Abstract
The function of lytic peptidoglycan transglycosylases is poorly understood. Single lytic transglycosylase mutants of Escherichia coli have no growth phenotype. By contrast, mutation of Neisseria gonorrhoeae ltgC inhibited cell separation without affecting peptidoglycan monomer production. Thus, LtgC has a dedicated function in gonococcal cell division.
Lytic transglycosylases act to cleave peptidoglycan (PG) and are thought to function in the removal of PG strands for cell wall remodeling during bacterial growth and division (9). However, mutations in single or multiple lytic transglycosylase genes do not inhibit growth or division in Escherichia coli (11). Only a strain with all known lytic transglycosylases mutated was reported to have a slight division phenotype, growing in groups of approximately three to eight cells (8). Neisseria gonorrhoeae is a gram-negative coccus that normally grows as a diplococcus or single coccus and releases soluble PG fragments during growth. The major fragments released are the 1,6-anhydrodisaccharide tripeptide monomer and the 1,6-anhydrodisaccharide tetrapeptide monomer (16). These PG fragments have potent biological effects, including killing ciliated fallopian tube cells (13), inducing inflammatory cytokine production (4), and causing arthritis (5). Previous studies in our laboratory found that mutations affecting lytic transglycosylases LtgA and LtgB lower PG monomer production but do not alter cell division (2; K. A. Cloud, E. T. Beck, and J. P. Dillard, submitted for publication). We are mutating and characterizing putative lytic transglycosylases in order to determine which enzymes are responsible for PG fragment production and release.
Identification of N. gonorrhoeae lytic transglycosylase C.
N. gonorrhoeae encodes a membrane-bound lytic transglycosylase A (MltA) homologue, which we have designated lytic transglycosylase C (LtgC). LtgC exhibits 21% identity and 31% similarity to E. coli MltA and contains a consensus lipoprotein site, suggesting that, like many other PG hydrolases, LtgC may be a lipoprotein. A close homologue of LtgC (known as GNA33) has been studied in Neisseria meningitidis. Jennings et al. determined that GNA33 has lytic transglycosylase activity and is a lipoprotein when expressed in E. coli (10).
Creation of an ltgC mutant and complemented strain.
A combination of positive and negative selection was used to create a 33-bp deletion at the 5′ end of ltgC that removed the putative start codon. ltgC was amplified from N. gonorrhoeae MS11 chromosomal DNA by using specific primers 5′ ATTGCCTGCCGCCGGTTTATAG 3′ and 5′ AAGAAACGCCATACCGACCAAG 3′ and inserted into pKC1 (2), forming pKC11 (Table 1). Through several steps, an ermC-rpsL cassette was inserted into an internally deleted ltgC, forming pKC17. This plasmid was transformed into N. gonorrhoeae by the method of Gunn and Stein (6), and transformants were selected with 10 μg of erythromycin/ml. A deletion in the 5′ end of ltgC was formed by digesting pKC11 with BsaXI, blunting it with T4 DNA polymerase, and ligating the DNA to form pKC19. This plasmid was transformed into the ltgC insertion strain in order to replace the ermC-rpsL cassette with the ltgC deletion. Streptomycin resistance at 100 μg/ml was used to select for loss of the original insertion.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Properties | Reference or source |
|---|---|---|
| pHSS6 | E. coli cloning vector (Kanr) | 15 |
| pKH23 | Gonococcal complementation vector (Camr) derived from pGCC6 | K. T. Hackett |
| pKC1 | N. gonorrhoeae insertion-duplication, positive-negative selection plasmid (Ermr Strs) | 2 |
| pKC11 | ltgC PCR fragment (MfeI; NruI) ligated into pKC1 at EcoRI and SmaI sites (Ermr Strs) | This work |
| pKC13 | ltgC internal deletion; pKC11 digested with StyI (Ermr Strs) | This work |
| pKC15 | ltgC internal deletion subcloned into pHSS6 (Kanr) | This work |
| pKC17 | ltgC disruption plasmid; ermC-rpsL cloned into FseI site of pKC15; Kanr Ermr Strs | This work |
| pKC19 | 5′ deletion of ltgC; pKC11 digested with BsaXI, blunted (Ermr Strs) | This work |
| pKC22 | ltgC complementation vector; ltgC cloned into NheI and NsiI sites of pKH23 (Camr) | This work |
| MS11 | Wild-type N. gonorrhoeae (Strr) | 14 |
| KC118 | MS11ltgC | This work |
| KC124 | MS11ltgC complemented (Camr) | This work |
A complemented strain was constructed by inserting a wild-type copy of ltgC at a distant location on the gonococcal chromosome. To create the complementation construct, ltgC and 211 bp of 5′ DNA were amplified by PCR with primers 5′ GACTAGTGACGGGCTTCGGACGGCA 3′ and 5′ GCGATGCATTAAACGCGAATGAACAAGG 3′ and cloned into pKH23, forming pKC22. The complementation plasmid is derived from pGCC6 and allows incorporation of the introduced gene into the gonococcal chromosome between aspC and lctP (12). Following transformation of KC118 with pKC22 and selection with chloramphenicol at 10 μg/ml, the desired strain (KC124) was identified using PCR by screening for both retention of the ltgC mutation and incorporation of ltgC at the alternate location.
Mutation of ltgC affects gonococcal growth.
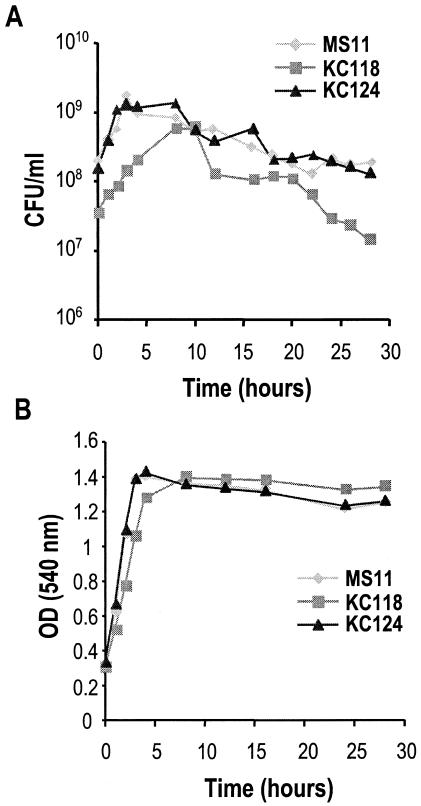
Colonies of KC118 appeared slightly smaller on an agar plate, and the mutant did not appear to grow rapidly when inoculated into liquid culture. Gonococci were grown with aeration in gonococcal base liquid medium as previously described (2). When inoculated at equivalent optical densities, both the number of CFU per milliliter (Fig. 1B) and the optical density of the mutant culture (Fig. 1A) were below those of the wild-type and complemented strains. Measurement of total protein in the cultures by the Bio-Rad protein assay showed that MS11ltgC and wild-type strains accumulated equivalent amounts of protein during the initial 4 h of log phase. Thus, the protein accumulation in the culture reflected the fact that the cells were growing to equivalent levels, whereas the numbers of CFU per milliliter differed by 5- to 10-fold. These results suggested that mutation of ltgC resulted in decreased cell viability or prevented cell division.
FIG. 1.
Mutation of ltgC affects growth characteristics. Aliquots of MS11, MS11ltgC, and MS11ltgC (complemented) cultures were taken, and CFU per milliliter (A) and optical density at 540 nm (B) were determined. Graphs shown are representative of three separate trials.
The differences in viable gonococci seen at various time points between MS11 and MS11ltgC could be due to an increased level of cell lysis in the mutant. MS11ltgC was found to undergo autolysis more than the wild type. Bacterial viability was determined by using a Live/Dead BacLight bacterial viability kit (Molecular Probes). Cultures were diluted to an optical density at 540 nm of 0.3, equivalent to 1.2 × 108 CFU/ml for the wild-type strain. Aliquots were washed in 0.1 M MOPS (morpholinepropanesulfonic acid)-1 mM MgCl2 (pH 7.2). After 2 h of growth, threefold-more dead bacteria were present in cultures of MS11ltgC (24.1% dead) than in those of the wild type (7.9% dead). Complementation of ltgC restored the wild-type phenotype; similar numbers of dead bacteria were seen in cultures of KC124 (4.2% dead) and MS11 (data not shown).
Mutation of ltgC alters septation and cell separation in N. gonorrhoeae.
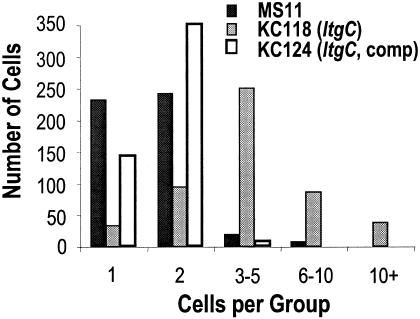
A disruption in cell division or separation would be one explanation for the decreased level of CFU per milliliter seen in cultures of MS11ltgC. To investigate this possibility, gonococci were grown overnight on gonococcal base plates and thin sections of each strain were prepared for transmission electron microscopy as described by Mehr et al. (12). Mutation of ltgC altered septation and division in N. gonorrhoeae, resulting in septa that were wavy (Fig. 2A) and thickened (Fig. 2B). Cells of MS11ltgC did not separate properly (Fig. 2B and E). Similar to the cell viability staining results, more lysed cells were seen in the preparations of MS11ltgC than in those of the wild type (Fig. 2E). Complementation of ltgC reversed these phenotypes (Fig. 2C and F); the morphology of the complemented strain was indistinguishable from that of MS11 (Fig. 2D). To quantify the cell separation defect, 500 cells of MS11, MS11ltgC, and the complemented strain were viewed. The number of cells per group and the number of groups of that size were counted (Fig. 3). Cells of MS11 and the complemented strain were mainly found growing in groups of one or two cells. Cells of MS11ltgC were mainly present in groups of three to five cells, and this was the only strain for which groups containing more than 10 cells were seen. Since thin sections were used for this analysis, additional bacteria outside the plane of the section were missed. Therefore, the number of bacteria in the aggregates is likely to exceed those counted.
FIG. 2.
Mutation of ltgC alters septation and cell separation. Thin-section transmission electron micrographs of MS11ltgC show irregular, wavy septa (A) or incomplete cell separation and thickened septa (B). Micrographs of MS11ltgC (complemented) (C) show that it is indistinguishable from the wild-type cell morphology of MS11 (D). Low-magnification micrographs of MS11ltgC (E) demonstrate that a majority of cells show cell separation defects; these are not seen in the ltgC-complemented strain (F).
FIG. 3.
Mutation of ltgC disrupts cell separation. Cells of MS11ltgC were seen growing in larger clusters than those of the wild type. Complementation of ltgC restored normal growth patterns.
Effects of ltgC mutation on PG fragment release.
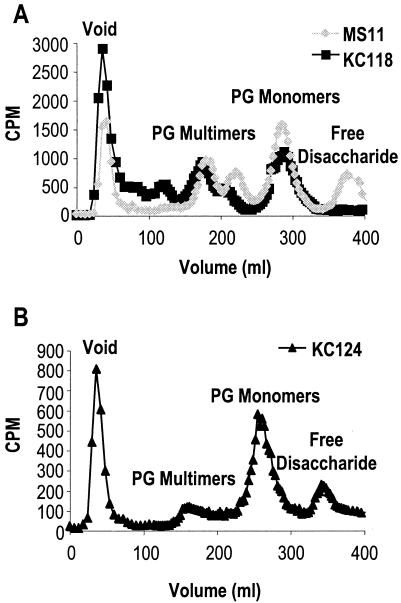
To determine if LtgC functions in the production or release of PG monomers, PG from MS11 and KC118 was metabolically labeled with [6-3H]glucosamine, and released PG fragments were collected and analyzed by size-exclusion chromatography as previously described (2). PG monomer release was not significantly reduced in the ltgC mutant, showing at most a slight decrease (Fig. 4A). Similarly, larger PG fragments were slightly increased. However, the most striking difference from the wild-type profile was the absence of released free disaccharide in the MS11ltgC profile (Fig. 4A). Free disaccharide release was restored by complementation of ltgC (Fig. 4B). Free disaccharides are predicted to be released by the combined action of a lytic transglycosylase and an amidase (7). Therefore, this result suggests that either the ltgC mutant is deficient in amidase activity or LtgC must first cleave the glycosidic bond before an amidase can act to remove the disaccharide from the peptide.
FIG. 4.
Mutation of ltgC alters the profile of released PG fragments. (A) 3H-labeled PG fragments were collected and separated by gel filtration chromatography. Mutation of ltgC inhibits the release of free disaccharide by growing gonococci. (B) Complementation of ltgC restores free disaccharide release.
If LtgC acts as a major contributor to PG fragment release, then ltgC mutants should show a lower rate of PG turnover, and more of the original PG should remain in the macromolecular PG than is maintained by the wild-type strain. However, soluble PG fragments were released into the medium at a higher rate in the ltgC mutant than in the wild type. PG turnover was measured for the wild-type, ltgC mutant, and complemented strains as described previously (2). After 4 h, only 51.2% ± 4.3% of macromolecular PG remained in KC118. This value was significantly different from that of the wild type (66.1% ± 8.0%) or the complemented strain (76.6% ± 3.3%) as determined by Student's t test, P < 0.05. These values are the averages of three experiments, and the error values are the standard errors. Although it is possible that the rate of turnover is enhanced in the ltgC mutant, we suspect that cell lysis during the first 4 h of growth accounts for this result. The macromolecular PG in lysed cells may not be as efficiently recovered by centrifugation as in whole cells. The appearance of high-molecular-weight PG fragments between the void peak and the PG multimer peaks in the profile of the ltgC mutant (Fig. 4A) is consistent with this idea.
Conclusions.
Mutation of ltgC resulted in cells that did not separate, had abnormal septa, and exhibited abnormal growth characteristics. Normal septation, growth, and cell separation were restored by the addition of a wild-type ltgC at a distant location on the chromosome. Thus, it is clear that ltgC is required for normal cell division and separation processes. Given its similarity to known lytic PG transglycosylases, we predict that LtgC functions in the removal of PG strands for splitting of the cell wall during cell division. Because of the severity of the defect seen in the thin-section electron micrographs, it is somewhat surprising that the mutants are viable and capable of exponential growth. The high degree of autolysis seen in the ltgC mutant may allow cells to split off from a cluster, thereby facilitating cell separation. In support of this hypothesis, many of the MS11ltgC cells in Fig. 2E have attached, lysed cells.
Recently, a report was published describing the effects of mutation of gna33 in N. meningitidis (1). The gna33 mutants did not separate well, released outer membrane proteins into the culture to a higher degree than the wild type did, and were avirulent in a rat model of septicemia (1). These results in N. meningitidis are similar to ours, suggesting that GNA33 and LtgC likely perform similar functions in these closely related species. The high degree of autolysis seen in ltgC mutants may also occur in gna33 mutants and could explain the additional protein release noted by Adu-Bobie et al (1).
The action of LtgC differs from that of the other gonococcal lytic transglycosylases that we have studied and from that of E. coli lytic transglycosylases. Mutation of ltgC does not greatly impact the release of PG monomers. Also, the other gonococcal lytic transglycosylase mutants are reduced, not increased, in autolysis (2, 3; Cloud et al., submitted). In E. coli, growth irregularities linked to the deletion of a single lytic transglycosylase have not been seen in the multiple investigations of these enzymes (11). By contrast, inactivation of LtgC alone alters gonococcal growth and inhibits cell separation. These data suggest that LtgC is a promising target for antimicrobials.
Acknowledgments
This work was supported by NIH grant AI47958 to J.P.D.
We thank Randall Massey and Ben August of the University of Wisconsin—Madison Medical School Electron Microscope Facility for producing the electron micrographs. We acknowledge the Gonococcal Genome Sequencing Project supported by USPHS-NIH grant AI38399 and B. A. Roe, L. Song, S. P. Lin, X. Yuan, S. Clifton, T. Ducey, L. Lewis, and D. W. Dyer of the University of Oklahoma.
REFERENCES
- 1.Adu-Bobie, J., P. Lupetti, B. Brunelli, D. Granoff, N. Norais, G. Ferrari, G. Grandi, R. Rappuoli, and M. Pizza. 2004. GNA33 of Neisseria meningitidis is a lipoprotein required for cell separation, membrane architecture, and virulence. Infect. Immun. 72:1914-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cloud, K. A., and J. P. Dillard. 2002. A lytic transglycosylase of Neisseria gonorrhoeae is involved in peptidoglycan-derived cytotoxin production. Infect. Immun. 70:2752-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dillard, J. P., and H. S. Seifert. 1997. A peptidoglycan hydrolase similar to bacteriophage endolysins acts as an autolysin in Neisseria gonorrhoeae. Mol. Microbiol. 25:893-901. [DOI] [PubMed] [Google Scholar]
- 4.Dokter, W. H. A., A. J. Dijkstra, S. B. Koopmans, B. K. Stulp, W. Keck, M. R. Halie, and E. Vellenga. 1994. G(Anh)MTetra, a natural bacterial cell wall breakdown product, induces interleukin-1β and interleukin-6 expression in human monocytes. J. Biol. Chem. 269:4201-4206. [PubMed] [Google Scholar]
- 5.Fleming, T. J., D. E. Wallsmith, and R. S. Rosenthal. 1986. Arthropathic properties of gonococcal peptidoglycan fragments: implications for the pathogenesis of disseminated gonococcal disease. Infect. Immun. 52:600-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunn, J. S., and D. C. Stein. 1996. Use of a nonselective transformation technique to construct a multiply restriction/modification-deficient mutant of Neisseria gonorrhoeae. Mol. Gen. Genet. 251:509-517. [DOI] [PubMed] [Google Scholar]
- 7.Heidrich, C., M. F. Templin, A. Ursinus, M. Merdanovic, J. Berger, H. Schwarz, M. A. de Pedro, and J.-V. Höltje. 2001. Involvement of N-acetylmuramyl-l-alanine amidases in cell separation and antibiotic-induced autolysis of Escherichia coli. Mol. Microbiol. 41:167-178. [DOI] [PubMed] [Google Scholar]
- 8.Heidrich, C., A. Ursinus, J. Berger, H. Schwarz, and J.-V. Höltje. 2002. Effects of multiple deletions of murein hydrolases on viability, septum cleavage, and sensitivity to large toxic molecules in Escherichia coli. J. Bacteriol. 184:6093-6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Höltje, J.-V. 1998. Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli. Microbiol. Mol. Biol. Rev. 62:181-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jennings, G. T., S. Savino, E. Marchetti, B. Arico, T. Kast, L. Baldi, A. Ursinus, J.-V. Höltje, R. A. Nicholas, R. Rappuoli, and G. Grandi. 2002. GNA33 from Neisseria meningitidis serogroup B encodes a membrane-bound lytic transglycosylase (MltA). Eur. J. Biochem. 269:3722-3731. [DOI] [PubMed] [Google Scholar]
- 11.Lommatzsch, J., M. F. Templin, A. R. Kraft, W. Vollmer, and J.-V. Höltje. 1997. Outer membrane localization of murein hydrolases: MltA, a third lipoprotein lytic transglycosylase in Escherichia coli. J. Bacteriol. 179:5465-5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehr, I. J., C. D. Long, C. D. Serkin, and H. S. Seifert. 2000. A homologue of the recombination-dependent growth gene, rdgC, is involved in gonococcal pilin antigenic variation. Genetics 154:523-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melly, M. A., Z. A. McGee, and R. S. Rosenthal. 1984. Ability of monomeric peptidoglycan fragments from Neisseria gonorrhoeae to damage human fallopian-tube mucosa. J. Infect. Dis. 149:378-386. [DOI] [PubMed] [Google Scholar]
- 14.Segal, E., E. Billyard, M. So, S. Storzbach, and T. F. Meyer. 1985. Role of chromosomal rearrangement in N. gonorrhoeae pilus phase variation. Cell 40:293-300. [DOI] [PubMed] [Google Scholar]
- 15.Seifert, H. S., E. Y. Chen, M. So, and F. Heffron. 1986. Shuttle mutagenesis: a method of transposon mutagenesis for Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 83:735-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinha, R. K., and R. S. Rosenthal. 1980. Release of soluble peptidoglycan from growing gonococci: demonstration of anhydro-muramyl-containing fragments. Infect. Immun. 29:914-925. [DOI] [PMC free article] [PubMed] [Google Scholar]