Abstract
White matter (WM) degeneration has been found during the course of cognitive decline in both Alzheimer's disease (AD) and amnestic mild cognitive impairment (aMCI), however, it is unclear whether there are different WM microstructural abnormalities between two subtypes of aMCI, including single domain aMCI (aMCI-s) and multiple domain aMCI (aMCI-m). Thirty-two patients of aMCI single-domain (aMCI-s), twenty-three patients of aMCI multiple-domain (aMCI-m) and twenty-three healthy normal controls (NC) participated in this study. Neuropsychological measures and diffusion tensor imaging (DTI) data were acquired from each subject and tract-based spatial statistics (TBSS) was implemented. It was found that both aMCI groups showed significantly reduced fractional anisotropy (FA) in the right superior longitudinal fasciculus (SLF) than NC. It was also identified that, as compared to aMCI-m, aMCI-s showed significantly decreased FA in the left SLF, left uncinate fasciculus (UF) and left inferior longitudinal fasciculus (ILF), while significantly increased FA in the left anterior thalamic radiation (ATR). The correlation analysis showed that FA values in the regions with group difference were significantly correlated with cognitive functions as measured by Boston naming test and trail making test. These results suggested that the variations of aMCI may be differentiated by FA indexes and DTI may help to understand why specific signs and symptoms occur in patients.
Introduction
Alzheimer’s disease (AD) is a common dementia in elderly populations, and amnestic mild cognitive impairment (aMCI) often represents a transitional stage between normal aging and early dementia [1,2]. Patients with aMCI are at higher risks of evolving toward AD (approximately 10%-15% per year), up to 80% of aMCI individuals would progress to dementia after 6 years [3]. There are two types of aMCI: single-domain of aMCI (aMCI-s) and multiple-domain of aMCI (aMCI-m). aMCI-s have isolated memory impairment, whereas aMCI-m have impairments in multiple cognitive domains including memory, language, executive functions, visuospatial skills, etc. [4]. It is necessary to differential diagnose of aMCI-s and aMCI-m based on objective imaging characteristics not only for the in-depth understanding of degenerative neural changes of aMCI but also for the different treatment and judgment of conversion for the two subtypes.
AD was widely reported to have the neurodegenerative effects on cerebral white matter (WM) microstructure [5,6]. Diffusion tensor imaging (DTI) has been demonstrated to be a useful tool for the early detection of AD as WM changes have been detected in early AD or prodromal AD stages which is known as MCI stages [7]. There are many studies in AD and MCI by DTI, and the differences of fractional anisotropy (FA) and mean diffusivity (MD) were observed in many cortical regions [8–13]. Furthermore, it was also found that WM abnormalities in patients were associated with various cognitive dysfunctions [14,15]. However, less study have focused on patients with aMCI by DTI [16–18] and the findings are heterogeneous. In particular, the WM changes of the two subtypes of aMCI, i.e., aMCI-s and aMCI-m, were not studied so far.
The main goal of this study is to investigate the difference of diffusion indices between aMCI-s and aMCI-m by using tract-based spatial statistics (TBSS), which could improve the sensitivity, objectivity and interpretability of diffusion imaging results at group level [19]. Given aMCI is a kind of disconnection syndrome and significant difference between aMCI-s and aMCI-m, it is hypothesized that both aMCI-s and aMCI-m may have significant alterations in DTI metrics than normal controls (NC), and some DTI indexes may differentiate between aMCI-s and aMCI-m.
Subjects and Methods
Subjects
Thirty-two aMCI-s (70.75±6.40 years old; 15 females) and 23 aMCI-m (70.91±8.07 years old; 10 females) patients were screened from the Department of Neurology of Xuanwu Hospital, Capital Medical University from January 2011 to March 2015. Twenty-three NC (64.61±9.11 years old; 13 females) were recruited from local residents. Written informed consents were obtained from all participants or their relatives before the MRI scan. This study was approved by the Institutional Review Board of Xuanwu Hospital, Capital Medical University.
The patients with aMCI were diagnosed according to Petersen’s criteria [4] and National Institute on Aging- Alzheimer’s Association criteria for MCI due to AD [20] as following: (a) memory complaint; (b) objective memory impairment; (c) near-normal performance on general cognition and preserved daily life activities (as measured by Activity of Daily Living Scale (ADL)); (d) Clinical Dementia Rating (CDR) score of 0.5; (e) failure to meet the criteria for dementia according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition, revised (DSM-IV) [21]; (f) hippocampal atrophy observed (as measured by the Medial Temporal lobe Atrophy scale (MTA-scale)) and (h) the Han nationality, right-handed (the Edinburgh handedness scale score > 40 points). The diagnosis of aMCI-s and aMCI-m were fulfilled according to Petersen’s diagnostic criteria [4].
Participants of NC were cognitively normal and had a CDR of 0. Subjects were excluded if they met the following clinical characteristics: (a) those who have a clear history of stroke (Hachinski Ischemic Scale score (HIS)> 7 points); (b) severe depression (Hamilton Depression Rating Scale score (HAMD) > 24 points); (c) cognitive impairment caused by traumatic brain injury; (d) other nervous system diseases, which could cause cognitive impairment; (e) systemic diseases, which could cause cognitive impairment; (f) a history of psychosis or congenital mental growth retardation; and (g) those who cannot corporate with neuropsychological tests or have any contraindication to Magnetic Resonance Imaging (MRI).
All participants underwent a series of neurological tests and a battery of neuropsychological assessments by two experienced neurologists (JL and YH), including mini-mental state examination (MMSE) [22], Montreal cognitive assessment (MoCA) [23,24], Auditory Verbal Learning Test (AVLT of Chinese version; short delay free recall) [25], Boston naming test (BNT) used by Cheung RW et al [26], trail making test (TMT) [27], clock drawing test (CDT; 3-point) [28] and CDR [29]. Specifically, TMT is a neuropsychological test of visual attention and task switching [27]. It consists of two parts in which the subject is instructed to connect a set of 25 dots as quickly as possible while still maintaining accuracy. There are two parts to the test: in the first part, the targets are all numbers (1, 2, 3, etc.) and the test taker needs to connect them in sequential order; in the second part, the subject alternates between numbers and letters (1, A, 2, B, etc.). The time taken to complete the test is used to be the primary performance metric. We then used 3-point CDT to test the visuospatial skill, TMT to evaluate the executive function, BNT to assess the language skill, and AVLT (short delay free recall) to measure the memory function. In addition, the prevalence of vascular factors such as hypertension, hypercholesterolemia, and heart attack, did not differ among the groups. All subjects had no history of a psychiatric or neuropsychological disease.
MRI Acquisition
MRI data acquisition was performed on a 3-Tesla scanner (Siemens Medical Solutions, Erlangen, Germany). MRI data were collected the day after the subjects finished the cognitive testing. Sedation was forbidden during the MRI scanning. Foam padding and headphones were used to limit head motion and reduce scanner noise. The 3D T1-weighted anatomical image was acquired with a magnetization-prepared rapid gradient echo (MPRAGE) method in the following parameters: repetition time (TR)/echo time (TE)/inversion time (TI) / flip angle (FA) = 1900 ms/2.2 ms/900 ms/9°, acquisition matrix = 224 × 256 × 176, voxel size = 1 × 1 × 1 mm3. DTI parameters were: 12 non-linear directions (b-value = 1000 s/mm2) with 1 non-diffusion weighting acquisition, TR/TE = 6000 ms /85 ms, 30 axial slices, slice thickness = 5 mm, FOV = 256 × 256 mm2, acquisition matrix = 128×128, number of averages = 4.
DTI Data Processing
DTI data were pre-processed and analyzed using the FMRIB's Diffusion Toolbox in FSL software (FMRIB Software Library, http://www.fmrib.ox.ac.uk/fsl) [30]. First, the original data was corrected for the effects of head movement and eddy currents using eddy correct command by applying an affine alignment of each diffusion-weighted image to the first b = 0 image. The non-brain tissue was removed by using the Brain Extraction Tool (BET) [31]. Then, the diffusion tensor elements were estimated and the corresponding FA and MD value of each voxel was calculated.
TBSS Analysis
Tract-based spatial statistics of FA and MD images were adopted by using TBSS in FSL software [19]. FA images from all individuals were registered to Montreal Neurological Institute (MNI) 152 template using FNIRT, the non-linear registration tool in FSL. A mean FA image of all aligned FA images was calculated and then 'skeletonized' to create the study-specific mean FA skeleton, which represents the centers of all white matter tracts common for all subjects. Then, each individual’s FA and MD images were projected onto the skeleton. Voxel-wise statistics across subjects were calculated for each point on the skeleton.
Statistical Analysis
Group statistical analysis was conducted only on voxels within the white-matter skeleton. Differences in FA and MD between aMCI-s, aMCI-m and NC were assessed by using analysis of covariance (ANCOVA), with age, gender and education level as covariates of no-interest. Nonparametric permutation test was used based on 5000 random permutations to generate a null distribution for each contrast. Threshold-free cluster enhancement (TFCE) was used in all comparisons to correct for multiple comparisons. The clusters with a TFCE-corrected P-value of less than 0.01 and a minimum cluster size of 50 contiguous voxels were reported. The John Hopkinson University (JHU) white-matter atlas [32] was applied to identify the names of WM bundles. Each cluster showed significant between-group difference was defined as a region of interest (ROI) and the confirmative analysis was then performed on each ROI to see the detailed relative patterns among the three groups. Finally, the partial correlation analyses were performed between mean WM index of each ROI and neuropsychological measures, including MMSE, MoCA, AVLT, BNT, TMT and CDT, with gender, age and education level as covariates. The threshold for the correlation analysis is corrected by using the Bonferroni corrections, i.e. (0.05/the number of ROIs).
Results
Demographic Information
As shown in Table 1, there were significant differences in age and education level among the three groups including NC, aMCI-s and aMCI-m, but no gender difference. There were also significant differences of AVLT, MMSE, MoCA (as well as CDR) among the three groups, however, these differences were mainly driven by the NC group while there were no significant difference between aMCI-s and aMCI-m. In particular, the significant group difference in BNT, TMT and CDT were found among the three groups, which then contributed to define the aMCI subtypes.
Table 1. Demographic characteristics of the three groups of participants including NC, aMCI-s and aMCI-m.
| NC | aMCI-s | aMCI-m | p value | |
|---|---|---|---|---|
| Subjects (m/f) | 23(10/13) | 32(17/15) | 23(13/10) | 0.661* |
| Age | 64.61±9.11 | 70.75±6.40 | 70.91±8.07 | 0.009# |
| Years of education | 11.43±3.31 | 8.16±3.74 | 10.26±3.56 | 0.005# |
| AVLT | 10.09±2.32 | 3.28±2.05 | 4.35±3.28 | <0.001# |
| MMSE | 28.43±1.50 | 24.09±3.30 | 24.48±3.93 | <0.001# |
| MoCa | 26.61±1.83 | 19.84±4.20 | 20.43±4.32 | <0.001# |
| CDR | 0±0 | 0.5±0 | 0.5±0 | - |
| BNT | 29.26±0.84 | 27.78±1.31 | 23.39±2.06 | <0.001# |
| TMT | 71.04±35.72 | 78.75±24.63 | 113.17±28.87 | <0.001# |
| CDT | 3±0 | 2.81±0.39 | 2.04±0.75 | <0.001# |
Values are means±SD. AVLT: the Auditory Verbal Learning Test (AVLT of Chinese version); MMSE: Mini-Mental State Examination; MoCA: the Montreal cognitive assessment; CDR: the Clinical Dementia Rating Scale; BNT: the Boston naming test; TMT: the trail making test; CDT: clock drawing test. The AVLT, MMSE, MoCA, CDT and BNT are based on number correct, CDR based on comprehensive rating scale, and TMT based on seconds.
* The p value was obtained using a Pearson x2 two-tailed test, with continuity correction for n < 5.
# The p value was obtained using one-way ANOVA.
DTI Results
ANCOVA results showed that there were significant FA differences among the three groups including NC, aMCI-s and aMCI-m in the bilateral superior longitudinal fasciculus (SLF), left uncinate fasciculus (UF), left anterior thalamic radiation (ATR) and left inferior longitudinal fasciculus (ILF) (Fig 1 and Table 2). No cluster showed significant MD difference among the three groups.
Fig 1. Regions showing significant FA differences among NC, aMCI-s and aMCI-m based on ANCOVA.
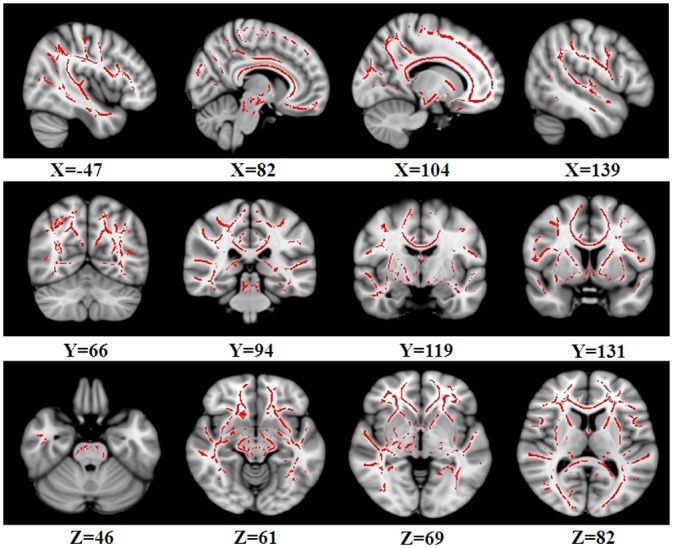
The threshold was set at a TFCE-corrected P-value of less than 0.01 and a minimum cluster size of 50 contiguous voxels. Left is the left.
Table 2. Regions showing significant FA differences among NC, aMCI-s and aMCI-m based on ANCOVA.
The clusters with a TFCE-corrected P-value of less than 0.01 and a minimum cluster size of 50 contiguous voxels were reported.
| Anatomical region | MNI | Voxels | p-value | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Superior longitudinal fasciculus R | 47 | 117 | 46 | 64041 | <0.001 |
| Uncinate fasciculus L | -82 | 131 | 61 | 368 | <0.001 |
| Superior longitudinal fasciculus L | -138 | 66 | 69 | 99 | 0.002 |
| Anterior thalamic radiation L | -104 | 94 | 82 | 82 | 0.004 |
| Inferior longitudinal fasciculus L | -144 | 119 | 53 | 64 | 0.003 |
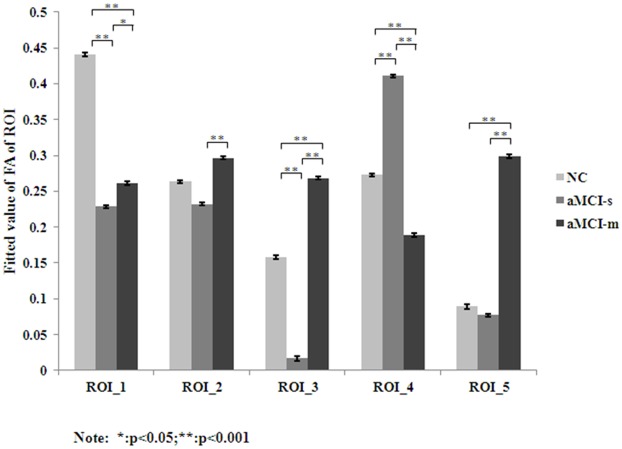
To further specify the variations in FA index, the activated clusters were defined as the regions of interest (ROI) and then post-hoc pair-wise comparisons were performed on mean FA values of each ROI (Fig 2). It was found that both aMCI-s and aMCI-m showed significant reduced FA than NC in the right SLF. It was also found that, in contrast to aMCI-m, aMCI-s showed significantly decreased FA in the left UF, left SLF and left ILF; while increased FA in left ATR.
Fig 2. Paired-wise comparison of FA values in different ROIs.
ROI were defined based on the activated clusters of ANCOVA (Table 1). * represents p<0.05; ** represents p < 0.001.
Correlations between FA and Neuropsychological Measurements
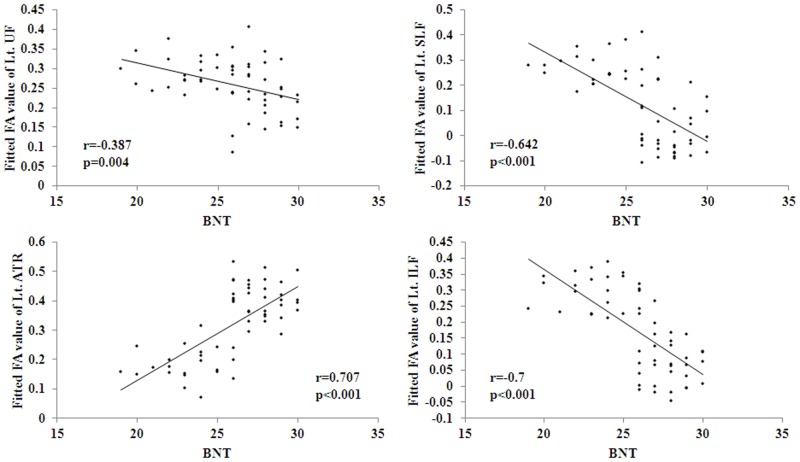
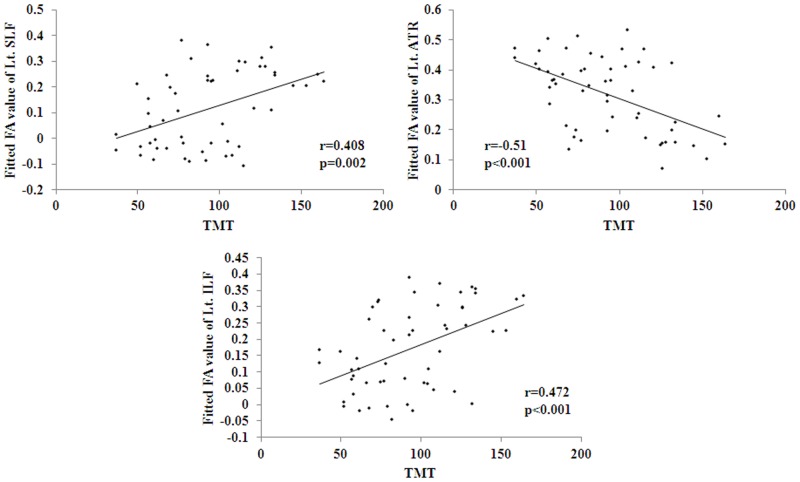
The partial correlation analyses with gender, age and education level as covariates were performed between mean FA of each ROI and neuropsychological measures including MMSE, MoCA, AVLT, BNT, TMT and CDT. It was found that BNT was negatively correlated with FA in the left UF, SLF and ILF, while positively correlated with FA in the left ATR (Fig 3). It was also detected that TMT was negatively correlated with FA in the left ATR, while positively correlated with FA in the left SLF and ILF (Fig 4). All the other correlations were not significant.
Fig 3. Significant correlations between FA values and BNT measures were found to be negative in Lt. UF, Lt. SLF and Lt. ILF, and positive in Lt. ATR. BNT: the Boston naming test.
Fig 4. Significant correlations between FA values and TMT scales were found to be negative in Lt. ATR, and positive in Lt. SLF and Lt. ILF. TMT: the trail making test.
Discussion
To our knowledge, this is the first study to examine the WM difference between the subtypes of aMCI. Our results showed that aMCI-s significantly differed from aMCI-m in some WM tracts, and the FA differences were associated with the cognitive deficits of patients. Particularly, some possible compensation effects were also identified. These results have added new evidence to the disconnection mechanism of aMCI [33–35], and further suggest that the two subtypes of aMCI had different structural connection damage, and FA may be a potential biomarker to differentiate aMCI-s from aMCI-m.
A larger cluster including the right SLF showed significant decreased FA in both aMCI-s and aMCI-m as compared to NC. This has added new evidences to the disconnection mechanism of MCI/AD. However, it was also found that a cluster in the left SLF indicated the different pattern that, in contrast to NC, aMCI-m showed increased FA while aMCI-s showed decreased FA. Specifically, FA values in the left SLF were significantly correlated with BNT and TMT, which suggested that the abnormality in the left SLF associated with the language and attention ability of aMCI patients. In particular, both the positive correlation between FA of the left SLF and TMT and the negative correlation between FA of the left SLF and BNT might indicate an effort of functional compensation (based on structural plasticity) in aMCI-m patients, although the language and attention ability of aMCI-m have been substantial damaged. This is totally congruent with the facts that SLF has a broad neuroanatomical extent, connecting the frontal, parietal, and temporal lobes [36], and plays an important role in higher-level cognitive functions including language [37–39]. However, we cannot exclude the possibility that white matter hyperintensity (WMH) in this area contribute for the abnormality in the left SLF, as the area is often affected by WMH.
This study found no significant alterations in the UF between NC and the two types of aMCI, although the UF was reported to be impaired in AD [40,41]. Particularly, FA values in the left UF might contribute to the differential diagnosis of the subtypes of aMCI, as there was significant FA difference between aMCI-s and aMCI-m. The UF is known for its involvement in human language functions [42–44], as it connects brain regions that have putative functions in language: the anterior temporal lobes and portions of the frontal lobes, both of which have been proposed to encode, store and retrieve semantic knowledge. In line with this, the significant increased FA in the UF in aMCI-m, in contrast to aMCI-s, might imply a kind of compensation mechanism, as evidenced by the negative correlation between FA in the UF and BNT.
The left ILF showed significant higher FA in aMCI-m as compared to aMCI-s and NC. It should be noted that the pattern in the left ILF was similar to that of the left UF. Actually, this might be explained by the facts that the ILF anteriorly joins the UF to relay information to the orbitofrontal brain [38], which means that the two fiber bundles linked and cooperated with each other to support some cognitive functions. In particular, the ILF is related to object cognition, verbal and visual memory as well as visuospatial cognition [8,45–47], and impairment of the ILF may induce thought disorders and cognitive impairment [48]. Thus, together with the significant correlations between FA in the left ILF and BNT as well as TMT, these results might also suggest a compensation mechanism in aMCI-m.
The ATR consists of fibers connecting mediodorsal and anterior thalamic nuclei to the prefrontal cortex and the anterior cingulate cortex. The left ATR in this study showed the different abnormal pattern from the other identified bundles. In contrast to NC, aMCI-m showed decreased FA while aMCI-s indicated increased FA. Further, the correlation between FA in the left ATR and BNT as well as TMT provided the possible explanation that the impairment of the left ATR contributes to the cognitive dysfunction in aMCI-m. Based on the identical logic to the aforementioned discussion, the relative higher FA in aMCI-s might indicate the compensation mechanism. These explanations were totally consist with the previous reports of the role of the ATR in cognition that the ATR is involved in working memory, executive function and planning complex behaviors [49,50]. This finding that the left ATR appears to be completely spared in aMCI-s but not in aMCI-m is also congruent with a previous work in CADASIL, which has shown that strategic lesions in this area can have consequences for impairments in multiple domains, including processing speed [51].
There were still some limitations in this study. First, the WMH within the white matter tracts assessed may substantially affect FA values. However, in this study, the WM lesions were not evaluated quantitatively and used as covariates to control for their effects when estimating the burden of FA on cognitive decline. Thus, it should be acknowledged that the findings are not necessarily independent of what is already known concerning the effects of WMH on cognitive performance. Second, given that the diffusion was 12 directions and 5mm thickness, which is not a optimized DTI protocol for high diffusion typically applied via TBSS. New experiment should be made to further validate the current findings with the optimized protocol. Third, this study was a cross-sectional design, and thus could not reveal the possible different progress characteristics of aMCI-s and aMCI-m. Furthermore, it was reported that, a novel technique, diffusion kurtosis imaging (DKI), enables characterization of non-Gaussian water diffusion behavior, and is considered to be more sensitive than DTI [52]. Given these advantages, we will also include DKI scan in the following studies. Finally, the current results were attained based on the Caucasian brain atlas. As the morphological characteristics of the western and Chinese populations are significantly different, in the future, the statistical Chinese brain atlas, such as Chinese2020 [53], may be used.
Conclusion
Although there are common FA abnormalities between aMCI-s and aMCI-m in contrast to NC, the two types of aMCI own their own DTI characteristics separately. The findings in this study suggest that DTI might (in addition to traditional imaging and neuropsychological testing) serve as a sensitive technique for the differential diagnosis of aMCI-s and aMCI-m.
Acknowledgments
This article was supported by National Natural Science Foundation of China (Grant Nos. 81301208, 81341088, 61473196, 31371007, 81430037), the Specialized Research Fund for the Doctoral Program of Higher Education (20131107120002), Foundation of Clinical and basic cooperation of Capital Medical University (No. 13JL70). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper.
Funding Statement
This article was supported by National Natural Science Foundation of China (Grant Nos. 81301208, 81341088, 61473196, 31371007, 81430037), the Specialized Research Fund for the Doctoral Program of Higher Education (20131107120002), Foundation of Clinical and Basic Cooperation of Capital Medical University (No. 13JL70), and Beijing Nova Program (Z12111000250000, Z131107000413120). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Petersen RC, Negash S. Mild cognitive impairment: an overview. CNS Spectr. 2008; 13: 45–53. [DOI] [PubMed] [Google Scholar]
- 2.Liang P, Xiang J, Liang H, Qi Z, Li K, Alzheimer's Disease NeuroImaging I. Altered amplitude of low-frequency fluctuations in early and late mild cognitive impairment and Alzheimer's disease. Curr Alzheimer Res. 2014; 11: 389–398. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999; 56: 303–308. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004; 256: 183–194. 10.1111/j.1365-2796.2004.01388.x [DOI] [PubMed] [Google Scholar]
- 5.Brun A, Englund E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann Neurol. 1986; 19: 253–262. 10.1002/ana.410190306 [DOI] [PubMed] [Google Scholar]
- 6.Englund E, Brun A, Alling C. White matter changes in dementia of Alzheimer's type. Biochemical and neuropathological correlates. Brain. 1988; 111 (Pt 6): 1425–1439. [DOI] [PubMed] [Google Scholar]
- 7.Rose SE, Janke AL, Chalk JB. Gray and white matter changes in Alzheimer's disease: a diffusion tensor imaging study. J Magn Reson Imaging. 2008; 27: 20–26. 10.1002/jmri.21231 [DOI] [PubMed] [Google Scholar]
- 8.Kiuchi K, Morikawa M, Taoka T, Nagashima T, Yamauchi T, Makinodan M, et al. Abnormalities of the uncinate fasciculus and posterior cingulate fasciculus in mild cognitive impairment and early Alzheimer's disease: a diffusion tensor tractography study. Brain Res. 2009; 1287: 184–191. 10.1016/j.brainres.2009.06.052 [DOI] [PubMed] [Google Scholar]
- 9.Pievani M, Agosta F, Pagani E, Canu E, Sala S, Absinta M, et al. Assessment of white matter tract damage in mild cognitive impairment and Alzheimer's disease. Hum Brain Mapp. 2010; 31: 1862–1875. 10.1002/hbm.20978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brueggen K, Dyrba M, Barkhof F, Hausner L, Filippi M, Nestor PJ, et al. Basal Forebrain and Hippocampus as Predictors of Conversion to Alzheimer's Disease in Patients with Mild Cognitive Impairment—A Multicenter DTI and Volumetry Study. J Alzheimers Dis. 2015; 48: 197–204. 10.3233/JAD-150063 [DOI] [PubMed] [Google Scholar]
- 11.Ito K, Sasaki M, Takahashi J, Uwano I, Yamashita F, Higuchi S, et al. Detection of early changes in the parahippocampal and posterior cingulum bundles during mild cognitive impairment by using high-resolution multi-parametric diffusion tensor imaging. Psychiatry Res. 2015; 231: 346–352. 10.1016/j.pscychresns.2015.01.020 [DOI] [PubMed] [Google Scholar]
- 12.Nowrangi MA, Rosenberg PB. The fornix in mild cognitive impairment and Alzheimer's disease. Front Aging Neurosci. 2015; 7: 1 10.3389/fnagi.2015.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishioka C, Poh C, Sun SW. Diffusion tensor imaging reveals visual pathway damage in patients with mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2015; 45: 97–107. 10.3233/JAD-141239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delbeuck X, Collette F, Van der Linden M. Is Alzheimer's disease a disconnection syndrome? Evidence from a crossmodal audio-visual illusory experiment. Neuropsychologia. 2007; 45: 3315–3323. 10.1016/j.neuropsychologia.2007.05.001 [DOI] [PubMed] [Google Scholar]
- 15.Fellgiebel A, Schermuly I, Gerhard A, Keller I, Albrecht J, Weibrich C, et al. Functional relevant loss of long association fibre tracts integrity in early Alzheimer's disease. Neuropsychologia. 2008; 46: 1698–1706. 10.1016/j.neuropsychologia.2007.12.010 [DOI] [PubMed] [Google Scholar]
- 16.Oishi K, Akhter K, Mielke M, Ceritoglu C, Zhang J, Jiang H, et al. Multi-modal MRI analysis with disease-specific spatial filtering: initial testing to predict mild cognitive impairment patients who convert to Alzheimer's disease. Front Neurol. 2011; 2: 54 10.3389/fneur.2011.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie Y, Cui Z, Zhang Z, Sun Y, Sheng C, Li K, et al. Identification of Amnestic Mild Cognitive Impairment Using Multi-Modal Brain Features: A Combined Structural MRI and Diffusion Tensor Imaging Study. J Alzheimers Dis. 2015; 47: 509–522. 10.3233/JAD-150184 [DOI] [PubMed] [Google Scholar]
- 18.Hiyoshi-Taniguchi K, Oishi N, Namiki C, Miyata J, Murai T, Cichocki A, et al. The Uncinate Fasciculus as a Predictor of Conversion from Amnestic Mild Cognitive Impairment to Alzheimer Disease. J Neuroimaging. 2015; 25: 748–753. 10.1111/jon.12196 [DOI] [PubMed] [Google Scholar]
- 19.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006; 31: 1487–1505. 10.1016/j.neuroimage.2006.02.024 [DOI] [PubMed] [Google Scholar]
- 20.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011; 7: 270–279. 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gmitrowicz A, Kucharska A. [Developmental disorders in the fourth edition of the American classification: diagnostic and statistical manual of mental disorders (DSM IV—optional book)]. Psychiatr Pol. 1994; 28: 509–521. [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975; 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 23.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005; 53: 695–699. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 24.Lu J, Li D, Li F, Zhou A, Wang F, Zuo X, et al. Montreal cognitive assessment in detecting cognitive impairment in Chinese elderly individuals: a population-based study. J Geriatr Psychiatry Neurol. 2011; 24: 184–190. 10.1177/0891988711422528 [DOI] [PubMed] [Google Scholar]
- 25.Guo Q, Zhao Q, Chen M, Ding D, Hong Z. A comparison study of mild cognitive impairment with 3 memory tests among Chinese individuals. Alzheimer Dis Assoc Disord. 2009; 23: 253–259. 10.1097/WAD.0b013e3181999e92 [DOI] [PubMed] [Google Scholar]
- 26.Cheung RW, Cheung MC, Chan AS. Confrontation naming in Chinese patients with left, right or bilateral brain damage. J Int Neuropsychol Soc. 2004; 10: 46–53. 10.1017/S1355617704101069 [DOI] [PubMed] [Google Scholar]
- 27.Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol. 1955; 19: 393–394. [DOI] [PubMed] [Google Scholar]
- 28.Sunderland T, Hill JL, Mellow AM, Lawlor BA, Gundersheimer J, Newhouse PA, et al. Clock drawing in Alzheimer's disease. A novel measure of dementia severity. J Am Geriatr Soc. 1989; 37: 725–729. [DOI] [PubMed] [Google Scholar]
- 29.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993; 43: 2412–2414. [DOI] [PubMed] [Google Scholar]
- 30.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004; 23 Suppl 1: S208–219. [DOI] [PubMed] [Google Scholar]
- 31.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002; 17: 143–155. 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008; 39: 336–347. 10.1016/j.neuroimage.2007.07.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang P, Wang Z, Yang Y, Jia X, Li K. Functional disconnection and compensation in mild cognitive impairment: evidence from DLPFC connectivity using resting-state fMRI. PLoS One. 2011; 6: e22153 10.1371/journal.pone.0022153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang P, Wang Z, Yang Y, Li K. Three subsystems of the inferior parietal cortex are differently affected in mild cognitive impairment. J Alzheimers Dis. 2012; 30: 475–487. 10.3233/JAD-2012-111721 [DOI] [PubMed] [Google Scholar]
- 35.Liang P, Li Z, Deshpande G, Wang Z, Hu X, Li K. Altered causal connectivity of resting state brain networks in amnesic MCI. PLoS One. 2014; 9: e88476 10.1371/journal.pone.0088476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005; 57: 8–16. 10.1002/ana.20319 [DOI] [PubMed] [Google Scholar]
- 37.Geldmacher DS, Quigg M, Elias WJ. MR tractography depicting damage to the arcuate fasciculus in a patient with conduction aphasia. Neurology. 2007; 69: 321; author reply 321–322. 10.1212/01.wnl.0000275278.38229.7b [DOI] [PubMed] [Google Scholar]
- 38.Catani M, Piccirilli M, Cherubini A, Tarducci R, Sciarma T, Gobbi G, et al. Axonal injury within language network in primary progressive aphasia. Ann Neurol. 2003; 53: 242–247. 10.1002/ana.10445 [DOI] [PubMed] [Google Scholar]
- 39.Wise RJ. Language systems in normal and aphasic human subjects: functional imaging studies and inferences from animal studies. Br Med Bull. 2003; 65: 95–119. [DOI] [PubMed] [Google Scholar]
- 40.Taoka T, Iwasaki S, Sakamoto M, Nakagawa H, Fukusumi A, Myochin K, et al. Diffusion anisotropy and diffusivity of white matter tracts within the temporal stem in Alzheimer disease: evaluation of the "tract of interest" by diffusion tensor tractography. AJNR Am J Neuroradiol. 2006; 27: 1040–1045. [PMC free article] [PubMed] [Google Scholar]
- 41.Yasmin H, Nakata Y, Aoki S, Abe O, Sato N, Nemoto K, et al. Diffusion abnormalities of the uncinate fasciculus in Alzheimer's disease: diffusion tensor tract-specific analysis using a new method to measure the core of the tract. Neuroradiology. 2008; 50: 293–299. 10.1007/s00234-007-0353-7 [DOI] [PubMed] [Google Scholar]
- 42.Schmahmann JD, Smith EE, Eichler FS, Filley CM. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci. 2008; 1142: 266–309. 10.1196/annals.1444.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Catani M, Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex. 2008; 44: 953–961. 10.1016/j.cortex.2008.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain. 2013; 136: 1692–1707. 10.1093/brain/awt094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005; 6: 691–702. 10.1038/nrn1747 [DOI] [PubMed] [Google Scholar]
- 46.Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009; 46: 530–541. 10.1016/j.neuroimage.2009.01.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shinoura N, Suzuki Y, Tsukada M, Katsuki S, Yamada R, Tabei Y, et al. Impairment of inferior longitudinal fasciculus plays a role in visual memory disturbance. Neurocase. 2007; 13: 127–130. 10.1080/13554790701399254 [DOI] [PubMed] [Google Scholar]
- 48.Chanraud S, Zahr N, Sullivan EV, Pfefferbaum A. MR diffusion tensor imaging: a window into white matter integrity of the working brain. Neuropsychol Rev. 2010; 20: 209–225. 10.1007/s11065-010-9129-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van der Werf YD, Jolles J, Witter MP, Uylings HB. Contributions of thalamic nuclei to declarative memory functioning. Cortex. 2003; 39: 1047–1062. [DOI] [PubMed] [Google Scholar]
- 50.Floresco SB, Grace AA. Gating of hippocampal-evoked activity in prefrontal cortical neurons by inputs from the mediodorsal thalamus and ventral tegmental area. J Neurosci. 2003; 23: 3930–3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duering M, Zieren N, Herve D, Jouvent E, Reyes S, Peters N, et al. Strategic role of frontal white matter tracts in vascular cognitive impairment: a voxel-based lesion-symptom mapping study in CADASIL. Brain. 2011; 134: 2366–2375. 10.1093/brain/awr169 [DOI] [PubMed] [Google Scholar]
- 52.Winston GP. The potential role of novel diffusion imaging techniques in the understanding and treatment of epilepsy. Quant Imaging Med Surg. 2015; 5: 279–287. 10.3978/j.issn.2223-4292.2015.02.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liang P, Shi L, Chen N, Luo Y, Wang X, Liu K, et al. Construction of brain atlases based on a multi-center MRI dataset of 2020 Chinese adults. Sci Rep. 2015; 5: 18216 10.1038/srep18216 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.