Abstract
Escherichia coli has four gluconate transporters, GntP, GntU, GntT, and IdnT, which are members of the major facilitator superfamily. The physiological function of GntP was previously unknown and is the subject of this study. GntP is not induced by gluconate, and despite being located adjacent to genes involved in glucuronate catabolism, gntP does not encode a glucuronate transporter. Here we identify gntP as the gene which encodes the fructuronate transporter. We show that gntP is induced by fructuronate and is a new member of the UxuR regulon: gntP is derepressed in an uxuR strain, UxuR binds in vitro specifically to an operator site that overlaps the gntP promoter, and UxuR binding is eliminated by fructuronate. Transcription of gntP requires activation by cyclic AMP (cAMP)-cAMP receptor protein. A gntP mutant cannot grow on fructuronate but grows normally on glucuronate and gluconate. Thus, the UxuR regulon is a module of sugar acid catabolism whose physiological role is for growth on fructuronate. Glucuronate, because it proceeds through a fructuronate intermediate, must induce the UxuR regulon and must also induce the ExuR regulon, which encodes the glucuronate transporter, ExuT, and the first step in its catabolism, UxaC. Thus, hexuronate catabolism in E. coli requires both the ExuR and UxuR regulons, while fructuronate catabolism requires only the UxuR regulon.
Escherichia coli grows on several hexonates and hexuronates (19), some that feed into the Entner-Doudoroff pathway (5, 7) and others that feed into the Ashwell pathway (1). The pathways involve at least 26 known genes in six regulons that have been studied extensively (19, 27). Still, there are some long-standing mysteries regarding the transport and catabolism of sugar acids by E. coli. In particular, we are interested in gntP, which was implicated as being important for colonization of the mouse intestine by a human commensal E. coli strain (30). gntP is one of four genes which encode gluconate transporters (20), but previous studies showed that it is not induced by gluconate (10).
The gntP gene is adjacent to and divergently transcribed from uxuAB (2, 10). The uxuA and uxuB genes are negatively regulated by the product of the downstream gene, uxuR, and are induced for growth on glucuronate (1, 2, 8, 23-27). The gntP gene is predicted to be induced by glucuronate (27), but this has not been established experimentally. However, GntP does not appear to be involved in glucuronate catabolism: GntP is not homologous to the known glucuronate transporters, ExuT and KdgT, and GntP does not appear to transport glucuronate (10). Since GntP does not transport glucuronate and is not induced by gluconate, we explored the possibility that GntP transports one of the other sugar acids that support growth of E. coli, i.e., fructuronate (17).
Fructuronate is the first intermediate of the glucuronate pathway and is the inducer of the UxuR regulon (8, 17, 21, 26). Growth on fructuronate does not require the hexuronate transporter, ExuT, but rather, depends on another transporter, the identity of which was unknown (17). Thus, we hypothesized that gntP encodes the E. coli fructuronate transporter.
Since fructuronate is not commercially available, we devised a way to biosynthesize it from glucuronate. This preparation was used to demonstrate that growth on fructuronate depends on functional GntP, strongly implicating GntP as the primary fructuronate transporter. We show here that gntP is induced by fructuronate, regulated by UxuR, and requires activation by cyclic AMP (cAMP)-cAMP receptor protein (CRP). Based upon these findings, we suggest that GntP functions to transport fructuronate and that the UxuR regulon alone is sufficient for the initial steps of fructuronate catabolism.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The E. coli strains and plasmids used for this study are listed in Table 1. E. coli strains were grown at 37°C in Luria-Bertani broth (LB) (12) or MOPS (morpholinepropanesulfonic acid) minimal medium (16) with or without added carbohydrate (0.2%). Cell growth was monitored spectrophotometrically, at 600 nm.
TABLE 1.
Plasmids and strains used
| Strain or plasmid | Relevant genotype | Source or reference |
|---|---|---|
| E. coli strains | ||
| MG1655Strr | K-12 wild type (CGSC 7740) Strr | 15 |
| W1485 | K-12 wild type (CGSC 5024) | CGSCa |
| ABN11 | MG1655Strr ΔuxuB::Kanr | This study |
| ABN12 | MG1655Strr ΔgntP::Kanr | This study |
| ABN13 | MG1655Strr ΔuxaC::Kanr | This study |
| ABN14 | MG1655Strr ΔuxuB::Camr ΔuxaB::Kanr | This study |
| ABN15 | MG1655Strr ΔuxaC::Camr ΔgntP::Kanr | This study |
| CU110 | W1485 gntP-lacZ | This study |
| CU111 | W1485 uxuR gntP-lacZ | This study |
| CU112 | W1485 Δcya gntP-lacZ | This study |
| CU113 | W1485 Δcrp gntP-lacZ | This study |
| I57 | uxuR | J. Robert-Boudouy |
| RH74 | MC4100 Δcya851 ilv::Tn10 | R. Hengge-Aronis |
| RH77 | MC4100 Δcya851Δcrp-zhd732::Tn10 | R. Hengge-Aronis |
| Plasmids | ||
| pABN13 | uxaC | This study |
| pQE30 | Expression vector, His6 affinity tag | 18 |
| pCU107 | uxuR | This study |
| pCU108 | gntP promoter, mutated operator | This study |
| pCU109 | gntP promoter, mutated operator | This study |
| pCU110 | gntP promoter 720-bp fragment | This study |
E. coli Genetic Stock Center.
Construction of mutants.
Transduction with bacteriophage P1 was described previously (11). E. coli I57 (26) served as the source of the uxuR allele. E. coli RH74 (13) was the source of the Δcya851 allele. RH77 (13) was the source of the Δcrp-zhd732::Tn10 allele. All other mutants were constructed by allelic replacement and antibiotic resistance markers were excised as described previously (6). All mutations were verified by DNA sequencing of genomic DNA preparations.
Construction of lacZ chromosomal fusions.
The gntP promoter region (720 bp) was amplified using primers 5′-GCGGATCCACCACCCAGAGAATGTTAAGCA-3′ and 5′-GCGGATCCGTATTCGAGGTCAGTACGGGTC-3′ and cloned into pRS551 (operon fusion vector) to make plasmid pCU110 (29). The plasmid construction was confirmed by restriction analysis. Single-copy chromosomal fusions were made by homologous recombination with the lambda phage λRS88 and integration of the lysogenic phage into the chromosome to create a single-copy gntP-lacZ fusion in the wild-type background, E. coli CU110. The copy number was confirmed as described previously (22). The same method was used to generate gntP-lacZ single-copy chromosomal fusions in E. coli CU111, CU112, and CU113.
β-Galactosidase assays.
β-Galactosidase activity was determined as described previously and is expressed in Miller units (14). Reported values are the average of three independent measurements.
RNA isolation and Northern blot analysis.
Total RNA was isolated by the hot phenol method (18) from E. coli W1485 grown to mid-log phase (optical density, 0.6). Contaminating DNA was removed by treatment with RNase-Free DNase (QIAGEN). RNA samples were electrophoresed on a 1.5% agarose-formaldehyde denaturing gel. RNA was transferred overnight onto a Nytran membrane using 20× SSC buffer (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). After prehybridization, hybridization with the radiolabeled probe was carried out overnight at 65°C. To synthesize radiolabeled RNA probes, internal fragments of gntP, uxuA, uxuB, and uxuR were cloned into pBluescript and confirmed by sequencing. The plasmids were linearized, and RNA was synthesized using T3 RNA polymerase and [α-32P] UTP. The membranes were washed, dried, and visualized by exposure to Kodak X-ray film at room temperature.
QPCR.
Transcript levels were confirmed by quantitative PCR (QPCR). Cells were grown on MOPS minimal medium to mid-log growth phase and total RNA was prepared by using RNAlater (Ambion) and purified using an RNeasy MinElute (QIAGEN) column (31). Taqman probes and primers were designed by using Primer Express software provided with the ABI Prism 7000 sequence detector (Applied Biosystems). Transcript levels were normalized to 16S rRNA as described previously (9), and the values were expressed relative to cells grown on glucose.
Cloning of uxuR and purification of UxuR.
UxuR was prepared by using the His tag modification system from QIAGEN. PCR primers were designed for amplification of the uxuR gene: 5′-CAAGCTTTGATGAAAATGCACC-3′ and 5′-CGGTACCAAATCTGCCACCTCT-3′. The amplified DNA fragment was digested with KpnI and HindIII and ligated into pQE30 to create pCU107. UxuR was overproduced and purified on a nickel-nitrilotriacetate column, as described previously (18).
Mobility shift binding assay.
The DNA probe used for the gel retardation assays was PCR amplified with primers 5′-TCATGGTTGTTGCTGCAAA-3′ and 5′-TTTCATAGTGTGCAGCAGCG-3′, which produced a 113-bp fragment containing the putative UxuR binding site upstream of gntP. The DNA fragment was end labeled using [δ-32P]ATP and T4 polynucleotide kinase. The binding mixture contained 1 μg of salmon sperm DNA, 0.66 mM dithiothreitol, the indicated amount of UxuR protein, 8 nmol of probe, and binding buffer (50 mM Tris, pH 7.5, 100 mM KCl, 10% glycerol, and 1 mM EDTA) in a total volume of 17.5 μl. The binding mixture was incubated at room temperature for 30 min, loaded onto a 5% nondenaturing agarose gel prepared in Tris-borate-EDTA buffer, and electrophoresed for 2 h. Dried gels were subjected to autoradiography. A small amount of pure fructuronate was provided by Hans J. Nelis from the University of Gent. Other sugars were purchased from Sigma-Aldrich Co.
To construct mutant fragments of the operator, PCR primers were designed to introduce two nucleotide replacements in the UxuR binding site. Primers for pCU108 were 5′-GATATGTTATGTAAATTAATCAACCATTGTTGCGATG-3′ and 5′-CATCGCAACAATGGTTGATTAATTTACATAACATATC-3′ and for pCU109 were 5′-GATATGTTATGTAAATTGGTCGGCCATTGTTGCGATG-3′ and 5′-CATCGCAACAATGGCCGACCAATTTACATAACATATC-3′, using pCU110 as a template. The mutant binding sites were confirmed by sequencing.
Biosynthesis of fructuronate.
Fructuronate was biosynthesized in cell extracts of E. coli ABN14 (pABN13) which overproduces glucuronate isomerase and is deficient for fructuronate oxidoreductase (Fig. 1). The mutant strain was grown in LB medium supplemented with 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside), harvested at mid-log phase, and lysed by sonication. The cell extract was then centrifuged and the supernatant incubated in a 20 mM solution of glucuronate at 37°C for 30 min. The resulting mixture of glucuronate and fructuronate was heat inactivated and filtered (0.45-μm pore size). To prepare fructuronate from this mixture, MOPS minimal medium was added to grow E. coli ABN12 (gntP mutant), which selectively consumed the glucuronate. Likewise, fructuronate was removed from the mixture by growth of E. coli ABN13 (uxaC mutant), leaving only glucuronate. The cells were centrifuged and the supernatants, which were significantly enriched for fructuronate or glucuronate, respectively, were collected and filter sterilized for use in growth experiments. Biochemical confirmation of fructuronate in the enriched sample was prevented by the cell extract components.
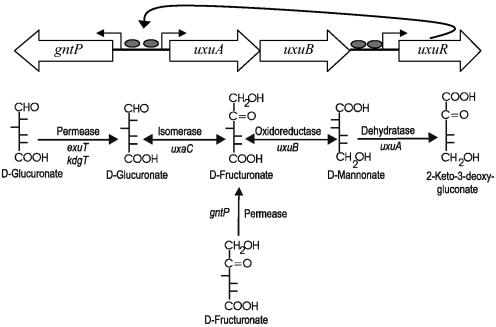
FIG. 1.
Gene map of gntP region (top). Grey ovals indicate putative UxuR binding sites (27). Bottom, proposed pathway for fructuronate metabolism in E. coli.
RESULTS
Transcriptional analysis of the UxuR regulon.
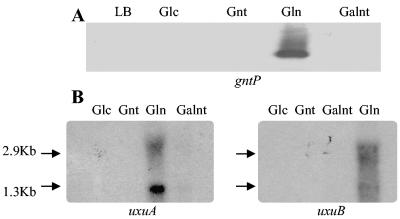
To identify the conditions in which gntP is induced, expression from a single-copy chromosomal gntP-lacZ operon fusion was measured (Table 2). In wild-type cells, gntP was induced fivefold by glucuronate but not by other sugar acids. Northern hybridization analysis confirmed induction of a 1.5-kb gntP transcript (10) by glucuronate (Fig. 2A). The uxuA and uxuB transcripts were also induced by growth on glucuronate (Fig. 2B). The presence of two transcripts that hybridized to the uxuA and uxuB probes suggests that the primary 2.9-kb transcript is processed. Glucuronate induction of gntP and uxuB, as well as eda, which encodes the Entner-Doudoroff aldolase, was confirmed by QPCR (Table 3). These results suggest that transcription of gntP, uxuA, and uxuB is coordinated. Thus, gntP behaves like other known glucuronate-induced genes.
TABLE 2.
Effects of cya, crp, and uxuR mutations on gntP::lacZ expressiona
| E. coli strain | Relevant genotype | β-Galactosidase activity (Miller units)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| None | Glc | Gnt | Galnt | Gln
|
Gln+Glc
|
||||
| None | +cAMP | None | +cAMP | ||||||
| CU110 | wt | 176 | 25 | 50 | 171.5 | 870 | 1,693 | 27 | 1,470 |
| CU111 | uxuR | 1,700 | ND | ND | ND | 394 | ND | ND | ND |
| CU112 | cya | 19 | ND | ND | ND | 13 | 1,985 | 0 | 1,565 |
| CU113 | crp | 8 | ND | ND | ND | 8 | 8 | 0 | 0 |
Growth conditions were in LB medium plus the relevant carbon source (Glc, glucose; Gnt, gluconate; Galnt, galacturonate; Gln, glucuronate; none, no added cAMP). wt, wild type; ND, results have not been determined.
FIG. 2.
A. Northern blot analysis of gntP transcription with a gntP-specific probe. RNA samples from E. coli W1485 grown in LB medium with indicated carbon sources (0.2%): LB, none; Glc, glucose; Gnt, gluconate; Gln, glucuronate; Galnt, galacturonate. B. Northern blot of the E. coli uxuA and uxuB transcripts with gene-specific probes. RNA samples were isolated from E. coli W1485 cells grown in LB medium with the indicated carbon sources (0.2%).
TABLE 3.
Transcript levels of key pathway genes measured by QPCRa
| Substrate |
gntP
|
uxuB
|
edd
|
eda
|
||||
|---|---|---|---|---|---|---|---|---|
| Δ Fold | SD | Δ Fold | SD | Δ Fold | SD | Δ Fold | SD | |
| Gluconate | 2.2 | 0.16 | 0.79 | 0.070 | 53 | 7.4 | 1.7 | 0.040 |
| Glucuronate | 120 | 6.4 | 140 | 7.2 | 0.87 | 0.090 | 1.5 | 0.15 |
| Frn | 88 | 2.0 | 400 | 43 | 0.63 | 0.10 | 4.4 | 0.52 |
| Frn + glucose | 11 | 0.92 | 180 | 27 | 3.5 | 0.35 | 9.5 | 1.1 |
Δ Fold, relative to cells grown on minimal glucose medium. Frn, fructuronate; SD, standard deviation.
Catabolite repression of gntP.
Sequence analysis indicated a CRP binding site in the gntP regulatory region. This location, at position −42 with respect to the previously mapped transcription start site (10), is indicative of class II activation (3). A gntP-lacZ operon fusion was repressed 7- and 3.5-fold in the presence of glucose or gluconate, respectively, by comparison to LB medium alone (Table 2). Also, addition of glucose to cells growing on LB-glucuronate resulted in 30-fold catabolite repression of gntP transcription compared to glucuronate alone (Table 2). QPCR confirmed that the gntP transcript is eightfold higher on minimal fructuronate medium compared with the mixture of fructuronate plus glucose (Table 3). Likewise, the uxuB transcript was more than twofold higher on fructuronate than on fructuronate plus glucose (Table 3). Exogenous cAMP relieved catabolite repression of glucuronate-dependent induction of gntP transcription caused by glucose, presumably by allowing formation of cAMP-CRP complex and activation by binding to the CRP site (Table 2). Exogenous cAMP also caused a twofold induction over that by glucuronate alone (Table 2).
To further examine cAMP-dependent catabolite repression, a gntP-lacZ fusion was constructed in cya and crp mutants. In the wild-type background expression of the gntP-lacZ fusion was approximately 100-fold higher than in either the crp or cya mutants, indicating that cAMP and CRP are required for activation (Table 2). As expected, addition of exogenous cAMP to the cya mutant, but not the crp mutant, relieved catabolite repression. These results demonstrate that transcription of gntP requires activation by the cAMP-CRP complex.
Effect of a uxuR mutation on gntP expression.
To investigate the role of UxuR on the expression of gntP, the gntP-lacZ chromosomal fusion was transduced into an uxuR mutant to create E. coli CU111. The gntP-lacZ fusion in E. coli CU111 was derepressed 10-fold in the absence of glucuronate, in comparison to the wild type (Table 2). Expression of the gntP-lacZ fusion was repressed approximately twofold in the presence of glucuronate compared with the fully induced level in the wild type grown on glucuronate. These results confirm that UxuR is the negative regulator of gntP and suggest that glucuronate, in addition to glucose and gluconate, may be a catabolite repressing sugar. However, there is no evidence that glucuronate is preferred over fructuronate, as growth of E. coli on the mixture does not result in diauxie (data not shown).
Binding of UxuR to the gntP operator.
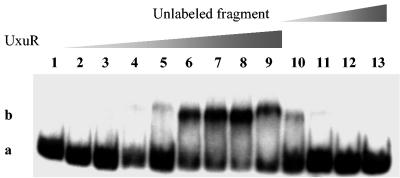
Since mutation of uxuR derepressed gntP expression, we measured binding of UxuR to the gntP regulatory region. Other researchers have searched all known genes involved in hexuronate catabolism to derive a putative UxuR operator consensus sequence, AAATTGGTNNACCAATTT (27). The gntP UxuR operator lies within the promoter region, centered at −22 with respect to the transcriptional start site (10). The UxuR operator of gntP has the sequence ACAATGGTTGACCAATTT, which matches the right-half site exactly and six of eight positions in the left-half site of the consensus binding site. To determine interactions between UxuR and the gntP operator and to identify the inducer of the regulon, gel mobility shift assays were performed. A DNA probe containing the gntP operator was end labeled with 32P and incubated with increasing amounts of UxuR protein. A single band with reduced mobility, corresponding to binding of UxuR, was observed (Fig. 3). Addition of a 200-fold excess of the unlabeled DNA fragment completely abolished the shift.
FIG. 3.
Gel retardation of a 32P-labeled gntP promoter probe by purified UxuR. All lanes contained 8 nmol of a radioactively labeled DNA fragment containing the UxuR operator. Lane 1, no protein. Lanes 2 to 9 contain increasing amounts of UxuR: 0.9, 1.8, 3.6, 7.2, 14.4, 28.8, 57.6, and 115.2 nmol, respectively. Lanes 10 to 13 represent competition experiments using unlabeled gntP fragments: lane 10, 25×; lane 11, 100×; lane 12, 200×; and lane 13, 300× unlabeled fragment (by weight). The shifted band (b) and free DNA (a) are indicated on the left.
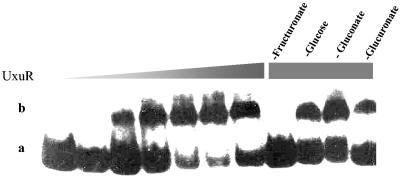
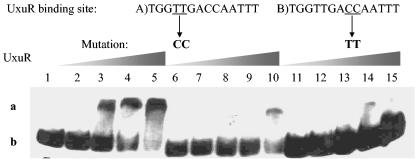
To determine the inducer of the UxuR regulon, various pure sugars were tested for their ability to inhibit formation of the UxuR-DNA complex (Fig. 4). Pure fructuronate, provided by Hans J. Nelis, was the only sugar that inhibited formation of the complex. To prove that UxuR binds specifically to the putative consensus UxuR operator sequence, we constructed mutants carrying two base pair substitutions in either the left- or right-half sites; both mutations drastically reduced binding (Fig. 5). These results indicate that UxuR binding to the UxuR operator is sequence specific and that binding is inhibited by fructuronate.
FIG. 4.
Binding of UxuR to the gntP operator in the presence of various sugars. All lanes contained 8 nmol of radioactively labeled DNA probe. Lane 1 contains no protein and lanes 2 to 7 contain increasing amounts of UxuR protein: 0.9, 1.8, 3.6, 7.2, 14.4, and 28.8 nmol, respectively. Lanes 8 to 11 contain 28.8 nmol of UxuR protein together with 100 mM d-fructuronate (lane 8), d-glucose (lane 9), d-gluconate (lane 10), or d-glucuronate (lane 11). The shifted band (b) and free DNA (a) are indicated on the left.
FIG. 5.
Effect of UxuR binding site mutations on formation of the repressor-operator complex. The wild-type UxuR binding site is shown at the top with the mutated base pairs within the binding sites underlined and the alterations shown in boldface below the wild-type binding site. Lane 1 contains the wild-type DNA probe and no protein. Lanes 2 to 5 contain the wild-type binding site and increasing amounts of UxuR: 0.9, 1.8, 3.6, and 7.2 nmol, respectively. Lanes 6 to 10 contain the DNA probe with a mutation in the left-half site (A) and increasing amounts of UxuR (same concentrations as lanes 2 to 5). Lanes 11 to 15 contain the DNA probe with a mutation in the right-half site (B) with increasing amounts of UxuR (same concentrations as lanes 2 to 5). The shifted band (b) and free DNA (a) are indicated on the left.
Induction of the UxuR regulon by fructuronate.
Since fructuronate inhibits UxuR binding to the gntP operator, we tested whether fructuronate was able to induce genes of the UxuR regulon. QPCR showed that gntP and uxuB were induced 88- and 400-fold, respectively, by fructuronate and 120- and 140-fold, respectively, by glucuronate compared to glucose (Table 3). In addition, fructuronate induced eda approximately fourfold, but edd, which is not in the Ashwell pathway, was not induced (Table 3). Thus, the results indicate that growth on fructuronate induces GntP and the enzymes involved in fructuronate catabolism. Since glucuronate does not inhibit binding of UxuR to the gntP operator, the results confirm that fructuronate formed from glucuronate is responsible for induction of the UxuR regulon during growth on glucuronate.
Mutation of gntP prevents growth on fructuronate.
The wild-type strain grew to a final density of 1.61 (A600 units) on the mixture of glucuronate and fructuronate, which was biosynthesized as described in Materials and Methods (Table 4). Diminished cell yield of the wild type on the glucuronate and fructuronate preparations provided evidence that the biosynthesis strategy worked to selectively remove the countercomponent of the mixture. The ratio of cell yields (A600 1.10 versus 0.49) on the enriched preparations indicated that the original mixture contained glucuronate and fructuronate in approximately a 2:1 ratio. Since the cell yield on the enriched preparations is roughly additive, it appears that E. coli uses the two sugars with similar efficiency. The uxaC mutant had final cell yields on the mixture (A600, 0.57) and on fructuronate (A600, 0.51) equivalent to that of the wild-type strain grown on fructuronate alone (Table 4). The uxaC mutant was unable to grow on glucuronate and grew on fructuronate with a generation time similar to that of the wild type (Table 5). The gntP mutant grew on the mixture and on glucuronate to final densities of A600 1.20 and 1.11, respectively, which is equivalent to that of the wild-type strain grown on glucuronate alone (Table 4). Furthermore, the gntP mutant grew on glucuronate at the same rate as the wild type but was unable to grow on fructuronate (Table 5). Growth of the gntP mutant was not affected on glucose or galacturonate. The results confirm the hypothesis that a gntP mutant cannot grow on fructuronate, presumably due to a defect in fructuronate transport.
TABLE 4.
Final growth yield of wild-type and mutant strains grown on fructuronate, glucuronate, and fructuronate plus glucuronatea
| Substrate | WT
|
uxaC
|
gntP
|
|||
|---|---|---|---|---|---|---|
| A600 | SD | A600 | SD | A600 | SD | |
| Gln | 1.10 | 0.045 | 0.10 | 0.042 | 1.11 | 0.06 |
| Frn | 0.49 | 0.024 | 0.51 | 0.030 | 0.09 | 0.02 |
| Frn + Gln | 1.61 | 0.023 | 0.57 | 0.089 | 1.20 | 0.04 |
Carbon sources biosynthesized and enriched as described in Materials and Methods. Frn, fructuronate; Gln, glucuronate; WT, wild type; SD, standard deviation.
TABLE 5.
Generation times of wild-type and mutant strains grown on sugar acidsa
| Substrate | WT
|
uxaC
|
gntP
|
|||
|---|---|---|---|---|---|---|
| G (min) | SD | G (min) | SD | G (min) | SD | |
| Glucose | 79 | 5 | 78 | 0.10 | 81 | 4.9 |
| Glucuronate | 81 | 8.9 | NG | 81 | 0.2 | |
| Galacturonate | 105 | 4.5 | NG | 106 | 4.3 | |
| Fructuronate | 96 | 1.1 | 105 | 25 | NG | |
G (min), generation time in minutes. NG, no growth; SD, standard deviation; WT, wild type.
DISCUSSION
The primary findings of the present study are that gntP is a member of the UxuR regulon, induced by fructuronate, and that GntP is required for growth on fructuronate. Since GntP is in the major facilitator superfamily of sugar transporters (28) and is a proven sugar acid transporter (10), we conclude that GntP is the transporter for fructuronate. The scheme for fructuronate transport and catabolism is shown in Fig. 1.
The gntP gene is adjacent to and divergently transcribed from the uxuAB operon (2, 10, 24). The results shown in Tables 2 and 3 and Fig. 2 demonstrate that transcription of gntP is induced by glucuronate and fructuronate. Genes in the UxuR regulon are known to be induced by glucuronate via its conversion to fructuronate and are under the negative control of UxuR (17, 26). Derepression of gntP in an uxuR mutant strongly suggests that it is a new member of the UxuR regulon (Table 2). UxuR is known to bind to a consensus operator site that is conserved in several species (27). We identified a putative UxuR binding site overlapping the gntP promoter and confirmed that this site is bound in vitro (Fig. 3 and 5). Furthermore, we demonstrated that binding of the UxuR operator in the gntP regulatory region is specifically inhibited by fructuronate (Fig. 4). Taken together, these results prove that gntP is a member of the UxuR regulon.
The data in Tables 2 and 3 indicate that gntP and, to a lesser extent, uxuB are catabolite repressed by glucose. As expected, cAMP-CRP is required to activate gntP transcription. These results indicate that E. coli prefers glucose over fructuronate and glucuronate. We have observed diauxic growth on a mixture of glucose and glucuronate but no diauxie for growth on the mixture of fructuronate and glucuronate (data not shown).
GntP is an inner membrane protein with 12 membrane-spanning domains (10). GntP was previously categorized as a high-affinity gluconate transporter, but growth on gluconate does not require GntP (data not shown) and gntP is not induced by gluconate (10). Despite the fact that gntP is induced by glucuronate, it does not appear to be the glucuronate transporter, since glucuronate does not compete with gluconate for uptake by GntP in E. coli (10). On the basis of these observations, we postulated that GntP is actually a fructuronate transporter. This hypothesis was confirmed by the finding that a gntP mutant is unable to grow on fructuronate as a sole carbon and energy source (Tables 4 and 5).
The UxuR regulon is best described as a sugar acid catabolism module whose role, first and foremost, is for growth on fructuronate. Fructuronate is the true inducer of the UxuR genes, including gntP, a member of the UxuR regulon that encodes the fructuronate transporter. Thus, catabolism of fructuronate requires only the UxuR regulon and the KdgR regulon, which lies downstream in the catabolic scheme (Fig. 1).
Although glucuronate also induces the UxuR regulon, it does so via its conversion to fructuronate (26). Transport of glucuronate is not mediated by a transporter in the UxuR regulon, but rather by ExuT, which is a member of the ExuR regulon (17). Glucuronate catabolism requires first the ExuR regulon, which controls transport of glucuronate and galacturonate and their conversion to fructuronate and tagaturonate, respectively, and then the UxuR and KdgR regulons. Thus, hexuronate catabolism in E. coli involves three distinct functional modules encoded by the ExuR, UxuR, and KdgR regulons. Within these modules, the pathway enzymes have overlapping functions as well as multiple target substrates. Repressors belonging to the three regulons also act cooperatively in inducing hexuronate metabolism. Previous studies showed that synthesis of enzymes encoded by uxuAB is principally regulated by UxuR and partially regulated by ExuR (26). Our finding that gntP is induced by both fructuronate and glucuronate is consistent with the interplay that exists within the hexuronate catabolic modules. Glucuronate, because it proceeds through a fructuronate intermediate, must induce the UxuR regulon and must also induce the ExuR regulon, which encodes the glucuronate transporter, ExuT, and the first step in its catabolism, UxaC. The UxuR regulon, on the other hand, can be thought of as a module that is principally involved in catabolism of fructuronate, which is transported by GntP.
Sweeney et al. suggested that a gntP mutation diminished the fitness of a human commensal E. coli strain for colonization of the mouse intestine (30). This finding suggested the possibility that fructuronate is important for E. coli colonization. Recently we completed a systematic nutritional characterization of E. coli MG1655 colonization with respect to its carbon nutrition requirements (4). In contrast to the earlier result, we found that a gntP mutant of E. coli MG1655 was perfectly fit for colonization of the mouse intestine. To our knowledge, fructuronate has not been found in the mouse intestine (19). In fact, there are no published reports that describe the natural occurrence of fructuronate. Thus, the ecological importance of GntP as a fructuronate transporter in E. coli remains to be established.
Acknowledgments
This work was supported by grants from the NSF (MCB-9723593) and NIH (RO1-AI48945).
REFERENCES
- 1.Ashwell, G. 1962. Enzymes of glucuronic and galacturonic acid metabolism in bacteria. Methods Enzymol. 5:190-208. [Google Scholar]
- 2.Blanco, C., P. Ritzenthaler, and A. Kolb. 1986. The regulatory region of the uxuAB operon in Escherichia coli K12. Mol. Gen. Genet. 202:112-119. [DOI] [PubMed] [Google Scholar]
- 3.Busby, S., and R. H. Ebright. 1999. Transcription activation by catabolite activator protein (CAP). J. Mol. Biol. 293:199-213. [DOI] [PubMed] [Google Scholar]
- 4.Chang, D. E., D. J. Smalley, D. L. Tucker, M. P. Leatham, W. E. Norris, S. J. Stevenson, A. B. Anderson, J. E. Grissom, D. C. Laux, P. S. Cohen, and T. Conway. 2004. Carbon nutrition of Escherichia coli in the mouse intestine. Proc. Natl. Acad. Sci. USA 101:7427-7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conway, T. 1992. The Entner-Doudoroff pathway: history, physiology and molecular biology. FEMS Microbiol. Rev. 9:1-27. [DOI] [PubMed] [Google Scholar]
- 6.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Entner, N., and M. Doudoroff. 1952. Glucose and gluconic acid oxidation of Pseudomonas saccharophila. J. Biol. Chem. 196:853-862. [PubMed] [Google Scholar]
- 8.Hugouvieux-Cotte-Pattat, N., and J. Robert-Baudouy. 1983. Regulation of expression of the uxu operon and of the uxuR regulatory gene in Escherichia coli K12. J. Gen. Microbiol. 129(Pt. 11):3345-3353. [DOI] [PubMed] [Google Scholar]
- 9.Huijsdens, X. W., R. K. Linskens, M. Mak, S. G. Meuwissen, C. M. Vandenbroucke-Grauls, and P. H. Savelkoul. 2002. Quantification of bacteria adherent to gastrointestinal mucosa by real-time PCR. J. Clin. Microbiol. 40:4423-4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klemm, P., S. Tong, H. Nielsen, and T. Conway. 1996. The gntP gene of Escherichia coli involved in gluconate uptake. J. Bacteriol. 178:61-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lennox, E. S. 1955. Transduction of linked genetic characters of the host by bacteriophage P1. Virology 1:190-206. [DOI] [PubMed] [Google Scholar]
- 12.Luria, S. E., and M. Delbruck. 1943. Mutations of bacteria from virus sensitivity to virus resistance. Genetics 28:491-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marschall, C., and R. Hengge-Aronis. 1995. Regulatory characteristics and promoter analysis of csiE, a stationary phase-inducible gene under the control of sigma S and the cAMP-CRP complex in Escherichia coli. Mol. Microbiol. 18:175-184. [DOI] [PubMed] [Google Scholar]
- 14.Miller, J. H. 1972. Experiments in molecular genetics. Cold Springs Harbor Laboratory Press, Cold Springs Harbor, N.Y.
- 15.Moller, A. K., M. P. Leatham, T. Conway, P. J. Nuijten, L. A. de Haan, K. A. Krogfelt, and P. S. Cohen. 2003. An Escherichia coli MG1655 lipopolysaccharide deep-rough core mutant grows and survives in mouse cecal mucus but fails to colonize the mouse large intestine. Infect. Immun. 71:2142-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nemoz, G., J. Robert-Baudouy, and F. Stoeber. 1976. Physiological and genetic regulation of the aldohexuronate transport system in Escherichia coli. J. Bacteriol. 127:706-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peekhaus, N., and T. Conway. 1998. Positive and negative transcriptional regulation of the Escherichia coli gluconate regulon gene gntT by GntR and the cyclic AMP (cAMP)-cAMP receptor protein complex. J. Bacteriol. 180:1777-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peekhaus, N., and T. Conway. 1998. What's for dinner?: Entner-Doudoroff metabolism in Escherichia coli. J. Bacteriol. 180:3495-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peekhaus, N., S. Tong, J. Reizer, M. H. Saier, Jr., E. Murray, and T. Conway. 1997. Characterization of a novel transporter family that includes multiple Escherichia coli gluconate transporters and their homologues. FEMS Microbiol. Lett. 147:233-238. [DOI] [PubMed] [Google Scholar]
- 21.Portalier, R., J. Robert-Baudouy, and F. Stoeber. 1980. Regulation of Escherichia coli K-12 hexuronate system genes: exu regulon. J. Bacteriol. 143:1095-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powell, B. S., M. P. Rivas, D. L. Court, Y. Nakamura, and C. L. Turnbough, Jr. 1994. Rapid confirmation of single copy lambda prophage integration by PCR. Nucleic Acids Res. 22:5765-5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ritzenthaler, P., and M. Mata-Gilsinger. 1983. Multiple regulation involved in the expression of the uxuR regulatory gene in Escherichia coli K-12. Mol. Gen. Genet. 189:351-354. [DOI] [PubMed] [Google Scholar]
- 24.Ritzenthaler, P., and M. Mata-Gilsinger. 1983. Use of uxu-lac fusion strains to study the regulation of the uxuAB operon in Escherichia coli K12. J. Gen. Microbiol. 129(Pt 11):3335-3343. [DOI] [PubMed] [Google Scholar]
- 25.Ritzenthaler, P., M. Mata-Gilsinger, and F. Stoeber. 1980. Construction and expression of hybrid plasmids containing Escherichia coli K-12 uxu genes. J. Bacteriol. 143:1116-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robert-Baudouy, J., R. Portalier, and F. Stoeber. 1981. Regulation of hexuronate system genes in Escherichia coli K-12: multiple regulation of the uxu operon by exuR and uxuR gene products. J. Bacteriol. 145:211-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodionov, D. A., A. A. Mironov, A. B. Rakhmaninova, and M. S. Gelfand. 2000. Transcriptional regulation of transport and utilization systems for hexuronides, hexuronates and hexonates in gamma purple bacteria. Mol. Microbiol. 38:673-683. [DOI] [PubMed] [Google Scholar]
- 28.Saier, M. H., Jr., J. T. Beatty, A. Goffeau, K. T. Harley, W. H. Heijne, S. C. Huang, D. L. Jack, P. S. Jahn, K. Lew, J. Liu, S. S. Pao, I. T. Paulsen, T. T. Tseng, and P. S. Virk. 1999. The major facilitator superfamily. J. Mol. Microbiol. Biotechnol. 1:257-279. [PubMed] [Google Scholar]
- 29.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 30.Sweeney, N. J., P. Klemm, B. A. McCormick, E. Moller-Nielsen, M. Utley, M. A. Schembri, D. C. Laux, and P. S. Cohen. 1996. The Escherichia coli K-12 gntP gene allows E. coli F-18 to occupy a distinct nutritional niche in the streptomycin-treated mouse large intestine. Infect. Immun. 64:3497-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tucker, D. L., N. Tucker, Z. Ma, J. W. Foster, R. L. Miranda, P. S. Cohen, and T. Conway. 2003. Genes of the GadX-GadW regulon in Escherichia coli. J. Bacteriol. 185:3190-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]