Abstract
Converging lines of research suggest that the hippocampal complex (HC) may have a role in the pathophysiology of major depressive disorder (MDD). Although postmortem studies show little cellular death in the HC of depressed patients, animal studies suggest that elevated glucocorticoid levels associated with MDD may negatively affect neurogenesis, cause excitotoxic damage or be associated with reduced levels of key neurotrophins in the HC. Antidepressant medications may counter these effects, having been shown to increase HC neurogenesis and levels of brain-derived neurotrophic factor in animal studies. Neuropsychological studies have identified deficits in hippocampus-dependent recollection memory that may not abate with euthymia, and such memory impairment has been the most reliably documented cognitive abnormality in patients with MDD. Finally, data from imaging studies suggest both structural changes in the volume of the HC and functional alterations in frontotemporal and limbic circuits that may be critical for mood regulation. The extent to which such functional and structural changes determine clinical outcome in MDD remains unknown; a related, but also currently unanswered, question is whether the changes in HC function and structure observed in MDD are preventable or modifiable with effective treatment for the depressive illness.
Medical subject headings: antidepressive agents; depression; glucocorticoids; hippocampus; models, animal; neurons
Abstract
Des pistes de recherche convergentes indiquent que le complexe hippocampique (CH) peut avoir un rôle à jouer dans la pathophysiologie du trouble dépressif majeur (TDM). Même si des études postmortem révèlent peu de mort cellulaire dans le CH de patients déprimés, des études animales indiquent que des concentrations élevées de glucocorticoïdes associées au TDM peuvent avoir un effet négatif sur la neurogénèse, causer des dommages excitotoxiques ou avoir un lien avec la baisse des concentrations de neurotrophines clés dans le CH. Les antidépresseurs peuvent contrer ces effets, car on a démontré au cours d'études animales qu'ils élèvent la neurogénèse dans le CH et les concentrations de facteurs neurotrophiques d'origine cérébrale. Des études neurophysiologiques ont révélé des déficits de la mémoire du souvenir dépendante de l'hippocampe que l'euthymie peut ne pas réduire, et ces déficits de la mémoire constituent les anomalies cognitives les mieux documentées chez les patients atteints de TDM. Enfin, des données tirées d'études d'imagerie indiquent à la fois des changements structurels du volume du CH et des altérations fonctionnelles des circuits frontotemporaux et limbiques qui peuvent jouer un rôle crucial dans la régulation de l'humeur. On ne sait toujours pas dans quelle mesure ces changements fonctionnels et structurels déterminent l'issue clinique du TDM. Une question connexe mais à laquelle il n'y a toujours pas de réponse vise à déterminer si les changements de la fonction et de la structure du CH observés dans des cas de TDM sont évitables ou modifiables par des traitements efficaces contre la maladie dépressive.
Introduction
The behavioural and mood abnormalities associated with mood disorders appear to arise as the result of disturbances in a temporolimbic–frontal–caudate network.1,2 Normal regulation of mood may depend on the integrity of pathways linking the paralimbic frontal cortex and the basal ganglia. Some investigators differentiate the orbitofrontal–amygdalar network that supports emotions and moods from the hippocampal–cingulate system that supports memory encoding and explicit processing, among other functions, but these 2 systems act in concert. Thus, a number of regions appear central to understanding the pathophysiology of mood disorders. The hippocampus is one region that has recently received significant attention in mood disorders research and, although almost certainly not solely responsible for the myriad of symptoms observed in depression, the highly plastic, stress-sensitive hippocampal region may play a central role in depressive illness.
Caudal to and intimately connected with the amygdala, the hippocampus is a bilaminar grey-matter structure that forms the floor of the inferior temporal horn of the lateral ventricle and extends from the anterior margin of the ventricular horn to the splenium of the corpus callosum. The laminae that make up the hippocampal complex (HC) consist of the dentate gyrus and the hippocampus proper, the cornu Ammonis. The latter, when observed in coronal sections, can be divided into regions, termed CA1–CA4, based on pyramidal neuron morphology and sensitivity to anoxia.3 The glutamatergic pyramidal and granule cells represent 90% of hippocampal neurons, and the remaining 10% are primarily γ-aminobutyric acid (GABA)-producing interneurons.4 Acetylcholine nicotinic receptors5 and noradrenaline and serotonin (5-HT)6 and 5-HT1A and 5-HT4 receptors7,8 in the CA1 region indicate the presence of other well-characterized neurotransmitters in the cornu Ammonis.9
The entorhinal cortex provides the major glutamatergic afferents to form synapses either directly on the pyramidal neurons of the CA1 region or to offer boutons to the dendrites of the granule cells of the dentate gyrus region. Mossy fibres originating from the granule neuron cells project to the dendrites of CA3 pyramidal cells, which in turn project to the CA1 region. Axons from the CA1 region contacted either directly or indirectly from the entorhinal cortex form at least 2 possible paths: (1) efferent projections to the subiculum, then through the fornix and the anterothalamic nucleus, or (2) efferent projections to the entorhinal cortex3 through which the hippocampal output fibres reach the inferior temporal association cortex, the prefrontal cortex and the temporal pole10 (Fig. 1). The HC also influences the ventral striatal loop via the nucleus accumbens.
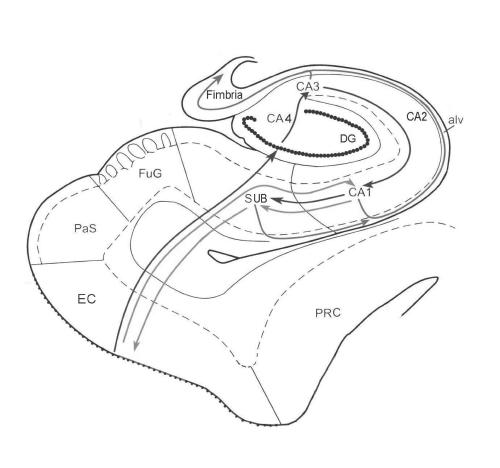
Fig. 1: Intrahippocampal pathways. The green line represents the direct intrahippocampal pathway from layer 3 of the entorhinal cortex (EC) to the CA1 region and then to the subiculum (SUB). From the subiculum, information can return to the entorhinal cortex or can enter the alveus (alv) and then the fimbria to influence neurotransmission in other cortical regions (red line). The afferent polysynaptic pathway is represented by the blue line. Axons from the entorhinal cortex enter the dentate gyrus (DG), and dendrites from the granule cells contact these glutamatergic axons. The mossy fibres of the granule cells project to the pyramidal neurons of the CA3 region, which then gives rise to efferent fibres to the alveus (in red) or Schaffer collateral fibres to the CA1 region. The pyramidal cells of the CA1 region, the primary output region of the cornu Ammonis, can project to either the subiculum (blue) or the alveus (red). Other regions displayed here include the perirhinal cortex (PRC), the parasubiculum (PaS) and the fusiform gyrus (FuG).
The hippocampus is involved in learning and in consolidation of explicit memories from short-term memory to cortical memory storage for the long term; its precise role in memory storage remains an active area of research and is beyond the scope of this review. Loss of pyramidal neurons of the CA1 region of the HC is sufficient to cause moderate-to-severe anterograde amnesia, and damage limited to the HC and the entorhinal cortex has been associated with retrograde and anterograde amnesia.11,12,13 The evidence suggests that the HC itself can be divided functionally along the septotemporal axis. Lesions of the dorsal HC of rats, corresponding to the posterior HC in primates, result in a greater impairment of spatial memory than corresponding lesions in the ventroanterior HC.14 Neurotoxic lesions of the ventral subiculum, an output region of the HC, appear to remove the negative feedback inhibition that the HC exerts on the hypothalamus, and elevated glucocorticoid levels are observed.15
Cellular studies of the hippocampus in depression
Grey-matter structures, including the HC, are vulnerable to atrophy in disorders such as schizophrenia,16,17,18,19,20,21,22 major depressive disorder (MDD),23,24,25,26,27,28,29,30 bipolar disorder31 and post-traumatic stress disorder.28 Volume reductions of the HC might be the result of remodelling of key cellular elements, involving retraction of dendrites, decreased neurogenesis in the dentate gyrus and loss of glial cells.2,32,33,34,35 Factors underlying this cellular remodelling include stress-induced elevated glucocorticoid levels, which are implicated in decreased neurogenesis36 and induce cell cycle arrest in the peripheral cells.37 Glucocorticoids eliminate activity-dependent increases in brain-derived neurotrophic factor (BDNF) levels,38 potentially making the neuron morphologically unresponsive to stimuli and inhibiting dendritic arborization. Increased activity of the hypothalamic–pituitary–adrenal axis resulting in elevated glucocorticoid levels combined with resistance to glucocorticoid-induced negative feedback control is commonly seen in MDD.39,40,41,42 This dysregulation of glucocorticoid secretion with increased activity of excitatory amino acid neurotransmitters could result in both potentially reversible remodelling and irreversible cell death in the HC of patients with MDD.43 As the hippocampus has a role in negative feedback inhibition of glucocorticoids,44 remodelling or neuronal damage may lead to less efficient inhibitory control of the corticotrophin-releasing hormone, producing cells of the hypothalamus, resulting in increased circulating glucocorticoids and further HC damage.45 Both elevated glucocorticoid levels15 and no significant changes in corticosterone concentrations46 have been observed in response to lesions of the HC, though the former study showed the increase specifically after lesions in the subiculum region of the HC. This suggests that there are regional differences within the HC tonic inhibition of adrenocortical activity. A decrease in survival and growth-promoting neurotrophic factors such as BDNF could lead to low HC volume and vulnerability to subsequent episodes of depression as a result of decreased neurogenesis, increased remodelling of dendrites and loss of glial cells or increased excitotoxicity.47
Animal models of depression
The action of antidepressant medications may at least partially be produced through their effects on the HC.48 Rat studies suggest that there may be a neuroprotective effect of antidepressant medications, because these agents can induce neuronal sprouting and neurogenesis.49 Antidepressant treatment results in increased levels of BDNF in the dentate gyrus and the CA3 region of the HC of treated rats that are stressed during early life by maternal separation.50 Long-term treatment with antidepressant medications is thought to act on monoamine systems to increase basal and stimulated adenylate cyclase activity,51 increase cyclic adenosine monophosphate (cAMP)-dependent phosphorylation52 and increase cAMP response element–binding protein (CREB) mRNA levels in the CA1, CA3 and dentate gyrus regions of the HC.53 Upregulation of CREB may be a common target of serotonergic and noradrenergic systems, the neurotransmitters that have to date been studied most with respect to MDD.54,55 CREB modulates BDNF production, and BDNF mRNA decreases in the HC after repeated stress.56 Antidepressant medications upregulate BDNF57 and its receptor trkB58 and block stress-related downregulation of BDNF.51 These effects occur after long-term treatment with antidepressant medications. Thus, animal and cellular studies suggest that antidepressant medications may protect HC integrity, but only 1 preliminary study in humans has suggested that these effects translate in patients into maintained HC structural integrity.59
Postmortem studies of patients with depression
Changes in glial cell density and decreased neuronal size have been reported in postmortem specimens from the prefrontal cortex of patients with MDD.60,61,62 Extreme stress and consequent severe glucocorticoid exposure are associated with changes in the gross cellular morphology of the HC in the rat and in nonhuman primates;63,64,65 however, evidence for gross hippocampal changes in human patients with MDD is lacking.66,67 It is not known whether this reflects limitations of the postmortem studies in failing to account for depressive burden, treatment history, age at onset of depression and heterogeneity in the causes of depression in samples studied to date. Postmortem studies of patients with schizophrenia, with changes in HC volume similar to those seen in MDD, have also failed to find reliable changes in HC neuronal number, density, orientation or size.68,21 Although moderate apoptosis has been observed in the dentate gyrus and the CA1 and CA4 regions of the HC of patients with MDD,66 this can only partially account for observed volumetric changes. These studies have not recorded whether the subjects were between depressive episodes; it is possible that patients experience some HC recovery in the inter-episode period, thus obscuring evidence of atrophy if death occurs when patients are relatively free of depressive symptoms. Studies of patients with Cushing's disease do suggest that HC volume and function can undergo recovery when glucocorticoid levels return to normal.69
Magnetic resonance imaging studies of the hippocampus in depression
In some ways the most provocative, but also the most controversial, data linking the HC to MDD have been from magnetic resonance imaging studies of the volume of the HC in patients with MDD; these studies are summarized in Table 1.23,24,25,26,27,29,30,70,71,72,73,74,75 Despite variation in the measurement techniques and patient samples, when we combined results from studies measuring the HC alone using a meta-analytic technique, we showed that depressed patients had significantly decreased left and right hippocampal volumes compared with controls (Fig. 2).76,77,78,79
Table 1
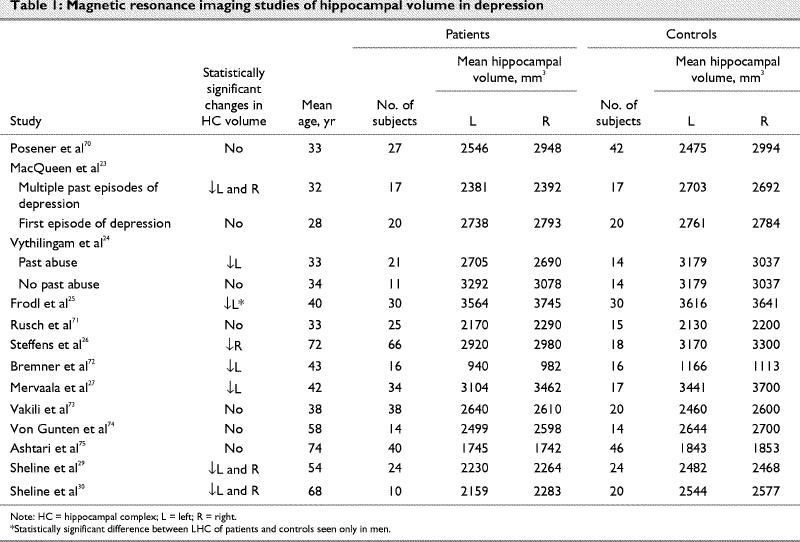
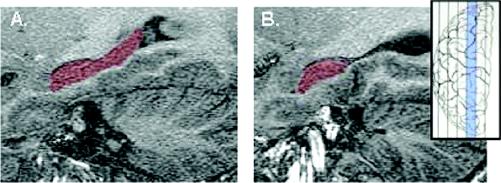
Fig. 2: Magnetic resonance spectroscopic images of the left hippocampus in a healthy control subject and in a patient with recurrent depression. The size of the difference shown here is unusually large, with most positive studies reporting a reduction in hippocampal complex (HC) volume of about 15% between cases and controls.78 Insert shows in blue the approximate sagittal level of the HC. Images were acquired on a 1.5-T GE Sigma Genesis–based EchoSpeed imager using previously published parameters.79
A: Sagittal view of the left HC, highlighted in red, of a healthy control subject whose left HC volume measured 3295 mm3.
B: The patient whose left HC is represented here, with an HC volume of 2015 mm3, was of the same age and sex as the control subject but had a long history of recurrent depression.
Genetic vulnerability, early abuse and chronic stress predispose individuals to depression. These factors might also predict a small HC in samples of depressed patients with these variables. The possibility that a small HC may predate onset of psychiatric illness has not been evaluated in samples of patients with strong family histories of MDD. The notion that a small HC may confer vulnerability to stress-associated disorders, however, has been recently assessed in a study of individuals exposed to combat who had nonexposed monozygotic twins. HC volume in the trauma-exposed and in the nonexposed, asymptomatic twin correlated negatively with severity of post-traumatic stress disorder in the twin who had been exposed to trauma.80 These data suggest that genetic factors lead to small HC volumes and confer vulnerability to psychiatric illness as a consequence of this reduction. Whether genetic vulnerability is expressed in part through a small HC that confers vulnerability to MDD is unknown, but the sample in which this may be most readily tested comprises individuals with strong family histories of MDD.
A literature beyond the scope of this summary has established the association between early trauma or abuse and depression.81 Several recent studies have focused on early abuse and HC function and volume in MDD. Vythilingam et al24 recently studied 21 women with a significant history of prepubertal physical or sexual abuse and current MDD. Prepubertal abuse is associated with long-term dysregulation of the hypothalamic–pituitary–adrenal axis, in contrast to postpubertal abuse, for which such an association has been less consistently described. Depressed women with past abuse had a 15% reduction in left HC volume compared with healthy control subjects; in contrast, women with MDD but no history of abuse had HC volumes similar to those of healthy controls. These data are consistent with those from other studies that examined women who had a history of prepubertal abuse; in general, past abuse is associated with reduced HC volume.24,81,82 Unfortunately, the samples of women studied to date have been heterogeneous with respect to past severity, duration and treatment history of depression. Therefore, although it appears that prepubertal abuse may be associated with reduced HC volume, it may also be the case that prepubertal abuse is associated with severe or recurrent depression, which results in reduced HC volume.
Chronic or recurrent depressive episodes may also lead to HC volume reduction. Sheline et al29 reported that total duration of past illness predicted degree of HC volume reduction when duration of illness was assessed as number of days spent ill; others have reported that volumetric reductions were greater in patients with a chronic course of depression and a large number of weeks spent ill compared with those who recovered fully with a shorter overall duration of illness,83 although not all investigators have reported such a relation.72 Our cross-sectional study of patients with either a first episode or multiple past episodes of depression suggested that patients with multiple episodes were more likely to have smaller HC volumes; a nonlinear relation between HC volume and number of past episodes further suggested that most of the volume loss occurred within the first few episodes.23 These data are consistent with recent reports that patients with schizophrenia have the greatest volumetric changes in the period shortly after onset of illness.84 A key related question is whether effective treatment starting early in the illness can slow or prevent the rate of volume loss in this illness. A recent study reported an association between HC volume loss and time spent with untreated depression but no association between HC volume and time during which depression was being treated, suggesting that volume loss may be arrested by antidepressant treatment.59 Further studies are necessary to clarify whether, in fact, effective treatment can minimize HC volume loss. Further studies are also required to reconcile the observed volume reductions in imaging studies with postmortem studies that have reported only moderate apoptosis in this region.66 It is possible, for example, that cell loss by apoptosis is a significant event over many years of illness, whereas retraction of the neuron's dendritic tree might be observed earlier in the course of illness and might partially account for the observed volume loss with the lack of significant cell death in depressed patients.
Neuropsychological studies of hippocampal function in depression
Discrete memory systems have now been classified.78,85 Recollection memory, which is analogous to explicit or declarative memory, requires the conscious recall of specific facts or events. In contrast, habit memory, implicit memory and nondeclarative memory all refer to a memory system that uses unconscious skills or strategies.78 The distinctions between these systems and the evidence that they are reliant on relatively independent brain structures have been extensively reviewed and debated. Evidence for the link between recollection memory and HC formation comes from studies of patients with HC damage,86,87,88animal studies89 and many functional imaging studies that have greatly clarified the role of frontal and temporal regions in various memory systems.90,91,92,93,94,95,96,97
An effect size analysis of cognitive functioning in 726 patients with MDD conducted using meta-analytic principles found that depression had the largest effect on recollection memory98 and little effect on habit memory, although evidence for this strict distinction has been controversial.99 Most studies report that depressed subjects perform as well as control subjects on implicit tasks,100,101,102,103 but one study reported implicit memory deficits in depressed subjects.104 Implicit memory tasks are potentially susceptible to the influence of explicit strategies, and differential use of explicit strategies in depressed and control groups may account for the apparent discrepancy on implicit tasks.
Further evidence for the proposal that recollection memory may be specifically impaired in depression comes from studies of working memory in depressed subjects. Working memory, that is, the ability to transiently maintain and use information, has been linked to the frontal cortex by both animal and human studies, and it has been reported to be abnormal in patients with psychotic disorders79,105,106 and obsessive–compulsive disorder.107 Studies of working memory in depressed patients, however, have failed to find differences in performance between patients and controls; working memory appears to be relatively spared in depression98 and other mood disorders.108,109 It is important to note that studies that assessed working memory and recollection memory tasks in the same subjects have found intact working memory but impaired recollection memory.110 Such studies highlight the fact that depressed patients do not show equal impairment across memory systems; rather, increasing evidence supports the hypothesis that depressed patients show differential impairment on recollection memory tasks that are dependent on the HC. This specificity of impairment also suggests that motivational impairment of depressed individuals cannot readily account for decreased test performance on recollection memory tasks. Furthermore, recollection memory impairments can persist when patients attain euthymia, with presumably full recovery of the motivational state.
Interestingly, a recent positron emission tomography (PET) study used [11C]WAY100635 to examine 5-HT1A receptors and assess their relation with memory function. Postsynaptic 5-HT1A receptors localized in the HC were found to have a negative influence on explicit memory function.111 These authors suggested that the antagonistic effect of postsynaptic 5-HT1A receptors in the HC could improve memory function, whereas administration of tandospirone, a 5-HT1A agonist, impaired explicit verbal memory in a dose-dependent manner. To date, there are no established strategies for treating the memory dysfunction associated with MDD, and this is particularly relevant given the evidence that such memory impairment persists into the euthymic state for many patients.
Functional imaging studies of hippocampal function in depression
PET has been used extensively to evaluate the metabolic activity of discrete brain regions in depressed patients compared with healthy controls by observing the increased glucose uptake (metabolism) and regional blood flow resulting from metabolically active tissues. Three recent studies of regional cerebral glucose metabolism in subjects with MDD did not find differences in activity in the HC of depressed patients compared with controls.112,113,114 Several reviews of earlier functional imaging studies in MDD do not discuss the HC in their overviews of regions that demonstrate cerebral blood flow changes in MDD.115,116,117 In contrast, 2 reports by Videbech et al118,119 demonstrate increased blood flow, and therefore increased regional metabolism, to the HC, the cerebellum, the anterior cingulate gyrus and the basal ganglia in depressed patients as compared with controls, with a correlation between the total Hamilton Depression Rating Scale score and blood flow to the HC.118 Depressed patients also showed increased activity of the HC compared with the healthy controls when the results were corrected for the influence of antidepressant medication.119
PET studies designed to evaluate regional glucose metabolism in the HC of depressed patients after treatment with antidepressant medications have shown either a relative decrease120,121 or no change,113 as compared with untreated patients. Interestingly, a recent study by Mayberg et al121 found a decrease in HC metabolism after treatment with fluoxetine that was not present in placebo-treated patients, although both groups of patients had equal numbers of responders. Other regions that showed fluoxetine-specific changes were the striatum and the brain stem. Overall, the extensive literature examining blood flow and cerebral metabolism in depressed patients has found a greater number of positive results for frontal and limbic regions, although some studies have also found parahippocampal changes. The significance of the studies that have reported metabolic changes to the HC in response to antidepressant treatment remains to be determined.
Summary
Several brain regions, including the prefrontal cortex and the limbic and cingulate regions, may be central to the pathology of MDD. Several lines of research have converged to support the notion of the HC being an important region in the pathophysiology of MDD. Data from animal and postmortem studies suggest that excitotoxic damage may occur to the HC after prolonged exposure to glucocorticoids and may result in long-lasting cellular alterations in this region. Data from volumetric imaging studies highlight the fact that clinical parameters of patients, such as early history, family history and burden of syndromal and subsyndromal illness, may make an important contribution to HC volume. Neuropsychological studies reliably report deficits in HC-dependent recollection memory that may not abate with euthymia. Functional imaging studies implicate frontotemporolimbic circuit changes in patients with MDD, but the results of these studies are variable with respect to observed changes in the HC. Despite these converging lines of evidence suggesting that the HC is important in the pathophysiology of MDD, including studies that suggest that there may be structural changes in this region, virtually nothing is known about whether appropriate early treatment of MDD can alleviate or even reverse some of these changes. Future studies will be important to clarify the association between HC integrity and clinical outcome and to examine potential strategies for maintaining HC integrity in patients with MDD and other psychiatric disorders.
Acknowledgments
Dr. MacQueen is a New Investigator with the Canadian Institutes of Health Research.
Footnotes
Competing interests: None declared.
Correspondence to: Dr. Glenda MacQueen, Department of Psychiatry and Behavioural Neurosciences, 4N77A, McMaster University Medical Centre, 1200 Main St. W, Hamilton ON L8N 3Z5; fax 905 304-5376; macqueng@mcmaster.ca
Submitted May 27, 2003; Revised Aug. 13, 2003; Oct. 29, 2003; Accepted Nov. 3, 2003
References
- 1.Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci 1999;877:614-37. [DOI] [PubMed]
- 2.Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, et al. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A 2001;98:12796-801. [DOI] [PMC free article] [PubMed]
- 3.Duvernoy HM. The human hippocampus: functional anatomy, vascularization and serial sections with MRI. 2nd ed. Berlin: Springer-Verlag; 1998.
- 4.Fritschy JM, Weinmann O, Wenzel A, Benke D. Synapse-specific localization of NMDA and GABA(A) receptor subunits revealed by antigen-retrieval immunohistochemistry. J Comp Neurol 1998;390:194-210. [PubMed]
- 5.Sudweeks SN, Yakel JL. Functional and molecular characterization of neuronal nicotinic ACh receptors in rat CA1 hippocampal neurons. J Physiol 2000;527(Pt 3):515-28. [DOI] [PMC free article] [PubMed]
- 6.Sudweeks SN, Hooft JA, Yakel JL. Serotonin 5-HT(3) receptors in rat CA1 hippocampal interneurons: functional and molecular characterization. J Physiol 2002;544:715-26. [DOI] [PMC free article] [PubMed]
- 7.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology 1999;38:1083-152. [DOI] [PubMed]
- 8.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, et al. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol Rev 1994;46(2):157-203. [PubMed]
- 9.Vizi ES, Kiss JP. Neurochemistry and pharmacology of the major hippocampal transmitter systems: synaptic and nonsynaptic interactions. Hippocampus 1998;8:566-607. [DOI] [PubMed]
- 10.Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus 1996;6:347-470. [DOI] [PubMed]
- 11.Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci 1986;6:2950-67. [DOI] [PMC free article] [PubMed]
- 12.Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J Neurosci 1996;16:5233-55. [DOI] [PMC free article] [PubMed]
- 13.Alvarez P, Squire LR. Memory consolidation and the medial temporal lobe: a simple network model. Proc Natl Acad Sci U S A 1994;91:7041-5. [DOI] [PMC free article] [PubMed]
- 14.Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus 1998;8:608-19. [DOI] [PubMed]
- 15.Herman JP, Dolgas CM, Carlson SL. Ventral subiculum regulates hypothalamo-pituitary-adrenocortical and behavioural responses to cognitive stressors. Neuroscience 1998;86:449-59. [DOI] [PubMed]
- 16.Bogerts B, Ashtari M, Degreef G, Alvir JM, Bilder RM, Lieberman JA. Reduced temporal limbic structure volumes on magnetic resonance images in first episode schizophrenia. Psychiatry Res 1990;35:1-13. [DOI] [PubMed]
- 17.Bogerts B, Lieberman JA, Ashtari M, Bilder RM, Degreef G, Lerner G, et al. Hippocampus-amygdala volumes and psychopathology in chronic schizophrenia. Biol Psychiatry 1993;33: 236-46. [DOI] [PubMed]
- 18.Breier A, Buchanan RW, Elkashef A, Munson RC, Kirkpatrick B, Gellad F. Brain morphology and schizophrenia: a magnetic resonance imaging study of limbic, prefrontal cortex, and caudate structures. Arch Gen Psychiatry 1992;49:921-6. [DOI] [PubMed]
- 19.Bryant NA, Buchanan RW, Vladar K, Breier A, Rothman M. Gender differences in temporal lobe structures of patients with schizophrenia: a volumetric MRI study. Am J Psychiatry 1999; 156: 603-9. [DOI] [PubMed]
- 20.Razi K, Greene KP, Sakuma M, Ge S, Kushner M, DeLisi LE. Reduction of the parahippocampal gyrus and the hippocampus in patients with chronic schizophrenia. Br J Psychiatry 1999; 174:512-9. [DOI] [PubMed]
- 21.Shenton ME, Kikinis R, Jolesz FA, Pollack SD, LeMay M, Wible CG, et al. Abnormalities of the left temporal lobe and thought disorder in schizophrenia. A quantitative magnetic resonance imaging study. N Engl J Med 1992;327:604-12. [DOI] [PubMed]
- 22.Wright IC, Rabe-Hesketh S, Woodruff PWR, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry 2000;157:16-25. [DOI] [PubMed]
- 23.MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A 2003;100:1387-92. [DOI] [PMC free article] [PubMed]
- 24.Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry 2002;159:2072-80. [DOI] [PMC free article] [PubMed]
- 25.Frodl T, Meisenzahl EM, Zetzche T, Born C, Groll C, Jager M, et al. Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry 2002;159:1112-8. [DOI] [PubMed]
- 26.Steffens DC, Byrum CE, McQuoid DR, Greenberg DL, Payne ME, Blitchington TF, et al. Hippocampal volume in geriatric depression. Biol Psychiatry 2000;48:301-9. [DOI] [PubMed]
- 27.Mervaala E, Fohr J, Kononen M, Valkonen-Korhonen M, Vainio P, Partanen K, et al. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med 2000;30: 117-25. [DOI] [PubMed]
- 28.Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry 1995;152:973-81. [DOI] [PMC free article] [PubMed]
- 29.Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci 1999;19:5034-43. [DOI] [PMC free article] [PubMed]
- 30.Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A 1996;93:3908-13. [DOI] [PMC free article] [PubMed]
- 31.Beyer JL, Krishnan KR. Volumetric brain imaging findings in mood disorders. Bipolar Disord 2002;4:89-104. [DOI] [PubMed]
- 32.Magarinos AM, Deslandes A, McEwen BS. Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. Eur J Pharmacol 1999;371:113-22. [DOI] [PubMed]
- 33.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 2000;20:9104-10. [DOI] [PMC free article] [PubMed]
- 34.Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry 2000; 48: 766-77. [DOI] [PubMed]
- 35.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci 1999;22:105-22. [DOI] [PubMed]
- 36.Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci 1999;2:894-7. [DOI] [PubMed]
- 37.Rogatsky I, Trowbridge JM, Garabedian MJ. Glucocorticoid receptor-mediated cell cycle arrest is achieved through distinct cell-specific transcriptional regulatory mechanisms. Mol Cell Biol 1997;17:3181-93. [DOI] [PMC free article] [PubMed]
- 38.Cosi C, Spoerri PE, Comelli MC, Guidolin D, Skaper SD. Glucocorticoids depress activity-dependent expression of BDNF mRNA in hippocampal neurones. Neuroreport 1993;4:527-30. [DOI] [PubMed]
- 39.Carroll BJ. The dexamethasone test for melancholia. Br J Psychiatry 1982;140:292-304. [DOI] [PubMed]
- 40.Kalin NH, Risch SC, Janowsky DS, Murphy DL. Use of dexamethasone suppression test in clinical psychiatry. J Clin Psychopharmacol 1981;1:64-9. [DOI] [PubMed]
- 41.Maes M, de Ruyter M, Suy E. Cortisol response to dexamethasone and noradrenergic function in depression. Acta Psychiatr Scand 1987;75:171-5. [DOI] [PubMed]
- 42.Young EA, Haskett RF, Grunhaus L, Pande A, Weinberg VM, Watson SJ, et al. Increased evening activation of the hypothalamic-pituitary-adrenal axis in depressed patients. Arch Gen Psychiatry 1994;51:701-7. [DOI] [PubMed]
- 43.Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biol Psychiatry 2000;48:755-65. [DOI] [PubMed]
- 44.Bremner JD. Brain pathways in stress processing. Biol Psychiatry 2002;51:91S-92S.
- 45.McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res 2000;886:172-89. [DOI] [PubMed]
- 46.Tuvnes FA, Steffenach HA, Murison R, Moser MB, Moser EI. Selective hippocampal lesions do not increase adrenocortical activity. J Neurosci 2003;23:4345-54. [DOI] [PMC free article] [PubMed]
- 47.Duman RS. Synaptic plasticity and mood disorders. Mol Psychiatry 2002;7(Suppl 1):S29-S34. [DOI] [PubMed]
- 48.Duman RS, Nakagawa S, Malberg J. Regulation of adult neurogenesis by antidepressant treatment. Neuropsychopharmacology 2001;25:836-44. [DOI] [PubMed]
- 49.Young LT. Neuroprotective effects of antidepressant and mood stabilizing drugs. J Psychiatry Neurosci 2002;27:8-9. [PMC free article] [PubMed]
- 50.MacQueen GM, Ramakrishnan K, Ratnasingan R, Chen B, Young LT. Desipramine treatment reduces the long-term behavioural and neurochemical sequelae of early maternal separation. Int J Neuropsychopharmacol 2003;6(4):391-6. [DOI] [PubMed]
- 51.Young LT, Bakish D, Beaulieu S. The neurobiology of treatment response to antidepressants and mood stabilizing medications. J Psychiatry Neurosci 2002;27:260-5. [PMC free article] [PubMed]
- 52.Stewart RJ, Chen B, Dowlatshahi D, MacQueen GM, Young LT. Abnormalities in the cAMP signaling pathway in post-mortem brain tissue from the Stanley Neuropathology Consortium. Brain Res Bull 2001;55:625-9. [DOI] [PubMed]
- 53.Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased temporal cortex CREB concentrations and antidepressant treatment in major depression. Lancet 1998;352:1754-5. [DOI] [PubMed]
- 54.Blier P, De Montigny C. Current advances and trends in the treatment of depression. Trends Pharmacol Sci 1994;15:220-6. [DOI] [PubMed]
- 55.Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci 1999;2:260-5. [DOI] [PubMed]
- 56.Rasmusson AM, Shi L, Duman R. Downregulation of BDNF mRNA in the hippocampal dentate gyrus after re-exposure to cues previously associated with footshock. Neuropsychopharmacology 2002;27:133-42. [DOI] [PubMed]
- 57.Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry 2001; 50: 260-5. [DOI] [PubMed]
- 58.Bayer TA, Schramm M, Feldmann N, Knable MB, Falkai P. Antidepressant drug exposure is associated with mRNA levels of tyrosine receptor kinase B in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2000;24:881-8. [DOI] [PubMed]
- 59.Sheline YI, Gado M, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry 2003;160:1-3. [DOI] [PubMed]
- 60.Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex 2002;12:386-94. [DOI] [PubMed]
- 61.Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A 1998;95:13290-5. [DOI] [PMC free article] [PubMed]
- 62.Rajkowska GR, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry 1999;45:1085-98. [DOI] [PubMed]
- 63.Sapolsky RM, Krey LC, McEwen BS. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J Neurosci 1985;5:1222-7. [DOI] [PMC free article] [PubMed]
- 64.Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci 1990;10:2897-902. [DOI] [PMC free article] [PubMed]
- 65.Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM. Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci 1989;9:1705-11. [DOI] [PMC free article] [PubMed]
- 66.Lucassen PJ, Muller MB, Holsboer F, Bauer J, Holtrop A, Wouda J, et al. Hippocampal apoptosis in major depression is a minor event and absent from subareas at risk for glucocorticoid overexposure. Am J Pathol 2001;158(2):453-68. [DOI] [PMC free article] [PubMed]
- 67.Muller MB, Lucassen PJ, Yassouridis A, Hoogendijk WJ, Holsboer F, Swaab DF. Neither major depression nor glucocorticoid treatment affects the cellular integrity of the human hippocampus. Eur J Neurosci 2001;14:1603-1612. [DOI] [PubMed]
- 68.Dwork AJ. Postmortem studies of the hippocampal formation in schizophrenia. Schizophr Bull 1997;23:385-402. [DOI] [PubMed]
- 69.Starkman MN, Giordani B, Gebarski SS, Berent S, Schork MA, Schteingart DE. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing's disease. Biol Psychiatry 1999;46:1595-602. [DOI] [PubMed]
- 70.Posener JA, Wang L, Price JL, Gado M, Province MA, Miller MI, et al. High-dimensional mapping of the hippocampus in depression. Am J Psychiatry 2003;160(1):83-9. [DOI] [PubMed]
- 71.Rusch BD, Abercrombie HC, Oakes TR, Schaefer SM, Davidson RJ. Hippocampal morphometry in depressed patients and control subjects: relations to anxiety symptoms. Biol Psychiatry 2001;50:960-4. [DOI] [PubMed]
- 72.Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry 2000;157:115-7. [DOI] [PubMed]
- 73.Vakili K, Pillay SS, Lafer B, Fava M, Renshaw PF, Bonello-Cintron CM, et al. Hippocampal volume in primary unipolar major depression: a magnetic resonance imaging study. Biol Psychiatry 2000;47:1087-90. [DOI] [PubMed]
- 74.Von Gunten A, Fox NC, Cipolotti L, Ron MA. A volumetric study of hippocampus and amygdala in depressed patients with subjective memory problems. J Neuropsychiatry Clin Neurosci 2000;12:493-8. [DOI] [PubMed]
- 75.Ashtari M, Greenwald BS, Kramer-Ginsberg E, Hu J, Wu H, Patel M, et al. Hippocampal/amygdala volumes in geriatric depression. Psychol Med 1999;29:629-38. [DOI] [PubMed]
- 76.Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry 2004;161(4):598-607. [DOI] [PubMed]
- 77.Pantel J, Schroder J, Essig M, Popp D, Dech H, Knopp MV, et al. Quantitative magnetic resonance imaging in geriatric depression and primary degenerative dementia. J Affect Disord 1997; 42:69-83. [DOI] [PubMed]
- 78.Zubenko GS, Sullivan P, Nelson JP, Belle SH, Huff J, Wolf GL. Brain imaging abnormalities in mental disorders of late life. Arch Neurol 1990;47:1107-11. [DOI] [PubMed]
- 79.Volz HP, Rzanny R, Rossger G, Hubner G, Kreitschmann-Andermahr I, Kaiser WA, et al. 31Phosphorus magnetic resonance spectroscopy of the dorsolateral prefrontal region in schizophrenics—a study including 50 patients and 36 controls. Biol Psychiatry 1998;44(6):399-404. [DOI] [PubMed]
- 80.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci 2002; 5:1242-7. [DOI] [PMC free article] [PubMed]
- 81.Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP. Developmental neurobiology of childhood stress and trauma. Psychiatr Clin North Am 2002;25:397-426. [DOI] [PubMed]
- 82.Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, et al. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—a preliminary report. Biol Psychiatry 1997;41:23-32. [DOI] [PMC free article] [PubMed]
- 83.Shah PJ, Ebmeier KP, Glabus MF, Goodwin GM. Cortical grey matter reductions associated with treatment resistant chronic unipolar depression. Controlled magnetic resonance imaging study. Br J Psychiatry 1998;172:527-32. [DOI] [PubMed]
- 84.McCarley RW, Shenton ME, Kasai K, Hirayasu Y, Salisbury DF. Progression of grey matter loss after first-episode in schizophrenia but not in bipolar psychoses. Biol Psychiatry 2002; 51: 11S.
- 85.McDonough L, Mandler JM, McKee RD, Squire LR. The deferred imitation task as a nonverbal measure of declarative memory. Proc Natl Acad Sci U S A 1995;92:7580-4. [DOI] [PMC free article] [PubMed]
- 86.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. 1957. J Neuropsychiatry Clin Neurosci 2000; 12: 103-13. [DOI] [PubMed]
- 87.Mishkin M, Suzuki WA, Gadian DG, Vargha-Khadem F. Hierarchical organization of cognitive memory. Philos Trans R Soc Lond B Biol Sci 1997;352:1461-7. [DOI] [PMC free article] [PubMed]
- 88.Mishkin M, Appenzeller T. The anatomy of memory. Sci Am 1987; 256:80-9. [DOI] [PubMed]
- 89.Zola-Morgan S, Squire LR, Ramus SJ. Severity of memory impairment in monkeys as a function of locus and extent of damage within the medial temporal lobe memory system. Hippocampus 1994;4:483-95. [DOI] [PubMed]
- 90.Buckner RL, Petersen SE, Ojemann JG, Miezin FM, Squire LR, Raichle ME. Functional anatomical studies of explicit and implicit memory retrieval tasks. J Neurosci 1995;15:12-29. [DOI] [PMC free article] [PubMed]
- 91.Schacter DL, Savage CR, Alpert NM, Rauch SL, Alpert MS. The role of hippocampus and frontal cortex in age-related memory changes: a PET study. Neuroreport 1996;7:1165-9. [DOI] [PubMed]
- 92.Bernard F, Desgranges B, Platel H, Baron JC, Eustache F. Contributions of frontal and medial temporal regions to verbal episodic memory: a PET study. Neuroreport 2001;12:1737-41. [DOI] [PubMed]
- 93.Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Staib LH, et al. Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biol Psychiatry 2003;53:879-89. [DOI] [PubMed]
- 94.Deckersbach T, Reilly-Harrington N, Clark L, Goodwin G, Savage CR, Dougherty DD, et al. Characteristics and functional anatomy of episodic memory impairment in bipolar disorder. Biol Psychiatry 2002;51:30S.
- 95.Owen AM, Doyon J, Petrides M, Evans AC. Planning and spatial working memory: a positron emission tomography study in humans. Eur J Neurosci 1996;8:353-64. [DOI] [PubMed]
- 96.Mayes AR, Montaldi D. Exploring the neural bases of episodic and semantic memory: the role of structural and functional neuroimaging. Neurosci Biobehav Rev 2001;25:555-73. [DOI] [PubMed]
- 97.Fletcher PC, Henson RN. Frontal lobes and human memory: insights from functional neuroimaging. Brain 2001;124:849-81. [DOI] [PubMed]
- 98.Zakzanis KK, Leach L, Kaplan E. On the nature and pattern of neurocognitive function in major depressive disorder. Neuropsychiatry Neuropsychol Behav Neurol 1998;11:111-9. [PubMed]
- 99.Weingartner H, Cohen RM, Murphy DL, Martello J, Gerdt C. Cognitive processes in depression. Arch Gen Psychiatry 1981; 38: 42-7. [DOI] [PubMed]
- 100.Roediger HL III, McDermott KB. Depression and implicit memory: a commentary. J Abnorm Psychol 1992;101:587-91. [DOI] [PubMed]
- 101.Bazin N, Perruchet P, De Bonis M, Feline A. The dissociation of explicit and implicit memory in depressed patients. Psychol Med 1994;24:239-45. [DOI] [PubMed]
- 102.Hertel PT, Hardin TS. Remembering with and without awareness in a depressed mood: evidence of deficits in initiative. J Exp Psychol Gen 1990;119:45-59. [DOI] [PubMed]
- 103.Watkins PC, Mathews A, Williamson DA, Fuller RD. Mood-congruent memory in depression: Emotional priming or elaboration? J Abnorm Psychol 1992;101:581-6. [DOI] [PubMed]
- 104.Danion JM, Willard-Schroeder D, Zimmermann MA, Grange D, Schlienger JL, Singer L. Explicit memory and repetition priming in depression. Preliminary findings. Arch Gen Psychiatry 1991;48:707-11. [DOI] [PubMed]
- 105.Buchanan RW, Vladar K, Barta PE, Pearlson GD. Structural evaluation of the prefrontal cortex in schizophrenia. Am J Psychiatry 1998;155:1049-55. [DOI] [PubMed]
- 106.Curtis VA, Bullmore ET, Brammer MJ, Wright IC, Williams SC, Morris RG, et al. Attenuated frontal activation during a verbal fluency task in patients with schizophrenia. Am J Psychiatry 1998;155:1056-63. [DOI] [PubMed]
- 107.Purcell R, Maruff P, Kyrios M, Pantelis C. Neuropsychological deficits in obsessive-compulsive disorder: a comparison with unipolar depression, panic disorder, and normal controls. Arch Gen Psychiatry 1998;55:415-23. [DOI] [PubMed]
- 108.Goodwin GM. Neuropsychological and neuroimaging evidence for the involvement of the frontal lobes in depression. J Psychopharmacol 1997;11:115-22. [DOI] [PubMed]
- 109.Clark L, Iversen SD, Goodwin GM. Sustained attention deficit in bipolar disorder. Br J Psychiatry 2002;180:313-9. [DOI] [PubMed]
- 110.Harmer CJ, Clark L, Grayson L, Goodwin GM. Sustained attention deficit in bipolar disorder is not a working memory impairment in disguise. Neuropsychologia 2002;40:1586-90. [DOI] [PubMed]
- 111.Yasuno F, Suhara T, Nakayama T, Ichimiya T, Okubo Y, Takano A, et al. Inhibitory effect of hippocampal 5-HT1A receptors on human explicit memory. Am J Psychiatry 2003; 160: 334-40. [DOI] [PubMed]
- 112.Kimbrell TA, Ketter TA, George MS, Little JT, Benson BE, Willis MW, et al. Regional cerebral glucose utilization in patients with a range of severities of unipolar depression. Biol Psychiatry 2002;51:237-52. [DOI] [PubMed]
- 113.Saxena S, Brody AL, Ho ML, Alborzian S, Maidment KM, Zohrabi N, et al. Differential cerebral metabolic changes with paroxetine treatment of obsessive-compulsive disorder vs major depression. Arch Gen Psychiatry 2002;59:250-61. [DOI] [PubMed]
- 114.Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Behav 2002;71:431-47. [DOI] [PubMed]
- 115.Grady CL, Keightley ML. Studies of altered social cognition in neuropsychiatric disorders using functional neuroimaging. Can J Psychiatry 2002;47:327-36. [DOI] [PubMed]
- 116.Liotti M, Mayberg HS. The role of functional neuroimaging in the neuropsychology of depression. J Clin Exp Neuropsychol 2001; 23:121-36. [DOI] [PubMed]
- 117.Videbech P. PET measurements of brain glucose metabolism and blood flow in major depressive disorder: a critical review. Acta Psychiatr Scand 2000;101:11-20. [DOI] [PubMed]
- 118.Videbech P, Ravnkilde B, Pedersen TH, Hartvig H, Egander A, Clemmensen K, et al. The Danish PET/depression project: clinical symptoms and cerebral blood flow. A regions-of-interest analysis. Acta Psychiatr Scand 2002;106:35-44. [DOI] [PubMed]
- 119.Videbech P, Ravnkilde B, Pedersen AR, Egander A, Landbo B, Rasmussen NA, et al. The Danish PET/depression project: PET findings in patients with major depression. Psychol Med 2001; 31:1147-58. [DOI] [PubMed]
- 120.Kennedy SH, Evans KR, Kruger S, Mayberg HS, Meyer JH, McCann S, et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry 2001; 158:899-905. [DOI] [PubMed]
- 121.Mayberg HS, Silva JA, Brannan SK, Tekell JL, Mahurin RK, McGinnis S, et al. The functional neuroanatomy of the placebo effect. Am J Psychiatry 2002;159:728-37. [DOI] [PubMed]


