Abstract
Background
Biphasic defibrillation has been practiced worldwide for >15 years. Yet, consensus does not exist on the best energy levels for optimal outcomes when used in patients with ventricular fibrillation (VF)/pulseless ventricular tachycardia (VT).
Methods
This prospective, randomized, controlled trial of 235 adult cardiac arrest patients with VF/VT was conducted in the emergency and cardiology departments. One group received low-energy (LE) shocks at 150–150–150 J and the other escalating higher-energy (HE) shocks at 200–300–360 J. If return of spontaneous circulation (ROSC) was not achieved by the third shock, LE patients crossed over to the HE arm and HE patients continued at 360 J. Primary end point was ROSC. Secondary end points were 24-hour, 7-day, and 30-day survival.
Results
Both groups were comparable for age, sex, cardiac risk factors, and duration of collapse and VF/VT. Of the 118 patients randomized to the LE group, 48 crossed over to the HE protocol, 24 for persistent VF, and 24 for recurrent VF. First-shock termination rates for HE and LE patients were 66.67% and 64.41%, respectively (P=0.78, confidence interval: 0.65–1.89). First-shock ROSC rates were 25.64% and 29.66%, respectively (P=0.56, confidence interval: 0.46–1.45). The 24-hour, 7-day, and 30-day survival rates were 85.71%, 74.29%, and 62.86% for first-shock ROSC LE patients and 70.00%, 50.00%, and 46.67% for first-shock ROSC HE patients, respectively. Conversion rates for further shocks at 200 J and 300 J were low, but increased to 38.95% at 360 J.
Conclusion
First-shock termination and ROSC rates were not significantly different between LE and HE biphasic defibrillation for cardiac arrest patients. Patients responded best at 150/200 J and at 360 J energy levels. For patients with VF/pulseless VT, consideration is needed to escalate quickly to HE shocks at 360 J if not successfully defibrillated with 150 or 200 J initially.
Keywords: defibrillation, sudden cardiac arrest, cardiopulmonary resuscitation, high-energy, low-energy
Introduction
Cardiac arrest occurs in ~300,000 people annually out-of-hospital in the US and ~1,400 in Singapore.1
The World Health Organization estimates ~7.3 million deaths from heart disease every year, with the top three countries contributing to this figure largely being the People’s Republic of China, with 1,505,300 deaths (109 per 100,000 population); India, with 1,215,400 deaths (98 per 100,000 population); and the Russian Federation, with 737,000 deaths (497 per 100,000 population).2 Approximately 40%–75% of these deaths are in the out-of-hospital environment. Survival rates from out-of-hospital cardiac arrest vary from 0% to 43%.3 The incidence and survival rates of in-hospital cardiac arrest are less well publicized.
The consistent use of prehospital cardiopulmonary resuscitation (CPR) and automated external defibrillation is not very prevalent in resource-challenged communities. In addition, optimal energy levels for biphasic electrical defibrillation for out-of-hospital cardiac arrest remain to be determined.
Currently available biphasic defibrillators provide either fixed low-energy (LE) defibrillation of up to 120–200 J or gradually escalating higher-energy (HE) of up to 360 J. These energy doses appear to be relatively effective and safe.
Biphasic energy level research was initially conducted in laboratory animals with otherwise normal hearts in which ventricular fibrillation (VF) had been induced for short time periods. LE biphasic defibrillation was shown to terminate the VF at least as effectively as HE monophasic shocks.4–12 Wide variations were reported in VF termination rates using LE biphasic shocks.13–15 Very few laboratory trials have been conducted to determine whether fixed LE or escalating HE protocols would provide the best chances for successful defibrillation.16,17 An out-of-hospital study demonstrated potential for good survival with escalating HE shocks.18 Another out-of-hospital prospective comparative study of fixed LE (150 J, 150 J, and 150 J) versus escalating HE (200 J, 300 J, and 360 J) biphasic defibrillation involving 221 patients demonstrated similar VF conversion and termination effect in both groups but greater termination and conversion rates with escalating HE shocks.19 A larger study showed that termination rates of VF declined when using repeated 200 J or 300 J shocks, unless an increased energy level (360 J) was selected.20 The out-of-hospital scene often presents problems in determining temporal cardiac arrest information and has more potential for less consistent CPR quality than the in-hospital environment.
Our objective was to perform an exploratory randomized controlled trial in an in-hospital environment (so that opportunities for a more consistent standard of CPR would be greater) comparing fixed LE and escalating HE protocols, using biphasic waveform defibrillation during manual external cardiac defibrillation, to determine likely optimal energy protocols for patients with VF or pulseless ventricular tachycardia (VT).
Patients and methods
The primary aim of this study was to compare the efficacy of escalating HE biphasic shocks (200–300–360 J) with that of LE biphasic shocks (150–150–150 J) for first and subsequent shock defibrillation success in an in-hospital environment.
Study design, interventions, and population
This was a multicenter, prospective, randomized, single-blinded, controlled trial with two treatment groups. One group (Group A) began with an escalating HE biphasic waveform protocol (200 J, 300 J, and 360 J). If termination including return of spontaneous circulation (ROSC) had not occurred by the third shock, further shocks would be delivered at 360 J until termination was obtained. If ROSC was attained at any stage of the study, the patients were followed up for primary and secondary end points. If ROSC was not attained and VF persisted or recurrence was observed, further shocks at the rate of 360 J would be given until either ROSC or final termination with asystole. The other group (Group B) started with a fixed LE protocol for the first three shocks delivered (150 J, 150 J, and 150 J) and if termination, including ROSC, was not achieved by then, the group was crossed over to the escalating HE protocol (200 J, 300 J, 360 J, 360 J, and 360 J) till ROSC or final termination, as described for Group A patients, occurred. The patients were recruited from January 2005 to November 2008 from seven sites in four general hospitals and one specialty heart center in Singapore.
Eligible participants were cardiac arrest patients of age ≥21 years with either VF or pulseless VT. All those aged <21 years or with trauma arrest or known to be pregnant were excluded. Patients with any other shockable rhythm (VT or supraventricular tachycardia) with a pulse and hemodynamically unstable were also excluded from the trial.
Randomization and masking
Owing to the time-critical nature of cardiac arrest management, a cluster block randomization by week was conducted within each study site. The allocation ratio of the two groups was 1:1 and carried out once weekly by sealed envelopes for each of the seven sites. Once randomized, the site would use the assigned treatment arm for the next 7 days until another randomization was conducted at each site. The assigned protocol for each site was mounted on a card on each of the defibrillators being used for the trial. The trial investigators and the resuscitation teams were not blinded to the assigned treatment arm.
Study setting
The study was conducted at four emergency departments (EDs) and three cardiology departments in Singapore, namely, the EDs at Changi General Hospital, National University Hospital, Singapore General Hospital and Tan Tock Seng Hospital, and the cardiology departments at Changi General Hospital, National Heart Centre, and Tan Tock Seng Hospital. The resuscitations were carried out by teams under the leadership of board-certified emergency physicians in the case of EDs and board-certified cardiologists in the case of cardiology departments. All doctors involved were currently certified in Basic and Advanced Cardiac Life Support as per the guidelines issued by the National Resuscitation Council Singapore (NRCS), which closely follow the International Liaison Committee on Resuscitation consensus and treatment recommendations. All nursing staff involved in the resuscitations were also currently certified in Basic Cardiac Life Support (BCLS), at the least, as per NRCS guidelines. Having current certification is a prerequisite for all staff working in these departments.
All study sites used the Physio-Control LIFEPAK® 12 Biphasic Defibrillator in the manual mode for all the patients recruited in the study so that defibrillation waveform differences would not be a factor affecting outcomes. If either VF or pulseless VT was detected, the patient would be included and the randomized defibrillation protocol for that site and that week would be instituted.
After the patient received the treatment, demographic data, time of collapse, rhythm at study entry, time and energy levels of the shocks utilized, ROSC, and disposition/death (as appropriate) were recorded. If resuscitation was successful, the patient was followed up and reviewed at 24 hours, 7 days, and 30 days.
The primary end point was ROSC, defined as restoration of a palpable carotid pulse consonant with the return of an organized rhythm within 60 seconds of shock administration. Blood pressure was recorded using an automated sphygmomanometer. The major secondary end points included termination of the shockable rhythm and survival status at 24 hours, 7 days, and 30 days. Termination was defined as disappearance of VF or pulseless VT for at least 5 seconds after shock administration, and included three types of defibrillation results – ROSC, pulseless electrical activity, and asystole.
Informed consent and good clinical practice issues
The trial was approved initially by the Clinical Trials Coordinating Committee of the Ministry of Health Singapore and subsequently by the respective institutional review boards (IRBs) of the participating institutions (SingHealth Centralised Institutional Review Board and the NHG Domain Specific Review Board). Waiver of informed consent was given for conducting the interventions at the time of cardiac arrest presentation by the Clinical Trial Coordinating Committee. Subsequently, within 6 weeks after the cardiac arrest, written approval to use the patient’s trial data and information from clinical records was obtained from the patients (if they survived) or from the next-of-kin or appropriate legal representative (in the event of nonsurvival). If unable to establish contact with the patient or the next-of-kin, as was appropriate, at least three documented attempts at contact including telephone call records and letters to the last known postal address were kept for potential inspection by the IRBs. The start of the trial was preceded by a series of meetings with local community and religious leaders to obtain feedback and advice on informed consent issues that affected each community group, a public forum to explain the trial to and obtain immediate feedback from members of the public followed by broadcasts about the trial through the print and broadcast media, and a period for further feedback from the public.
Data collection
The patient’s resuscitation record was obtained using the LIFEPAK 12 device. This was kept with the trial registration form and the patient’s case notes. Data from this, the trial registration form, and the patient’s case notes were subsequently transferred to anonymized case record forms (CRFs). The completed CRFs were sent to a primary data management, monitoring, and coordination center. The center conducted in-house central monitoring to ensure the completeness of CRF entries. Audited CRFs were used to create electronic data files for data analysis. This trial was registered on www.clinicaltrials.gov (NCT00429611).
Statistical analysis
The primary end point was ROSC. Statistical significance for the primary outcome was defined as P<0.05. Although an initial target ROSC difference of 10% was set, it was later amended to 16% in view of other interventions being implemented for cardiac arrest management during the study period that would have likely influenced outcomes. To detect this anticipated ROSC difference with a power of 80% in the final analysis, the study required at least 244 patients, ie, 122 patients in each treatment group in the intention-to-treat population.
Continuous variables were summarized with mean and standard deviation, while categorical variables were summarized with number and percentage. Comparisons of demographic characteristics between treatment groups were carried out by two-sample t-test for continuous variables and Fisher’s exact test for categorical variables. Fisher’s exact test was used to compare the efficacy of HE biphasic shocks and LE biphasic shocks on the primary end point (ROSC), while logistic regression was used to study treatment efficacy by adjusting for potential confounding factors such as age, duration of collapse, and duration of VF/pulseless VT. Similar analyses were carried out on ROSC and termination for first shock and subsequent shock effect, as well as survival status at 24 hours, 7 days, and 30 days. ROSC, termination, and survival status were further explored by the energy level at individual shocks in the HE and LE groups.
All statistical analyses were performed using SAS, Version 9.1 (SAS Institute Inc., Cary, NC, USA). All the tests were two-sided, and statistical significance was assumed if P-value <0.05. All confidence intervals (CIs) were at 95% level and were the approximated CIs for odds ratio (OR), except for 95% CIs for outcomes based on patients with persistent or recurrent VF in the LE group who crossed over to the HE protocol.
Results
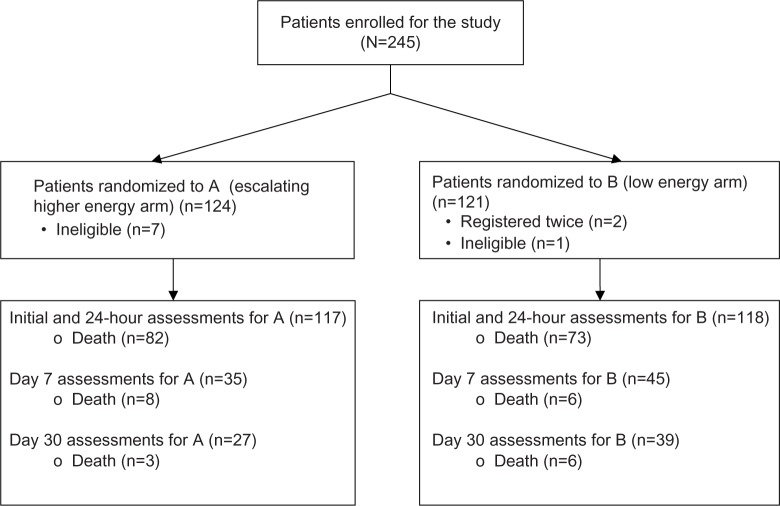
A total of 245 patients were recruited during the study period (Figure 1), 124 into the escalating HE arm (A) and 121 into the LE arm (B). Of these, eight patients were considered ineligible (seven in Group A and one in Group B) as review of their rhythm at study registration showed VT with pulse. Another two patients (both in Group B) were inadvertently registered twice, already having been registered earlier for the same episode. The total number of patients analyzed was 235 (117 in Group A and 118 in Group B).
Figure 1.
Consort table for all enrolled patients in HILOBED.
Abbreviation: HILOBED, higher biphasic versus low biphasic energy defibrillations.
The demographic and preintervention clinical characteristics of these patients are shown in Table 1. These were not significantly different between the two treatment groups.
Table 1.
Demographic characteristics of HILOBED study patients
| Characteristic | Group A (n=117) | Group B (n=118) | P-valuea |
|---|---|---|---|
| Age (years), mean (SD) | 62.07 (15.71) | 62.62 (15.43) | 0.79 |
| Male, n (%) | 88 (75.21) | 84 (71.19) | 0.56 |
| Ethnic group n (%) | |||
| Chinese | 76 (64.96) | 81 (68.64) | 0.86 |
| Malay | 19 (16.24) | 19 (16.10) | |
| Indian | 17 (14.53) | 15 (12.71) | |
| Others | 5 (4.27) | 3 (2.54) | |
| Weight (kg), mean (SD) | 63.53 (11.86) | 63.70 (11.36) | 0.93 |
| Height (cm), mean (SD) | 164.02 (7.62) | 163.92 (8.72) | 0.95 |
| BMIb (kg/m2), mean (SD) | 23.80 (4.07) | 23.41 (3.51) | 0.59 |
| Any medical historyc (%) | 76 (78.35) | 83 (79.05) | 1.00 |
| Known hypertensionc (%) | 52 (54.17) | 62 (59.05) | 0.57 |
| Known diabetes mellitusc (%) | 28 (29.17) | 35 (33.33) | 0.55 |
| Known hyperlipidemiac (%) | 40 (41.23) | 38 (36.19) | 0.47 |
| Known ischemic heart diseasec (%) | 49 (51.04) | 52 (49.52) | 0.89 |
| Location cardiac arrest occurred, n (%) | |||
| In-hospital | 48 (41.03) | 41 (35.04) | 0.42 |
| Out-of-hospital | 69 (58.97) | 76 (64.96) | |
| Duration of collapse prior to the first shock (minutes), mean (SD) | 22.48 (19.86) | 24.04 (19.91) | 0.55 |
| Duration of VF/pulseless VT prior to the first shock (minutes), mean (SD) | 2.63 (2.37) | 2.29 (3.12) | 0.36 |
| Rhythm at study entry, n (%) | |||
| VF | 101 (86.32) | 104 (88.14) | 0.70 |
| Pulseless VT | 16 (13.68) | 14 (11.86) |
Notes:
The P-value in the table is from (two-sided) Fisher’s exact test for categorical variables and from (two-sided) two-sample t-test for continuous variables.
Actual weight and height were used to calculate BMI. Where actual weight and height measurements could not be made, estimated weight and height recorded in the case report form were used. BMI was calculated according to the formula: BMI = weight (kg)/height (m)2.
The information on preexisting comorbidities was obtained, whenever possible, by Day 7.
Abbreviations: BMI, body mass index; CI, confidence interval; HILOBED, higher biphasic versus low biphasic energy defibrillations; SD, standard deviation; VF, ventricular fibrillation; VT, ventricular tachycardia.
Outcomes based on intention-to-treat perspective
Table 2 shows the number of patients (with percentages) who had first-shock termination with ROSC and the survival rates of these patients at 24 hours, 7 days, and 30 days for each of the treatment groups. There was no difference in VF/pulseless VT termination rates or ROSC rates for first-shock effect between the two groups, neither was there any difference in total ROSC rate. Even for patients who did not achieve ROSC after the first defibrillation shock, no significant difference was observed in the rate of subsequent ROSC between the two treatment groups. At 24 hours, survival rates appeared to veer toward superiority in Group B. This effect was more pronounced and appeared significant at 7 days. This benefit was not sustained at Day 30.
Table 2.
Outcomes summary for the two treatment groups by intention-to-treat perspective
| Outcomes | Escalating higher energy (Group A) | Fixed lower energy group (Group B) | Odds ratioa, P-valueb (95% approximated CI) | Adjusted odds ratioa, P-valueb (95% approximated CI) |
|---|---|---|---|---|
| Total number per group n (%) | 117 (100) | 118 (100) | – | – |
| VF termination after first shock, n (%) | 78 (66.67) | 76 (64.41) | 1.11, 0.78 (0.65, 1.89) | 1.09, 0.76 (0.62, 1.94) |
| ROSC after first shock, n (%) | 30 (25.64) | 35 (29.66) | 0.82, 0.56 (0.46, 1.45) | 0.85, 0.64 (0.44, 1.65) |
| Total ROSC, n (%) | 64 (54.70) | 65 (55.08) | 0.98, 1.00 (0.59, 1.65) | 1.16, 0.65 (0.61, 2.22) |
| 24-hour survival, n (%) | 35 (29.91) | 45 (38.14) | 1.44, 0.22 (0.84, 2.49) | 1.68, 0.13 (0.86, 3.27) |
| 7-day survival, n (%) | 27 (23.08) | 39 (33.05) | 1.65, 0.11 (0.92, 2.93) | 2.31, 0.03 (1.10, 4.85) |
| 30-day survival, n (%) | 24 (20.51) | 33 (27.97) | 1.50, 0.22 (0.82, 2.75) | 2.01, 0.07 (0.94, 4.30) |
| Subgroup: No-ROSC after first shock, n (%) | 87 (74.36) | 83 (70.34) | – | – |
| ROSC after subsequent shocks, n (%) | 34 (39.08)c [29.06]d | 30 (36.14)c [25.42]d | 1.13, 0.75e (0.61, 2.11) | 1.23, 0.58e (0.59, 2.56) |
Notes:
The odds ratio is the success odds ratio of A vs B for termination after first shock, ROSC after first shock, total ROSC, and ROSC after subsequent shocks, and is the death odds ratio of A vs B for all survival calculations.
The P-value for odds ratio is from two-sided Fisher’s exact test. The P-value for adjusted odds ratio is from multiple logistic regression that controls potential confounders of age, collapse duration, and duration of VF/pulseless VT.
The percentages for ROSC after subsequent shocks are derived from No-ROSC patients after first shock in Group A/B as the denominator.
The percentages in the brackets are the total of patients originally in Groups A/B as the denominator.
The odds ratio, P-value, and the 95% CI with or without adjustment for ROSC after subsequent shocks are referred to the subgroup of No-ROSC after first shock in Groups A/B.
Abbreviations: CI, confidence interval; ROSC, return of spontaneous circulation; VF, ventricular fibrillation; VT, ventricular tachycardia.
For patients who did not attain ROSC, the duration of VF or pulseless VT was very similar in both groups (Group A =3.67±2.98 minutes and Group B =3.66±4.00 minutes, P=0.99, 95% CI: −1.39 to −1.40). However, for those who did attain ROSC, the duration of VF/pulseless VT (1.22±1.53 minutes) was slightly lower in the LE group (Group B) than Group A (1.80±1.25 minutes; P=0.02, 95% CI: 0.09–1.07), indicating a potential slight baseline advantage for the LE biphasic group.
The termination and ROSC rates on a per energy level basis in the LE and escalating HE groups are presented in Table 3. For the 118 patients in the LE protocol, cumulative ROSC rates increased from 29.66% to 40.68% from the first to the third 150 J shock. Forty-eight patients (40.68%) crossed over to the escalating HE protocol, 24 for persistent (resistant) VF and 24 for recurrent VF. Recurrent (R) VF patients had a 41.67% ROSC rate versus 29.17% for those with persistent (P) VF (OR 1.73, 95% CI: 0.52–5.74, P=0.55). Survival rates at 24 hours, 7 days, and 30 days for these two subgroups were 20.83% (R) versus 12.50% (P) (OR 1.84, 95% CI: 0.31–13.32, P=0.70), 12.50% (R) versus 12.50% (P) (OR 1.00, 95% CI: 0.12–8.35, P=1.00), and 8.33% (R) versus 12.50% (P) (OR 0.64, 95% CI: 0.05–6.20, P=1.00), respectively. For those initially shocked at 150 J, a fourth shock at 200 J produced only a 6.25% conversion rate. Shocks at 300 J also resulted in relatively low conversion rates. However, for patients shocked at 360 J, the total ROSC rate was 37.50%.
Table 3.
Outcomes summary for patients on LE and escalating HE protocolsa (intention-to-treat analysis)
| Energy delivered (J) | Number of patients at each energy level | Number of patients with first termination at each energy level (%) | Number of patients with no further VF/pulseless VT after this energy level (%) | Number of patients with ROSC after the shock (%) | Number of patients with 24-hour survival after ROSC (%) | Number of patients with 7-day survival after ROSC (%) | Number of patients with 30-day survival after ROSC (%) |
|---|---|---|---|---|---|---|---|
| Group A (higher-energy patients) | |||||||
| First shock at 200 J | 117 | 78 (66.67) | 39 (33.33) | 30 (25.64) | 21 (17.95) | 15 (12.82) | 14 (11.97) |
| Second shock at 300 J | 78 | 8 (10.26) | 15 (19.23) | 9 (11.54) | 2 (2.56) | 2 (2.56) | 2 (2.56) |
| Third shock at 360 J | 63 | 5 (7.94) | 7 (11.11) | 3 (4.76) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Summary of first three HE shocks | 117 | 91 (77.78) | 61 (52.14) | 42 (35.90) | 23 (19.66) | 17 (14.53) | 16 (13.68) |
| All shocks at 360 J | 63 | 31 (49.21) | 63 (100.00) | 25 (39.68) | 12 (19.05) | 10 (15.87) | 8 (12.70) |
| Group B (low-energy patients) | |||||||
| First shock at 150 J | 118 | 76 (64.41) | 43 (36.44) | 35 (29.66) | 30 (25.42) | 26 (22.03) | 22 (18.64) |
| Second shock at 150 J | 75 | 11 (14.67) | 19 (25.33) | 9 (12.00) | 4 (5.33) | 4 (5.33) | 4 (5.33) |
| Third shock at 150 J | 56 | 7 (12.50) | 8 (14.29) | 4 (7.14) | 3 (5.36) | 3 (5.36) | 3 (5.36) |
| Summary of first three LE shocks | 118 | 94 (79.66) | 70 (59.32) | 48 (40.68) | 37 (31.36) | 33 (27.97) | 29 (24.58) |
| Fourth shock at 200 J | 48 | 6 (12.50) | 8 (16.67) | 3 (6.25) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Fifth shock at 300 J | 40 | 5 (12.50) | 8 (20.00) | 2 (5.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| All shocks at 360 J | 32 | 13 (40.63) | 32 (100.00) | 12 (37.50) | 8 (25.00) | 6 (18.75) | 5 (15.63) |
Notes:
The percentages in this table used the second column as the denominator.
Abbreviations: HE, higher-energy; LE, low-energy; ROSC, return of spontaneous circulation; VF, ventricular fibrillation; VT, ventricular tachycardia.
For the escalating HE group, the cumulative ROSC rate from the first shock at 200 J to the third shock at 360 J increased from 25.64% to 35.90%. For shocks delivered at the highest energy available, viz 360 J, this group produced a total ROSC rate of 39.68%.
Outcomes based on individual shocks administered (as treated analysis)
For all patients recruited in the study (Table 4), the best ROSC rates were in those given 150 J energy (40.68%) and 360 J energy (38.95%). Patients shocked at 200 J had an ROSC rate of 20.00%, whereas those shocked at 300 J had only 9.32%. Survival data for these various energy levels, whether at 24 hours, 7 days, or 30 days, were seemingly better for those who were shocked at an earlier phase of the protocol cycle (ie, better at 150 J than at 200 J and better at 200 J than at 300 J). However, at 360 J there was an upward survival trend across all three time periods.
Table 4.
Outcomes summary for all patients shocked at different energy levels (per energy level analysis)
| Energy delivered | Number of patients | Number of ROSC after the shock (%) | Duration of collapse prior to first shock (minutes)a
|
Duration of VF/pulseless VT prior to first shock at the energy level (minutes)b
|
24-hour survival (%)d | 7-day survival (%)d | 30-day survival (%)d | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ROSC no, mean (SD) | ROSC yes, mean (SD) | 95% CI of difference (P-valuec) | ROSC no, mean (SD) | ROSC yes, mean (SD) | 95% CI of difference (P-valuec) | ||||||
| First shock at 150 J | 118 | 35 (29.66) | 30.01 (18.75) | 10.57 (15.51) | 12.27–26.61 (P<0.01) | 2.84 (3.51) | 1.06 (1.33) | 0.88–2.68 (P<0.01) | 30 (85.71) | 26 (74.29) | 22 (62.86) |
| First shock at 200 J | 117 | 30 (25.64) | 25.95 (20.18) | 12.63 (15.34) | 5.30–21.34 (P<0.01) | 2.94 (2.57) | 1.73 (1.41) | 0.41–1.96 (P<0.01) | 21 (70.00) | 15 (50.00) | 14 (46.67) |
| Total patients shocked at 150 J | 118 | 48 (40.68) | 32.27 (18.75) | 12.32 (15.16) | 13.40–26.50 (P<0.01) | 3.13 (3.71) | 1.09 (1.27) | 1.08–3.02 (P<0.01) | 37 (77.08) | 33 (68.75) | 29 (60.42) |
| Total patients shocked at 200 J | 165 | 33 (20.00) | 27.74 (19.31) | 13.94 (16.13) | 6.59–21.01 (P<0.01) | 3.16 (3.31) | 1.64 (1.41) | 0.77–2.27 (P<0.01) | 21 (63.64) | 15 (45.45) | 14 (42.42) |
| Total patients shocked at 300 J | 118 | 11 (9.32) | 26.74 (19.15) | 30.73 (19.58) | −16.00–8.06 (P=0.51) | 3.22 (3.47) | 1.64 (1.03) | 0.66–2.51 (P<0.01) | 2 (18.18) | 2 (18.18) | 2 (18.18) |
| Total patients shocked at 360 J | 95 | 37 (38. 95) | 29.29 (15.40) | 18.30 (16.65) | 4.29–17.68 (P<0.01) | 4.18 (4.24) | 1.89 (1.61) | 1.04–3.53 (P<0.01) | 20 (54.05) | 16 (43.24) | 13 (35.14) |
Notes:
The duration of collapse prior to first shock is the time duration from collapse to the first shock.
The duration of VF/pulseless VT is the duration from onset of VF/pulseless VT to the first shock.
The P-value is from (two-sided) two-sample t-test.
The denominator of the survival outcome percentage is the number of ROSC patients.
Abbreviations: CI, confidence interval; ROSC, return of spontaneous circulation; SD, standard deviation; VF, ventricular fibrillation; VT, ventricular tachycardia.
Table 4 also indicates that those with ROSC had a significantly shorter duration of collapse than those who did not achieve ROSC for most shocks. Similarly, VF/pulseless VT duration was also shorter in those who achieved ROSC. It must be noted, though, that the duration of collapse (as well as the duration of VF/pulseless VT) prior to the shock at the HE levels, viz at 200 J, 300 J, and 360 J in the case of the LE group, and at 300 J and 360 J in the case of the HE group, would have had been longer than durations prior to the first shock for these groups. These longer durations were due to the defibrillation protocols used, all of which included graduated escalating energy at ~2-minute intervals if the patients could not achieve VF termination at lower energy levels.
Discussion
This is the first in-hospital study evaluating the efficacy of LE versus escalating HE biphasic electrical defibrillation for cardiac arrest patients. The study was conducted in the in-hospital environment, which allowed greater scope for better determination of cardiac arrest time intervals through interaction with paramedics, family members, and departmental documentation. More consistent standards of CPR performance were assured in the static mode with less interruptions to chest compressions. The use of in-hospital manual defibrillation allowed greater opportunities for shorter interruptions to chest compressions during rhythm analysis. Patient identification for subsequent contact was also enhanced.
From an intention-to-treat perspective, no significant difference in terms of ROSC rates was demonstrated between LE and escalating HE biphasic defibrillation. A trend toward slightly better outcomes noted across the board for patients treated initially with LE biphasic defibrillations may have been due to shorter VF durations in that group. First-shock termination rates were very similar in both treatment groups; so were the ROSC rates after first-shock termination. Initiating defibrillation either at 150 J or at 200 J biphasic waveform energy does not result in any significant difference in outcomes for first-shock effect. An animal study comparing first shock of 150 J versus 360 J showed no difference in ROSC rates.16 A randomized comparison of fixed lower (150 J) versus escalating HE (beginning at 200 J) levels for defibrillation in out-of-hospital cardiac arrest (BIPHASIC Trial. 2007) showed very similar first-shock effect, though there was a tendency toward higher conversion rates with escalating HE after completion of the full resuscitation protocol for both groups.19
This study also showed that ROSC rates fell from 29.66% with the first 150 J shock to only 7.14% with the third 150 J shock. These rates remained low in the initial phase of cross over to the HE range surging to 37.50% with repeated use of 360 J shocks. A similar phenomenon was noted in the HE group, when repeated use of 360 J pushed ROSC rates up to 39.68%. This suggests that repeated use of 360 J shocks may have a role in cardioversion of patients who do not appear to respond to LE shocks initially. Similar observations were also made in the BIPHASIC Trial 2007.19 A recent alert by the US Food and Drug Administration (FDA) highlighted a concern about nonconversion of arrhythmias at ≤200 J.21 The FDA reported 14 events over a 3-year period since 2006 in which 200 J biphasic defibrillators were ineffective in converting patients, whereas subsequent shocks from a different 360 J biphasic defibrillator resulted in immediate defibrillation/cardioversion. This is especially relevant in our study with the 40.68% who had either persistent or recurrent VF after a few LE shocks. Among these, an additional 35.42% achieved ROSC when crossing to escalating HE, especially at 360 J. This suggests that some patients require higher levels of energy for successful conversion of VF.
An escalating HE model may, thus, be optimal for cardiac arrest patients with VF, with the initial shock being at 150 J or 200 J, and if termination is not achieved, then immediately escalating to a significantly HE level might be required. Low-energy biphasic energy (150–200 J) is useful in converting a significant number of cardiac arrest victims. There remains a group of patients (~40%) who appear resistant to termination with LE biphasic shocks and required higher levels of energy. The study also demonstrated, as expected, that patients with longer duration of collapse or VF/pulseless VT had lower ROSC and hence, lower survival rates. This may have been owing to protocols allowing HE levels to be administered later in the resuscitation cycle. This has been noted in a previous study.22
The lower ROSC and survival rates at intermediate energy levels such as 200 J/300 J may indicate that patients not converting with LE (150 J–200 J) may merit consideration to immediate escalation to significantly HE levels, such as at 360 J. The mechanism of VF within the first few minutes of onset is likely due to intramural reentry wave fronts, as opposed to propagation of intramural foci in the later minutes for maintenance of the fibrillation wavefront.23,24 The energies required to address these different wave front mechanisms may be different. Heart muscles exposed to longer periods of cardiac arrest, hypoxia, and acidosis may be less viable than within the first few minutes. Failure to obtain ROSC at this late stage provides a fertile ground for increased mortality. Once ROSC is achieved, other pathophysiological mechanisms that typify the postcardiac arrest myocardium and different additional treatments would all play a part in survival.24 This study was not powered to compare the effect of shocks on patients with persistent versus recurrent VF.
A similar but larger trial comparing outcomes of VF or pulseless VT patients defibrillated initially and, perhaps, repeatedly, at either 150 J–200 J or at 360 J may better illustrate whether the real difference between LE and HE defibrillation would be best demonstrated by focusing on these extremes of present-day acceptable energy levels and minimizing the delay in institution of higher energy would enhance survival.
Limitations
This study had the limitation of a relatively small sample size (though this was still the largest clinical trial comparing low with escalating HE biphasic defibrillation in a clinical environment).
In addition, since chest compressions were ongoing when shocks were administered, the unavailability of impedance signals to indicate initial termination could have resulted in an incorrect failed termination decision in some instances.
Conclusion
This study showed no significant difference in first-shock termination and ROSC rates between 150 J and 200 J during biphasic defibrillation of cardiac arrest patients managed in an in-hospital environment. Patients seemingly responded best at 150 J–200 J and 360 J energy levels. Consideration needs to be given for patients with VF/pulseless VT to escalate quickly to HE shocks at 360 J if they are not successfully defibrillated with 150–200 J in the first instance.
Acknowledgments
The authors wish to place on record the assistance and cooperation of the medical and nursing staff of the four emergency and three cardiology departments who participated in this study. The authors also thank the Project Management and Data Management Departments of the Singapore Clinical Research Institute for their assistance in trial and data management. The study was funded by the National Medical Research Council, Singapore, Medtronics Pvt Ltd., and the Clinical Trials, Research and Epidemiological Unit, Singapore. The sponsors had no role in the design, analysis, interpretation, or comments on the trial and no role in the drafting or writing of the manuscript.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Neumar RW, Barnhart JM, Berg RA, et al. Implementation strategies for improving survival after out-of-hospital cardiac arrest in the united states: consensus recommendations from the 2009 American Heart Association Cardiac Arrest Survival Summit. Circulation. 2011;123(24):2898–2910. doi: 10.1161/CIR.0b013e31821d79f3. [DOI] [PubMed] [Google Scholar]
- 2.WHO [webpage on the Internet] Estimated Deaths by Cause, Sex and WHO Member State (1), 2012. Geneva: World Health Organization (WHO) Department of Health Statistics and Information Systems; 2012. [Accessed May 2, 2016]. Available from: http://www.who.int/healthinfo/statistics/mortality_rawdata/en/ [Google Scholar]
- 3.Vukmir RB. Survival and outcome from prehospital cardiac arrest. Intern J Rescue Disaster Med. 2004;4(1):1–28. [Google Scholar]
- 4.Winkle RA, Mead RH, Ruder MA, et al. Improved low energy defibrillation efficacy in man with the use of a biphasic truncated exponential waveform. Am Heart J. 1989;117(1):122–127. doi: 10.1016/0002-8703(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 5.Yamanouchi Y, Brewer JE, Donohoo AM, Mowrey KA, Wilkoff BL, Tchou PJ. External exponential biphasic versus monophasic shock waveform: efficacy in ventricular fibrillation of longer duration. Pacing Clin Electrophysiol. 1999;22(10):1481–1487. doi: 10.1111/j.1540-8159.1999.tb00352.x. [DOI] [PubMed] [Google Scholar]
- 6.Swartz JF, Fletcher RD, Karasik PE. Optimisation of biphasic waveforms for human nonthoracotomy defibrillation. Circulation. 1993;88(6):2646–2654. doi: 10.1161/01.cir.88.6.2646. [DOI] [PubMed] [Google Scholar]
- 7.Olsovsky MR, Hodgson DM, Shorofsky SR, Kavesh NG, Gold MR. Effect of biphasic waveforms on transvenous defibrillation thresholds in patients with coronary artery disease. Am J Cardiol. 1997;80(8):1098–1100. doi: 10.1016/s0002-9149(97)00615-2. [DOI] [PubMed] [Google Scholar]
- 8.Bardy GH, Ivey TD, Allen MD, Johnson G, Mehra R, Grene HL. A prospective, randomized evaluation of biphasic versus monophasic waveform pulses on defibrillation efficacy in humans. J Am Coll Cardiol. 1989;64(3):728–733. doi: 10.1016/0735-1097(89)90118-6. [DOI] [PubMed] [Google Scholar]
- 9.Leng CT, Paradis NA, Calkins H, et al. Resuscitation after prolonged ventricular fibrillation with use of monophasic and biphasic waveform pulses for external defibrillation. Circulation. 2000;101(25):2968–2974. doi: 10.1161/01.cir.101.25.2968. [DOI] [PubMed] [Google Scholar]
- 10.Tovar OH, Jones JL. Electrophysiologic deterioration after one-minute fibrillation increases relative biphasic defibrillation efficacy. J Cardiovasc Electrophysiol. 2000;11(6):645–651. doi: 10.1111/j.1540-8167.2000.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 11.Higgins SL, Herre JM, Epstein AE, et al. A comparison of biphasic and monophasic shocks for external defibrillation. Physio-Control Biphasic Investigations. Prehosp Emerg Care. 2000;4(4):305–313. doi: 10.1080/10903120090941001. [DOI] [PubMed] [Google Scholar]
- 12.Mittal S, Ayati S, Stein KM, et al. Comparison of a novel rectilinear biphasic waveform with a damped sine wave monophasic waveform for transthoracic ventricular defibrillation. Zoll Investigators. J Am Coll Cardiol. 1999;34(5):1595–1601. doi: 10.1016/s0735-1097(99)00363-0. [DOI] [PubMed] [Google Scholar]
- 13.van Alem AP, Chapman FW, Lank P, Hart AAM, Koster RW. A prospective, randomised and blinded comparison of first shock success of monophasic and biphasic waveforms in out-of-hospital cardiac arrest. Resuscitation. 2003;58(1):17–24. doi: 10.1016/s0300-9572(03)00106-0. [DOI] [PubMed] [Google Scholar]
- 14.Walsh SJ, McClelland AJ, Owens CG, et al. Efficacy of distinct energy delivery protocols comparing two biphasic defibrillators for cardiac arrest. Am J Cardiol. 2004;94(3):378–380. doi: 10.1016/j.amjcard.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 15.Stothert JC, Hatcher TS, Gupton CL, Love JE, Brewer JE. Rectilinear biphasic waveform defibrillation of out-of-hospital cardiac arrest. Prehosp Emerg Care. 2004;8(4):388–392. doi: 10.1016/j.prehos.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Walcott GP, Melnick SB, Killingsworth CR, Ideker RE. Comparison of low-energy versus high-energy biphasic defibrillation shocks following prolonged ventricular fibrillation. Prehosp Emerg Care. 2010;14(1):62–70. doi: 10.3109/10903120903349838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walcott GP, Killingsworth CR, Smith WM, Ideker RE. Biphasic waveform external defibrillation thresholds for spontaneous ventricular fibrillation secondary to acute ischemia. J Am Coll Cardiol. 2002;39(2):359–365. doi: 10.1016/s0735-1097(01)01723-5. [DOI] [PubMed] [Google Scholar]
- 18.Chapman FW, Walker RG, Koster RW. Abstract: O-33. Use of 360 joule biphasic shocks for initial and recurrent ventricular fibrillation in prehospital cardiac arrest. Resuscitation. 2006;69:49–50. [Google Scholar]
- 19.Stiell IG, Walker RG, Nesbitt LP, et al. BIPHASIC Trial: a randomized comparison of fixed lower versus escalating higher energy levels for defibrillation in out-of-hospital cardiac arrest. Circulation. 2007;115(12):1511–1517. doi: 10.1161/CIRCULATIONAHA.106.648204. [DOI] [PubMed] [Google Scholar]
- 20.Koster RW, Walker RD, Chapman FW. Recurrent ventricular fibrillation during advanced life support care of patients with prehospital cardiac arrest. Resuscitation. 2008;78(3):252–257. doi: 10.1016/j.resuscitation.2008.03.231. [DOI] [PubMed] [Google Scholar]
- 21.US Food and Drug Administration [webpage on the Internet] Energy Levels in External Biphasic Defibrillators: Initial Communication. Medical Device Safety Alert Notice dated November 5, 2009. 2011. [Accessed November 7, 2011]. Available from: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm189259.htm.
- 22.Li L, Jin Q, Huang J, Cheng KA, Ideker RE. Intramural foci during long-duration fibrillation in the pig ventricle. Circ Res. 2008;102(10):1256–1264. doi: 10.1161/CIRCRESAHA.107.170399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trayanova NA. The long and the short of long and short duration ventricular fibrillation. Circ Res. 2008;102(10):1151–1152. doi: 10.1161/CIRCRESAHA.108.177303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neumar RW, Nolan JP, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation. Circulation. 2008;118(23):2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]