Abstract
Background
Nasal carriage with Staphylococcus aureus is a common risk factor for invasive infections, indicating the necessity to monitor prevalent strains, particularly in the vulnerable paediatric population. This surveillance study aims to identify carriage rates, subtypes, antimicrobial susceptibilities and virulence markers of nasal S. aureus isolates collected from children living in the Ashanti region of Ghana.
Methods
Nasal swabs were obtained from children < 15 years of age on admission to the Agogo Presbyterian Hospital between April 2014 and January 2015. S. aureus isolates were characterized by their antimicrobial susceptibility, the presence of genes encoding for Panton-Valentine leukocidin (PVL) and toxic shock syndrome toxin-1 (TSST-1) and further differentiated by spa-typing and multi-locus-sequence-typing.
Results
Out of 544 children 120 (22.1%) were colonized with S. aureus, with highest carriage rates during the rainy seasons (27.2%; p = 0.007), in females aged 6–8 years (43.7%) and males aged 8–10 years (35.2%). The 123 isolates belonged to 35 different spa-types and 19 sequence types (ST) with the three most prevalent spa-types being t355 (n = 25), t84 (n = 18), t939 (n = 13), corresponding to ST152, ST15 and ST45. Two (2%) isolates were methicillin-resistant S. aureus (MRSA), classified as t1096 (ST152) and t4454 (ST45), and 16 (13%) were resistant to three or more different antimicrobial classes. PVL and TSST-1 were detected in 71 (58%) and 17 (14%) isolates respectively.
Conclusion
S. aureus carriage among Ghanaian children seems to depend on age, sex and seasonality. While MRSA rates are low, the high prevalence of PVL is of serious concern as these strains might serve not only as a source for severe invasive infections but may also transfer genes, leading to highly virulent MRSA clones.
Background
Staphylococcus aureus contributes significantly to morbidity and mortality worldwide, causing a broad spectrum of diseases [1,2]. S. aureus nasal colonization has been identified as the most important risk factor for subsequent invasive infections [3]. An estimated 30% of humans are nasal carriers of S. aureus [4], however carriage rates vary with geographic location, seasonality, age and sex [1]. Studies among Dutch children revealed a decreasing carriage rate during the first year of life, remaining stable at 20–30% until it increases again to 40–50% between the age of 6 to 12 years [5,6]. In West Africa those rates might be considerably different due to co-colonization with other pathogens or particular living conditions, such as large family sizes and lower sanitary standards, which are all associated with S. aureus nasal carriage [7,8]. Studies from West and Central Africa show carriage rates ranging from 21% in Ghana [9] to 29% in Gabon [10] and 36% in Senegal [11], but none of these studies focus on children.
A high prevalence of Panton-Valentine Leukocidin (PVL), a cytolytic pore forming toxin, has been reported among clinical S. aureus isolates from Ghana (60%) and other West African countries [12,13]. Other toxins, such as the Toxic-shock-syndrome-toxin 1 (TSST-1) are often neglected, although remarkably prevalent in some African settings, as demonstrated in the Congo (18%) [14]. In case of autoinfection or transmission to other children those highly virulent isolates may cause severe infections in paediatric populations.
Similarly, regular surveillance of multidrug resistant (MDR) or methicillin resistant S. aureus (MRSA) is essential for clinicians, as second line therapies are often not available or contraindicated in children. Within West Africa MRSA rates from clinical samples range from 3% in Ghana to 20% in Nigeria [12,15] and a substantial number of MRSA (23%) from this region have been characterized as community-acquired MRSA (CA-MRSA) [16].
This surveillance study aims to identify demographic and season specific carriage rates, clonal types, antimicrobial susceptibilities and virulence markers of nasal S. aureus isolates collected from children living in the Ashanti Region of Ghana.
Methods
Isolation of bacterial strains
Study site was the Agogo Presbyterian Hospital, a district hospital with approximately 250 patient beds located in the Asante Akim North municipality in Ghana. The Asante Akim North municipal area has a population of approximately 142,400 inhabitants, spread over an area of 1,160 square kilometres. The region has a tropical climate and is mainly covered by secondary rain forest and cultivated land. The area is holoendemic for malaria transmission.
Nasal swabs from both anterior nares were taken from all children admitted to the pediatric ward between April 2014 and January 2015. Swabs were taken during the admission process, stored at 4°C and plated within a few hours on Columbia and Mannitol Salt Agar plates (all Oxoid, Basingstoke, UK) and incubated at 37°C for 24 hours. For each sample, all morphologically different colonies indicative for S. aureus were selected and phenotypically confirmed by standard gram staining and the 4–24 hour tube test for free coagulase in rabbit-citrate-plasma (Becton and Dickinson®; Heidelberg, Germany). S. aureus ATCC 33592 (MRSA; American Type Culture Collection, Wesel, Germany) and S. epidermidis DSM 20044 (German Collection of Microorganisms and cell cultures, Braunschweig, Germany) served as positive and negative controls.
The Committee on Human Research, Publications and Ethics, School of Medical Science, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana provided ethical approval for this study. All participants were informed about the study’s purpose and procedures. Written informed consent was obtained from the parents or the guardian on behalf of the study children prior to study enrolment.
Determination of antimicrobial susceptibility
All strains were analysed for antimicrobial susceptibility with the Kirby-Bauer disk diffusion method on Mueller-Hinton agar including the antibiotics cefoxitin, clindamycin, erythromycin, penicillin, linezolid, ciprofloxacin, tetracycline, trimethoprim-sulfamethoxazole, tigecycline, gentamicin, and vancomycin (Oxoid, Basingstoke, United Kingdom) according to the 2015 European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines (www.eucast.org). MRSA isolates were additionally tested for vancomycin resistance by E-test [17]. Isolates exhibiting resistance to three or more antimicrobial classes were defined as multidrug resistant (MDR), as described before [18].
Molecular typing of S. aureus strains and the presence of virulence factors
S. aureus isolates were discriminated by spa-typing as described before [19]. The spa-types and clonal complexes (CC) were determined using the StaphType software and the Ridom SpaServer (http://spaserver.ridom.de/). Unknown spa-types were uploaded and assigned to new spa-types by the Ridom StaphType software (http://spaserver.ridom.de/). Multi-locus-sequence-typing (MLST) was performed on all isolates with new spa-types and on those, which could not be assigned to a CC by the Ridom spa server. For MLST typing, the DNA sequence analyses were carried out employing the S. aureus MLST site (http://saureus.mlst.net/).
For spa-typing, a cell suspension was heated for 10 minutes at 95°C. Cell debris was pelleted by centrifugation (5 min, 20,000 rpm, RT). Amplification of the spa gene was achieved by using standard primers as previously described [19].
For MLST PCRs, chromosomal DNA was purified using the PrestoSpin D BUG Kit (Molzym, Bremen, Germany) according to the suppliers’ instructions. For cell lysis, the buffer was supplemented with 10 μl lysostaphin (5 mg/ml; Genmedics, Reutlingen, Germany). Gene amplification was executed as previously described [20].
All strains were additionally tested by PCR for the presence of the toxic shock syndrome toxin (TSST) and the lukFS-genes encoding both subunits of the Panton-Valentine leukocidin (PVL) [21,22]. Typing of the Staphylococcal cassette chromosome mec (SCCmec) and screening for the presence of mecA and mecC has been performed for all MRSA isolates by PCR as previously described [23,24].
In all PCR reactions the high fidelity Phusion polymerase (Finnzymes, New England Biolabs, Ipswich, USA) was used according to the suppliers’ manual. Prior to sequencing (Seqlab, Göttingen, Germany), all PCR products were purified with the GeneJET gel extraction kit (Thermo Scientific, Rochester, USA).
Epidemiological analysis
Categorical variables were described as frequencies and percentages. Continuous variables were described using medians and their corresponding interquartile ranges (IQRs). The Student’s t-test and chi-square test were used to compare means and proportions respectively, with a p-value <0.05 being considered statistically significant. All data analyses were performed with Stata 14 (StataCorp LP, College Station, USA).
Results
Nasal swabs from 544 children were collected during the study period. The most common causes for admission were malaria (57.7%), lower respiratory tract infections (31.1%), urinary tract infections (16.7%) and gastrointestinal infections (13.6%). The median age of patients was 2 (IQR: 1–5) years and 255 (46.9%) study children were female. In total 120 (22.1%) swabs were positive for S. aureus of which 3 revealed two different S. aureus isolates, resulting in a total of 123 S. aureus isolates. S. aureus carriage was similarly distributed (p = 0.33) among girls (n = 61/255; 23.9%) and boys (n = 59/289; 20.4%) (Table 1).
Table 1. Demographic characteristics of study participants.
| Characteristics | Total (%) | S. aureus carrier (%) | non-S.aureus carrier (%) |
|---|---|---|---|
| (N = 544) | (N = 120) | (N = 424) | |
| Sex, female (%) | 255 (46.9) | 61 (50.8) | 194 (45.8) |
| Age, median months (IQR) | 32 (17–60) | 41 (24–78) | 29 (15–52) |
IQR, interquartile range.
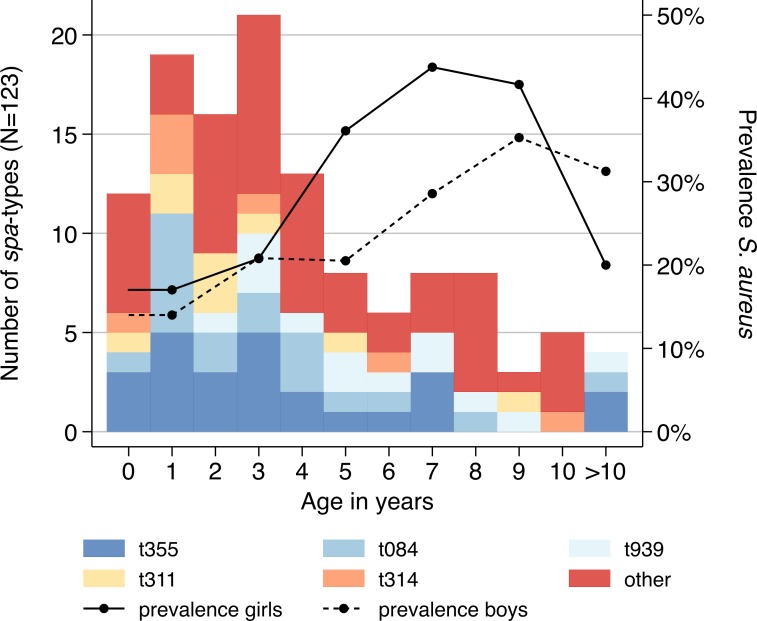
S. aureus carriers had a higher median age of 3 years (IQR: 2–6) compared to children without S. aureus colonization (median = 2 years; IQR = 1–4; p = 0.004). In both, girls and boys, the prevalence of S. aureus increased with age, however female patients seemed to be colonised at an earlier age (Fig 1). However, case numbers for each age group were not sufficient to perform significance testing. The highest S. aureus prevalence in girls was observed in the age-group 6–8 years (n = 7; 43.7%) and in boys in the age-group 8–10 years (n = 6; 35.2%). Above the age of 10, carriage rates dropped in both sexes. Apart from nine carriers with not further characterized skin infections, there were no indications for a link between S. aureus carriage and an underlying infection.
Fig 1. Age and sex distribution of spa-types.
Distribution of spa-types (n = 123) and nasal Staphylococcus aureus prevalence by 2-years age-groups and sex (n = 544). Girls (continuous line) are colonized when they are approximately 2 years younger than boys (dashed line).
The 123 isolates were classified into 35 different spa-types and 19 sequence types (ST). The most commonly detected spa-types (i.e., n >5) were t355 (n = 25; 20.3%), t084 (n = 18; 14.6%), t939 (n = 13; 10.6%), t311 (n = 9; 7.3%) and t314 (n = 7; 5.7%), corresponding to ST152, ST15, ST45 and ST121 (Table 2). Two isolates were newly assigned to the two spa-types t15727 and t15728, which belong to ST508 and ST707, respectively. Based on spa-typing, the following CCs were identified: CC152 (n = 36), CC15 (n = 21), CC45 (n = 19), CC121 (n = 19), CC1 (n = 6), CC30 (n = 6), CC8 (n = 5), CC88 (n = 3), CC5 (n = 1). All spa-types were randomly distributed throughout age, except t939, which only occurred in children above the age of 1 year (Fig 1).
Table 2. Characterization of nasal Staphylococcus aureus isolates (n = 123).
| CC | ST | spa types (n) | MRSA [n(%)] | MDR [n(%)] | PVL [n(%)] | TSST [n(%)] |
|---|---|---|---|---|---|---|
| CC1 | ST1 | t127 (3), t591 (1) | 0 (0.0) | 0 (0.0) | 2 (66.7) | 2 (66.7) |
| ST72 | t537 (2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (50.0) | |
| CC121 | ST121 | t311 (9), t314 (7), t645 (1), t1114 (2) | 0 (0.0) | 3 (15.8) | 13 (68.4) | 2 (10.5) |
| CC15 | ST15 | t84 (18), t085 (1), t346 (1), t385 (1) | 0 (0.0) | 2 (9.5) | 14 (66.7) | 3 (14.3) |
| CC152 | ST152 | t1096 (3), t355 (25), t1123 (2), t1172 (1), t1299 (4) | 1 (2.9) | 7 (20.0) | 30 (85.7) | 3 (8.6) |
| ST377 | t5047 (1) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | |
| CC30 | ST30 | t318 (1), t363 (3), t2147 (1) | 0 (0.0) | 0 (0.0) | 4 (80.0) | 0 (0.0) |
| ST30, ST33, ST55 | t21 (1) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | |
| CC45 | ST45 | t939 (13), t157 (1), t4454 (5) | 1 (5.3) | 1 (5.3) | 3 (15.8) | 1 (5.3) |
| CC508 | ST508 | t15727 (1), t1510 (1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (100.0) |
| CC5 | ST5 | t450 (1) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) |
| CC707 | ST707 | t15728 (1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) |
| CC8 | ST8 | t8 (3), t1476 (1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| ST18 | t451 (1) | 0 (0.0) | 1 (100.0) | 1 (100.0) | 0 (0.0) | |
| CC88 | ST88 | t186 (1), t4104 (2) | 0 (0.0) | 0 (0.0) | 3 (100.0) | 0 (0.0) |
| singleton | ST3248 | t6063 (2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (100.0) |
| singleton | ST944 | t616 (2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
CC: Clonal Complex; ST: Sequence Type; MRSA: Methicillin-resistant Staphylococcus aureus; MDR: Multidrug resistant; PVL: Panton-Valentine leukocidin; TSST: Toxic shock syndrome toxin.
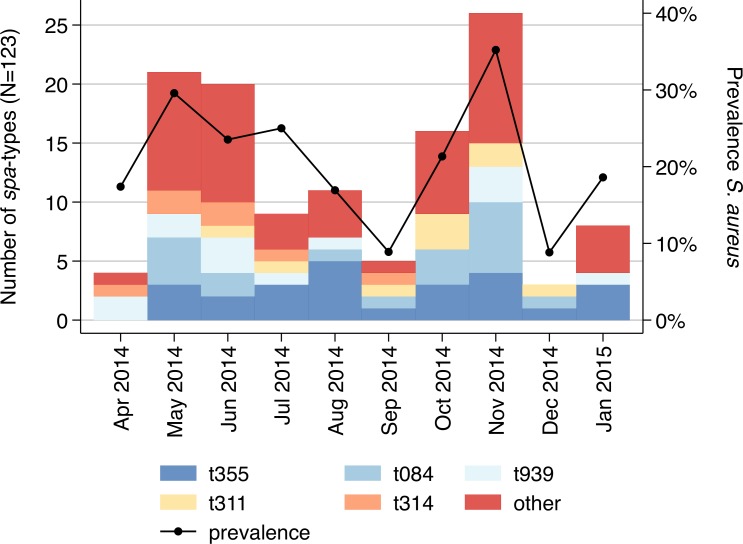
The S. aureus prevalence revealed seasonal fluctuations in nasal carriage (Fig 2). The highest carriage rates were observed during May (n = 21; 29.6%) and November (n = 25; 35.2%), which corresponds to peaks of the two rainy seasons within the study area (p = 0.007). Notably, the frequent spa-types (i.e., n >5) occurred throughout the whole study period, while the more uncommon spa-types were primarily observed during the months of the rainy seasons.
Fig 2. Temporal distribution of spa-types.
Distribution of spa-types (n = 123) by month. The black line indicates the Staphylococcus aureus prevalence (n = 544).
PVL and TSST-1 were detected in 71 (57.7%) and 17 (13.8%) isolates respectively. Among all STs, which contain five or more isolates, ST152 (n = 30; 85.7%) and ST30 (n = 4; 80.0%) revealed the highest PVL positivity and ST15 the highest proportion of TSST-1 positive isolates (n = 3; 14.3%).
Two (1.6%) isolates were MRSA (SCCmec type IVa), classified as t1096 (ST152) and t4454 (ST45). MDR, defined as resistance to three or more different antimicrobial classes, was detected in 16 (13%) isolates. High MDR prevalences existed in ST152 (n = 7; 20.0%) and ST121 (n = 3; 15.8%). The highest resistance rates were found for penicillin (n = 117; 95.1%), tetracycline (n = 66; 53.7%) and erythromycin (n = 14; 11.4%) (Table 3). All isolates were susceptible to linezolid and vancomycin.
Table 3. Antibiotic resistance profile of nasal Staphylococcus aureus isolates (n = 123).
| Antibiotic | Resistance [n (%)] | |
|---|---|---|
| Penicillin | 117 | (95.1) |
| Tetracycline | 66 | (53.7) |
| Erythromycin | 14 | (11.4) |
| Clindamycin | 7 | (5.7) |
| Trimethoprim-sulfamethoxazole | 4 | (3.3) |
| Gentamicin | 3 | (2.4) |
| Cefoxitin | 2 | (1.6) |
| Ciproflocacin | 2 | (1.6) |
| Linezolid | 0 | (0.0) |
| Tigecycline | 0 | (0.0) |
| Vancomycin | 0 | (0.0) |
| Multidrug resistance§ | 16 | (13.0) |
§ Resistance to three or more antimicrobial classes.
Discussion
The present study identifies an age- (p = 0.004) and season-dependent (p = 0.007) nasal S. aureus colonisation and genotype pattern among children living in rural Ghana. Similar age-dependent parabolic carriage rates have been described in the Netherlands, however peak incidences occurred at a slightly older age (10 years) [5]. Earlier colonisation after the first year of life might be caused by different exposures in rural Ghana compared to industrialized countries. For instance large family sizes and lower socio-economic development have been associated with S. aureus carriage [7,25]. However, the total rate of S. aureus carriers in the present study (22.1%) tends to be lower than in industrialized countries, such as the United States (36.9%; age-group 1–19 years) and the Netherlands (36,0%; age-group 1–19 years) [5,26]. Children aged 2–15 years from The Gambia revealed a carriage rate of 27.3% [27]. Other carriage studies from West Africa do not allow a comparison, as either children were not included or rates were not stratified by age-groups [9,11,28].
Differences might also be attributed to the variable nasal carriage of Streptococcus pneumoniae and Haemophilus influenzae, which previously showed a negative correlation for co-colonisation with S. aureus [5,8,29,30]. This association has been discussed in the context of lower S. aureus carriage rates among underprivileged populations, such as indigenous Australians, Hispanic Americans and Bedouin Israelis compared to the rest of the population [8,26,30].
Co-colonisation might play a role in rural Ghana, in particular for the observed seasonal distribution, with prevalence peaks during the two rainy seasons, which occur from May to June and from October to November (http://www.meteo.gov.gh). In Israel the highest S. aureus prevalence was observed during summer, when S. pneumoniae and H. influenzae carriage were the lowest [8]. In Sweden and the United Kingdom prevalence rates culminated in spring and autumn, respectively, when viral respiratory infections are on the rise [31,32]. Similarly, an increase of enveloped respiratory viruses occurs during the rainy seasons in Ghana [33]. To our knowledge no seasonality data for S. aureus colonisation is yet available for West Africa.
This study reports equal S. aureus carriage rates for boys and girls, the latter colonized when they are approximately two years younger than male study participants. Interestingly, male sex has been repeatedly identified as a risk factor for carriage in industrialized countries [5,8,26], while most studies from sub-Sahara Africa did not observe any difference [9,10,27].
The two most frequently detected spa-types, t355 and t084, have been described before as the major S. aureus lineages in asymptomatic carriers as well as in clinical isolates from Ghana [9,12]. Indeed, those spa-types have always been among the most frequently detected in nasal carriage studies throughout West Africa [11,28]. Spa-type t939, with a prevalence of 11% being the third most common in this study, has only been detected sporadically in other West and Central African countries, such as Angola, Gabon, Nigeria and São Tomé and Príncipe [34–37]. In Ghana, t939 isolates have been only reported once from a nasal carrier and from the wounds of two patients with Buruli ulcer [9,38]. In the past spa-type t939 has been associated with transmission among pig farms in the Netherlands [39]. Livestock associated transmission might be one explanation for the exclusive detection of this spa-type in children above one year in the present study.
The MRSA prevalence among nasal carriers is below two percent, which is similar to what has been previously described in Ghana [9,12]. The detected MRSA spa-types t1096 and t4454 belong to CC152 and CC45 respectively, which are well-described MRSA CCs, circulating on the African continent [40]. However, the spa-type t1096 has so far only been detected in a methicillin susceptible S. aureus (MSSA) isolate in Ghana whereas a methicillin resistant t4454 S. aureus has not been described before in Africa. Compared to a nasal carriage study from Ghana, conducted between 2011 and 2012, MDR rates seem to increase from 6% reported previously to 13% detected in the present study [9]. Similarly, penicillin and erythromycin resistance rate increase from 91% to 95% and from 2% to 11%, respectively [9]. These two studies have been conducted in different areas; therefore those trends must be interpreted cautiously. A high tetracycline resistance has been previously found in a rural community (50%) in Ghana when compared to an urban setting (18%) [9]. The authors suggested the common use of tetracycline on Ghanaian livestock farms to be correlated to this finding.
PVL is highly prevalent (58%) among the collected isolates, similar to those from a remote Gabonese Pigmy population (56%) [41]. Other carriage studies from sub-Sahara Africa reported a lower PVL prevalence ranging from 8% in Angola to 36% in São Tomé and Príncipe [9,11,36,37]. Even lower PVL rates have been reported from the United States with 1% among MSSA [42]. The observed high number of PVL-positive MSSA in combination with an increased risk of human-to-human transmission due to poor sanitary conditions and overcrowding in rural Ghana could pose a serious threat for the generation of PVL-positive MRSA clones through horizontal gene transfer.
The study presented here has a few limitations. The recruitment was hospital-based and only children admitted to the paediatric ward were included into the study, which may therefore not be representative for the local healthy community. Antibiotic consumption, previous hospital stays and other risk factors, which may explain the increasing MDR rates, have not been assessed in this study. The cross-sectional design of the study with a single nasal culture does not allow the classification of individuals as persistent or intermittent carriers. This distinction may be relevant, as persistent carriers have a higher risk to acquire S. aureus infections and patients with a negative nasal culture might actually be intermittent carriers.
Conclusion
The results of the present study suggest that nasal S. aureus carriage among children in Ghana is dependent on age and seasonality, with children above one year being colonized at a younger age compared to previous studies from industrialized countries. To what extent socioeconomic conditions, climatic factors or co-colonization with other pathogens play a role for nasal S. aureus carriage in this geographic region needs to be investigated in further studies. High PVL rates can be considered a serious threat for the development of virulent MRSA in the future. Already today, physicians must be aware of increasing antibiotic resistance among paediatric S. aureus isolates when comparing to previous Ghanaian studies.
Supporting Information
(TXT)
Acknowledgments
The authors thank Lisa Reigl and Thalea Tamminga for the excellent administrative support. We also thank Anna Jaeger for organizing the data management.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the German Center for Infection Research (Deutsches Zentrum für Infektionsforschung, DZIF). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sollid JUE, Furberg AS, Hanssen AM, Johannessen M. Staphylococcus aureus: determinants of human carriage. Infect Genet Evol. 2014;21: 531–41. 10.1016/j.meegid.2013.03.020 [DOI] [PubMed] [Google Scholar]
- 2.Nagel M, Dischinger J, Türck M, Verrier D, Oedenkoven M, Ngoubangoye B, et al. Human-associated Staphylococcus aureus strains within great ape populations in Central Africa (Gabon). Clin Microbiol Infect. 2013;19: 1072–1077. 10.1111/1469-0691.12119 [DOI] [PubMed] [Google Scholar]
- 3.Wertheim HFL, Vos MC, Ott A, van Belkum A, Voss A, Kluytmans JAJW, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet (London, England). 364: 703–5. [DOI] [PubMed] [Google Scholar]
- 4.Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7: 629–41. 10.1038/nrmicro2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogaert D, van Belkum A, Sluijter M, Luijendijk A, de Groot R, Rümke HC, et al. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet (London, England). 2004;363: 1871–2. [DOI] [PubMed] [Google Scholar]
- 6.Lebon A, Labout JAM, Verbrugh HA, Jaddoe VW V, Hofman A, van Wamel W, et al. Dynamics and determinants of Staphylococcus aureus carriage in infancy: the Generation R Study. J Clin Microbiol. 2008;46: 3517–21. 10.1128/JCM.00641-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mollema FPN, Richardus JH, Behrendt M, Vaessen N, Lodder W, Hendriks W, et al. Transmission of methicillin-resistant Staphylococcus aureus to household contacts. J Clin Microbiol. 2010;48: 202–7. 10.1128/JCM.01499-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewnard JA, Givon-Lavi N, Huppert A, Pettigrew MM, Regev-Yochay G, Dagan R, et al. Epidemiological markers for interactions among Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus in upper respiratory tract carriage. J Infect Dis. 2016;213: 1596–605. 10.1093/infdis/jiv761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egyir B, Guardabassi L, Esson J, Nielsen SS, Newman MJ, Addo KK, et al. Insights into nasal carriage of Staphylococcus aureus in an urban and a rural community in Ghana. PLoS One. 2014;9: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ateba Ngoa U, Schaumburg F, Adegnika AA, Kösters K, Möller T, Fernandes JF, et al. Epidemiology and population structure of Staphylococcus aureus in various population groups from a rural and semi urban area in Gabon, Central Africa. Acta Trop. 2012;124: 42–7. 10.1016/j.actatropica.2012.06.005 [DOI] [PubMed] [Google Scholar]
- 11.Fall C, Richard V, Dufougeray A, Biron A, Seck A, Laurent F, et al. Staphylococcus aureus nasal and pharyngeal carriage in Senegal. Clin Microbiol Infect. 2014;20: O239–41. 10.1111/1469-0691.12385 [DOI] [PubMed] [Google Scholar]
- 12.Egyir B, Guardabassi L, Sørum M, Nielsen SS, Kolekang A, Frimpong E, et al. Molecular epidemiology and antimicrobial susceptibility of clinical Staphylococcus aureus from healthcare institutions in Ghana. PLoS One. 2014;9: 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaumburg F, Alabi AS, Peters G, Becker K. New epidemiology of Staphylococcus aureus infection in Africa. Clin Microbiol Infect. 2014;20: 589–596. 10.1111/1469-0691.12690 [DOI] [PubMed] [Google Scholar]
- 14.De Boeck H, Vandendriessche S, Hallin M, Batoko B, Alworonga J-P, Mapendo B, et al. Staphylococcus aureus nasal carriage among healthcare workers in Kisangani, the Democratic Republic of the Congo. Eur J Clin Microbiol Infect Dis. 2015;34: 1567–72. 10.1007/s10096-015-2387-9 [DOI] [PubMed] [Google Scholar]
- 15.Ghebremedhin B, Olugbosi MO, Raji AM, Layer F, Bakare RA, König B, et al. Emergence of a community-associated methicillin-resistant Staphylococcus aureus strain with a unique resistance profile in Southwest Nigeria. J Clin Microbiol. 2009;47: 2975–80. 10.1128/JCM.00648-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breurec S, Zriouil SB, Fall C, Boisier P, Brisse S, Djibo S, et al. Epidemiology of methicillin-resistant Staphylococcus aureus lineages in five major African towns: emergence and spread of atypical clones. Clin Microbiol Infect. 2011;17: 160–5. 10.1111/j.1469-0691.2010.03219.x [DOI] [PubMed] [Google Scholar]
- 17.Fitzgibbon MM, Rossney AS, O’Connell B. Investigation of reduced susceptibility to glycopeptides among methicillin-resistant Staphylococcus aureus isolates from patients in Ireland and evaluation of agar screening methods for detection of heterogeneously glycopeptide-intermediate S. aureus. J Clin Microbiol. 2007;45: 3263–9. 10.1128/JCM.00836-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magiorakos A-P, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18: 268–81. 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 19.Koreen L, Ramaswamy S V, Graviss EA, Naidich S, Musser JM, Kreiswirth BN. Spa typing method for discriminating among Staphylococcus aureus isolates: implications for use of a single marker to detect genetic micro- and macrovariation. J Clin Microbiol. 2004;42: 792–9. 10.1128/JCM.42.2.792-799.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. 2000;38: 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell SJ, Deshmukh HS, Nelson CL, Bae I-G, Stryjewski ME, Federspiel JJ, et al. Genotypic characteristics of Staphylococcus aureus isolates from a multinational trial of complicated skin and skin structure infections. J Clin Microbiol. 2008;46: 678–84. 10.1128/JCM.01822-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becker K, Roth R, Peters G. Rapid and specific detection of toxigenic Staphylococcus aureus: use of two multiplex PCR enzyme immunoassays for amplification and hybridization of staphylococcal enterotoxin genes, exfoliative toxin genes, and toxic shock syndrome toxin 1 gene. J Clin Microbiol. 1998;36: 2548–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghaznavi-Rad E, Nor Shamsudin M, Sekawi Z, Van Belkum A, Neela V. A simplified multiplex PCR assay for fast and easy discrimination of globally distributed staphylococcal cassette chromosome mec types in meticillin-resistant Staphylococcus aureus. J Med Microbiol. 2010;59: 1135–1139. 10.1099/jmm.0.021956-0 [DOI] [PubMed] [Google Scholar]
- 24.Cuny C, Layer F, Strommenger B, Witte W. Rare occurrence of methicillin-resistant Staphylococcus aureus CC130 with a novel mecA homologue in humans in Germany. PLoS One. 2011;6: e24360 10.1371/journal.pone.0024360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wertheim H, Melles D. The Role of Nasal Carriage in Staphylococcus aureus Infections. Lancet Infect Dis. 2005;5: 751–762. 10.1016/S1473-3099(05)70295-4 [DOI] [PubMed] [Google Scholar]
- 26.Kuehnert MJ, Kruszon-Moran D, Hill HA, McQuillan G, McAllister SK, Fosheim G, et al. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001–2002. J Infect Dis. 2006;193: 172–9. 10.1086/499632 [DOI] [PubMed] [Google Scholar]
- 27.Ebruke C, Dione MM, Walter B, Worwui A, Adegbola RA, Roca A, et al. High genetic diversity of Staphylococcus aureus strains colonising the nasopharynx of Gambian villagers before widespread use of pneumococcal conjugate vaccines. BMC Microbiol. 2016;16: 38 10.1186/s12866-016-0661-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayepola OO, Olasupo NA, Egwari LO, Becker K, Schaumburg F. Molecular characterization and antimicrobial susceptibility of Staphylococcus aureus isolates from clinical infection and asymptomatic carriers in Southwest Nigeria. PLoS One. 2015;10: e0137531 10.1371/journal.pone.0137531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwambana BA, Barer MR, Bottomley C, Adegbola RA, Antonio M. Early acquisition and high nasopharyngeal co-colonisation by Streptococcus pneumoniae and three respiratory pathogens amongst Gambian new-borns and infants. BMC Infect Dis. 2011;11: 175 10.1186/1471-2334-11-175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watson K, Carville K, Bowman J, Jacoby P, Riley TV, Leach AJ, et al. Upper respiratory tract bacterial carriage in Aboriginal and non-Aboriginal children in a semi-arid area of Western Australia. Pediatr Infect Dis J. 2006;25: 782–90. 10.1097/01.inf.0000232705.49634.68 [DOI] [PubMed] [Google Scholar]
- 31.Harrison LM, Morris JA, Telford DR, Brown SM, Jones K. The nasopharyngeal bacterial flora in infancy: effects of age, gender, season, viral upper respiratory tract infection and sleeping position. FEMS Immunol Med Microbiol. 1999;25: 19–28. [DOI] [PubMed] [Google Scholar]
- 32.Nilsson P, Ripa T. Staphylococcus aureus throat colonization is more frequent than colonization in the anterior nares. J Clin Microbiol. 2006;44: 3334–9. 10.1128/JCM.00880-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Annan A, Ebach F, Corman VM, Krumkamp R, Adu-Sarkodie Y, Eis-Hübinger AM, et al. Similar Virus Spectra and Seasonality in Paediatric Patients with Acute Respiratory Disease, Ghana and Germany. Clin Microbiol Infect. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaumburg F, Alabi a S, Mombo-Ngoma G, Kaba H, Zoleko RM, Diop D a, et al. Transmission of Staphylococcus aureus between mothers and infants in an African setting. Clin Microbiol Infect. 2013; 1–7. [DOI] [PubMed] [Google Scholar]
- 35.Shittu AO, Okon K, Adesida S, Oyedara O, Witte W, Strommenger B, et al. Antibiotic resistance and molecular epidemiology of Staphylococcus aureus in Nigeria. BMC Microbiol. 2011;11: 92 10.1186/1471-2180-11-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conceição T, Santos Silva I, de Lencastre H, Aires-de-Sousa M. Staphylococcus aureus nasal carriage among patients and health care workers in São Tomé and Príncipe. Microb Drug Resist. 2014;20: 57–66. 10.1089/mdr.2013.0136 [DOI] [PubMed] [Google Scholar]
- 37.Conceição T, Coelho C, Santos-Silva I, de Lencastre H, Aires-de-Sousa M. Epidemiology of methicillin-resistant and -susceptible Staphylococcus aureus in Luanda, Angola: first description of the spread of the MRSA ST5-IVa clone in the African continent. Microb Drug Resist. 2014;20: 441–9. 10.1089/mdr.2014.0007 [DOI] [PubMed] [Google Scholar]
- 38.Amissah NA, Glasner C, Ablordey A, Tetteh CS, Kotey NK, Prah I, et al. Genetic diversity of Staphylococcus aureus in Buruli ulcer. PLoS Negl Trop Dis. 2015;9: e0003421 10.1371/journal.pntd.0003421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Duijkeren E, Ikawaty R, Broekhuizen-Stins MJ, Jansen MD, Spalburg EC, de Neeling AJ, et al. Transmission of methicillin-resistant Staphylococcus aureus strains between different kinds of pig farms. Veterinary Microbiology. 2008. [DOI] [PubMed] [Google Scholar]
- 40.Abdulgader SM, Shittu AO, Nicol MP, Kaba M. Molecular epidemiology of Methicillin-resistant Staphylococcus aureus in Africa: a systematic review. Front Microbiol. 2015;6: 348 10.3389/fmicb.2015.00348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaumburg F, Köck R, Friedrich AW, Soulanoudjingar S, Ngoa UA, von Eiff C, et al. Population structure of Staphylococcus aureus from remote African Babongo Pygmies. PLoS Negl Trop Dis. 2011;5: e1150 10.1371/journal.pntd.0001150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuehnert MJ, Kruszon-Moran D, Hill HA, McQuillan G, McAllister SK, Fosheim G, et al. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001–2002. J Infect Dis. 2006;193: 172–9. 10.1086/499632 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TXT)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.