Diabetic retinopathy (DR) is the most common complication of diabetes and a leading cause of vision loss worldwide (1). Unfortunately, there are no treatments targeting early stages of the disease prior to the onset of sight-threatening vascular defects such as macular edema or neovascularization. A better understanding of the etiology of DR is needed to identify therapeutic targets to halt early disease progression. To this end, numerous studies demonstrated that a low-grade inflammation occurs in retinas of diabetic animal models and suggest that inflammation contributes a role in DR progression. Various mechanisms leading to retinal inflammation in DR have been described, with the majority of studies implicating retinal Müller glial cells and microglia as the initiators of retinal inflammation (for review, see ref. 2). However, seldom does a study make a strong connection between these two cell types. In this issue of Diabetes, Portillo et al. (3) describe a mechanism in which retinal inflammation in diabetic mice is dependent upon expression of the cluster of differentiation gene 40 (CD40) receptor by Müller cells (Fig. 1). The study suggests that CD40 activation induces Müller cells to release ATP, leading to activation of P2X7 purinergic receptors on retinal microglia and their subsequent expression of inflammatory cytokines. Importantly, the requirement of Müller cell–specific CD40 expression to recapitulate the appearance of acellular capillaries in diabetic retinas also suggests that inflammation is necessary for the loss of vascular cells associated with DR pathology.
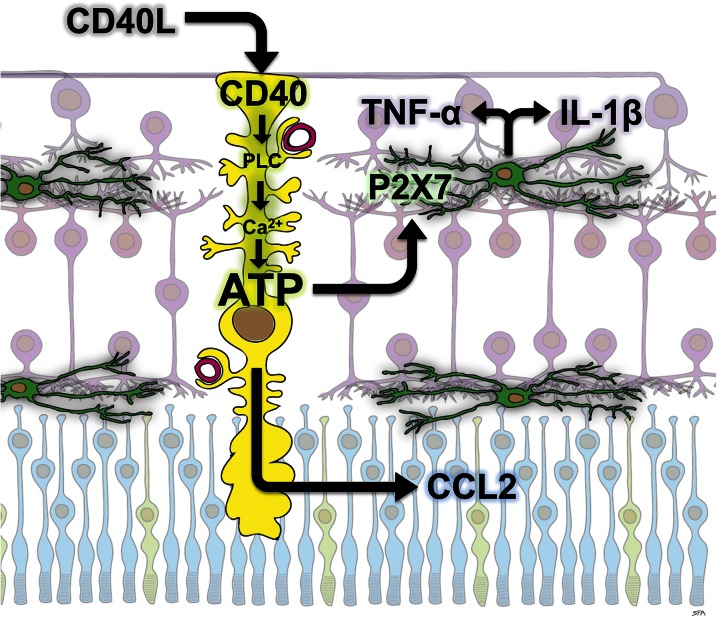
Figure 1.
Proposed role of CD40 and P2X7 receptors in diabetic retinal neuroinflammation. The CD40 receptor on Müller cells (yellow) is activated in retinas of diabetic mice, presumably by binding to CD40L. CD40 activation triggers phospholipase Cγ1 (PLC) activation, leading to an increase in intracellular calcium (Ca2+) resulting in release of ATP. CD40 activation also causes Müller cells to express the chemokine CCL2. Extracellular ATP activates P2X7 receptors on a subset of retinal microglia (green), which is necessary for their expression of the cytokines TNF-α and IL-1β. Thus, CD40 activation on Müller cells links macroglial and microglial inflammatory responses in DR.
CD40 is mainly known as an immune costimulatory molecule, and interactions between CD40 and its major ligand (CD40L) play key roles in immunological licensing of antigen-presenting cells by CD4+ T cells and for B-cell activation, proliferation, class switching, and immunoglobin production (4). In a previous study Portillo et al. (5) found that germline deletion of the CD40 gene blocked intracellular adhesion molecule 1 expression, leukostasis, and the appearance of acellular capillaries in the retinas of diabetic mice. These authors also found that in the retina Müller glial cells express CD40, as do endothelial cells, microglia, and retinal ganglion cells (RGC) (6). Since CD40 was deleted in all cells of the germline knockout mice, including circulating immune cells, the mechanism and cell type in which CD40 contributed to DR pathology were unclear. In the current study CD40 was “added back” to the knockout mice in such a way that it was expressed exclusively by Müller cells, which was sufficient to restore the diabetes-induced inflammation and vascular pathology.
Müller cell–targeted transgenic add-back of CD40 represents an elegant means of testing the hypothesis that CD40 expression by this cell type is sufficient for low-grade retinal inflammation in this diabetic model. However, the choice of targeting these glial cells is not obvious. Müller cells span radially across retina layers providing structural, metabolic, and neurotrophic support necessary for homeostasis (7). CD40 is an immune costimulatory molecule, and a role for CD40–CD40L interaction in Müller cell pathophysiology is unprecedented. Furthermore, one might have expected leukostasis and capillary dropout in the diabetic retina to be dependent on CD40 expression on endothelial cells, since the interaction of luminal CD40 with CD40L on activated platelets causes endothelial activation and adhesion molecule upregulation leading to leukocyte adherence (8,9).
Importantly, Portillo et al. (3) provide compelling evidence that Müller cells initiate retinal inflammation in the diabetic retina and signal to microglia to elicit their participation. The study concludes that diabetes triggers retinal neuroinflammation directly through CD40 stimulation on Müller cells and indirectly through ATP release by Müller cells, leading to stimulation of P2X7 purinergic receptors on microglia/macrophages. One might have expected inflammatory responses to be dependent on direct CD40 stimulation on microglia. Microglia represent the major resident innate immune cells of the retina and other neuronal tissues and are uniquely equipped to mount inflammatory responses to infection and tissue damage. In the retina, the conventional view is that microglia are the first responders, initiating an inflammatory response that leads to Müller cell reactive gliosis (10). Furthermore, in the brain, CD40 is mainly found on microglia, where CD40 deficiency or neutralization of CD40L inhibits microglial activation, alleviates brain pathology, and improves cognitive performance in mouse models of Alzheimer disease (11).
The ATP-mediated mechanism described by Portillo et al. (3) may have implications for other aspects of DR pathology as well. Several purinergic receptors, including P2X7, play key roles in retinal physiology and pathophysiology, including modulation of retinal neurotransmission, control of vascular tone, and Müller cell swelling and gliosis, as well as RGC apoptosis (12). Diabetes was found to increase the susceptibility of retinal microvessels to transmembrane pore formation in response to P2X7 activation, suggesting that extracellular ATP may cause mural cell loss in DR (13). In addition, P2X7 and P2X4 receptor antagonists inhibited the induction of endothelial cell inflammation and permeability by high glucose (14). Release of ATP by Müller cells could also contribute to the death of RGC observed in DR, as evidence suggests that the release of ATP by gliotic Müller cells induces RGC apoptosis through P2X7 activation (15,16).
Finally, the current study suggests the possibility that CD40–CD40L and ATP–P2X7 interactions represent promising new therapeutic targets for prevention of DR progression. Although the study did not establish the source of CD40L in diabetic retinas, plasma levels of soluble CD40L were significantly increased in the diabetic mice, suggesting a systemic influence (platelet activation, perhaps) on DR pathology in this model. Plasma levels of soluble CD40L are also elevated in patients with type 1 and type 2 diabetes (17,18). Several systemic treatments targeting the CD40–CD40L system have been developed to treat cancer, inflammation, and autoimmune disease (4). Although clinical trials of monoclonal antibodies blocking CD40L failed due to occurrence of thromboembolisms (19), such a complication is unlikely with intravitreal applications of these biologics. Also, development of blood-brain-barrier–penetrant P2X7 antagonists for treatment of neuroinflammation has been under way for some time (20). Although the role for this receptor in normal retinal function suggests caution, it is possible that inhibition of P2X7 on retinal microglia could modulate their function to prevent neuroinflammation from progressing toward sight-threatening DR.
Article Information
Funding. S.F.A. is funded by National Eye Institute grants R01EY007739 and R01EY020582 and National Institute of Diabetes and Digestive and Kidney Diseases grant R24DK082841.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying article, p. 483.
References
- 1.Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond) 2015;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sorrentino FS, Allkabes M, Salsini G, Bonifazzi C, Perri P. The importance of glial cells in the homeostasis of the retinal microenvironment and their pivotal role in the course of diabetic retinopathy. Life Sci 2016;162:54–59 [DOI] [PubMed] [Google Scholar]
- 3.Portillo J-AC, Corcino YL, Miao Y, et al. CD40 in retinal Müller cells induces P2X7-dependent cytokine expression in macrophages/microglia in diabetic mice and development of early experimental diabetic retinopathy. Diabetes 2017;66:483–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang B, Wu T, Chen M, Zhou Y, Yi D, Guo R. The CD40/CD40L system: a new therapeutic target for disease. Immunol Lett 2013;153:58–61 [DOI] [PubMed] [Google Scholar]
- 5.Portillo J-AC, Greene JA, Okenka G, et al. CD40 promotes the development of early diabetic retinopathy in mice. Diabetologia 2014;57:2222–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Portillo J-AC, Okenka G, Kern TS, Subauste CS. Identification of primary retinal cells and ex vivo detection of proinflammatory molecules using flow cytometry. Mol Vis 2009;15:1383–1389 [PMC free article] [PubMed] [Google Scholar]
- 7.Reichenbach A, Bringmann A. New functions of Müller cells. Glia 2013;61:651–678 [DOI] [PubMed] [Google Scholar]
- 8.Ramirez SH, Fan S, Dykstra H, et al. Dyad of CD40/CD40 ligand fosters neuroinflammation at the blood-brain barrier and is regulated via JNK signaling: implications for HIV-1 encephalitis. J Neurosci 2010;30:9454–9464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henn V, Slupsky JR, Gräfe M, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 1998;391:591–594 [DOI] [PubMed] [Google Scholar]
- 10.Wang M, Wong WT. Microglia-Müller cell interactions in the retina. Adv Exp Med Biol 2014;801:333–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen K, Huang J, Gong W, Zhang L, Yu P, Wang JM. CD40/CD40L dyad in the inflammatory and immune responses in the central nervous system. Cell Mol Immunol 2006;3:163–169 [PubMed] [Google Scholar]
- 12.Reichenbach A, Bringmann A. Purinergic signaling in retinal degeneration and regeneration. Neuropharmacology 2016;104:194–211 [DOI] [PubMed] [Google Scholar]
- 13.Sugiyama T, Kobayashi M, Kawamura H, Li Q, Puro DG. Enhancement of P2X(7)-induced pore formation and apoptosis: an early effect of diabetes on the retinal microvasculature. Invest Ophthalmol Vis Sci 2004;45:1026–1032 [DOI] [PubMed] [Google Scholar]
- 14.Sathanoori R, Swärd K, Olde B, Erlinge D. The ATP receptors P2X7 and P2X4 modulate high glucose and palmitate-induced inflammatory responses in endothelial cells. PLoS One 2015;10:e0125111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue Y, Xie Y, Xue B, et al. Activated Müller cells involved in ATP-induced upregulation of P2X7 receptor expression and retinal ganglion cell death. Biomed Res Int 2016;2016:9020715 [DOI] [PMC free article] [PubMed]
- 16.Xue B, Xie Y, Xue Y, et al. Involvement of P2X7 receptors in retinal ganglion cell apoptosis induced by activated Müller cells. Exp Eye Res 2016;153:42–50 [DOI] [PubMed]
- 17.El-Asrar MA, Adly AA, Ismail EA. Soluble CD40L in children and adolescents with type 1 diabetes: relation to microvascular complications and glycemic control. Pediatr Diabetes 2012;13:616–624 [DOI] [PubMed] [Google Scholar]
- 18.Seijkens T, Kusters P, Engel D, Lutgens E. CD40-CD40L: linking pancreatic, adipose tissue and vascular inflammation in type 2 diabetes and its complications. Diab Vasc Dis Res 2013;10:115–122 [DOI] [PubMed] [Google Scholar]
- 19.Law CL, Grewal IS. Therapeutic interventions targeting CD40L (CD154) and CD40: the opportunities and challenges. Adv Exp Med Biol 2009;647:8–36 [DOI] [PubMed] [Google Scholar]
- 20.Rech JC, Bhattacharya A, Letavic MA, Savall BM. The evolution of P2X7 antagonists with a focus on CNS indications. Bioorg Med Chem Lett 2016;26:3838–3845 [DOI] [PubMed] [Google Scholar]