Abstract
β-Cell failure in type 2 diabetes (T2D) was recently proposed to involve dedifferentiation of β-cells and ectopic expression of other islet hormones, including somatostatin and glucagon. Here we show that gastrin, a stomach hormone typically expressed in the pancreas only during embryogenesis, is expressed in islets of diabetic rodents and humans with T2D. Although gastrin in mice is expressed in insulin+ cells, gastrin expression in humans with T2D occurs in both insulin+ and somatostatin+ cells. Genetic lineage tracing in mice indicates that gastrin expression is turned on in a subset of differentiated β-cells after exposure to severe hyperglycemia. Gastrin expression in adult β-cells does not involve the endocrine progenitor cell regulator neurogenin3 but requires membrane depolarization, calcium influx, and calcineurin signaling. In vivo and in vitro experiments show that gastrin expression is rapidly eliminated upon exposure of β-cells to normal glucose levels. These results reveal the fetal hormone gastrin as a novel marker for reversible human β-cell reprogramming in diabetes.
Introduction
Failure of pancreatic β-cells to compensate for increased demand is a central event in the pathogenesis of type 2 diabetes (T2D). It is thought that a vicious cycle of glucotoxicity harms β-cells and further increases glucose levels and metabolic load, but the underlying mechanisms remain incompletely understood. β-Cell failure may result from chronic endoplasmic reticulum (ER) stress or oxidative stress, leading to stunned β-cells that fail to secrete bioactive insulin (1,2). Alternatively, β-cell failure was proposed to result from β-cell death or failed β-cell replication, leading to reduced β-cell mass. This view is supported by autopsy studies, which suggested that people with T2D have, on average, a 50% reduction in β-cell mass compared with BMI-matched control subjects without T2D (3). More recently, Talchai et al. (4) proposed that β-cell failure occurs to a large extent via dedifferentiation, causing an apparent decrease of β-cell mass. According to this model, most β-cells remain alive in T2D but lose the ability to express insulin and other hallmarks of differentiation and revert to a fetal-like state characterized by expression of the endocrine progenitor regulator neurogenin3 (NeuroG3), subsequently gaining expression of other islet hormones such as glucagon and somatostatin (4). The idea of β-cell dedifferentiation, followed by expression of noninsulin hormones, was supported by several additional studies, which also showed that normalization of glycemia reverses the phenomenon (5,6). However, controversy remains, in particular regarding the existence and magnitude of the phenomenon in human diabetes (7,8). Notably, all solid demonstrations of dedifferentiation so far have been based on analysis of genetically engineered mouse models, where genetic lineage tracing could prove that preexisting β-cells are losing cell-specific identity and turning on non–β-cell genes. Current evidence for dedifferentiation in spontaneous models of diabetes in rodents and humans is indirect, relying mostly on observations of cells coexpressing insulin and glucagon or somatostatin, a phenomenon that could be explained in multiple ways (e.g., preexisting α- or δ-cells gaining expression of insulin) (9).
We previously characterized the developmental determinants of pancreatic G cells expressing the hormone gastrin (10). These cells form abundantly during embryonic development of the pancreas from the same NeuroG3+ endocrine progenitor cells that give rise to all islet cells. Around birth, however, all pancreatic gastrin+ cells disappear and are never seen in the adult pancreas other than in rare pancreatic gastrinomas.
Here we report that gastrin expression is induced in β-cells in multiple settings of diabetes, including human T2D. We demonstrate that gastrin expression depends on glucose metabolism acting via membrane depolarization and calcineurin signaling and is reversible upon normalization of glycemia. We also show that dedifferentiation to a fetal progenitor state is not involved. In addition to these molecular insights, gastrin expression provides a valuable biomarker for β-cell reprogramming, or loosened identity, in human T2D.
Research Design and Methods
Immunostaining
Primary antibodies used in this study included rabbit anti-gastrin (1:200; Cell Marquee), guinea pig anti-insulin (1:400; Dako), mouse anti-glucagon (1:800; Abcam), mouse anti-somatostatin (1:400; BCBC), goat anti–green fluorescent protein (GFP) (1:400; Abcam), mouse anti-nkx6.1 (1:200; BCBC), rabbit anti-mafA (1:300; Bethyl), goat anti-pdx1 (1:2,500, a gift from Chris Wright), and mouse anti-NeuroG3 (1:500; Hybridoma Bank). Secondary antibodies were from Jackson ImmunoResearch. Fluorescent images were taken on a Nikon C1 confocal microscope at original magnification ×40.
Proximity Ligation Assay
After incubation with primary antibodies rabbit anti-gastrin (1:1,500) and mouse anti-insulin (1:10,000; Abcam), proximity ligation assay (PLA) was performed (Duolink In Situ Orange Starter Kit Mouse/Rabbit, DUO92102; Sigma-Aldrich) according to the manufacturer’s instructions. Briefly, slides were washed and incubated in PLA solution for 1 h at 37°C. Slides were washed, and ligation was performed at 37°C for 30 min, followed by incubation in amplification-polymerase solution for 100 min at 37°C. Secondary antibodies were added and incubated at room temperature for 2 h. Slides were washed and mounted with Duolink In Situ Mounting Medium with DAPI and visualized as described above.
Real-Time PCR
RNA was isolated and purified from fresh islets with TRI Reagent (Sigma-Aldrich) and an RNeasy Micro Kit (Qiagen). cDNA was prepared from 50 ng RNA by a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). For quantitative real-time PCR, we used SYBR Green mix (Quanta Biosciences) and the following primers:
Gastrin (5′-GCTGGGCTCAGCCTCTCA-3′, 5′-TGCTTCTTGGACAGGTCTGCTA-3′),
NeuroG3 (5′-ACTGACCTGCTGCTCTCTATTCTTT-3′, 5′-GGCGCCATCCTAGTTCTCC-3′),
Oct4 (5′-AGAGGGAACCTCCTCTGAAGC-3′, 5′-CCTGGGAAAGGTGTCCTGTA-3′),
Nanog (5′-CTGAGGAAGCATCGAATTCTG-3′, 5′-TGAAGAGGCAGGTCTTCAGAGG −3′), and
β-actin (5′-CACAGCTTCTTTGCAGCTCCT-3′, 5′-GTCATCCATGGCGAACTGG-3′).
Reactions were performed in triplicate in 96-well plates using the CFX96 Real-Time System (Bio-Rad). Reactions were performed in triplicate with biological replicates. The relative amount of mRNA was calculated using the comparative Ct method after normalization to β-actin. Gastrin cycles in purified islets ranged between 23.2 and 24.2 (db/db 3 months old) and 28.4 and 30.7 (controls), 23.2 (Akita) and 25.6 and 29.4 (controls), 26 and 29 (S961), and 31 and 32.2 (PBS). Actin cycles were between 21 and 25. Gastrin cycles in cultured islets ranged from 29.1 to 30.5 (25 mmol/L glucose) to 32.4 to 34 (5 mmol/L glucose), whereas actin in these conditions was seen after 24.2–25 cycles.
Animals
Mouse strains used in this study included lepRmut/mut (db/db) (The Jackson Laboratory), Ins2WT/C96Y (Akita) (The Jackson Laboratory), Insulin-rtTA;TET-DTA (BDTA) (11), Insulin-CreER (12), NeuroG3lox/lox (13), Rosa26-LSL-Kir6.2-V59M (14), and Rosa26-LSL-YFP (15). As controls we used wild-type or single transgenic littermates. Psammomys obesus (desert gerbil) were raised on low- or high-calorie diets, as described (16). The joint ethics committee (Institutional Animal Care and Use Committee) of the Hebrew University and Hadassah Medical Center approved the study protocol for animal welfare. The Hebrew University is an Association for Assessment & Accreditation of Lab Animal Care International accredited institute.
Human Samples
We used paraffin sections that were obtained from the pancreata of brain-dead patients, after institutional review board permission was granted. Maximal warm ischemia time before fixation was 6 h. Patient details are presented in Supplementary Table 1.
Mouse Procedures
Tamoxifen (20 mg/mL in corn oil; Sigma-Aldrich) was injected subcutaneously to normoglycemic adult mice (1 month old). Two daily doses of 8 mg were used to achieve GFP marking and/or deletion of NeuroG3 or activation of βKir6.2-V59M transgene in β-cells.
Insulin receptor antagonist S961 was a gift from Novo Nordisk. Vehicle (PBS) or S961 (12 nmol) was loaded into an ALZET osmotic pump (model 2001) and implanted subcutaneously on the back of 6-week-old ICR mice. Diabetes-prone male P. obesus (Hebrew University Colony; Harlan, Jerusalem, Israel) were fed low-energy normoglycemia-maintaining diet (9.96 kJ/g; Koffolk, Petach-Tikva, Israel) or a high-energy diet (14.23 kJ/g; Teklad Global Diets) leading to diabetes (defined as glucose levels >200 mg/dL).
Doxycycline (Dexon) was given to 1-month-old male Insulin-rtTA;TET-DTA mice in the drinking water (200 µg/mL doxycycline, 2% w/v sucrose) for 7 days. Severely hyperglycemic mice (blood glucose >500 mg/dL) were implanted subcutaneously with insulin pellets (LinBit implants; LinShin, Scarborough, ON, Canada; two implants for the first 20 g body weight and another pellet for each additional 5 g) or left untreated. Mice were sacrificed 2 months after implantation.
Long-term insulin detemir (Levemir) was injected subcutaneously to diabetic db/db mice, twice daily for 8–16 days, to normalize glycemia. Insulin doses were adjusted depending on measured blood glucose levels.
Islet Procedures
Islets were isolated using collagenase P (Roche Applied Science) injected to the pancreatic duct, followed by Histopaque (1119 and 1077; Sigma-Aldrich) gradient. Islets were incubated overnight in standard RPMI-1640 medium (Biological Industries) supplemented with 10% FBS, L-glutamine, and penicillin-streptomycin in a 37°C, 5% CO2 incubator. Hand-picked islets (30–70 islets) were placed for 42–48 h in 5 mmol/L or 25 mmol/L glucose RPMI medium and treated with 325 μmol/L diazoxide (Sigma-Aldrich), 10 μmol/L nifedipine (Alomone Labs), 37 nmol/L tacrolimus (Astellas Pharma), 0.5 μmol/L glyburide (Sigma-Aldrich), or 3 μmol/L Bay-K8644 (Alomone Labs).
Results
Gastrin Expression in β-Cells of Diabetic db/db Mice
Immunostaining for gastrin revealed abundant expression in islets of diabetic db/db mice, which lack the leptin receptor (Fig. 1A). The vast majority of gastrin-expressing cells were β-cells, as judged by coexpression with insulin, proinsulin, Pdx1, and Nkx6.1 (Fig. 1B and Supplementary Fig. 1). No gastrin staining was observed in age-matched wild-type controls and in young, normoglycemic db/db mice, suggesting gastrin expression is linked to metabolic status rather than being caused by a deficiency in leptin signaling (Fig. 1C). Gastrin protein was found in ∼9% of β-cells in diabetic db/db mice (Fig. 1D). The induction of gastrin expression was validated by real-time PCR of islet cDNA (Fig. 1E). Islets of young, mildly hyperglycemic db/db mice showed a small elevation of gastrin mRNA, although we were not able to observe gastrin protein staining in these mice.
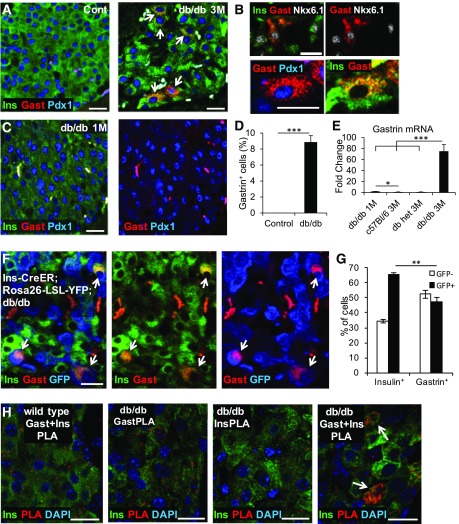
Figure 1.
Gastrin is expressed in β-cells of diabetic db/db mice. A: Costaining for gastrin (Gast), insulin (Ins), and Pdx1 in islets of 3-month-old (3M) control and diabetic db/db mice. Arrows point to gastrin+ cells. B: High magnification confocal images show that gastrin is coexpressed with insulin, Nkx6.1, and Pdx1 in db/db mice. C: Gastrin is not expressed in β-cells of 1-month-old (1M) db/db mice, before the onset of hyperglycemia. Right panel shows only the gastrin and Pdx1 channels, emphasizing the lack of gastrin staining. D: Percentage of β-cells stained positive for gastrin in 3- to 4-month-old control mice and diabetic db/db mice. ***P < 0.001. E: Quantitative real-time PCR analysis of gastrin mRNA expression in islets of 1-month-old db/db mice and in 3-month-old db/+ (heterozygous) mice, C57/BL6 mice, and diabetic db/db mice (n ≥ 4 in each group). Results were normalized to β-actin. *P < 0.05; ***P < 0.001. F: Lineage tracing experiment demonstrates that gastrin is expressed in β-cells that existed before diabetes. Immunostaining for insulin, gastrin, and GFP in the pancreas of db/db;Insulin-CreER;Rosa26-LSL-YFP mice injected with tamoxifen at 1 month and sacrificed when severely diabetic at 3.5 months of age. Arrows point to gastrin+YFP+ cells. YFP was detected using an anti-GFP antibody. G: Efficiency of β-cell Cre-mediated labeling (percentage of insulin+ cells that stain for GFP) and fraction of gastrin+ cells that carry the mark of preexisting β-cells in db/db mice. Four mice were analyzed, and >3,000 insulin+ and >130 gastrin+ cells were counted per mouse. **P < 0.01. H: PLA to assess proximity of gastrin and insulin proteins in diabetic db/db mice. GastPLA and InsPLA are controls where only the indicated primary antibody was added. Scale bars = 20 μm.
To determine the cellular origins of gastrin-expressing cells, we traced their lineage using the Cre-lox system. We generated db/db mice that express tamoxifen-dependent Cre recombinase in β-cells (Insulin-CreER) (17), as well as a fluorescent Cre reporter (Rosa26-LSL-YFP) (15). We injected tamoxifen at 1 month of age to permanently pulse-label β-cells with YFP and sacrificed the animals 2.5 months later, after they had developed diabetes. Half of the gastrin+ cells expressed YFP, proving that they derived from preexisting β-cells (Fig. 1F and G). The gastrin+ cells that were not labeled with YFP could theoretically derive from non–β-cells or from β-cells that were not labeled at the time of the tamoxifen injection. Costaining for gastrin, insulin, glucagon, and somatostatin revealed that gastrin colocalized only with insulin, further supporting its exclusive association with β-cells (Supplementary Fig. 1). To determine whether insulin and gastrin reside in the same cellular compartment, we used PLA, an immunostaining technique that indicates whether two proteins are located less than 40 nm apart (18). Insulin and gastrin antibodies generated a clear signal in the β-cells of diabetic db/db mice (Fig. 1H), suggesting colocalization, likely within the same granules (average granule diameter, 300 nm [19]). These results reveal that β-cells in diabetic db/db mice turn on expression of gastrin mRNA and protein.
Gastrin Expression in Multiple Rodent Models of Hyperglycemia
We conducted experiments to determine whether the induction of gastrin expression in β-cells was specific to the db/db model or a generalized response to hyperglycemia. Conditional ablation of the majority of β-cells in mice using doxycycline-induced expression of diphtheria toxin, led to severe hyperglycemia (11), and induced gastrin expression in ∼5% of surviving β-cells as early as 10 weeks after the onset of hyperglycemia (Fig. 2A and B). A subset of β-cells in hyperglycemic Akita mice, a model of diabetes resulting from misfolding of insulin (20), also expressed gastrin protein, and Akita islets had more gastrin mRNA than islets of control mice (Fig. 2C–E). Further, treatment of wild-type adult mice with the insulin receptor antagonist S961, which causes severe insulin resistance and hyperglycemia (21), induced gastrin expression in a similar subset of β-cells (Fig. 2F and G). Finally, P. obesus, a model of acute diet-induced diabetes (22), showed gastrin expression in β-cells when fed a high-calorie but not a low-calorie diet (Fig. 2H). Thus, expression of gastrin in a subset of β-cells appears to be a general feature of rodent β-cells exposed to hyperglycemia.
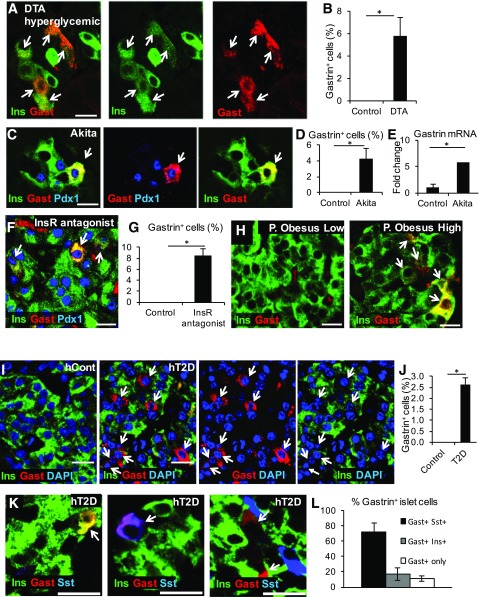
Figure 2.
Expression of gastrin in other diabetes models and in patients with T2D. A: Gastrin (Gast) expression in β-cells of diabetic Insulin-rtTA;TET-DTA mice, 11.5 weeks after administration of doxycycline for 1 week. Arrows in all panels point to gastrin+ cells. B: Quantification of the percentage of β-cells expressing gastrin in Insulin-rtTA;TET-DTA (n = 4 mice). In each mouse, >1,800 β-cells were counted. Blood glucose levels were >580 mg/dL. *P < 0.05. C: Expression of gastrin in islets of 5.5-month-old diabetic Akita mice. Gastrin is coexpressed with both insulin (Ins) and Pdx1. D: Percentage of β-cells stained for gastrin in 3- to 5.5-month-old Akita mice. *P < 0.05. E: Quantitative real-time PCR analysis of gastrin mRNA in islets isolated from diabetic Akita mice (n = 3; average blood glucose, 591 mg/dL) and control mice (n = 3; average blood glucose, 170 mg/dL). Results were normalized to β-actin. *P < 0.05. F: Gastrin expression after administration of insulin receptor (InsR) antagonist S961 to ICR mice. Mice received 12 nmol of the drug via osmotic minipumps for 7 days before sacrifice. Average blood glucose level at sacrifice, 515 mg/dL. G: Quantification of the percentage of β-cells expressing gastrin after administration of the insulin receptor antagonist S961. *P < 0.05. H: Gastrin expression in islets of P. obesus fed with a low- or high-energy diet. The latter animals were hyperglycemic. I: Gastrin expression in islets of human patients with (hT2D) but not in islets of control subjects (hCont). J: Quantification of the percentage of gastrin+ cells in islets of control subjects and humans with T2D (n = 3 subjects were analyzed, and <200 islet cells were counted in each). *P < 0.05. K: Examples of islet cells expressing gastrin alone or with insulin or somatostatin (Sst). Paraffin sections from humans with T2D were used. L: Percentage of gastrin+ islet cells from humans with T2D that were costained for somatostatin, insulin, or none of these hormones. Scale bars = 20 μm.
Gastrin Expression in Islets of Humans With T2D
We asked whether islet gastrin expression occurs also in humans. Although no gastrin expression was found in islets of individuals without T2D, we observed gastrin expression in ∼2.5% of islet cells in patients with T2D (Fig. 2I and J). These findings are consistent with a significant elevation of gastrin mRNA in one published transcriptome of islets from humans with T2D (23) (false discovery rate = 0.04274, P = 0.00969), although no significant change was observed in two other data sets (24,25).
Costaining experiments revealed that 72% of gastrin+ cells in human T2D islets coexpressed somatostatin, whereas 17% coexpressed insulin and 11% were negative for both insulin and somatostatin (Fig. 2K and L).
Thus, expression of gastrin marks a subset of islet cells in human T2D. We could not determine the origins of human gastrin+ islet cells, but the coexpression patterns suggest that, unlike the situation in mice (likely pure β-cell origins), human gastrin+ islet cells may derive from both δ- and β-cells. Because gastrin is not expressed in islets of healthy adult humans, its presence provides a novel and convenient biomarker for deregulated hormone expression in T2D islets, including β-cells.
NeuroG3 Is Not Required for Gastrin Expression in β-Cells of Adult db/db Mice
Previous studies suggested that β-cell dedifferentiation in diabetes involves the expression, and presumably the activity, of the embryonic progenitor cell determinant NeuroG3 (4–6). That β-cell dedifferentiation involved the pluripotency markers Nanog and Oct4 was also proposed (4). To examine this idea, we investigated the expression of these factors in models of spontaneous diabetes in mice. We did not observe expression of Oct4 or Nanog mRNA in islets of db/db mice, Akita mice, and wild-type mice treated with S961, nor in islets cultured in high glucose, although we did detect high-level expression in embryonic stem cells (Supplementary Fig. 2). Similarly, expression of NeuroG3 was undetectable in islets of db/db mice, using both immunostaining and quantitative real-time PCR (Fig. 3A and B), in islets of Akita and S961-treated mice, and in islets cultured in high glucose (Supplementary Fig. 2), whereas NeuroG3 protein and mRNA were readily detectable in the fetal pancreas.
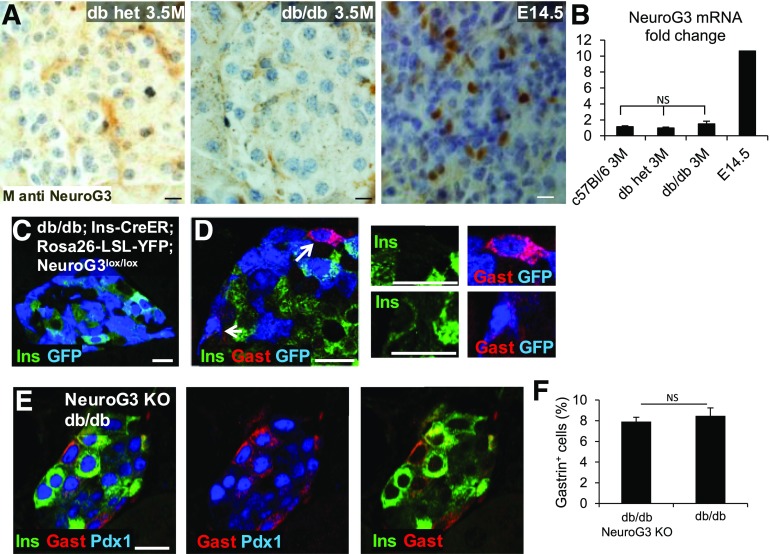
Figure 3.
NeuroG3 is not required for β-cell expression of gastrin in diabetic db/db mice. A: Immunostaining for NeuroG3 in islets of 3.5-month-old (3.5M) control and diabetic db/db mice and in the pancreas of an E14.5 wild-type mouse embryo serving as a positive control. B: Quantification of NeuroG3 mRNA expression in islets of control and diabetic db/db mice and in an E14.5 pancreas serving as a positive control. P > 0.05. Results were normalized to β-actin. C: Evidence for efficient tamoxifen-induced Cre-mediated recombination in β-cells, indicating parallel NeuroG3 deletion. Most β-cells in db/db;Insulin-CreER;Rosa26-LSL-YFP;Neurog3lox/lox are stained for GFP. D: Staining for gastrin (Gast), insulin (Ins), and GFP in a db/db mouse after Cre-mediated deletion of NeuroG3 combined with activation of a YFP permanent tracer. Gastrin+ β-cells are GFP+, suggesting that they have lost the NeuroG3 gene. Right panels show high magnification of cells marked by arrows in the left panel. E: Staining for gastrin, Pdx1, and insulin in the pancreas of db/db mice with adult β-cell–specific deletion of NeuroG3. F: Quantification of the percentage of islet cells stained for gastrin in db/db mice with intact (db/db) or deficient (knockout [KO]) NeuroG3. NeuroG3 was deleted using tamoxifen injection at 1 month of age, and mice were sacrificed at 2.5–3 months of age. Control and NeuroG3-deleted db/db mice (n = 3 per group) were comparably hyperglycemic at sacrifice (average blood glucose, 557 mg/dL). Scale bars = 20 μm.
The inability to detect NeuroG3 in db/db islets argues against involvement of this factor in β-cell dedifferentiation or reprogramming but does not rule out this possibility because transient expression of NeuroG3 could have a biological effect and still be missed by expression analysis. We examined this idea more rigorously by deleting Neurog3 in β-cells of db/db mice before the onset of hyperglycemia (or gastrin expression). We generated db/db mice that contained a floxed allele of NeuroG3 (NeuroG3lox/lox), an Insulin-CreER transgene, and a Rosa26-LSL-YFP lineage reporter. Tamoxifen injection at 4 weeks of age should result in β-cell–specific deletion of NeuroG3 and expression of YFP. We examined the pancreas for evidence of recombination and gastrin expression 5–8 weeks after the tamoxifen injection, when the mice were severely diabetic. Widespread expression of YFP indicated efficient Cre- mediated recombination and hence NeuroG3 deletion in β-cells (Fig. 3C). Islets of diabetic NeuroG3-deleted db/db mice had abundant gastrin+ cells (Fig. 3D and E), and quantification revealed that the numbers of gastrin+ cells were similar to diabetic db/db animals, which have an intact NeuroG3 gene (Fig. 3F). To more directly look at β-cells that have lost NeuroG3, we costained for insulin, YFP, and gastrin. YFP+gastrin+ cells were readily detected, including some that were negative for insulin. This result further indicates that NeuroG3-deficient β-cells are capable of turning on gastrin expression (Fig. 3D).
Gastrin Expression in β-Cells Is Triggered by Glucose and Requires Membrane Depolarization, Calcium Influx, and Calcineurin Signaling
To understand how diabetes causes gastrin expression in β-cells, we tested the role of elevated glucose. We incubated wild-type mouse islets in normal and high concentrations of glucose and determined gastrin expression by quantitative real-time PCR. As early as 48 h after exposure to high glucose (25 mmol/L), there was a massive upregulation of gastrin mRNA in islets, which increased further after 72 h (Fig. 4A). These results show that high levels of glucose are sufficient to trigger gastrin expression in islet cells.
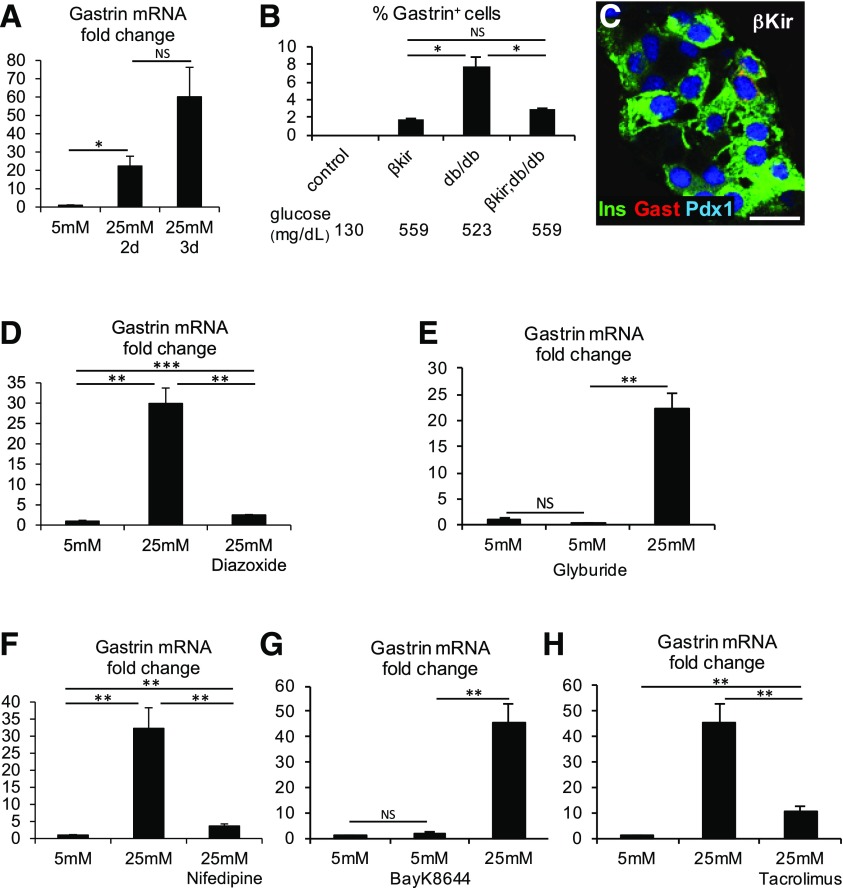
Figure 4.
Glucose, membrane depolarization, and calcium signaling drive gastrin expression in β-cells. A: Exposure of wild-type islets to a high glucose concentration is sufficient to induce gastrin mRNA expression. Islets of 2-month-old ICR mice (70 islets per preparation; experiments performed using 4 mice) were incubated for 2 or 3 days in RPMI medium containing the indicated concentration of glucose. *P < 0.05. Results were normalized to β-actin. B: Genetic evidence that membrane depolarization is necessary for gastrin expression in β-cells of diabetic db/db mice. Insulin-CreER;Rosa26-LSL-Kir6.2-V59M mice (βKir) contain more gastrin+ cells than controls, potentially derived from non-recombined cells caused by the effect of hyperglycemia. Expression of the βKir transgene on a db/db background reduces the fraction of β-cells expressing gastrin, despite severe hyperglycemia (n = 5 mice for βΚir, 2 mice for βΚir;db/db, and 5 mice for db/db; >3,000 β-cells were counted per mouse). *P < 0.05. C: Costaining for gastrin (Gast), insulin (Ins), and Pdx1 in islets of βKir mice. Scale bar = 20 μm. D: Pharmacological evidence that membrane depolarization is necessary for glucose-induced gastrin mRNA expression in islets. Islets cultured in 25 mmol/L glucose for 2 days were cotreated with 325 μmol/L diazoxide. E: Membrane depolarization is not sufficient for gastrin mRNA expression. Wild-type islets were treated with 0.5 μmol/L glyburide. F: Calcium influx is necessary for glucose-induced gastrin expression. Islets cultured in 25 mmol/L glucose were treated with 10 μmol/L nifedipine, a calcium channel blocker. G: Calcium influx is not sufficient for gastrin mRNA expression. Islets cultured in 5 mmol/L glucose were treated with 3 μmol/L Bay-K8644, an L-type calcium channel opener. H: Calcineurin signaling is necessary for glucose-induced gastrin expression. Islets cultured in 25 mmol/L glucose were treated with 37 nmol/L tacrolimus, a calcineurin inhibitor. For panels D–H: **P < 0.01, ***P < 0.001.
To further examine how glucose drives gastrin expression, we examined the role of membrane depolarization, a key step in the pathway leading from glucose uptake to insulin secretion. We generated db/db mice with a β-cell–specific, conditional allele that causes hyperactivation of the KATP channel (Insulin-CreER;Rosa26-LSL-Kir6.2-V59M). β-Cells expressing the mutant KATP channel do not depolarize when exposed to glucose, and hence fail to activate voltage-gated calcium channels. Consequently, they fail to secrete insulin (14). Tamoxifen injection in 1-month-old db/db mice containing both Insulin-Cre and Kir6.2 transgenes led to immediate hyperglycemia, similar to that which gradually developed in unmanipulated db/db mice. Strikingly, the presence of mutant Kir6.2 reduced the fraction of gastrin+ β-cells in diabetic db/db mice by 70% (Fig. 4B and C). Mice expressing the Kir6.2 mutant in the background of wild-type leptin receptor still had a small fraction of gastrin+ cells (compared with zero in wild-type mice), potentially resulting from the effect of systemic hyperglycemia on non-recombined β-cells (Fig. 4B). Further supporting these findings, treatment of cultured wild-type islets with diazoxide, a KATP channel opener, prevented glucose-stimulated expression of gastrin (Fig. 4D). Finally, we incubated islets with glyburide, a drug that forces KATP channel closure. This treatment was not sufficient to induce gastrin expression on the background of 5 mmol/L glucose (Fig. 4E). These findings indicate that closure of KATP channels is a necessary but not sufficient step in the pathway by which high glucose stimulates gastrin expression in β-cells.
Downstream of KATP channels, the glucose-stimulated insulin secretion pathway in β-cells involves calcium influx. The calcium channel blocker nifedipine blocked glucose-induced gastrin expression in islets (Fig. 4F), but the calcium channel opener BayK8644 did not induce gastrin expression in islets cultured in 5 mmol/L glucose (Fig. 4G). Thus, calcium influx is necessary but not sufficient for gastrin expression in adult islets. Finally, we treated islets with tacrolimus, an inhibitor of calcineurin, a major signaling pathway that acts downstream to calcium. Tacrolimus effectively inhibited glucose-stimulated gastrin expression (Fig. 4H).
These findings reveal a pathway for gastrin expression in islets involving glucose metabolism, membrane depolarization, calcium entry, and calcineurin signaling.
Gastrin Expression Is Reversible Upon Normalization of Glycemia
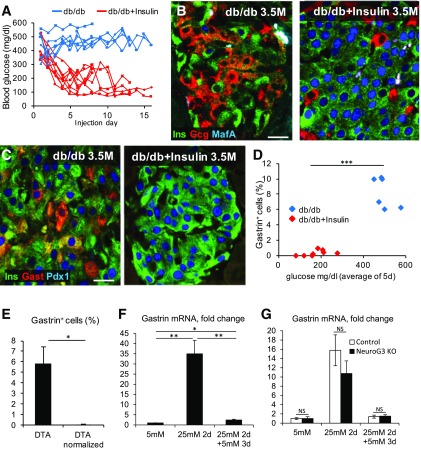
Previous reports showed that several aspects of β-cell dedifferentiation—specifically the appearance of insulin+glucagon+ cells and NeuroG3+ cells—are reversible upon normalization of blood glucose levels (5,6). To determine whether gastrin expression in diabetic β-cells in vivo is similarly reversible, we treated 3.5-month-old diabetic db/db mice (which had been severely diabetic for ∼4 weeks) with insulin injections to lower their blood glucose levels. We examined their islets 8 or 16 days later (Fig. 5A). Insulin treatment led to a dramatic reduction in the islet area staining for glucagon as well as increased expression of MafA, as previously reported (Fig. 5B). Strikingly, gastrin expression was almost completely eliminated after 8 days of insulin treatment (Fig. 5C). Insulin injections in db/db mice led to a variable degree of glycemic correction. Plotting the percentage of gastrin+ cells as a function of glucose suggested a cutoff where islets of mice with blood glucose levels <300 mg/dL had much less gastrin expression than islets of mice with blood glucose >400 mg/dL (Fig. 5D).
Figure 5.
Reversal of gastrin expression in islets. A: Blood glucose levels of diabetic db/db mice injected daily with insulin (2.87 units/g/day). B: Treatment of 3.5-month-old (3.5M) db/db mice with insulin causes the disappearance of insulin+glucagon+ cells and induces MafA expression in β-cells. C: Insulin treatment causes the disappearance of gastrin expression from islets of diabetic db/db mice. D: Correlation between blood glucose levels and the percentage of islet cells expressing gastrin. Blue, diabetic db/db mice; red, insulin-injected db/db mice. ***P < 0.001. E: Treatment of DTA mice with insulin pellets causes the disappearance of gastrin+ cells in islets. Average blood glucose level at sacrifice of DTA 580 vs. normalized DTA 244 mg/dL (n = 4 diabetic DTA mice, 3 normalized DTA mice; >1,000 cells were counted per mouse). *P < 0.05. F: Glucose-induced gastrin mRNA expression is reversible. Islets of 2-month-old ICR mice (40 islets per preparation; experiments performed using 6 mice) were incubated for 2 days in RPMI medium containing 25 mmol/L glucose, followed by 3 more days of RPMI medium containing 5 mmol/L glucose. Results were normalized to β-actin. *P < 0.05; **P < 0.01. G: NeuroG3 is not required for elimination of gastrin expression upon normalization of glucose. Isolated islets from NeuroG3 knockout (KO) mice induce and extinguish gastrin expression depending on glucose levels in the medium, similarly to islets of control mice (n = 3 mice per genotype). Results were normalized to β-actin. Scale bars = 20 μm. Gast, gastrin; Gcg, glucagon; Ins, insulin.
We further examined the reversibility of gastrin expression in the Insulin-rtTA;TET-DTA model. We treated severely hyperglycemic DTA mice with insulin pellets and examined gastrin expression. Insulin-treated DTA mice had average glucose levels of 244 mg/dL compared with 580 mg/dL in untreated mice. No gastrin+ cells were observed in the treated mice (Fig. 5E), consistent with the findings in db/db mice. We also asked whether glucose-induced gastrin expression in cultured islets was reversible. Islets from wild-type mice cultured for 2 days in 25 mmol/L glucose showed a dramatic elevation in gastrin mRNA, which was reversed upon shifting to 5 mmol/L glucose for an additional 3 days (Fig. 5F). Finally, cultured islets from mice with NeuroG3-deficient β-cells induced gastrin and reversed its expression similarly to wild-type islets (Fig. 5G), indicating that NeuroG3 is not involved in gastrin induction or its silencing. In all of these experiments, reversal to normal glucose levels did not seem to involve massive β-cell death. Thus, although we cannot exclude the possibility that some gastrin+ cells die after normalization of glucose, it is more likely that they remain alive and shut off gastrin expression. We conclude that β-cell gastrin expression in diabetes is readily reversible upon normalization of glycemia.
Discussion
We demonstrate in this report that in T2D, β-cells may turn on expression of the fetal islet hormone gastrin. The molecular mechanism involves glucose metabolism leading to membrane depolarization, calcium influx via voltage-dependent calcium channels, and calcineurin signaling, is independent of the endocrine progenitor cell marker NeuroG3 and the stem cell factors Oct4 and Nanog, and is fully reversible upon normalization of glucose levels. The process is conserved from rodents to humans, although in human T2D, many gastrin+ cells coexpress somatostatin rather than insulin, suggesting both β- and δ-cell origins. These findings add to a growing body of evidence that diabetes, and specifically hyperglycemia, compromises the identity of β-cells (4–7,26). They also provide insights into the molecular nature of the process and offer novel tools to study it, in particular in the context of human T2D.
G cells appearing in the embryonic pancreas derive from NeuroG3+ endocrine progenitor cells, and their formation depends on the presence of an intact NeuroG3 gene (10). Our results show that in sharp contrast, gastrin expression in adult β-cells is independent of NeuroG3. We speculate that more generally, the phenomenon of reversible β-cell reprogramming in diabetes does not involve a reversion to a fetal progenitor cell state but rather represents a direct change of identity. Hence the term dedifferentiation, implying a switch to undifferentiated stem or progenitor cells, may not be appropriate to describe the process. We prefer reprogramming (when cell identity is profoundly altered) or loosening of identity (when changes in “identity” genes are limited in scope).
Our work reveals part of the molecular signaling involved in gastrin expression and opens up many interesting questions. For example, although the glucose to calcineurin pathway is necessary for gastrin expression, it is not sufficient, as evident by the inability of glyburide or the calcium channel opener BayK8644 to trigger gastrin expression in normal glucose, suggesting a complex regulation of expression. A more detailed analysis of gastrin expression in human islets is also warranted given the coexpression with somatostatin that is not seen in rodents. Lastly, it will be interesting to examine why only a subset of the β-cells exposed to hyperglycemia turn on gastrin expression. We speculate that this response is a reflection of the recognized but little understood heterogeneity of β-cells (27–30).
Identifying the molecular players underlying gastrin expression in β-cells may also illuminate the puzzling observation that gastrin and ghrelin are transiently expressed in the fetal pancreas but normally disappear after birth. We speculate that this phenomenon relates to the evolutionary roots of islet cells. Is it possible that β-cell reprogramming in diabetes reflects a recapitulation of some phylogenetic elements of islet cell development?
The reversibility of gastrin expression upon normalization of glucose is consistent with previous reports on reversibility of glucagon expression in β-cells (5,6). More broadly, the reacquisition of a normal β-cell phenotype is reminiscent of clinical observations that β-cell function improves after normalization of glycemia (31–33). An important open question is whether reprogrammed β-cells in diabetes remain permanently capable of reversing to a normal phenotype or whether dysfunction/reprogramming become permanent at some point or under certain metabolic conditions. The expression of gastrin in metabolically stressed β-cells in vivo and in vitro provides a convenient experimental system to address this question, which has clear implications for the understanding of β-cell failure in T2D.
The ability to detect gastrin expression in metabolically stressed β-cells provides a novel and convenient biomarker for the study of β-cell reprogramming in T2D, in particular in humans where lineage tracing approaches are not available. Gastrin may not be just a marker, however. It is a biologically active peptide that may influence islet biology in a paracrine manner. The receptor for gastrin (CCKB-R) is reported to be expressed in α- and δ-cells (34,35), and gastrin was reported to influence glucagon expression and secretion (34,36). Gastrin was also proposed to promote β-cell replication and β-cell neogenesis (37–40), although these effects remain controversial. Future work will determine whether β-cells expressing gastrin also process and secrete it and under which conditions and whether locally produced gastrin affects islet biology.
Finally, our results highlight the competence of islet cells to change aspects of their terminal differentiation, namely, the specific hormone that they produce. Notably, reprogrammed islet cells tend to retain an endocrine identity. The underlying reason for the conservation of endocrine phenotype is likely the extensive similarity of the transcriptome, chromatin structure, and DNA methylation patterns among different islet cell types, which puts a lower bar for changes of terminal identity. Although this phenomenon may partly explain β-cell failure in diabetes, it also presents opportunities for therapeutic approaches using non–β-cells as a source for the generation of new β-cells (9,41).
Supplementary Material
Article Information
Acknowledgments. The authors thank Louis Philipson (The University of Chicago) for the generous gift of Insulin-CreER mice (17), Guoqiang Gu (Vanderbilt University) for floxed NeuroG3 mice (13), and the staff of the interdepartmental equipment unit of the Hebrew University Faculty of Medicine for expert technical help.
Funding. O.Z. was supported by a New York Stem Cell Foundation Druckenmiller Fellowship. This study was supported by grants from JDRF, the Beta Cell Biology Consortium and the Human Islet Research Network of the National Institute of Diabetes and Digestive and Kidney Diseases (DK-104216 and DK-104119), The Leona M. and Harry B. Helmsley Charitable Trust, the European Research Council (BetaToBeta ERC consolidator grant), Britain Israel Research and Academic Exchange Partnership Regenerative Medicine Initiative (14BX14NHBG), the DON Foundation, the Israel Science Foundation, The Soyka Pancreatic Cancer Fund, and Israeli Centers for Research Excellence Program of The Israel Science Foundation #41.11 (to Y.D.). This work was supported in part by a grant from United States Agency for International Development's American Schools and Hospitals Abroad Program for the purchase of a Miseq sequencer and a Zeiss confocal microscope.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. T.D., O.Z., E.H., H.Z., J.L., and A.S. performed experiments. T.D., O.Z., E.H., B.G., and Y.D. designed the experiments. T.D., B.G., and Y.D. wrote the manuscript. G.L., F.M.A., and P.I.V. contributed essential reagents. Y.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-0641/-/DC1.
O.Z. is currently affiliated with The Faculty of Medicine in the Galilee, Bar-Ilan University, Safed, Israel.
References
- 1.Leibowitz G, Kaiser N, Cerasi E. β-Cell failure in type 2 diabetes. J Diabetes Investig 2011;2:82–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halban PA, Polonsky KS, Bowden DW, et al. β-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care 2014;37:1751–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 [DOI] [PubMed] [Google Scholar]
- 4.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 2012;150:1223–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brereton MF, Iberl M, Shimomura K, et al. Reversible changes in pancreatic islet structure and function produced by elevated blood glucose. Nat Commun 2014;5:4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z, York NW, Nichols CG, Remedi MS. Pancreatic β cell dedifferentiation in diabetes and redifferentiation following insulin therapy. Cell Metab 2014;19:872–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cinti F, Bouchi R, Kim-Muller JY, et al. Evidence of β-cell dedifferentiation in human type 2 diabetes. J Clin Endocrinol Metab 2016;101:1044–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butler AE, Dhawan S, Hoang J, et al. β-cell deficit in obese type 2 diabetes, a minor role of β-cell dedifferentiation and degranulation. J Clin Endocrinol Metab 2016;101:523–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thorel F, Népote V, Avril I, et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 2010;464:1149–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suissa Y, Magenheim J, Stolovich-Rain M, et al. Gastrin: a distinct fate of neurogenin3 positive progenitor cells in the embryonic pancreas. PLoS One 2013;8:e70397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest 2007;117:2553–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004;429:41–46 [DOI] [PubMed] [Google Scholar]
- 13.Wang S, Jensen JN, Seymour PA, et al. Sustained Neurog3 expression in hormone-expressing islet cells is required for endocrine maturation and function. Proc Natl Acad Sci U S A 2009;106:9715–9720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girard CA, Wunderlich FT, Shimomura K, et al. Expression of an activating mutation in the gene encoding the KATP channel subunit Kir6.2 in mouse pancreatic beta cells recapitulates neonatal diabetes. J Clin Invest 2009;119:80–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srinivas S, Watanabe T, Lin CS, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 2001;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaiser N, Cerasi E, Leibowitz G. Diet-induced diabetes in the sand rat (Psammomys obesus). Methods Mol Biol 2012;933:89–102 [DOI] [PubMed] [Google Scholar]
- 17.Wicksteed B, Brissova M, Yan W, et al. Conditional gene targeting in mouse pancreatic ß-cells: analysis of ectopic Cre transgene expression in the brain. Diabetes 2010;59:3090–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gullberg M, Gústafsdóttir SM, Schallmeiner E, et al. Cytokine detection by antibody-based proximity ligation. Proc Natl Acad Sci U S A 2004;101:8420–8424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou JC, Min L, Pessin JE. Insulin granule biogenesis, trafficking and exocytosis. Vitam Horm 2009;80:473–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izumi T, Yokota-Hashimoto H, Zhao S, Wang J, Halban PA, Takeuchi T. Dominant negative pathogenesis by mutant proinsulin in the Akita diabetic mouse. Diabetes 2003;52:409–416 [DOI] [PubMed] [Google Scholar]
- 21.Vikram A, Jena G. S961, an insulin receptor antagonist causes hyperinsulinemia, insulin-resistance and depletion of energy stores in rats. Biochem Biophys Res Commun 2010;398:260–265 [DOI] [PubMed] [Google Scholar]
- 22.Gross DJ, Leibowitz G, Cerasi E, Kaiser N. Increased susceptibility of islets from diabetes-prone Psammomys obesus to the deleterious effects of chronic glucose exposure. Endocrinology 1996;137:5610–5615 [DOI] [PubMed] [Google Scholar]
- 23.Bugliani M, Liechti R, Cheon H, et al. Microarray analysis of isolated human islet transcriptome in type 2 diabetes and the role of the ubiquitin-proteasome system in pancreatic beta cell dysfunction. Mol Cell Endocrinol 2013;367:1–10 [DOI] [PubMed] [Google Scholar]
- 24.Marselli L, Thorne J, Dahiya S, et al. Gene expression profiles of beta-cell enriched tissue obtained by laser capture microdissection from subjects with type 2 diabetes. PLoS One 2010;5:e11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fadista J, Vikman P, Laakso EO, et al. Global genomic and transcriptomic analysis of human pancreatic islets reveals novel genes influencing glucose metabolism. Proc Natl Acad Sci U S A 2014;111:13924–13929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dor Y, Glaser B. β-cell dedifferentiation and type 2 diabetes. N Engl J Med 2013;368:572–573 [DOI] [PubMed] [Google Scholar]
- 27.Bader E, Migliorini A, Gegg M, et al. Identification of proliferative and mature β-cells in the islets of Langerhans. Nature 2016;535:430–434 [DOI] [PubMed] [Google Scholar]
- 28.Dorrell C, Schug J, Canaday PS, et al. Human islets contain four distinct subtypes of β cells. Nat Commun 2016;7:11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang YJ, Schug J, Won KJ, et al. Single-cell transcriptomics of the human endocrine pancreas. Diabetes 2016;65:3028–3038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnston NR, Mitchell RK, Haythorne E, et al. Beta cell hubs dictate pancreatic islet responses to glucose. Cell Metab 2016;24:389–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ilkova H, Glaser B, Tunçkale A, Bagriaçik N, Cerasi E. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients by transient intensive insulin treatment. Diabetes Care 1997;20:1353–1356 [DOI] [PubMed] [Google Scholar]
- 32.Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet 2008;371:1753–1760 [DOI] [PubMed] [Google Scholar]
- 33.Ryan EA, Imes S, Wallace C. Short-term intensive insulin therapy in newly diagnosed type 2 diabetes. Diabetes Care 2004;27:1028–1032 [DOI] [PubMed] [Google Scholar]
- 34.Leung-Theung-Long S, Roulet E, Clerc P, et al. Essential interaction of Egr-1 at an islet-specific response element for basal and gastrin-dependent glucagon gene transactivation in pancreatic alpha-cells. J Biol Chem 2005;280:7976–7984 [DOI] [PubMed] [Google Scholar]
- 35.Morisset J, Julien S, Lainé J. Localization of cholecystokinin receptor subtypes in the endocine pancreas. J Histochem Cytochem 2003;51:1501–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boushey RP, Abadir A, Flamez D, et al. Hypoglycemia, defective islet glucagon secretion, but normal islet mass in mice with a disruption of the gastrin gene. Gastroenterology 2003;125:1164–1174 [DOI] [PubMed] [Google Scholar]
- 37.Wang TC, Bonner-Weir S, Oates PS, et al. Pancreatic gastrin stimulates islet differentiation of transforming growth factor alpha-induced ductular precursor cells. J Clin Invest 1993;92:1349–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Téllez N, Montanya E. Gastrin induces ductal cell dedifferentiation and β-cell neogenesis after 90% pancreatectomy. J Endocrinol 2014;223:67–78 [DOI] [PubMed] [Google Scholar]
- 39.Song I, Patel O, Himpe E, Muller CJ, Bouwens L. Beta cell mass restoration in alloxan-diabetic mice treated with EGF and gastrin. PLoS One 2015;10:e0140148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang M, Lin Q, Qi T, et al. Growth factors and medium hyperglycemia induce Sox9+ ductal cell differentiation into β cells in mice with reversal of diabetes. Proc Natl Acad Sci U S A 2016;113:650–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chera S, Baronnier D, Ghila L, et al. Diabetes recovery by age-dependent conversion of pancreatic δ-cells into insulin producers. Nature 2014;514:503–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.