Abstract
The liver extracellular matrix (ECM) expands with high-fat (HF) feeding. This finding led us to address whether receptors for the ECM, integrins, are key to the development of diet-induced hepatic insulin resistance. Integrin-linked kinase (ILK) is a downstream integrin signaling molecule involved in multiple hepatic processes, including those related to differentiation, wound healing, and metabolism. We tested the hypothesis that deletion of ILK in mice on an HF diet would disrupt the ECM-integrin signaling axis, thereby preventing the transformation into the insulin-resistant liver. To determine the role of ILK in hepatic insulin action in vivo, male C57BL/6J ILKlox/lox mice were crossed with Albcre mice to produce a hepatocyte-specific ILK deletion (ILKlox/loxAlbcre). Results from this study show that hepatic ILK deletion has no effect on insulin action in lean mice but sensitizes the liver to insulin during the challenge of HF feeding. This effect corresponds to changes in the expression and activation of key insulin signaling pathways as well as a greater capacity for hepatic mitochondrial glucose oxidation. This demonstrates that ILK contributes to hepatic insulin resistance and highlights the previously undefined role of integrin signaling in the pathogenesis of diet-induced hepatic insulin resistance.
Introduction
Insulin resistance is a hallmark of metabolic syndrome and it precedes the development of type 2 diabetes (1,2). The liver is integral to maintaining whole-body glucose homeostasis. Under healthy conditions, insulin robustly suppresses glucose production by the liver and lipolysis in adipocytes. In the insulin-resistant state, insulin-mediated suppression of hepatic glucose production and adipocyte lipolysis is impaired, leading to increased blood glucose and elevated circulating nonesterified fatty acid (NEFA) levels. Elevated NEFAs promote hepatic triglyceride (TG) synthesis and storage that results in increased hepatic lipid accumulation (3,4) and liver damage (5) that is associated with increases in extracellular matrix (ECM) proteins (6).
Integrins communicate changes in the ECM to the intracellular space through protein-protein interactions and signaling events (7). In mammals, there are 24 distinct integrins each composed of an α and β subunit. Studies suggest that integrins regulate glucose and lipid metabolism in several key metabolic organs, including skeletal muscle (8) and liver (6,9). Specifically, it was recently determined that integrin α1β1 preserves hepatic insulin action during states of nutrient overload (6). However, integrin signaling is complex and multifaceted. It requires the recruitment of many unique signaling and/or adaptor proteins to the cytoplasmic β tails (10). Thus each integrin expressed on the cell surface may bind different signaling proteins that serve a unique function.
Perhaps the most central of the integrin binding proteins is integrin-linked kinase (ILK). ILK is a highly conserved intracellular scaffolding protein. It is a component of the ILK-PINCH-parvin (IPP) complex located at focal adhesions and interacts with the β1-, β2-, and β3-integrin cytoplasmic domains in addition to numerous cytoskeleton-associated proteins such as actin (10,11). Interestingly, ILK was originally identified as a kinase due to the significant sequence homology of the C terminus to other Ser/Thr protein kinases (12). Since then, several lines of evidence have confirmed that ILK does not possess an active kinase domain and it is now classified as a bona fide pseudokinase (13,14). The pseudokinase domain of ILK is an essential domain for the recruitment of several adaptor proteins and signaling molecules, including proteins involved in insulin action such as PKB/Akt, PDK1, and GSK-3β. Therefore, although ILK lacks intrinsic kinase activity, it has been implicated in the regulation of numerous cell signaling cascades (15–17).
ILK is a major mediator of integrin signaling and is a key regulator of ECM-cell interactions. Extensive research has been conducted on ILK signaling and function in cellular systems (10,18,19). These studies helped to identify ILK as a signaling molecule at the intersection of many critical signaling pathways, including those involved in insulin action (20,21). Few studies, however, have focused on the role of ILK in the liver and even fewer have focused on the role of hepatic ILK in vivo. Previous studies in mice described a role for ILK in the regulation of liver fibrosis (22), differentiation (23), and wound healing. For example, the genetic deletion of ILK from hepatocytes leads to enhanced liver regeneration after insult (23–26). ILK protein expression is increased in a rodent model of liver fibrosis (22), and ILK has also been shown to be involved in the regulation of matrix-induced hepatocyte differentiation in vitro and in vivo (23). Collectively, these studies show that ILK is critical for the activation of insulin signaling molecules, such as Akt, in addition to liver regeneration and differentiation in response to injury. Nonetheless, the role of ILK in response to the injury and hepatic metabolic derangements associated with overnutrition is a glaring deficit.
The current study tests whether the biochemical link of ILK to insulin signaling contributes to hepatic metabolic dysregulation in the high fat (HF)–fed mouse. The advantage of studying ILK is that it is more efficient and meaningful than studying the numerous integrin receptors. ILK is common to a broad range of integrin signaling pathways and is the best representation of the integrated response to activation of the numerous integrin receptors. Herein, we hypothesize that the deletion of hepatic ILK protects the liver against diet-induced impairments in hepatic insulin action. The conclusions that follow show that ILK is a major contributor to both hepatic insulin resistance and lipid accumulation in the HF-fed state. The results from these studies establish a new paradigm for hepatic insulin resistance and emphasize that integrin signaling represents a novel therapeutic target to treat the underlying hepatic insulin resistance associated with type 2 diabetes.
Research Design and Methods
Mouse Models
The global deletion of ILK is embryonic lethal (27). Thus, mice with a hepatocyte-specific deletion of ILK were used. C57BL/6J mice carrying alleles with ILK genes flanked by loxP sites (ILKlox/lox) (28) were crossed to transgenic mice expressing Cre recombinase under control of the albumin (Alb) promoter (The Jackson Laboratory) to generate ILKlox/loxAlbcre mice and wild-type littermates, ILKlox/lox mice. Animal protocols were approved by the Vanderbilt University Institutional Animal Care and Use Committee and conducted in Association for Assessment and Accreditation of Laboratory Animal Care–accredited facilities. See Supplementary Data for details.
Hepatocyte Isolation
Livers of anesthetized mice were perfused as previously described (6). Cell fractions were collected, washed, and resuspended in lysis buffer prior to protein extraction.
Hyperinsulinemic-Euglycemic Clamp (Insulin Clamp)
Carotid artery and jugular vein catheters were implanted for sampling and infusion 5 days prior to the study (29). Mice were fasted for 5 h, and [3-3H]glucose was primed (2.4 μCi) and infused for a 90-min equilibration period (0.04 μCi/min) before the insulin infusion. Baseline measurements were collected at 15 and 5 min prior to the insulin infusion for analysis of glucose, [3-3H]glucose, free fatty acids (FFAs), and insulin. At t = 0, insulin (4 mU/kg−1/min−1) was infused at a fixed rate and glucose (D50 mixed with [3-3H]glucose) was infused at a variable rate to maintain euglycemia. This minimizes deviations in specific activity during the insulin clamp (30). Glucose was clamped at euglycemia (130–140 mg/dL). Heparinized saline-washed erythrocytes were infused to prevent a fall in hematocrit. Blood was taken at 80–120 min for the determination of [3-3H]glucose, insulin, TGs, and FFA.
Processing of Insulin Clamp Plasma Samples
Radioactivity of [3-3H]glucose was determined by scintillation counting (29). Ra and Rd were calculated using non–steady-state equations (31). Arterial insulin was determined by ELISA (ALPCO). FFAs were assessed using an enzymatic assay (NEFA C Kit; Wako Chemicals).
Measurement of Plasma and Hepatic TGs
Plasma and liver TGs were quantified using the Triglycerides-GPO Reagent Set according to the manufacturer’s protocol (Pointe Scientific, Inc.).
Measurement of Hepatic Glycogen Content
Liver glycogen content was determined as described previously (32).
Immunoprecipitation and Immunoblotting
Frozen tissue was homogenized as previously described (8). Imaging was performed using the ImageJ processing program and Odyssey imaging system (LI-COR Biosciences).
Real-Time PCR
RNA was extracted using the RNeasy Mini Kit (Qiagen). RNA was reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad). Quantitative PCR (qPCR) was performed using TaqMan Universal PCR Master Mix and TaqMan Assays (Applied Biosystems) for Srebp1c, Acc1, and Fas. Gene expression for Dgat1 and Dgat2 was measured using SYBR Green as previously described (33). Data were normalized to the 18S ribosomal protein (TaqMan) or cyclophilin (SYBR Green) and analyzed using the 2−ΔΔCt method (34).
Kinexus Assay
The Kinex Kam-850 Antibody Microarray Kit (Kinexus) was used as per the manufacturer’s instructions (www.kinexus.ca). See Supplementary Data for details.
Statistical Analysis
Data are presented as means ± SEM. Statistical analyses were performed using Student t test or two-way ANOVA followed by Tukey post hoc tests as appropriate. The significance level was P ≤ 0.05.
Results
Hepatocyte Adaptations to the Genetic Deletion of ILK and HF Diet
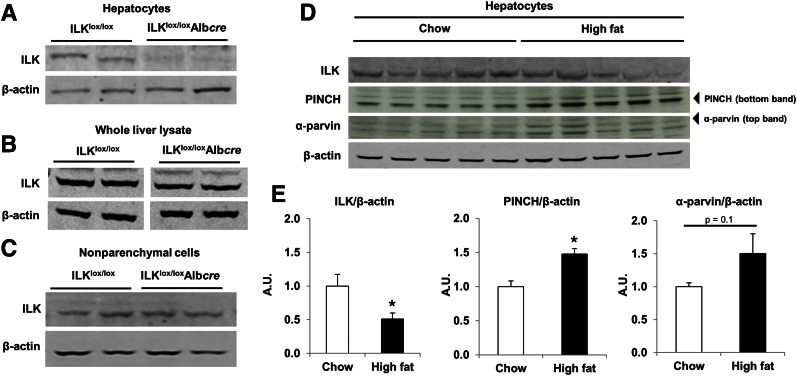
Hepatocytes were separated from nonparenchymal cells as described in research design and methods. The deletion of ILK in hepatocytes was confirmed in ILKlox/loxAlbcre mice by Western blot (Fig. 1A). ILK was highly expressed in whole-liver lysate and in the nonparenchymal fraction (Fig. 1B and C). This is consistent with another study confirming the high expression of ILK in other liver cell types, including endothelial cells, Kupffer cells, stellate cells, and biliary cells (35). It has been shown that when ILK expression is downregulated, other components of the IPP complex are also decreased (36). Thus, we determined PINCH and α-parvin protein expression in homogenate from whole liver, hepatocytes, and nonparenchymal cells (Supplementary Fig. 1). We found that both PINCH and α-parvin protein expression were downregulated in hepatocytes from ILKlox/loxAlbcre mice.
Figure 1.
ILK is absent in hepatocytes isolated from ILKlox/loxAlbcre mice, and members of the IPP complex are differentially expressed in hepatocytes isolated from HF-fed mice. Western blot analysis for ILK protein expression from hepatocytes (A), whole-liver lysate (B), and nonparenchymal cells (C) isolated from ILKlox/lox and ILKlox/loxAlbcre mice. D: Western blot analysis for members of the IPP complex in isolated hepatocytes. E: Quantitative analysis of data from D. Integrated intensities were obtained by the Odyssey and ImageJ software. Protein expression was normalized to β-actin. Data are represented as means ± SEM; n = 5/group. *P < 0.05 chow vs. HF fed.
To determine the role of ILK in hepatic insulin action in vivo, ILKlox/lox and ILKlox/loxAlbcre mice were placed on either a chow or HF diet. HF feeding increased body weight and fat mass (Table 1). There were no differences in body weight, fat mass, or lean mass between genotypes on either a chow or HF diet. HF feeding can differentially regulate protein expression in hepatocytes; thus, we examined ILK, PINCH, and α-parvin protein expression via Western blot in hepatocytes isolated from chow- and HF-fed mice. We found that ILK protein expression was decreased and PINCH and α-parvin protein expression were increased in hepatocytes isolated from HF-fed mice (Fig. 1D and E). Previous studies (23,24,35) described changes in the size of the liver upon ILK deletion. Liver wet weight was determined and there was no difference between the genotypes on an HF diet (Supplementary Fig. 2).
Table 1.
Metabolic characteristics of mice with a hepatocyte-specific deletion of ILK (ILKlox/loxAlbcre) and wild-type mice (ILKlox/lox) fed either a chow or HF diet (60% kcal from fat)
| Chow |
HF |
|||
|---|---|---|---|---|
| ILKlox/lox | ILKlox/loxAlbcre | ILKlox/lox | ILKlox/loxAlbcre | |
| Weight (g) | 29.5 ± 1.2 | 29.7 ± 1.1 | 41.3 ± 2.1* | 38.0 ± 1.8* |
| Adiposity (%) | 10.6 ± 1.8 | 7.4 ± 0.4 | 31.8 ± 2.0* | 32.3 ± 3.1* |
| Absolute lean mass (g) | 20.8 ± 0.7 | 22.6 ± 0.6 | 26.0 ± 0.9 | 24.1 ± 0.9 |
| Age at study (weeks) | 19 | 19 | 19 | 19 |
| Duration on diet (weeks) | 16 | 16 | 16 | 16 |
| Blood glucose (mg/dL) | ||||
| Basal | 136 ± 4 | 136 ± 3 | 140 ± 18 | 121 ± 10 |
| Clamp | 135 ± 2 | 140 ± 4 | 135 ± 2 | 130 ± 4 |
Body weight, body composition, and fasting blood glucose were determined in basal 5 h–fasted mice. Data are represented as means ± SEM; n = 5–8/group.
*P < 0.05 compared with chow-fed mice of the same genotype.
Hepatic Insulin Action Is Improved in HF ILKlox/loxAlbcre Mice
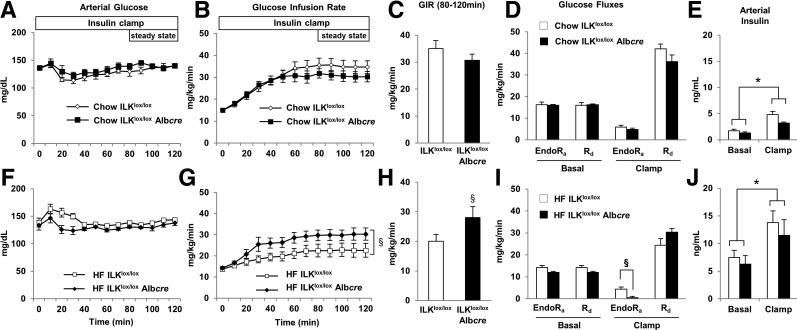
Insulin action was assessed in ILKlox/lox and ILKlox/loxAlbcre mice on both a chow and HF diet using the insulin clamp. During the insulin clamp, blood glucose was maintained between 130 and 140 mg/dL (Fig. 2A and F). There was no difference in the glucose infusion rate (GIR), endogenous glucose production (endoRa), or glucose utilization (Rd) (Fig. 2B–D) during the insulin clamp between the chow-fed ILKlox/lox and ILKlox/loxAlbcre mice. In contrast, the GIR was increased in HF-fed ILKlox/loxAlbcre mice by 50% compared with HF-fed ILKlox/lox mice (Fig. 2G and H). Basal endoRa was not different between genotypes (Fig. 2I). Suppression of endoRa during the insulin clamp was attenuated in ILKlox/lox mice by the HF diet, whereas full suppression of endoRa was restored in HF-fed ILKlox/loxAlbcre mice (Fig. 2I). There was no difference in Rd during the insulin clamp (Fig. 2I). Basal 5 h–fasted plasma insulin values were not different between the genotypes and plasma insulin was raised during the insulin clamp (Fig. 2E and J).
Figure 2.
Hepatic insulin sensitivity is improved in HF-fed ILKlox/loxAlbcre mice. Arterial glucose (A and F) and the GIR (B, C, G, and H) were measured during the hyperinsulinemic-euglycemic (insulin) clamp. EndoRa and whole-body disappearance (Rd) (D and I) were determined during the steady-state period of the insulin clamp. Arterial insulin (E and J) was determined in the basal 5 h–fasted state and during the insulin clamp. Data are represented as means ± SEM; n = 5–8/group. *P < 0.05 compared with the basal 5 h–fasted state and §P < 0.05 compared with HF-fed ILKlox/loxAlbcre mice.
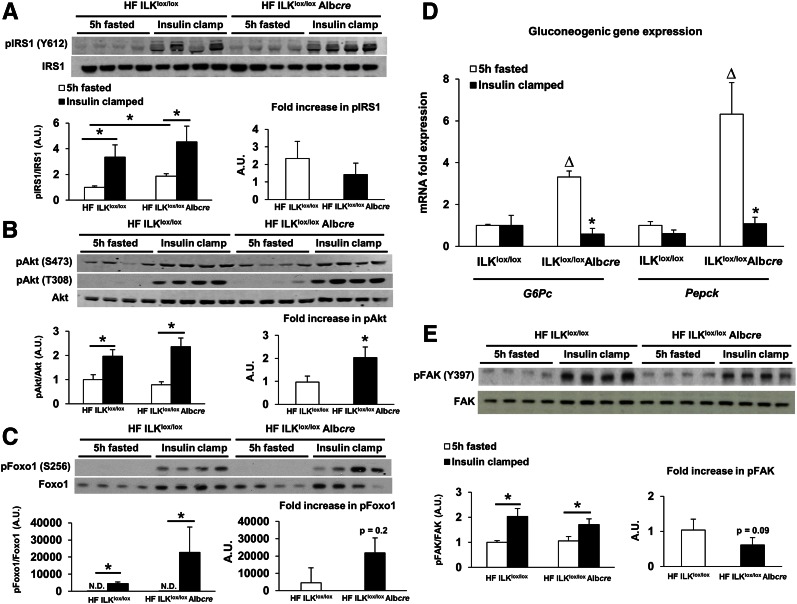
ILK has been shown to interact with several modulators of the insulin signaling cascade, such as Akt (37). To determine whether the hepatocyte-specific deletion of ILK affected insulin signaling, markers of insulin signaling were assessed in 5 h–fasted and insulin-stimulated livers from HF-fed ILKlox/lox and ILKlox/loxAlbcre mice via Western blot (Fig. 3). IRS1, Akt, and focal adhesion kinase (FAK) phosphorylation were increased in the liver with an insulin stimulus regardless of genotype (Figs. 3A, B, and E). Consistent with the hepatic glucose flux measurements, the fold increase in Akt phosphorylation at S473 was elevated in insulin-stimulated HF-fed ILKlox/loxAlbcre mice and there was a slight, nonsignificant elevation in FoxO1 S256 phosphorylation. To further determine the role of ILK in insulin signaling within the liver, an antibody microarray was used to identify several phosphoproteins that were changing in insulin-stimulated livers from HF-fed ILKlox/loxAlbcre mice compared with HF-fed ILKlox/lox mice. These phosphoproteins are represented in Supplementary Table 1. In support of the improved insulin sensitivity and Western blot analysis in HF-fed ILKlox/loxAlbcre mice, FoxO1 phosphorylation at S256 was increased.
Figure 3.
Hepatic insulin action is increased in HF-fed ILKlox/loxAlbcre mice. Western blot analysis was performed on liver homogenates from basal 5 h–fasted and 5 h–fasted insulin-clamped mice (A, B, C, and E). A quantitative analysis of Western blots is depicted below each series of blots. Fold increase of insulin-stimulated activation was calculated relative to wild-type (ILKlox/lox) mice. Integrated intensities were obtained by the Odyssey and ImageJ software. D: mRNA was extracted from both basal 5 h–fasted and 5 h–fasted insulin-clamped livers. qPCR was performed to determine gene expression of the gluconeogenic genes G6pc and Pepck. Data are represented as means ± SEM; n = 5–8/group. *P < 0.05 compared with basal 5 h–fasted HF-fed ILKlox/lox mice; △P < 0.05 compared with basal 5 h–fasted HF-fed ILKlox/loxAlbcre mice.
To determine the effect of ILK on hepatic gluconeogenic gene expression in the HF-fed state, qPCR was used to determine gene expression of glucose-6-phosphatase (G6pc) and phosphoenolpyruvate carboxylase (Pepck) in the livers from 5 h–fasted and insulin-clamped mice (Fig. 3D). G6pc and Pepck gene expression were increased in 5 h–fasted HF-fed ILKlox/loxAlbcre mice. There was no suppression of either G6pc or Pepck gene expression in the HF-fed ILKlox/lox mice. In contrast, and in support of the insulin clamp data and Akt signaling, G6pc and Pepck expression were greatly suppressed with an insulin stimulus in HF-fed ILKlox/loxAlbcre mice. Mitochondrial dysfunction is associated with the development of diet-induced hepatic insulin resistance (38). To test the hypothesis that mitochondrial function was improved in the HF-fed ILKlox/loxAlbcre mice, respirometry was performed using isolated mitochondria (Supplementary Fig. 3). Consistent with our hypothesis, glutamate/malate-stimulated state 3 respiration was increased in ILKlox/loxAlbcre mice (Supplementary Fig. 3A).
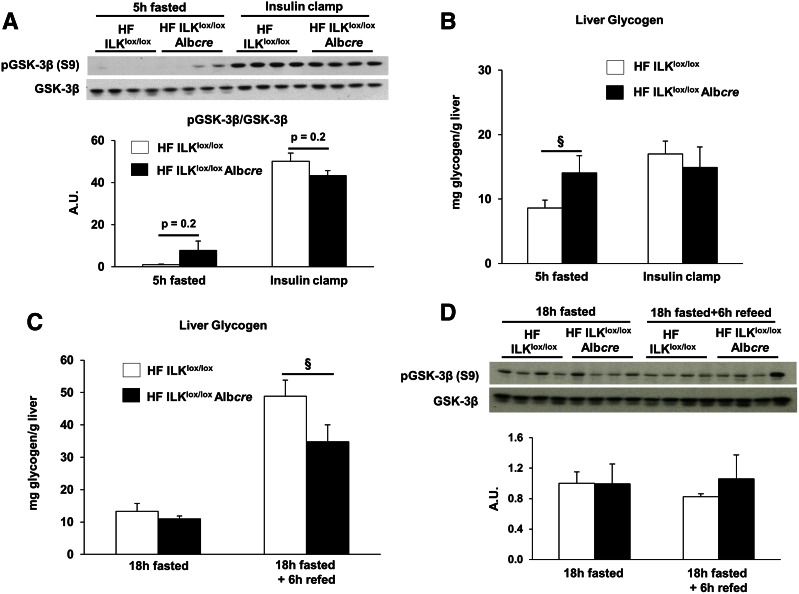
ILK is a known effector of GSK-3β (20). Thus, GSK-3β signaling and liver glycogen were determined in several conditions known to perturb glycogen metabolism. There was a slight, but insignificant, elevation in GSK-3β phosphorylation in the 5 h–fasted state (Fig. 4A). Short-term fasting glycogen levels were increased in HF-fed ILKlox/loxAlbcre mice (Fig. 4B). Insulin stimulated glycogen accumulation in the livers of insulin-clamped HF-fed ILKlox/lox mice; however, no net accumulation was observed in the HF-fed ILKlox/loxAlbcre mice (Fig. 4B). Liver glycogen content was increased when mice were subjected to an 18-h fast and a 6-h refeeding period in ILKlox/lox mice (Fig. 4C), but this effect was blunted in ILKlox/loxAlbcre mice. There was no difference in the phosphorylation of GSK-3β between the genotypes in response to the 18-h fast or the 18-h fast and 6-h refeeding period (Fig. 4D).
Figure 4.
Effect of hepatocyte ILK deletion of glycogen metabolism. A: Western blot analysis for GSK-3β and pGSK-3β in the 5 h–fasted and insulin-clamped conditions. Liver glycogen (B) was assessed in livers from basal 5 h–fasted and insulin-clamped mice. C: Liver glycogen assessed in livers from either 18 h–fasted and 18 h–fasted and refed mice. D: Western blot analysis for GSK-3β and pGSK-3β in the livers from either 18 h–fasted or 18 h–fasted and refed mice. Data are represented as means ± SEM; n = 7–8/group. §P < 0.05 compared with HF-fed ILKlox/loxAlbcre mice.
Hepatic TG Content Was Decreased in Mice With a Genetic Deletion of ILK
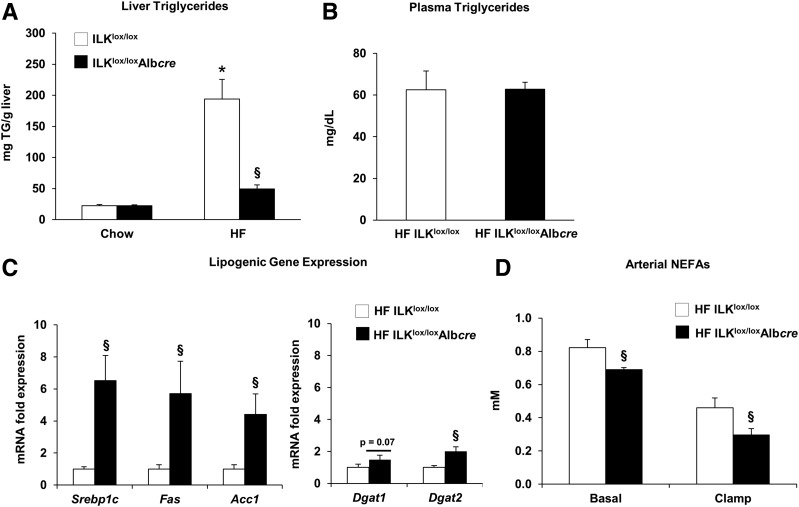
HF feeding leads to the development of fatty liver and hepatic insulin resistance (4). To test the hypothesis that improved hepatic insulin action in HF-fed ILKlox/loxAlbcre mice was associated with decreased TG accumulation, markers of lipid metabolism were measured. Hepatic TG content was decreased in livers from 5 h–fasted HF-fed ILKlox/loxAlbcre mice (Fig. 5A). There was no difference in circulating 5 h–fasted plasma TGs (Fig. 5B). Lipogenic gene expression was determined using qPCR (Fig. 5C). Despite decreased TG accumulation, gene expression for the lipogenic genes Srebp-1c, Fas, Acc1, Dgat1, and Dgat2 was increased. Circulating FFAs are the main substrate for the synthesis and accumulation of hepatic TGs (39). Consistent with decreased liver TG content, circulating FFAs were decreased in HF-fed ILKlox/loxAlbcre mice in both the 5 h–fasted and insulin-clamped states (Fig. 5D).
Figure 5.
ILK promotes hepatic lipid accumulation in HF-fed mice. Liver TG content (A) was determined in livers from 5 h–fasted mice. B: Circulating plasma TGs in basal 5 h–fasted mice. C: mRNA was extracted from basal 5 h–fasted livers, and qPCR was used to determine gene expression of several lipogenic genes. D: Arterial NEFAs in basal 5 h–fasted and insulin-clamped mice. Data are represented as means ± SEM; n = 5–8/group. *P < 0.05 compared with chow-fed ILKlox/lox mice; §P < 0.05 compared with HF-fed ILKlox/loxAlbcre mice.
Discussion
The goal of this study was to determine whether the integrin signaling molecule ILK contributes to hepatic insulin resistance and lipid accumulation in the insulin-resistant state. Here we show that the selective deletion of hepatic ILK in vivo results in the complete restoration of hepatic insulin sensitivity in HF-fed mice. Strikingly, deletion of hepatic ILK in chow-fed mice was without an observable phenotype difference compared with littermate controls. The increase in insulin action in HF-fed mice corresponds to changes in the activation of key signaling pathways, a greater capacity for hepatic mitochondrial glucose oxidation, and decreased hepatic lipid accumulation.
Hepatocytes are the most abundant cell type in the liver and a major site of hepatic insulin action and lipid metabolism (40). Our data illustrate that ILK was selectively deleted from hepatocytes in the ILKlox/loxAlbcre mice. The liver is composed of several different cell types, including stellate cells, Kupffer cells, and endothelial cells. ILK expression in liver lysates from ILKlox/loxAlbcre mice is consistent with other reports that show high expression of ILK in stellate cells. Differences between our study and others may arise from the different mouse models used to drive the deletion of ILK (22,35).
ILK protein expression was decreased in hepatocytes isolated from HF-fed mice compared with chow-fed mice. We propose that ILK protein expression is downregulated as a consequence of overnutrition and may have an impact on its downstream targets, yet this downregulation does not fully protect the hepatocyte and is not enough to prevent hepatic insulin resistance in wild-type HF-fed mice. The complete removal of ILK signaling from the hepatocyte was necessary for the full protective effects. Perhaps it is not solely the downregulation of ILK in the presence of an HF diet that alters hepatic insulin action but the upregulation of PINCH and α-parvin in combination with the downregulation of ILK. The observation that PINCH and α-parvin are downregulated when ILK is deleted has been described elsewhere (36) and is supported by data here. Hence when ILK is deleted and PINCH and α-parvin are subsequently downregulated, it is the downregulation of the entire IPP complex, and not just ILK, that causes the liver to become more sensitized to insulin in the context of overnutrition.
Hepatic insulin resistance is characterized by normal or increased hepatic glucose output despite high levels of circulating insulin. In our studies, the insulin clamp was used to determine the role of ILK in hepatic insulin action in vivo. Here we show that the GIR was increased in HF-fed ILKlox/loxAlbcre mice by 50% compared with HF-fed ILKlox/lox mice. There was no difference in GIR or endoRa in chow-fed mice during the insulin clamp. In HF-fed mice, basal endoRa was not different between genotypes. The insulin-mediated suppression of endoRa during the insulin clamp was attenuated in ILKlox/lox mice fed an HF diet, and the suppression of endoRa was fully restored by the deletion of ILK. Despite improved indicators of insulin action, there was a marked increase in fasting insulin in HF ILKlox/loxAlbcre mice compared with chow-fed mice. Thus, it is possible that the HF ILKlox/loxAlbcre mice are insulin resistant compared with chow-fed mice. This is reflected by the decrease in Rd in HF mice compared with chow-fed mice within each genotype. It is also possible that differences in insulin clearance exist with HF diet (41–43) that the deletion of ILK does not correct. There was no difference in insulin-stimulated Rd between the genotypes on an HF diet, indicating that the difference in GIR was solely attributable to differences in hepatic insulin action between genotypes.
ILK has been implicated in the regulation of both integrin and growth factor signaling (16,20,44). It was once postulated that ILK was a Ser/Thr kinase capable of directly phosphorylating Akt (12,17). This led to the notion that ILK directly activates Akt in vitro and in vivo. This has since been refuted. It is now recognized that ILK is an adaptor protein that is part of a multiprotein complex that recruits kinases and promotes the phosphorylation of Akt through an indirect mechanism (16,44). However, no studies to date have addressed the role of ILK in the phosphorylation of hepatic Akt, or other key insulin signaling molecules such as IRS1, after a prolonged insulin stimulus in vivo. In support of the insulin clamp data, herein we show by Western blot and protein microarray that insulin-mediated phosphorylation of Akt Ser473 and FoxO1 Ser256 was enhanced in HF-fed ILKlox/loxAlbcre mice. This finding is supported by a study showing that treatment of ILK-deficient cells with insulin resulted in the phosphorylation of Akt (27). There was no difference in IRS1 phosphorylation between genotypes, suggesting that the deletion of hepatic ILK improves insulin signaling downstream of IRS1. The results from the phosphoprotein microarray (Supplementary Fig. 1) highlight the differences between the genotypes upon insulin stimulation. Although these data are descriptive in nature, they provide insight into the complex signaling pathways that exist at the intersection of integrin and insulin signaling. We also found that FAK phosphorylation is increased in the liver after an insulin clamp. FAK is an integrin-associated cytoplasmic tyrosine kinase implicated in the regulation of hepatic insulin signaling (45–47). This is consistent with other studies showing that insulin stimulates hepatic FAK phosphorylation (47,48). In further support of the insulin clamp data, the increase in hepatic insulin signaling in HF-fed ILKlox/loxAlbcre mice led to greater insulin-mediated suppression of the gluconeogenic genes G6pc and Pepck. The basal transcript levels of G6pc and Pepck were higher in the HF-fed ILKlox/loxAlbcre mice, and this may be a result of any number of factors, including differences in circulating glucagon and/or corticosterone levels or a component of the complex integrin-metabolic signaling network that is as yet undefined. Collectively, measurements of gene expression, downstream insulin signaling, and glucose fluxes combine to show that hepatocyte ILK deletion protects against hepatic insulin resistance during overnutrition. However, the precise mechanism whereby ILK signaling interfaces with insulin signaling remains unknown. This demonstrates the complexity of working with whole-tissue homogenates designed to mimic physiological settings that simply cannot be replicated or achieved in cell culture. Future studies are necessary to fully define how ILK and insulin signaling intersect to promote hepatic insulin action.
GSK-3β is a proposed downstream target of ILK signaling (12,17); thus, we examined GSK-3β phosphorylation and glycogen content in livers from HF-fed ILKlox/lox and ILKlox/loxAlbcre mice under different conditions. Glycogen accumulation after an insulin clamp and fasting/refeeding was impaired by ILK deletion. This appeared to be independent of GSK-3β Ser9 phosphorylation as this was not significantly affected under any condition. The reason for the apparent lower rate of glycogen synthesis upon insulin stimulation or during fasting and refeeding may result from reduced glycogen deposition from gluconeogenic carbons as Pepck was reduced in insulin-stimulated ILKlox/loxAlbcre mice. It is noteworthy that fasting liver glycogen stores were actually elevated in ILKlox/loxAlbcre mice. This may be due to the lower rate of liver glycogen breakdown or increased shunting of glucose precursors into glycogen.
One of the most interesting findings was that the improvement in insulin action in the HF-fed ILKlox/loxAlbcre mice coincided with decreased hepatic lipid accumulation. This is consistent with the observation that hepatic lipid accumulation is associated with insulin resistance (4). There was no difference in liver wet weight between the genotypes on an HF diet that could explain this effect. This is in contrast to previous studies (23,24,35) and is likely due to differences in several factors, including diet, background strain, method of ILK deletion, and time at which the mice were studied.
A large fraction of liver TGs are derived from circulating FFAs (39,49). Circulating FFAs were decreased in both the 5 h–fasted state and during the insulin clamp in the HF-fed ILKlox/loxAlbcre mice. Hence, these data strongly suggest that the decrease in hepatic TGs is due, at least in part, to decreases in circulating NEFAs under both basal and insulin-stimulated conditions. The decrease in liver TGs in HF-fed ILKlox/loxAlbcre mice occurred in the presence of increased gene expression for several lipogenic genes. These data are consistent with our previous demonstration that HF-fed integrin-α1 null mice have decreased hepatic TG accumulation despite increased lipogenic gene expression (6). This supports the idea that during times of dietary lipid excess, the liver promotes the incorporation of lipid metabolites into neutral lipid droplets to protect against the deleterious effects of an HF diet. It remains unknown whether de novo lipogenic flux and/or VLDL secretion is different between the genotypes, and one outstanding question is how a liver knockout decreases peripheral FFA release. One could speculate the existence of a “hepatokine” that decreases lipolysis in HF-fed ILKlox/loxAlbcre mice or that decreased FFAs are a reflection of improved metabolic health in HF ILKlox/loxAlbcre mice due to differences in circulating levels of cytokines, such as TNF-α or IL-6, known to affect adipose tissue health and FFA release.
In summary, we demonstrate for the first time that hepatic ILK contributes to insulin resistance during the challenge of HF feeding and promotes hepatic lipid accumulation in vivo. The selective deletion of ILK from hepatocytes leads to a marked improvement in hepatic insulin action in HF-fed, but not chow-fed, mice. This improvement was associated with increased insulin signaling, a greater capacity for hepatic mitochondrial glucose oxidation, and decreased hepatic lipid accumulation. It is feasible that the deletion of hepatic ILK in the context of overnutrition improves hepatic health by either 1) attenuating the ability of the ECM to transmit signals inside the cell or 2) facilitating intracellular signaling to promote insulin action and downregulate lipogenic processes. ILK has numerous binding partners. Thus, ILK may contribute to the pathogenesis of diet-induced hepatic insulin resistance by causing multiple defects, and the phosphoproteome (Supplementary Table 1) provides initial insight into possible pathways affected. Overall, this study emphasizes the importance of the ECM and integrin signaling in the regulation of hepatic macronutrient metabolism in the diet-induced obese state. These findings combined with our earlier work support the notion that integrins represent novel therapeutic targets to treat the underlying insulin resistance associated with type 2 diabetes.
Supplementary Material
Article Information
Acknowledgments. The authors thank Reinhard Fässler (Max Planck Institute of Biochemistry, Martinsried, Germany) for generously providing the ILK floxed mice in addition to the PINCH and α-parvin antibodies for Western blot analysis and the Vanderbilt University Hormone Assay Core for insulin analysis.
Funding. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (RO1 DK095761 to A.P., R01 DK083187 to R.Z., R01 DK075594 to R.Z., R01 DK069221 to R.Z., R37 DK050277 to D.H.W., R01 DK054902 to D.H.W., and U24 DK059637 to D.H.W.) and Veterans Affairs Merit Review, Center for Integrated Healthcare, U.S. Department of Veterans Affairs (1I01BX002025-01 to A.P. and 1I01BX002196 to R.Z.). This work was also supported by NIDDK DK20593 (the Diabetes Research and Training Center) and the Molecular Endocrinology Training Program at Vanderbilt University.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.S.W. researched data, contributed to discussion, and wrote the manuscript. E.T. and L.L. researched data, contributed to discussion, and edited the manuscript. C.A.G., D.P.B., and F.D.J. researched data. A.P., R.Z., and D.H.W. contributed to discussion, reviewed data, and edited the manuscript. A.S.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Data contained within this article were presented at the 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 13–17 June 2014, and the 75th Scientific Sessions of the American Diabetes Association, Boston, MA, 5–9 June 2015.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-0484/-/DC1.
References
- 1.Pehling G, Tessari P, Gerich JE, Haymond MW, Service FJ, Rizza RA. Abnormal meal carbohydrate disposition in insulin-dependent diabetes. Relative contributions of endogenous glucose production and initial splanchnic uptake and effect of intensive insulin therapy. J Clin Invest 1984;74:985–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeFronzo RA, Del Prato S. Insulin resistance and diabetes mellitus. J Diabetes Complications 1996;10:243–245 [DOI] [PubMed] [Google Scholar]
- 3.Puri P, Baillie RA, Wiest MM, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 2007;46:1081–1090 [DOI] [PubMed] [Google Scholar]
- 4.Samuel VT, Liu ZX, Qu X, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem 2004;279:32345–32353 [DOI] [PubMed] [Google Scholar]
- 5.Li ZZ, Berk M, McIntyre TM, Feldstein AE. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J Biol Chem 2009;284:5637–5644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams AS, Kang L, Zheng J, et al. Integrin α1-null mice exhibit improved fatty liver when fed a high fat diet despite severe hepatic insulin resistance. J Biol Chem 2015;290:6546–6557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hynes RO. The extracellular matrix: not just pretty fibrils. Science 2009;326:1216–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang L, Ayala JE, Lee-Young RS, et al. Diet-induced muscle insulin resistance is associated with extracellular matrix remodeling and interaction with integrin alpha2beta1 in mice. Diabetes 2011;60:416–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams AS, Kang L, Wasserman DH. The extracellular matrix and insulin resistance. Trends Endocrinol Metab 2015;26:357–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wickström SA, Lange A, Montanez E, Fässler R. The ILK/PINCH/parvin complex: the kinase is dead, long live the pseudokinase! EMBO J 2010;29:281–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Legate KR, Fässler R. Mechanisms that regulate adaptor binding to beta-integrin cytoplasmic tails. J Cell Sci 2009;122:187–198 [DOI] [PubMed] [Google Scholar]
- 12.Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, et al. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature 1996;379:91–96 [DOI] [PubMed] [Google Scholar]
- 13.Lange A, Wickström SA, Jakobson M, Zent R, Sainio K, Fässler R. Integrin-linked kinase is an adaptor with essential functions during mouse development. Nature 2009;461:1002–1006 [DOI] [PubMed] [Google Scholar]
- 14.Fukuda K, Gupta S, Chen K, Wu C, Qin J. The pseudoactive site of ILK is essential for its binding to alpha-Parvin and localization to focal adhesions. Mol Cell 2009;36:819–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qian F, Zhang ZC, Wu XF, Li YP, Xu Q. Interaction between integrin alpha(5) and fibronectin is required for metastasis of B16F10 melanoma cells. Biochem Biophys Res Commun 2005;333:1269–1275 [DOI] [PubMed] [Google Scholar]
- 16.Hill MM, Feng J, Hemmings BA. Identification of a plasma membrane Raft-associated PKB Ser473 kinase activity that is distinct from ILK and PDK1. Curr Biol 2002;12:1251–1255 [DOI] [PubMed] [Google Scholar]
- 17.Persad S, Attwell S, Gray V, et al. Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: critical roles for kinase activity and amino acids arginine 211 and serine 343. J Biol Chem 2001;276:27462–27469 [DOI] [PubMed] [Google Scholar]
- 18.Di-Poï N, Tan NS, Michalik L, Wahli W, Desvergne B. Antiapoptotic role of PPARbeta in keratinocytes via transcriptional control of the Akt1 signaling pathway. Mol Cell 2002;10:721–733 [DOI] [PubMed] [Google Scholar]
- 19.Plante I, Charbonneau M, Cyr DG. Activation of the integrin-linked kinase pathway downregulates hepatic connexin32 via nuclear Akt. Carcinogenesis 2006;27:1923–1929 [DOI] [PubMed] [Google Scholar]
- 20.Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci U S A 1998;95:11211–11216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Troussard AA, Mawji NM, Ong C, Mui A, St -Arnaud R, Dedhar S. Conditional knock-out of integrin-linked kinase demonstrates an essential role in protein kinase B/Akt activation. J Biol Chem 2003;278:22374–22378 [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Ikegami T, Honda A, et al. Involvement of integrin-linked kinase in carbon tetrachloride-induced hepatic fibrosis in rats. Hepatology 2006;44:612–622 [DOI] [PubMed] [Google Scholar]
- 23.Gkretsi V, Mars WM, Bowen WC, et al. Loss of integrin linked kinase from mouse hepatocytes in vitro and in vivo results in apoptosis and hepatitis. Hepatology 2007;45:1025–1034 [DOI] [PubMed] [Google Scholar]
- 24.Apte U, Gkretsi V, Bowen WC, et al. Enhanced liver regeneration following changes induced by hepatocyte-specific genetic ablation of integrin-linked kinase. Hepatology 2009;50:844–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shafiei MS, Rockey DC. The role of integrin-linked kinase in liver wound healing. J Biol Chem 2006;281:24863–24872 [DOI] [PubMed] [Google Scholar]
- 26.Gkretsi V, Bowen WC, Yang Y, Wu C, Michalopoulos GK. Integrin-linked kinase is involved in matrix-induced hepatocyte differentiation. Biochem Biophys Res Commun 2007;353:638–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakai T, Li S, Docheva D, et al. Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes Dev 2003;17:926–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terpstra L, Prud’homme J, Arabian A, et al. Reduced chondrocyte proliferation and chondrodysplasia in mice lacking the integrin-linked kinase in chondrocytes. J Cell Biol 2003;162:139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 2006;55:390–397 [DOI] [PubMed] [Google Scholar]
- 30.Finegood DT, Bergman RN, Vranic M. Modeling error and apparent isotope discrimination confound estimation of endogenous glucose production during euglycemic glucose clamps. Diabetes 1988;37:1025–1034 [DOI] [PubMed] [Google Scholar]
- 31.Steele R, Wall JS, De Bodo RC, Altszuler N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol 1956;187:15–24 [DOI] [PubMed] [Google Scholar]
- 32.Chan TM, Exton JH. A rapid method for the determination of glycogen content and radioactivity in small quantities of tissue or isolated hepatocytes. Anal Biochem 1976;71:96–105 [DOI] [PubMed] [Google Scholar]
- 33.Koliwad SK, Streeper RS, Monetti M, et al. DGAT1-dependent triacylglycerol storage by macrophages protects mice from diet-induced insulin resistance and inflammation. J Clin Invest 2010;120:756–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 35.Gkretsi V, Apte U, Mars WM, et al. Liver-specific ablation of integrin-linked kinase in mice results in abnormal histology, enhanced cell proliferation, and hepatomegaly. Hepatology 2008;48:1932–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moik D, Böttcher A, Makhina T, et al. Mutations in the paxillin-binding site of integrin-linked kinase (ILK) destabilize the pseudokinase domain and cause embryonic lethality in mice. J Biol Chem 2013;288:18863–18871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu C, Dedhar S. Integrin-linked kinase (ILK) and its interactors: a new paradigm for the coupling of extracellular matrix to actin cytoskeleton and signaling complexes. J Cell Biol 2001;155:505–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vial G, Dubouchaud H, Couturier K, et al. Effects of a high-fat diet on energy metabolism and ROS production in rat liver. J Hepatol 2011;54:348–356 [DOI] [PubMed] [Google Scholar]
- 39.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005;115:1343–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest 2008;118:829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strömblad G, Björntorp P. Reduced hepatic insulin clearance in rats with dietary-induced obesity. Metabolism 1986;35:323–327 [DOI] [PubMed] [Google Scholar]
- 42.Svedberg J, Strömblad G, Wirth A, Smith U, Björntorp P. Fatty acids in the portal vein of the rat regulate hepatic insulin clearance. J Clin Invest 1991;88:2054–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshii H, Lam TK, Gupta N, et al. Effects of portal free fatty acid elevation on insulin clearance and hepatic glucose flux. Am J Physiol Endocrinol Metab 2006;290:E1089–E1097 [DOI] [PubMed] [Google Scholar]
- 44.Lynch DK, Ellis CA, Edwards PA, Hiles ID. Integrin-linked kinase regulates phosphorylation of serine 473 of protein kinase B by an indirect mechanism. Oncogene 1999;18:8024–8032 [DOI] [PubMed] [Google Scholar]
- 45.Lebrun P, Mothe-Satney I, Delahaye L, Van Obberghen E, Baron V. Insulin receptor substrate-1 as a signaling molecule for focal adhesion kinase pp125(FAK) and pp60(src). J Biol Chem 1998;273:32244–32253 [DOI] [PubMed] [Google Scholar]
- 46.Bisht B, Srinivasan K, Dey CS. In vivo inhibition of focal adhesion kinase causes insulin resistance. J Physiol 2008;586:3825–3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheung AT, Wang J, Ree D, Kolls JK, Bryer-Ash M. Tumor necrosis factor-alpha induces hepatic insulin resistance in obese Zucker (fa/fa) rats via interaction of leukocyte antigen-related tyrosine phosphatase with focal adhesion kinase. Diabetes 2000;49:810–819 [DOI] [PubMed] [Google Scholar]
- 48.Huang D, Cheung AT, Parsons JT, Bryer-Ash M. Focal adhesion kinase (FAK) regulates insulin-stimulated glycogen synthesis in hepatocytes. J Biol Chem 2002;277:18151–18160 [DOI] [PubMed] [Google Scholar]
- 49.Murthy VK, Shipp JC. Synthesis and accumulation of triglycerides in liver of diabetic rats. Effects of insulin treatment. Diabetes 1979;28:472–478 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.