the secondary prevention of osteoporotic fragility fractures in postmenopausal women. NICE technology appraisal guidance 161. 2008 [Google Scholar]
- 8.Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD. Osteoporosis Medication Use after Hip Fracture in U.S. Patients between 2002 and 2011. J Bone Miner Res. 2014 doi: 10.1002/jbmr.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollingworth W, Rooshenas L, Busby J, et al. Using clinical practice variations as a method for commissioners and clinicians to identify and prioritise opportunities for disinvestment in health care: a cross-sectional study, systematic reviews and qualitative study. Health Services and Delivery Research. 2015;3:1–172. [PubMed] [Google Scholar]
- 10.Herrett E, Gallagher A, Bhaskaran K, Forbes H, Mathur R, van Staa T, Smeeth L. Data Resource Profile: Clinical Practice Research Datalink (CPRD) International Journal of Epidemiology. 2015;44:827–836. doi: 10.1093/ije/dyv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawley S, Javaid MK, Prieto-Alhambra D, Lippett J, Sheard S, Arden NK, Cooper C, Judge A. Clinical effectiveness of orthogeriatric and fracture liaison service models of care for hip fracture patients: population-based longitudinal study. Age and ageing. 2016;45:236–242. doi: 10.1093/ageing/afv204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 13.Fine JPG, RJ A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 14.Glover JA. The Incidence of Tonsillectomy in School Children: (Section of Epidemiology and State Medicine) Proc R Soc Med. 1938;31:1219–1236. [PMC free article] [PubMed] [Google Scholar]
- 15.Sinner MF, Piccini JP, Greiner MA, Walkey AJ, Wallace ER, Heckbert SR, Benjamin EJ, Curtis LH. Geographic variation in the use of catheter ablation for atrial fibrillation among Medicare beneficiaries. Am Heart J. 2015;169:775–782.e772. doi: 10.1016/j.ahj.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams MV, Drinkwater KJ. Geographical variation in radiotherapy services across the UK in 2007 and the effect of deprivation. Clin Oncol (R Coll Radiol) 2009;21:431–440. doi: 10.1016/j.clon.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Lawlor DA, Bedford C, Taylor M, Ebrahim S. Geographical variation in cardiovascular disease, risk factors, and their control in older women: British Women's Heart and Health Study. J Epidemiol Community Health. 2003;57:134–140. doi: 10.1136/jech.57.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaur B, Anderson HR, Austin J, Burr M, Harkins LS, Strachan DP, Warner JO. Prevalence of asthma symptoms, diagnosis, and treatment in 12-14 year old children across Great Britain (international study of asthma and allergies in childhood, ISAAC UK) Bmj. 1998;316:118–124. doi: 10.1136/bmj.316.7125.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wennberg JE, Barnes BA, Zubkoff M. Professional uncertainty and the problem of supplier-induced demand. Soc Sci Med. 1982;16:811–824. doi: 10.1016/0277-9536(82)90234-9. [DOI] [PubMed] [Google Scholar]
- 20.Skinner J, Staiger D. Technology Diffusion and Productivity Growth in Health Care. The review of economics and statistics. 2015;97:951–964. doi: 10.1162/REST_a_00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reames BN, Shubeck SP, Birkmeyer JD. Strategies for reducing regional variation in the use of surgery: a systematic review. Ann Surg. 2014;259:616–627. doi: 10.1097/SLA.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2008;19:385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hippisley-Cox J, Coupland C. Predicting risk of osteoporotic fracture in men and women in England and Wales: prospective derivation and validation of QFractureScores. BMJ. 2009;339:b4229. doi: 10.1136/bmj.b4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanis JA, McCloskey EV, Johansson H, Strom O, Borgstrom F, Oden A. Case finding for the management of osteoporosis with FRAX®—assessment and intervention thresholds for the UK. Osteoporosis International. 2008;19:1395–1408. doi: 10.1007/s00198-008-0712-1. [DOI] [PubMed] [Google Scholar]
- 25.Shepstone L, Fordham R, Lenaghan E, et al. A pragmatic randomised controlled trial of the effectiveness and cost-effectiveness of screening older women for the prevention of fractures: rationale, design and methods for the SCOOP study. Osteoporos Int. 2012;23:2507–2515. doi: 10.1007/s00198-011-1876-7. [DOI] [PubMed] [Google Scholar]
- 26.Javaid MK, Rai S, Schoo R, Stanley R, Vasilakis N, Tsang C. Fracture Liaison Service (FLS) Database facilities audit. FLS breakpoint: opportunities for improving patient care following a fragility fracture. Royal College of Physicians; London: 2016. [Google Scholar]
- 27.Hiligsmann M, Evers SM, Ben Sedrine W, Kanis JA, Ramaekers B, Reginster JY, Silverman S, Wyers CE, Boonen A. A systematic review of cost-effectiveness analyses of drugs for postmenopausal osteoporosis. Pharmacoeconomics. 2015;33:205–224. doi: 10.1007/s40273-014-0231-1. [DOI] [PubMed] [Google Scholar]
- 28.Services PaPC. Quality and Outcomes Framework – Prevalence, Achievements and Exceptions Report: England, 2013-14. In: Centre HaSCI, editor. Health and Social Care Information Centre; 2014. [Google Scholar]
- 29.Compston J. Overdiagnosis of osteoporosis: fact or fallacy? Osteoporos Int. 2015;26:2051–2054. doi: 10.1007/s00198-015-3220-0. [DOI] [PubMed] [Google Scholar]
- 30.Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 31.Reynolds K, Muntner P, Cheetham TC, Harrison TN, Morisky DE, Silverman S, Gold DT, Vansomphone SS, Wei R, O'Malley CD. Primary non-adherence to bisphosphonates in an integrated healthcare setting. Osteoporos Int. 2013;24:2509–2517. doi: 10.1007/s00198-013-2326-5. [DOI] [PubMed] [Google Scholar]
- 32.Li L, Roddam A, Gitlin M, Taylor A, Shepherd S, Shearer A, Jick S. Persistence with osteoporosis medications among postmenopausal women in the UK General Practice Research Database. Menopause. 2012;19:33–40. doi: 10.1097/gme.0b013e318221bacd. [DOI] [PubMed] [Google Scholar]
- 33.Klop C, Welsing PM, Elders PJ, Overbeek JA, Souverein PC, Burden AM, van Onzenoort HA, Leufkens HG, Bijlsma JW, de Vries F. Long-term persistence with anti-osteoporosis drugs after fracture. Osteoporos Int. 2015;26:1831–1840. doi: 10.1007/s00198-015-3084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drew S, Judge A, Cooper C, Javaid MK, Farmer A, Gooberman-Hill R. Secondary prevention of fractures after hip fracture: a qualitative study of effective service delivery. Osteoporos Int. 2016 doi: 10.1007/s00198-015-3452-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tafaro L, Nati G, Leoni E, Baldini R, Cattaruzza MS, Mei M, Falaschi P. Adherence to anti-osteoporotic therapies: role and determinants of "spot therapy". Osteoporos Int. 2013;24:2319–2323. doi: 10.1007/s00198-013-2283-z. [DOI] [PubMed] [Google Scholar]
- 36.Curtis JR, Cai Q, Wade SW, Stolshek BS, Adams JL, Balasubramanian A, Viswanathan HN, Kallich JD. Osteoporosis medication adherence: physician perceptions vs. patients' utilization. Bone. 2013;55:1–6. doi: 10.1016/j.bone.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bianchi ML, Duca P, Vai S, et al. Improving adherence to and persistence with oral therapy of osteoporosis. Osteoporos Int. 2015;26:1629–1638. doi: 10.1007/s00198-015-3038-9. [DOI] [PubMed] [Google Scholar]
- 38.Solomon DH, Iversen MD, Avorn J, et al. Osteoporosis telephonic intervention to improve medication regimen adherence: a large, pragmatic, randomized controlled trial. Arch Intern Med. 2012;172:477–483. doi: 10.1001/archinternmed.2011.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tuzun S, Akyuz G, Eskiyurt N, et al. Impact of the training on the compliance and persistence of weekly bisphosphonate treatment in postmenopausal osteoporosis: a randomized controlled study. International journal of medical sciences. 2013;10:1880–1887. doi: 10.7150/ijms.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silverman SL, Siris E, Kendler DL, et al. Persistence at 12 months with denosumab in postmenopausal women with osteoporosis: interim results from a prospective observational study. Osteoporos Int. 2015;26:361–372. doi: 10.1007/s00198-014-2871-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hadji P, Papaioannou N, Gielen E, et al. Persistence, adherence, and medication-taking behavior in women with postmenopausal osteoporosis receiving denosumab in routine practice in Germany, Austria, Greece, and Belgium: 12-month results from a European non-interventional study. Osteoporos Int. 2015;26:2479–2489. doi: 10.1007/s00198-015-3164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marsh D, Akesson K, Beaton DE, Bogoch ER, Boonen S, Brandi ML, McLellan AR, Mitchell PJ, Sale JE, Wahl DA. Coordinator-based systems for secondary prevention in fragility fracture patients. Osteoporos Int. 2011;22:2051–2065. doi: 10.1007/s00198-011-1642-x. [DOI] [PubMed] [Google Scholar]
- 43.Eisman JA, Bogoch ER, Dell R, Harrington JT, McKinney RE, Jr, McLellan A, Mitchell PJ, Silverman S, Singleton R, Siris E. Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Miner Res. 2012 doi: 10.1002/jbmr.1698. [DOI] [PubMed] [Google Scholar]
- 44.Association BO. The care of patients with fragility fractures. 2007 [Google Scholar]
- 45.Akesson K, Marsh D, Mitchell PJ, McLellan AR, Stenmark J, Pierroz DD, Kyer C, Cooper C, Group IOFFW Capture the Fracture: a Best Practice Framework and global campaign to break the fragility fracture cycle. Osteoporos Int. 2013;24:2135–2152. doi: 10.1007/s00198-013-2348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.