Abstract
Background/Aim
Lipitor is a cholesterol-lowering drug and Celebrex is a cyclooxygenase 2 inhibitor. We investigated the effects of Lipitor and Celebrex on human prostate cancer VCaP cells cultured in vitro and grown as orthotopic xenograft tumors in SCID mice.
Materials and Methods
Apoptosis was measured by morphological assessment and caspase-3 assay. Nuclear factor kappa B (NF-κB) activation was determined by luciferase reporter assay. B cell lymphoma 2 (Bcl2) was measured by western blotting and immunohistochemistry. Orthotopic prostate tumors were monitored by the IVIS imaging system.
Results
the combination of Lipitor and Celebrex had stronger effects on the growth and apoptosis of VCaP cells than either drug alone. The combination more potently inhibited activation of NFκB and expression of Bcl2 than either drug alone. The growth of orthotopic VCaP prostate tumors was strongly inhibited by treatment with the drug combination.
Conclusion
administration of Lipitor and Celebrex in combination may be an effective strategy for inhibiting the growth of prostate cancer.
Keywords: Prostate cancer, statin, NSAIDs, orthotopic, NFκB
INTRODUCTION
Prostate cancer represents one of the most frequently diagnosed malignancies in men in the United States (1). Although anti-androgen and chemotherapy options are available for patients with advanced prostate cancer, these treatment options are only temporarily effective (2-4). In addition, anti-androgen and chemotherapy are considerably toxic to the patients. Therefore, novel and less toxic approaches for delaying the progression of prostate cancer to androgen independence would be of great benefit for patients.
Epidemiological studies showed that use of statin drugs was associated with a reduced risk of advanced prostate cancer (5-8). Recent clinical studies found that statin use was associated with a reduction in the risk of biochemical recurrence in patients with prostate cancer (9) and a decreased risk of cancer mortality (10). Statin drugs including Lipitor (atorvastatin) were found to inhibit proliferation and induce apoptosis of prostate cancer cells (11-12). Celebrex (celecoxib) is a selective Cyclooxygenase 2 (COX2) inhibitor. Many studies indicate that Celebrex has activity in prostate cancer (13-16). Studies from our laboratory showed that Lipitor and Celebrex in combination synergistically inhibited the growth and induced apoptosis of cultured prostate cancer cells. This combination inhibited the progression of androgen-dependent LNCaP tumors to androgen independence and the growth of androgen-independent PC-3 prostate tumors in SCID mice more effectively than either agent alone (17, 18). Additional studies showed that the combination of Lipitor and Celebrex had a stronger effect on decreasing the levels of interleukin 6 (IL6) in cultured prostate cancer cells and in xenograft prostate tumors (19). We also found that survivin, a downstream effector of IL6/signal transducer and activator of transcription 3 (STAT3) signaling pathway, was decreased in cultured prostate cancer cells and in xenograft tumors when treated with Lipitor and Celebrex (19). Since nuclear factor kappa B (NFκB) is known to activate IL6 expression (20), it is of interest to determine the effect of Lipitor and Celebrex on activation of NFκB and its downstream gene expression in prostate cancer cells. In the present study, we determined the effect of Lipitor and Celebrex alone and in combination on activation of NFκB and on expression of its downstream gene B cell lymphoma 2 (Bcl2). We found that the combination of Lipitor and Celebrex had a more potent inhibitory effect on NFκB activation and Bcl2 expression than either drug alone. This drug combination also strongly inhibited the growth of orthotopic VCaP prostate tumors and decreased the level of Bcl2 in the tumors.
MATERIALS AND METHODS
Cells and reagents
VCaP cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). Lipitor and Celebrex were provided by the National Cancer Institute’s Repository. Matrigel was obtained from BD Biosciences (Bedford, MA, USA). RPMI-1640 tissue culture medium, penicillin-streptomycin, L-glutamine and fetal bovine serum (FBS) were from Gibco (Grand Island, NY, USA). VCaP cells were maintained in RPMI-1640 culture medium containing 10% FBS that was supplemented with penicillin (100 units/ml)-streptomycin (100 μg/ml) and L-glutamine (300 μg/ml). Cultured cells were grown at 37°C in a humidified atmosphere of 5% CO2 and were passaged twice a week. Proliferating VCaP cells at about 70% confluence were used for the animal experiment.
Determination of the number of viable cells
The number of viable cells after each treatment was determined using a hemacytometer under a light microscope (Nikon Optiphot; Nikon Co., Tokyo, Japan). Cell viability was determined by the trypan blue exclusion assay, which was done by mixing 80 μl of cell suspension and 20 μl of 0.4% trypan blue solution for 2 min. Blue cells were counted as dead cells and the cells that did not absorb dye were counted as live cells.
Assessment of apoptotic cells by morphology and by activation of caspase-3
Apoptosis was determined by morphological assessment in cells stained with propidium iodide (21, 22). Briefly, cytospin slides were prepared after each experiment and cells were fixed with acetone/methanol (1:1) for 10 min at room temperature, followed by 10 min with propidium iodide staining [1 μg/ml in phosphate buffered-saline (PBS)] and analyzed using a fluorescence microscope (Nikon Eclipse TE200, Nikon Co., Tokyo, Japan). Apoptotic cells were identified by classical morphological features including nuclear condensation, cell shrinkage, and formation of apoptotic bodies (21, 22).
Caspase-3 activation was measured using an EnzoLyte AMC Caspase-3 Assay Fluorimetric kit (AnaSpec, Fremont, CA, USA) following the manufacturer instructions (23). Briefly, 1×105 cells were plated in triplicate in a flat-bottomed 96-well plate. After treatment with Lipitor/Celebrex, caspase-3 substrate was added to each well. Plates were incubated for 30 min at room temperature. Fluorescence intensity was measured in a Tecan Inifinite M200 plate reader (Tecan US Inc., Durham NC, USA).
NFκB-dependent reporter gene expression assay
NFκB transcriptional activity was measured by the NFκB-luciferase reporter gene expression assay. An NFκB luciferase construct was stably transfected into VCaP cells and a single stable clone, VCaP/N (24), was used in the present study. In brief, VCaP/N cells were treated with Lipitor or Celebrex alone and in combination for 24 h, and the NFκB-luciferase activity was measured using the luciferase assay kit from Promega (Madison, WI, USA). After treatment, the cells were washed with ice-cold PBS and harvested in 1× reporter lysis buffer. After centrifugation, 10 μl aliquots of the supernatants were measured for luciferase activity by using a Luminometer from Turner Designs Instrument (Sunnyvale, CA, USA). The luciferase activity was normalized against known protein concentrations and expressed as the percentage of luciferase activity in the control cells, which were treated with dimethyl sulfoxide (DMSO) solvent. The protein level was determined by Bio-Rad protein assay kits (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instructions.
Western blotting
After treatment, the cells were washed with ice-cold PBS and lysed with lysis buffer (10 mmol/l Tris-HCl (pH 7.4), 50 mmol/l sodium chloride, 30 mmol/l sodium pyrophosphate, 50 mmol/l sodium fluoride, 100 μmol/l sodium orthovanadate, 2 mmol/l iodoacetic acid, 5 mmol/l ZnCl2, 1 mmol/l phenylmethylsulfonyl fluoride, and 0.5% Triton X-100). The lysates were centrifuged at 12,000 ×g at 4°C for 25 min, and protein concentrations in the supernatant fractions were determined with a Bio-Rad protein assay kit according to the manufacturer’s instructions. Proteins were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) at 100 V for 110 min on 4% to 12% gradient gels. Separated proteins were transferred to nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ, USA). After blocking nonspecific binding sites with blocking buffer, the membrane was incubated overnight at 4°C with primary antibody to Bcl-2 (05-729, Millipore Co, Billerica, MA, USA). β-Actin was used as a loading control. Following removal of the primary antibody, the membrane was washed three times with TBS (PBS containing 0.05% tween 20) buffer at room temperature and then incubated with fluorochrome-conjugated secondary antibody (Santa Cruz Biotechnology Inc., Santa Cruz CA, USA). The membrane was then washed with TBS three times. Final detection was carried out with an Odyseey infrared imaging system (Li-Cor Co., Lincoln, NE, USA).
Orthotopic xenograft VCaP tumors in SCID mice
Male SCID mice were obtained from Taconic Farms Inc. (Germantown, NY, USA). The animals were housed in sterile filter-capped microisolator cages and were provided with sterilized 5010 rodent diet and water. VCaP cells were transfected with a luciferase expressing vector using the Lipofectamine™ 2000 (LF2000; Invitrogen Life Technology, San Diego, CA, USA). A single clone of VCaP cells that stably expressed luciferase (VCaP-luc) was obtained and used in the present study. VCaP-luc cells (1×106 cells/0.05 ml/mouse) suspended in 50% Matrigel (Collaborative Research, Bedford, MA, USA) in RPMI-1640 medium were injected into the prostatic capsule of SCID mice. Four weeks after the injection, mice were examined by the IVIS imaging system to monitor the growth of orthotopic prostate tumors. Mice with orthotopic tumors were fed AIN76A diet (9 mice) or AIN76A diet containing 0.02% Lipitor and 0.05% Celebrex (10 mice) for 28 days. Growth of the orthotopic prostate tumors was determined by the IVIS imaging system. The animal experiment was carried out under an Institutional Animal Care and Use Committee approved protocol (RU 02-001).
Statistical analyses
Analysis of variance (ANOVA) method with the Tukey-Kramer test was used for the comparison of growth inhibition and apoptosis. Student t-test was used to determine the difference of tumor size between the control group and the treated group.
RESULTS
Effects of Lipitor and Celebrex alone or in combination on the growth and apoptosis of human prostate cancer VCaP cells
The effects of different concentrations of Lipitor and Celebrex on the growth and death of VCaP cells were determined using the trypan blue assay. VCaP cells were treated with different concentrations of Lipitor (2-10 μM) or Celebrex (2-10 μM) for 96 h. We found that treatment of VCaP cells with Lipitor or Celebrex inhibited cell growth and caused cell death in a concentration-dependent manner. Treatment with Lipitor (2 μM) or Celebrex (2 μM) alone had little effect on the growth and death of VCaP cells, while their combination caused a 27% decrease in the number of viable cells as compared with the control (Figure 1). Treatment with Lipitor (5 μM) or Celebrex (5 μM) alone caused a 14% and 18% decrease in the number of viable cells, respectively, and a combination of Lipitor and Celebrex (both at 5 μM) caused a 43% decrease in viable cells (Figure 1). At a higher concentration (10 μM), Lipitor and Celebrex in combination also caused a stronger decrease in cell viability than either agent alone (Figure 1).
Figure 1.
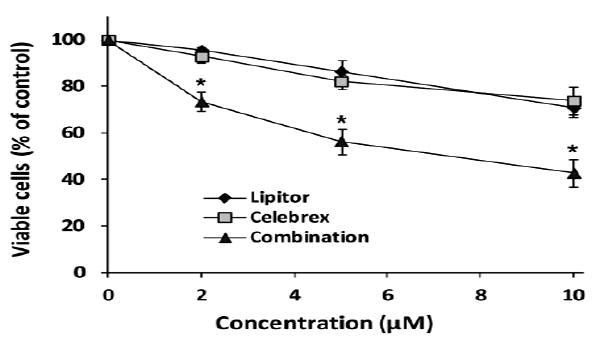
Effect of Lipitor and Celebrex alone and in combination on the growth of VCaP prostate cancer cells. VCaP cells were seeded at a density of 0.2×105 cells/ml in 35-mm tissue culture dishes (2 ml/dish) and incubated for 24 h. The cells were then treated with different concentrations of Lipitor or Celebrex alone and in combination for 96 h. The number of viable cells was determined by the trypan blue assay and expressed as a percentage that of solvent-treated control. Each value is the mean ± S.E. from three separate experiments. Differences for the number of viable cells between a combination group and a single agent-treated group were analyzed by ANOVA with the Tukey-Kramer multiple comparison test.*p<0.001 as compared to Lipitor or Celebrex alone.
Morphological assessment and caspase-3 assay were used to determine the effect of Lipitor and Celebrex on stimulating apoptosis in VCaP cells. As shown in Table I, treatment of VCaP cells with Lipitor or Celebrex alone resulted in about 15% and 13% apoptotic cells, respectively. A combination of Lipitor and Celebrex increased the proportion of apoptotic cells to 38%. This drug combination also caused a stronger increase in caspase-3 activation than either drug alone (Table I).
Table I.
Effect of Lipitor and Celebrex on apoptosis of VCaP cells. VCaP cells were seeded at a density of 0.2×105 cells/ml and incubated for 24 h. The cells were then treated with Lipitor (10 μM) or Celebrex (10 μM) alone or in combination for 96 h. Apoptosis was determined by morphological assessment and caspase-3 assay. Each value is the mean ± S.E from three experiments. Differences for the number of apoptotic cells and caspase-3 activation between a combination group and a single agent-treated group were analyzed by ANOVA with the Tukey-Kramer multiple comparison test.
| Treatment | Apoptotic cells (%) | Caspase-3 activity |
|---|---|---|
| Control | 2.3 ± 0.3 | 1.0 |
| Lipitor | 15.2 ± 2.6 | 6.7 ± 1.2 |
| Celecoxib | 12.7 ± 3.6 | 6.4 ± 2.1 |
| Combination | 37.8 ± 6.1* | 18.1 ± 3.4* |
p<0.001 as compared to Lipitor or Celebrex alone.
Effects of Lipitor and Celebrex on NF-κB transcriptional activity
An NF-κB-luciferase reporter gene expression assay was used to investigate the effect of Lipitor and Celebrex on activation of NF-κB. In the experiments, VCaP/N cells were treated with Lipitor (10 µM) and Celebrex (10 µM) alone or in combination for 24 h. Treatment of VCaP/N cells with Lipitor (10 µM) or Celebrex (10 μM) alone caused a modest decrease in luciferase activity, and the combination of Lipitor and Celebrex (both at 10 μM) had a stronger effect than either agent alone (Figure 2).
Figure 2.
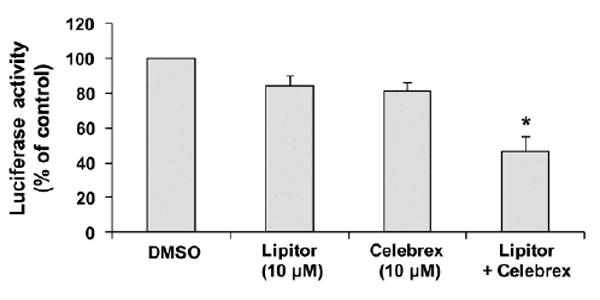
Inhibitory effect of Lipitor and Celebrex alone or in combination on NF- B activation in VCaP prostate cancer cells. VCaP/N cells were seeded at a density of 0.2×105 cells/ml of medium in 12-well plates and incubated for 24 h. The cells were then treated with Lipitor (10 μM) alone or in combination with Celebrex (10 μM) for 24 h. The NF- B transcriptional activity was measured by a luciferase activity assay. Differences in the NF- B transcriptional activity between the combination group and single agent-treated group were analyzed by ANOVA with the Tukey-Kramer multiple comparison test.*p<0.001 as compared to Lipitor or Celebrex alone.
Effects of Lipitor and Celebrex on the level of Bcl2
The level of Bcl2 in VCaP cells was determined by the western blot analysis using an antibody to Bcl2 obtained from Millipore Co. In the experiments, VCaP cells were treated with Lipitor (10 μM) or Celebrex (10 μM) alone or in combination for 24 h and analyzed by Western blotting. Treatment of VCaP cells with Lipitor (10 μM) or Celebrex (10 μM) alone resulted in little or no change in the level of Bcl2, and the combination of Lipitor and Celebrex (both at 10 μM) caused a stronger decrease in the level of Bcl2 than either agent alone (Figure 3). The extent of protein loading was determined by blotting for β-actin.
Figure 3.
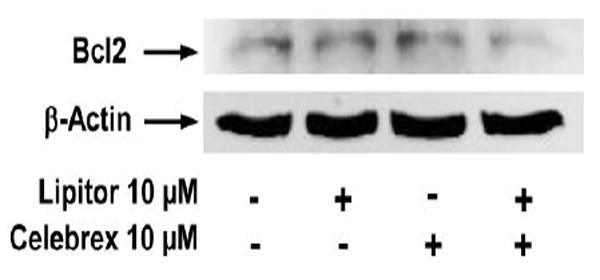
Effect of Lipitor and Celebrex on the level of B cell lymphoma 2 (Bcl2) in VCaP prostate cancer cells. VCaP cells were seeded at a density of 1×105 cells/ml of medium and incubated for 24 h. The cells were then treated with Lipitor (10 μM) or Celebrex (10 μM) alone and in combination for 48 h. Bcl2 was determined by western blotting using an antibody to Bcl2 (05-729, Millipore Co, Billerica, MA, USA).
Effect of Lipitor and Celebrex on orthotopic VCaP xenograft tumors
In this experiment, mice with established orthotopic VCaP tumors as determined by the IVIS imaging system were fed AIN76A diet or AIN76A diet containing 0.02% Lipitor and 0.05% Celebrex for 28 days. Growth of the orthotopic VCaP tumors was monitored by the IVIS imaging system. We found that treatment of the mice with Lipitor combined with Celebrex significantly inhibited the growth of orthotopic VCaP tumors (Figure 4). Representative IVIS images of a mouse in the control group before and after treatment, and a mouse in the Lipitor and Celebrex-treated group before and after treatment are shown in Figure 4. Expression of Bcl2 in the tumors was determined by immunohistochemistry. We found that treatment of the mice with Lipitor and Celebrex reduced the levels of Bcl2 immunostatining in the tumors. Representative micrographs of Bcl2 immunohistochemical staining in a VCaP tumor from the control group (Figure 4E) and a VCaP tumor from the Lipitor and Celebrex-treated group (Figure 4F) are shown.
Figure 4.
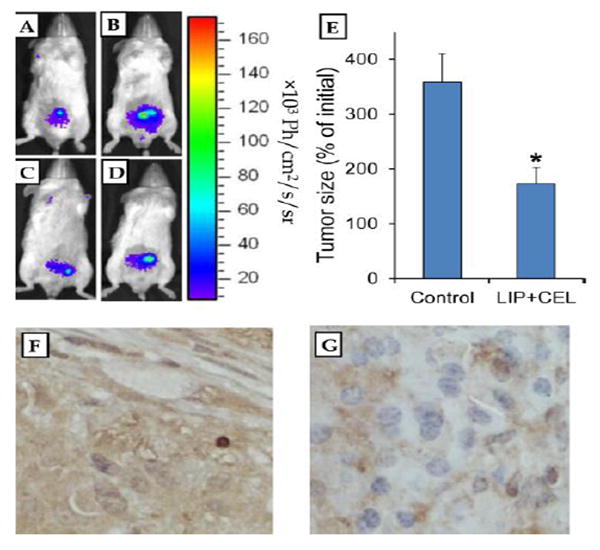
Effect of dietary Lipitor and Celebrex on the growth of orthotopic VCaP prostate cancer tumors in SCID mice and expression of B cell lymphoma 2 (Bcl2) in the tumors. SCID mice were injected orthotopically with VCaP-luc cells (1×106 cells/mouse). After four weeks, mice with established VCaP tumors were fed AIN76A diet (9 mice) or AIN76A diet containing 0.02% Lipitor and 0.05% Celebrex (10 mice) for 28 days. Tumor size in each mouse was determined once a week using the IVIS system. Representative images are shown. A and B: A mouse of the control group before and after treatment; C and D: a mouse of the group treated with Lipitor and Celebrex before and after treatment. E: Percentage of initial tumor size in the control group and the group treated with Lipitor (LIP) and Celebrex (CEL) . Each value represents the mean± S.E. Differences in the tumor size between the control group and the treatment group was analyzed by Student t-test. *p<0.001. F: A representative micrograph of immunohistochemistry in a tumor from the control group shows brown staining of Bcl2. G: A representative micrograph of immunohistochemistry in a tumor from the Lipitor and Celebrex-treated group shows reduced brown staining of Bcl2.
DISCUSSION
In the present study, we found that Lipitor and Celebrex in combination had a stronger inhibitory effect than either drug alone on the growth of cultured VCaP prostate cancer cells. This drug combination also strongly inhibited the transcriptional activity of NF-κB in the cells. In addition, Lipitor in combination with Celebrex more potently reduced the level of Bcl2 expression in VCaP cells than either drug used individually. NF-κB is an important cellular regulator of growth and apoptosis in a variety of cells including prostate cancer cells (25-27). NF-κB signaling has been shown to play an important role in prostate cancer growth, angiogenesis, tumorigenesis and metastatic progression (28-31). Inhibition of NF-κB was shown to increase cell transmembrane receptor Fas-mediated (32) and 12-O-tetradecanoylphorbol-13-acetate induced (33) apoptosis of PC-3 cells, and to reverse the resistance of prostate cancer cells to docetaxel (34). Inhibition of NF-κB signaling may be an effective strategy for enhancing anticancer treatment in patients with prostate cancer.
In the animal experiment, we found that oral administration of 0.02% Lipitor and 0.05% Celebrex in the AIN76A diet to male SCID mice for four weeks resulted in a strong inhibition in the growth of VCaP orthotopic tumors. Our previous study showed that oral administration of 0.02% Lipitor in AIN76A diet to male SCID mice for two weeks resulted in a serum concentration of 6.1 ng/ml (35). This serum drug concentration is comparable to the level of ~7 ng/ml reported in humans who had an oral administration of 20 mg Lipitor (36). After oral administration of Lipitor (20 mg) once a day for 14 days to humans, the peak plasma level was 15 ng/ml (37). It was also reported that oral administration of Celebrex (200 mg) to humans resulted in a peak plasma level of 600-1300 ng/ml (38). Our previous study showed that oral administration of Celebrex (0.05% in the AIN76A diet) for two weeks in male SCID mice resulted in a plasma level of 1090 ng/ml (35). Taken together, our results indicate that the serum levels of Lipitor and Celebrex associated with their inhibitory effect on the growth of VCaP prostate tumors in SCID mice are achievable in humans.
In summary, the results of the present study demonstrate that a combination of Lipitor and Celebrex has a potent inhibitory effect on the growth of cultured prostate cancer VCaP cells than either drug alone. Inhibition of proliferation and stimulation of apoptosis in VCaP cells are associated with a decrease in the transcriptional activity of NF-κB, and with a decrease in the expression of Bcl2, a downstream gene of the NF-κB signaling pathway. In vivo study demonstrates that oral administration of Lipitor with Celebrex strongly inhibits the growth of VCaP orthotopic tumors and the expression of Bcl2. Our results suggest that Lipitor in combination with Celebrex may be an effective regimen for inhibiting prostate cancer.
Acknowledgments
The present study was supported by grants from the Chinese National Science Foundation (81272452, 21272043 and 21102020) and the Rutgers Cancer Institute of New Jersey (CCSG P30-CA072720 RSD).
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Stein MN, Goodin S, Dipaola RS. Abiraterone in prostate cancer: a new angle to an old problem. Clin Cancer Res. 2012;18:1848–1854. doi: 10.1158/1078-0432.CCR-11-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seruga B, Tannock IF. Chemotherapy-based treatment for castration-resistant prostate cancer. J Clin Oncol. 2011;29:3686–3694. doi: 10.1200/JCO.2010.34.3996. [DOI] [PubMed] [Google Scholar]
- 4.Thomas BM, Smith C, Evans J, Button MR, Kumar S, Palaniappan N, Staffurth J, Tanguay JS, Lester JF. Time to prostate-specific antigen (PSA) nadir may predict rapid relapse in men with metastatic castration-resistant prostate cancer (CRPC) receiving docetaxel chemotherapy. Med Oncol. 2013;30:719–725. doi: 10.1007/s12032-013-0719-0. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs EJ, Rodriguez C, Bain EB, Wang Y, Thun MJ, Calle EE. Cholesterol-lowering drugs and advanced prostate cancer incidence in a large U.S. cohort. Cancer Epidemiol Biomarkers Prev. 2007;16:2213–2217. doi: 10.1158/1055-9965.EPI-07-0448. [DOI] [PubMed] [Google Scholar]
- 6.Flick ED, Habel LA, Chan KA, Van Den Eeden SK, Quinn VP, Haque R, Orav EJ, Seeger JD, Sadler MC, Quesenberry CP, Jr, Sternfeld B, Jacobsen SJ, Whitmer RA, Caan BJ. Statin use and risk of prostate cancer in the California Men’s Health Study Cohort. Cancer Epidemiol Biomarkers Prev. 2007;16:2218–2225. doi: 10.1158/1055-9965.EPI-07-0197. [DOI] [PubMed] [Google Scholar]
- 7.Murtola T, Tammela TLJ, Lahtela J, Auvinen A. Cholesterol-lowering drugs and prostate cancer risk: a population-based case–control study. Cancer Epidemiol Biomarkers Prev. 2007;16:2226–2232. doi: 10.1158/1055-9965.EPI-07-0599. [DOI] [PubMed] [Google Scholar]
- 8.Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, Willett WC, Giovannucci E. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98:1819–1825. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton RJ, Banez LL, Aronson WJ, Terris MK, Platz EA, Kane CJ, Presti JC, Jr, Amling CL, Freedland SJ. Statin medication use and the risk of biochemical recurrence after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) Database. Cancer. 2010;116:3389–3398. doi: 10.1002/cncr.25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu O, Eberg M, Benayoun S, Aprikian A, Batist G, Suissa S, Azoulay L. Use of statins and the risk of death in patients with prostate cancer. J Clin Oncol. 2014;32:5–11. doi: 10.1200/JCO.2013.49.4757. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Tao W, Wang Y, Bikow J, Lu B, Keating A, Verma S, Parker TG, Han R, Wen XY. Rosuvastatin, identified from a zebrafish chemical genetic screen for antiangiogenic compounds, suppresses the growth of prostate cancer. Eur Urol. 2010;58:418–426. doi: 10.1016/j.eururo.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 12.Park YH, Seo SY, Lee E, Ku JH, Kim HH, Kwak C. Simvastatin induces apoptosis in castrate resistant prostate cancer cells by deregulating nuclear factor-κB pathway. J Urol. 2013;189:1547–1552. doi: 10.1016/j.juro.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 13.Abedinpour P, Baron VT, Welsh J, Borgström P. Regression of prostate tumors upon combination of hormone ablation therapy and Celebrex in vivo. Prostate. 2010;71:813–823. doi: 10.1002/pros.21297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris RE. Cyclooxygenase-2 (COX-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung. Inflammopharmacology. 2009;17:55–67. doi: 10.1007/s10787-009-8049-8. [DOI] [PubMed] [Google Scholar]
- 15.Carles J, Font A, Mellado B, Domenech M, Gallardo E, González-Larriba JL, Catalan G, Alfaro J, Gonzalez Del Alba A, Nogué M, Lianes P, Tello JM. Weekly administration of docetaxel in combination with estramustine and Celebrex in patients with advanced hormone-refractory prostate cancer: final results from a phase II study. Br J Cancer. 2007;97:1206–1210. doi: 10.1038/sj.bjc.6604030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adhami VM, Malik A, Zaman N, Sarfaraz S, Siddiqui IA, Syed DN, Afaq F, Pasha FS, Saleem M, Mukhtar H. Combined inhibitory effects of green tea polyphenols and selective cyclooxygenase-2 inhibitors on the growth of human prostate cancer cells both in vitro and in vivo. Clin Cancer Res Mar. 2007;13:1611–1619. doi: 10.1158/1078-0432.CCR-06-2269. [DOI] [PubMed] [Google Scholar]
- 17.Zheng X, Cui XX, Gao Z, Zhao Y, Lin Y, Shih WJ, Huang M-T, Liu Y, Rabson A, Reddy B, Yang CS, Conney AH. Lipitor and Celebrex in combination inhibits the progression of androgen-dependent LNCaP xenograft prostate tumors to androgen independence. Cancer Prev Res (Phila Pa) 2010;3:114–24. doi: 10.1158/1940-6207.CAPR-09-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng X, Cui XX, Avila GE, Huang MT, Liu Y, Patel J, Kong AN, Paulino R, Shih WJ, Lin Y, Rabson AB, Reddy BS, Conney AH. Lipitor and Celebrex inhibit prostate PC-3 tumors in immunodeficient mice. Clin Cancer Res. 2007;13:5480–5487. doi: 10.1158/1078-0432.CCR-07-0242. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Cui XX, Goodin S, Ding N, Van Doren J, Du Z, Huang MT, Liu Y, Cheng X, Dipaola RS, Conney AH, Zheng X. Inhibition of IL-6 expression in LNCaP prostate cancer cells by a combination of Lipitor and Celebrex. Oncol Rep. 2014;31:835–841. doi: 10.3892/or.2013.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao W, Hodge DR, Wang L, Yang X, Zhang X, Farrar WL. NF-κB activates IL-6 expression through cooperation with c-Jun and IL6-AP1 site, but is independent of its IL6-NFκB regulatory site in autocrine human multiple myeloma cells. Cancer Biol Ther. 2004;3:1007–1017. doi: 10.4161/cbt.3.10.1141. [DOI] [PubMed] [Google Scholar]
- 21.Ploszaj T, Motyl T, Orzechowski A, Zimowska W, Wareski P, Skierski J, Zwierzchowski L. Antiapoptotic action of prolactin is associated with up-regulation of Bcl2 and down-regulation of Bax in HC11 mouse mammary epithelial cells. Apoptosis. 1998;3:295–304. doi: 10.1023/a:1009669427662. [DOI] [PubMed] [Google Scholar]
- 22.Zheng X, Chang RL, Cui XX, Avila GE, Lee S, Lu YP, Lou YR, Shih WJ, Lin Y, Reuhl K, Newmark H, Rabson A, Conney AH. Inhibitory effect of 12-O-tetradecanoylphorbol-13-acetate alone or in combination with all-trans-retinoic acid on the growth of LNCaP prostate tumors in immunodeficient mice. Cancer Res. 2004;64:1811–1820. doi: 10.1158/0008-5472.can-03-2848. [DOI] [PubMed] [Google Scholar]
- 23.Wei X, Du ZY, Cui XX, Verano M, Mo RQ, Tang ZK, Conney AH, Zheng X, Zhang K. Effects of cyclohexanone analogues of curcumin on growth, apoptosis and NF-κB activity in PC-3 human prostate cancer cells. Oncol Lett. 2012;4:279–284. doi: 10.3892/ol.2012.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou DY, Zhang K, Conney AH, Ding N, Cui XX, Wang H, Verano M, Zhao SQ, Fan YX, Zheng X, Du ZY. Synthesis and evaluation of curcumin-related compounds containing benzyl piperidone for their effects on human cancer cells. Chem Pharm Bull (Tokyo) 2013;61:1149–1155. doi: 10.1248/cpb.c13-00507. [DOI] [PubMed] [Google Scholar]
- 25.Jain G, Cronauer MV, Schrader M, Möller P, Marienfeld RB. NF-κB signaling in prostate cancer: a promising therapeutic target? World J Urol. 2012;30:303–310. doi: 10.1007/s00345-011-0792-y. [DOI] [PubMed] [Google Scholar]
- 26.Suh J, Payvandi F, Edelstein LC, Amenta PS, Zong WX, Gélinas C, Rabson AB. Mechanisms of constitutive NF-κB activation in human prostate cancer cells. Prostate. 2002;52:183–200. doi: 10.1002/pros.10082. [DOI] [PubMed] [Google Scholar]
- 27.Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajasekhar VK, Studer L, Gerald W, Socci ND, Scher HI. Tumour-initiating stem-like cells in human prostate cancer exhibit increased NF-κB signalling. Nat Commun. 2011;2:162. doi: 10.1038/ncomms1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Altuwaijri S, Deng F, Chen L, Lal P, Bhanot UK, Korets R, Wenske S, Lilja HG, Chang C, Scher HI, Gerald WL. NF-κB regulates androgen receptor expression and prostate cancer growth. Am J Pathol. 2009;175:489–499. doi: 10.2353/ajpath.2009.080727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seo SI, Song SY, Kang MR, Kim MS, Oh JE, Kim YR, Lee JY, Yoo NJ, Lee SH. Immunohistochemical analysis of NF-κB signaling proteins IKKε, p50/p105, p52/ p100 and RelA in prostate cancers. APMIS. 2009;117:623–628. doi: 10.1111/j.1600-0463.2009.02506.x. [DOI] [PubMed] [Google Scholar]
- 31.Jin RJ, Lho Y, Connelly L, Wang Y, Yu X, Saint Jean L, Case TC, Ellwood-Yen K, Sawyers CL, Bhowmick NA, Blackwell TS, Yull FE, Matusik RJ. The nuclear factor- κB pathway controls the progression of prostate cancer to androgen- independent growth. Cancer Res. 2008;68:6762–6769. doi: 10.1158/0008-5472.CAN-08-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimada K, Nakamura M, Ishida E, Kishi M, Matsuyoshi S, Konishi N. The molecular mechanism of sensitization to Fas-mediated apoptosis by 2-methoxyestradiol in PC3 prostate cancer cells. Mol Carcinog. 2004;39:1–9. doi: 10.1002/mc.10158. [DOI] [PubMed] [Google Scholar]
- 33.Zheng X, Chang RL, Cui XX, Avila G, Huang MT, Liu Y, Kong AN, Rabson AB, Conney AH. Inhibition of NF-κB by(E)3-[(4-methylphenyl)-sulfonyl]-2-propenenitrile (BAY11-7082; BAY) is associated with enhanced 12-O-tetradecanoylphorbol-13-acetate-induced growth suppression and apoptosis in human prostate cancer PC-3 cells. Int J Oncol. 2008;32:257–264. [PubMed] [Google Scholar]
- 34.O’Neill AJ, Prencipe M, Dowling C, Fan Y, Mulrane L, Gallagher WM, O’Connor D, O’Connor R, Devery A, Corcoran C, Rani S, O’Driscoll L, Fitzpatrick JM, Watson RW. Characterisation and manipulation of docetaxel-resistant prostate cancer cell lines. Mol Cancer. 2011;10:126. doi: 10.1186/1476-4598-10-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng X, Cui XX, Gao Z, Zhao Y, Shi Y, Huang MT, Liu Y, Wagner GC, Lin Y, Shih WJ, Rao CV, Yang CS, Conney AH. Inhibitory effect of dietary Lipitor and Celebrex together with voluntary running wheel exercise on the progression of androgen-dependent LNCaP prostate tumors to androgen independence. Exp Ther Med. 2011;2:221–228. doi: 10.3892/etm.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cilla DD, Jr, Whitfield LR, Gibson DM, Sedman AJ, Posvar EL. Multiple-dose pharmacokinetics, pharmacodynamics, and safety of Lipitor, an inhibitor of HMG-CoA reductase, in healthy subjects. Clin Pharmacol Ther. 1996;60:687–95. doi: 10.1016/S0009-9236(96)90218-0. [DOI] [PubMed] [Google Scholar]
- 37.Lennernäs H. Clinical pharmacokinetics of Lipitor. Clin Pharmacokinet. 2003;42:1141–1160. doi: 10.2165/00003088-200342130-00005. [DOI] [PubMed] [Google Scholar]
- 38.Davies NM, McLachlan AJ, Day RO, Williams KM. Clinical pharmacokinetics and pharmacodynamics of Celebrex: a selective cyclo-oxygenase-2 inhibitor. Clin Pharmacokinet. 2000;38:225–242. doi: 10.2165/00003088-200038030-00003. [DOI] [PubMed] [Google Scholar]


