Abstract
Objective
Some antipsychotic medications prescribed for the treatment of psychoses, mood disorders or post-traumatic stress disorder in patients with coexisting substance dependence disorders (SDD) have reduced substance dependence. We studied the potential benefits of quetiapine in the treatment of SDD.
Methods
We conducted a retrospective chart review of data for 9 patients who were admitted to a 28-day residential rehabilitation program designed for individuals with SDD during a 3-month period from January 2003 through March 2003 and treated with quetiapine for nonpsychotic anxiety. These patients also met the Diagnostic and Statistical Manual of Mental Disorders, fourth edition, criteria for alcohol, cocaine and/or methamphetamine dependence and substance-induced anxiety disorder. The patients were assessed using the Hamilton-D Rating Scale for Depression (Ham-D), a 10-point Likert scale to measure alcohol or drug cravings, and random Breathalyzer and urine drug screens.
Results
Quetiapine was generally well tolerated. Only 1 of the 9 patients stopped taking the medication because of increased anxiety. Other patients reported improvement in sleep and anxiety. The mean decrease in Ham-D score at discharge for the responders was 18.5 (p < 0.005). The biggest decreases on the Ham-D occurred on the subscales of insomnia, agitation, somatic anxiety, psychologic anxiety, hypochondriasis and obsessional symptoms. The mean decrease in the Likert 10-point craving scale was 5.9 for the responders (p < 0.005). These patients' periodic Breathalyzer and urine test results suggested that they remained abstinent from alcohol and other drug use.
Conclusion
Quetiapine was beneficial in the treatment of SDD in patients with nonpsychotic anxiety.
Medical subject headings: antipsychotic agents, quetiapine, substance-related disorders
Abstract
Objectif
Quelques antipsychotiques prescrits pour traiter des psychoses, des troubles de l'humeur ou le syndrome de stress posttraumatique conjugués à des troubles coexistants de dépendance de substances (TDS) ont réduit la dépendance à l'égard des substances. Nous avons étudié les avantages que pourrait offrir la quétiapine pour traiter des TDS.
Méthodes
Au cours d'une étude rétrospective de dossiers, nous avons analysé les données portant sur neuf patients admis en trois mois, soit de janvier à mars 2003, dans un programme de réadaptation en résidence d'une durée de 28 jours conçu pour les personnes qui avaient un TDS et nous avons traité les patients à la quétiapine pour une anxiété non psychotique. Ces patients satisfaisaient aux critères de la quatrième édition du Diagnostic and Statistical Manual of Mental Disorders qui ont trait à la dépendance de l'alcool, de la cocaïne ou des méthamphétamines et à un trouble anxieux provoqué par une substance. On a évalué les patients en fonction de l'échelle de dépression de Hamilton-D (Ham-D), d'une échelle de Likert à 10 points afin de mesurer l'état de manque d'alcool ou de drogue, et procédé à des dépistages aléatoires au moyen de l'alcootest et d'analyses d'urine.
Résultats
La quétiapine est en général bien tolérée. Un seul des neuf patients a cessé de prendre le médicament parce que son anxiété s'est aggravée. D'autres patients ont signalé qu'ils dormaient mieux et que leur anxiété avait diminué. La diminution moyenne du résultat Ham-D au moment du congé chez les sujets répondants a atteint 18,5 (p < 0,005). Les diminutions les plus importantes du résultat Ham-D se sont produites au niveau des sous-échelles de l'insomnie, de l'agitation, de l'anxiété somatique, de l'anxiété psychologique, de l'hypochondrie et des symptômes obsessionnels. La diminution moyenne sur l'échelle de 10 points de Likert indiquant l'état de besoin s'est établie à 5,9 chez les sujets répondants (p < 0,005). Les résultats des tests périodiques de dépistage à l'alcootest et aux analyses d'urine chez ces patients ont indiqué qu'ils continuaient de s'abstenir d'alcool et de drogues.
Conclusion
La quétiapine a eu un effet bénéfique dans le traitement des TDS chez des patients qui avaient une anxiété non psychotique.
Introduction
Treatment of substance dependence disorders (SDD) is complex and multidisciplinary and involves efforts by the patient to maintain sobriety, structured living and attendance at self-help group meetings along with individual, group and family therapy. Pharmacotherapy might provide some benefit, but there is disagreement about the potential benefits of various pharmacologic agents for the treatment of SDD. The potential benefit of antipsychotic drugs for the treatment of substance use disorders has been seen in patients with schizophrenia or other psychotic illness who also reported concurrent substance dependence disorders. Even though the medications were prescribed primarily for the treatment of the underlying psychoses, patients taking these medications reported a significant reduction in substance use.1,2,3,4,5,6,7,8,9,10,11,12,13,14 However, the evidence for the potential benefit of the antipsychotic medications in reducing substance use has not been consistent. Although some studies suggest that their use decreases substance use, other researchers contend that the substance use might increase with the use of typical antipsychotic medications.15,16,17
A review of the literature suggests that the differences in efficacy might occur because of differences in the mechanism of action of these antipsychotic medications. Antipsychotic medications with a lower potency (such as chlorpromazine) might appear to reduce substance use, whereas those with higher potency (haloperidol) might appear to have limited benefits and perhaps may increase the substance use. Green et al18 suggested that this might occur because of the antipsychotic medications' dopamine antagonism in the mesocorticolimbic neurons of the “reward pathway.” The higher-potency antipsychotic medications exert more of this antagonistic effect, whereas those with a lower potency show less dopamine antagonism. The antipsychotic medications with lower potency, such as chlorpromazine and thioridazine, have been used for the treatment of patients with substance use disorders but no psychotic disorder; however, because of the potential for adverse effects, which include tardive dyskinesia, neuroleptic malignant syndrome and extrapyramidal side effects, antipsychotic medications have never achieved widespread use in the treatment of substance use disorders.
Novel antipsychotic medications show significantly less dopamine antagonism and exert their clinical effects through their actions on the serotonin, histamine and norepinephrine pathways. Clozapine, olanzapine and quetiapine are a few of the novel antipsychotic medications that share this mechanism of action. Because of the minimal dopamine blockade with these novel antipsychotics, they might provide some benefit for patients with SDD.
Zimmet et al19 reported that in a sample of 38 patients with schizophrenia and SDD, 3 years of clozapine treatment decreased the cravings for alcohol and drugs by 85%. Drake et al20 conducted a prospective study of 151 patients with schizophrenia and SDD and found that 79% of patients who were prescribed clozapine achieved complete sobriety after 3 years, versus only 33% of patients taking other typical antipsychotic drugs. Tsuang et al21 also reported benefits of clozapine in decreasing SDD. Several other reports in the literature suggest benefits of novel antipsychotics in decreasing SDD in patients with a dual diagnosis.22,23,24,25,26,27
Littrell et al28 conducted a 12-month open-label trial of olanzapine in 30 patients with schizophrenia and SDD and found that 70% of patients achieved sobriety by the twelfth month. Brown et al29 studied the effects of quetiapine in a 12-week prospective study in patients with bipolar disorder and SDD (n = 12) and found that quetiapine improved mood symptoms and decreased drug cravings. We recently reported similar benefits of quetiapine in reducing alcohol use and cravings in post-traumatic stress disorder (PTSD),30 suggesting that quetiapine might improve SDD in patients with a nonpsychotic, nonbipolar disorder as well. Although the use of quetiapine in patients with a nonpsychotic disorder is increasing, there are few data to suggest that this medication might be beneficial in substance users with no psychotic or bipolar disorder. We conducted a retrospective chart review to study the potential benefits of quetiapine for the treatment of substance dependence.
Method
This is a retrospective chart review of data from patients who were admitted to a 28-day residential rehabilitation program designed for individuals with substance use disorders during a 3-month period from January 2003 through March 2003 and received pharmacotherapy with quetiapine for a nonpsychotic condition. Nine patients were identified who were treated with quetiapine for nonpsychotic anxiety. These patients also met the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV),31 criteria for alcohol, cocaine and/or methamphetamine dependence and substance-induced anxiety disorder. All these patients were admitted for voluntary residential treatment. As part of their treatment, they received individual and group therapy and self-help therapy with Alcoholics Anonymous meetings. Pharmacotherapy was started after patients reported continued symptoms of anxiety. Initially, these patients were prescribed therapeutic doses of antidepressant medications (such as selective serotonin reuptake inhibitors), anti-anxiety medications (such as buspirone, gabapentin or propranolol) and trazodone to treat their nonpsychotic symptoms of anxiety. However, when they reported minimal improvement even after 1–2 weeks of treatment, other psychiatric and medical conditions were ruled out (including drug and alcohol intoxication or withdrawal) and adjunctive treatment with the antipsychotic medication quetiapine was recommended by the treating psychiatrists. Other treatments that the patients were receiving were continued. As part of regular treatment, these patients also received periodic assessment with standardized testing such as the Hamilton-D Rating Scale for Depression (Ham-D),32 which is a highly reliable and valid, clinician-administered, 21-item screening instrument designed to measure the severity and progress of depression, a 10-point Likert scale to assess cravings for alcohol or the drug of choice (ranging from 1 = no craving to 10 = maximum craving) and random Breathalyzer and urine drug screens. These tests were routinely conducted at intake, at weekly follow-up meetings with the psychiatrist and at discharge from the 28-day residential program. We reviewed the test scores of the 9 selected patients from the time of admission into the program, the point of starting the quetiapine and after approximately 1 week of quetiapine treatment.
Results
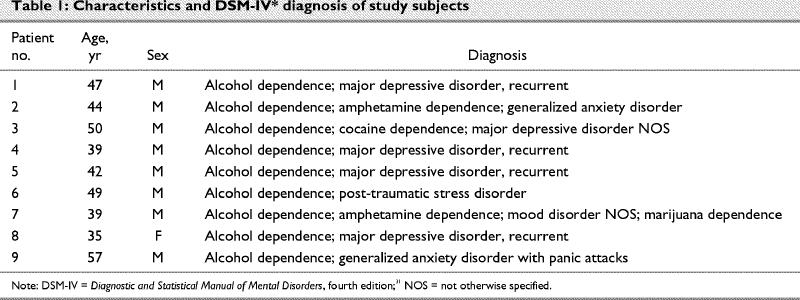
Nine patients (8 male, 1 female) were identified who received quetiapine treatment for their nonpsychotic anxiety. Their mean age was 45 (range 39–57) years, and they were all single and white. All the patients met DSM-IV criteria for alcohol dependence, and 3 met the DSM-IV criteria for drug dependence (cocaine and methamphetamine). The patients' mean age at first incidence of SDD was 14.4 years. On average, patients had attended 3 previous treatments for their SDD (Table 1).
Table 1
Quetiapine was started on average 17 days after admission into the 28-day residential program. Other treatment that patients were referred to was continued. The initial dose of quetiapine for all 9 patients was 50 mg at bedtime and was gradually increased to tolerance. The dose of quetiapine ranged from 50 mg to a maximum of 300 mg per day (the average dose was 153 mg per day).
Quetiapine was generally well tolerated. Only 1 of the 9 patients stopped taking quetiapine because of increased anxiety. Other patients reported improvement in sleep and anxiety. The change in Ham-D scores from before the start of the quetiapine to the time of discharge varied from a decrease of 29 points to an increase of 2 points for the nonresponder. The mean decrease in Ham-D for the responders was 18.5 points (p < 0.005) (Fig. 1). The biggest decreases on the Ham-D occurred on the subscales of insomnia, agitation, somatic anxiety, psychologic anxiety, hypochondriasis and obsessional symptoms. The change in the Likert 10-point craving scale varied from a decrease of 8 points to an increase of 1 point. The mean decrease in Likert score was 5.9 for the responders (p < 0.005) (Fig. 2). Further, these patients' periodic Breathalyzer and urine test results suggested that they remained abstinent from alcohol and other drug use.
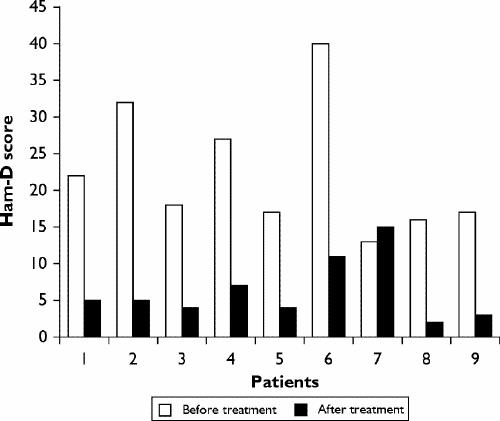
Fig. 1: Hamilton-D Rating Scale for Depression (Ham-D) scores before and after treatment with quetiapine.
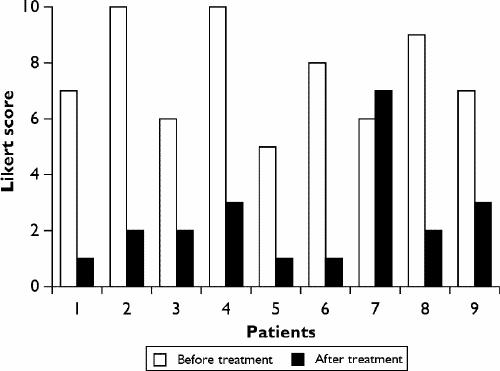
Fig. 2: Likert scores for cravings for alcohol or the drug of choice before and after treatment with quetiapine.
All 8 responders completed the 28-day residential program and were discharged to structured-living or outpatient programs. They continued to take quetiapine and at last contact had achieved 6 months of abstinence.
Discussion
This study adds to the growing literature on the potential benefits of the atypical antipsychotic medication quetiapine in reducing substance abuse in patients with nonpsychotic or nonbipolar disorders.33 In all 8 cases, the use of quetiapine was adjunctive with other pharmacologic and nonpharmacologic treatment. Therefore, symptom improvement could be attributed to concomitant medications, because of increased duration of treatment or increased serum levels through drug–drug interaction or because of increased duration of nonpharmacologic interventions. However, the rapid improvement in symptoms after the addition of quetiapine suggests causality. Further, these findings are similar to previous reports of quetiapine's beneficial effects on SDD in patients with psychotic and bipolar disorders. In addition, 2 other recently published studies suggest a benefit of quetiapine in patients with schizophrenia and bipolar disorder along with substance abuse,34,35 although only the study cited earlier in this paragraph, by Monnelly et al,33 was of patients with nonpsychotic and nonbipolar disorders.
Drugs and alcohol reportedly exert their addictive properties through the limbic system's reward pathway, probably mediated by the release of the neurotransmitter dopamine.36 Medications that block dopamine may inhibit this pathway, possibly leading to efforts to counter this blockade through increased substance use. This might explain the increased substance use seen after treatment with typical antipsychotic drugs, which block dopamine D2 receptors. Novel antipsychotic drugs, such as quetiapine, have significantly lower dopamine blockade, which might not lead to any increases in substance use. Further, quetiapine, in particular, only transiently blocks the dopamine receptors in the limbic system, which might facilitate a minimal blockade of dopamine in the reward pathway.37 In addition, quetiapine binds to histamine H1 receptors and produces sedation, which might also decrease anxiety and improve sleep. Patients with SDD often show fixed obsessive thinking that revolves around drugs. The antipsychotic effects of quetiapine may decrease this fixed thinking and allow patients to look beyond their substance use. Quetiapine's beneficial effects in decreasing SDD may be explained by Khantzian's self-medication hypothesis,38 which suggests that substances are abused to overcome anxiety or the distressing effects of illness or its treatment. However, there may also be some other, yet unexplained, mechanism that causes quetiapine to decrease substance dependence.
Even though quetiapine potentially has benefits in the treatment of SDD, it should be used cautiously because movement disorders such as tardive dyskinesia can occur more frequently in individuals with substance use disorders.39 Further, other serious adverse effects such as extrapyramidal side effects, metabolic irregularities, weight gain and neuroleptic malignant syndrome have been reported with antipsychotic medications. Alternatives should be tried before considering quetiapine. Benzodiazepine treatment in individuals with substance use disorders is generally discouraged except for acute detoxification, because of its potential to trigger relapse into alcohol and drug use. Because of these concerns, physicians who treat such patients often shy away from prescribing benzodiazepines, which might provide much-needed relief. This can hurt the doctor–patient relationship and increase treatment dropout rates and might even lead to the patient's relapse into using alcohol and drugs. For such patients, quetiapine offers another option. Quetiapine may provide an immediate subjective relief of anxiety (similar to the effects of drugs or alcohol) and has so far not shown any potential for abuse. Further, its use during the initial stages of treatment provides the patient with an opportunity to participate in treatment longer and facilitates the development of an improved treatment alliance, while allowing patients to learn new relapse prevention techniques. The risks of extrapyramidal side effects, neuroleptic malignant syndrome and tardive dyskinesia can be minimized with close supervision and the use of minimal doses for brief periods of treatment. The benefits of long-term quetiapine therapy in patients with no psychotic disorder have not been evaluated, and quetiapine should be used with caution over extended periods. Patients should be educated about the risks of novel antipsychotic drugs versus the risks of continuing substance abuse. In cases where the risks and consequences of SDD are greater than the risks associated with quetiapine, its use may be justified.
It is not known whether the benefits of quetiapine in the treatment of SDD in patients with a nonpsychotic or nonbipolar disorder result from improvement of anxiety or a decrease in craving independent of the anxiety. Further, it is not known whether quetiapine has similar benefits in individuals with substance use disorders in the absence of anxiety disorders. More work is needed before quetiapine becomes an established treatment for SDD. In the meantime, for a select group of patients, quetiapine may offer some advantages in preventing relapse.
Footnotes
Competing interests: Dr. Sattar has been a paid consultant to AstraZeneca, Eli Lilly and Abbott Laboratories and has received honoraria, speaker fees and research funding from AstraZeneca, Eli Lilly, Bristol-Myers Squibb and Abbott Laboratories. Dr. Bhatia has been a paid consultant to Bristol-Myers Squibb and Eli Lilly and has received honoraria, speaker fees and travel assistance from AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Janssen-Ortho. Dr. Petty has been a paid consultant to Eli Lilly and AstraZeneca and has received honoraria, speaker fees and travel assistance from AstraZeneca and Eli Lilly.
Correspondence to: Dr. S. Pirzada Sattar, 3528 Dodge St., Omaha, NE 68131; fax 402 449-0677; syed.sattar@med.va.gov
Submitted Aug. 1, 2003; Revised Dec. 12, 2003; Accepted Mar. 29, 2004
References
- 1.Gastager H. Chlormethiazole treatment of abstinence symptoms after drug withdrawal. Preliminary reports. 3. Acta Psychiatr Scand Suppl 1966;192:193-6. [PubMed]
- 2.Karkalas J, Lal H. A comparison of haloperidol with methadone in blocking heroin-withdrawal symptoms. A pilot study. Int Pharmacopsychiatry 1973;8(4):248-51. [DOI] [PubMed]
- 3.Maltbie AA. Haloperidol treatment of a sixty-year narcotic addiction: case report. Mil Med 1979;144(4):251-2. [PubMed]
- 4.Itil TM, Wadud A. Treatment of human aggression with major tranquilizers, antidepressants, and newer psychotropic drugs. J Nerv Ment Dis 1975;160(2-1):83-99. [DOI] [PubMed]
- 5.Gawin FH, Allen D, Humblestone B. Outpatient treatment of ‘crack’ cocaine smoking with flupenthixol decanoate. A preliminary report. Arch Gen Psychiatry 1989;46(4):322-5. [DOI] [PubMed]
- 6.Soyka M, De Vry J. Flupenthixol as a potential pharmacotreatment of alcohol and cocaine abuse/dependence. Eur Neuropsychopharmacol 2000;10(5):325-32. [DOI] [PubMed]
- 7.Levin FR. Pergolide mesylate for cocaine abuse: a controlled preliminary trial. Am J Addict 1999;8(2):120-7. [DOI] [PubMed]
- 8.Carlsson C, Gullberg B. A double blind study with melperone and placebo in the treatment of chronic alcoholics. Int J Clin Pharmacol Biopharm 1978;16(7):331-2. [PubMed]
- 9.Hochenegg L. Clopentixol decanoate in the treatment of chronic alcoholism. Acta Psychiatr Belg 1981;81(2):121-7. [PubMed]
- 10.Overall JE. Drug treatment of anxiety and depression in detoxified alcoholic patients. Arch Gen Psychiatry 1973;29(2):218-25. [DOI] [PubMed]
- 11.Simeon J, Wadud A, Itil T. A comparison of phenothiazines in managing aggressive episodes in schizophrenic patients. Hosp Community Psychiatry 1975;26(9):574. [DOI] [PubMed]
- 12.Janssen PA. Addiction and the potential for therapeutic drug development. EXS 1994;71:361-70. [DOI] [PubMed]
- 13.Rubio VG, Casas BM. Treatment of schizophrenia in subjects with substance use disorders. A review [in Spanish]. Actas Espanolas de Psiquiatria 2001;29(2):124-30. [PubMed]
- 14.Block C, Borendal-Jansson B, Carlsson C. A double-blind crossover comparison between clothiapine and diazepam in the treatment of mental symptoms in chronic alcoholics. Int J Clin Pharmacol 1974;9(4):321-5. [PubMed]
- 15.Bowers MB Jr, Mazure CM, Nelson JC, Jatlow PI. Psychotogenic drug use and neuroleptic response. Schizophr Bull 1990; 16(1):81-5. [DOI] [PubMed]
- 16.Siris SG. Pharmacological treatment of substance-abusing schizophrenic patients. Schizophr Bull 1990;16(1):111-22. [DOI] [PubMed]
- 17.McEvoy JP, Freudenreich O, Levin ED, Rose JE. Haloperidol increases smoking in patients with schizophrenia. Psychopharmacology (Berl) 1995;119(1):124-6. [DOI] [PubMed]
- 18.Green AI, Zimmet SV, Strous RD, Schildkraut JJ. Clozapine for comorbid substance use disorder and schizophrenia: Do patients with schizophrenia have a higher reward deficiency syndrome that can be ameliorated by clozapine? Harv Rev Psychiatry 1999;6(6):287-96. [DOI] [PubMed]
- 19.Zimmet SV, Strous RD, Burgess ES, Kohnstamm SAB, Green AI. Effects of clozapine on substance use in patients with schizophrenia and schizoaffective disorders. A retrospective study. J Clin Psychopharm 2000;20(1):94-8. [DOI] [PubMed]
- 20.Drake RE, Xie H, McHugo GJ, Green AI. The effects of clozapine on alcohol and drug use disorders among patients with schizophrenia. Schizophr Bull 2000;26(2):441-9. [DOI] [PubMed]
- 21.Tsuang JW, Eckman TE, Shaner A, Marder SR. Clozapine for substance abusing schizophrenic patients. Am J Psychiatry 1999;156(7):1119-20. [DOI] [PubMed]
- 22.Buckley PF. Novel antipsychotic medications and treatment of co-morbid substance abuse in schizophrenia. J Subst Abuse 1998;15(2):113-6. [DOI] [PubMed]
- 23.Treatment of special populations with the atypical antipsychotics. Collaborative Working Group of Clinical Trials Evaluations. J Clin Psychiatry 1998;59(Suppl 12):46-52. [PubMed]
- 24.Volvaka J. The effects of clozapine on aggression and substance abuse in schizophrenic patients. J Clin Psychiatry 1999; 60(Suppl 12):43-6. [PubMed]
- 25.Wilkins JN. Pharmacotherapy of schizophrenia patients with co-morbid substance abuse. Schizophr Bull 1997;23(2):215-28. [DOI] [PubMed]
- 26.Miller NS, Guttman JC. The integration of pharmacological therapy for co-morbid psychiatric and addictive disorders. J Psychoactive Drugs 1997;29(3):249-54. [DOI] [PubMed]
- 27.Rubio VG, Casas BM. Treatment of schizophrenia in subjects with substance use disorders. A review [in Spanish]. Actas Espanolas de Psiquiatria 2001;29(2):124-30. [PubMed]
- 28.Littrell KH, Petty RG, Hilligoss NM, Peabody CD, Johnson CG. Olanzapine treatment of patients with schizophrenia and substance abuse. J Subst Abuse Treat 2001;21(4):217-21. [DOI] [PubMed]
- 29.Brown ES, Nejtek VA, Perantie DC, Bobadilla L. Quetiapine in patients with bipolar disorder and cocaine dependence [poster]. Fourth International Conference on Bipolar Disorder; 2001 June 14–16; Pittsburgh, PA.
- 30.Sattar SP, Ucci B, Grant K, Bhatia SC, Petty F. Quetiapine as an adjunct in a substance abusing veteran with PTSD. Ann Pharmacother 2002;36(12):1875-8. [DOI] [PubMed]
- 31.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington: The Association; 1994.
- 32.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56-62. [DOI] [PMC free article] [PubMed]
- 33.Monnelly EP, Ciraulo DA, Knapp C, LoCastro J, Sepulveda I. Quetiapine for treatment of alcohol dependence. J Clin Psychopharmacol 2004;24:532-5. [DOI] [PubMed]
- 34.Longoria J, Brown ES, Perantie DC, Bobadilla L, Nejtek VA. Quetiapine for alcohol use and craving in bipolar disorder. J Clin Psychopharmacol 2004;24(1):101-2. [DOI] [PubMed]
- 35.Weisman RL. Quetiapine in the successful treatment of schizophrenia with comorbid alcohol and drug dependence: a case report. Int J Psychiatry Med 2003;33(1):85-9. [DOI] [PubMed]
- 36.Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, et al. Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs 2000; 32(Suppl):i-iv, 1-112. [DOI] [PubMed]
- 37.Kapur S, Zipursky R, Jones C, Shamm C, Remington G, Seeman P. A positron emission tomography study of quetiapine in schizophrenia: a preliminary finding of an antipsychotic effect with only transiently high dopamine D2 receptor occupancy. Arch Gen Psychiatry 2000;57(6):553-9. [DOI] [PubMed]
- 38.Khantzian E. The self-medication hypothesis of addictive disorders. Am J Psychiatry 1985;142:1259-64. [DOI] [PubMed]
- 39.Bailey LG, Maxwell S, Brandabur MM. Substance abuse as a risk factor for tardive dyskinesia: a retrospective analysis of 1,027 patients. Psychopharmacol Bull 1997;33(1):177-81. [PubMed]


