Abstract
Pharmacological activation of the hypothalamic glucagon-like peptide 1 (GLP-1) receptor (GLP-1R) promotes weight loss and improves glucose tolerance. This demonstrates that the hypothalamic GLP-1R is sufficient but does not show whether it is necessary for the effects of exogenous GLP-1R agonists (GLP-1RA) or endogenous GLP-1 on these parameters. To address this, we crossed mice harboring floxed Glp1r alleles to mice expressing Nkx2.1-Cre to knock down Glp1r expression throughout the hypothalamus (GLP-1RKDΔNkx2.1cre). We also generated mice lacking Glp1r expression specifically in two GLP-1RA–responsive hypothalamic feeding nuclei/cell types, the paraventricular nucleus (GLP-1RKDΔSim1cre) and proopiomelanocortin neurons (GLP-1RKDΔPOMCcre). Chow-fed GLP-1RKDΔNkx2.1cre mice exhibited increased food intake and energy expenditure with no net effect on body weight. When fed a high-fat diet, these mice exhibited normal food intake but elevated energy expenditure, yielding reduced weight gain. None of these phenotypes were observed in GLP-1RKDΔSim1cre and GLP-1RKDΔPOMCcre mice. The acute anorectic and glucose tolerance effects of peripherally dosed GLP-1RA exendin-4 and liraglutide were preserved in all mouse lines. Chronic liraglutide treatment reduced body weight in chow-fed GLP-1RKDΔNkx2.1cre mice, but this effect was attenuated with high-fat diet feeding. In sum, classic homeostatic control regions are sufficient but not individually necessary for the effects of GLP-1RA on nutrient homeostasis.
Introduction
Glucagon-like peptide 1 (GLP-1) is a gut-secreted peptide that augments glucose-dependent insulin secretion via a pancreatic GLP-1 receptor (GLP-1R) (1). Long-acting GLP-1R agonists (GLP-1RA) are used for treating type 2 diabetes (2). GLP-1RA also reduce food intake and body weight primarily by targeting the central nervous system (CNS) (3). Intracerebroventricular (ICV) injection of GLP-1RA reduce food intake (4–8) and body weight (9) in rodents. Furthermore, CNS deletion of the Glp1r attenuates the anorectic effect of the GLP-1RA liraglutide (3). Hypothalamic GLP-1R signaling has received particular attention as this region regulates energy balance as well as glucose and lipid metabolism (10,11). Peripheral administration of GLP-1RA stimulates hypothalamic neuronal activity (7,12,13). Injection of GLP-1RA to hypothalamic nuclei suppresses feeding in rats (5,14–16), and recent evidence suggests that the arcuate nucleus (ARC) mediates the anorectic effects of liraglutide (17). Furthermore, targeting liraglutide to the ventromedial hypothalamic (VMH) nucleus stimulates brown adipose tissue thermogenesis and adipocyte browning, suggesting that hypothalamic GLP-1R signaling also controls body weight via regulation of energy expenditure (EE) in rodents (15).
The studies described above primarily entailed pharmacological activation of the GLP-1R. Although this demonstrates that the hypothalamic GLP-1R is sufficient to modulate energy balance, it does not address whether it is necessary for the effects of endogenous GLP-1 or clinically utilized GLP-1RA on phenotypes associated with energy balance. ICV delivery of the GLP-1R antagonist exendin (9-39) (Ex9) stimulates food intake in fed rats and blocks the anorectic effects of peripherally administered GLP-1RA, suggesting that the CNS GLP-1R is necessary for the feeding effects of endogenous and exogenous GLP-1RA (7,18). However, this does not identify the specific CNS regions that mediate the satiety effects of GLP-1 or therapeutic GLP-1RA. Furthermore, acute pharmacological approaches do not assess the long-term, day-to-day regulation of energy balance by the CNS GLP-1R. To address this, we generated mice lacking hypothalamic Glp1r expression by crossing mice expressing floxed Glp1r alleles with Nkx2.1-Cre mice. Nkx2.1-Cre mice exhibit Cre expression throughout the hypothalamus including in the ARC, paraventricular (PVN), VMH, dorsomedial (DMH), and lateral (LH) nuclei (19). As the PVN and ARC have been identified as GLP-1RA–responsive hypothalamic nuclei (14,17), we also generated mice lacking Glp1r expression specifically in the PVN and in proopiomelanocortin (POMC) neurons (ARC Glp1r expression is primarily in POMC neurons [14]) by crossing floxed Glp1r mice with Sim1-Cre and POMC-Cre mice, respectively (20,21). Using these models, we tested the hypothesis that disruption of hypothalamic Glp1r expression dysregulates energy balance and that the anorectic effects of peripherally administered GLP-1RA require the hypothalamic GLP-1R. As pharmacological studies suggest a role for the hypothalamic GLP-1R in the maintenance of glucose homeostasis (14), we also tested the hypothesis that disruption of hypothalamic Glp1r expression impairs glucose tolerance.
We demonstrate that knockdown of the hypothalamic Glp1r has no overall effects on net energy balance, glucose homeostasis, or the response to peripherally administered GLP-1RA. These findings highlight the complexity of CNS GLP-1R–mediated signals modulating feeding behavior and glucose handling and indirectly support a role for extrahypothalamic brain regions in GLP-1R–dependent control of energy homeostasis.
Research Design and Methods
Animals and Housing
We generated mouse lines lacking Glp1r expression in the hypothalamus (GLP-1RKDΔNkx2.1cre), PVN (GLP-1RKDΔSim1cre), and POMC neurons (GLP-1RKDΔPOMCcre) by breeding floxed Glp1r mice (GLP-1Rf/f), generated as described previously (22), with Nk2 homeobox 1 (Nkx2.1)-Cre, single-minded homolog 1 (Sim1)-Cre, and POMC-Cre mice (The Jackson Laboratory, Bar Harbor, ME), respectively (19–21). Experiments were performed in male mice. Chow diet (Harlan Teklad #2016) studies were performed in 3- to 4-month-old animals. Following energy balance assessment, mice were placed on a 60% high-fat diet (HFD) (D12492; Research Diets, New Brunswick, NJ) for 12 weeks, such that HFD-fed studies were performed in 7- to 8-month-old animals. Animals were maintained on a 12:12-h light:dark cycle in facilities at Sanford Burnham Prebys Medical Discovery Institute (SBP) at Lake Nona or the University of Cincinnati (UC). All protocols were approved by the SBP and UC Institutional Animal Care and Use Committees.
Cannula Implantation
Cannulae were implanted as previously described (4). Guide cannulae (Plastics One, Roanoke, VA) targeted the PVN (0.7 mm caudal, 0.3 mm from midline, 5.0 mm ventral), ARC (2.3 mm caudal, 1.1 mm from midline, 5.6 mm ventral, 10° angle), or cortex (0.46 mm caudal, 2.0 mm from midline, 1.75 mm ventral). Verification of proper coordinates was made histologically following injection of 1% Evans-Blue dye. Mice recovered for 7 days before experimentation.
Validation of Glp1r Knockdown
Glp1r expression was assessed in GLP-1RKDΔNkx2.1cre, GLP-1Rf/f, and whole-body GLP-1R knockout mice (GLP-1RKO) mice by qRT-PCR. Total RNA was extracted from the hypothalamus using a column-based method (Zymo Research, Irvine, CA) and converted to cDNA (High Capacity RNA-to-cDNA kit, Applied Biosystems, Foster City, CA). Glp1r and ribosomal protein L32 mRNA transcript levels were determined using gene-specific probes in accordance with manufacturer’s instructions using a StepOnePlus qPCR instrument (Applied Biosystems). qPCR primers (5′→3′) were as follows: Glp1r, F:GATGCTGCCCTCAAGTGGAT, R:ATGAGCAGGAACACCAGTCG and L32, F:ACATTTGCCCTGAATAGTGGT, R:ATCCTCTTGCCCTGATCCTT. qPCR was also performed in whole-brain homogenates excluding the hypothalamus. Gene expression levels were calculated as relative quantification using the 2-ΔΔCT method.
Glp1r knockdown in the PVN and ARC of GLP-1RKDΔSim1cre and GLP-1RKDΔPOMCcre mice was confirmed by RNA in situ hybridization. Whole brains were harvested, and tissue was formalin-fixed (24-h, 10% neutral buffered formalin) and paraffin-embedded (FFPE) and sectioned using a microtome. Five-micrometer coronal sections were stained with RNAscope probes (Advanced Cell Diagnostics, Newark, CA), according to manufacturer’s instructions. Sections were treated with test (Glp1r) and control (positive, peptidylprolyl isomerase B; negative, dihydrodipicolinate reductase) RNA probes using a single-plex chromogenic-RED RNAscope 2.5 assay kit on a Bond RX automated in situ hybridization slide staining system (Leica Biosystems, Buffalo Grove, IL). The automated protocol included heat retrieval at 88°C for 15 min and protease retrieval at room temperature for 15 min. Stained slides were coverslipped and scanned using an Aperio ScanScope XT instrument (Leica Biosystems).
Body Composition Analysis and Assessment of Energy Balance
Lean, fat, and fluid mass were measured in 5-h fasted mice using a LF90II-TD NMR (Bruker, Billerica, MA). Body weights were recorded weekly over the 12-week HFD feeding period. Energy balance was assessed using a Comprehensive Lab Animal Monitoring System (CLAMS) (Columbus Instruments, Columbus, OH). Animals were acclimated to the CLAMS 24 h prior to the start of experimentation. Forty-eight hour food intake, water intake, locomotor activity, EE, and respiratory exchange ratio (RER) were measured at 15-min intervals as previously described (4).
Food Intake Response to Centrally and Peripherally Administered GLP-1RA
Following cannulation, recovery, and acclimation, 18-h food intake was measured after PVN- or ARC-targeted delivery of 0.0025, 0.005, or 0.025 µg/100 nL GLP-1 or exendin-4 (Ex4) (R&D Systems, Minneapolis, MN) or artificial cerebrospinal fluid (ACSF) (100 nL bilateral) vehicle (Harvard Apparatus, Holliston, MA) in 5-h fasted mice just prior to the onset of the dark cycle at 1800. For peripheral administration, 16-h food intake was measured in mice treated with Ex4 (3 µg/kg BW, i.p.), liraglutide (200 µg/kg BW, s.c.; Novo Nordisk, Copenhagen, Denmark), or saline vehicle. Peripheral doses were chosen based on previously demonstrated anorectic efficacy (3,23).
Glucose Tolerance Tests
Glucose tolerance tests (GTTs) were performed on 5-h fasted mice. In chow-fed mice, 2 g/kg BW glucose was administered orally or intraperitoneally. In HFD-fed animals, 1 g/kg BW glucose was administered orally. GTTs were also performed in animals that received PVN- or ARC-targeted injections of Ex4 (0.025 µg/100 nL, bilateral) concurrent with an intraperitoneal glucose dose at t = 0. In additional experiments, GLP-1RKDΔNkx2.1cre, GLP-1RKDΔSim1cre, and GLP-1Rf/f mice were dosed with the GLP-1R antagonist Ex9 (50 µg, i.p.; American Peptide) or liraglutide (400 µg/kg, s.c.) 15 min (Ex9 studies) or 120 min (liraglutide studies) prior to an intraperitoneal GTT. Animals were fasted for 4 h, and glucose was administered at a fixed dose (200 µL 25% dextrose, Ex9 studies) or at 2 g/kg BW for liraglutide studies. Blood glucose measurements were made at the indicated time points. Glucose tolerance was calculated as area under the curve above baseline.
Body Weight Response to Peripheral Administration of Liraglutide
Liraglutide (200 μg/kg BW s.c., BID as described by Secher et al. [17]) or isotonic saline was administered for 14 days in chow- and HFD-fed mice, and body weight was measured daily. Body weight was also measured daily during a 7-day recovery period, over which animals received no injection.
Statistical Analyses
Data are presented as mean ± SEM. Differences between groups were determined by one-way ANOVA followed by Tukey or Newman-Keuls multiple comparison post hoc tests or by two-tailed t tests as appropriate. Statistical significance was set to P < 0.05.
Results
Targeted Delivery of GLP-1 or Ex4 to the PVN and ARC Dose Dependently Suppresses Food Intake
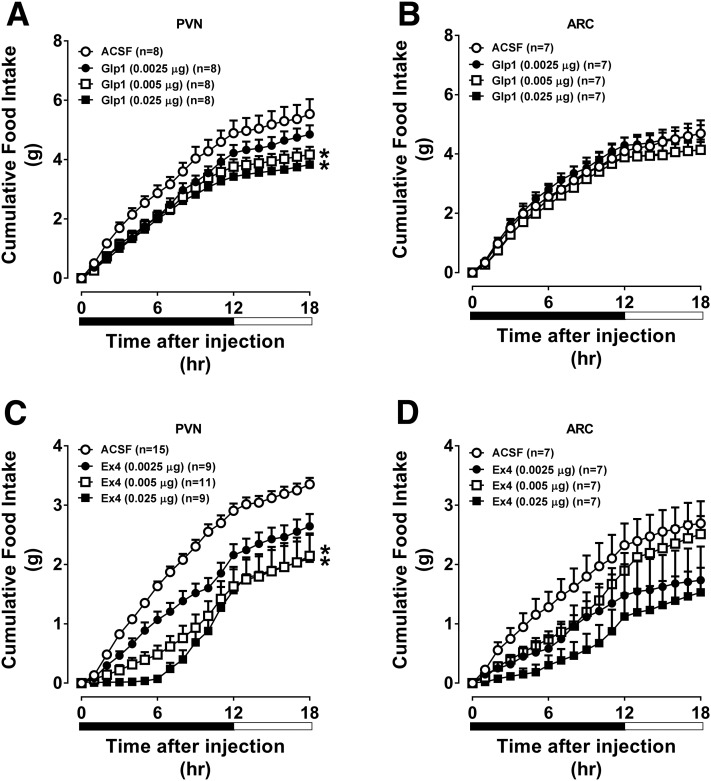
Consistent with previous studies in rats (5,14,15), targeting GLP-1 to the PVN (Fig. 1A) but not the ARC (Fig. 1B) significantly reduced food intake in mice. Targeting Ex4 to the PVN (Fig. 1C) or ARC (Fig. 1D) potently suppressed food intake, particularly during the first 12 h after administration. These findings demonstrate that pharmacological activation of the PVN or ARC GLP-1R reduces food intake in mice, although the magnitude and duration of the response depend on the GLP-1RA administered and region targeted.
Figure 1.
Direct injection of Ex4 into the PVN or ARC of the hypothalamus robustly suppresses food intake, whereas GLP-1 only modestly suppresses food intake. The values are mean ± SEM and represent the time-course of cumulative 18-h food intake in chow-fed C57BL/6J mice receiving GLP-1 or Ex4 (0.0025, 0.005, or 0.025 µg) or ACSF (100 nL bilateral [PVN] or unilateral [ARC]) in the PVN (A and C) or ARC (B and D). n = 7–15 mice/group. *P < 0.05 vs. ACSF.
Gene Expression of the Hypothalamic Glp1r Is Reduced by Cre-Lox Recombination
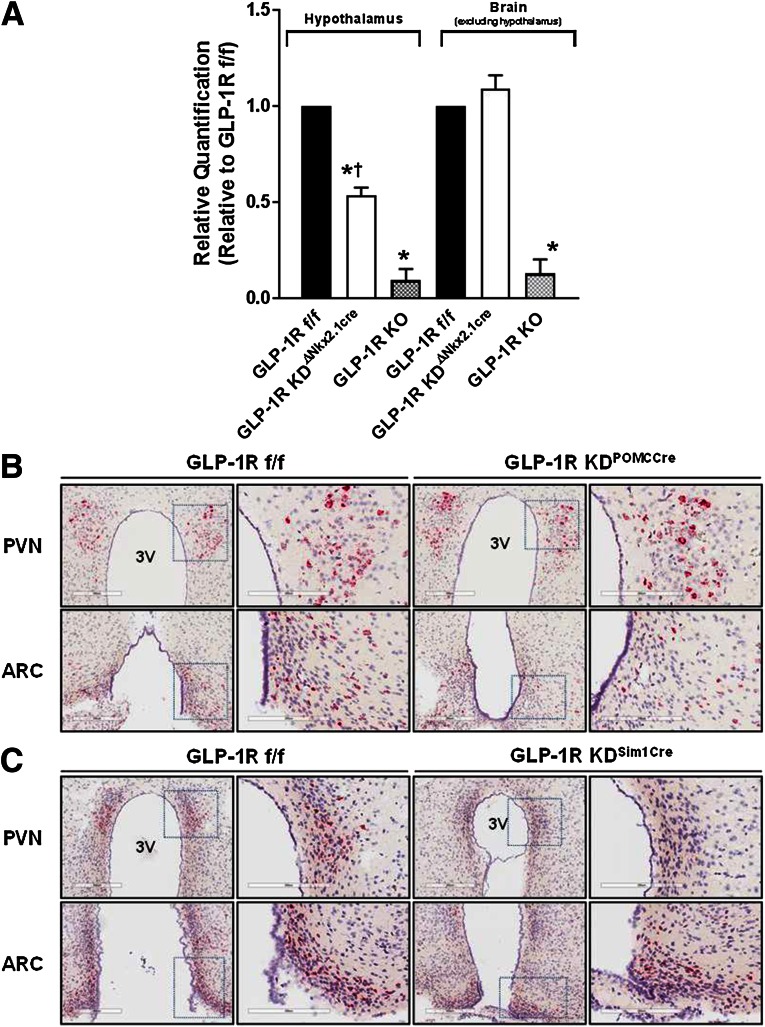
To test whether the hypothalamic GLP-1R is necessary for chronic maintenance of nutrient homeostasis, we bred floxed Glp1r mice with a line expressing Cre recombinase in multiple hypothalamic regions, Nkx2.1-Cre mice (19), to generate hypothalamic Glp1r knockout (GLP-1RKDΔNkx2.1cre) mice. Glp1r mRNA levels were significantly reduced in hypothalami from GLP-1RKDΔNkx2.1cre mice, although not to the same extent as in GLP-1RKO mice (Fig. 2A). Glp1r mRNA levels were unaffected in the cortex (nonhypothalamic control) of GLP-1RKDΔNkx2.1cre mice (Fig. 2A). As the PVN and ARC have been shown to mediate the anorectic effects of pharmacological GLP-1RA, we also generated mice lacking Glp1r expression in the PVN (GLP-1RKDΔSim1cre mice) and POMC neurons (GLP-1RKDΔPOMCcre mice). Selective deletion in POMC neurons was chosen because the Glp1r is expressed in anorexigenic POMC but not in orexigenic NPY/AgRP neurons of the ARC (14). Knockdown of the Glp1r in GLP-1RKDΔSim1cre mice was restricted to the PVN (Fig. 2C), and knockdown of the Glp1r in GLP-1RKDΔPOMCcre mice was restricted to the ARC (Fig. 2B).
Figure 2.
A: Cre-recombinase site-selectively knocks down the Glp1r in the hypothalamus. The values are mean ± SEM and represent gene expression levels of the Glp1r in RNA isolates from the hypothalamus or extrahypothalamic brain tissue of GLP-1Rf/f, GLP-1RKDΔNkx2.1cre, and GLP-1RKO mice as determined by qRT-PCR. n = 5 mice/group. *P < 0.05 vs. GLP-1Rf/f. †P < 0.05 vs. GLP-1RKO. Representative histology images showing Glp1r RNA expression in PVN and ARC sections of GLP-1RKDΔPOMCcre (B) and GLP-1RKDΔSim1cre (C) mice, each compared with GLP-1Rf/f controls. Glp1r RNA expression is indicated as a red, chromogenic signal. Images are shown at both 200 μm and 300 μm (magnified area designated by the dashed box). Scale bars are 200 μM and 300 μM for the lower and higher magnification panels, respectively. 3V, third ventricle.
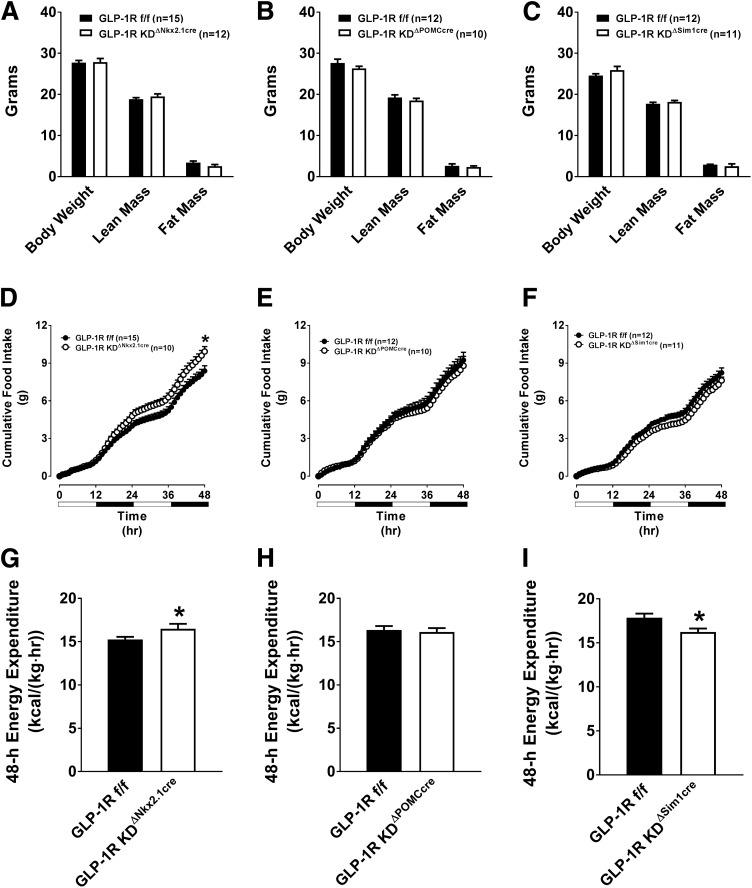
Disruption of Glp1r Expression in Nkx2.1-Expressing Neurons Elevates Food Intake and EE With No Alterations in Body Weight or Composition in Chow-Fed Mice
Total, lean, and fat mass were unaltered in chow-fed GLP-1RKDΔNkx2.1cre mice (Fig. 3A), although there was a tendency (P = 0.07) for reduced fat mass relative to total body mass as shown by ANCOVA (Supplementary Table 1). Disruption of Glp1r expression in chow-fed GLP-1RKDΔPOMCcre (Fig. 3B) and GLP-1RKDΔSim1cre (Fig. 3C) mice also had no effect on body weight or composition. GLP-1RKDΔNkx2.1cre mice displayed significantly elevated 48-h food (Fig. 3D) and water (Supplementary Fig. 1A) intake. Basal 48-h EE was also elevated in GLP-1RKDΔNkx2.1cre mice (Fig. 3G). This was not due to an appreciable increase in locomotor activity (Supplementary Fig. 1G), nor was there any change in the RER (Supplementary Fig. 1D). Increased EE in GLP-1RKDΔNkx2.1cre mice occurred independently from food intake or total body mass (Supplementary Table 1). These results indicate that the hypothalamic Glp1r plays a role in the regulation of basal food intake and EE.
Figure 3.
Chow-fed GLP-1RKDΔNkx2.1cre mice exhibit elevated food intake and EE, compared with GLP-1Rf/f controls. The values are mean ± SEM and represent 5-h–fasted body composition (body weight, lean mass, and fat mass), cumulative 48-h food intake, and 48-h EE in GLP-1RKDΔNkx2.1cre (n = 12) (A, D, and G), GLP-1RKDΔPOMCcre (n = 10) (B, E, and H), and GLP-1RKDΔSim1cre (n = 11) (C, F, and I) mice, compared with GLP-1Rf/f controls (n = 12–15). *P < 0.05 vs. GLP-1Rf/f.
Targeted Disruption of Glp1r Expression in POMC Neurons and the PVN Elicits Minor Effects on Energy Balance in Chow-Fed Mice
We next determined whether the elevated food intake and EE in GLP-1RKDΔNkx2.1cre mice was due to loss of Glp1r expression in POMC neurons or the PVN. Knockdown of Glp1r within POMC neurons conferred no changes in basal food intake (Fig. 3E) or EE (Fig. 3H). Although knockdown of the GLP-1R specifically within the PVN conferred no changes in basal food intake (Fig. 3F), there was a significant decrease in EE (Fig. 3I). There were no significant effects on water intake, RER, or locomotor activity in GLP-1RKDΔPOMCcre or GLP-1RKDΔSim1cre mice (Supplementary Fig. 1).
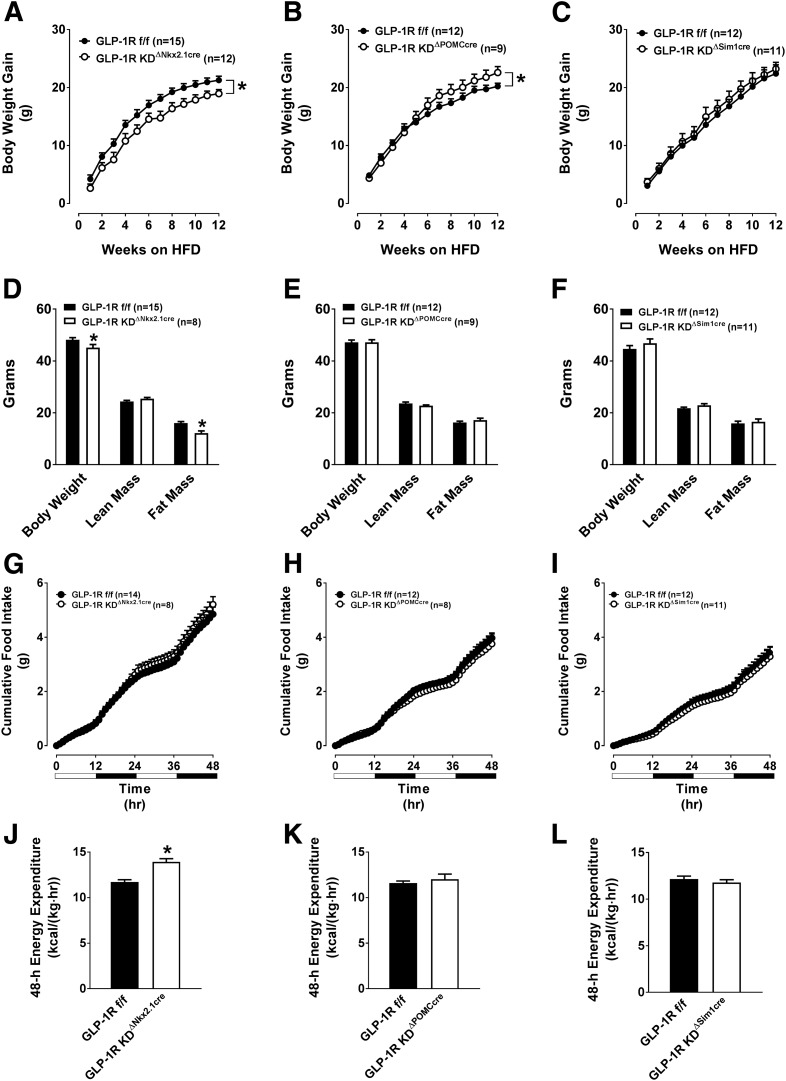
Glp1r Knockdown in Nkx2.1-Expressing Neurons Protects Mice From HFD-Induced Weight Gain and Increased Fat Mass, and This Phenotype Is Associated With Elevated EE
We next determined whether the body composition and energy balance phenotypes observed in chow-fed GLP-1RKDΔNkx2.1cre mice persist after HFD feeding. GLP-1RKDΔNkx2.1cre mice displayed a significantly reduced HFD-induced weight gain and fat mass (Fig. 4A and D, respectively). In contrast to chow-fed animals, HFD-fed GLP-1RKDΔNkx2.1cre mice did not exhibit increased 48-h food intake (Fig. 4G). Despite this, 48-h water intake was significantly elevated in GLP-1RKDΔNkx2.1cre mice (Supplementary Fig. 2A). Forty-eight hour EE remained significantly elevated in GLP-1RKDΔNkx2.1cre mice (Fig. 4J), and this was independent of the difference in total body weight, compared with controls (Supplementary Table 1). Elevated EE was not due to an appreciable difference in locomotor activity (Supplementary Fig. 2G). There was no difference in the RER (Supplementary Fig. 2D). Thus, loss of Glp1r expression in Nkx2.1-expressing neurons increases EE regardless of diet and independently of effects on food intake.
Figure 4.
Disruption of the GLP-1R in GLP-1RKDΔNkx2.1cre, GLP-1RKDΔPOMCcre, and GLP-1RKDΔSim1cre mice differentially alters HFD-induced weight gain, body composition, and EE. The values are mean ± SEM and represent HFD-induced body weight gain, body composition (body weight, lean mass, and fat mass), cumulative 48-h food intake, and 48-h EE in GLP-1RKDΔNkx2.1cre (n = 12) (A, D, G, and J), GLP-1RKDΔPOMCcre (n = 9) (B, E, H, and K), and GLP-1RKDΔSim1cre (n = 11) (C, F, I, and L) mice, compared with GLP-1Rf/f controls (n = 12–15). *P < 0.05 vs. GLP-1Rf/f.
Disruption of Glp1r Expression in POMC Neurons and the PVN Elicits Variable Effects on Energy Balance in HFD-Fed Mice
We assessed whether the protection from HFD-induced obesity and increased EE in GLP-1RKDΔNkx2.1cre mice was due to loss of Glp1r expression in POMC neurons or the PVN. Contrary to GLP-1RKDΔNkx2.1cre mice, GLP-1RKDΔPOMCcre mice displayed increased HFD-induced weight gain (Fig. 4B). However, there were no significant differences in lean or fat mass between genotypes (Fig. 4E). No differences in HFD-induced weight gain or body composition were observed in GLP-1RKDΔSim1cre mice (Fig. 4C and F). Additional parameters of energy balance in these mice were unaltered (Fig. 4H, I, K, and L and Supplementary Fig. 2). These findings suggest that the energy balance phenotypes observed in GLP-1RKDΔNkx2.1cre mice are not due to loss of Glp1r expression in the PVN or POMC neurons.
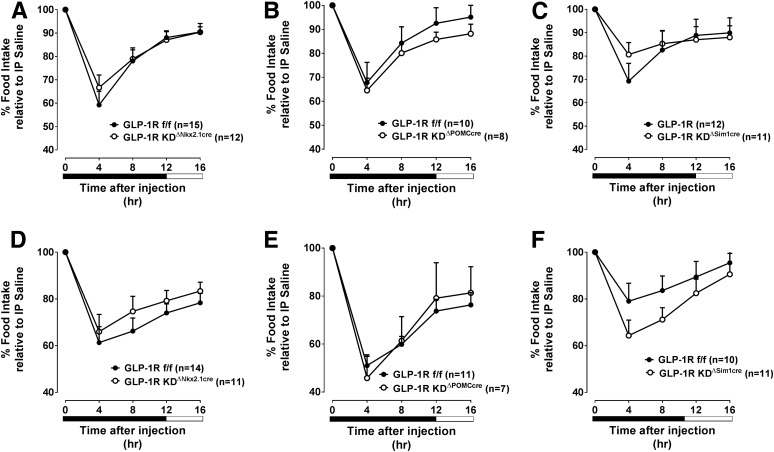
Hypothalamic Glp1r Knockdown Mice Exhibit a Normal Anorectic Response to Acute, Peripheral Administration of Ex4
We next tested the hypothesis that the loss of hypothalamic Glp1r expression blunts the acute anorectic effect of peripherally dosed GLP-1RA. Suppression of food intake by Ex4 was equivalent in chow-fed GLP-1RKDΔNkx2.1cre, GLP-1RKDΔPOMCcre, and GLP-1RKDΔSim1cre (Fig. 5A–C), compared with their respective controls. Ex4 also suppressed food intake similarly in HFD-fed GLP-1RKDΔNkx2.1cre, GLP-1RKDΔPOMCcre, and GLP-1RKDΔSim1cre (Fig. 5D–F) mice, compared with controls. These findings show that the GLP-1R in Nkx2.1-expressing neurons and classic hypothalamic feeding centers (i.e., PVN and POMC neurons) is not required for the acute anorectic effects of peripherally administered GLP-1RA.
Figure 5.
Disruption of the GLP-1R in Nkx2.1 neurons, POMC neurons, or the PVN does not blunt the food intake–suppressive effect of peripherally dosed Ex4. The values are mean ± SEM and represent 16-h food intake following treatment with Ex4 (3 µg, i.p.) in chow- or HFD-fed (top and bottom panels, respectively) GLP-1RKDΔNkx2.1cre (n = 11–12) (A and D), GLP-1RKDΔPOMCcre (n = 7–8) (B and E), and GLP-1RKDΔSim1cre (n = 11) (C and F) mice, compared with GLP-1Rf/f controls (n = 10–15). Data are shown at 4-h intervals and expressed as a percentage of the food intake response observed following treatment with vehicle.
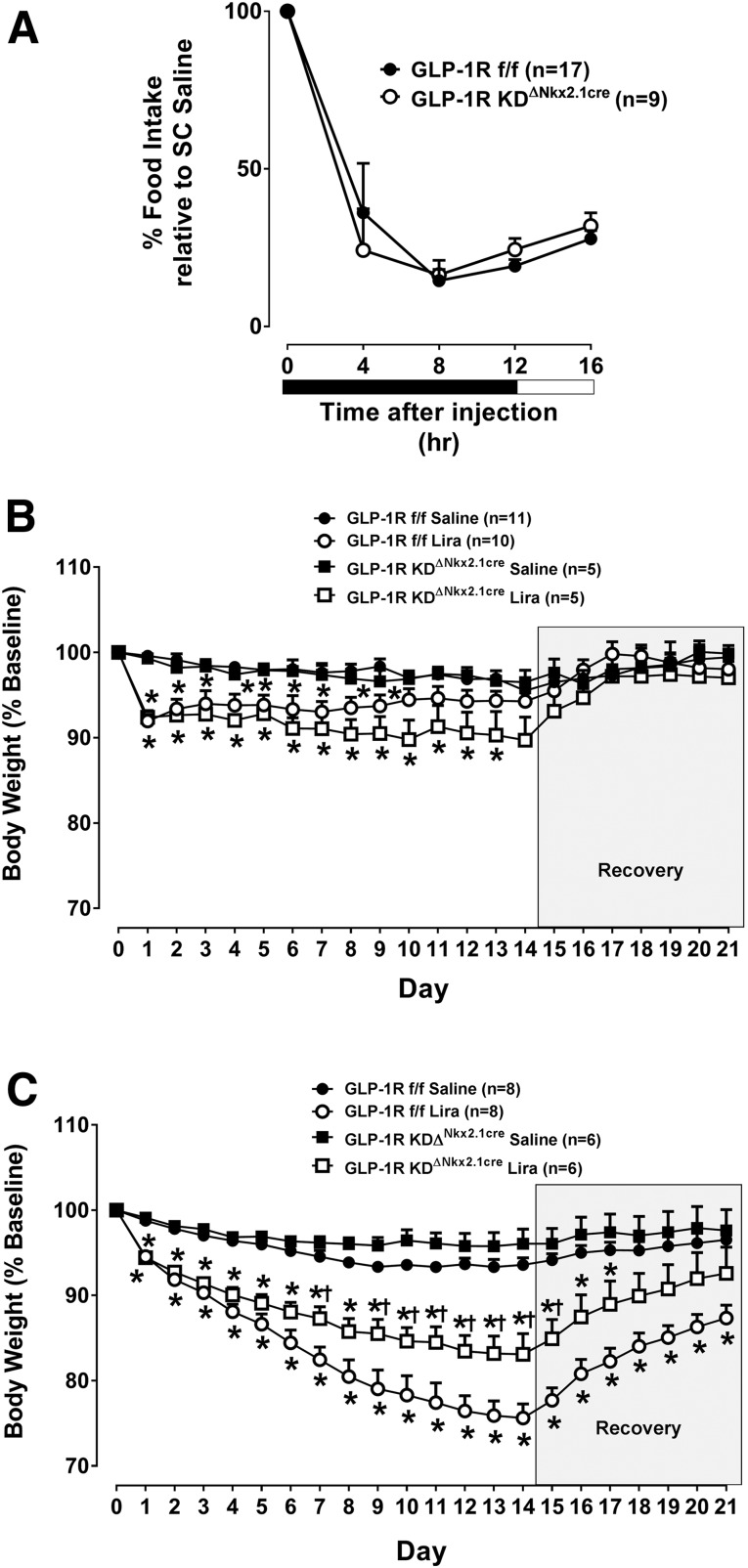
Loss of the Glp1r in Nkx2.1-Expressing Neurons Attenuates the Body Weight–Reducing Effect of Chronic Liraglutide Treatment Only in HFD-Fed Mice
Liraglutide does not reduce 24-h food intake in mice with CNS deletion of the GLP-1R (3). We tested the hypothesis that this is due to loss of hypothalamic Glp1r expression. Loss of Glp1r expression in Nkx2.1-expressing neurons did not affect the acute food intake–suppressive effect of liraglutide (Fig. 6A). We then tested whether loss of hypothalamic Glp1r expression attenuates the body weight–reducing effects of a chronic dosing regimen of liraglutide. Secher et al. (17) recently demonstrated that the infusion of the GLP-1R antagonist Ex9 into the ARC partially attenuates the anorectic effects of a 14-day liraglutide treatment. We show that the body weight–reducing effect of liraglutide was unaffected in chow-fed GLP-1RKDΔNkx2.1cre mice (Fig. 6B). However, loss of hypothalamic Glp1r expression attenuated the weight-reducing effect of liraglutide in HFD-fed mice (Fig. 6C), suggesting that the hypothalamic GLP-1R is required for peripherally dosed liraglutide to exert its body weight–reducing effect during the metabolic stress of an HFD.
Figure 6.
Disruption of the GLP-1R in Nkx2.1 neurons does not blunt the food intake–suppressive effect of peripherally dosed liraglutide but does impact the compound’s body weight–lowering effect in HFD-fed mice. A: The values are mean ± SEM and represent 16-h food intake following treatment with liraglutide (Lira) (200 µg, s.c.) in chow-fed GLP-1RKDΔNkx2.1cre (n = 9) mice, compared with GLP-1Rf/f controls (n = 17). Data are shown at 4-h intervals and expressed as a percentage of the food intake response observed following treatment with vehicle. B and C: The values are mean ± SEM and represent daily body weight over the course of 21 days in chow-fed (B) or HFD-fed (C) GLP-1RKDΔNkx2.1cre (n = 5–6), compared with GLP-1Rf/f controls (n = 8–11) treated with liraglutide. Data are expressed as a percentage of baseline (i.e., prior to liraglutide treatment) body weight. On days 0–13, morning body weight was measured, and animals received a twice-daily injection of liraglutide (200 µg/kg BW, s.c.) or vehicle. On recovery days 14–21, only morning body weight was measured. *P < 0.05 vs. saline. †P < 0.05 vs. GLP-1Rf/f.
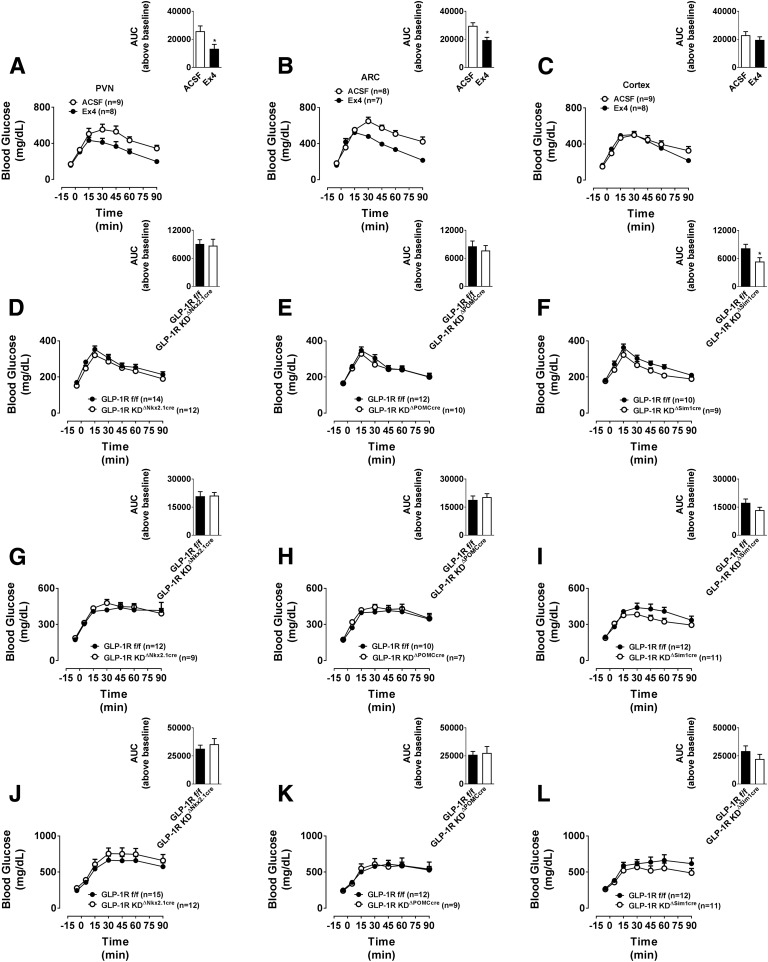
Glucose Tolerance Is Improved by Pharmacological Activation of the GLP-1R in the PVN and ARC, but Loss of Glp1r Expression in These Brain Regions Does Not Impair Glucose Tolerance
Brain GLP-1R signaling modulates peripheral glucose production and/or utilization (14,24–27). We assessed the effect of targeted delivery of Ex4 to specific hypothalamic nuclei on glucose tolerance. An anorectic dose of Ex4 (0.025 µg) targeted to the PVN (Fig. 7A) or ARC (Fig. 7B), but not the cortex (Fig. 7C), improved glucose tolerance.
Figure 7.
Direct injection of Ex4 into the PVN or ARC of the hypothalamus robustly improves glucose tolerance, whereas disruption of the hypothalamic GLP-1R in GLP-1RKDΔNkx2.1cre, GLP-1RKDΔPOMCcre, and GLP-1RKDΔSim1cre mice does not affect glucose tolerance. The values are mean ± SEM and represent glucose excursion in chow-fed C57BL/6J mice following a gavage of glucose (2 g/kg BW, i.p.) and treatment with Ex4 (0.025 µg) or ACSF (100 nL) in the PVN (n = 8–9) (A), ARC (n = 7–8) (B), or cortex (n = 8–9) (C) at t = 0 min. Inset: Area under the curve (AUC) above baseline for each group. *P < 0.05 vs. ACSF. The values are mean ± SEM and represent glucose excursion in chow- or HFD-fed GLP-1RKDΔNkx2.1cre (n = 9–12) (D, G, and J), GLP-1RKDΔPOMCcre (n = 7–10) (E, H, and K), and GLP-1RKDΔSim1cre (n = 9–11) (F, I, and L) mice, compared with GLP-1Rf/f controls (n = 10–15). D, E, and F: chow diet, oral GTT; G, H, and I: chow diet, intraperitoneal GTT; J, K, and L: HFD, oral GTT. Inset: AUC above baseline for each group. *P < 0.05 vs. ACSF.
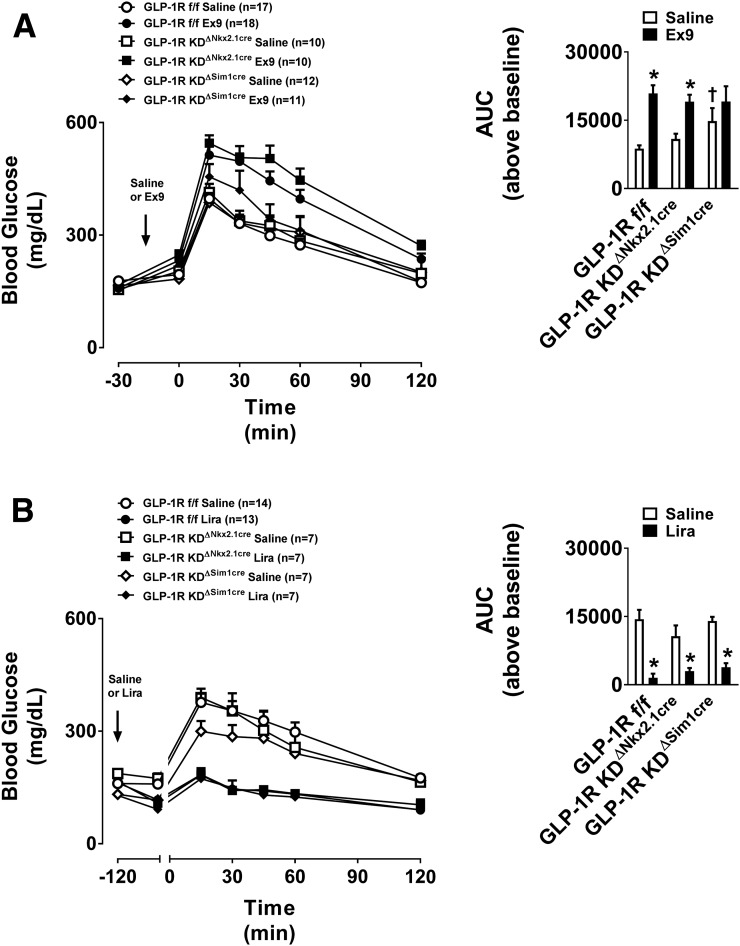
We then assessed whether the loss of Glp1r expression in the Nkx2.1 neurons, PVN, or POMC neurons impairs glucose handling. Oral (Fig. 7D–F) and intraperitoneal (Fig. 7G–I) glucose tolerance was identical in chow-fed GLP-1RKDΔNkx2.1cre, GLP-1RKDΔPOMCcre, and GLP-1RKDΔSim1cre mice, compared with respective controls. Similarly, oral glucose tolerance was unaffected by the loss of Glp1r expression in these brain regions in HFD-fed mice (Fig. 7J–L). Pretreatment with the GLP-1R antagonist Ex9 impaired glucose tolerance similarly in GLP-1RKDΔNkx2.1cre and GLP-1RKDΔSim1cre mice (Fig. 8A). Thus, although pharmacological activation of the hypothalamic GLP-1R improves glucose tolerance, GLP-1R in this brain region is not necessary for glucose handling.
Figure 8.
Disruption of the GLP-1R in GLP-1RKDΔNkx2.1cre and GLP-1RKDΔSim1cre mice does not impact glucose tolerance following pretreatment with Ex9 or liraglutide (Lira). The values are mean ± SEM and represent glucose excursion in chow-fed GLP-1RKDΔNkx2.1cre (n = 7–10) and GLP-1RKDΔSim1cre (n = 7–12) pretreated with Ex9 (50 µg, i.p.) vs. vehicle (A) or liraglutide (400 µg/kg BW, s.c.) vs. vehicle (B) 15 min (for Ex9 studies) or 120 min (for liraglutide studies) prior to an intraperitoneal glucose challenge. Inset: Area under the curve (AUC) above baseline for each group. *P < 0.05 vs. saline. †P < 0.05 vs. GLP-1Rf/f.
Liraglutide Improves Glucose Tolerance Independently of the Hypothalamic GLP-1R
Neuronal-wide deletion of the GLP-1R does not affect the ability of liraglutide to improve glucose tolerance (3). However, global CNS deletion of GLP-1R could mask the contributions of specific brain regions to the regulation of glucose tolerance. Pretreatment with liraglutide improves intraperitoneal glucose tolerance similarly in both GLP-1RKDΔNkx2.1cre and GLP-1RKDΔSim1cre mice (Fig. 8B), suggesting that the hypothalamic GLP-1R is not required for the glucoregulatory effect of peripherally administered liraglutide.
Discussion
GLP-1RA suppress food intake and reduce body weight via food intake–regulatory brain regions including the hypothalamus and brain stem (17,28). We and others have shown that GLP-1RA targeted to the hindbrain (29) and hypothalamic nuclei, including the PVN (30), DMH (5), and ARC, reduce food intake. However, these acute pharmacological interventions do not reflect the physiological, long-term regulation of feeding by brain GLP-1R. The present studies assessed the impact of selectively knocking down hypothalamic Glp1r expression on overall energy balance. Glp1r expression was disrupted in Nkx2.1-expressing neurons, PVN, or POMC neurons to address whether these regions are necessary for the anorectic and glucoregulatory effects of GLP-1RA. We report that loss of Glp1r expression in these three regions does not affect energy balance or responsiveness to peripheral administration of GLP-1RA. This is surprising as targeted administration of GLP-1RA to these regions potently reduces food intake and improves glucose tolerance. These findings demonstrate that classic GLP-1RA–responsive hypothalamic neurons are not necessary for the anorectic and glucoregulatory effects of GLP-1RA.
The GLP-1R is expressed in extrahypothalamic food intake–regulatory regions, including the central amygdala, hindbrain, and area postrema (31). Several studies have also shown that targeting GLP-1RA to reward regions, including the ventral tegmental area (32), nucleus accumbens (33), and lateral parabrachial nucleus (LPBN) (34), suppresses food intake. Thus, CNS GLP-1R signaling could reduce food intake and body weight by decreasing the hedonic value of food (35). Anorectic effects of GLP-1RA may also be secondary to homeostatic stress (36,37). GLP-1 signaling in the PVN and hindbrain regulates the behavioral, autonomic, and neuroendocrine responses to stress by activating both the “fight or flight” response acutely and the hypothalamic-pituitary-adrenal axis chronically (36–40). Moreover, the central GLP-1R system is activated in response to stressful stimuli, which can themselves reduce food intake and elevate blood glucose (37,41). The GLP-1R is also expressed in peripheral vagal afferent neurons (42), yet the extent to which these neurons relay gut-derived GLP-1R signals to the brain to mediate satiating and glucoregulatory responses remains controversial. Kanoski et al. (18) demonstrated that the anorectic effects of peripherally dosed Ex4 and liraglutide involve the activation of GLP-1R both on vagal afferents and in the CNS, whereas Secher et al. (17) report that subdiaphragmatic vagal afferent deafferentation does not impact the food intake– and body weight–lowering effects of peripherally dosed liraglutide.
GLP-1RKDΔNkx2.1cre mice display elevated 48-h food intake and EE. The net effect likely explains the absence of a body weight effect in these mice. Knockdown of the GLP-1R in the PVN or POMC neurons did not recapitulate the increased food intake and EE phenotypes observed in GLP-1RKDΔNkx2.1cre mice, suggesting that alternative Glp1r-expressing hypothalamic regions, such as the VMH, DMH, or LH (31), may be involved in regulating these processes. We then hypothesized that the loss of hypothalamic Glp1r would exacerbate the metabolic derangements of HFD feeding. On the contrary, GLP-1RKDΔNkx2.1cre mice were protected from HFD-induced weight and fat mass gain. This was not due to reduced food intake. Instead, the elevated EE observed in chow-fed GLP-1RKDΔNkx2.1cre mice persisted with HFD feeding, demonstrating that loss of hypothalamic Glp1r expression increases EE independently of food intake. This is surprising because ICV or hypothalamic administration of GLP-1RA increases EE (15,43). Loss of hypothalamic Glp1r expression may increase sensitivity to GLP-1 in other brain regions, thus elevating EE. However, whole-body Glp1r KO mice also exhibit increased EE (44). Paradoxically, loss of POMC Glp1r expression increased HFD-induced weight gain, suggesting distinct roles for GLP-1R signaling in different hypothalamic regions. Elevated HFD-induced weight gain in GLP-1RKDΔPOMCcre mice was not associated with significant changes in body composition, food intake, or EE. One potential explanation is that weight gain was measured in ad libitum–fed conditions, whereas body composition was measured in 5-h fasted mice. Thus, POMC GLP-1R may regulate the handling of nutrient stores during fasting. Unlike Nkx2.1 neuron- and POMC-specific Glp1r knockdown models, GLP-1RKDΔSim1cre mice displayed no effects on body composition or energy balance parameters. These observations highlight the complexity of brain GLP-1R actions on the maintenance of energy balance and suggest that different hypothalamic nuclei mediate distinct effects of GLP-1, consistent with previous findings demonstrating nuclei-specific effects of GLP-1R signaling in visceral illness and hypothalamic-pituitary-adrenal axis activation (16).
Peripheral administration of GLP-1RA reduces food intake and body weight, likely due, in part, to their ability to cross the blood-brain barrier (17,45). Indeed, disruption of pan-neuronal Glp1r expression inhibits the anorectic effects of peripherally administered liraglutide (3). We hypothesized that this is due to the loss of hypothalamic Glp1r expression. Surprisingly, the acute anorectic effects of peripherally dosed Ex4 and liraglutide were preserved in our hypothalamic GLP-1RKO models. We then tested whether the hypothalamic GLP-1R mediates the long-term weight loss effects of GLP-1RA. Targeted delivery of the GLP-1R antagonist Ex9 to the ARC attenuates the weight loss effect of a 14-day liraglutide dosing regimen (17). In the present studies, chow-fed GLP-1RKDΔNkx2.1cre mice displayed similar weight loss as controls following a 14-day liraglutide treatment but were refractory to liraglutide-induced weight loss when fed an HFD. Importantly, in both the present studies and those by Secher et al. (17), pharmacological or genetic blockade of the ARC or POMC GLP-1R did not prevent the initial weight loss induced by liraglutide. These findings implicate other hypothalamic regions and/or extrahypothalamic regions as mediators of the anorexigenic effects of GLP-1RA.
Modulation of the brain GLP-1R affects glucose production and utilization under hyperinsulinemic conditions (24,46). We show that glucose tolerance is identical in GLP-1RKDΔNkx2.1cre, GLP-1RKDΔSim1cre, and GLP-1RKDΔPOMCcre mice, compared with controls. Moreover, Ex9 impaired glucose tolerance in GLP-1RKDΔNkx2.1cre and GLP-1RKDΔSim1cre mice, suggesting that these GLP-1R populations do not mediate Ex9-induced impairments in glucose homeostasis. This is distinct from observations in β-cell GLP-1RKO mice in which Ex9 does not impair glucose tolerance (47). Liraglutide improved glucose tolerance in GLP-1RKDΔNkx2.1cre and GLP-1RKDΔSim1cre mice to similar degrees as their respective controls. These observations were surprising considering that targeted delivery of Ex4 to the PVN and ARC improved glucose tolerance. Nevertheless, these results support observations by Sisley et al. (3) that loss of CNS GLP-1R expression does not affect glucose tolerance or the glucoregulatory effects of liraglutide.
Although the Cre lines in these studies primarily target hypothalamic neurons, there are interpretative caveats, including incomplete Cre expression and off-target effects. We achieved only a ∼50% reduction in Glp1r expression in GLP-1RKDΔNkx2.1cre mice. One potential explanation is that Nkx2.1 is expressed in GABAergic but not glutamatergic neurons (48), raising the possibility that GLP-1R may remain expressed in hypothalamic glutamatergic neurons. Sim1 is not only expressed in the PVN, but it is also enriched in the cerebellum, midbrain, and hippocampus (21). Although enriched in the ARC, POMC is also expressed in the hippocampus and hindbrain (20). Moreover, there are non-POMC and non-NPY/AgRP ARC neurons that are GLP-1R positive (14).
These studies demonstrate that GLP-1R expression within two hypothalamic regions typically assigned a prominent role in regulating energy balance, the ARC and PVN, is sufficient but not necessary for the effects of GLP-1RA on energy balance and highlights the importance of combining targeted pharmacological and genetic approaches to unravel the complex mechanisms by which GLP-1RA maintain nutrient homeostasis. The identification of overlapping CNS sites of action and signaling pathways for GLP-1R–mediated regulation of homeostatic and hedonic aspects of feeding behavior may inform the development of more effective therapies for type 2 diabetes and obesity.
Supplementary Material
Article Information
Acknowledgments. The authors would like to recognize John Shelley (SBP at Lake Nona) for his assistance with the RNA in situ hybridization studies.
Funding. D.J.D. is supported by the Canada Research Chairs program and Canadian Institutes of Health Research grant 123391.This work was supported by National Institutes of Health grants 7R01DK082480 (to D.A.Sa.), 1R01DK107652 (to R.J.S.), and 4R01DK097361 (to Ju.E.A.).
Duality of Interest. D.J.D. has received consulting honoraria related to GLP-1 from MedImmune and Novo Nordisk. D.A.Sa. has received research support from Ethicon Endo-Surgery, Novo Nordisk, and Boehringer Ingelheim. R.J.S. has received research support from Ethicon Endo-Surgery, Novo Nordisk, Sanofi, and Janssen; has served on scientific advisory boards for Ethicon Endo-Surgery, Daiichi Sankyo, Janssen, Novartis, Nestle, Takeda, Boehringer Ingelheim, Sanofi, and Novo Nordisk; and is also a paid speaker for Ethicon Endo-Surgery. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.A.B. and Ju.E.A. designed the experiments. M.A.B., Je.E.A., H.S., and A.L.-R. collected and analyzed the data. D.J.D., D.A.St., D.A.Sa., and R.J.S. provided essential research tools. M.A.B., Je.E.A., J.D.B., D.A.Sa., and Ju.E.A. wrote the manuscript. All authors reviewed and edited the manuscript. M.A.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-1102/-/DC1.
References
- 1.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–2157 [DOI] [PubMed] [Google Scholar]
- 2.Fonseca VA, Zinman B, Nauck MA, Goldfine AB, Plutzky J. Confronting the type 2 diabetes epidemic: the emerging role of incretin-based therapies. Am J Med 2010;123:S2–S10 [DOI] [PubMed] [Google Scholar]
- 3.Sisley S, Gutierrez-Aguilar R, Scott M, D’Alessio DA, Sandoval DA, Seeley RJ. Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. J Clin Invest 2014;124:2456–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burmeister MA, Ayala J, Drucker DJ, Ayala JE. Central glucagon-like peptide 1 receptor-induced anorexia requires glucose metabolism-mediated suppression of AMPK and is impaired by central fructose. Am J Physiol Endocrinol Metab 2013;304:E677–E685 [DOI] [PubMed] [Google Scholar]
- 5.Schick RR, Zimmermann JP, vorm Walde T, Schusdziarra V. Peptides that regulate food intake: glucagon-like peptide 1-(7-36) amide acts at lateral and medial hypothalamic sites to suppress feeding in rats. Am J Physiol Regul Integr Comp Physiol 2003;284:R1427–R1435 [DOI] [PubMed] [Google Scholar]
- 6.Tang-Christensen M, Larsen PJ, Göke R, et al. . Central administration of GLP-1-(7-36) amide inhibits food and water intake in rats. Am J Physiol 1996;271:R848–R856 [DOI] [PubMed] [Google Scholar]
- 7.Turton MD, O’Shea D, Gunn I, et al. . A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 1996;379:69–72 [DOI] [PubMed] [Google Scholar]
- 8.Donahey JC, van Dijk G, Woods SC, Seeley RJ. Intraventricular GLP-1 reduces short- but not long-term food intake or body weight in lean and obese rats. Brain Res 1998;779:75–83 [DOI] [PubMed] [Google Scholar]
- 9.Meeran K, O’Shea D, Edwards CM, et al. . Repeated intracerebroventricular administration of glucagon-like peptide-1-(7-36) amide or exendin-(9-39) alters body weight in the rat. Endocrinology 1999;140:244–250 [DOI] [PubMed] [Google Scholar]
- 10.Roh E, Song K, Kim MS. Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp Mol Med 2016;48:e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Picard A, Moullé VS, Le Foll C, et al. . Physiological and pathophysiological implications of lipid sensing in the brain. Diabetes Obes Metab 2014;16(Suppl. 1):49–55 [DOI] [PubMed] [Google Scholar]
- 12.Barrera JG, D’Alessio DA, Drucker DJ, Woods SC, Seeley RJ. Differences in the central anorectic effects of glucagon-like peptide-1 and exendin-4 in rats. Diabetes 2009;58:2820–2827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pannacciulli N, Le DS, Salbe AD, et al. . Postprandial glucagon-like peptide-1 (GLP-1) response is positively associated with changes in neuronal activity of brain areas implicated in satiety and food intake regulation in humans. Neuroimage 2007;35:511–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandoval DA, Bagnol D, Woods SC, D’Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes 2008;57:2046–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beiroa D, Imbernon M, Gallego R, et al. . GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 2014;63:3346–3358 [DOI] [PubMed] [Google Scholar]
- 16.Kinzig KP, D’Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci 2002;22:10470–10476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Secher A, Jelsing J, Baquero AF, et al. . The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest 2014;124:4473–4488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology 2011;152:3103–3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ring LE, Zeltser LM. Disruption of hypothalamic leptin signaling in mice leads to early-onset obesity, but physiological adaptations in mature animals stabilize adiposity levels. J Clin Invest 2010;120:2931–2941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balthasar N, Coppari R, McMinn J, et al. . Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 2004;42:983–991 [DOI] [PubMed] [Google Scholar]
- 21.Balthasar N, Dalgaard LT, Lee CE, et al. . Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 2005;123:493–505 [DOI] [PubMed] [Google Scholar]
- 22.Wilson-Pérez HE, Chambers AP, Ryan KK, et al. . Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like peptide 1 receptor deficiency. Diabetes 2013;62:2380–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL. Peripheral exendin-4 and peptide YY(3-36) synergistically reduce food intake through different mechanisms in mice. Endocrinology 2005;146:3748–3756 [DOI] [PubMed] [Google Scholar]
- 24.Burmeister MA, Ferre T, Ayala JE, King EM, Holt RM, Ayala JE. Acute activation of central GLP-1 receptors enhances hepatic insulin action and insulin secretion in high-fat-fed, insulin resistant mice. Am J Physiol Endocrinol Metab 2012;302:E334–E343 [DOI] [PubMed] [Google Scholar]
- 25.Daniele G, Iozzo P, Molina-Carrion M, et al. . Exenatide regulates cerebral glucose metabolism in brain areas associated with glucose homeostasis and reward system. Diabetes 2015;64:3406–3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tudurí E, Beiroa D, Porteiro B, López M, Diéguez C, Nogueiras R. Acute but not chronic activation of brain glucagon-like peptide-1 receptors enhances glucose-stimulated insulin secretion in mice. Diabetes Obes Metab 2015;17:789–799 [DOI] [PubMed] [Google Scholar]
- 27.Sandoval D, Sisley SR. Brain GLP-1 and insulin sensitivity. Mol Cell Endocrinol 2015;418:27–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 2009;150:2654–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alhadeff AL, Grill HJ. Hindbrain nucleus tractus solitarius glucagon-like peptide-1 receptor signaling reduces appetitive and motivational aspects of feeding. Am J Physiol Regul Integr Comp Physiol 2014;307:R465–R470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katsurada K, Maejima Y, Nakata M, et al. . Endogenous GLP-1 acts on paraventricular nucleus to suppress feeding: projection from nucleus tractus solitarius and activation of corticotropin-releasing hormone, nesfatin-1 and oxytocin neurons. Biochem Biophys Res Commun 2014;451:276–281 [DOI] [PubMed] [Google Scholar]
- 31.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 1999;403:261–280 [DOI] [PubMed] [Google Scholar]
- 32.Mietlicki-Baase EG, Ortinski PI, Rupprecht LE, et al. . The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. Am J Physiol Endocrinol Metab 2013;305:E1367–E1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci 2011;31:14453–14457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swick JC, Alhadeff AL, Grill HJ, et al. . Parabrachial nucleus contributions to glucagon-like peptide-1 receptor agonist-induced hypophagia. Neuropsychopharmacology 2015;40:2001–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skibicka KP. The central GLP-1: implications for food and drug reward. Front Neurosci 2013;7:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghosal S, Myers B, Herman JP. Role of central glucagon-like peptide-1 in stress regulation. Physiol Behav 2013;122:201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsen PJ, Tang-Christensen M, Jessop DS. Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology 1997;138:4445–4455 [DOI] [PubMed] [Google Scholar]
- 38.Gil-Lozano M, Romaní-Pérez M, Outeiriño-Iglesias V, et al. . Corticotropin-releasing hormone and the sympathoadrenal system are major mediators in the effects of peripherally administered exendin-4 on the hypothalamic-pituitary-adrenal axis of male rats. Endocrinology 2014;155:2511–2523 [DOI] [PubMed] [Google Scholar]
- 39.Tauchi M, Zhang R, D’Alessio DA, Seeley RJ, Herman JP. Role of central glucagon-like peptide-1 in hypothalamo-pituitary-adrenocortical facilitation following chronic stress. Exp Neurol 2008;210:458–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto H, Lee CE, Marcus JN, et al. . Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest 2002;110:43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holt MK, Trapp S. The physiological role of the brain GLP-1 system in stress. Cogent Biol 2016;2:1229086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katsurada K, Yada T. Neural effects of gut- and brain-derived glucagon-like peptide-1 and its receptor agonist. J Diabetes Investig 2016;7(Suppl. 1):64–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lockie SH, Heppner KM, Chaudhary N, et al. . Direct control of brown adipose tissue thermogenesis by central nervous system glucagon-like peptide-1 receptor signaling. Diabetes 2012;61:2753–2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hansotia T, Maida A, Flock G, et al. . Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure. J Clin Invest 2007;117:143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J Mol Neurosci 2002;18:7–14 [DOI] [PubMed] [Google Scholar]
- 46.Knauf C, Cani PD, Perrin C, et al. . Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J Clin Invest 2005;115:3554–3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith EP, An Z, Wagner C, et al. . The role of β cell glucagon-like peptide-1 signaling in glucose regulation and response to diabetes drugs. Cell Metab 2014;19:1050–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Magno L, Catanzariti V, Nitsch R, Krude H, Naumann T. Ongoing expression of Nkx2.1 in the postnatal mouse forebrain: potential for understanding NKX2.1 haploinsufficiency in humans? Brain Res 2009;1304:164–186 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.