Abstract
Seed germination is the key step for successful establishment, growth and further expansion of population especially for alien plants with annual life cycle. Traits like better adaptability and germination response were thought to be associated with plant invasion. However, there are not enough empirical studies correlating adaptation to environmental factors with germination response of alien invasive plants. In this study, we conducted congeneric comparisons of germination response to different environmental factors such as light, pH, NaCl, osmotic and soil burials among four alien amaranths that differ in invasiveness and have sympatric distribution in Jiangsu Province, China. The data were used to create three-parameter sigmoid and exponential decay models, which were fitted to cumulative germination and emergence curves. The results showed higher maximum Germination (Gmax), shorter time for 50% germination (G50) and the rapid slope (Grate) for Amaranthus blitum (low-invasive) and A. retroflexus (high-invasive) compare to intermediately invasive A. spinosus and A. viridis in all experimental regimes. It indicated that germination potential does not necessarily constitute a trait that can efficiently distinguish highly invasive and low invasive congeners in four Amaranthus species. However, it was showed that the germination performances of four amaranth species were more or less correlated with their worldwide distribution area. Therefore, the germination performance can be used as a reference indicator, but not an absolute trait for invasiveness. Our results also confirmed that superior germination performance in wide environmental conditions supplementing high seed productivity in highly invasive A. retroflexus might be one of the reasons for its prolific growth and wide distribution. These findings lay the foundation to develop more efficient weed management practice like deep burial of seeds by turning over soil and use of tillage agriculture to control these invasive weed species.
Introduction
The effects of invasive alien species include altering ecosystem, threatening the existence of native species, reducing biodiversity, and degrading the environment. The identification of the 0.1% of harmful invasives among important plant species within a country or a region and prioritizing control efforts according to their specific threat are the challenges we have to face [1]. It is critical, despite its difficulty to determine which exotic plant species may well become invasive ones to control them in their native ecosystem [2]. As a result, unraveling what makes a species invasive and identifying what characteristics are associated with successful establishment for invasive alien plant species are the major objectives of invasion ecology and still represents an ultimate goal of invasion ecologists [3–10].
In previous studies on identification for the traits associated with invasiveness of plant species, the comparative studies among congeneric invasive and non-invasive alien species are recognized as an effective and direct approach [5], [11], [7–9], [12]. Congeneric species are referred to the species that belong to same genus. The congeneric comparison can eliminate or reduce the biasness and variation associated with phylogenetic distance and habitat affinities of the species compared and is the better approach for identifying the traits associated with successful invaders [5], [13], [14], [6], [7], [11]. If the comparative studies were conducted by controlling for life-form, introduction history, native range of origin and habitat preferences of the study species, the results will supply better reference for invasiveness.
Seed germination is the first step of plant life cycle and the germination proportion and germination timing of seeds, major life-history traits, likely to play an important role in biological invasions [14]. Successful germination within favorable time period is crucial for establishing a population and further expansion especially in annual invasive species. [15]. In previous studies, only few experimental comparisons related to germination and early establishment were reported on some invasive and non-invasive or naturalized alien [16–18], [9], [14] and also among invasive and native [12], alien and native [5], [10], and populations of invasive species in the native and introduced ranges [19–21], [15]. Such previous comparisons highlighted different performances in germination characteristics such as rapidity and extent of germination that can be used, at least partly, to separate successful and unsuccessful invaders and deemed to be the most useful traits of evaluating potentially troublesome species[16], [22], [5], [6], [9], [10]. However, other studies also showed that there was not much difference in germination characteristic between successful and unsuccessful invaders [17], [18], [12]. Therefore to identify and further characterize any potential differences in germination traits, large number of empirical multi-species comparative studies is needed among invasive and non-invasive congeners in a common environment. [7], [11], [14].
The traits that allow a species to adapt to a wider range of environmental factors seem to be favorable for a successful invasion [4]; therefore, the invasive species that can be adapted to various conditions are more likely to have better chances to be dispersed and higher chances of invasiveness. Some experimental results of reproductive traits of congeneric pairs demonstrated that rare species were less tolerant to environmental factors than their congeneric widespread species [23–25] based on a comparative analysis of 25 ecological and biological traits in 20 congeneric pairs of endemic and widespread plant species occurring in the French Mediterranean flora. The authors found that morphological and eco-physiological traits of widespread species are often more stress-tolerant than their narrow endemics congeners.
Amaranthus species, commonly referred to as ‘‘pigweeds,” are among the most troublesome weeds in many crop production systems. Some results of germination ecology and response to environmental factors were reported by weed scientists [26–32]. Amaranthus is also the genus with several naturalized weeds including the highly invasive A. retroflexus species in China [33]. There are a few alien species among the genus differing in invasiveness, so it is an ideal studying objective for congeneric comparison for alien plants. A. retroflexus was listed as a highly invasive plant, with a risk index value of 62 and the risk rank as second grade [33]. It has been listed as one of the most noxious invasive plants in China [34]. A. spinosus L. and A. viridis L. are listed as intermediately invasive plants. Their risk indices values are 59 and 52, respectively, and the risk ranks are both third grade [33]. A. blitum didn’t appear in some inventories of invasive plants in China [35], [36], but is listed as an invasive weed with wide distribution in other inventories [37]. We defined it as a low-invasive plant.
In previous studies, the data on the influence of environmental conditions on certain germination characteristics of some Amaranthus spp. were reported. Thomas et al. [31] and Chauhan and Johnson [32] demonstrated the seedling emergence responses of spiny amaranth (A. spinosus) and slender amaranth (A. viridis) to temperature, light, pH solution, moisture stress, salt stress and depth of emergence. The comparative experiments on effects of temperature on seed germination of redroot pigweed (A. retroflexus), Palmer amaranth (A. palmeri), and common waterhemp (A. rudis) in regards to their population in America were showed by Guo and Al-Khatib [27], and a similar report of nine amaranth in America was published by Steckel et al. [30]. Although above mentioned data on the influence of environmental conditions on certain germination characteristics of some Amaranthus spp. are available, little is known on their relative invasiveness.
In this study, four congeneric alien amaranths having similar distribution area, life-form and introduction history, native range of origin and habitat preferences but differing in invasiveness were selected to study their germination and emergence responses to different environmental factors such as photoperiod, pH, osmotic potential, NaCl solution, and burial depth. The objectives of this study were (1) to test the correlation of seed response with invasiveness by comparing germination response data among four amaranths and (2) to supply the empirical evidence for considering whether seed germination response to environmental factors can be included as an indicator of risk assessment protocols for plant invasion.
Materials and Methods
Plant material
According to the results of Records of World Weeds [38], invasive risk indexes and ranks in China [33], invasive categories in China [37], four alien amaranths were selected which are sympatric distribution naturally in Jiangsu Province, China and have similar life-form, introduction history, native range of origin and habitat preference, but differ in invasiveness. They were redroot pigweed (A. retroflexus), spiny amaranth (A. spinosus), slender amaranth (A. viridis), and livid amaranth (A. blitum). All the selected species are annual and introduced into China in the middle of the eighteenth century (Table 1).
Table 1. Origin, introduction history, distribution and invasive status of four Amaranthus species.
| Species | Origina[37] | First Record [37] | Distribution Province in China [37] | Records of World Weedsb [38] | Global Recordc | Global Occurencec | Risk Rank in China [33] | Risk Index in China [33] | Invasive Status |
|---|---|---|---|---|---|---|---|---|---|
| A. retroflexus | Am | 1753 | 32 | S = 16, P = 16, C = 2, X = 12, F = 0 | 31183 | 36070 | 2nd | 62 | High |
| A. spinosus | TAm | 1753 | 30 | S = 7, P = 11, C = 18, X = 21, F = 0 | 2108 | 4847 | 3th | 59 | Intermediate |
| A. viridis | SAm | 1763 | 31 | S = 8, P = 14, C = 9, X = 13, F = 4 | 4458 | 6891 | 3th | 52 | Intermediate |
| A. blitum | TAm, Mediterranean region Europe, Asia and North Africa | 1753 | 30 | S = 0, P = 5, C = 9, X = 17, F = 0 | 13557 | 16639 | - | - | Low |
aAm = America, SAm = South America, TAm = Tropical America (Global Biodiversity Information Facility, www.gbif.org, accessed on 2016-10-7),
bS: Serious weed; P: Principal weed; C: Common weed; X: Present as a weed (the species is present and behave as a weed, but its rank of importance is unknown; F: Flora (the species is known to be present in the flora of the county, but confirming evidence is needed that the plant behave as a weed);
cGlobal Biodiversity Information Facility, www.gbif.org, accessed on 2016-10-7.
Seeds of four amaranths were collected from road side and abandoned areas in Yancheng (34°0′36″N, 119°49′48″E) and Nanjing (31°39′37″N, 119°15′19″E), Jiangsu Province, China in July 2011. As our four experimental species commonly grows as wild population in open areas, no legal permission needed for sampling. Seeds collected from many randomly selected plants were stored at room temperature (25°C) in paper bags until used in the experiments in February and April of 2012. The 1,000-seed weights of A. retroflexus, A. spinosus, A. viridis and A. blitum were recorded as 312mg, 129mg, 388mg, and 378mg, respectively.
Germination tests
Germination response was determined by placing fifty seeds evenly in a 90 mm dia. Petri dish containing two pieces of filter paper. Distilled water (control set and light experiment) or the appropriate treatment solution was added to the filter paper as needed. Dishes containing seeds were incubated at 30°C-14h/25°C-10h of alternating temperatures and at 12h light/12h dark (in light experiment). This alternating temperature regime was found to be optimum among several temperature conditions tested previously in four amaranths (data not shown). Daily germination counts were made for 15 days. Each seedling was removed when a visible radicle could be discerned. The germination experiments were conducted in growth chambers with three tier racks illuminated with cool white fluorescent light (40μmm-2s-1, Philips) with 70% relative humidity (RH).
Photoperiod treatment
The effect of light on germination was determined by incubating seeds of four Amaranths in light/dark regimes of 0h/24h, 8h/16h, 12h/12h and 16h/8h. For germination in complete darkness, dishes were wrapped in a layer of aluminum foil.
pH treatment
The effect of a pH buffered solution on germination was determined by incubating seeds in dishes containing solution of pH4-pH10, which were prepared as described by Burke et al. [39] or Chauhan et al. [40]. A 100 mM Potassium hydrogen phthalate buffer solution was adjusted to pH 4, pH 5, and pH 6 with 0.1N HCl or 0.1N NaOH. A 200mM KH2PO4 buffer solution was adjusted to pH 7 or 8 with 0.1N HCl or 0.1N NaOH. A 50 mM Sodium borate buffer solution was similarly adjusted to pH 9 or 10 with 0.1N HCl or 0.1N NaOH.
NaCl treatment
The effects of salinity on germination response were determined by placing seeds in dishes containing aqueous solution of 0, 25, 50, 100, 150, and 200mM Sodium chloride (NaCl).
Water potential treatments
The effect of osmotic stress on germination was determined by incubating seeds in solutions with osmotic potentials of 0, -0.2, -0.4, -0.6 and -0.8Mpa which were prepared by dissolving 0, 112.38, 172.41, 218.13, and 256.13 g of polyethylene glycol (PEG6000) in 300 ml of distilled water [41].
Seed burial depth treatment
The effect of seed burial depth on seedling emergence was investigated in a greenhouse. Fifty seeds of each species were covered with soil to depths of 0, 1, 2, 3, 4, 5, and 6 cm in plastic pots (15 cm in diameter). Pots were watered initially with an overhead mist sprinkler and later sub-irrigated. Plants were watered throughout the study when the soil surfaces were dried. Seedlings were considered emerged when a cotyledon was visible on the soil surface. Emerged seedlings were counted every day up to 30 days after sowing (DAS).
Statistical analysis
Germination performance across all treatments in all four species were analysed statistically. Each treatment had four replicates in each species. Data were analyzed using the nonlinear regression model of Sigma Plot (SigmaPlot version 11.0, from Systat Software, Inc., San Jose California USA). A three-parameter sigmoid function is used for curve-fitting to the germination data of photoperiod, pH, NaCl and water potential treatments. The formulation was as following:
| (1) |
G is the cumulative percentage germination at time x, Gmax is the maximum germination (%), T50 is the time (d) required for 50% of maximum germination, and Grate indicates the slope.
A four-parameter sigmoid model was fitted to the germination data of seedling emergence (%) at different depths. The model fitted was as following:
| (2) |
E represents cumulative emergence (%) at time x, E0 is the minimum emergence (%), Emax is the difference of maximum and minimum emergence (%), T50 is the time (d) required for 50% of maximum emergency, and Erate indicates the slope.
The main effects of differences among species and treatments were analyzed using the univariate analysis of general linear model. The significant differences among different treatments and among species were analyzed by One-Way ANOVA using SPSS 16. The significant difference among different treatments × species were analyzed by Two-Way ANOVA using SPSS16. For all analyses, differences at p<0.05 was considered significant, while p<0.01 was considered highly significant. When the test results of variances homogeneity were >0.05, the Tukey HSD were selected for multiple comparisons. When the test results of variances homogeneity were <0.05, the Dunnett C were selected for multiple comparisons.
Results
Light treatment
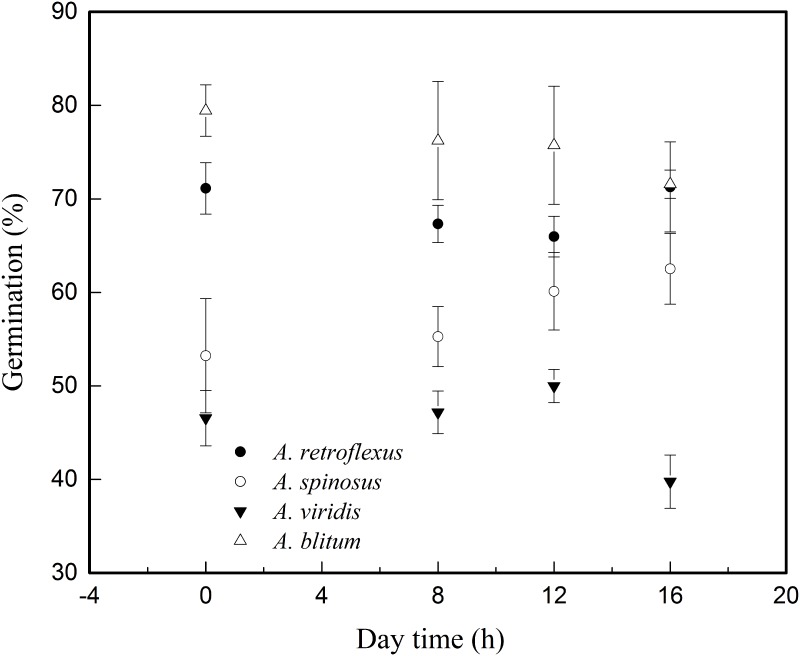
Irrespective of presence or absence of light, all the four amaranths were able to germinate in our experiment (Table 2 and Fig 1); indicating that light is not an absolute requirement for their germination. The analysis of the results of main effects of general linear model showed that there was no significant difference in seed germinations (Gmax) among four photoperiods (DF = 3, F = 0.183, p>0.05), but highly significant difference noted in seed germination among four species (DF = 3, F = 62.225, p<0.01). There was no significant difference in Gmax among photoperiod×species (DF = 9, F = 1.457, p>0.05). The analysis using multiple comparisons showed that there were no significant differences among four photoperiod treatments in all of A. retroflexus (DF = 3, F = 0.783, p>0.05), A. spinosus (DF = 3, F = 1.049, p>0.05) and A. blitum (DF = 3, F = 0.422, p>0.05). Among the four amaranth species, the only difference in light treatment was recorded in A. viridis (DF = 3, F = 3.406, p<0.05), where the seed germination response at 16/8 (day/night) light exposure were lower than that of 12/12 (day/night) light, as evident from the longer time period required for 50% germination (T50) and relatively high Grates value showing that perhaps over-exposure to light period depressed the germination response of A. viridis seeds. Compared to other three amaranths, the time required for 50% germination (T50) of A. viridis were longer and the Grates values were higher (Table 2).
Table 2. Effect of photoperiod on the germination of four Amaranthus species, incubated at 30/25°C in light/dark.
| Species | Parameters | Day time (h) | |||
|---|---|---|---|---|---|
| 0 | 8 | 12 | 16 | ||
| A. retroflexus | Gmax (%) | 72.90(2.74)a,k | 70.40(1.98)a,kl | 67.50(2.17)a,kl | 72.70(4.81)a,k |
| Grate | 0.32(0.04) | 0. 14(0.16) | 0.16(0.08) | 0.16(0.10) | |
| T50(d) | 1.96(0.03) | 1.78(0.25) | 1.82(0.09) | 1.80 (0.12) | |
| R2 | 0.9962 | 0.9937 | 0.9967 | 0.9954 | |
| A. spinosus | Gmax (%) | 54.50 (6.10)a,l | 61.20 (3.21)a,lm | 60.50 (4.14)a,lm | 66.30 (3.79)a,k |
| Grate | 0.37 (0.02 | 0.22 (0.12) | 0.12 (0.12) | 0.20 (0.05) | |
| T50 (d) | 2.22 (0.02) | 1.79 (0.13) | 1.80 (0.19) | 1.89 (0.03) | |
| R2 | 0.9985 | 0.9711 | 0.9990 | 0.9978 | |
| A. viridis | Gmax (%) | 52.40 (2.95)ab,l | 54.70 (2.29)ab,m | 58.60 (1.78)a,m | 46.0 (2.85)b,l |
| Grate | 0.79 (0.09) | 1.47 (0.12) | 1.27 (0.18) | 2.23 (0.16) | |
| T50 (d) | 4.51 (0.10) | 6.06 (0.14) | 4.15 (0.21) | 8.92 (0.24) | |
| R2 | 0.9913 | 0.9920 | 0.9739 | 0.9944 | |
| A. blitum | Gmax (%) | 79.20 (2.75)a,k | 78.20 (6.32)a,k | 77.00 (6.31)a,k | 72.50 (1.51)a,k |
| Grate | 0.34 (0.05) | 0.10 (0.23) | 0.08 (0.69) | 0.11 (0.24) | |
| T50 (d) | 2.48 (0.02) | 1.78 (0.48) | 1.82 (1.62) | 1.77 (0.48) | |
| R2 | 0.9994 | 0.9991 | 0.9999 | 0.9970 | |
Table showing parameter estimates [Gmax, maximum germination (%); Grate, slope; T50, time to reach 50% of maximum germination (days)] of seed germination.
Values represent mean and standard error (parentheses).
Significant differences in Gmax were indicated by letters a-b, among photoperiod treatments within same species (comparison within row) and letters k-m, among species within same photoperiod treatment (comparison within column).
Fig 1. Plot showing three-parameter sigmoid model fitted data of Germination percentage (Gmax) in four Amaranthus species with respect to different light treatments.
pH treatment
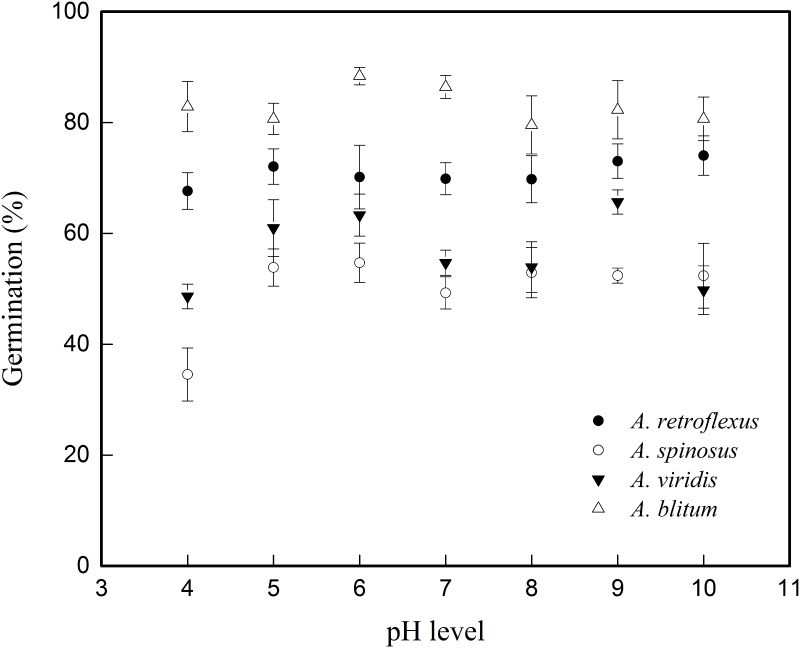
The results of seed germination at different pH treatments in amaranths showed that all four species can germinate at pH 4-pH10 solutions (Table 3 and Fig 2), demonstrating their broad adaptability to germinate in a wide range of soil pH conditions. The results of ANOVA analysis showed that there were significant differences in seed germination (Gmax) among treatments, from pH 4 to pH 10 levels (DF = 6, F = 2.890, p<0.05), and highly significant differences among four amaranth species (DF = 3, F = 74.070, p<0.01). However, there were no significant difference in seed germinations (Gmax) among pH×species (DF = 18, F = 1.136, p>0.05). Amaranthus blitum and A. retroflexus recorded best germination response than other two species. A. spinosus recorded the lowest germination response in all the pH ranges. The analysis of the results of multiple comparisons showed that there were no significant differences among 7 pH levels in both A. retroflexus (DF = 6, F = 0.346, p>0.05) and A. blitum (DF = 6, F = 0.733, p>0.05), but there were significant differences among pH levels in both A. spinosus (DF = 6, F = 3.438, p<0.05) and A. viridis (DF = 6, F = 3.131, p<0.05). The A. retroflexus and A. blitum had higher and quicker germination response than A. spinosus and A. viridis at pH 4–10 solution (Fig 2). Both A. retroflexus and A. blitum recorded highest Gmax of 77.3% and 88.9%, respectively along with relatively lower T50 of 1.79–2.58 for A. retroflexus and between 1.78–1.90 for A. blitum. On the contrary, the highest Gmax of A. spinosus and A. viridis were below 58.6% and 74.9% respectively with T50 ranging from 2.35–4.26 for A. spinosus and 4.9–26.77 for A. viridis. Except for A. blitum, the values of T50 of the other three amaranths were longer in pH4, pH 9, and pH10 than those in pH 5-pH8 (Table 3).
Table 3. Effect of pH on the germination of four Amaranthus species, incubated at 30/25°C in light/dark.
| Species | Parameters | pH level | ||||||
|---|---|---|---|---|---|---|---|---|
| 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| A. retroflexus | Gmax (%) | 69.90 (3.31)a,l | 73.50 (3.20)a,kl | 74.30 (5.75)a,kl | 76.60 (2.89)a,l | 77.30 (4.24)a,kl | 76.80 (3.10)a,kl | 76.00 (3.56)a,k |
| Grate | 0.35 (0.05) | 0.13 (0.15) | 0.13 (0.17) | 0.33 (0.07) | 0.12 (0.14) | 0.42 (0.04) | 0.041 (0.04) | |
| T50 (d) | 1.97 (0.04) | 1.79 (0.24) | 1.81 (0.25) | 1.90 (0.06) | 1.79 (.024) | 2.44 (0.05) | 2.58 (0.05) | |
| R2 | 0.9939 | 0.9979 | 0.9967 | 0.9861 | 0.9982 | 0.9938 | 0.9934 | |
| A. spinosus | Gmax (%) | 34.55 (4.76)b,n | 55.00 (3.36)a,l | 58.60 (3.54)a,l | 52.00 (2.90)ab,m | 55.00 (4.52)a,l | 55.10 (1.36)a,m | 57.60 (5.84)a,l |
| Grate | 0.61 (0.04) | 0.61 (0.07) | 0.46 (0.06) | 0.42 (0.05) | 0.39 (0.03) | 0.87 (0.10) | 0.66 (0.06) | |
| T50 (d) | 4.26 (0.05) | 2.55 (0.08) | 2.53 (0.07) | 2.79 (0.06) | 2.35 (0.04) | 3.66 (0.12) | 3.32 (0.07) | |
| R2 | 0.9972 | 0.9886 | 0.9886 | 0.9926 | 0.9967 | 0.9874 | 0.9930 | |
| A. viridis | Gmax (%) | 48.70 (2.20)b,m | 66.60 (5.13)ab,l | 70.50 (3.79)ab,l | 64.80 (2.29)ab,m | 59.80 (4.59)ab,l | 74.90 (2.18)a,l | 67.30 (4.40)ab, l |
| Grate | 0.81 (0.05) | 1.10 (0.13) | 0.72 (0.06) | 0.61 (0.11) | 0.98 (0.09) | 1.49 (0.17) | 1.41 (0.20) | |
| T50 (d) | 5.64 (0.06) | 5.10 (0.15) | 4.92 (0.07) | 5.05 (0.13) | 5.39 (011) | 6.77 (0.20) | 6.52 (0.24) | |
| R2 | 0.9973 | 0.9876 | 0.9956 | 0.9839 | 0.9927 | 0.9864 | 0.9789 | |
| A. blitum | Gmax (%) | 84.00 (4.53)a,k | 83.20 (2.82)a,k | 88.90 (1.56)a,k | 87.00 (2.07)a,k | 80.40 (5.26)a,k | 85.30 (5.25)a,k | 80.70 (3.93)a, k |
| Grate | 0.25 (0.05) | 0.12 (0.12) | 0.09 (0.23) | 0.09 (0.31) | 0.12 (0.13) | 0.20 (0.08) | 0.19 (0.04) | |
| T50 (d) | 1.90 (0.03) | 1.80 (0.21) | 1.80 (0.52) | 1.78 (0.73) | 1.80 (0.21) | 1.84 (0.07) | 1.88 (0.03) | |
| R2 | 0.9967 | 0.9992 | 0.9999 | 0.9994 | 0.9987 | 0.9938 | 0.9993 | |
Table showing parameter estimates [Gmax, maximum germination (%); Grate, slope; T50, time to reach 50% of maximum germination (days)] of seed germination.
Values represent mean and standard error (parentheses).
Different lowercase letters (a-b) after the value of Gmax indicated significant difference among treatments within same species (comparison within row).
Different lower case letters (k-n) after the value of Gmax indicated significant differences among species within same treatment (comparison within column).
Fig 2. Figure showing three-parameter sigmoid model fitted data of Germination percentage (Gmax) in four Amaranthus species versus varying pH levels tested (pH4-pH10).
NaCl treatment
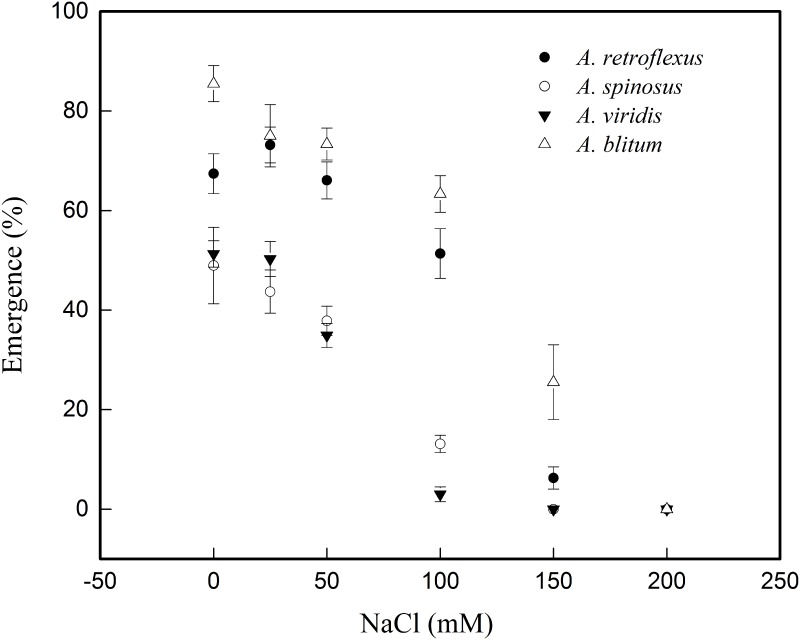
The results of seed germination at different NaCl treatments of four amaranths (Table 4 and Fig 3) showed that their Gmax decreased and T50 increased with increasing NaCl concentrations, except in A. retroflexus and A. blitum that showed a little increase in Gmax and at 150mM NaCl relative to other species. Although A. retroflexus recorded relatively lower Gmax estimates than A. blitum at 150mM NaCl, it took less time to achieve 50% germination (T50). We observed highly significant difference in seed germinations (Gmax), both among different species (DF = 3, F = 110.403, p<0.01) and at different NaCl concentrations used (DF = 5, F = 242.173, p<0.01). There were highly significant difference in seed germinations (Gmax) among NaCl×species (DF = 15, F = 10.261, p<0.01). The NaCl concentrations which in which the Gmax significantly decreased, with respect to control (0mM) were 100mM for A. retroflexus, 100mM for A. spinosus, 50mM for A. viridis, and 150mM for A. blitum, respectively. The A. retroflexus and A. blitum recorded higher and quicker germinations response than A. spinosus and A. viridis at all concentrations (Table 4).
Table 4. Effect of sodium chloride concentrations on the germination of four Amaranthus species, incubated at 30/25°C in light/dark.
| Species | Parameters | NaCl Concentrations (mM) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 25 | 50 | 100 | 150 | 200 | ||
| A. retroflexus | Gmax (%) | 69.50 (4.00)a,l | 74.70 (3.61)a,k | 68.20 (3.72)a,k | 59.00 (5.00)b,l | 7.00 (2.24)c,l | 0c |
| Grate | 0.26 (0.07) | 0.15 (0.10) | 0.30 (0.07) | 1.08 (0.18) | 1.54 (0.28) | 0 | |
| T50 (d) | 1.86 (0.05) | 1.81 (0.12) | 1.87 (0.05) | 3.59 (0.21) | 6.21 (0.33) | 0 | |
| R2 | 0.9913 | 0.9967 | 0.9883 | 0.9682 | 0.9623 | 0 | |
| A. spinosus | Gmax (%) | 51.50 (7.67)a,lm | 45.80 (4.35)a,l | 38.00 (2.94)a,l | 13.00 (1.74)b,m | 0b,l | 0c |
| Grate | 0.40 (0.05) | 0.70 (0.05) | 1.39 (0.19) | 1.23 (0.14) | 0 | 0 | |
| T50 (d) | 2.05 (0.05) | 2.99 (0.06) | 4.01 (0.21) | 4.77 (0.16) | 0 | 0 | |
| R2 | 0.9931 | 0.9958 | 0.9748 | 0.9851 | 0 | 0 | |
| A. viridis | Gmax (%) | 63.70 (2.65)a,m | 58.40 (3.52)a,l | 47.10 (2.43)b,l | 3.50 (1.49)c,m | 0c,l | 0c |
| Grate | 0.85 (0.05) | 1.58 (0.22) | 1.01 (0.13) | 0.55 (0.07) | 0 | 0 | |
| T50 (d) | 5.57 (0.06) | 6.66 (0.26) | 7.39 (0.15) | 11.06 (0.08) | 0 | 0 | |
| R2 | 0.9976 | 0.9787 | 0.9884 | 0.9923 | 0 | 0 | |
| A. blitum | Gmax (%) | 87.30 (3.62)a,k | 76.30 (6.23)a,k | 74.30 (3.21)a,k | 70.50 (3.68)a,k | 25.90 (7.51)b,k | 0c |
| Grate | 0.16 (0.10) | 0.16 (0.07) | 0.26 (0.03) | 0.25 (0.07) | 0.14 (0.39) | 0 | |
| T50 (d) | 1.80 (0.12) | 1.83 (0.08) | 1.96 (0.05) | 2.67 (0.11) | 11.26 (0.72) | 0 | |
| R2 | 0.9957 | 0.9985 | 0.9987 | 0.9778 | 0.9674 | 0 | |
Table showing parameter estimates [Gmax, maximum germination (%); Grate, slope; T50, time to reach 50% of maximum germination (days)] of seed germination.
Values represent mean and standard error (parentheses).
Different lowercase letters (a-c) after the value of Gmax indicated significant differences among treatment within same species (comparison within row).
Different lower case letters (k-m) after the value of Gmax indicated significant differences among species within same treatment (comparison within column).
Fig 3. Figure showing effect of NaCl on germination response of four Amaranthus species fitted to three-parameter sigmoid model.
Osmotic treatment
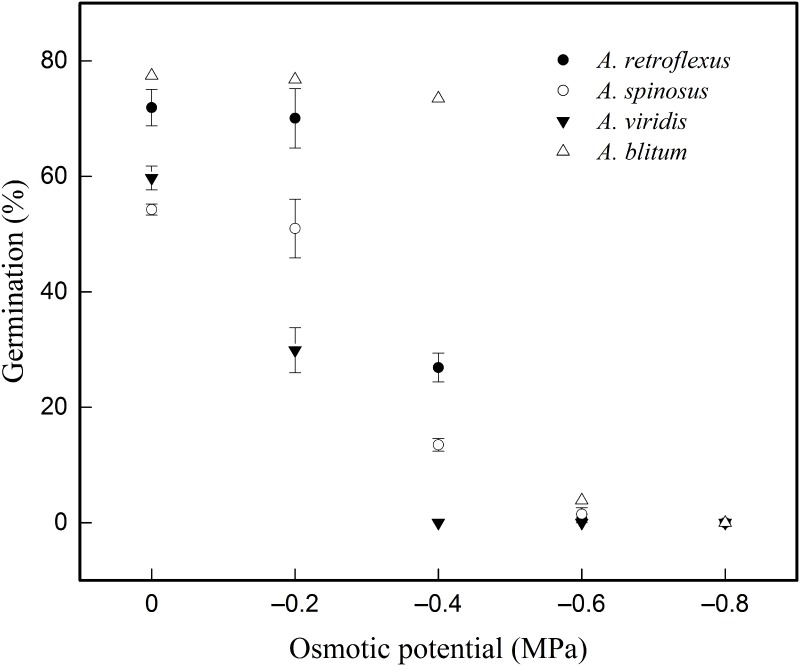
The results of seed germination at different osmotic potential treatments in four amaranths showed that (Table 5 and Fig 4) in overall their Gmax decreased and T50 increased with increasing osmotic potential. The analysis of main effects of general linear model showed that there were extremely significant difference in seed germinations (Gmax) in both among species (DF = 3, F = 103.972, p<0.01) and at different osmotic potentials (DF = 4, F = 482.920, p<0.01). Highly significant difference in seed germinations (Gmax) was recorded among osmotic potential×species (DF = 12, F = 26.118, p<0.01). The osmotic potentials at which the Gmax significantly decreased compared to 0 potential were -0.4MPa for A. retroflexus and A. spinosus, -0.2MPa for A. viridis, and -0.6MPa for A. blitum respectively. No germination appeared at -0.4MPa for A. viridis while other three congeners having high or different degree of germination rate at the same potential, demonstrating lowest resistance to the moisture deficit for A. viridis. Noticeably A. retroflexus and A. blitum had similar higher and quicker germinations than A. spinosus and A. viridis at all potentials (Table 5).
Table 5. Effect of osmotic potential on the germination of four Amaranthus species incubated at 30/25°C in light/dark.
| Species | Parameters | Osmotic potential (MPa) | ||||
|---|---|---|---|---|---|---|
| 0 | -0.2 | -0.4 | - 0.6 | -0.8 | ||
| A. retroflexus | Gmax (%) | 72.30 (3.15)a,k | 73.00 (5.13)a,kl | 29.20 (2.50)b,l | 0.00 (0.74)c,lm | 0c |
| Grate | 0.14 (0.10) | 0.43 (0.04) | 0.58 (0.11) | 0.55 (0.14) | 0 | |
| T50 (d) | 1.81 (1.15) | 2.30 (0.04) | 3.63 (0.12) | 7.97 (0.16) | 0 | |
| R2 | 0.9983 | 0.9956 | 0.9799 | 0.9759 | 0 | |
| A. spinosus | Gmax (%) | 56.30 (0.95)a,l | 53.90 (5.08)a,l | 13.50 (1.10)b,m | 1.50 (1.10)c,l | 0c |
| Grate | 0.47 (0.07) | 0.70 (0.12) | 0.56 (0.02) | 0.04 | 0 | |
| T50 (d) | 2.15 (0.07) | 2.81 (0.14) | 3.98 (0.02) | 6.97 | 0 | |
| R2 | 0.9881 | 0.9740 | 0.9996 | 1.0000 | 0 | |
| A. viridis | Gmax (%) | 64.90 (2.06)a,l | 41.20 (3.91)b,m | 0c,n | 0c,l | 0c |
| Grate | 1.23 (0.10) | 1.95 (0.25) | 0 | 0 | 0 | |
| T50 (d) | 5.20 (0.12) | 7.83 (0.33) | 0 | 0 | 0 | |
| R2 | 0.9923 | 0.9812 | 0 | 0 | 0 | |
| A. blitum | Gmax (%) | 79.30 (2.73)a,k | 80.10 (1.78)a,k | 74.70 (1.68)a,k | 4.00 (0.82)b,k | 0b |
| Grate | 0.14 (0.10) | 0.28 (0.07) | 0.31 (0.02) | 0.24 (0.05) | 0 | |
| T50 (d) | 1.80 (0.14) | 1.87 (0.05) | 3.79 (0.02) | 3.46 (0.09) | 0 | |
| R2 | 0.9982 | 0.9888 | 0.9990 | 0.9852 | 0 | |
Table showing parameter estimates [Gmax, maximum germination (%); Grate, slope; T50, time to reach 50% of maximum germination (days)] of seed germination.
Values represent mean and standard error (parentheses).
Different lowercase letters (a-c) after the value of Gmax indicated significant differences among treatment within same species (comparison within row).
Different letters (k-n) after the value of Gmax indicated significant differences among species within same treatment (comparison within column).
Fig 4. Effect of different Osmotic potential on germination response of Amaranthus species fitted to three-parameter sigmoid model.
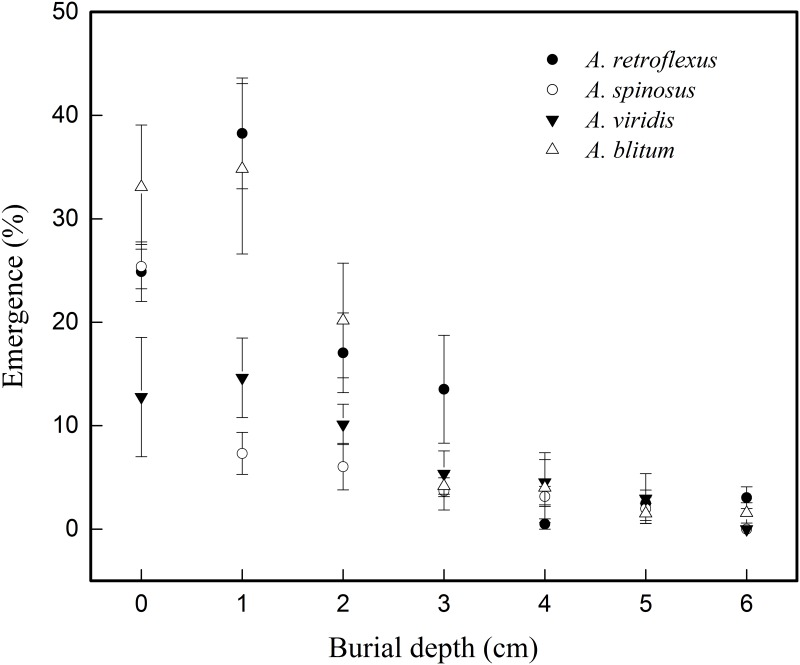
Burial depth
The results of seed germination at different soil burial treatments of four amaranths (Table 6 and Fig 5) showed that the Emax’s increased at depth of 1 cm for A. retroflexus, A. viridis, and A. blitum. Their Emax’s decreased with the increase of burial depths when burial depths were more than 1 cm. On the contrary, highest Emax at soil surface recorded in A. spinosus, and that decreased with the increasing burial depths. The analysis of the result of general linear model showed that there were highly significant differences in seedling emergence (Emax) among different species (DF = 3, F = 8.277, p<0.01) and also at different burial depths (DF = 6, F = 32.796, p<0.01). Our results also detected highly significant difference in seed seedling emergence (Emax) among burial depths×species (DF = 18, F = 2.870, p<0.01). The burial depths in which the Emax’s significantly decreased compared to soil surface were 4 cm for A. retroflexus, 1cm for A. spinosus, 6 cm for A. viridis, and 3 cm for A. blitum, respectively (Table 6).
Table 6. Effect of burial depths on the seedling emergence of four Amaranthus species.
| Species | Parameters | Burial depths (cm) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | ||
| A. retroflexus | Emax (%) | 26.00 (2.89)a,kl | 39.00 (5.35)a,k | 17.00 (3.86)a,k | 13.50 (0.09)ab,k | 0.50 (1.00)b,k | 2.50 (1.29)b,k | 3.04 (1.05)b,k |
| Erate | 1.67 (0.09) | 1.39 (0.08) | 1.90 (0.18) | 1.05 (0.04) | 0.02 | 0.78 (0.06) | 1.77 (0.18) | |
| T50 (d) | 17.72 (0.10) | 17.31 (0.10) | 17.92 (0.20) | 17.97 (0.04) | 17.50 | 18.22 (0.07) | 15.20 (0.20) | |
| Emin | -0.07 (0.19) | 0.00 (0.30) | -0.16 (0.24) | -0.05 (0.05) | -4.25 | -0.05 (0.02) | -0.04 (0.05) | |
| R2 | 0.9968 | 0.9961 | 0.9898 | 0.9991 | 1.0000 | 0.9967 | 0.9886 | |
| A. spinosus | Emax (%) | 27.50 (2.21)a,kl | 7.00 (2.03)b,l | 6.04 (2.25)bc,k | 3.50 (1.84)bc,k | 3.00 (0.97)bc,k | 2.00 (1.13)bc,k | 0c,k |
| Erate | 1.66 (0.14) | 3.43 (0.38) | 1.84 (0.15) | 4.94 (1.06) | 4.39 (0.84) | 0.77 (0.11) | 0 | |
| T50 (d) | 18.66 (0.16) | 17.63 (0.39) | 18.28 (0.17) | 14.66 (0.88) | 14.97 (0.75) | 13.66 (0.13) | 0 | |
| Emin | 0.20 (0.28) | 0.07 (0.16) | 0.16 (0.07) | -0.33 (0.28) | -0.15 (0.19) | -0.02 (0.03) | 0 | |
| R2 | 0.9924 | 0.9852 | 0.9924 | 0.9609 | 0.9634 | 0.9882 | 0 | |
| A. viridis | Emax (%) | 13.00 (5.76)a,l | 15.00 (3.84)a,l | 10.50 (1.95)ab,k | 5.50 (2.21)ab,k | 4.53 (2.19)ab,k | 3.00 (2.41)ab,k | 0b,k |
| Erate | 2.83 (0.58) | 3.06 (0.26) | 2.52 (0.28) | 1.00 (0.07) | 2.65 (0.30) | 0.34 (0.07) | 0 | |
| T50 (d) | 17.25 (0.05) | 22.02 (0.34) | 23.87 (0.40) | 22.39 (0.04) | 21.35 (0.37) | 20.51 (0.10) | 0 | |
| Emin | -1.07 (0.40) | -0.23 (0.17) | 0.05 (0.12) | -0.20 (0.04) | -0.07 (0.07) | 0.07 (0.04) | 0 | |
| R2 | 0.9421 | 0.9915 | 0.9858 | 0.9955 | 0.9841 | 0.9850 | 0 | |
| A. blitum | Emax (%) | 33.50 (6.00)a,k | 35.50 (8.24)a,k | 20.00 (5.54)ab,k | 4.00 (0.80)b,k | 4.00 (2.19)b,k | 1.50 (0.97)b,k | 1.50 (0.98)b,k |
| EGrate | 1.51 (0.07) | 1.09 (0.04) | 1.57 (0.05) | 2.08 (0.23) | 1.22 (0.08) | 1.14 (0.15) | 1.93 (0.41) | |
| T50 (d) | 17.67 (0.08) | 17.20 (0.05) | 18.01 (0.05) | 20.33 (0.26) | 19.10 (0.09) | 19.06 (0.18) | 19.14 (0.46) | |
| Emin | -0.19 (0.21) | 0.02 (0.16) | -0.09 (0.08) | 0.03 (0.06) | -0.05 (0.03) | 0.00 (0.02) | 0.05 (0.05) | |
| R2 | 0.9977 | 0.9988 | 0.9989 | 0.9854 | 0.9965 | 0.9851 | 0.9493 | |
Table showing parameter estimates [Emax, the difference of maximum and minimum emergence (%); Erate, slope; T50, time (d) required for 50% of maximum seedling emergence, Emin, minimum seedling emergence (%)] of seedling emergence.
Values represent mean and standard error (parentheses).
Different lowercase letters (a-c) after the value of Emax indicated significant differences among treatments within same species (comparison within row).
Different letters (k-l) after the value of Emax indicated significant differences among species within same treatment (comparison within column).
Fig 5. Figure showing effect of burial depth on germination response measured by seedling emergence (Emax) in four Amaranthus species fitted to three-parameter sigmoid model.
Discussion
Seed germination is an integrated process influenced by biotic and abiotic factors as well as dependent on the genetic and physiological state of readiness [42, 43]. Germination characteristics affect plant propagation and distribution especially for annual species reproducing exclusively by seed [44]. This study demonstrated the effects of some environmental factors on the germination response in four wild Amaranthus species. Our experimental findings provide evidence that Amaranthus species have variable potential to grow in a variety of environmental regime. The results showed that the A. blitum and A. retroflexus have better overall germination performances (higher Gmax, rapider Grate and shorter T50) compare to A. spinosus and A. viridis in various treatments. Low invasive weed A. blitum recorded the best overall germination performance, with the highest Gmax, the rapidest Grate, and the shortest T50. Highly invasive A. retroflexus did not differ significantly from A. blitum in most treatments, except for pH (pH4-pH7), NaCl (0mM, 100mM, 150mM) and osmotic potential (-0.4 and -0.6MPa). The overall germination performances of A. spinosus and A. viridis were similar and consistently low across all treatments. In most treatments, the Gmax of A. spinosus and A. viridis were not extremely different except for photoperiod 16/8, pH 4, pH 9, osmotic potential -0.2 and -0.4MPa.
The germination results of the present study were mostly in accordance with the previous studies in A. retroflexus [26], A. spinosus [32], and A. viridis [31], [32]. In a study by Ghorbani et al. [26], the germination of A. retroflexus found to decrease with increasing moisture deficit, and the germination rates (Gmax) at 25°C were 68%, 52%, 50%, 33%, 14%, 8%, and 0% at 0MPa, -0.1MPa, -0.2MPa, -0.3MPa, -0.4MPa, -0.5MPa, and -1MPa osmotic potential respectively. The seedlings of A. retroflexus emerged only at depths of lower than 5cm. In the study of Chauhan and Johnson [32], germination of A. viridis was more sensitive to increasing salt and water stress than A. spinosus. Our present results confirmed their observation. The Gmax of A. viridis declined sharply from 47.1% at 50mM to 3.5% at 100mM NaCl concentration, whereas A. spinosus declined slowly from 38.0% at 50mM to 13.0% at 100mM NaCl. Meanwhile, the Gmax of A. spinosus was 13.5% at -0.4MPa osmotic potential, whereas seeds of A. viridis did not germinate at all at this potential. Similar germination response of A. viridis to water stress was also demonstrated in the study of Thomas et al. [31]. In their study, extremely low germination (1.25% at 30/20°C) at -0.4MPa was reported which was the lethal osmotic potential causing complete inhibition in our study. The only difference observed is in the germination response of A. viridis to different pH values between our study and that of Thomas et al. [31]. In their study, the average values of Gmax from pH3 to pH9 were 68.8%, 63.8%, 79.5%, 58.1%, 49.0%, 39.6%, and 52.8%, respectively. They concluded that the germination was greater with acidic than with basic pH. The average values of Gmax from pH 4 to pH 10 in our study were recorded as 48.7%, 66.6%, 70.5%, 64.8%, 59.8%, 74.9%, and 67.3% in A. viridis, respectively. Our results showed that there were no significant differences among different pH values, except between pH 4 with pH 9 suggesting they are adapted to both acidic and basic pH ranges, but with lower germination response at pH 4 and relatively higher at pH 9. The results of burial depth were also in conformity to Chauhan and Johnson’s [32], and showed that emergence of A. spinosus was affected to greater extent by increasing seed burial depths. The germination of A. spinosus was the highest at soil surface (27.5% in our study and 56.0% in Chauhan and Johnson study). The emergence rate significantly declined when seeds were covered by soil, even if at 0.5 cm depth (7.0% in ours as well as in Chauhan and Johnson’s study). However, there were no significant differences for germination at depths from 0 cm to 3 cm for A. retroflexus, at depths from 0 cm to 5 cm for A. viridis and at depths from 0 cm to 2 cm for A. blitum. The reason for this likely related to the seed size. Seed size was associated with germination traits and seedling growth in non-competitive cover [45]. Lower seedling emergence of seeds at deep depths may be linked to limited seed reserves [45, 46]. Larger seeds often have greater reserves and are able to emerge from greater depths [46]. The seeds of A. spinosus were significantly smaller and relatively lighter (129mg for 1000-seed weights) than those of the other three amaranths (312mg for A. retroflexus, 388mg for A. viridis, and 378g for A. blitum respectively) and that might be a reason for A. spinosus which had highest emergence rate on the soil surface and emergence decreased with increasing burial depth.
According to Pyšek and Richardson [47] germination of alien invasive species was more rapid, higher and successful across more environmental conditions than that of congeneric native/noninvasive taxa. However, in our present study, it appeared that invasiveness of amaranth was not always positively correlated, or at least partly, with their germination performance in wide environmental conditions as demonstrated in two amaranth species (A. retroflexus and A. blitum) with contrasting invasiveness but exhibiting equivalently high germination performance. However, when considering the global records and occurrences of these four amaranths, it showed that these two species with higher germination performances (A. retroflexus and A. blitum) have more respective global records (31183 & 13557) and occurrences (36070 & 16639) than the other two congeners (A. spinosus and A. viridis) with lower germination performances (Table 1). This implied that high germination potential and adaptability to a wide soil condition might play a key role contributing to population establishment and colonization and one of the reasons for widespread distribution of A. retroflexus and A. blitum.
Rapid distribution and invasion by weeds and invasive plants is an increasingly serious problem and has attracted considerable attention worldwide [48]. Physiochemical properties of soil influenced by growing microbial communities and other edaphic factors cause change in pH, salinity, and nutrient level that have profound effect on seedling growth and emergence of weedy/invasive species [49]. Besides germination performance, other reproductive traits such as seed production, seed viability, and dynamics of soil seed bank were important ones which may influence the distribution and invasion success in the new region [17, 4, 5]. In our common garden experiment on four amaranth species, the invasiveness (Table 1) was positively correlated with the seed production (78,063±18,013 for A. retroflexus, 22,777±9,451 for A. spinosus, and 9152± 4407 for A. viridis). However, the seed production of A. blitum (5357± 2104) was relatively less and only found to be 6.9% of A. retroflexus, 23.5% of A. spinosus and 58.5% of A. viridis. Therefore, the seed germination potential, maybe along with high seed production, plays the union role in wide distribution and invasion success of highly invasive A. retroflexus.
Our experimental results confirmed that the superior germination potential under a wide spectrum of environmental conditions is likely to make it difficult to control A. retroflexus, in agricultural field and in non-native habitat. Practices like deep burial of seeds by turning over the top soil and using of tillage agriculture system are some of the potential options to inhibit the emergence and growth of these weed species.
Conclusion
According to our congeneric comparative results in four amaranths, the germination performances among different species differing in invasiveness didn’t show complete positive correlation with the invasiveness. Therefore, the germination performances can’t be used directly or solely as an indicator of invasiveness, but could be considered as a reference indicator. An integrated consideration of the role of germination and emergence response to various environmental factors combined with other factors is necessary to assess the weediness and invasive characteristics of weedy/invasive plants. In addition, it shall be noted that large scale multi-species empirical comparative experiments involving invasive, non-invasive and native species was imperative for the understanding of invasion mechanism and risk assessment of plant invasion.
Acknowledgments
Authors are grateful to Dr. B.S Chauhan, Center for Plant Science, University of Queensland, Australia for his encouragement and helpful suggestions. We thank Li-Jun Wei, Yan Cheng High School, China for collecting seeds of Amaranthus. Authors are grateful to Chaobin Zhang, School of Life Sciences, Nanjing University for his help in preparation of figures. Authors are also thankful to two anonymous reviewers for critical evaluation and suggestions to improve the manuscript.
Data Availability
All relevant data and related information on experimental material, procedure and methodologies to replicate the experiment are present in the manuscript.
Funding Statement
The present work received funding from following sources: 1. Natural Science Foundation of Jiangsu, China - Grant No-BK20131192; 2. National Natural Science Foundation of China - Grant No.-31370548; 3. Science and Technology Plan Project of General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Grant No.- JSCIQ_2014IK013; 4. Suzhou Science and Technology Plan Projects. Grant No- SYN201304.
References
- 1.Jose S, Singh H P, Batish D R. Kohli RK, Bardhan S. Invasive plant ecology: the horse behind the cart? In: Jose S, Singh H P, Batish D R, Kohli RK editors. Invasive plant ecology. Boca Raton: CRC Press; 2013. pp. 1–6. [Google Scholar]
- 2.Holzmueller EJ, Jose S. What makes alien plants so successful? In: Jose S, Singh H P, Batish D R, Kohli RK editors. Invasive plant ecology. Boca Raton: CRC Press; 2013. pp. 7–15. [Google Scholar]
- 3.Rejmánek M, Richardson DM, Higgins SI, Pitcairn MJ, Grotkopp E. Ecology of invasive plants: state of the art In: Mooney HA, McNeelly JA, Neville L, Schei PJ, Waage J, editors. Invasive alien species: a new synthesis, Washington, DC: Island Press; 2005. pp. 104–162. [Google Scholar]
- 4.Pyšek P, Richardson DM. Traits associated with invasiveness in alien plants: where do we stand? In: Nentwig W, ed. Biological invasions, Section II. Berlin: Springer; 2007. pp. 97–125. [Google Scholar]
- 5.Perglová I, Pergl J, Skálováa H, Moravcováa L, Jarošík V, Pyšek P. Differences in germination and seedling establishment of alien and native Impatiens species. Preslia.2009; 81: 357–375. [Google Scholar]
- 6.van Kleunen M, Johnson SD. South African Iridaceae with rapid and profuse seedling emergence are more likely to become naturalized in other regions. J Ecol. 2007; 95: 674–681. [Google Scholar]
- 7.van Kleunen M, Weber E, Fischer M. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol Lett. 2010b; 13: 235–245. [DOI] [PubMed] [Google Scholar]
- 8.van Kleunen M, Schlaepfer DR, Glättli M, Fischer M. Pre-adapted for invasiveness: do species traits or their plastic responses to shading differ between invasive and non-invasive plant species in their native range? J Biogeogr. 2011; 38: 1294–1304. [Google Scholar]
- 9.Moravcová L, Pyšek P, Jarošík V, Havlíčková V, Zákravský P. Reproductive characteristics of neophytes in the Czech Republic: traits of invasive and non-invasive species. Preslia. 2010; 82: 365–390 [Google Scholar]
- 10.Chrobock T, Kempel A, Fischer M, van Kleunen M. Introduction bias: cultivated plant species germinate faster and more abundantly than native species in Switzerland. Basic Appl Ecol. 2011; 12: 244–250. [Google Scholar]
- 11.van Kleunen M, Dawson W, Schlaepfer DR, Jeschke JM, Fischer M. Are invaders different? A conceptual framework of comparative approaches for assessing determinants of invasiveness. Ecol Lett. 2010a; 13: 947–958. [DOI] [PubMed] [Google Scholar]
- 12.Skálová H, Moravcová L, Pyšek P. Germination dynamics and seedling frost resistance of invasive and native Impatiens species reflect local climatic conditions. Perspect Plant Ecol. 2011; 13: 173–180. [Google Scholar]
- 13.Wang K, Yang J, Chen J. The applications of congeneric comparisons in plant invasion ecology. Biodivers Sci. 2009; 17: 353–361. [Google Scholar]
- 14.Schlaepfer DR, Glattli M, Fischer M, van Kleunen M. A multi-species experiment in their native range indicates pre-adaptation of invasive alien plant species. New Phytol. 2010; 185: 1087–1099. 10.1111/j.1469-8137.2009.03114.x [DOI] [PubMed] [Google Scholar]
- 15.Leiblein-Wild MC, Kaviani R, Tackenberg O. Germination and seedling frost tolerance differ between the native and invasive range in common ragweed. Oecologia. 2014; 174: 739–750. 10.1007/s00442-013-2813-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forcella F, Wood JT, Dillon SP. Characteristics distinguishing invasive weeds within Echium (Bugloss). Weed Res. 1986; 26: 351–364. [Google Scholar]
- 17.Radford IJ, Cousens RD. Invasiveness and comparative life history traits of exotic and indigenous Senecio species in Australia. Oecologia. 2000; 125: 531–542. [DOI] [PubMed] [Google Scholar]
- 18.Mandák B. Germination requirements of invasive and non-invasive Atriplex species: a comparative study. Flora. 2003; 198: 45–54. [Google Scholar]
- 19.Kudoh H, Nakayama M, Lihova′ J. Marhold K. Does invasion involve alternation of germination requirements? A comparative study between native and introduced strains of an annual Brassicaceae, Cardamine hirsuta.Ecol Res. 2007; 22: 869–875. [Google Scholar]
- 20.Li YP, Feng YL. Differences in seed morphometric and germination traits of crofton weed (Eupatorium adenophorum) from different elevations. Weed Sci. 2009; 57: 26–30. [Google Scholar]
- 21.Beckmann M, Bruelheide H, Erfmeier A. Germination responses of three grassland species differ between native and invasive origins. Ecol Res. 2011; 26: 763–771. [Google Scholar]
- 22.Dreyer GD, Baird LM, Fickler C. Celastrus scandens and Celastrus orbiculatus: comparisons of reproductive potential between a native and an introduced woody vine. Bull Torrey Bot Club. 1987; 114: 260–264. [Google Scholar]
- 23.Young AS, Chang S, Rebecca R, Sharitz RR. Reproductive ecology of a federally endangered legume, Baptisia arachnifera, and its more widespread congener B. Lanceolata (Fabaceae). Am J Bot. 2007; 94: 228–236. 10.3732/ajb.94.2.228 [DOI] [PubMed] [Google Scholar]
- 24.Mattana E, Daws MI, Bacchetta G.Comparative germination ecology of the endemic Centranthus amazonum (Valerianaceae) and its widespread congener Centranthus ruber. Plant Sp Biol. 2010; 25: 165–172. [Google Scholar]
- 25.Lavergne S, Thompson JD, Garnier E, Debussche M. The biology and ecology of narrow endemic and widespread plants: a comparative study of trait variation in 20 congeneric pairs. Oikos. 2004; 107: 505–518. [Google Scholar]
- 26.Ghorbani R, Seel W, Leifert C. Effects of environmental factors on germination and emergence of Amaranthus retroflexus. Weed Sci. 1999; 47: 505–510. [Google Scholar]
- 27.Guo P, Al-Khatib K. Temperature effects on germination and growth of redroot pigweed (Amaranthus retroflexus), Palmer amaranth (A. palmeri), and common waterhemp (A. rudis). Weed Sci. 2003; 51: 869–875. [Google Scholar]
- 28.Sellers BA, Smeda RJ, Johnson WG, Kendig JA, Ellersieck MR. Comparative growth of six Amaranthus species in Missouri. Weed Sci. 2003; 51: 329–333. [Google Scholar]
- 29.Costea M, Weaver S and Tardif FJ. The biology of Canadian weeds. 130. Amaranthus retroflexus L., A. powellii S. Watson and A. hybridus L. (update). Can J Plant Sci. 2004; 84: 631–668. [Google Scholar]
- 30.Steckel LE, Sprague CL, Stoller EW, Wax LM. Temperature effects on germination of nine Amaranthus species. Weed Sci. 2004; 52: 217–221. [Google Scholar]
- 31.Thomas WE, Burke IC, Spears JF, Wilcut JW. Influence of environmental factors on slender amaranth (Amaranthus viridis) germination. Weed Sci. 2006; 54: 316–320. [Google Scholar]
- 32.Chauhan BS, Johnson DE. Germination ecology of spiny (Amaranthus spinosus) and slender amaranth (A. viridis): troublesome weeds of direct-seeded rice. Weed Sci. 2009; 57(4): 379–385. [Google Scholar]
- 33.Feng J, Zhu Youyong. Alien invasive plants in China: risk assessment and spatial patterns. Biodivers Conserv. 2010; 19: 3489–3497 [Google Scholar]
- 34.Huang QQ, Wu JM, Bai YY, Zhou L, Wang GX. Identifying the most noxious invasive plants in China: role of geographical origin, life form and means of introduction. Biodivers Conserv. 2009; 18: 305–316. [Google Scholar]
- 35.Weber E, Guo SG, Li B. Invasive alien plants in China: diversity and ecological insights. Biol Invas. 2008; 10: 1411–1429. [Google Scholar]
- 36.Xu HG, Qiang S. Inventory of invasive alien species in China. 1st ed Beijing: China Environmental Science Press; 2004. [Google Scholar]
- 37.Ma J. The checklist of the Chinese invasive plants. 1st ed Beijing: China Higher Education Press; 2013. [Google Scholar]
- 38.Holm LG, Pancho JV, Herberger JP, Plucknett DL. A geographical atlas of world weeds. 1st ed New York: John Wiley & Sons; 1979. [Google Scholar]
- 39.Burke IC, Thomas WE, Spears JF, Wilcut JW. Influence of environmental factors on broadleaf signal grass (Brachia riaplatyphylla) germination. Weed Sci. 2003; 51: 683–689. [Google Scholar]
- 40.Chauhan BS, Gill G and Preston C. Influence of environmental factorson seed germination and seedling emergence of rigid ryegrass (Lolium rigidum). Weed Sci. 2006; 54: 1004–1012. [Google Scholar]
- 41.Michel B.E. Evaluation of the Water Potentials of Solutions of Polyethylene Glycol 8000. Plant Physiol. 1983; 72: 66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noe Gregory B, Zedler JoyB. Differential effects of four abiotic factors on the germination of salt marsh annuals. Am. J. Bot. 2000; 87:1679–1692. [PubMed] [Google Scholar]
- 43.Marc A. Cohn. Seed development, dormancy and germination. Annual Plant Reviews, Vol 27. Ann Bot. 2008; 102 (5): 877–878. [Google Scholar]
- 44.van Acker RC. Weed biology serves practical weed management. Weed Res. 2009; 49: 1–5. [Google Scholar]
- 45.Gross KL. Effects of seed size and growth form on seedling establishment of six monocarpic perennial plants. J Ecol. 1984; 72: 369–387. [Google Scholar]
- 46.Tobe K, Zhang L, Omasa K. Seed size effects on seedling emergence of desert psammophytes in China. Arid Land Research and Management. 2007; 21: 181–192. [Google Scholar]
- 47.Pyšek P, Richardson DM. Traits associated with invasiveness in alien plants: where do we stand? In: Nentwig W (ed) Biological invasions, Ecological Studies. 193: 97–126; Springer-Verlag, Berlin & Heidelberg, Germany; 2007. [Google Scholar]
- 48.Vilà M, Espinar JL, Hejda M, Hulme PE, Jarošík V, Maron JL, Pergl J, Schaffner U, Sun Y, Pyšek P. Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecology Letters. 2011; 14: 702–708. 10.1111/j.1461-0248.2011.01628.x [DOI] [PubMed] [Google Scholar]
- 49.Novoa A, Rodríguez R, Richardson D, González L. Soil quality: a key factor in understanding plant invasion? The case of Carpobrotus edulis (L.) N.E.Br. Biol Invasions. 2014; 16: 429. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data and related information on experimental material, procedure and methodologies to replicate the experiment are present in the manuscript.