Abstract
Aims
To compare use of alternative nicotine products, smoking behavior and tobacco biomarker exposure in smokers unwilling to quit who were randomly assigned to normal nicotine content (NNC) cigarettes, or very low nicotine content (VLNC) cigarettes.
Design
Randomized, parallel arm 8-week study with assignment to VLNC (VLNC 1, N =53) or NNC (NNC, N=27) with access to non-cigarette combusted and non-combusted tobacco/nicotine products or to VLNC with access to only non-combusted products (VLNC2; N=56).
Setting
Clinics in Minnesota, USA
Participants
Smokers uninterested in quitting smoking with a mean (SD) age of 44 (14) years and smoking 16 (7) cigarettes/day; 51% female, 72% White.
Measurements
During the experimental period the measures taken included: rate of alternative products used, amount of and abstinence from combusted tobacco used, and tobacco exposure biomarkers.
Findings
There were higher rates of non-combustible alternative tobacco/nicotine product use in both VLNC conditions versus the NNC condition (rate ratio [RR]=2.18, 95% CI =1.94, 2.46 and RR=1.64, 95% CI=1.46, 1.85, respectively) and in VLNC1 versus VLNC2 condition (RR=1.33, 95% CI=1.23, 1.44), accompanied by reduced biomarkers of exposure primarily in VLNC2 condition (ps < 0.05). Fewer combusted products were smoked at almost all visits (ps ≤ 0.02) and there were higher rates of abstinence for both VLNC conditions compared with the NNC condition (VLNC1 versus NNC: RR = 9.96, 95% CI = 5.01, 19.81; VLNC2 versus NNC: RR = 11.23, 95% CI = 5.74, 21.97).
Conclusion
The offer of, and instructions to use, reduced nicotine content cigarettes over an 8 week period led to greater use of alternative tobacco/nicotine products compared with continued use of normal nicotine cigarettes and also reductions in smoking rates.
Keywords: reduced nicotine content cigarettes, smokeless tobacco, electronic cigarettes, medicinal nicotine, exposure biomarkers
Introduction
Although comprehensive tobacco control measures have had a significant impact in reducing smoking, 42 million U.S. adults still smoke (1). Globally, about a billion people smoke cigarettes, with evidence of increasing rates in countries such as China, the Eastern Mediterranean region, and potentially Africa (2). Therefore, novel tobacco control approaches to reduce combusted tobacco product use are needed (3). A powerful tool granted to the U.S. Food and Drug Administration through the 2009 Family Smoking Prevention and Tobacco Control Act is the authority to establish product standards, which includes reducing levels of nicotine content to render the cigarette minimally addictive. Similarly, Article 9 of the Framework Convention on Tobacco Control also authorizes the regulation of tobacco contents and emissions, including nicotine (4). While constituents other than nicotine are responsible for the death and disease caused by smoking (5), this regulatory policy could have a profound impact on public health by minimizing the transition from experimental cigarette use to dependence in youth, facilitating quit attempts, and reducing the occurrence of relapse. Thus, public health benefit would be the result of reducing smoking prevalence, not by reducing the harmfulness of smoking.
To date, studies examining the effects of reducing nicotine to very low levels have shown promising results including a reduction in smoking and dependence relative to normal nicotine content cigarettes and an increase in quit attempts (6–8). However, the majority of these studies, although strong experimentally, were not conducted in the context of a marketplace where many types of tobacco and nicotine-containing products are available. Thus, extrapolation of the results from these studies to the real world may be limited. If a nicotine product standard is implemented, then the likelihood that smokers will seek alternative sources of nicotine could be high. For example, prior studies have shown that a significant number of smokers who were assigned to reduced nicotine content cigarettes and asked to solely use these products were “non-complaint” (7–10). In a large study conducted by Donny et al. (6), a secondary analysis of product non-compliance demonstrated that about three-fourths of participants using the 0.4 mg nicotine content cigarettes (0.04 mg machine determined nicotine yield in smoke) were confirmed biochemically to be using some non-study tobacco products based on levels of their urinary total nicotine equivalents (TNE, 11). This non-compliance could have contributed to the less than anticipated reductions in study cigarette consumption and dependence with the very low nicotine content cigarettes.
The overall primary aims of this exploratory study were to determine the amount of use of alternative tobacco/nicotine products and subsequent effects on smoking behaviors and biomarkers of exposures when smokers were randomly assigned to very low nicotine content (VLNC) vs. normal nicotine content (NNC) cigarettes. Another primary aim was to determine if access to both combusted and non-combusted alternative tobacco/nicotine products vs. only non-combusted products would differentially affect smoking behaviors and biomarkers of exposure. Finally, a secondary aim was to determine the relationship between amount of non-combusted product use with the use of combusted products, days abstinent and biomarkers of exposure.
Methods
Experimental Cigarettes
Study cigarettes (Spectrum) were ordered through the National Institute on Drug Abuse. The NNC non-menthol cigarettes had 16.6 mg nicotine/g tobacco (NRC600; 0.83 mg nicotine and 10.4 mg tar yields as determined by ISO smoking machine method) and menthol cigarettes had 16.08 mg nicotine/g tobacco (NRC601; 0.75 mg nicotine and 10.4 mg tar yields). These nicotine levels were chosen because they reflect the nicotine delivery of commercially available cigarettes. The VLNC non-menthol and menthol cigarettes were both 1.3 mg nicotine/g tobacco (NRC200 and NRC201, respectively, with 0.07 mg nicotine and 8.0 and 8.2 mg tar yields, respectively). These nicotine levels were chosen because prior studies have shown that at this dose, there is a significant reduction in cigarettes smoked/day and biomarkers of nicotine exposure compared to NNC cigarettes (6, 12). Participants were free to choose either non-menthol or menthol study cigarettes.
Design
Participants were randomized to one of three conditions for eight weeks in a parallel arm study design: 1) VLNC cigarettes with access to non-cigarette combusted products1 and non-combusted tobacco and nicotine (including medicinal) products,2 referred to as VLNC1; 2) VLNC cigarettes with access to only non-combusted products, referred to as VLNC2; or 3) NNC cigarettes with access to non-cigarette combusted and non-combusted products, simulating the current marketplace, referred to as NNC. One of the primary comparisons was between VLNC1 and VLNC2 vs. NNC conditions to determine the effects of nicotine content in cigarettes on rate of use of alternative tobacco/nicotine products and the effects of cigarette type and use of these alternative products on smoking behaviors, smoking abstinence rates and biomarkers of tobacco exposure. Another primary comparison was between VLNC1 vs. VLNC2 to determine the effect of access to combusted and non-combusted products vs. non-combusted products alone on similar outcome measures.
Participants
Cigarette smokers (targeted n=125) were recruited to the University of Minnesota, Minneapolis-St. Paul and Duluth campuses, U.S.A, by placing advertisements through a variety of media outlets including the internet. They contacted the respective research clinics and were screened for eligibility over the telephone. Participants were recruited if they were ≥ 18 years of age and smoked ≥ 5 cigarettes daily for ≥ 1 year and had made no serious quit attempts in the last 3 months. Moreover, they needed to demonstrate stable medical and psychiatric status and no current alcohol or drug abuse problems. Participants were excluded if they were regular users of tobacco or nicotine products other than cigarettes, had a planned quit date in the next two months, were experiencing an unstable living situation and if female, were pregnant or breastfeeding or were soon planning to be pregnant.
Procedure
Potential participants attended an orientation visit to be further informed about the study protocol, provide consent and be confirmed for eligibility. Participants were told that the study was examining the effects of a reduced nicotine cigarette on smoking behavior and the possible use of other nicotine containing products. After obtaining consent, tobacco use history including the Fagerstrom Test for Nicotine Dependence (13), demographics, and medical and psychiatric history were collected. Women received pregnancy testing. Eligibility was established after reviewing all the information.
Eligible participants then underwent 2 weeks of baseline smoking of usual brand cigarettes and attended two visits (Week −1 and 0). During this time, participants recorded the amount of cigarette or other tobacco/nicotine intake on a daily basis using an interactive voice response (IVR) system via the telephone. During the baseline clinic visits, participants completed a number of subjective measures and were assessed for exhaled carbon monoxide (CO), vital signs and weight. Additionally, on the second baseline visit, first morning void urine samples were collected for analysis of biomarkers of tobacco exposure.
After baseline assessment at Week 0, participants were assigned to either VLNC1, VLNC2, or NNC conditions (2:2:1 ratio using permuted block (size 5 or 10) randomization stratified by site, performed by the study statistician). Participants were blinded to the nicotine content of the cigarettes to which they were assigned and provided the cigarettes at no cost. During this period, participants were instructed to smoke as many of their assigned cigarettes as they wanted. They were provided 150% of the number of usual brand smoked to accommodate any compensatory smoking behavior.
At the initial visit when cigarettes were dispensed, participants were asked to smoke one of the assigned cigarettes in the laboratory to experience the effects from the cigarette so they could make an informed decision as to whether or not supplemental tobacco/nicotine products would be desired or needed. At this and subsequent visits, participants were dispensed study cigarettes and given 25 points worth US$1.00 per point and provided an opportunity to exchange their points for non-cigarette tobacco/nicotine products. Points were deducted each time they exchanged them for one of the products. To promote obtaining products from our marketplace and to minimize hoarding of the points, products were discounted at about 66% of the retail price. If participants ran out of products between visits and wanted to obtain more products using their banked points, they could schedule a visit at the clinic. Any points that were not expended were converted to cash at the end of the study. Participants were strongly discouraged from purchasing or using any tobacco or nicotine products (including usual brand cigarettes) other than those we offered or provided to them. The importance of honest reporting was stressed. In the event that participants non-study tobacco or nicotine products, they were asked to report the type and amount of product use.
Participants continued to report tobacco and other nicotine product use via the daily IVR. At every clinic visit, Timeline Follow Back measures (14, 15) were obtained to confirm the daily IVR reports. In addition, vital signs, weight, and exhaled CO were assessed and subjective measures to determine responses to cigarettes (not reported in this paper) were administered. The exception to these procedures was at Weeks 5 and 7 when only study cigarettes were dispensed, access to products provided and records of product use checked. Participants brought in a first morning urine specimen to each visit (except weeks 5 and 7), although only baseline, weeks 4 and 8 samples were analyzed for biomarkers of exposure.
At the end of the eight-week experimental period, no more study products were provided and participants were advised to quit using all tobacco products (including e-cigarettes) and set a quit date. A treatment manual for cessation was provided and participants were encouraged to call the state telephone quit line.
Follow-up visit occurred at 12 weeks after the end of the experimental period as a safety check. Tobacco/nicotine product use, amount of cigarettes smoked and biomarkers of exposure were assessed to determine if there were any increases in these values compared to baseline.
Participants were compensated for transportation costs and their time.
Outcome Measures
Alternative tobacco/nicotine product use (non-study cigarettes, non-cigarette combusted products and non-combusted products), amount of study cigarette use, amount of combusted product use (study cigarettes, non-study cigarettes, and non-cigarette combusted products), and abstinence from any combusted products were assessed each day during the eight-week experimental period using the IVR system. Use was defined as any uptake of a particular product. Biomarkers of tobacco exposure included CO and analyses of urine for nicotine exposure as assessed by total nicotine equivalents (TNE), the sum of total nicotine, nicotine N-oxide, total cotinine, and total 3’-hydroxycotinine (16), which accounts for ≥ 85% of the nicotine dose (17). Additionally, carcinogen exposures were determined by analyzing NNK metabolites, total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (total NNAL; 18) and NNN metabolites, total N’-nitrosonornicotine (NNN; 19, 20). For each analyte, total refers to the analyte plus its glucuronide conjugate(s). Biomarkers of exposure were adjusted per mg creatinine.
Statistical Analysis
Participants’ baseline demographics and smoking characteristics were summarized using mean, standard deviation (SD), frequency and percentage, as appropriate. As a measure of treatment compliance, the percent of participants in each condition who used assigned sudy cigarettes by week were examined and compared using Chi-square test.
The number of days using alternative nicotine products other than the assigned study cigarettes and the number of 24-hour attempts of abstinence from any combusted products were analyzed using Poisson regression with an offset term equal to the logarithm of the total number of days under intervention, adjusting for baseline demographics and smoking characteristics (age, gender, race, education, income, FTND total score, cigarettes/day, and past 30 days’ use of alternative tobacco/nicotine products prior to randomization). The amount of assigned study cigerettes used/day, combusted products used/day, and CO were summarized by week using mean and 95% confidence interval (CI). TNE, total NNAL and total NNN, adjusted per creatinine, were summarized by week using geometric mean and 95% CI. Linear mixed model was performed for these repeated measures outcomes (or log-transformed outcomes including TNE, total NNAL, and total NNN) with covariates of week, intervention condition, and their interaction, adjusting for their baseline levels. We tested and found no evidence for heterogeneity of effect across the two sites in any of the outcomes. Hence, site was not included in the final models.
The cross-sectional associations of non-combusted products use with combusted product use, the number of days of abstinence from combusted product use, and biomarkers (log-transformed as appropriate) at different visits were examined using marginal generaized estimating equations (GEE) model (21, Chapter 12) with the non-combusted product use as the independent variable, adjusting for week, experimental condition, and the baseline level of the dependent variable when available.
All analyses were intent-to-treat and using observed data only. All tests were two-sided and p-values <0.05 were considered statistically significant. All analyses were performed in SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Power Calculation
This study was an exploratory trial, and therefore sample size and power calculations were not crucial. Nevertheless, we found that the target sample size (n=50, 50, and 25 for VLNC1, VLNC2, and NNC, respectively) was powered to detect the difference between VLNC1 and NNC in rate of alternative products use (i.e., proportion of days using alternative products during intervention) if the rates of two arms were 0.60 and 0.20, respectively. No power analyses were performed for other aims. The unbalanced sample size (i.e., more subjects in the VLNC conditions) was proposed in order to get more precise estimates for the VLNC conditions for use in future power calculations for a full-sized trial.
Results
Demographics
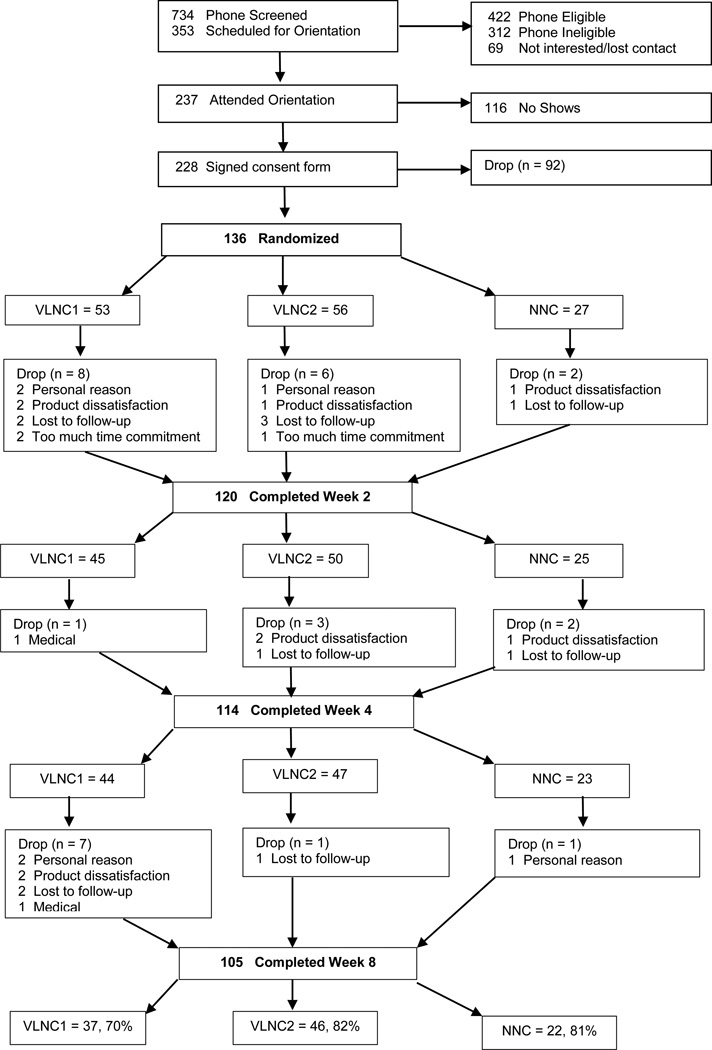
Of the 228 smokers who signed consent, 136 were randomized (N=40 Duluth, N=96 Twin Cities) and 105 completed the study. Figure 1 presents the flow of participants and reasons for study withdrawal. The three conditions did not differ significantly in the likelihood of completing the end of intervention or Week 8 assessment (p=0.31). Table 1 shows baseline demograhic and smoking history information of randomized participants.
Figure 1.
Participant flow from screening to end of intervention.
Table 1.
Baseline demographics and smoking history
| Variable | Overall N = 136 |
VLNC1 N = 53 |
VLNC 2 N = 56 |
NNC N = 27 |
|---|---|---|---|---|
| Age, Mean (SD) | 44 (14) | 44 (15) | 45 (15) | 43 (14) |
| Gender, Female (N, %) | 69 (51%) | 29 (55%) | 29 (52%) | 11 (41%) |
| Race, 3-category (N, %) | ||||
| White | 98 (72%) | 38 (72%) | 41 (73%) | 19 (70%) |
| Black | 26 (19%) | 9 (17%) | 11 (20%) | 6 (22%) |
| Other | 12 (9%) | 6 (11%) | 4 (7%) | 2 (7%) |
| Education, 2-category (N, %) | ||||
| High School Graduate or Less | 52 (39%) | 19 (37%) | 22 (39%) | 11 (41%) |
| Some College or More | 83 (61%) | 33 (63%) | 34 (61%) | 16 (59%) |
| FTND† total score, Mean (SD) | 5 (2) | 5 (2) | 5 (2) | 5 (3) |
| Cigarettes/day | 16 (7) | 15 (6) | 16 (7) | 16 (7) |
| Alternative nicotine product use†† (N, %) |
29 (21%) | 10 (19%) | 12 (21%) | 7 (26%) |
VLNC1: Very low nicotine content cigarettes with access to non-cigarette combusted and non-combusted tobacco/nicotine products
VLNC2: Very low nicotine content cigarettes with access to only non-combusted tobacco/nicotine products.
NNC: Normal nicotine content cigarettes with access to non-cigarette combusted and non-combusted tobacco/nicotine products
Fagerstrom Test for Nicotine Dependence
Alternative tobacco/nicotine products included e-cigarettes, cigars, cigarillos, little cigars, pipe, snus, snuff, chewing tobacco, hookah, and nicotine gum, patch, and lozenges. Use was defined as past 30 day use (participants excluded if greater than weekly use
Study cigarette use
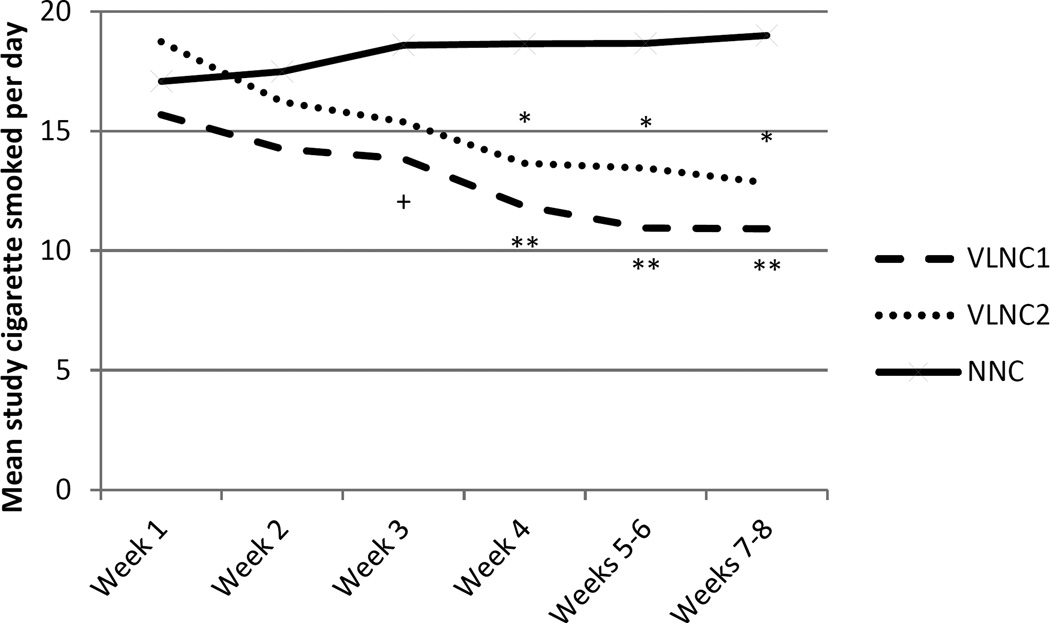
During any week, there was no significant difference in percent who used assigned study cigarettes among the three conditions. The percent of participants using study cigarette by week ranged from 88% to 100% for both VLNC1 and VLNC2 conditions, with the highest rates of use during the initial weeks. For the NNC condition, the rates ranged from 96% to 100% with no particular pattern of rate of use across weeks. Figure 2 shows the number of study cigarettes smoked/day. Significant differences were observed between VLNC1 vs. NNC from Weeks 3 through 8 and VLNC2 vs. NNC from Weeks 4 through 8.
Figure 2.
Mean number of study cigarettes smoked/day across experimental conditions (VLNC1: Very Low Nicotine Content (VLNC) cigarettes with access to non-cigarette combusted and non-combusted tobacco/nicotine products; VLNC2: VLNC cigarettes with access to non-combusted tobacco/nicotine products only; NNC: normal nicotine content (NNC) cigarettes with access to non-cigarette combusted and non-combusted tobacco/nicotine products) during the 8-week intervention period. Significant difference compared with the NNC condition (+p<0.05, *p <0.01, **p<0.001) based on adjusted repeated measures analysis.
Amount and pattern of tobacco/nicotine product use
Table 2 shows the number of days of use and proportion of days using alternative tobacco/nicotine products. Adjusted and unadjusted analyses show that participants assigned to VLNC1 and VLNC2 conditions reported significantly higher percent of days of alternative product use compared to those in the NNC condition and VLNC1 had a higher rate of use compared to VLNC2.
Table 2.
Mean number of days and proportion of days (number of days using divided by number of participant intervention days) using alternative nicotine-containing products across intervention conditions unadjusted and adjusted for baseline levels
| Variable | VLNC1 N=53 Mean (SD) |
VLNC2 N=56 Mean (SD) |
NNC N=27 Mean (SD) |
VLNC1 vs NNC | VLN2 vs NNC | VLNC1 vs VLNC2 | |||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted RR (95% CI) p-value |
Adjusted RR (95% CI) p-value |
Unadjusted RR (95% CI) p-value |
Adjusted RR (95% CI) p-value |
Unadjusted RR (95% CI) p-value |
Adjusted RR (95% CI) p-value |
||||
| Number of days using alternative products |
26 (19) | 20 (19) | 14 (17) | 2.04 (1.82, 2.29) <0.0001 |
2.18 (1.94, 2.46) <0.0001 |
1.53 (1.36, 1.72) <0.0001 |
1.64 (1.46, 1.85) <0.0001 |
1.34 (1.24, 1.46) <0.0001 |
1.33 (1.23, 1.44) <0.0001 |
| Proportion of days using alternative products |
0.59 (0.37) | 0.45 (0.35) | 0.32 (0.35) | ||||||
VLNC1: Very low nicotine content cigarettes with access to non-cigarette combusted and non-combusted tobacco/nicotine products
VLNC2: Very low nicotine content cigarettes with access to only non-combusted tobacco/nicotine products
NNC: Normal nicotine content cigarettes with access to non-cigarette combusted and non-combusted tobacco/nicotine products
Unadjusted: Poisson regression with the only covariate of study arm
Adjusted: Poisson regression adjusted for baseline age, gender, race, education, income, FTND total score, cigarettes per day, and use of alternative nicotine products in the past 30 days
RR: Rate ratio
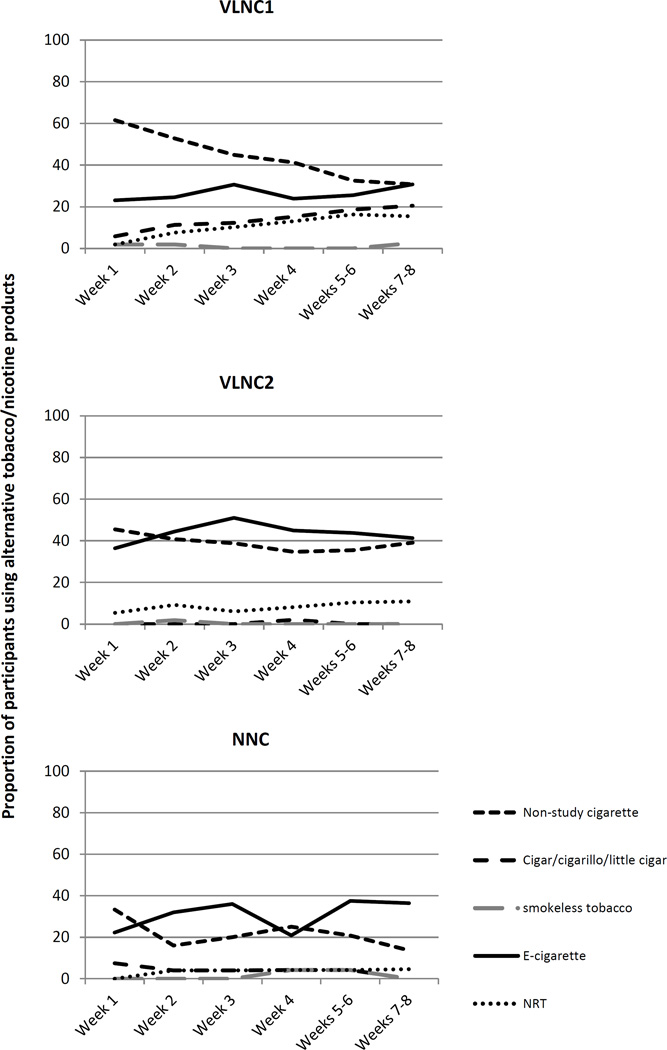
Figure 3 shows the proportion of participants in each of the conditions that used the various alternative products including non-study cigarettes. The predominant product that was chosen across all conditions, other than non-study cigarettes, was e-cigarettes. Non-study cigarettes were used particularly during the early experimental phase. The mean (SD) number of non-study cigarettes smoked on days when they were smoked was 5.1 (4.6), with no significant differences across conditions (p=0.38).
Figure 3.
Percent use of alternative products in each of the experimental conditions (VLNC1: Very Low Nicotine Content (VLNC) cigarettes with access to non-cigarette combusted and non-combusted tobacco/nicotine products; VLNC2: VLNC cigarettes with access to non-combusted tobacco/nicotine products only; NNC: normal nicotine content (NNC) cigarettes with access to non-cigarette combusted and non-combusted tobacco/nicotine products) during the 8-week intervention period.
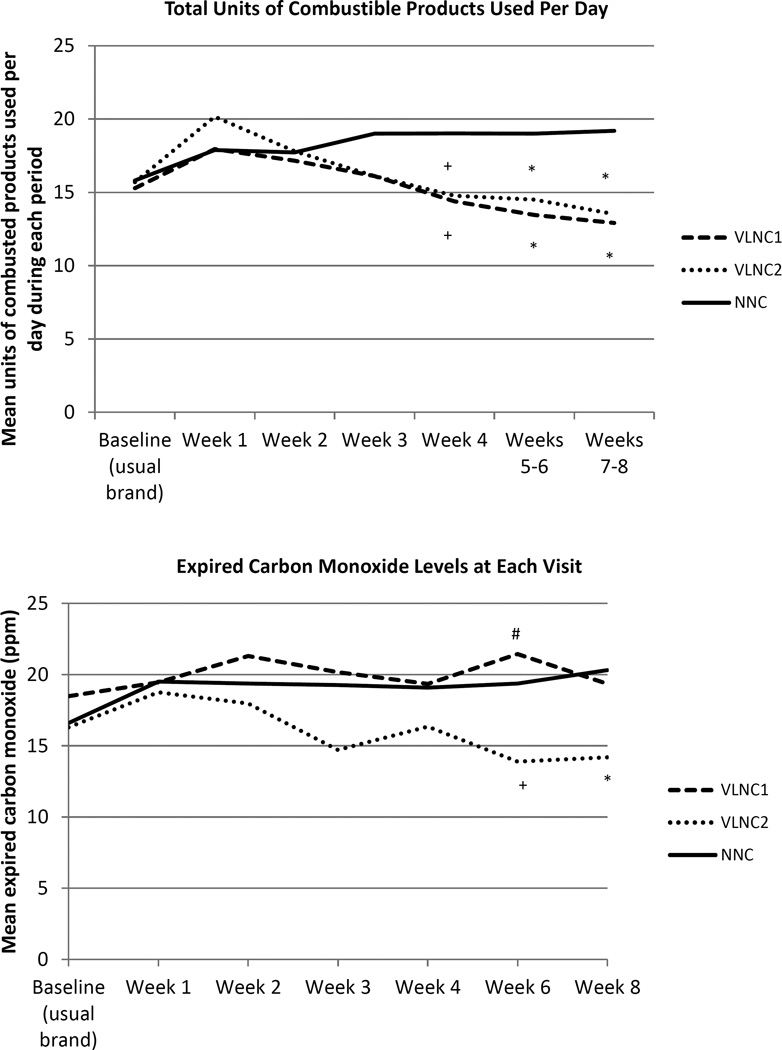
Figure 4 shows the mean number of study and non-study combusted tobacco products smoked/day by visit. Repeated measures analysis shows that participants in both VLNC1 and VLNC2 vs. NNC conditions smoked significantly fewer combusted products/day between Weeks 3 and 8. No significant differences were observed between VLNC1 vs. VLNC2 conditions. Mean (SD) number of days abstinent from study and non-study combusted products during intervention in the VLNC1, VLNC2, and NNC conditions were 2.5 (7.9), 4.0 (9.9), and 0.3 (1.2), respectively. The rate of 24-h abstinence attempts were 23% vs. 27% vs. 7%, respectively. The adjusted analysis confirms that VLNC1 and VLNC2 participants had significantly higher rates of abstinence attempts compared to NNC participants (VLNC1 vs. NNC: RR = 9.96, 95% CI = 5.01, 19.81, p<0.0001; VLNC2 vs. NNC: RR = 11.23, 95% CI = 5.74, 21.97, p<0.0001), while VLNC1 and VLNC2 participants were not significantly different (VLNC2 vs. VLNC1: RR = 1.13, 95% CI = 0.89, 1.43, p=0.32).
Figure 4.
Mean number of combusted products used per day and carbon monoxide levels at each visit across experimental conditions (VLNC1: Very Low Nicotine Content (VLNC) cigarettes with access to non-cigarette combusted and non-combusted tobacco/nicotine products; VLNC2: VLNC cigarettes with access to non-combusted tobacco/nicotine products only; NNC: normal nicotine content (NNC) cigarettes with access to non-cigarette combusted and non-combusted tobacco/nicotine products). Baseline refers to ad libitum smoking of usual brand cigarettes. Significant differences compared with the NNC condition (+p<0.05 and *p=0.01) and compared with VLNC2 (#p<0.01) based on adjusted repeated measures analysis.
Biomarkers of tobacco exposure
Figure 4 also shows CO levels among participants and Table 3 shows the geometric means for creatinine adjusted TNE, total NNAL and total NNN across the experimental conditions. Participants experienced lower mean CO levels if assigned to VLNC2 vs. NNC at Weeks 6 and 8 and VLNC2 vs. VLNC1 at Week 6. For TNE, participants in VLNC1 and VLNC2 vs. NNC showed significantly lower levels at both Weeks 4 and 8, respectively. Significantly lower TNE levels were also observed for VLNC2 vs. VLNC1 at Week 4. For total NNAL, significantly lower levels were observed in VLNC2 vs. NNC at Weeks 4 and 8 and VLNC2 vs. VLNC1 at week 4. For total NNN, only marginally significant effects were observed for VLNC2 vs. NNC at Week 8.
Table 3.
Significant differences in biomarkers of exposure across intervention conditions unadjusted and adjusted for baseline levels
| VLNC1 N=53 Geometric mean (95% CI) |
VLNC2 N=56 Geometric mean (95% CI) |
NNC N=27 Geometric mean (95% CI) |
VLNC1 vs NNC | VLNC2 vs NNC | VLNC1 vs VLNC2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted p-value |
Adjusted p-value |
Unadjusted p-value |
Adjusted p-value |
Unadjusted p-value |
Adjusted p-value |
|||||
|
TNE (nmol/mg creatinine) |
||||||||||
| Baseline | 34.5 (28.5, 41.7) |
34.0 (28.9, 39.9) |
36.9 (29.2, 46.8) |
|||||||
| Week 4 | 14.5 (9.4, 22.3) |
7.9 (5.4, 11.6) |
34.6 (27.7, 43.1) |
0.02 | 0.02 | <0.0001 | 0.02 | 0.02 | <0.0001 | |
| Week 8 | 13.9 (7.9, 24.4) |
10.8 (7.0, 16.8) |
39.9 (32.5, 49.0) |
<0.01 | <0.01 | <0.001 | <0.001 | |||
|
Total NNAL (pmol/mg creatinine) |
||||||||||
| Baseline | 1.33 (0.96, 1.83) |
1.17 (0.88, 1.56) |
1.54 (1.09, 2.17) |
|||||||
| Week 4 | 0.99 (0.63, 1.54) |
0.50 (0.36, 0.70) |
1.22 (0.92, 1.63) |
<0.01 | <0.01 | <0.01 | <0.01 | |||
| Week 8 | 0.71 (0.41, 1.23) |
0.51 (0.35, 0.74) |
1.22 (0.91, 1.65) |
<0.01 | <0.01 | |||||
|
Total NNN (pmol/mg creatinine) |
||||||||||
| Baseline | 0.039 (0.023, 0.064) |
0.022 (0.016, 0.031) |
0.031 (0.018, 0.051) |
|||||||
| Week 4 | 0.025 (0.015, 0.040) |
0.020 (0.013, 0.031) |
0.035 (0.020, 0.063) |
|||||||
| Week 8 | 0.025 (0.014, 0.046) |
0.015 (0.010, 0.022) |
0.035 (0.019, 0.068) |
0.02 | 0.04 | |||||
VLNC1: Very low nicotine content cigarettes with access to non-cigarette combusted and non-combusted tobacco/nicotine products
VLNC2: Very low nicotine content cigarettes with access to only non-combusted tobacco/nicotine products
NNC: Normal nicotine content cigarettes with access to non-cigarette combusted and non-combusted tobacco/nicotine products
TNE: Total nicotine equivalents, biomarker for nicotine exposure
Total NNAL: Biomarker for exposure to carcinogen, NNK
Total NNN: Biomarker for exposure to carcinogen, NNN
Unadjusted: Linear mixed effects model with the covariates of study arm, week, and their interaction.
Adjusted: Linear mixed effects model with the covariates of study arm, week, and their interaction, adjusting for the baseline level of the corresponding biomarker.
Relationship between use of alternative non-combusted products on combusted tobacco use
Adjusted repeated measures analysis showed that the amount of non-combusted product use during the 8 weeks was significantly, negatively associated with the amount of study and non-study combusted product use (p<0.01) and the level of total NNAL (p<0.001), and significantly, positively associated with the number of abstinence days (p<0.001). However, the amount of non-combusted product use was not significantly associated with CO or TNE levels. See Appendices A–E for results from statistical analyses.
Follow-up
Follow-up data did not reveal any significant safety issues. The mean CO and percent use of alternative nicotine products were not significantly higher than baseline values. Self-reported number of cigarettes/day decreased but TNE slightly increased (p=0.02) compared to baseline (see Appendix F).
Discussion
The results from this study showed that compared to smokers assigned to normal nicotine content cigarettes, those assigned to very low nicotine content cigarettes used significantly more alternative nicotine products, smoked fewer combusted tobacco products, had higher number of smoking quit attempts and experienced less nicotine exposure. Less toxicant exposure (CO, total NNAL) and greater reductions in nicotine exposure was experienced in the very low nicotine condition with access to only non-combusted products. The findings also showed a negative association between the amount of non-combusted product used with amount of tobacco product smoked and with levels of toxicant exposure, and a negative association with number of abstinent days.
These results are concordant with past studies demonstrating that smokers who were switched to VLNC cigarettes experienced reduced smoking, nicotine and toxicant exposure, and increased quit attempts relative to NNC cigarettes (6, 7, 9, 10, 22). Nevertheless, despite these findings, several concerns have been raised regarding a regulation to reduce nicotine in cigarettes including the occurrence of compensatory smoking, manifestation of cigarette withdrawal symptoms, lack of data on vulnerable populations (e.g., smokers with mental illness) and potential for illicit trade. Furthermore, reducing nicotine in cigarettes has been considered to be a type of prohibition, which could result in a number of negative consequences (23, 24). One way to mitigate these concerns is to provide alternative less harmful sources of nicotine to smokers who experience difficulties when switched to low nicotine cigarettes. Indeed, the findings from this study indicate that reducing nicotine content in cigarettes could lead to a significant proportion of smokers using alternative nicotine containing products. Smokers, particularly those who are highly dependent, may seek out other sources of nicotine as a way to reduce withdrawal symptoms that they may experience when switching from NNC cigarettes to VLNC cigarettes. Some smokers may also seek other sources of nicotine to replace certain reinforcing effects that are experienced with NNC cigarettes or as a means to enhance the reinforcing effects of other reinforcers (25). The availability of less harmful alternative nicotine products might also facilitate cessation of cigarettes among populations with the highest rates of smoking (e.g., smokers with co-morbid disorders). Furthermore, if alternative nicotine products are readily made available, illicit trade may be reduced and consumer perceptions of “prohibition” mitigated. Nevertheless, countries would need sufficient resources to implement are regulatory policy for nicotine reduction (4).
Ideally, nicotine would be substantially reduced in all combusted products. Reductions in CO and other biomarkers of exposures were only seen in the condition in which smokers were switched to VLNC cigarettes with access to only non-combusted tobacco/nicotine products. Thus, the likely outcome of a nicotine regulatory approach for all combusted products could be shifting some smokers from the most harmful tobacco products - cigarettes and other combusted products - to products that are lower in the continuum of harm (26, e.g., non-combusted tobacco products, 27).
The optimal goal would be to have cigarette smokers quit use of all products or, if necessary, shift to using only medicinal nicotine products (26). However, smokers will more likely choose electronic nicotine delivery systems (ENDS) over medicinal nicotine because of their ability to provide some of the sensory effects of smoking (28, 29), potential for higher and more rapid delivery of nicotine (30–33), and greater accessibility and lower cost per unit dose. ENDS are considered to pose considerably less harm than cigarettes and potentially be an effective smoking cessation tool (5). Another alternative to cigarette use is smokeless tobacco. However, the use of smokeless tobacco or snus is historically lower in the U.S. because of their lack of appeal to smokers (34–37). In this study, smokers indeed chose e-cigarettes over medicinal nicotine and smokeless tobacco or snus. The possible substitution of ENDS for VLNC cigarettes indicates the importance for government agencies to implement product standards and require quality control measures for these systems (38).
This study was exploratory with a small sample size, limiting the generalizability of the results. Additionally, participants were blind to the dose, which would not happen if the nicotine content in cigarettes is reduced as a national regulatory policy. Study cigarettes were provided free of charge and price is an important factor that affects smoking behavior (39). The strength of this study was a better simulation of tobacco/nicotine use behaviors because it was conducted in the context of a more complex tobacco and nicotine product marketplace.
In summary, this study adds support for establishing regulations on levels of nicotine in cigarettes and preferably all combusted products to protect public health. This study also provides an important step towards understanding the role of other nicotine-containing but less harmful products if the nicotine in cigarettes is reduced minimally addictive levels. Such a policy may increase the use of other tobacco or nicotine products, but public health benefit would still be realized by the decreased use of combusted, more harmful products and especially if the non-combusted tobacco and non-therapeutic nicotine delivery systems are appropriately regulated. Conversely, some countries and tobacco control scientists have advocated for the use of reduced risk alternative nicotine products (e.g., e-cigarettes) as a way to shift smokers away from combusted products (5, 40, 41). This study shows that reducing nicotine in cigarettes would nudge smokers to these products and potentially expedite the demise of deadly combusted products.
Supplementary Material
Acknowledgments
Declaration of competing interests: Dr. Shields does litigation consulting in smoking cases on behalf of plaintiffs
NCT: 02000921
Many thanks to Joni Jensen for overseeing the study, Alex Johnson for the figures, Lori Strayer for developing the data base, and Kathy Longley for her assistance with the manuscript preparation. This study was funded by National Cancer Institute, and U.S. Food and Drug Administration (U19CA157345). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration.
Footnotes
The non-cigarette combusted brands included White Owl (premium cigar), Swisher Sweets (little cigars), Black and Milds (cigarillos).
Non-combusted brands included Copenhagen, Kodiak, Grizzly, Camel Snus (smokeless tobacco and snus); NJOY and Blu (electronic cigarettes) and Nicoderm patch, Nicorette gum and lozenge (over-the counter nicotine medications).
All products were available in different flavors, doses and number of units per package.
References
- 1.Jamal A, Homa DM, O'connor E, Babb SD, Caraballo RS, Singh T, et al. Current Cigarette Smoking Among Adults - United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2015;64:1233–1240. doi: 10.15585/mmwr.mm6444a2. [DOI] [PubMed] [Google Scholar]
- 2.Eriksen MP, Mackay J, Schluger N, Islami F, Drope J. The Tobacco Atlas. Atlanta, GA: American Cancer Society; 2015. [Google Scholar]
- 3.Warner KE. An endgame for tobacco? Tob Control. 2013;22(Suppl 1):i3–i5. doi: 10.1136/tobaccocontrol-2013-050989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. WHO Study Group on Tobacco Product Regulation (TobReg) Geneva, Switzerland: World Health Organization; 2015. Advisory Note: Global Nicotine Reduction Strategy. [Google Scholar]
- 5.Royal College of Physicians. Nicotine without smoke: Tobacco harm reduction. London: RCP; 2016. [Google Scholar]
- 6.Donny EC, Denlinger RL, Tidey JW, Koopmeiners JS, Benowitz NL, Vandrey RG, et al. Randomized trial of reduced-nicotine standards for cigarettes. N Engl J Med. 2015;373:1340–1349. doi: 10.1056/NEJMsa1502403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatsukami DK, Kotlyar M, Hertsgaard LA, Zhang Y, Carmella SG, Jensen JA, et al. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction. 2010;105:343–355. doi: 10.1111/j.1360-0443.2009.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatsukami DK, Hertsgaard LA, Vogel RI, Jensen JA, Murphy SE, Hecht SS, et al. Reduced nicotine content cigarettes and nicotine patch. Cancer Epidemiol Biomarkers Prev. 2013;22:1015–1024. doi: 10.1158/1055-9965.EPI-12-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P., 3rd Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidemiol Biomarkers Prev. 2007;16:2479–2485. doi: 10.1158/1055-9965.EPI-07-0393. [DOI] [PubMed] [Google Scholar]
- 10.Benowitz NL, Dains KM, Hall SM, Stewart S, Wilson M, Dempsey D, et al. Smoking behavior and exposure to tobacco toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiol Biomarkers Prev. 2012;21:761–769. doi: 10.1158/1055-9965.EPI-11-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nardone N, Donny EC, Hatsukami DK, Koopmeiners JS, Murphy SE, Strasser AA, et al. Estimations and predictors of non-compliance in switchers to reduced nicotine content cigarettes. Addiction. 2016 doi: 10.1111/add.13519. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatsukami DK, Heishman SJ, Vogel RI, Denlinger RL, Roper-Batker AN, Mackowick KM, et al. Dose-response effects of spectrum research cigarettes. Nicotine Tob Res. 2013;15:1113–1121. doi: 10.1093/ntr/nts247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 14.Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav. 2014;28:154–162. doi: 10.1037/a0030992. [DOI] [PubMed] [Google Scholar]
- 15.Sobell LC, Sobell MB. Timeline Follow-back. A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption Psychosocial and Biochemical Methods. New York: Springer-Verlag; 1992. pp. 41–72. [Google Scholar]
- 16.Murphy SE, Park SS, Thompson EF, Wilkens LR, Patel Y, Stram DO, et al. Nicotine N-glucuronidation relative to N-oxidation and C-oxidation and UGT2B10 genotype in five ethnic/racial groups. Carcinogenesis. 2014;35:2526–2533. doi: 10.1093/carcin/bgu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scherer G, Engl J, Urban M, Gilch G, Janket D, Riedel K. Relationship between machine-derived smoke yields and biomarkers in cigarette smokers in Germany. Regul Toxicol Pharmacol. 2007;47:171–183. doi: 10.1016/j.yrtph.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Carmella SG, Ming X, Olvera N, Brookmeyer C, Yoder A, Hecht SS. High throughput liquid and gas chromatography-tandem mass spectrometry assays for tobacco-specific nitrosamine and polycyclic aromatic hydrocarbon metabolites associated with lung cancer in smokers. Chem Res Toxicol. 2013;26:1209–1217. doi: 10.1021/tx400121n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan JM, Knezevich AD, Wang R, Gao YT, Hecht SS, Stepanov I. Urinary levels of the tobacco-specific carcinogen N'-nitrosonornicotine and its glucuronide are strongly associated with esophageal cancer risk in smokers. Carcinogenesis. 2011;32:1366–1371. doi: 10.1093/carcin/bgr125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stepanov I, Hecht SS. Detection and quantitation of N'-nitrosonornicotine in human toenails by liquid chromatography-electrospray ionization-tandem mass spectrometry. Cancer Epidemiol Biomarkers Prev. 2008;17:945–948. doi: 10.1158/1055-9965.EPI-07-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diggle PJ, Heagerty P, Liang K-Y, Zeger SL. Analysis of Longitudinal Data. Oxford: Oxford University Press; 2002. [Google Scholar]
- 22.Hatsukami DK, Perkins KA, Lesage MG, Ashley DL, Henningfield JE, Benowitz NL, et al. Nicotine reduction revisited: Science and future directions. Tob Control. 2010;19:e1–e10. doi: 10.1136/tc.2009.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozlowski LT. Cigarette prohibition and the need for more prior testing of the WHO TobReg's global nicotine-reduction strategy. Tob Control. 2016 doi: 10.1136/tobaccocontrol-2016-052995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borland R. Paying more attention to the 'elephant in the room'. Tob Control. 2016 doi: 10.1136/tobaccocontrol-2016-053150. [DOI] [PubMed] [Google Scholar]
- 25.Rupprecht LE, Smith TT, Schassburger RL, Buffalari DM, Sved AF, Donny EC. Behavioral mechanisms underlying nicotine reinforcement. Curr Top Behav Neurosci. 2015;24:19–53. doi: 10.1007/978-3-319-13482-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeller M, Hatsukami D. The Strategic Dialogue on Tobacco Harm Reduction: a vision and blueprint for action in the US. Tob Control. 2009;18:324–332. doi: 10.1136/tc.2008.027318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hatsukami DK, Joseph AM, Lesage M, Jensen J, Murphy SE, Pentel PR, et al. Developing the science base for reducing tobacco harm. Nicotine Tob Res. 2007;9(Suppl 4):S537–S553. doi: 10.1080/14622200701679040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Etter JF. Electronic cigarettes: a survey of users. BMC Public Health. 2010;10:231. doi: 10.1186/1471-2458-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mcrobbie H, Bullen C, Hartmann-Boyce J, Hajek P. Electronic cigarettes for smoking cessation and reduction. The Cochrane database of systematic reviews. 2014;12 doi: 10.1002/14651858.CD010216.pub2. CD010216. [DOI] [PubMed] [Google Scholar]
- 30.Vansickel AR, Eissenberg T. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine Tob Res. 2013;15:267–270. doi: 10.1093/ntr/ntr316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez AA, Hiler MM, Soule EK, Ramoa CP, Karaoghlanian NV, Lipato T, et al. Effects of Electronic Cigarette Liquid Nicotine Concentration on Plasma Nicotine and Puff Topography in Tobacco Cigarette Smokers: A Preliminary Report. Nicotine Tob Res. 2015 doi: 10.1093/ntr/ntv182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramoa CP, Hiler MM, Spindle TR, Lopez AA, Karaoghlanian N, Lipato T, et al. Electronic cigarette nicotine delivery can exceed that of combustible cigarettes: a preliminary report. Tob Control. 2015 doi: 10.1136/tobaccocontrol-2015-052447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shihadeh A, Eissenberg T. Electronic cigarette effectiveness and abuse liability: predicting and regulating nicotine flux. Nicotine Tob Res. 2015;17:158–162. doi: 10.1093/ntr/ntu175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biener L, Roman AM, Mc Inerney SA, Bolcic-Jankovic D, Hatsukami DK, Loukas A, et al. Snus use and rejection in the USA. Tob Control. 2014 doi: 10.1136/tobaccocontrol-2013-051342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mcmillen R, Maduka J, Winickoff J. Use of emerging tobacco products in the United States. J Environ Public Health. 2012;2012:989474. doi: 10.1155/2012/989474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King BA, Dube SR, Tynan MA. Current tobacco use among adults in the United States: findings from the National Adult Tobacco Survey. Am J Public Health. 2012;102:e93–e100. doi: 10.2105/AJPH.2012.301002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berg CJ, Haardoerfer R, Escoffery C, Zheng P, Kegler M. Cigarette users' interest in using or switching to electronic nicotine delivery systems for smokeless tobacco for harm reduction, cessation, or novelty: a cross-sectional survey of US adults. Nicotine Tob Res. 2015;17:245–255. doi: 10.1093/ntr/ntu103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brandon TH, Goniewicz ML, Hanna NH, Hatsukami DK, Herbst RS, Hobin JA, et al. Electronic nicotine delivery systems: a policy statement from the American Association for Cancer Research and the American Society of Clinical Oncology. Clin Cancer Res. 2015;21:514–525. doi: 10.1158/1078-0432.CCR-14-2544. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization. Raising taxes on tobacco. Geneva, Switzerland: World Health Organization; 2015. WHO report on the global tobacco epidemic, 2015. [Google Scholar]
- 40.Cobb NK, Abrams DB. The FDA, e-cigarettes, and the demise of combusted tobacco. N Engl J Med. 2014;371:1469–1471. doi: 10.1056/NEJMp1408448. [DOI] [PubMed] [Google Scholar]
- 41.Mcneill A, Brose LS, Calder R, Hitchman SC, Hajek P, Mcrobbie H. E-cigarettes: an evidence update. A report commissioned by Public Health England. London: Public Health England; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.