Abstract
Tat is among the required regulatory genes of human immunodeficiency virus type 1 (HIV-1). Tat functions both within infected cells as a transcription factor and as an extracellular factor that binds and alters bystander cells. Some functions of extracellular Tat can be neutralized by immune serum or monoclonal antibodies. In order to understand the antibody response to Tat, we are defining antibody epitopes and the effects of natural Tat sequence variation on antibody recognition. The dominant Tat epitope in macaque sera is within the first 15 amino acids of the protein amino terminus. Together with a subdominant response to amino acids 57 to 60, these two regions account for most of the macaque response to linear Tat epitopes and both regions are also sites for the binding of neutralizing antibodies. However, the dominant and subdominant epitope sequences differ among virus strains, and this natural variation can preclude antibody binding and Tat neutralization. We also examined serum samples from 31 HIV-positive individuals that contained Tat binding antibodies; 23 of the 31 sera recognized the amino terminus peptide. Similar to binding in macaques, human antibody binding to the amino terminus was affected by variations at positions 7 and 12, sequences that are distinct for clade B compared to other viral clades. Tat-neutralizing antibodies to the dominant amino terminus epitope are affected by HIV clade variation.
The Tat protein of human immunodeficiency virus type 1 (HIV-1) is the product of two exons within the second half of the viral genome. The first exon codes for amino acids 1 to 72, and the second exon has open reading frames of various lengths, resulting in Tat proteins ranging from 86 to 102 amino acids and having molecular sizes of up to 14 kDa. Tat is expressed early after infection and forms a complex with host nuclear proteins and the viral RNA (vRNA) stem-loop structure, called a transactivation response element, to relieve a block to transcript elongation (for a review, see references 14, 17, and 32). The Tat protein is also released from infected cells and functions as an extracellular factor (for a review, see references 15 and 27) that modifies bystander cells and favors virus spread.
Extracellular Tat binds chemokine receptors, integrins, or CD26 to induce cellular signaling (27). The in vitro effects of Tat include altered cytokine secretion (6), cellular anergy (43, 47), apoptosis (26, 45, 48), chemokine receptor induction (24, 38), and T-cell activation (29, 39, 46). Only the effects on cytokines and chemokine receptors were verified in vivo in a nonhuman primate model of AIDS (30).
Evidence for the role of extracellular Tat in HIV-1 pathogenesis, along with studies showing a correlation between Tat immunity and prognosis, prompted a search for vaccines against Tat. Protein, peptides, and DNA constructs have been used to immunize nonhuman primates. Most of these studies used an HIV Tat protein antigen (7, 8, 30, 40) or a carboxy-methylated form demonstrated to be biologically inert, called Tat toxoid (30, 40). Macaques immunized with Tat or Tat toxoid protein developed antibody and lymphoproliferative responses (7, 30, 40). Some macaque antisera neutralized the in vitro activity of Tat (4, 41). Plasmid DNA constructs were used to elicit cytotoxic T-lymphocyte (CTL) responses to the simian immunodeficiency virus (SIV) Tat protein. The CTL response to SIV Tat was reported to be strong enough to select for Tat escape variants in infected animals (2), but a preexisting CTL response to Tat had no effect on SIV infection (1). Overall, the results of published Tat vaccine studies in nonhuman primates range from complete protection against a challenge virus (7-9, 13) to disease attenuation (18, 30, 42) to no effect (1, 40). In the first clinical studies employing a Tat vaccine, HIV-infected (22) or noninfected control individuals (21) were vaccinated with Tat toxoid, and the vaccine was immunogenic, eliciting proliferative responses and Tat-binding antibodies.
Animal immunization studies showed that the dominant Tat epitope was contained within the amino terminus (5, 10, 36, 41). Clinical studies of sera from HIV-infected individuals also identified antibodies to this region (12), but the effect of sequence variation on binding or Tat neutralization was not well studied. Here, we identify amino acids that are essential for antibody binding to the amino terminus and show how natural sequence variation distinguishes clade B Tat from Tat proteins produced by other viral clades.
MATERIALS AND METHODS
Immune sera from rhesus macaques.
Four healthy rhesus macaques were immunized by intradermal and intramuscular injection of Tat toxoid for a previous study of vaccine effects (30). Antigen doses ranged from 20 to 60 μg of protein for each injection. Sera were collected 8 to 12 days after the last immunization and stored at −130°C until used.
HIV-infected individuals.
Sera were also obtained from 31 HIV-positive individuals receiving antiretroviral therapy. All had vRNA levels of <50 copies/ml of plasma and CD4+-T-cell counts of >200 cells/mm3 at the time of sampling. Volunteers provided informed consent, and the protocol was approved by the Institutional Review Board for the University of Maryland, Baltimore.
Monoclonal antibodies.
The TR1 murine monoclonal antibody was derived in our laboratory (41). The C3.2.D7 murine monoclonal antibody was isolated in the laboratory of Chandra (11). Both antibodies are of the immunoglobulin G2a (IgG2a) isotype, both bind Tat protein at the amino terminus epitope, and both neutralize Tat transactivation.
ELISA.
Peptides (1 μg/well) or 86-amino-acid Tat protein (kindly provided by Aventis Pasteur, Inc. Toronto, Canada) at a concentration of 100 ng/well were adsorbed to enzyme-linked immunosorbent assay (ELISA) plates (Costar, Cambridge, Mass.) by overnight incubation at 4°C in 100 mM carbonate buffer, pH 9.5. The coated plates were washed and then treated with 50 mM Tris HCl (pH 7.8), 0.15 M NaCl, 0.05% Tween 20, and 1% bovine serum albumin (BSA) (ELISA buffer). The wells were filled with monoclonal antibody solution at a concentration of 0.5 μg/ml or monkey serum diluted in ELISA buffer and incubated for 1 h. After four washes with ELISA buffer, secondary antibodies (anti-mouse IgG-alkaline phosphatase conjugate for the monoclonal antibody samples and anti-monkey IgG alkaline phosphate conjugate for the monkey serum samples) were added for 1 h at a 1:5,000 dilution. After four more washes, p-nitrophenyl phosphate substrate solution was added, and plates were incubated at 37°C for 30 min before the optical density was measured at 405 nm. The baseline was determined for BSA-coated wells without peptide or Tat that were overlaid with each serum dilution. Positive wells in the ELISA had A405 values of ≥2 standard deviations above the mean of background wells after subtracting the mean background values.
Peptide competition assay.
A total of 100 ng of an 86-amino-acid Tat protein (Aventis Pasteur) was incubated overnight at 4°C to bind this protein to plastic 96-well assay plates. Dilutions of sera (1:100 to 1:100,000) from Tat-immunized rhesus macaques were incubated with 50 μg of peptide (N-terminal peptide, MEPVDPRLEPWKHPGSQPKT; basic domain peptide, SYGSKKRRQRRRPPQDNQTH; scrambled N-terminal peptide, DPGTVEPKPLHPERKQMPWS; or scrambled basic domain peptide, QKRHRQHTGRAQYRSRSKRN) per ml for 30 min before the serum-peptide mix was added to the Tat-coated plate. Unbound antibodies were removed by washing. Secondary antibody incubation and color development were the same as for the standard ELISA.
Peptide arrays.
Two peptide arrays were generated. The first set was used for fine mapping of the N-terminal antibody response and included the N-terminal clade B 15-mer peptide, along with five peptides generated by sequentially substituting three amino acids at a time with alanine residues as follows: control (Tat sequence), MEPVDPRLEPWKHPG; first substitution, AAAVDPRLEPWKHPG; second substitution, MEPAAARLEPWKHP; third substitution, MEPVDPAAAPWKHPG; fourth substitution, MEPVDPRLEAAAHPG; and fifth substitution, MEPVDPRLEPWKAAA. The second peptide array contained five N-terminal 20-mer peptides; three represent clade B Tat sequences, and two are clade C sequences (B.-.NL43E9, MEPVDPRLEPWKHPGSQPKT; B.AU.MBCD36, MEPVDPKLEPWKHPGSQPRT; B.US.SF2, MEPVDPNLEPWKHPGSQPRT; consensus C, MEPVDPNLEPWNHPGSQPKT; and C.BW.96BW17, MDPVDPSLEPWNHPGSQPKT [Los Alamos HIV database]). A sixth, negative control peptide (DPGTVEPKPLHPERKQMPWS) was generated by randomly scrambling the 20 N-terminal amino acids from the consensus clade B sequence so that no sequence of three amino acids or longer in the scrambled peptide matched any of the other five corresponding peptide sequences. Peptides were synthesized by using 9-fluorenylmethoxy carbonyl chemistry at the Biopolymer Core Facility, Department of Microbiology and Immunology, University of Maryland School of Medicine.
Tat neutralization assay.
In order to measure transactivation by extracellular Tat, we used HeLa cells containing an HIV-1 provirus lacking Tat (kindly provided by Barbara Felber and George Pavlakis [37]). Cells were seeded into a 96-well plate at 20,000 cells per well and incubated overnight. The cells were then washed three times with warmed serum-free RPMI medium before they were overlaid with RPMI medium containing 0.1% ultrapure BSA (Panvera, Madison, Wis.) and 500 ng of HIV Tat protein per well for 90 min. Other experimental samples involved the addition of Tat that was preincubated for 30 min with monoclonal antibodies at a 2.5-fold molar excess (12.5 μg of Tat to 1 μg of TR1 monoclonal antibody or control IgG2a) or the preincubations of the monoclonal antibodies with B or C clade N-terminal peptides at a 100-fold molar excess (20 μg of peptide to 12.5 μg of antibody) for 30 min prior to the addition of the Tat protein. All treatments were overlaid on the indicator cells for 90 min and then removed and replaced with Dulbecco's modified Eagle medium containing 10% heat inactivated fetal bovine serum. Culture fluids were collected 72 h later, and cell-free virus was detected with a commercial antigen capture ELISA for the p24 capsid protein (R & D Systems, Minneapolis, Minn.).
RESULTS
Linear epitopes in the Tat amino terminus.
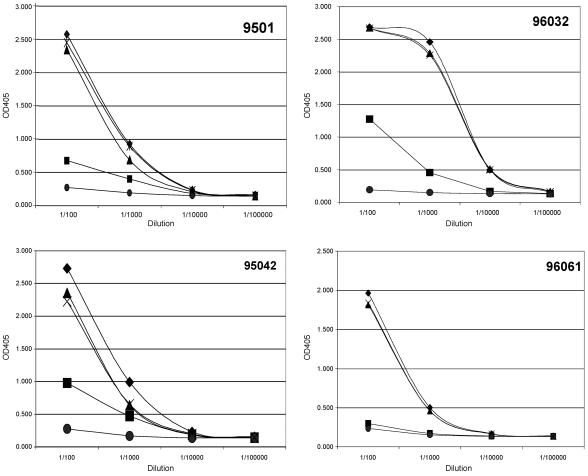
Four rhesus macaques were immunized with Tat toxoid for a previous study (30). These sera bound strongly to Tat and to N-terminal sequences (41). We established a competition binding assay by using soluble peptides to compete with whole Tat antigen on the plate to measure the proportion of antibodies that bind the linear amino terminus epitope peptide. We also tested whether peptides for a second major epitope (basic region, amino acids 57 to 60) would compete with full-length Tat antigen for serum antibody binding. Lastly, we combined the two peptides and competed for antibody binding to Tat protein. In all four animals, preincubating sera with an N-terminal peptide matched to the immunogen sequence eliminated most of the antibody binding to Tat protein on the plate (Fig. 1). The basic peptide alone had less capacity to block antibody binding. When we combined the amino terminus and basic region epitope peptides, serum antibody binding to Tat protein was virtually eliminated. Scrambled (control) peptides having the same composition but with different sequences did not compete for antibody binding to Tat (Fig. 1). Serum antibodies to a linear N-terminal region comprised the dominant response in immunized rhesus macaques, and together with a subdominant basic region epitope, accounted for nearly all of the macaque antibody responses to Tat linear epitopes.
FIG. 1.
Peptide competition of Tat toxoid immunized monkey sera binding to Tat. Monkey sera at dilutions between 1:100 and 1:1,000 were preincubated with no peptide (♦) or peptides corresponding to the N-terminal region (▪), the basic domain (▴), both peptides (×), or scrambled peptides corresponding to both regions (•). The optical density at 405 nm (x axis) was measured after an ELISA on plates coated with an 86-amino-acid Tat protein. Animals are identified by number.
Fine mapping of amino terminus epitopes with monoclonal antibodies.
We decided to fine map the N terminus epitope by using two mouse monoclonal antibodies against Tat. The TR1 (41) and C3.2.D7 (11) antibodies were tested on a peptide array where each peptide was modified by the substitution of three sequential alanines for the immunogen sequence. Both of the monoclonal antibodies reacted to whole Tat protein and to the 15-amino-acid clade B N-terminal sequence (Table 1). Substitution of the first three amino acids with alanine did not affect binding for either of the monoclonal antibodies. Substitution of the second triplet eliminated peptide binding by the TR1 antibody but did not affect binding of C3.2.D7. Neither monoclonal antibody was able to bind when the third triplet of amino acids was replaced with alanines. However, some reactivity of the TR1 antibody but not the C3.2.D7 antibody was observed when the fourth triplet was replaced. Peptides with substitutions at the fifth triplet were bound by both antibodies. For the TR1 monoclonal antibody, amino acid residues 4 to 9 were most important for binding to the peptide epitope; for C3.2.D7, residues 7 to 12 were most important (Table 1). These studies indicate the presence of at least two overlapping, linear antibody epitopes within the N-terminal 15 amino acids of the Tat protein.
TABLE 1.
Monoclonal antibody binding to peptide arrays
| Sequence or protein | Monoclonal antibody binding (OD405)a
|
|
|---|---|---|
| TR1 | C3.2.D7 | |
| Alanine peptide arrayb | ||
| MEPVDPRLEPWKHPG | 2.69 | 2.61 |
| AAAVDPRLEPWKHPG | 2.70 | 2.66 |
| MEPAAARLEPWKHPG | 0.00 | 2.61 |
| MEPVDPAAAPWKHPG | 0.00 | 0.01 |
| MEPVDPRLEAAAHPG | 0.46 | 0.01 |
| MEPVDPRLEPWKAAA | 2.73 | 2.63 |
| Tat | 2.70 | 2.70 |
| N-terminal peptide arrayc | ||
| MEPVDPRLEPWKHPGSQPKT | 2.70 | 2.62 |
| MEPVDPKLEPWKHPGSQPRT | 2.55 | 2.66 |
| MEPVDPNLEPWKHPGSQPRT | 0.02 | 2.50 |
| MEPVDPNLEPWNHPGSQPKT | 0.00 | 0.00 |
| MDPVDPSLEPWNHPGSQPKT | 0.02 | 0.01 |
| DPGTVEPKPLHPERKQMPWS | 0.00 | 0.00 |
| Tat | 2.78 | 2.71 |
OD405, optical density at 405 nm based on ELISA. Values in boldface are ≥2 standard deviations above the mean of background wells after subtracting the mean background values.
Substituted triplets are in boldface.
Amino acids 4 to 12 (in boldface) comprise an important region for monoclonal antibody binding.
In order to define individual amino acids that impacted monoclonal antibody binding, we used an array of peptides with amino acid substitutions reflecting natural variation in the Tat N terminus. We used three clade B and two clade C peptides, as well as a scrambled control peptide. Within the region important for binding these monoclonal antibodies (amino acids 4 to 12), there were frequent substitutions at two positions, amino acids 7 and 12. Results of the peptide scan (Table 1) showed that both of these amino acid positions were critical for monoclonal antibody binding. Substitutions at position 7 eliminated binding of the TR1 monoclonal antibody, and substitutions at position 12 eliminated binding of the C3.2.D7 antibody. We also noted that amino acids 7 and 12 are highly variable in Tat sequences (19, 41).
Macaque sera and monoclonal antibodies recognize similar N terminus epitopes.
The specificity of immune macaque serum was tested further by binding to peptides that were systematically altered. Sera were diluted 1:100 or 1:1,000 and tested. Results of the alanine scan peptide array are shown in Table 2. Dilutions of 1:100 of sera from all four macaques reacted strongly with whole Tat protein and to the clade B amino terminus epitope peptide. Alanine substitutions for amino acids 4, 5, and 6 reduced binding with sera from two animals (numbers 95011 and 96061). However, binding for all sera was reduced by alanine substitutions of amino acids 7 to 9 or 10 to 12, showing that positions 7 to 12 are critical for antibody recognition of the Tat amino terminus. When these positions were altered, binding was reduced to baseline levels. When the final three amino acids in the 15-mer peptide were replaced with alanine, binding was not affected. This pattern mimicked the performance of murine monoclonal antibodies and confirmed the importance of amino acids 4 to 12 for macaque serum antibody binding to the N terminus of Tat.
TABLE 2.
Monkey Tat antisera reactivity on peptide arrays
| Sequence or protein (dilution) | OD405 for antiserum from animal:a
|
|||
|---|---|---|---|---|
| 95011 | 96032 | 95042 | 96061 | |
| Alanine peptide array (1:100)b | ||||
| MEPVDPRLEPWKHPG | 1.17 | 2.61 | 2.34 | 0.80 |
| AAAVDPRLEPWKHPG | 1.11 | 2.68 | 2.48 | 0.70 |
| MEPAAARLEPWKHPG | 0.13 | 2.06 | 1.80 | 0.03 |
| MEPVDPAAAPWKHPG | 0.03 | 0.29 | 0.08 | 0.18 |
| MEPVDPRLEAAAHPG | 0.02 | 0.00 | 0.01 | 0.01 |
| MEPVDPRLEPWKAAA | 1.17 | 2.54 | 2.07 | 0.54 |
| Tat | 2.74 | 2.70 | 2.65 | 2.57 |
| N-terminal peptide array (1:1,000)c | ||||
| MEPVDPRLEPWKHPGSQPKT | 1.64 | 2.59 | 1.54 | 0.50 |
| MEPVDPKLEPWKHPGSQPRT | 0.53 | 2.22 | 0.86 | 0.22 |
| MEPVDPNLEPWKHPGSQPRT | 0.55 | 1.76 | 0.47 | 0.20 |
| MEPVDPNLEPWNHPGSQPKT | 1.10 | 2.32 | 0.22 | 0.14 |
| MDPVDPSLEPWNHPGSQPKT | 1.06 | 2.12 | 0.05 | 0.08 |
| Tat | 2.07 | 2.73 | 1.76 | 1.29 |
OD405, optical density at 405 nm based on ELISA.
Substituted triplets are in boldface.
Amino acids 4 to 12 (in boldface) comprise an important region for monoclonal antibody binding.
The same sera were screened on the cross-clade peptide array at a 1:1,000 dilution; these peptides reflect natural sequence variation within the Tat amino terminus. Previous results with this cohort showed a high degree of cross-reactivity to N-terminal peptides at a 1:100 dilution (41). At a 1:1,000 dilution, differences in reactivity were observed (Table 2). Each serum sample bound the clade B (immunogen) peptide. Two distinct patterns were observed for binding to variant N-terminal peptides. Sera from two macaques (animals 95011 and 96032) had higher binding compared to other clade B or consensus clade C sequences to a peptide matching the immunogen. Despite having some binding to all Tat sequences, the highest binding was to the clade B immunogen sequence. Sera from the remaining two macaques (animals 95042 and 96061) had lower binding to heterologous N-terminal sequences but, overall, had higher reactivity to clade B than to clade C sequences. To further investigate the differences in reactivity, endpoint titrations were done for the two macaques with the highest reactivity to Tat (animals 95011 and 96032). These data show that amino acid changes at positions 7 and 12 caused up to eightfold differences in binding (Table 3).
TABLE 3.
Heterologous N-terminal array endpoint titers for immunized macaques 95011 and 96032
| Sequence or proteina | Maximum dilution for animal:b
|
|
|---|---|---|
| 95011 | 96032 | |
| MEPVDPRLEPWKHPGSQPKT | 1:16,000 | 1:32,000 |
| MEPVDPKLEPWKHPGSQPRT | 1:2,000 | 1:16,000 |
| MEPVDPNLEPWKHPGSQPRT | 1:2,000 | 1:8,000 |
| MEPVDPNLEPWNHPGSQPKT | 1:8,000 | 1:16,000 |
| MDPVDPSLEPWNHPGSQPKT | 1:4,000 | 1:8,000 |
| Tat | 1:32,000 | 1:128,000 |
Amino acids 4 to 12 (in boldface) comprise an important region for monoclonal antibody binding.
Values listed are the maximum dilution before loss of reactivity with the peptide. All values twofold higher than baseline (binding to scrambled peptide) were considered positive.
Sequence-specific Tat neutralizing antibodies.
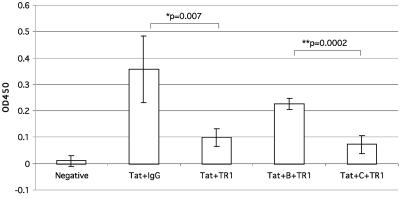
We next tested whether antibody neutralization of Tat function was affected by amino terminus variation. We used CD4+ HeLa cells with an integrated provirus lacking Tat function to measure viral transactivation by extracellular clade B Tat protein (Fig. 2). When Tat protein was preincubated with the TR1 monoclonal antibody, transactivation was reduced, as has been reported previously (41). We then competed for antibody neutralization by preincubating TR1 with a B clade amino terminus peptide and showed that transactivation was restored. Substituting a C clade peptide having asparagine at both positions 7 and 12 in place of the arginine or lysine found in clade B failed to block antibody neutralization of Tat (Fig. 2).
FIG. 2.
Peptide inhibition of Tat neutralization by monoclonal antibody TR1. The optical density at 450 nm (OD450) was measured by a p24 ELISA 72 h after the addition of samples to a HeLa cell line with an integrated provirus lacking Tat.
Antibodies from HIV-positive individuals recognize the Tat amino terminus.
We tested sera from 31 HIV-positive individuals receiving antiretroviral therapy with vRNA levels of <50 copies/ml of plasma and CD4+-T-cell counts of >200 cells/mm3. We detected Tat binding antibodies in every specimen; 23 of the 31 sera also had positive binding to amino terminus peptides. The consensus clade B sequence (peptide 1) was recognized most frequently (Table 4). The other B clade sequences (peptides 2 and 3) with changes at either position 7 or 12 were recognized by 32 or 45% of sera, respectively, and the clade C sequences (peptides 4 and 5) were recognized by less than 30% of the serum samples.
TABLE 4.
The proportion of human antisera that recognize each of five variant amino terminus epitope peptidesa
| Peptide | Sequence | Proportion (%) of antisera recognizing peptide (no. of positives/total no. of samples)b |
|---|---|---|
| Peptide 1 clade B | MEPVDPRLEPWKHPG | 68 (21/31) |
| Peptide 2 clade B | MEPVDPKLEPWKHPG | 32 (10/31) |
| Peptide 3 clade B | MEPVDPNLEPWKHPG | 45 (14/31) |
| Peptide 4 clade C | MEPVDPNLEPWNHPG | 29 (9/31) |
| Peptide 5 clade C | MEPVDPSLEPWNHPG | 19 (6/31) |
The peptides reflect natural sequence variations of Tat among several virus clades, with changes at positions 7 and 12 (in boldface).
Positive binding was defined as optical density at 405 nm of ≥2 standard deviations of the mean of the background after subtracting the mean background value.
The peptide recognition pattern showed that changes at amino acids 7 and 12 affected human antibody binding to the Tat amino terminus. The highest binding occurred when these amino acids were arginine and lysine, respectively. Importantly, the sequence containing Arg7 and Lys12 is characteristic of clade B Tat and is not found in consensus sequences from other clades (Table 5). The most frequent sequences in non-B clades are Asn7 and Asn12. As shown in Table 4, both of these changes reduced binding by the human sera (from individuals presumed to have clade B virus infections in Baltimore), showing a focus on the B clade sequence and a lack of cross-reaction with more common amino terminus sequences found in non-B clade viruses.
TABLE 5.
Tat amino terminus sequences from six virus clades and one circulating recombinant form
| Clade | na | N-terminal sequence (amino acids 1 to 15)b |
|---|---|---|
| A | 53 | MDPVDPNLEPWNHPGSQP?T |
| B | 455 | MEPVDPRLEPWKHPGSQPKT |
| C | 159 | MEPVDPNLEPWNHPGSQPKT |
| D | 10 | MDPVDPNLEPWNHPGSQPRT |
| F1/F2 | 9 | MELVDPNLDPWNHPGSQPTT |
| G | 8 | MDPVDPNLEPWNHPGSQPKT |
| CRF02 | 14 | MEPVDPSLEPWNHPGSQPTT |
n, number of sequence entries that were examined.
Positions 7 and 12 are highlighted.
DISCUSSION
We characterized the dominant antibody epitope in HIV-1 Tat protein by using mouse monoclonal antibodies, immunized macaque sera, and sera from HIV-positive volunteers. We defined two overlapping epitopes in the amino terminus. Natural variation in Tat protein sequences includes substitutions at amino acids 7 and 12, both of which lowered antibody recognition by monoclonal, macaque, and human antibodies. The consensus clade B sequence includes Arg7 and Lys12, making clade B Tat distinct from all other clade consensus sequences at these critical antibody recognition sites. Antibodies directed at the amino terminus can neutralize Tat protein functions in vitro, and natural sequence variation will affect both antibody binding and neutralization. Our data suggest that other immunogen sequences, likely including Asn7 and Asn12, may be needed to elicit antibodies against this dominant epitope in non-B clade viruses.
We know from previous studies (16, 25, 33-35) that the Tat amino terminus is critical for its function. N-terminal deletions (between residues 2 to 6, 3 to 19, or 2 to 35) render Tat defective for transactivation (25), and substitutions for proline within this domain also reduced activity (33, 34). Of direct interest to our studies, substitution of glutamine for Arg7 or Lys12 had little effect on Tat transactivation (34). Naturally occurring variant viruses with similar substitutions at these positions still have functional Tat and cause disease worldwide.
Recently obtained nuclear magnetic resonance structures placed the N terminus of Tat within the core of the Tat protein (3, 20, 31). However, apparent burying of the N terminus did not preclude the generation of antibodies to this immunodominant domain, and the pattern of epitope dominance seems to be independent of the route for immunization. Dominant N terminus responses were observed in mice immunized with either Tat protein (5) or DNA expression constructs (23). Several groups reported this pattern for immunized macaques or HIV-infected individuals (10, 12, 36, 41).
The present study provides a quantitative analysis for the proportion of macaque antibodies that recognize the N terminus and depend on amino acid sequences at positions 4 to 12. Changing amino acids (positions 7 and 12) within this region eliminated antibody binding. This result indicated the presence of two, overlapping linear epitopes or a single, complex epitope within the N terminus. Goldstein et al. demonstrated that antisera from monkeys immunized with the N-terminal sequence VDPNLEPWKHPGS showed only 20% cross-reactivity to VDPRLEWPWK (19). Conversely, immunization with VDPNLEPWNHPGS resulted in only 1% cross-reactivity with a VDPRLEPWK peptide (19); bold type indicates amino acid positions 7 and 12. Our results agree with these findings and show similar results for HIV-positive individuals. Rabbit antisera to a clade B 101-amino-acid Tat protein showed only low binding to heterologous Tat variants (28), further supporting the idea that Tat antibodies recognizing linear, neutralizing epitopes may not show broad cross-reactivity.
N terminus antibodies prevent Tat uptake and transactivation in vitro (11, 41), and their levels were correlated with better prognoses for infected individuals (35). In the five studies that reported protection against simian-human immunodeficiency virus (SHIV) challenge after Tat immunization (7, 9, 18, 30, 42), the antibody responses were shown (10, 18, 41), or presumed to be, mainly focused on the amino terminus. However, the one published study showing no protection from SHIV after Tat immunization also found antibody responses to the amino terminus (40), but they were not as dominant as the responses we observed in macaques that were protected by Tat toxoid immunization (30). At present, the discrepant findings have not been explained and could involve differences in the Tat-neutralizing capacity of each serum or aspects of the cellular immune response.
Our goal is to develop Tat antigens that can be combined with virus structural antigens to create a more effective HIV vaccine. Immunity to Tat may lessen the destructive capacity of HIV by decreasing the levels of immunosuppressive cytokines and preventing up-regulation of chemokine receptors that facilitate virus spread (30). It is important to note a recent paper that explored the efficacy of complex vaccines in macaques. By adding a Tat-Nef fusion protein to a gp120 immunization regimen, macaques were protected from SHIV challenge even though neither antigen was protective on its own (44). The possibility of achieving similar results for HIV vaccines in humans encourages the further development of safe and effective Tat antigens.
Acknowledgments
This work was supported by PHS grant AI49805 (C.D.P.).
We are grateful to Maria Salvato for critical reading of the manuscript.
REFERENCES
- 1.Allen, T. M., L. Mortara, B. R. Mothe, M. Liebl, P. Jing, B. Calore, M. Piekarczyk, R. Ruddersdorf, D. H. O'Connor, X. Wang, C. Wang, D. B. Allison, J. D. Altman, A. Sette, R. C. Desrosiers, G. Sutter, and D. I. Watkins. 2002. Tat-vaccinated macaques do not control simian immunodeficiency virus SIVmac239 replication. J. Virol. 76:4108-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, T. M., D. H. O'Connor, P. Jing, J. L. Dzuris, B. R. Mothe, T. U. Vogel, E. Dunphy, M. E. Liebl, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, D. B. Allison, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407:386-390. [DOI] [PubMed] [Google Scholar]
- 3.Bayer, P., M. Kraft, A. Ejchart, M. Westendorp, R. Frank, and P. Rosch. 1995. Structural studies of HIV-1 Tat protein. J. Mol. Biol. 247:529-535. [DOI] [PubMed] [Google Scholar]
- 4.Belliard, G., A. Romieu, J. F. Zagury, H. Dali, O. Chaloin, R. Le Grand, E. Loret, J. P. Briand, B. Roques, C. Desgranges, and S. Muller. 2003. Specificity and effect on apoptosis of Tat antibodies from vaccinated and SHIV-infected rhesus macaques and HIV-infected individuals. Vaccine 21:3186-3199. [DOI] [PubMed] [Google Scholar]
- 5.Brake, D. A., J. Goudsmit, W. J. Krone, P. Schammel, N. Appleby, R. H. Meloen, and C. Debouck. 1990. Characterization of murine monoclonal antibodies to the Tat protein from human immunodeficiency virus type 1. J. Virol. 64:962-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buonaguro, L., G. Barillari, H. K. Chang, C. A. Bohan, V. Kao, R. Morgan, R. C. Gallo, and B. Ensoli. 1992. Effects of the human immunodeficiency virus type 1 Tat protein on the expression of inflammatory cytokines. J. Virol. 66:7159-7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cafaro, A., A. Caputo, C. Fracasso, M. T. Maggiorella, D. Goletti, S. Baroncelli, M. Pace, L. Sernicola, M. L. Koanga-Mogtomo, M. Betti, A. Borsetti, R. Belli, L. Akerblom, F. Corrias, S. Butto, J. Heeney, P. Verani, F. Titti, and B. Ensoli. 1999. Control of SHIV-89.6P-infection of cynomolgus monkeys by HIV-1 Tat protein vaccine. Nat. Med. 5:643-650. [DOI] [PubMed] [Google Scholar]
- 8.Cafaro, A., A. Caputo, M. T. Maggiorella, S. Baroncelli, C. Fracasso, M. Pace, A. Borsetti, L. Sernicola, D. R. Negri, P. Ten Haaft, M. Betti, Z. Michelini, I. Macchia, E. Fanales-Belasio, R. Belli, F. Corrias, S. Butto, P. Verani, F. Titti, and B. Ensoli. 2000. SHIV89.6P pathogenicity in cynomolgus monkeys and control of viral replication and disease onset by human immunodeficiency virus type 1 Tat vaccine. J. Med. Primatol. 29:193-208. [DOI] [PubMed] [Google Scholar]
- 9.Cafaro, A., F. Titti, C. Fracasso, M. T. Maggiorella, S. Baroncelli, A. Caputo, D. Goletti, A. Borsetti, M. Pace, E. Fanales-Belasio, B. Ridolfi, D. R. Negri, L. Sernicola, R. Belli, F. Corrias, I. Macchia, P. Leone, Z. Michelini, P. ten Haaft, S. Butto, P. Verani, and B. Ensoli. 2001. Vaccination with DNA containing tat coding sequences and unmethylated CpG motifs protects cynomolgus monkeys upon infection with simian/human immunodeficiency virus (SHIV89.6P). Vaccine 19:2862-2877. [DOI] [PubMed] [Google Scholar]
- 10.Caselli, E., M. Betti, M. P. Grossi, P. G. Balboni, C. Rossi, C. Boarini, A. Cafaro, G. Barbanti-Brodano, B. Ensoli, and A. Caputo. 1999. DNA immunization with HIV-1 tat mutated in the trans activation domain induces humoral and cellular immune responses against wild-type Tat. J. Immunol. 162:5631-5638. [PubMed] [Google Scholar]
- 11.Demirhan, I., A. Chandra, O. Hasselmayer, and P. Chandra. 1999. Intercellular traffic of human immunodeficiency virus type 1 transactivator protein defined by monoclonal antibodies. FEBS Lett. 445:53-56. [DOI] [PubMed] [Google Scholar]
- 12.Demirhan, I., A. Chandra, F. Mueller, H. Mueller, P. Biberfeld, O. Hasselmayer, and P. Chandra. 2000. Antibody spectrum against the viral transactivator protein in patients with human immunodeficiency virus type 1 infection and Kaposi's sarcoma. J. Hum. Virol. 3:137-143. [PubMed] [Google Scholar]
- 13.Ensoli, B., and A. Cafaro. 2000. Control of viral replication and disease onset in cynomolgus monkeys by HIV-1 TAT vaccine. J. Biol. Regul. Homeost. Agents 14:22-26. [PubMed] [Google Scholar]
- 14.Frankel, A. D., and J. A. Young. 1998. HIV-1: fifteen proteins and an RNA. Annu. Rev. Biochem. 67:1-25. [DOI] [PubMed] [Google Scholar]
- 15.Gallo, R. C. 1999. Tat as one key to HIV-induced immune pathogenesis and Tat [correction of Pat] toxoid as an important component of a vaccine. Proc. Natl. Acad. Sci. USA 96:8324-8326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia, J. A., D. Harrich, L. Pearson, R. Mitsuyasu, and R. B. Gaynor. 1988. Functional domains required for tat-induced transcriptional activation of the HIV-1 long terminal repeat. EMBO J. 7:3143-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gatignol, A., and K. T. Jeang. 2000. Tat as a transcriptional activator and a potential therapeutic target for HIV-1. Adv. Pharmacol. 48:209-227. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein, G., K. Manson, G. Tribbick, and R. Smith. 2000. Minimization of chronic plasma viremia in rhesus macaques immunized with synthetic HIV-1 Tat peptides and infected with a chimeric simian/human immunodeficiency virus (SHIV33). Vaccine 18:2789-2795. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein, G., G. Tribbick, and K. Manson. 2001. Two B cell epitopes of HIV-1 Tat protein have limited antigenic polymorphism in geographically diverse HIV-1 strains. Vaccine 19:1738-1746. [DOI] [PubMed] [Google Scholar]
- 20.Gregoire, C., J. M. Peloponese, Jr., D. Esquieu, S. Opi, G. Campbell, M. Solomiac, E. Lebrun, J. Lebreton, and E. P. Loret. 2001. Homonuclear (1)H-NMR assignment and structural characterization of human immunodeficiency virus type 1 Tat Mal protein. Biopolymers 62:324-335. [DOI] [PubMed] [Google Scholar]
- 21.Gringeri, A., E. Santagostino, M. Muca-Perja, H. Le Buanec, B. Bizzini, A. Lachgar, J. F. Zagury, J. Rappaport, A. Burny, R. C. Gallo, and D. Zagury. 1999. Tat toxoid as a component of a preventive vaccine in seronegative subjects. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 20:371-375. [DOI] [PubMed] [Google Scholar]
- 22.Gringeri, A., E. Santagostino, M. Muca-Perja, P. M. Mannucci, J. F. Zagury, B. Bizzini, A. Lachgar, M. Carcagno, J. Rappaport, M. Criscuolo, W. Blattner, A. Burny, R. C. Gallo, and D. Zagury. 1998. Safety and immunogenicity of HIV-1 Tat toxoid in immunocompromised HIV-1-infected patients. J. Hum. Virol. 1:293-298. [PubMed] [Google Scholar]
- 23.Hinkula, J., C. Svanholm, S. Schwartz, P. Lundholm, M. Brytting, G. Engstrom, R. Benthin, H. Glaser, G. Sutter, B. Kohleisen, V. Erfle, K. Okuda, H. Wigzell, and B. Wahren. 1997. Recognition of prominent viral epitopes induced by immunization with human immunodeficiency virus type 1 regulatory genes. J. Virol. 71:5528-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang, L., I. Bosch, W. Hofmann, J. Sodroski, and A. B. Pardee. 1998. Tat protein induces human immunodeficiency virus type 1 (HIV-1) coreceptors and promotes infection with both macrophage-tropic and T-lymphotropic HIV-1 strains. J. Virol. 72:8952-8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuppuswamy, M., T. Subramanian, A. Srinivasan, and G. Chinnadurai. 1989. Multiple functional domains of Tat, the trans-activator of HIV-1, defined by mutational analysis. Nucleic Acids Res. 17:3551-3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, C. J., D. J. Friedman, C. Wang, V. Metelev, and A. B. Pardee. 1995. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science 268:429-431. [DOI] [PubMed] [Google Scholar]
- 27.Noonan, D., and A. Albini. 2000. From the outside in: extracellular activities of HIV Tat. Adv. Pharmacol. 48:229-250. [DOI] [PubMed] [Google Scholar]
- 28.Opi, S., J. M. Peloponese, Jr., D. Esquieu, G. Campbell, J. de Mareuil, A. Walburger, M. Solomiac, C. Gregoire, E. Bouveret, D. L. Yirrell, and E. P. Loret. 2002. Tat HIV-1 primary and tertiary structures critical to immune response against non-homologous variants. J. Biol. Chem. 277:35915-35919. [DOI] [PubMed] [Google Scholar]
- 29.Ott, M., S. Emiliani, C. Van Lint, G. Herbein, J. Lovett, N. Chirmule, T. McCloskey, S. Pahwa, and E. Verdin. 1997. Immune hyperactivation of HIV-1-infected T cell mediated by Tat and the CD28 pathway. Science 275:1481-1485. [DOI] [PubMed] [Google Scholar]
- 30.Pauza, C. D., P. Trivedi, M. Wallace, T. J. Ruckwardt, H. Le Buanec, W. Lu, B. Bizzini, A. Burny, D. Zagury, and R. C. Gallo. 2000. Vaccination with tat toxoid attenuates disease in simian/HIV-challenged macaques. Proc. Natl. Acad. Sci. USA 97:3515-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peloponese, J. M., Jr., C. Gregoire, S. Opi, D. Esquieu, J. Sturgis, E. Lebrun, E. Meurs, Y. Collette, D. Olive, A. M. Aubertin, M. Witvrow, C. Pannecouque, E. De Clercq, C. Bailly, J. Lebreton, and E. P. Loret. 2000. 1H-13C nuclear magnetic resonance assignment and structural characterization of HIV-1 Tat protein. C R Acad. Sci. III 323:883-894. [DOI] [PubMed] [Google Scholar]
- 32.Rana, T. M., and K. T. Jeang. 1999. Biochemical and functional interactions between HIV-1 Tat protein and TAR RNA. Arch. Biochem. Biophys. 365:175-185. [DOI] [PubMed] [Google Scholar]
- 33.Rappaport, J., S. J. Lee, K. Khalili, and F. Wong-Staal. 1989. The acidic amino-terminal region of the HIV-1 Tat protein constitutes an essential activating domain. New Biol. 1:101-110. [PubMed] [Google Scholar]
- 34.Reddy, M. V., M. Desai, J. Jeyapaul, D. D. Prasad, T. Seshamma, D. Palmeri, and S. A. Khan. 1992. Functional analysis of the N-terminal domain of Tat protein of the human immunodeficiency virus type 1. Oncogene 7:1743-1748. [PubMed] [Google Scholar]
- 35.Rice, A. P., and F. Carlotti. 1990. Structural analysis of wild-type and mutant human immunodeficiency virus type 1 Tat proteins. J. Virol. 64:6018-6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richardson, M. W., J. Mirchandani, P. Silvera, E. G. Regulier, C. Capini, P. M. Bojczuk, J. Hu, E. J. Gracely, J. D. Boyer, K. Khalili, J. F. Zagury, M. G. Lewis, and J. Rappaport. 2002. Immunogenicity of HIV-1 IIIB and SHIV 89.6P Tat and Tat toxoids in rhesus macaques: induction of humoral and cellular immune responses. DNA Cell Biol. 21:637-651. [DOI] [PubMed] [Google Scholar]
- 37.Sadaie, M. R., E. Tschachler, K. Valerie, M. Rosenberg, B. K. Felber, G. N. Pavlakis, M. E. Klotman, and F. Wong-Staal. 1990. Activation of tat-defective human immunodeficiency virus by ultraviolet light. New Biol. 2:479-486. [PubMed] [Google Scholar]
- 38.Secchiero, P., D. Zella, S. Capitani, R. C. Gallo, and G. Zauli. 1999. Extracellular HIV-1 Tat protein up-regulates the expression of surface CXC-chemokine receptor 4 in resting CD4+ T cells. J. Immunol. 162:2427-2431. [PubMed] [Google Scholar]
- 39.Secchiero, P., D. Zella, S. Curreli, P. Mirandola, S. Capitani, R. C. Gallo, and G. Zauli. 2000. Pivotal role of cyclic nucleoside phosphodiesterase 4 in Tat-mediated CD4+ T cell hyperactivation and HIV type 1 replication. Proc. Natl. Acad. Sci. USA 97:14620-14625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silvera, P., M. W. Richardson, J. Greenhouse, J. Yalley-Ogunro, N. Shaw, J. Mirchandani, K. Khalili, J. F. Zagury, M. G. Lewis, and J. Rappaport. 2002. Outcome of simian-human immunodeficiency virus strain 89.6p challenge following vaccination of rhesus macaques with human immunodeficiency virus Tat protein. J. Virol. 76:3800-3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tikhonov, I., T. J. Ruckwardt, G. S. Hatfield, and C. D. Pauza. 2003. Tat-neutralizing antibodies in vaccinated macaques. J. Virol. 77:3157-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verrier, B., R. Le Grand, Y. Ataman-Onal, C. Terrat, C. Guillon, P. Y. Durand, B. Hurtrel, A. M. Aubertin, G. Sutter, V. Erfle, and M. Girard. 2002. Evaluation in rhesus macaques of Tat and Rev-targeted immunization as a preventive vaccine against mucosal challenge with SHIV-BX08. DNA Cell Biol. 21:653-658. [DOI] [PubMed] [Google Scholar]
- 43.Viscidi, R. P., K. Mayur, H. M. Lederman, and A. D. Frankel. 1989. Inhibition of antigen-induced lymphocyte proliferation by Tat protein from HIV-1. Science 246:1606-1608. [DOI] [PubMed] [Google Scholar]
- 44.Voss, G., K. Manson, D. Montefiori, D. I. Watkins, J. Heeney, M. Wyand, J. Cohen, and C. Bruck. 2003. Prevention of disease induced by a partially heterologous AIDS virus in rhesus monkeys by using an adjuvanted multicomponent protein vaccine. J. Virol. 77:1049-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Westendorp, M. O., R. Frank, C. Ochsenbauer, K. Stricker, J. Dhein, H. Walczak, K. M. Debatin, and P. H. Krammer. 1995. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 375:497-500. [DOI] [PubMed] [Google Scholar]
- 46.Westendorp, M. O., M. Li-Weber, R. W. Frank, and P. H. Krammer. 1994. Human immunodeficiency virus type 1 Tat upregulates interleukin-2 secretion in activated T cells. J. Virol. 68:4177-4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zagury, D., A. Lachgar, V. Chams, L. S. Fall, J. Bernard, J. F. Zagury, B. Bizzini, A. Gringeri, E. Santagostino, J. Rappaport, M. Feldman, A. Burny, and R. C. Gallo. 1998. Interferon alpha and Tat involvement in the immunosuppression of uninfected T cells and C-C chemokine decline in AIDS. Proc. Natl. Acad. Sci. USA 95:3851-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zauli, G., and D. Gibellini. 1996. The human immunodeficiency virus type-1 (HIV-1) Tat protein and Bcl-2 gene expression. Leuk. Lymphoma 23:551-560. [DOI] [PubMed] [Google Scholar]