Abstract
Induction of mucosal anti-human immunodeficiency virus type 1 (HIV-1) T-cell responses in males and females will be important for the development of a successful HIV-1 vaccine. An HIV-1 envelope peptide, DNA plasmid, and recombinant modified vaccinia virus Ankara (rMVA) expressing the H-2Dd-restricted cytotoxic T lymphocyte P18 epitope were used as immunogens to test for their ability to prime and boost anti-HIV-1 T-cell responses at mucosal and systemic sites in BALB/c mice. We found of all prime-boost combinations tested, an HIV-1 Env peptide subunit mucosal prime followed by systemic (intradermal) boosting with rMVA yielded the maximal induction of gamma interferon (IFN-γ) spot-forming cells in the female genital tract and colon. However, this mucosal prime-systemic rMVA boost regimen was minimally immunogenic for the induction of genital, colon, or lung anti-HIV-1 T-cell responses in male mice. We determined that a mucosal Env subunit immunization could optimally prime an rMVA boost in female but not male mice, as determined by the magnitude of antigen-specific IFN-γ responses in the reproductive tracts, colon, and lung. Defective mucosal priming in male mice could not be overcome by multiple mucosal immunizations. However, rMVA priming followed by an rMVA boost was the optimal prime-boost strategy for male mice as determined by the magnitude of antigen-specific IFN-γ responses in the reproductive tract and lung. Thus, prime-boost immunization strategies able to induce mucosal antigen-specific IFN-γ responses were identified for male and female mice. Understanding the cellular and molecular basis of gender-determined immune responses will be important for optimizing induction of anti-HIV-1 mucosal immune responses in both males and females.
Development of an human immunodeficiency virus type 1 (HIV-1) vaccine is a global priority (65). HIV-1 can be transmitted either mucosally as a sexually transmitted disease or parenterally by blood administration or intravenous drug use (65). Studies with the rhesus macaque-simian immunodeficiency virus (SIV) model have indicated that dendritic cells residing in the lamina propria of the vagina were the first cellular targets of SIV after intravaginal inoculation with cell-free SIVmac251 (35, 55). SIV was detectable in the internal iliac lymph node and in the blood within 2 and 5 days, respectively, of intravaginal infection with SIV (55). Therefore, HIV-1 initiates infection at the mucosal surface by infecting dendritic cells present in the lamina propria adjacent to the mucosal surface. HIV-1-infected dendritic cells then spread to regional lymph nodes where HIV-1 is disseminated (24). Anti-HIV-1 T- and B-cell mucosal immune responses are desired because they may be able to prevent systemic HIV-1 infection by eliminating the infection at the mucosal surfaces or keep the infection localized by containing the infection at the regional lymph nodes (30, 31).
A successful HIV-1 vaccine must be able to induce protective anti-HIV-1 systemic and mucosal immunity in both males and females. Gender differences in immune responses to vaccines have been reported in mice immunized with T-dependent antigens BSA (64), keyhole limpet hemocyanin (67), OVA (67), and hen egg lysozyme (20), as well as the T-independent antigen polyvinyl pyrrolidone (13). Gender has also been reported to affect immune responses after pseudorabies virus vaccination of swine (8). Vaccine-induced antibody responses were significantly greater in women than men (3, 48), and a herpes simplex virus (HSV) vaccine was protective in females but not in males (60). Measurement of antigen-specific immune responses by enzyme-linked immunosorbent assay, neutralizing antibody, lymphocyte proliferation, and gamma interferon IFN-γ secretion assays in the HSV vaccine trial did not reveal any differences in the magnitude of vaccine-induced immunity between males and females, although significant differences in efficacy were observed (60).
In this study, we evaluated three model HIV-1 immunogens, a Th-cytotoxic T lymphocyte (CTL) peptide (ThD) containing the HIV-1 IIIB gp120 P18 CTL epitope, a DNA vaccine expressing HIV-1 IIIB Env, and a recombinant modified vaccinia virus Ankara (rMVA) expressing the HIV-1 IIIB envelope ThD epitope, for their ability to prime and boost anti-HIV-1 T-cell responses at mucosal sites. We found that the optimal regimen for induction of HIV-1-specific T cells in multiple female mucosal tissues utilized a mucosal peptide prime, followed by a systemic MVA boost. Surprisingly, this immunization regimen was only weakly immunogenic in males, due to an inability of mucosal immunization to prime T-cell responses in male mice. However, a systemic MVA prime followed by an MVA boost was optimal for the induction of HIV-1-specific T cells in the reproductive tract and lungs of male mice.
MATERIALS AND METHODS
Animals.
Female and male BALB/c mice (18 to 20 g, 6 to 8 weeks of age) were purchased from the Frederick Cancer Research and Development Center, National Cancer Institute, Frederick, Md. Mice were housed in filter top cages and provided food and water ad libitum. All procedures were approved by the Duke University Institutional Animal Care and Use Committee.
Immunogens.
An rMVA containing a linear array of the following HIV-1 helper and CTL epitopes, including the P18 CTL epitope (RIHIGPGRAFYTTKN) (5, 22, 63), was used for immunizations: HAGPIAPGQMREPRG (66)/ KQIINMWQEVGKAMYA (54), KEKVYLAWVPAHKGIG (21)/MYAPPIGGQI (11), LLFIHFRIGCRHSR (53)/DRVIEVVQGAYRAIR (54), and the ThD sequences EQMHEDIISLWDQSL/RIHIGPGRAFYTTKN (5, 22, 63). ThD and P18 peptides were synthesized by SynPep (Dublin, Calif.). Peptides were purified by high-performance liquid chromatography and verified to be >95% pure by mass spectroscopy. The DNA vaccine plasmid was constructed with the PMV vector backbone, provided by Wyeth-Lederle Vaccines (Pearl River, N.Y.). A plasmid DNA vaccine expressing HIV-1 IIIB gp120 (PMV-gp120) was used for immunizing mice in these experiments. The empty PMV vector was used as a sham plasmid. Since all immunogens contained the dominant P18 epitope and we have assays available for monitoring P18-specific responses via enzyme-linked immunospot (ELISpot) and H-2Dd-P18 tetramers (56), all immune responses followed in this study were to the P18 epitope.
Immunizations.
A Th-CTL HIV-1 Env peptide (ThD) containing the HIV-1 IIIB CTL P18 epitope was used for intranasal (i.n.) and subcutaneous (s.c.) peptide immunizations. For nasal immunization, mice were anesthetized with Isofluorane (Abbott Laboratories, North Chicago, Ill.), and 50 μg of peptide with 1 μg of cholera toxin (List Biological, Campbell, Calif.) was administered in saline for a total volume of 15 μl (7.5 μl per nare) (4, 44, 56-59). For s.c. peptide immunizations, mice were immunized at the base of the tail with 50 μg of peptide, and 25 μg of RC-529 (a synthetic toll-like receptor 4 ligand) (23, 43) obtained from Wyeth was used as the adjuvant. Mice immunized with MVA were anesthetized with ketamine-xylazine (173 and 7 mg/kg of body weight) before 10 μl of saline containing MVA (107 PFU) was injected into the left ear pinna (first dose) or right ear pinna (second dose) intradermally (i.d.) with a Gastight syringe with a 31-gauge needle (Hamilton Co., Reno, Nev.). DNA immunizations were performed by injecting 50 μl of saline containing plasmid DNA (25 μg) intramuscularly (i.m.), into both hind-leg quadriceps muscles for a total of 50 μg of plasmid DNA (51, 52).
Optimization of peptide, MVA, and DNA immunizations.
In dosing studies, we found that maximal CTL and IFN-γ spot-forming cell (SFC) responses were obtained using 50 μg of ThD HIV-1 Env subunit peptide per immunization, given as a mucosal i.n. prime dose and three i.n. boosts. Therefore, this dose and immunization regimen was used for subunit immunizations throughout. Similar dosing studies showed that the optimal dose of MVA given ID was 107 PFU. Finally, the optimal dose of HIV-1 IIIB gp120 DNA was previously determined to be 50 μg of DNA IM per immunization (51, 52). In addition to the concentration of MVA to be used for optimal induction of P18-specific cell-mediated immunity, we also determined if inoculation with MVA by different routes affected the induction of P18-specific IFN-γ SFC responses. Groups of BALB/c mice (four mice per group) were immunized intraperitoneally (i.p.), i.n., or intradermally (i.d.) with MVA at 106 or 107 PFU on day 0 and boosted 18 days later with the same dose and via the same route. After the second immunization, splenocytes were assayed for P18-specific IFN-γ SFCs. A prime-boost regimen of MVA via the i.d. route was significantly more effective than i.p. or i.n. immunization (P < 0.01).
Isolation of mucosal lymphocytes.
Lymphocytes were isolated from mucosal tissues as previously described (56), except that calcium- and magnesium-free phosphate-buffered saline was used for whole-body perfusion prior to usual tissue harvest. The female genital tract included the ovaries, fallopian tubes, uterus, and vagina. The male genital tract included the preputial gland, testicles, epididymis, vas deferens, prostate, seminal vesicles, and coagulating glands. Colon tissues included the ascending and descending colon. All tissues were digested with RPMI-1640 with 5% fetal bovine serum, penicillin-streptomycin, HEPES, and 2.5 mg of collagenase type A (Roche catalogue number 1088 785)/ml and 5 units of DNase I (Roche catalogue number 104 159)/ml. Mucosal tissues were pooled from four mice/immunization group to provide sufficient cells to perform replicates of each experiment (IFN-γ ELISpot) from each tissue to allow for accurate measurement of immune responses in each immunization group. The average yield of cells per mouse was 2.9 × 106 cells/female reproductive tract, 10.8 × 106 cells/male reproductive tract, 2.2 × 106 cells/colon, and and 7.9 × 106 cells/lung.
IFN-γ and IL-2 ELISpot and CTL assays.
ELISpot and CTL assays were performed as previously described (56), except that anti-interleukin-2 (IL-2) capture antibody (JES6-1A12) and biotin anti-IL-2 detection antibody (JES6-5H4) (both from BD PharMingen, San Diego, Calif.) were used in the IL-2 ELISpot assay. ELISpot plates were scanned into an ImmunoSpot Series I analyzer, and spots were quantitated with ImmunoSpot 2.1 software (CTL Analyzers, Cleveland, Ohio). Controls included cells cultured in medium in the absence of peptide stimulation. The frequency of IFN-γ SFCs in control wells (typically <10 IFN-γ SFCs/106 cells) was subtracted from the frequency of IFN-γ SFCs detected in the peptide-stimulated cells for calculation of antigen-specific IFN-γ responses. For CTL assays, spontaneous release was <10% of maximum release. The percent specific cytotoxicity was measured as follows: (experimental release − spontaneous release)/(maximum release − spontaneous release) × 100.
Tetramer analysis of antigen-specific CD8+ T cells.
H-2Dd-P18 tetramers were prepared and used as described (56). A total of 0.1 to 0.2 μg of phycoerythrin-labeled tetrameric H-2Dd-P18 complexes with allophycocyanin (APC)-labeled anti-mouse CD8 (Ly-2; Caltag, South San Francisco, Calif.) monoclonal antibody was used to identify P18-specific CD8+ T cells. Samples were analyzed with tetrameric H-2Dd-P18 complexes for the percentage of CD8+ T cells by two-color flow cytometry with a FACScalibur (Becton Dickinson, Mountain View, Calif.) system.
CD8 depletion.
CD8+ lymphocytes were depleted from spleen and mucosal samples with magnetically activated cell sorting CD8α (Ly-2) MicroBeads (Miltenyi, Auburn, Calif.), following the protocol provided with the MicroBeads. Briefly, single-cell suspensions were incubated with MicroBeads for 15 min at 6°C. The cells were then washed with 5 ml of phosphate-buffered saline, 0.5% fetal bovine serum, and 2 mM EDTA. The pellet was then resuspended in 500 μl of wash buffer and placed onto a prewetted MS+ selection column (Miltenyi) in the separator. Following the separation, the column was washed three times, and the negatively selected cells were washed and pelleted before being counted and adjusted to a proper concentration for the ELISpot assay.
Statistics.
Data for each assay were compared by analysis of variance (ANOVA) and Student's t test. If significant differences between the groups were identified by ANOVA, multiple comparison procedures (Tukey) were performed to determine differences between specific groups (4, 12). To improve the clarity of the figures, significant differences between genders within the same immunization group are indicated by brackets and the P value.
RESULTS
Prime-boost regimens including a boost with rMVA are optimal for the induction of antigen-specific immunity. We first determined the optimal prime-boost immunization regimens for induction of antigen-specific (gp120 IIIB P18) immunity in female mice (Table 1). This analysis revealed that maximal antigen-specific IFN-γ SFC and tetramer-positive CD8+ T cells were induced by DNA prime-rMVA boost, peptide prime-rMVA boost, and rMVA prime-rMVA boost.
TABLE 1.
Immunization regimens
| Prime (dose)/route | Adjuvant | Boost (dose)/route | Adjuvant (dose) | na | P18-specific IFN-γ SFCb | P18-specific tetramer + CD8 cellsc |
|---|---|---|---|---|---|---|
| Peptide (50 μg)/i.n. | Cholera toxin (1 μg) | Peptide (50 μg × 3)/i.n. | Cholera toxin (1 μg) | 9 | 116.2 ± 32.9 | 2.8 ± 0.28 |
| Peptide (50 μg)/i.n. | Cholera toxin (1 μg) | MVA (107 PFU)/i.d. | None | 12 | 502.3 ± 99.9d | 4.8 ± 0.53d |
| Peptide (50 μg)/i.n. | Cholera toxin (1 μg) | DNA (50 μg)/i.m. | None | 4 | 19.1 ± 6.4 | 1.3 ± 0.09 |
| None/i.n. | Cholera toxin (1 μg) | None/i.n. | Cholera toxin (1 μg) | 4 | 5 ± 2.0 | 0.5 ± 0.10 |
| MVA (107 PFU)/i.d. with minigene | None | Peptide (50 μg)/i.n. | Cholera toxin (1 μg) | 4 | 243 ± 62.0 | 2.1 ± 0.16 |
| MVA (107 PFU)/i.d. with minigene | None | MVA (107 PFU)/i.d. with minigene | None | 12 | 438.6 ± 77.6e | 8.4 ± 0.81e |
| MVA (107 PFU)/i.d. with minigene | None | DNA (50 μg)/i.m. | None | 12 | 113.6 ± 21.4 | 3.1 ± 0.40 |
| MVA (107 PFU)/i.d. with minigene | None | None | None | 4 | 176 ± 41.0 | 2.4 ± 0.19 |
| MVA (107 PFU)/i.d. without minigene | None | MVA (107 PFU)/i.d. without minigene | None | 4 | 5 ± 2.0 | 0.5 ± 0.10 |
| DNA (50 μg)/i.m. | None | Peptide (50 μg)/i.n. | Cholera toxin (1 μg) | 10 | 11.4 ± 3.8 | 0.8 ± 0.06 |
| DNA (50 μg)/i.m. | None | Peptide (50 μg × 3)/i.n. | Cholera toxin (1 μg) | 12 | 103 ± 31.5 | 2.6 ± 0.18 |
| DNA (50 μg)/i.m. | None | MVA (107 PFU)/i.d. with minigene | None | 12 | 380 ± 76.0f | 9.4 ± 0.96f |
| DNA (50 μg)/i.m. | None | DNA (50 μg)/i.m. | None | 4 | 8.3 ± 3.1 | 1.1 ± 0.08 |
| DNA (50 μg)/i.m. | None | None | None | 3 | 11.6 ± 2.6 | 0.8 ± 0.09 |
| PMV plasmid (50 μg)/i.m. | None | PMV plasmid (50 μg)/i.m. | None | 4 | 6.3 ± 2.0 | 0.4 ± 0.02 |
n, number of mice.
Data are means ± standard error, per 1, million cells.
Data are means ± standard errors.
Compared to all regimens primed with peptide, peptide prime-rMVA boost induced significantly more IFN-γ SFCs and tetramer-specific cells when compared by t test (P < 0.05). Analysis by ANOVA was comparable in the IFN-γ ELISpot assay (P = 0.05), but did not reveal highly significant differences in tetramer-specific cells between peptide-rMVA and peptide-peptide three-dose regimens or peptide-rMVA and peptide-DNA.
Compared to all regimens primed with rMVA rMVA prime-rMVA boosting induced significantly more tetramer-specific cells when compared by t test (P < 0.05) and by ANOVA (P = 0.05). Analysis by ANOVA was comparable but did not reveal highly significant differences between rMVA-rMVA and rMVA-peptide or rMVA-rMVA and rMVA-no-treatment regimens by the ELISpot assay.
Compared to all regimens primed with DNA, DNA-rMVA induced significantly more IFN-γ SFCs and tetramer-specific cells when compared by t test (P < 0.05). Analysis by ANOVA was comparable in the tetramer binding assay; however, ANOVA only demonstrated highly significant results (P = 0.05) when DNA-rMVA was compared to DNA-peptide by the IFN-γ ELISpot assay.
Because gender differences in response to vaccines have been reported, (3, 8, 13, 20, 48, 64, 67), we examined these three best immunization strategies, DNA prime-rMVA boost, peptide prime-rMVA boost and rMVA prime-rMVA boost for their ability to induce antigen-specific T-cell responses in spleens of both female and male BALB/c mice (Fig. 1). Using a P18-specific major histocompatibility complex class I H-2Dd-restricted tetramer, IFN-γ ELISpot, and chromium-51release assay, we determined the magnitude of antigen-specific CD8+ lymphocytes in the spleens of male and female mice.
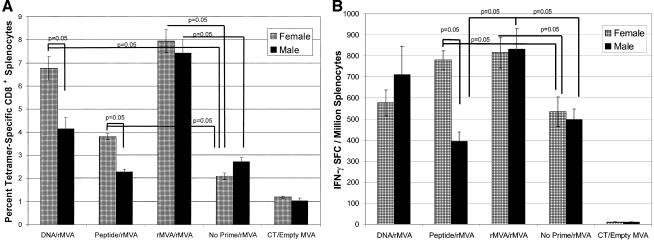
FIG. 1.
Antigen-specific CD8+ responses in splenocytes from female and male BALB/c mice following various prime-boost regimens. Female and male mice were primed with either DNA (50 μg) i.m., peptide (50 μg) with cholera toxin i.n., or rMVA (107 PFU) i.d. All animals were boosted with rMVA (107 PFU) i.d. (see Materials and Methods for immunization details). Spleens were removed 20 days after the rMVA boosting and assayed for the frequency of P18 tetramer-specific CD8+ splenocytes (A) and antigen-specific IFN-γ-secreting cells (B) by ELISpot. (A) When evaluated by tetramer analysis, significant differences were observed between the genders in animals primed with DNA and boosted with rMVA (41 females and 23 males; P = 0.05) and animals primed with peptide and boosted with rMVA (86 females and 60 males; P = 0.05). Other significant differences were observed in females (n = 40) and males (n = 24) primed with rMVA and boosted with rMVA; their tetramer responses were significantly greater than any other immunization regimen (P = 0.05). Females in the DNA-rMVA, peptide-rMVA, and rMVA-rMVA regimes had significantly increased tetramer responses when compared to female mice in the no prime-rMVA group (P = 0.05). Only males primed and boosted with rMVA showed an increased frequency of tetramer-specific responses when compared to the no prime-rMVA immunization group (P = 0.05). (B) Gender differences were observed in mice primed with peptide and boosted with rMVA (86 female mice and 60 male mice; P = 0.05). Other significant differences were detected in females primed with peptide and boosted with rMVA (86 mice) or primed with rMVA and boosted with rMVA (40 mice); these groups had a significantly higher frequency of IFN-γ SFCs than did females receiving only one immunization of rMVA (47 mice) (P = 0.05). Males primed with rMVA and boosted with rMVA (24 mice) had a significantly higher frequency of IFN-γ SFCs than did males primed with peptide and boosted with rMVA (60 mice) or males receiving only one immunization of rMVA (38 mice) (P = 0.05).
When mice were primed with DNA i.m. or with peptide i.n. and boosted systemically with rMVA, female mice had a significantly greater frequency of tetramer-specific CD8+ lymphocytes than male mice. For DNA prime-rMVA boosting, female mice had 6.77 ± 0.51 tetramer+ CD8+ lymphocytes versus 4.15 ± 0.48 in male mice (P = 0.05). With peptide prime-rMVA boosting, female mice had 3.81 ± 0.19 tetramer+ CD8+ lymphocytes versus 2.28 ± 0.11 tetramer+ CD8+ lymphocytes in male mice (P = 0.05) (Fig. 1A). In addition, rMVA prime-rMVA boosting gave the highest frequency of tetramer-specific CD8+ splenocytes with no differences between males and females (Fig. 1A).
Control groups of female and male mice that received only one immunization of rMVA were immunized at the times when all other groups were boosted with rMVA and are thus referred to as no prime-rMVA boost groups. Females primed with DNA (6.77 ± 0.51 tetramer+ CD8+ lymphocytes), peptide (3.81 ± 0.19 tetramer+ CD8+ lymphocytes), or rMVA (7.97 ± 0.49 tetramer+ CD8+ lymphocytes) and boosted with rMVA had significantly more tetramer-specific CD8+ splenocytes than did females receiving only one immunization of rMVA (2.09 ± 0.94 tetramer+ CD8+ lymphocytes) (P = 0.05). In contrast, males primed with DNA (4.15 ± 0.48 tetramer+ CD8+ lymphocytes) or peptide (2.28 ± 0.11) and boosted with rMVA had no difference in tetramer-specific CD8+ splenocytes compared to males receiving only one immunization of rMVA (2.72 ± 0.18 tetramer+ CD8+ lymphocytes). Only males that were primed and boosted with rMVA showed an increased frequency of tetramer-specific binding compared to only one immunization with rMVA (7.44 ± 0.56 versus 2.72 ± 0.18 tetramer+ CD8+ lymphocytes; P = 0.05). Thus, males had a defect in mucosal priming with peptide.
Tetramer analysis identifies antigen-specific CD8+ T cells but does not evaluate antigen-specific cell functional activity. Therefore, we utilized IFN-γ ELISpots and chromium release assays to monitor the functional activity of antigen-specific responses induced using the prime-boost immunization regimens. Following DNA prime-rMVA boost immunization, male mice exhibited the same frequency of IFN-γ SFCs/106 cells (710 ± 134) compared to female mice (576 ± 62; P values were not significant). However, as with tetramer analysis, gender differences in the peptide-rMVA group were significant with females having a significantly higher frequency of IFN-γ SFC than males (780 ± 44 versus 393 ± 45; P = 0.05). Furthermore, females primed with peptide and boosted with rMVA had significantly higher frequencies of IFN-γ SFC than female mice receiving only one immunization of rMVA (780 ± 44 versus 533 ± 79, respectively; P = 0.05), indicating that peptide would prime for an rMVA boost in females. IFN-γ SFCs in these assays were CD8+, since depletion of CD8+ lymphocytes decreased the frequency of antigen-specific IFN-γ responses by 89% (data not shown).
Immunization with rMVA prime-rMVA boost resulted in similar IFN-γ SFC responses in both females and males (816 ± 74 and 832 ± 97 IFN-γ SFC/106 cells). The rMVA-rMVA immunization strategy induced a significantly higher frequency of IFN-γ SFCs in the spleens of both females (Fig. 1B) and males (Fig. 1B) than did female and male mice receiving only one immunization of rMVA (female mice, 533 ± 79; male mice, 496 ± 52) (P = 0.05). Thus, rMVA could prime for an rMVA boost in both males and female mice. In contrast, as with tetramer responses, mucosal peptide primed for an rMVA boost only in females, as there was no significant difference between peptide prime-rMVA boosting and no prime-rMVA boosting in males (P values were not significant) while the difference was significant in females (P = 0.05) (Fig. 1B). In addition, chromium release assays were performed and gender differences in antigen-specific lytic responses matched the profile of the IFN-γ ELISpot assay. At effector-to-target ratios of 5:1, 10:1, 20:1, and 40:1, splenocytes of female mice primed with peptide and boosted with rMVA induced significantly more target cell lysis than did splenocytes in male mice primed and boosted with the same regimen (P = 0.05); there were no gender differences in target cell lysis by splenocytes from mice primed with DNA or rMVA and boosted with rMVA (data not shown).
In addition to the IFN-γ SFC responses, we examined P18-specific production of IL-2 with a P18-specific IL-2 ELISpot assay. As with IFN-γ SFC responses, there were also gender differences in the IL-2 SFC responses in mice in the peptide-rMVA group (P = 0.05; data not shown). Thus, the gender differences in mucosal prime-rMVA boosting were also seen with IL-2 SFC generation as well as with IFN-γ responses. Among these assays, there was consistent inability of mucosal peptide immunization to prime for rMVA boosting in male, but not in female, mice.
Gender differences in mucosal P18-specific IFN-γ SFC.
Since antigen-specific T-cell responses at mucosal tissues may contribute to vaccine-induced protection against HIV-1, we evaluated antigen-specific T-cell responses in the reproductive tract, colon, and lung to quantitate these responses and determine if gender differences in antigen-specific T-cell responses existed in mucosal tissues. We limited our analysis of the mucosal responses to mice immunized utilizing the peptide prime (i.n.)-rMVA boost (i.d.) and rMVA prime (i.d.)-rMVA boost (i.d.) immunization regimens.
Antigen-specific IFN-γ responses in the reproductive tract.
We first evaluated antigen-specific IFN-γ SFC responses in the reproductive tracts of male and female mice immunized with the peptide prime (i.n.)-rMVA boost (i.d.) and rMVA prime (i.d.)-rMVA boost (i.d.) immunization regimen. We also included the control group that did not receive a priming immunization, but did receive the rMVA boost immunization (no prime-rMVA).
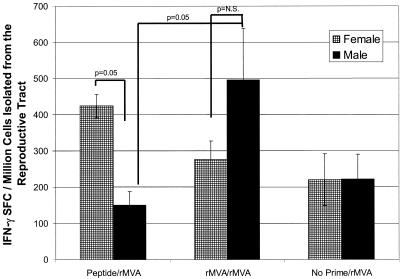
We found a gender difference in antigen-specific P18 responses in the reproductive tract with peptide-rMVA immunization (424 ± 33 SFCs versus 151 ± 37 SFCs, respectively; P = 0.05) but not with rMVA-rMVA immunization (P values were not significant) (Fig. 2). Male reproductive tract IFN-γ responses detected in the peptide-rMVA group were no different than those detected in the no prime-rMVA group, demonstrating that mucosal peptide priming was not effective in male mice.
FIG. 2.
P18-specific IFN-γ SFCs in the genital tracts of female and male BALB/c mice following prime-boost immunization. BALB/c mice were primed with peptide (50 μg) with cholera toxin i.n. or MVA (107 PFU) i.d. All animals were boosted with MVA (107 PFU) i.d. Twenty days after the MVA boost, genital tract lymphocytes were isolated and assayed for the frequency of P18-specific IFN-γ SFCs by ELISPot. Due to low yields of cells from individual mice, cells isolated from four mice were pooled. Each bar represents the mean ± standard error of the mean of six pooled sets of genital tract tissue (specimens from 24 mice). Lymphocytes isolated from the genital tract of females primed with peptide and boosted with rMVA had a significantly higher frequency of IFN-γ SFCs than did lymphocytes isolated from the genital tracts of males primed with peptide and boosted with rMVA (P = 0.05). P18-specific IFN-γ SFC responses were significantly greater in mice immunized with rMVA prime-rMVA boosting than in male mice immunized with peptide prime-rMVA boosting (496 ± 142 SFCs versus 151 ± 37 SFCs, respectively) (P = 0.05).
P18-specific IFN-γ SFC responses were significantly greater in male mice immunized with rMVA prime-rMVA boosting than in male mice immunized with peptide prime-rMVA boosting (496 ± 142 SFCs versus 151 ± 37 SFCs, respectively; P = 0.05) (Fig. 2). Thus, i.d. systemic priming with rMVA overcame male mouse hypo-responsiveness to peptide priming for P18 IFN-γ responses in the genital tract.
Antigen-specific IFN-γ responses in the colon.
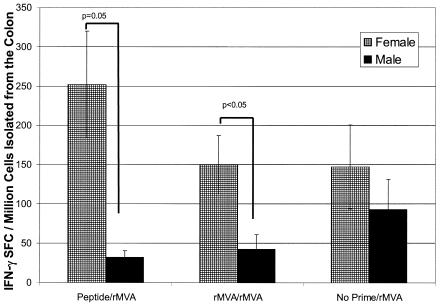
Although the reproductive tracts of males and females both exhibit immunologic characteristics indicating they are tissues of the mucosal immune system (9, 10, 26, 28, 29, 40-42, 46), the male and female reproductive tracts are obviously anatomically and functionally distinct. Therefore, we evaluated antigen-specific IFN-γ responses in the colons of mice immunized with our prime-boost immunization strategies. As with the IFN-γ responses in spleens and reproductive tracts, we also observed gender differences in IFN-γ responses in the colon. Female mice immunized with peptide-rMVA have significantly greater IFN-γ responses in the colon than the responses detected in male mice immunized with the same regimen (252 ± 67 SFCs versus 32 ± 8 SFCs, respectively; P = 0.05) (Fig. 3). The colon IFN-γ response of peptide-rMVA-imunized female mice, but not male mice, was elevated when compared to the IFN-γ response in the colons of mice immunized using the no prime-rMVA regimen. These results are similar to the results observed for the reproductive tract and again supported the notion that there is a defect in the mucosal nasal priming in male, but not female, mice.
FIG. 3.
Antigen-specific IFN-γ-producing lymphocytes isolated from the colon of female and male BALB/c mice following prime-boost immunization. Female and male mice were primed with either peptide (50 μg) with cholera toxin i.n. or MVA (107 PFU) i.d. All animals were boosted with MVA (107 PFU) i.d. Twenty days after MVA boosting, colon lymphocytes were isolated and assayed for the frequency of P18-specific IFN-γ-producing cells by ELISpot. Due to low yields of cells from individual mice, cells isolated from four mice were pooled. Each bar represents the mean ± standard error of six pooled sets of colon tissue (specimens from 24 mice). Lymphocytes isolated from the colons of females primed with peptide and boosted with rMVA had a significantly higher frequency of IFN-γ SFCs than did lymphocytes isolated from the colons of males primed and boosted via the same regimen (P = 0.05 by ANOVA). (Although ANOVA revealed no significance between females and males receiving rMVA-rMVA, Student's t test showed P values of <0.05).
In contrast to the results detected in the reproductive tract of mice immunized with rMVA-rMVA, female mice contained a greater frequency of colon IFN-γ SFCs than male mice (149 ± 37 SFCs versus 42 ± 19 SFCs) (Fig. 3). Though the differences are not statistically significant by ANOVA, Student's t test reveals P values of <0.05. Additionally, mice immunized with the rMVA-rMVA regimen had colon IFN-γ SFC responses that were no better those detected in the female and male mice immunized with the no prime-rMVA regimen (147 ± 52 SFCs versus 93 ± 38 SFCs). These results suggested that rMVA priming was not able to overcome the priming defect observed in male mice immunized with peptide-rMVA, with regard to colon IFN-γ responses.
Antigen-specific IFN-γ responses in the lung.
While the lungs are not a site for HIV-1 transmission, they are an additional mucosal site with similar organ structure in males and females. Of interest was the observation that the lung had the highest frequency of vaccine induced antigen-specific IFN-γ SFCs of any tissue evaluated.
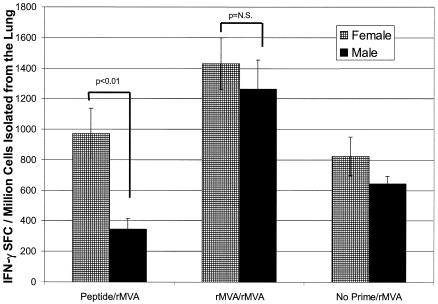
Gender differences were again detected between female and male mice immunized with peptide i.n. and boosted with rMVA i.d. (972 ± 163 SFCs and 345 ± 69 SFCs, respectively) (Fig. 4). Though the trend was not significant by ANOVA, the Student's t test analysis showed a significance of P < 0.01. Additionally, peptide priming of males did not increase the IFN-γ response over that observed in mice immunized with no prime-rMVA, again demonstrating a male defect in mucosal priming. In contrast, i.d. rMVA priming was an effective method of priming male and female mice for lung IFN-γ T-cell responses.
FIG. 4.
Antigen-specific IFN-γ-producing lymphocytes isolated from the lungs of female and male BALB/c mice following prime-boost immunization. Groups of female and male BALB/c mice were immunized with peptide (50 μg) i.n. with cholera toxin or MVA (107 PFU) i.d. All animals were boosted with MVA (107 PFU) i.d. Twenty days after the MVA boost, lymphocytes from the lungs were isolated and assayed for the frequency of P18-specific IFN-γ-producing cells by ELISpot. Cells isolated from four mice were pooled. Each bar represents the mean ± standard error of six pooled sets of colon tissue (24 mice). Though ANOVA revealed no significance between females and males receiving peptide-rMVA, Student's t test showed P values of <0.01).
Inability to overcome defective mucosal priming in males by repeated i.n. immunizations.
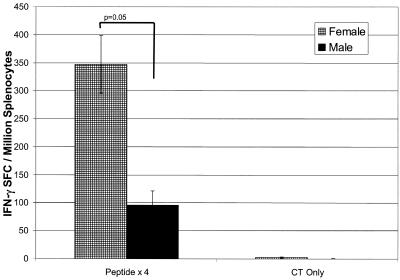
Our results suggested that the gender difference observed in mice immunized with the peptide-rMVA regimen was due to ineffective nasal priming of male mice. To determine if the mucosal priming defect in male mice could be overcome by multiple mucosal immunizations, we immunized female and male BALB/c mice with an i.n. peptide prime on day 0, followed by three i.n. peptide boosts on days 7, 14, and 28 (56-59). On day 40, splenocytes were isolated and tested for their ability to produce IFN-γ in response to in vitro peptide stimulation (Fig. 5). Splenocytes from female mice immunized four times i.n. with peptide contained a significantly greater frequency of P18-specific IFN-γ SFCs than male mice (347 ± 51 SFCs versus 96 ± 26 SFCs, respectively; P = 0.05). As with the peptide prime-rMVA immunization strategy, there was a gender difference in the antigen-specific IFN-γ response after nasal peptide priming done once with a peptide boost done three times, suggesting that nasal immunization of male mice is defective regardless of the number of times of immunization.
FIG. 5.
Antigen-specific IFN-γ SFCs isolated from the spleens of female and male BALB/c mice following repeated i.n. peptide immunizations. Groups of female and male BALB/c mice were immunized i.n. with 50 μg of peptide plus 1 μg of cholera toxin on days 0, 7, 14, and 28. On day 40, spleens were isolated and assayed by ELISpot for the frequency of P18-specific IFN-γ-secreting cells. Each bar is representative of the mean ± standard error of results from 12 mice. P values were 0.05 when P18-specific IFN-γ-secreting responses from females were compared to males.
i.n. but not s.c. peptide priming enhances antigen-specific IFN-γ responses in female mice.
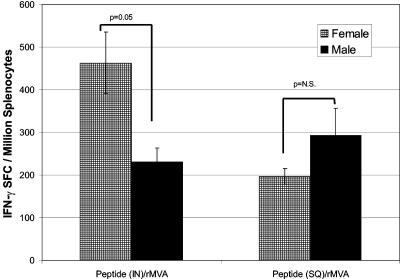
To determine whether the gender difference observed in the IFN-γ SFC responses was due to the route of priming or due to the nature of the immunogen itself, we evaluated antigen-specific IFN-γ SFC responses in spleens of female and male mice primed i.n. versus s.c. with peptide and then boosted i.d. with rMVA. Confirming our observations presented above (Fig. 1B), i.n. peptide priming and rMVA boosting produced significantly more spleen IFN-γ SFCs in females than in males (463 ± 73 SFCs versus 231 ± 33 SFCs; P = 0.05) (Fig. 6). When groups of female and male mice were primed with peptide delivered s.c. with RC-529 adjuvant and later boosted with rMVA, the IFN-γ responses were not significantly different in female mice versus male mice (196 ± 19 SFCs and 292 ± 64 SFCs; P values were not significant) (Fig. 6). Thus, there was no male s.c. priming defect, as responses were identical in males and females with the same antigen administered s.c. The difference in female SFC responses i.n. versus SFC responses s.c. can be attributed to the strength of the adjuvant used for s.c. immunization. The important point here is that with s.c. dosing, there were no gender differences, whereas with i.n. immunization there were gender differences. Taken together, our results suggested that the gender differences observed in the splenocyte IFN-γ SFC responses after i.n. peptide priming and i.d. rMVA boosting are associated with as-yet-undefined differences in immune recognition in the nasally associated lymphoid tissue (NALT) between male and female mice.
FIG. 6.
Enhanced antigen-specific responses in female BALB/c mice are dependent upon the route of peptide delivery. Groups of female and male BALB/c mice were immunized i.n. with 50 μg of peptide plus 1 μg of cholera toxin or immunized s.c. with 50 μg of peptide plus 25 μg of RC-529. Both groups were boosted with 107 PFU of rMVA i.d. Spleens were removed and assayed for the frequency of IFN-γ-producing cells by the ELISpot assay. Each bar is representative of the mean and standard error of results with 10 mice. P values were 0.05 when females primed with peptide i.n. and boosted with rMVA were compared to male mice primed i.n. with peptide and boosted with rMVA. When female and male mice immunized with peptide-rMVA s.c. were compared by ANOVA or Student's t test, there was no difference in the number of IFN-γ SFCs (P values were not significant and P = 0.17, respectively).
DISCUSSION
In this study, we have determined that an immunization strategy optimized for the induction of HIV-1-specific T-cell-mediated immune responses in female systemic and mucosal lymphoid tissues failed to induce optimal HIV-1-specific systemic and mucosal T-cell responses in males. The inability of the immunization strategy to induce HIV-1-specific responses in male mice was associated with defective T-cell priming after nasal immunization. Interestingly, the gender-dependent differences in the vaccine-induced immune responses in the systemic compartment could be overcome by utilizing alternative priming immunization strategies such as i.d. rMVA.
The induction of maximal immune responses in the systemic compartment (spleen), reproductive tract, and colon of females was dependent upon a nasal peptide prime followed by an rMVA boost. However, this immunization strategy was not optimal in male mice. In male mice, an i.d. rMVA prime followed by an i.d. rMVA boost was optimal for the induction of maximal immune responses in the spleen, reproductive tract, and lungs. Our results suggest that priming by the nasal route is effective in female mice but not male mice and that separate immunization strategies may be needed for optimal induction of immune responses in males and females. The efficacy of peptide priming by the nasal route in female mice is supported by our previous observations on the ability of nasal peptide immunization to induce humoral and cell-mediated immune responses in systemic and mucosal tissues (4, 44, 56, 58, 59).
Although we have not previously evaluated nasal immunization in male mice, others have reported that male, but not female, CBA/J mice are unresponsive to allergic sensitization by nasal immunization with allergen (71). The unresponsiveness to allergic sensitization in CBA/J mice can be reversed by castration, indicating that androgens were modulating the induction of antigen-specific responses after nasal antigen exposure (71). There was no gender difference in the induction of allergen-specific immunoglobulin E in BALB/c mice, the mouse strain used in our studies, suggesting that the host genotype also influences the induction of antigen-specific responses after nasal antigen exposure (71). It is important to mention that the allergic sensitization studies were performed by nasal immunization with allergen in the absence of adjuvant (71), while our studies have utilized the potent mucosal adjuvant cholera toxin. Nasal immunization with the B subunit of cholera toxin is an effective method of immunization in male humans (50) while nasal immunization of male rabbits utilizing cholera toxin as an adjuvant induced protective immunity against a nasal challenge with Pasteurella multocida (7). Taken together, these results suggested that a number of factors can contribute to the lack of efficacy of the nasal peptide prime-rMVA boost utilized in our study.
i.d. immunization with rMVA was utilized to boost mice that had been primed by the i.n. (peptide) or i.d. (rMVA) route. Although the rMVA was administered by a systemic route, the tissue distribution of the rMVA after systemic delivery may affect the immune responses induced in mucosal and systemic tissues (27, 47). For example, i.p. or s.c. administration of rMVA expressing luciferase (rMVAluc) to female mice resulted in the detection of luciferase activity in both systemic and mucosal tissues including the spleen, lung, ovaries, inguinal, and cervical and hilus lymph nodes but not the small intestine or NALT (47). In contrast, nasal administration of rMVAluc resulted in luciferase detection in the lung, NALT and hilus lymph nodes, while gastric administration of rMVAluc resulted in detectable luciferase activity only in the NALT (47). Therefore, systemic administration of rMVAluc resulted in antigen expression in both systemic (spleen and lymph nodes) and mucosal tissues (ovaries and lung), while mucosal administration resulted in a very limited expression of antigen in only mucosal tissues (NALT and lung). In this study, the tissue distribution of rMVA in male mice was not evaluated. A separate report that monitored T-cell-mediated immune responses in the ovaries and testes utilized i.p. administration of vaccinia virus to female mice (since vaccinia virus, like MVA, has a propensity to disseminate to murine ovaries) but used direct injection of vaccinia virus into the testes of mice, since infection of the testis could only be achieved by microinjection (27). These studies suggested that rMVA, like vaccinia virus, has a propensity to localize to tissues of the female reproductive tract but not the male reproductive tract. If local antigen expression is associated with the magnitude of antigen-specific responses induced in genital tract tissues, it seems likely that the combination of a nasal peptide prime (a route of immunization known to induce responses in the female reproductive tract) (1, 4, 39, 49, 58, 59) and a systemic rMVA boost (that results in antigen expression in the female reproductive tract) would result in maximal antigen-specific responses in mucosal tissues including the female reproductive tract. This conclusion may seem to contradict our findings with the reproductive tracts of male mice, where rMVA-rMVA boosting was superior to all other immunization regimens tested. However, since MVA disseminates widely through systemic tissues, induction of antigen-specific immune responses in the male reproductive tract may be less dependent upon activation of the mucosal immune system and more dependent upon effective systemic immunization. Our antigen-specific IFN-γ results with colon tissue support the conclusion that rMVA dissemination affects the magnitude of antigen-specific immune responses detected in mucosal tissues. Antigen-specific IFN-γ responses in the colon after rMVA-rMVA immunization were greater in female than in male mice. It seems likely that dissemination of rMVA to and antigen expression in the female reproductive tract activated the mucosal immune system, resulting in augmented IFN-γ responses in other mucosal tissues such as the colon.
Gender influences on the induction of multiple types of antigen-specific immune responses have been known for many years. In 1968, Terres et al. reported that male Swiss albino mice had significantly lower antibody responses than female mice after immunization with bovine serum albumin (64). Additional studies with mice have documented gender-dependent differences in response to keyhole limpet hemocyanin (67), OVA (67), hen egg lysozyme (20), and polyvinyl pyrrolidone (13), with female mice mounting more potent antigen-specific immune responses than males. Similar gender-dependent differences in vaccinated swine and humans have been reported (3, 8, 48). Importantly, an HSV experimental vaccine was protective in human females but not in males (60), and a pseudorabies virus vaccine resulted in significantly lower virus excretion in female swine than in male swine (8).
Numerous factors likely contribute to the differences in vaccine-induced immune responses between males and females. First, the age of the vaccinee may affect the response. There were no differences in postimmunization vaccine-specific antibody titers between genders in infants vaccinated with acellular or whole-cell pertussis vaccines (6), while vaccination of adult females resulted in antigen-specific immune responses significantly greater than those induced in adult males (3, 48, 60). In contrast, with the inactivated influenza vaccine, there was no significant difference in vaccine-specific immune responses between adult men and women (2, 6). Second, gender-dependent APC production of IL-12 and IL-10 has been reported (68). Female SJL mice preferentially developed Th1 immune responses, while male SJL mice developed Th2 immune responses. This was due to gender-dependent production of IL-12 by APCs, with APCs from female mice producing more IL-12 than APCs from male mice (68). Third, the social stresses of housing of male animals could decrease immunogen-induced immune responses, compared to immunogen-induced responses in females (8, 20). Individual housing of male mice was associated with significantly increased antigen-specific immune responses, compared to group-housed males (8, 20). Fourth, the presence of estrogen could affect the induction of antigen-specific immune responses (32). The inability of the peptide nasal immunization to induce comparable levels of antigen-specific IFN-γ responses in male mice may be associated with the ability of estrogen to augment the induction of cell-mediated immune responses, including T-lymphocyte proliferation and IFN-γ production in female mice (32). Alternatively, the enhanced induction of HIV-1-specific IFN-γ SFC responses in the genital tracts of female mice after nasal peptide prime/MVA boost may represent augmented antigen presentation in the female upper respiratory tract, via estrogen-dependent mechanisms, since estrogen has been reported to augment the APC activity of mucosal epithelial cells (45, 69, 70).
It is of interest that MVA priming was effective in male mice while i.n. peptide priming was not. The strength of peptide-induced CTL responses is significantly increased by the presence of T-helper responses (62). Although our peptide immunogen contained a T-helper epitope, the rMVA vaccine expressed numerous other HIV-1 Env or MVA T-helper epitopes that may provide superior T-cell help for the induction of vaccine-specific IFN-γ responses and overcome the defect observed in male mice after i.n. peptide immunization. Additionally, it has been reported that persistence of antigen influences the induction of CD8-restricted T-cell responses with greater CD8-restricted T-cell responses occurring in the setting of persistent antigen (61). With i.n. peptide subunit priming, the antigen may not persist as long as antigen produced by rMVA immunization, resulting in a less effective priming immunization. A decreased density of T-helper epitope and/or decreased persistence of antigen after mucosal immunization combined with gender differences in APC function (68) or estrogen augmentation of epithelial cell APC activity (45, 69, 70) may explain the defective mucosal peptide priming observed with male mice. However, these explanations do not explain the effectiveness of nasal peptide priming with female mice.
A fundamental question is whether a mucosal prime and boost is needed to generate protective anti-HIV-1 immune responses at mucosal sites. We and others have previously reported that nasal immunization is an effective method of immunization for the induction of antigen-specific humoral immune responses in the female genital tract (1, 4, 16, 18, 37, 38, 57-59). In the present study, all immunization strategies using systemic i.d. MVA induced detectable HIV-1-specific IFN-γ SFC responses in the mucosal tissues of the lung, genital tract, and colon, although a mucosal peptide prime followed by a systemic MVA boost was optimal for the induction of HIV-1-specific female genital tract and colon IFN-γ SFC responses. Others have suggested that mucosal priming is critical for generating mucosal responses, since the use of a nasal recombinant vaccinia virus prime followed by a systemic or mucosal DNA vaccine boost was superior to systemic prime boost immunization strategies for the induction of optimal vaccine-induced immunoglobulin A in the female genital tract and antigen-specific IFN-γ SFC in the spleen and regional lymph nodes (14). It has also been reported that i.n. and parenteral immunization with BCG induced similar levels of antigen-specific responses in the spleen while only the i.n.-immunized mice had specific T-cell responses in the lung lymph nodes, suggesting that a mucosal route of immunization was required for the induction of mucosal T cells (19). Others have reported that mucosal priming followed by i.d. MVA boosting of macaques enhanced SIV Gag-specific CD8+ T-cell responses in the colon when compared to animals immunized with i.d. MVA alone (15). Our results are in agreement with reports documenting the detection of CD8+ T-cell responses in multiple mucosal sites after induction by systemic immunizations (17, 25, 33, 34, 36). However, until the correlates of protective immune responses to HIV-1 challenge are known and the level of those immune responses needed for protection are determined, it will not be conclusively known if a systemic immunization will provide adequate anti-HIV-1 immunity at mucosal sites.
In conclusion, we have found that significant differences in vaccine-induced, HIV-1-specific immune responses in the spleen, genital tract, and colon may exist between males and females. It will be important to determine if the levels of mucosal immunity induced by systemic immunization are protective in males and females in nonhuman primates and humans. If a given strategy is not protective, it may be important to use distinct immunization strategies in males and females to induce optimal protective immune responses.
Acknowledgments
This work was supported by NIH Integrated Preclinical/Clinical AIDS Vaccine Development grant 5 P01 AI35351-09. J.W.P. is supported by the Interdisciplinary Research Training Program in AIDS, NIH grant 5T32 AI07392-14. Flow cytometry was performed by Patrice McDermott and John F. Whitesides in the Duke Human Vaccine Institute Flow Cytometry Facility, which is supported by the National Institutes of Health award AI-51445.
REFERENCES
- 1.Bergquist, C., E.-L. Johansson, T. Lagergard, J. Holmgren, and A. Rudin. 1997. Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infect. Immun. 65:2676-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beyer, W. E., A. M. Palache, R. Kerstens, and N. Masurel. 1996. Gender differences in local and systemic reactions to inactivated influenza vaccine, established by a meta-analysis of fourteen independent studies. European J. Clin. Microbiol. Infect. Dis. 15:65-70. [DOI] [PubMed] [Google Scholar]
- 3.Bock, H. L., J. Kruppenbacher, R. Sanger, W. Hobel, R. Clemens, and W. Jilg. 1996. Immunogenicity of a recombinant hepatitis B vaccine in adults. Arch. Intern. Med. 156:2226-2231. [PubMed] [Google Scholar]
- 4.Bradney, C. P., G. D. Sempowski, H.-X. Liao, B. F. Haynes, and H. F. Staats. 2002. Cytokines as adjuvants for the induction of anti-human immunodeficiency virus peptide immunoglobulin G (IgG) and IgA antibodies in serum and mucosal secretions after nasal immunization. J. Virol. 76:517-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casement, K. S., P. N. Nehete, R. B. Arlinghaus, and K. J. Sastry. 1995. Cross-reactive cytotoxic t lymphocytes induced by V3 loop synthetic peptides from different strains of human immunodeficiency virus type 1. Virology 211:261-267. [DOI] [PubMed] [Google Scholar]
- 6.Christy, C., M. E. Pichichero, G. F. Reed, M. D. Decker, E. L. Anderson, M. B. Rennels, J. A. Englund, K. M. Edwards, and M. C. Steinhoff. 1995. Effect of gender, race, and parental education on immunogenicity and reported reactogenicity of acellular and whole-cell pertussis vaccines. Pediatrics 96:584-587. [PubMed] [Google Scholar]
- 7.Confer, A. W., M. A. Suckow, M. Montelongo, S. M. Dabo, L. J. Miloscio, A. J. Gillespie, and G. L. Meredith. 2001. Intranasal vaccination of rabbits with Pasteurella multocida A:3 outer membranes that express iron-regulated proteins. Am. J. Vet. Res. 62:697-703. [DOI] [PubMed] [Google Scholar]
- 8.de Groot, J., M. A. Ruis, J. W. Scholten, J. M. Koolhaas, and W. J. Boersma. 2001. Long-term effects of social stress on antiviral immunity in pigs. Physiol. Behav. 73:145-158. [DOI] [PubMed] [Google Scholar]
- 9.D'Hooghe, T. M., J. Pudney, and J. A. Hill. 2001. Immunobiology of the reproductive tract in a female baboon. Am. J. Primatol. 53:47-54. [DOI] [PubMed] [Google Scholar]
- 10.Dondero, F., A. Radicioni, L. Gandini, and A. Lenzi. 1984. Immunoglobulins in human seminal plasma. Andrologia 16:228-236. [DOI] [PubMed] [Google Scholar]
- 11.Duarte, E. A., G. Eberl, and G. Corradin. 1996. Specific tolerization of active cytolytic T lymphocyte responses in vivo with soluble peptides. Cell. Immunol. 169:16-23. [DOI] [PubMed] [Google Scholar]
- 12.Egan, M. A., S. Y. Chong, M. Hagen, S. Megati, E. B. Schadeck, P. Piacente, B.-J. Ma, D. C. Montefiori, B. F. Haynes, Z. R. Israel, J. H. Eldridge, and H. F. Staats. 2004. A comparative evaluation of nasal and parenteral vaccine adjuvants to elicit systemic and mucosal HIV-1 peptide-specific humoral immune responses in cynomolgus macaques. Vaccine 22:3774-3788. [DOI] [PubMed] [Google Scholar]
- 13.Eidinger, D., and T. J. Garrett. 1972. Studies of the regulatory effects of the sex hormones on antibody formation and stem cell differentiation. J. Exp. Med. 136:1098-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eo, S. K., M. Gierynska, A. A. Kamar, and B. T. Rouse. 2001. Prime-boost immunization with DNA vaccine: mucosal route of administration changes the rules. J. Immunol. 166:5473-5479. [DOI] [PubMed] [Google Scholar]
- 15.Evans, D. T., L.-M. Chen, J. Gillis, K.-C. Lin, B. Harty, G. P. Mazzara, R. O. Donis, K. G. Mansfield, J. D. Lifson, R. C. Desrosiers, J. E. Galán, and R. P. Johnson. 2003. Mucosal priming of simian immunodeficiency virus-specific cytotoxic T-lymphocyte responses in rhesus macaques by the Salmonella type III secretion antigen delivery system. J. Virol. 77:2400-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furr, P. M., and D. Taylor-Robinson. 1993. Mycoplasma pulmonis infection of the murine oropharynx protects against subsequent vaginal colonization. Epidemiol. Infect. 111:307-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallichan, W. S., and K. L. Rosenthal. 1996. Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. J. Exp. Med. 184:1879-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallichan, W. S., and K. L. Rosenthal. 1998. Long-term immunity and protection against herpes simplex virus type 2 in the murine female genital tract after mucosal but not systemic immunization. J. Infect. Dis. 177:1155-1161. [DOI] [PubMed] [Google Scholar]
- 19.Goonetilleke, N. P., H. McShane, C. M. Hannan, R. J. Anderson, R. H. Brookes, and A. V. S. Hill. 2003. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guérin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J. Immunol. 171:1602-1609. [DOI] [PubMed] [Google Scholar]
- 20.Grewal, I. S., M. Heilig, A. Miller, and E. E. Sercarz. 1997. Environmental regulation of T-cell function in mice: group housing of males affects accessory cell function. Immunology 90:165-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haas, G., R. David, R. Frank, H. Gausepohl, C. Devaux, J. M. Claverie, and M. Pierres. 1991. Identification of a major human immunodeficiency virus-1 reverse transcriptase epitope recognized by mouse CD4+ T lymphocytes. Eur. J. Immunol. 21:1371-1377. [DOI] [PubMed] [Google Scholar]
- 22.Hale, P. M., K. B. Cease, R. A. Houghten, C. Ouyang, S. Putney, K. Javaherian, H. Margalit, J. L. Cornette, J. L. Spouge, C. DeLisi, et al. 1989. T cell multideterminant regions in the human immunodeficiency virus envelope: toward overcoming the problem of major histocompatibility complex restriction. Int. Immunol. 1:409-415. [DOI] [PubMed] [Google Scholar]
- 23.Ismaili, J., J. Rennesson, E. Aksoy, J. Vekemans, B. Vincart, Z. Amraoui, F. Van Laethem, M. Goldman, and P. M. Dubois. 2002. Monophosphoryl lipid A activates both human dendritic cells and T cells. J. Immunol. 168:926-932. [DOI] [PubMed] [Google Scholar]
- 24.Kahn, J. O., and B. D. Walker. 1998. Acute human immunodeficiency virus type 1 infection. N. Engl. J. Med. 339:33-39. [DOI] [PubMed] [Google Scholar]
- 25.Kim, S. K., K. S. Schluns, and L. Lefrancois. 1999. Induction and visualization of mucosal memory CD8 T cells following systemic virus infection. J. Immunol. 163:4125-4132. [PubMed] [Google Scholar]
- 26.Kondi-Paphitis, A., H. Carvounis, E. Kairi, M. Frangou, A. Papayanopoulou, and H. Deligeorgi. 1999. Expression of a local immune defense system in the female genital tract. An immunohistochemical study. Eur. J. Gynaecol. Oncol. 20:141-143. [PubMed] [Google Scholar]
- 27.Kundig, T. M., H. Hengartner, and R. Zinkernagel. 1993. T cell-dependent IFN-γ exerts an antiviral effect in the central nervous system but not in peripheral solid organs. J. Immunol. 150:2316-2321. [PubMed] [Google Scholar]
- 28.Kutteh, W. H., R. E. Blackwell, H. Gore, C. C. Kutteh, B. R. Carr, and J. Mestecky. 1990. Secretory immune system of the female reproductive tract. II. Local immune system in normal and infected fallopian tube. Fertil. Steril. 54:51-55. [PubMed] [Google Scholar]
- 29.Kutteh, W. H., K. D. Hatch, R. E. Blackwell, and J. Mestecky. 1988. Secretory immune system of the female reproductive tract: I. Immunoglobulin and secretory component-containing cells. Obstet. Gynecol. 71:56-60. [PubMed] [Google Scholar]
- 30.Lehner, T., L. A. Bergmeier, C. Panagiotidi, L. Tao, R. Brookes, L. S. Klavinskis, P. Walker, J. Walker, R. G. Ward, L. Hussain, et al. 1992. Induction of mucosal and systemic immunity to a recombinant simian immunodeficiency viral protein. Science 258:1365-1369. [DOI] [PubMed] [Google Scholar]
- 31.Lehner, T., L. Tao, C. Panagiotidi, L. S. Klavinskis, R. Brookes, L. Hussain, N. Meyers, S. E. Adams, A. J. Gearing, and L. A. Bergmeier. 1994. Mucosal model of genital immunization in male rhesus macaques with a recombinant simian immunodeficiency virus p27 antigen. J. Virol. 68:1624-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maret, A., J. D. Coudert, L. Garidou, G. Foucras, P. Gourdy, A. Krust, S. Dupont, P. Chambon, P. Druet, F. Bayard, and J. C. Guery. 2003. Estradiol enhances primary antigen-specific CD4 T cell responses and Th1 development in vivo. Essential role of estrogen receptor alpha expression in hematopoietic cells. Eur. J. Immunol. 33:512-521. [DOI] [PubMed] [Google Scholar]
- 33.Masopust, D., J. Jiang, H. Shen, and L. Lefrancois. 2001. Direct analysis of the dynamics of the intestinal mucosa CD8 T cell response to systemic virus infection. J. Immunol. 166:2348-2356. [DOI] [PubMed] [Google Scholar]
- 34.Masopust, D., V. Vezys, A. L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291:2413-2417. [DOI] [PubMed] [Google Scholar]
- 35.Miller, C. J. 1994. Mucosal transmission of simian immunodeficiency virus. Curr. Top. Microbiol. Immunol. 188:107-122. [DOI] [PubMed] [Google Scholar]
- 36.Musey, L., Y. Ding, M. Elizaga, R. Ha, C. Celum, and M. J. McElrath. 2003. HIV-1 vaccination administered intramuscularly can induce both systemic and mucosal T cell immunity in HIV-1-uninfected individuals. J. Immunol. 171:1094-1101. [DOI] [PubMed] [Google Scholar]
- 37.Pal, S., T. J. Fielder, E. M. Peterson, and L. M. de la Maza. 1994. Protection against infertility in a BALB/c mouse salpingitis model by intranasal immunization with the mouse pneumonitits biovar of Chlamydia trachomatis. Infect. Immun. 62:3354-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pal, S., E. M. Peterson, and L. M. de la Maza. 1996. Intranasal immunization induces long-term protection in mice against a Chlamydia trachomatis genital challenge. Infect. Immun. 64:5341-5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parr, E. L., and M. B. Parr. 1999. Immune responses and protection against vaginal infection after nasal or vaginal immunization with attenuated herpes simplex virus type-2. Immunology 98:639-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parr, M. B., and E. L. Parr. 1989. Immunohistochemical investigation of secretory component and immunoglobulin A in the genital tract of the female rat. J. Reprod. Fertil. 85:105-113. [DOI] [PubMed] [Google Scholar]
- 41.Parr, M. B., and E. L. Parr. 1989. Immunohistochemical localization of secretory component and immunoglobulin A in the urogenital tract of the male rodent. J. Reprod. Fertil. 85:115-124. [DOI] [PubMed] [Google Scholar]
- 42.Parr, M. B., H. P. Ren, L. D. Russell, G. S. Prins, and E. L. Parr. 1992. Urethral glands of the male mouse contain secretory component and immunoglobulin A plasma cells and are targets of testosterone. Biol. Reprod. 47:1031-1039. [DOI] [PubMed] [Google Scholar]
- 43.Persing, D. H., R. N. Coler, M. J. Lacy, D. A. Johnson, J. R. Baldridge, R. M. Hershberg, and S. G. Reed. 2002. Taking toll: lipid A mimetics as adjuvants and immunomodulators. Trends Microbiol. 10:S32-S37. [DOI] [PubMed] [Google Scholar]
- 44.Porgador, A., H. F. Staats, B. Faiola, E. Gilboa, and T. J. Palker. 1997. Intranasal immunization with CTL epitope peptides from HIV-1 or ovalbumin and the mucosal adjuvant cholera toxin induces peptide-specific CTLs and protection against tumor development in vivo. J. Immunol. 158:834-841. [PubMed] [Google Scholar]
- 45.Prabhala, R. H., and C. R. Wira. 1995. Sex hormone and IL-6 regulation of antigen presentation in the female reproductive tract mucosal tissues. J. Immunol. 155:5566-5573. [PubMed] [Google Scholar]
- 46.Pudney, J., and D. J. Anderson. 1995. Immunobiology of the human penile urethra. Am. J. Pathol. 147:155-165. [PMC free article] [PubMed] [Google Scholar]
- 47.Ramirez, J. C., D. Finke, M. Esteban, J. P. Kraehenbuhl, and H. Acha-Orbea. 2003. Tissue distribution of the Ankara strain of vaccinia virus (MVA) after mucosal or systemic administration. Arch. Virol. 148:827-839. [DOI] [PubMed] [Google Scholar]
- 48.Reutter, J., P. A. Bart, P. Francioli, A. Safary, and P. C. Frei. 1998. Production of antibody to hepatitis A virus and hepatitis B surface antigen measured after combined hepatitis A/hepatitis B vaccination in 242 adult volunteers. J. Viral Hepat. 5:205-211. [DOI] [PubMed] [Google Scholar]
- 49.Rudin, A., E.-L. Johansson, C. Bergquist, and J. Holmgren. 1998. Differential kinetics and distribution of antibodies in serum and nasal and vaginal secretions after nasal and oral vaccination of humans. Infect. Immun. 66:3390-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rudin, A., G. C. Riise, and J. Holmgren. 1999. Antibody responses in the lower respiratory tract and male urogenital tract in humans after nasal and oral vaccination with cholera toxin B subunit. Infect. Immun. 67:2884-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santra, S., D. H. Barouch, S. S. Jackson, M. J. Kuroda, J. E. Schmitz, M. A. Lifton, A. H. Sharpe, and N. L. Letvin. 2000. Functional equivalency of B7-1 and B7-2 for costimulating plasmid DNA vaccine-elicited CTL responses. J. Immunol. 165:6791-6795. [DOI] [PubMed] [Google Scholar]
- 52.Santra, S., D. H. Barouch, A. H. Sharpe, and N. L. Letvin. 2000. B7 co-stimulatory requirements differ for induction of immune responses by DNA, protein and recombinant pox virus vaccination. Eur. J. Immunol. 30:2650-2659. [DOI] [PubMed] [Google Scholar]
- 53.Sarobe, P., J. J. Lasarte, J. J. Golvano, I. Prieto, A. Gullon, M. J. Soto, P. Labarga, J. Prieto, and F. Borras-Cuesta. 1994. Induction of neutralizing antibodies against human immunodeficiency virus type 1 using synthetic peptide constructs containing an immunodominant T-helper cell determinant from vpr. J. Acquir. Immune Defic. Syndr. 7:635-640. [PubMed] [Google Scholar]
- 54.Shirai, M., C. D. Pendleton, and J. A. Berzofsky. 1992. Broad recognition of cytotoxic T cell epitopes from the HIV-1 envelope protein with multiple class I histocompatibility molecules. J. Immunol. 148:1657-1667. [PubMed] [Google Scholar]
- 55.Spira, A. I., P. A. Marx, B. K. Patterson, J. Mahoney, R. A. Koup, S. M. Wolinsky, and D. D. Ho. 1996. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 183:215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Staats, H. F., C. P. Bradney, W. M. Gwinn, S. S. Jackson, G. D. Sempowski, H.-X. Liao, N. L. Letvin, and B. F. Haynes. 2001. Cytokine requirements for induction of systemic and mucosal cytotoxic T lymphocytes after nasal immunization. J. Immunol. 167:5386-5394. [DOI] [PubMed] [Google Scholar]
- 57.Staats, H. F., and F. A. Ennis. 1999. IL-1 is an effective adjuvant for mucosal and systemic immune responses when coadministered with protein immunogens. J. Immunol. 162:6141-6147. [PubMed] [Google Scholar]
- 58.Staats, H. F., S. P. Montgomery, and T. J. Palker. 1997. Intranasal immunization is superior to vaginal, gastric, or rectal immunization For the induction of systemic and mucosal anti-HIV antibody responses. AIDS Res. Hum. Retrovir. 13:945-952. [DOI] [PubMed] [Google Scholar]
- 59.Staats, H. F., W. G. Nichols, and T. J. Palker. 1996. Mucosal immunity to HIV-1: systemic and vaginal antibody responses after intranasal immunization with the HIV-1 C4/V3 peptide T1SP10 MN(A). J. Immunol. 157:462-472. [PubMed] [Google Scholar]
- 60.Stanberry, L. R., S. L. Spruance, A. L. Cunningham, D. I. Bernstein, A. Mindel, S. Sacks, S. Tyring, F. Y. Aoki, M. Slaoui, M. Denis, P. Vandepapeliere, and G. Dubin. 2002. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 347:1652-1661. [DOI] [PubMed] [Google Scholar]
- 61.Storni, T., C. Ruedl, W. A. Renner, and M. F. Bachmann. 2003. Innate immunity together with duration of antigen persistence regulate effector T cell induction. J. Immunol. 171:795-801. [DOI] [PubMed] [Google Scholar]
- 62.Swiniarski, H., S. F. Wolf, K. Sturmhoefel, R. L. Peterson, A. J. Dorner, and M. O'Toole. 2000. IL-12-dependent enhancement of CTL response to weak class I-restricted peptide immunogens requires coimmunization with T helper cell immunogens. Clin. Immunol. 94:200-211. [DOI] [PubMed] [Google Scholar]
- 63.Takahashi, H., J. Cohen, A. Hosmalin, K. B. Cease, R. Houghten, J. L. Cornette, C. DeLisi, B. Moss, R. N. Germain, and J. A. Berzofsky. 1988. An immunodominant epitope of the human immunodeficiency virus envelope glycoprotein gp160 recognized by class I major histocompatibility complex molecule-restricted murine cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. USA 85:3105-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Terres, G., S. L. Morrison, and G. S. Habicht. 1968. A quantitative difference in the immune response between male and female mice. Proc. Soc. Exp. Biol. Med. 127:664-667. [DOI] [PubMed] [Google Scholar]
- 65.UNAIDS. 2002. AIDS epidemic update: December 2002. Joint United Nations Programme on HIV/AIDS (UNAIDS), Geneva, Switzerland.
- 66.Vaslin, B., J. M. Claverie, O. Benveniste, F. C. Barre-Sinoussi, and D. Dormont. 1994. Nef and Gag synthetic peptide priming of antibody responses to HIV type 1 antigens in mice and primates. AIDS Res. Hum. Retrovir. 10:1241-1250. [DOI] [PubMed] [Google Scholar]
- 67.Weinstein, Y., S. Ran, and S. Segal. 1984. Sex-associated differences in the regulation of immune responses controlled by the MHC of the mouse. J. Immunol. 132:656-661. [PubMed] [Google Scholar]
- 68.Wilcoxen, S. C., E. Kirkman, K. C. Dowdell, and S. A. Stohlman. 2000. Gender-dependent IL-12 secretion by APC is regulated by IL-10. J. Immunol. 164:6237-6243. [DOI] [PubMed] [Google Scholar]
- 69.Wira, C. R., and R. M. Rossoll. 1995. Antigen-presenting cells in the female reproductive tract: influence of sex hormones on antigen presentation in the vagina. Immunology 84:505-508. [PMC free article] [PubMed] [Google Scholar]
- 70.Wira, C. R., and R. M. Rossoll. 1995. Antigen-presenting cells in the female reproductive tract: influence of the estrous cycle on antigen presentation by uterine epithelial and stromal cells. Endocrinology 136:4526-4534. [DOI] [PubMed] [Google Scholar]
- 71.Yamatomo, T., M. Okano, T. Ono, E. Nakayama, T. Yoshino, A. R. Satoskar, D. A. Harn, Jr., and K. Nishizaki. 2001. Sex-related differences in the initiation of allergic rhinitis in mice. Allergy 56:525-531. [DOI] [PubMed] [Google Scholar]