Abstract
Reovirus-induced apoptosis is associated with activation of the proapoptotic mitogen-activated protein kinase c-Jun N-terminal kinase (JNK) and the JNK-associated transcription factor c-Jun. Here we show that reovirus-induced apoptosis and activation of caspase 3 are inhibited in cells deficient in MEK kinase 1, an upstream activator of JNK in reovirus-infected cells. Inhibition of JNK activity following reovirus infection delays the release of proapoptotic mitochondrial factors and the subsequent onset of apoptosis. In contrast, reovirus-induced apoptosis is not blocked by infection with adenovirus expressing dominant-negative c-Jun, and c-Jun activation does not correlate with apoptosis in reovirus-infected cells. This is the first report demonstrating that JNK is associated with regulation of mitochondrial pathways of apoptosis following viral infection.
The c-Jun N-terminal kinases (JNKs) (also called stress-activated protein kinases) are mitogen-activated protein kinases (MAPKs) that are activated by stress stimuli, such as growth factor withdrawal and UV irradiation, and which function to communicate growth-inhibitory and apoptotic signals within cells (11, 14, 23). Members of the JNK family phosphorylate and activate members of the AP-1 group of transcription factors (c-Jun, JunB, and JunD) and the AP-1-related transcription factor ATF2, events which are likely to mediate, at least in part, the effects of JNK signaling pathways (9).
Gene disruption studies have shown that JNK is required for the release of proapoptotic molecules from the mitochondria in response to UV irradiation, indicating a role for JNK in mitochondrial pathways of apoptosis, although the exact mechanisms by which JNK facilitates this process remain incompletely understood (39). Mitochondrial apoptotic pathways are regulated by the Bcl-2 family of proteins. During mitochondrion-dependent apoptosis, the proapoptotic multidomain Bcl-2 family members (Bax and Bak) undergo a conformational change and redistribute from the cytoplasm to the mitochondria, where they are thought to mediate the release of proapoptotic molecules, including cytochrome c and Smac (15). The finding that Bax and Bak are essential for JNK-stimulated release of cytochrome c and apoptosis suggests that JNK influences mitochondrial apoptotic pathways through Bcl-2 family proteins (26).
Reovirus is a double-stranded RNA virus that induces apoptosis both in cultured cells and in target tissues (6). In the central nervous system and heart, virus-induced apoptosis correlates with pathology and is a critical mechanism by which disease is triggered in the host (10, 27, 32). In a variety of human epithelial cell lines and in primary neuronal cultures, reovirus-induced apoptosis is mediated by death ligands, including tumor necrosis factor, tumor necrosis factor-related apoptosis-inducing ligand, and Fas ligand (3, 4, 32). Ligand binding induces the activation of the initiator caspase, caspase 8, and the subsequent activation of the effector caspases (3, 20). Mitochondrial apoptotic pathways are also activated following reovirus infection by the caspase 8-dependent cleavage of Bid (20, 21). Consequently, reovirus-induced apoptosis is inhibited by overexpression of Bcl-2 (33), which blocks the release of proapoptotic mitochondrial factors, including cytochrome c and Smac, and the activation of caspase 3 in reovirus-infected cells (21). Although both Smac and cytochrome c function to promote caspase 3 activity, it is the release of Smac and the subsequent inhibition of cellular inhibitors of apoptosis that play the most significant role during reovirus-induced apoptosis (21).
We have shown that JNK and its associated transcription factor, c-Jun, are activated following reovirus infection and that JNK activation is mediated by MEK kinase I (MEKK1) (43). Activation of JNK and c-Jun are associated with reovirus-induced apoptosis (5). Thus, apoptotic viral strains induce the activation of JNK and c-Jun, whereas nonapoptotic viral strains do not (5). In addition, strain-specific differences in JNK activation are determined by the reovirus S1 and M2 gene segments, which encode viral outer capsid proteins (σ1 and μ1c) involved in receptor binding and host cell membrane penetration (5). These same gene segments also determine differences in the capacity of reovirus strains to induce apoptosis (40, 41). We now show that inhibition of JNK activity inhibits apoptosis in reovirus-infected cells. In contrast, inhibition of c-Jun does not affect reovirus-induced apoptosis, and c-Jun phosphorylation does not correlate with apoptosis in reovirus-infected cells. We further show that JNK inhibition delays the release of the proapoptotic mitochondrial factors Smac and cytochrome c from the mitochondria of infected cells. This report represents the first demonstration of JNK-induced regulation of mitochondrial apoptotic pathways in virus-infected cells.
MATERIALS AND METHODS
Cells and virus.
HEK293 cells (ATCC CRL1573) were grown in Dulbecco's modified Eagle's medium supplemented with 100 U each of penicillin and streptomycin/ml and containing 10% fetal bovine serum. HeLa cells (ATCC CCL2) were grown in Eagle's minimal essential medium supplemented with 2.4 mM l-glutamine, nonessential amino acids, and 60 U each of penicillin and streptomycin/ml and containing 10% fetal bovine serum (Gibco BRL). MEKK1−/− and wild-type (WT) embryonic stem (ES) cells and HEK293 cells expressing kinase-inactive MEK kinase 1, MEKK1KM (13), have previously been described. Reovirus strain type 3 Abney was used for all experiments. Reovirus strain type 3 Abney is a laboratory stock that has been plaque purified and passaged (twice) in L929 (ATCC CCL1) cells to generate working stocks. Virus infections and growth assays were performed as previously described (3, 41). Adenovirus expressing dominant-negative (DN) c-Jun (28) was provided by K. Heidenreich (University of Colorado).
Reagents.
JNK inhibitor I (JNKI1) was obtained from Alexis, and JNK inhibitor II (420119), PD98059 (MEK inhibitor), and SB203580 (p38 inhibitor) were purchased from Calbiochem. Each inhibitor was used at a concentration of 10 μM. Cells were pretreated with inhibitors for 2 h before viral infection and were added back to media following infection.
Apoptosis assays.
Cells were assayed for apoptosis by staining them with acridine orange, for determination of nuclear morphology, and ethidium bromide, to distinguish cell viability, at a final concentration of 1 μg/ml each. Following staining, cells were examined by epifluorescence microscopy (Labophot-2: B-2A filter, excitation, 450 to 490 nm; barrier, 520 nm; dichroic mirror, 505 nm; Nikon). The percentage of cells containing condensed nuclei and/or marginated chromatin in a population of 100 cells was recorded. The specificity of this assay has been previously established with reovirus-infected cells by using DNA laddering techniques and electron microscopy (4, 41).
Caspase 3 activity assays.
Caspase 3 activation assays were performed by using a kit obtained from Clontech. Cells (106) were centrifuged at 200 × g for 10 min, supernatants were removed, and cell pellets were frozen at −70°C until collections were made for all time points. Assays were performed with 96-well plates, and analysis was done by using a fluorescence plate reader (CytoFluor 4000; PerSeptive Biosystems). Cleavage of DEVD-AFC, a synthetic caspase-3 substrate, was used to measure caspase 3 activation in reovirus-infected cells. Cleavage after the second Asp residue produces free AFC that can be detected using a fluorescence plate reader. The amount of fluorescence detected is directly proportional to the amount of caspase 3 activity.
Western blot analysis.
Following infection with reovirus, cells were pelleted by centrifugation and washed twice with ice-cold phosphate-buffered saline (PBS). For whole-cell extracts, the cell pellet was lysed by sonication in 150 μl of a buffer containing 1% Triton X-100, 10 mM triethanolamine-HCl, 150 mM NaCl, 5 mM EDTA (pH = 8.0), 1 mM phenylmethylsulfonyl fluoride, and 2.5 μl of 25× complete protease inhibitor cocktail mix (catalog no. 1-697-498; Roche)/ml. The lysates were then cleared by centrifugation at 14,000 × g for 3 min, mixed 1:1 with 2× Laemmli buffer (catalog no. S-3401; Sigma), boiled for 5 min, and stored at −70°C. For mitochondrial extracts, the cell pellet was resuspended in 300 μl of a buffer containing 220 mM mannitol, 68 mM sucrose, 50 mM piperazine-N,N′-bis(2-ethanesulfonic acid)-KOH (pH = 7.4), 50 mM KCl, 5 mM EGTA, 2 mM MgCl2, and 1 mM dithiothreitol. The resuspended pellet was incubated on ice for 30 min and then homogenized with a B-pestle in a 2-ml dounce homogenizer for 40 strokes. The lysates were then cleared by centrifugation at 14,000 × g for 15 min. The supernatant, the mitochondrium-free fraction, was mixed 1:1 with 2× Laemmli Buffer (Sigma catalog no. S-3401), boiled for 5 min, and stored at −70°C. The pellet, the cytoplasmic fraction, was then lysed by sonication in 100 μl of whole-cell lysis buffer (above), mixed 1:1 with 2× Laemmli buffer, boiled for 5 min, and stored at −70°C.
Proteins were electrophoresed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, either through 10% Tris-tricine gels (phospho-c-Jun and c-Jun) or through 15% Tris-tricine gels (Smac and cytochrome c), and probed with antibodies directed against phospho-c-Jun (catalog no. 9261; 1:1,000 in 1% nonfat dry milk-Tris-buffered saline [TBS]; Cell Signaling), c-Jun (catalog no. 9162; 1:1,000 in 1% nonfat dry milk-TBS; Cell Signaling), Smac (catalog no. 567365; 1:1,000 in 1% nonfat dry milk-TBS; Calbiochem), and cytochrome c (catalog no. 556433; 1:1,000 in 1% nonfat dry milk-TBS; BD Pharmingen). All lysates were standardized for protein concentration with antibodies directed against actin (catalog no. CP-01; 1:10,000 in 1% nonfat dry milk-TBS; Calbiochem).
Immunocytochemistry.
Cells were plated in eight-well chamber slides. After infection, cells were fixed (3.7% formaldehyde-PBS) for 30 min at room temperature (RT). Cells were then washed with PBS and made permeable with PBS-0.1% Triton X-100 (PBSX) for 1 h at RT, blocked in 5% bovine serum albumin-PBSX for 2 h at RT, and then incubated overnight (4°C) with primary antibody (1:100) in 3% bovine serum albumin-PBSX. Primary antibodies used were directed against cleaved (active) caspase 3 (catalog no. 9661L; Cell Signaling). Following incubation, cells were washed three times (3 min each wash) with PBSX before being incubated with antirabbit or antirat immunoglobulin G conjugated with fluorescein isothiocyanate (1:100) for 1 h at RT. Fluorescent immunoglobulin Gs were obtained from Vector Laboratories. Incubation with secondary antibody and all subsequent steps were performed in the dark. Cells were then washed with PBSX (three times, 3 min each wash), incubated for a further 5 min with Hoechst-PBS (1:1000; Molecular Probes), washed again with PBSX (three times, 3 min each wash), and mounted with Vectashield (Vector Laboratories). After mounting, slides were sealed with clear nail polish and stored at 4°C. Labeling of mitochondria was performed using Cy3-labeled streptavidin (ImmunoResearch Laboratories) for 15 to 20 min prior to Hoechst staining.
RESULTS
MEKK1 is required for reovirus-induced apoptosis.
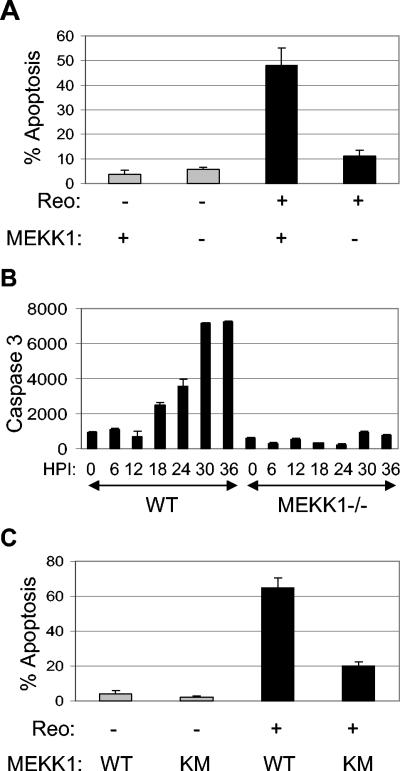
Reovirus-induced activation of JNK and c-Jun is associated with apoptosis in infected cells (5). We have shown that MEKK1 acts as an upstream activator of JNK signaling (43). We thus investigated the role of MEKK1 in reovirus-induced apoptosis. MEKK1−/− mouse embryo fibroblasts were infected with reovirus (multiplicity of infection[MOI], 10). At 48 h postinfection (p.i.), 48% of reovirus-infected wild-type (WT) cells contained apoptotic nuclei (Fig. 1A). This number was significantly reduced (P < 0.05) to 11% in MEKK1−/− cells (Fig. 1A). We next looked at reovirus-induced activation of caspase 3 in MEKK1−/− cells, using fluorogenic substrate assays. In WT cells, increases in caspase 3 activity were seen as early as 18 h p.i. and peaked at 30 h p.i., after which times levels remained high (Fig. 1B). In contrast, there was no increase in caspase 3 activity in MEKK1−/− cells following reovirus infection.
FIG. 1.
Reovirus-induced apoptosis is inhibited in MEKK1−/− cells and in cells expressing MEKK1KM. MEKK1−/− cells were infected with reovirus (MOI of 10; black bars) or were mock infected (gray bars), and the percentage of cells containing apoptotic nuclei at 48 h p.i. was determined (A). The graph shows the mean percentage of apoptotic cells from three independent experiments. Error bars represent standard errors of the mean. Reovirus-induced activation of caspase 3 was also determined for MEKK1−/− cells and controls (WT) at various times p.i. by fluorogenic substrate assay (B). The graph shows the mean fluorescence (arbitrary units) from three wells of an individual experiment, which represents caspase 3 activity, and is representative of three separate experiments. Cells expressing MEKK1KM and controls were infected with reovirus (MOI of 100; black bars) or were mock infected (gray bars), and the percentage of cells containing apoptotic nuclei at 48 h p.i. was determined (C). The graph shows the mean percentage of apoptotic cells from three independent experiments. Error bars represent standard errors of the mean.
Reovirus-induced apoptosis was also investigated in HEK293 cells expressing a kinase-inactive form of MEKK1 (MEKK1KM). Cells were infected with reovirus (MOI of 100). Forty-eight hours p.i., reovirus induced 65% apoptosis in cells expressing vector alone. This was significantly (P < 0.05) reduced to 20% in cells expressing MEKK1KM (Fig. 1C). These results indicate that reovirus-induced apoptosis is inhibited in MEKK1−/− cells and in cells expressing MEKK1KM, compared to the case with controls expressing WT MEKK1.
JNK is required for efficient apoptosis in reovirus-infected cells.
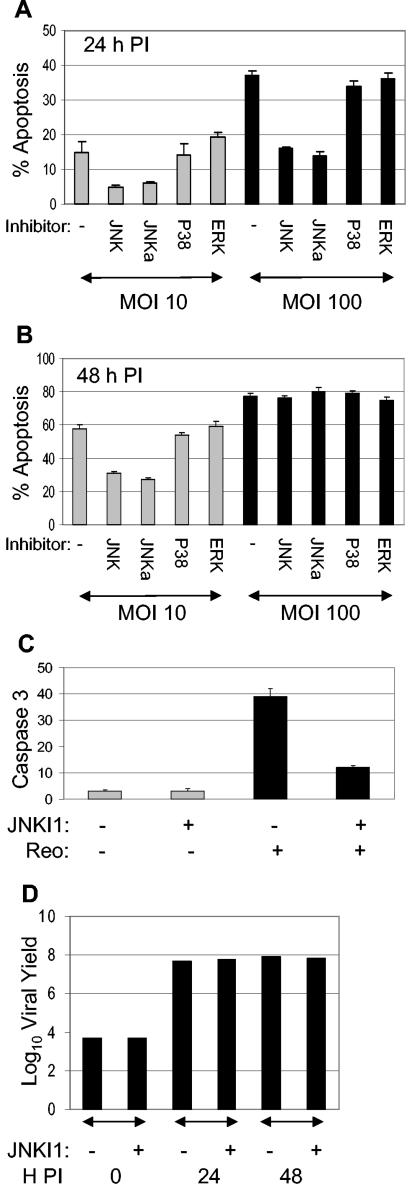
Having shown that reovirus-induced apoptosis requires MEKK1 and is inhibited in MEKK1−/− cells, which show reduced reovirus-induced JNK activation (43), we next investigated the effects of specific JNK inhibitors on reovirus-induced apoptosis. Two different inhibitors were used. The first, JNKI1, is a peptide inhibitor that blocks JNK binding to its substrate, and the second, JNK inhibitor II, inhibits JNK kinase activity. Since both HEK293 cells and HeLa cells have been used extensively in the analysis of reovirus-induced apoptosis, we used both of these cell lines in our experiments. Cells were treated with JNK inhibitors for 2 h before being infected with reovirus at a MOI of 10 or 100. Cells were harvested 24 and 48 h p.i., and the percentage of cells with apoptotic nuclear morphology was determined (Fig. 2A and B). In HEK293 cells, an inhibitor of JNK binding (JNKI1) inhibited reovirus (MOI of 10)-induced apoptosis at both 24 (a 67% reduction) and 48 (a 47% reduction; P < 0.001) h p.i. At a MOI of 100, inhibition of JNK binding delayed reovirus-induced apoptosis in HEK293 cells. Thus, at 24 h p.i., a 57% (P < 0.001) reduction in reovirus (MOI of 100)-induced apoptosis was observed. However, at 48 h p.i., JNKI1 was not able to protect HEK293 cells from reovirus (MOI of 100)-induced apoptosis, and high (>70%) levels of apoptosis were seen in both treated and untreated cells. Similar results were obtained by inhibition of JNK kinase activity. In contrast, inhibitors of the proapoptotic MAPK P38 (16, 24, 34) and the antiapoptotic extracellular signal-related kinases (ERK) (7) had no effect on reovirus-induced apoptosis (Fig. 2A and B), as previously shown (5). These results indicate that JNK, but not P38 or ERK, is required for efficient apoptosis in reovirus-infected HEK293 cells. Apoptosis in mock-infected cells, with or without inhibitor, was less than 5%. A similar effect on apoptosis was seen after treatment of HeLa cells with JNK and ERK inhibitors (data not shown). However, an inhibitor of P38 was able to reduce reovirus-induced apoptosis in these cells at 24 h p.i. (data not shown).
FIG. 2.
Reovirus-induced apoptosis requires JNK. HEK293 cells were treated with inhibitors of JNK (JNK, JNKa), P38 (P38), or ERK (ERK) for 2 h before being infected with reovirus (MOI of 10 [grey bars] or MOI of 100) ([black bars]). Specifically, JNKI1 (JNK) blocks JNK binding to its substrate, and JNK inhibitor II (JNKa) inhibits JNK kinase activity. Cells were harvested 24 (A) or 48 (B) h p.i., and the percentage of cells with apoptotic nuclear morphology was determined. The graphs show the mean percentages of apoptotic cells fromthree independent experiments. Error bars represent standard errors of the mean. Cells harvested at 18 h p.i. were fixed and stained with Hoechst 33342 (blue) to identify nuclei and with fluorescein isothiocyanate-labeled antibodies directed against active caspase 3 (green) to detect active caspase 3. The percentages of mock-infected (gray bars) and reovirus (Reo)-infected (black bars) cells with active caspase 3 staining were determined (C). The graph shows the mean percentages of apoptotic cells of three independent fields. Error bars represent standard errors of the means. One-step growth curves of reovirus in the presence or absence of JNKI1 are also presented (D). HEK293 cells were infected with reovirus (MOI of 1) and were harvested and assayed for growth at 24 and 48 h p.i. The graph shows the log10 virus yield over time.
The effect of JNK inhibition on apoptosis was also investigated by immunocytochemistry, using antibody directed against the cleaved (active) form of caspase 3. HeLa cells were incubated with JNKI1 for 2 h before being infected with reovirus. Cells were then incubated for a further 18 h before immunocytochemistry was performed (Fig. 2C). Active caspase 3 staining was seen in 39% of cells infected with reovirus, compared to 3% of mock-infected controls. Activation of caspase 3 was significantly (P < 0.001) reduced in the presence of JNKI1. These experiments suggest that JNK activity is required for efficient apoptosis and activation of caspase 3 in reovirus-infected cells.
The observed differences in the ability of reovirus to induce apoptosis were not caused by differences in viral growth, since one-step growth curves indicated that reovirus grows efficiently and equivalently in HEK293 cells in the presence or absence of JNKI1 (Fig. 2D).
c-Jun activation is not required for reovirus-induced apoptosis.
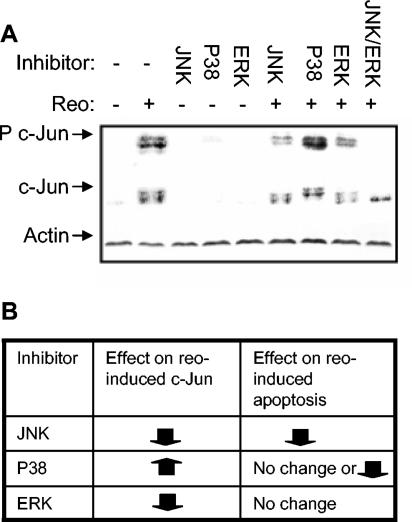
JNK activates members of the AP-1 group of transcription factors, including c-Jun, JunB, and JunD (9). We have previously shown that c-Jun is activated after reovirus infection, and this activation has been associated with reovirus-induced apoptosis. In other systems, JNK has been shown to influence apoptosis by its ability to activate the AP-1 transcription factor. We therefore investigated the role of AP-1 in reovirus-induced apoptosis. First, the role of MAPKs, including JNK, in c-Jun phosphorylation was determined in reovirus-infected cells. c-Jun is activated by 12 h p.i. in reovirus-infected HEK293 cells, and activation peaks around 18 h p.i. (5). We therefore investigated c-Jun phosphorylation in reovirus-infected HEK293 cells at 18 h p.i. in the presence or absence of inhibitors of JNK binding and P38 and ERK activity. Cells were pretreated with inhibitor for 2 h prior to infection (MOI of 100). Eighteen hours p.i., cells were harvested and screened by Western blot analysis for the presence of both the phosphorylated (active) and nonphosphorylated form of c-Jun. Reovirus infection resulted in the phosphorylation of c-Jun (Fig. 3A), as has previously been shown (5). The detection of multiple bands by the c-Jun antibodies indicates the multiple phosphorylation sites present on the c-Jun protein. Reovirus-induced activation of c-Jun was partially blocked by inhibition of JNK or ERK and was completely blocked when both JNK and ERK inhibitors were used together. In contrast, an inhibitor of P38 appeared to increase c-Jun phosphorylation after reovirus infection (Fig. 3A). Levels of total c-Jun indicated that reovirus also induced an increase in levels of total c-Jun in HEK293 cells (Fig. 3A). The presence of multiple bands again indicated that c-Jun was phosphorylated at multiple sites following reovirus infection. Increased c-Jun levels were not affected by inhibition of MAPK activity. Similar results were seen in HeLa cells (results not shown).
FIG. 3.
c-Jun is activated by JNK and ERK following reovirus infection. Cells were pretreated for 2 h with inhibitor of JNK binding (JNK) or p38 (p38) or ERK (ERK) activity. In addition, a combination of JNK and ERK inhibitors was used. Cells were then infected with reovirus (MOI of 100). Eighteen hours after infection, cells were harvested for Western blot analysis, using antibodies directed against phosphorylated c-Jun (P c-Jun), c-Jun, and actin (A). The effects of inhibitors of JNK, p38, and ERK on reovirus-induced activation and apoptosis were compared (B).
These results indicate that JNK and ERK, but not P38, contribute to c-Jun activation following reovirus infection. They also suggest that c-Jun activation does not correlate with apoptosis in reovirus-infected cells, since JNK inhibition reduces c-Jun phosphorylation and decreases apoptosis, ERK inhibition reduces c-Jun phosphorylation and has no effect on apoptosis, and P38 inhibition increases c-Jun phosphorylation yet has no effect on apoptosis in HEK293 cells and decreases apoptosis in HeLa cells (Fig. 3B).
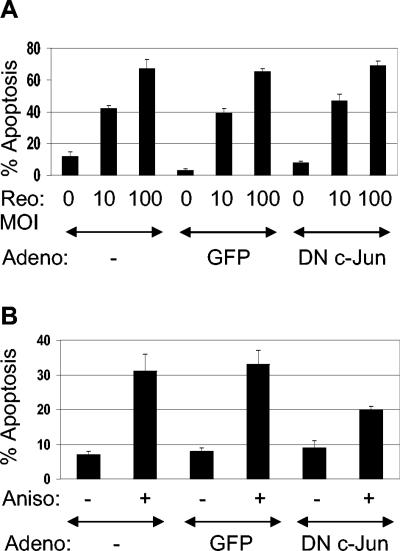
To conclusively determine the role of c-Jun/AP-1 in reovirus-induced apoptosis, we used adenovirus expressing dominant-negative (DN) c-Jun, which blocks AP-1 activity (28). HEK293 cells were infected with adenovirus (MOI of 50) expressing either DN c-Jun or green fluorescent protein (GFP) under the control of the cytomegalovirus promoter and were incubated for 36 h to allow expression of the DN c-Jun phenotype. Cells were then infected with reovirus (MOI of 10 and 100) for 48 h before being harvested. Reovirus infection at both a MOI of 10 and a MOI of 100 resulted in apoptosis (42 and 67%, respectively) in HEK293 cells that had not been previously treated with adenovirus (Fig. 4A). Reovirus induced apoptosis was not affected by treatment with adenovirus expressing either GFP or DN c-Jun (Fig. 4A). In contrast, anisomycin-induced apoptosis, which requires c-Jun, was reduced in HEK293 cells infected with adenovirus expressing DN c-Jun (Fig. 4B), indicating that c-Jun expression is not required for reovirus-induced apoptosis and suggesting that JNK exerts its proapoptotic effects in reovirus-infected cells through an alternate mechanism.
FIG. 4.
c-Jun is not required for reovirus-induced apoptosis. Cells were infected with adenovirus (Adeno) expressing GFP or DN c-Jun (MOI of 50) for 36 h. Cells were then infected with reovirus (MOI of 10 or 100) and were assayed for apoptosis after a further 48 h (A). Alternatively, cells were treated with anisomycin (1 μM) and were assayed for apoptosis after a further 24 h (B). The graphs show the mean percentages of apoptotic cells from three independent experiments. Error bars represent standard errors of the mean.
JNK is required for efficient release of Smac and cytochrome c after reovirus infection.
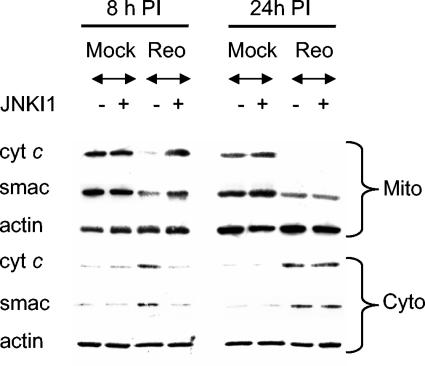
JNK is required for the release of proapoptotic molecules from the mitochondria in response to UV irradiation (39). Reovirus also activates mitochondrial apoptotic pathways following infection, resulting in the release of the proapoptotic mitochondrial factors cytochrome c and Smac (21). We thus investigated the role of JNK in the release of Smac and cytochrome c from the mitochondria during reovirus-induced apoptosis. HeLa cells were incubated with JNKI1 for 2 h before being infected with reovirus. Cells were then harvested at 8 and 24 h p.i. Mitochondrial pellet and mitochondrion-free fractions were prepared and analyzed by Western blot analysis, using antibodies directed against Smac and cytochrome c. Reovirus induced the release of both Smac and cytochrome c from the mitochondria of infected cells (Fig. 5A), as previously described (20, 21). Levels of Smac and cytochrome c thus dropped in mitochondrial pellet fractions and rose in mitochondrion-free fractions. JNKI1 delayed the release of both mitochondrial Smac and cytochrome c following reovirus infection.
FIG. 5.
JNK is required for the release of Smac and cytochrome c from the mitochondria of reovirus (Reo)-infected cells. Cells were pretreated with an inhibitor of JNK activity (JNKI1) for 2 h before being infected with reovirus (MOI of 100). Eight and twenty-four hours p.i., cells were harvested. Mitochondrial (Mito) and mitochondrium-free (Cyto) fractions were then prepared and used as lysates for Western blot analysis. Lysates were probed with antibodies directed against Smac and cytochrome c. Levels of actin were used to control for protein loading.
DISCUSSION
We have previously shown that reovirus-induced activation of JNK and its associated transcription factor, c-Jun, is associated with reovirus-induced apoptosis (5). We now show that reovirus-induced apoptosis is blocked in MEKK1−/− cells, in which reovirus-induced JNK activation is inhibited (43), and in cells in which MEKK1 kinase activity is blocked. In addition, specific inhibitors of JNK that block JNK-substrate binding or kinase activity block reovirus-induced apoptosis at a low MOI and significantly delay reovirus-induced apoptosis at a high MOI. These results indicate that JNK is required for efficient apoptosis in reovirus-infected cells (Fig. 6). The more-effective reduction in apoptosis seen in MEKK1−/− cells and cells expressing MEKK1KM compared to that in cells treated with an inhibitor of JNK suggests that MEKK1 may influence reovirus-induced apoptosis by JNK-independent, as well as JNK-dependent, pathways. Inhibition of P38 had no effect on reovirus-induced apoptosis in HEK293 cells, as has been previously shown (5). However, in HeLa cells, P38 inhibition decreased reovirus-induced apoptosis, suggesting cell type differences in MAPK involvement in reovirus-induced apoptosis. In contrast, inhibition of ERK had no effect on reovirus-induced apoptosis in either cell line, indicating specificity in the role of MAPKs in reovirus-induced apoptosis.
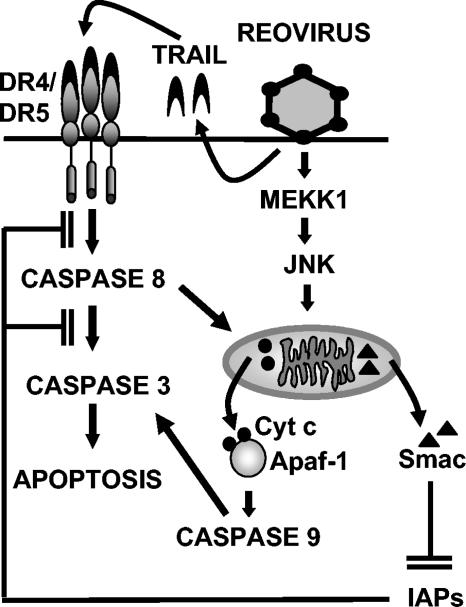
FIG. 6.
Model of apoptotic signaling pathways triggered by reovirus infection. Following reovirus infection, MEKK1 activates JNK, which promotes the release of the proapoptotic factors Smac and cytochrome c (Cyt c) from the mitochondria. Smac influences apoptosis by inhibiting cellular inhibitors of apoptosis (IAPs), whereas cytochrome c influences apoptosis by activating caspase 9 in conjunction with Apaf-1. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) receptor signaling is also induced following reovirus infection, and the reovirus-induced caspase 8-dependent cleavage of Bid also promotes the mitochondrial release of Smac and cytochrome c (20, 21).
It is possible that JNK regulation of the AP-1 transcription factor mediates, in part, the effects of JNK signaling pathways (9). JNK-mediated regulation of AP-1 has been reported to increase the expression of the BH3-only proteins Bim and HRK, which are critical for Bax-dependent cytochrome c release, caspase activation, and apoptosis in neurons (17, 30, 42), although in fibroblasts JNK does not increase Bim expression (25). To investigate the role of AP-1 in reovirus-induced apoptosis, we used adenovirus expressing DN c-Jun (TAM67), which prevents formation of a functional AP-1 transcription factor (28). Our studies indicate that expression of DN c-Jun does not block reovirus-induced apoptosis in fibroblasts. The expression of Bim also remains unaltered in reovirus-infected cells (data not shown). In addition, c-Jun phosphorylation does not correlate with reovirus-induced apoptosis. These results indicate that JNK-mediated activation of the c-Jun/AP-1 transcription factor is not required for reovirus-induced apoptosis and that other JNK-dependent pathways are involved.
We have previously demonstrated that caspase 8 activity is required for cleavage of the proapoptotic Bcl-2 family member, Bid, following reovirus infection (20). We now show that JNK activity is also required for the efficient release of the proapoptotic mitochondrial factors Smac and cytochrome c following reovirus infection. Since JNK activity in reovirus-infected cells does not appear to require death receptor signaling (5), caspase 8-dependent cleavage of Bid and the subsequent redistribution of Bcl-2 family proteins in the mitochondrial membrane provide a mechanism for Smac and cytochrome c release in the presence of JNKI1 (Fig. 6).
Apoptosis is a mechanism used by the host as part of the antiviral response, and deregulation of this host-pathogen interaction can alter the course of viral replication and explain aspects of viral disease. It is not surprising that many viruses have evolved to manipulate cellular apoptotic pathways. In many cases, inhibition of apoptosis by viruses serves to prevent premature death of the host cell. Inhibitors of apoptosis also may enhance virus production, enable efficient emergence from latency, facilitate persistent infection, and contribute to the avoidance of immune surveillance (8). Since mitochondrial apoptotic pathways are activated following infection with a wide variety of viruses, including human immunodeficiency virus (12, 18, 19), influenza A virus (2), herpes simplex virus 1 (44), human herpesvirus 8 (35), hepatitis B virus (38), and West Nile virus (29), and since many forms of virus-induced apoptosis are inhibited by antiapoptotic members of the Bcl-2 family (1, 31, 36, 37), a common method of viral inhibition of apoptosis is the expression of viral homologs of Bcl-2 (8) or the induction of antiapoptotic members of the Bcl-2 family (22).
In addition to contributing to the host's antiviral response, apoptosis can also influence viral pathogenesis and may facilitate rapid virus release and cell-to cell spread. Reovirus-induced apoptosis requires both death receptor and mitochondrial apoptotic pathways (6). Here we show that efficient activation of mitochondrial apoptotic pathways in reovirus-infected cells requires JNK. This is the first report linking JNK to the activation of mitochondrial apoptotic pathways in virus-infected cells. Since the proapoptotic Bcl-2 proteins Bax and Bak are essential for JNK-stimulated release of cytochrome c and apoptosis (26), further investigation of reovirus-induced mitochondrial apoptotic pathways may provide valuable insight into the pathogenesis of viruses that utilize Bcl-2 family proteins to promote apoptosis (22) or that are blocked by the overexpression of Bcl-2 (1, 31, 36, 37).
Acknowledgments
This work was supported by Public Health Service grant 1RO1AG14071 from the National Institutes of Health (K.L.T.), Merit and REAP grants from the Department of Veterans Affairs (K.L.T.), a U.S. Army Medical Research and Materiel Command grant, DAMD17-98-1-8614 (K.L.T.), the Reuler-Lewin Family Professorship of Neurology (K.L.T.), and the Ovarian Cancer Research Fund (P.C.).
Microscope assistance was provided by Ron Bouchard.
REFERENCES
- 1.Carthy, C. M., B. Yanagawa, H. Luo, D. J. Granville, D. Yang, P. Cheung, C. Cheung, M. Esfandiarei, C. M. Rudin, C. B. Thompson, D. W. Hunt, and B. M. McManus. 2003. Bcl-2 and Bcl-xL overexpression inhibits cytochrome c release, activation of multiple caspases, and virus release following coxsackievirus B3 infection. Virology 313:147-157. [DOI] [PubMed] [Google Scholar]
- 2.Chen, W., P. A. Calvo, D. Malide, J. Gibbs, U. Schubert, I. Bacik, S. Basta, R. O'Neill, J. Schickli, P. Palese, P. Henklein, J. R. Bennink, and J. W. Yewdell. 2001. A novel influenza A virus mitochondrial protein that induces cell death. Nat. Med. 7:1306-1312. [DOI] [PubMed] [Google Scholar]
- 3.Clarke, P., S. M. Meintzer, S. Gibson, C. Widmann, T. P. Garrington, G. L. Johnson, and K. L. Tyler. 2000. Reovirus-induced apoptosis is mediated by TRAIL. J. Virol. 74:8135-8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke, P., S. M. Meintzer, A. C. Spalding, G. L. Johnson, and K. L. Tyler. 2001. Caspase 8-dependent sensitization of cancer cells to TRAIL-induced apoptosis following reovirus-infection. Oncogene 20:6910-6919. [DOI] [PubMed] [Google Scholar]
- 5.Clarke, P., S. M. Meintzer, C. Widmann, G. L. Johnson, and K. L. Tyler. 2001. Reovirus infection activates JNK and the JNK-dependent transcription factor c-Jun. J. Virol. 75:11275-11283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke, P., and K. L. Tyler. 2003. Reovirus-induced apoptosis: a minireview. Apoptosis 8:141-150. [DOI] [PubMed] [Google Scholar]
- 7.Cobb, M. H., J. E. Hepler, M. Cheng, and D. Robbins. 1994. The mitogen-activated protein kinases, ERK1 and ERK2. Semin. Cancer Biol. 5:261-268. [PubMed] [Google Scholar]
- 8.Cuconati, A., and E. White. 2002. Viral homologs of BCL-2: role of apoptosis in the regulation of virus infection. Genes Dev. 16:2465-2478. [DOI] [PubMed] [Google Scholar]
- 9.Davis, R. J. 2000. Signal transduction by the JNK group of MAP kinases. Cell 103:239-252. [DOI] [PubMed] [Google Scholar]
- 10.DeBiasi, R. L., C. L. Edelstein, B. Sherry, and K. L. Tyler. 2001. Calpain inhibition protects against virus-induced apoptotic myocardial injury. J. Virol. 75:351-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derijard, B., M. Hibi, I. H. Wu, T. Barrett, B. Su, T. Deng, M. Karin, and R. J. Davis. 1994. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 76:1025-1037. [DOI] [PubMed] [Google Scholar]
- 12.Ferri, K. F., E. Jacotot, J. Blanco, J. A. Este, N. Zamzami, S. A. Susin, Z. Xie, G. Brothers, J. C. Reed, J. M. Penninger, and G. Kroemer. 2000. Apoptosis control in syncytia induced by the HIV type 1-envelope glycoprotein complex: role of mitochondria and caspases. J. Exp. Med. 192:1081-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson, S. B., R. Oyer, A. C. Spalding, S. M. Anderson, and G. L. Johnson. 2000. Increased expression of death receptors 4 and 5 synergizes the apoptosis response to combined treatment with etoposide and TRAIL. Mol. Cell. Biol. 20:205-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graves, J. D., J. S. Campbell, and E. G. Krebs. 1995. Protein serine/threonine kinases of the MAPK cascade. Ann. N. Y. Acad. Sci. 766:320-343. [DOI] [PubMed] [Google Scholar]
- 15.Gross, A., J. M. McDonnell, and S. J. Korsmeyer. 1999. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 13:1899-1911. [DOI] [PubMed] [Google Scholar]
- 16.Han, J., J. D. Lee, L. Bibbs, and R. J. Ulevitch. 1994. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265:808-811. [DOI] [PubMed] [Google Scholar]
- 17.Harris, C. A., and E. M. Johnson, Jr. 2001. BH3-only Bcl-2 family members are coordinately regulated by the JNK pathway and require Bax to induce apoptosis in neurons. J. Biol. Chem. 276:37754-37760. [DOI] [PubMed] [Google Scholar]
- 18.Jacotot, E., K. F. Ferri, C. El Hamel, C. Brenner, S. Druillennec, J. Hoebeke, P. Rustin, D. Metivier, C. Lenoir, M. Geuskens, H. L. Vieira, M. Loeffler, A. S. Belzacq, J. P. Briand, N. Zamzami, L. Edelman, Z. H. Xie, J. C. Reed, B. P. Roques, and G. Kroemer. 2001. Control of mitochondrial membrane permeabilization by adenine nucleotide translocator interacting with HIV-1 viral protein rR and Bcl-2. J. Exp. Med. 193:509-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacotot, E., L. Ravagnan, M. Loeffler, K. F. Ferri, H. L. Vieira, N. Zamzami, P. Costantini, S. Druillennec, J. Hoebeke, J. P. Briand, T. Irinopoulou, E. Daugas, S. A. Susin, D. Cointe, Z. H. Xie, J. C. Reed, B. P. Roques, and G. Kroemer. 2000. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J. Exp. Med. 191:33-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kominsky, D. J., R. J. Bickel, and K. L. Tyler. 2002. Reovirus-induced apoptosis requires both death receptor- and mitochondrial-mediated caspase-dependent pathways of cell death. Cell Death Differ. 9:926-933. [DOI] [PubMed] [Google Scholar]
- 21.Kominsky, D. J., R. J. Bickel, and K. L. Tyler. 2002. Reovirus-induced apoptosis requires mitochondrial release of Smac/DIABLO and involves reduction of cellular inhibitor of apoptosis protein levels. J. Virol. 76:11414-11424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotelkin, A., E. A. Prikhod'ko, J. I. Cohen, P. L. Collins, and A. Bukreyev. 2003. Respiratory syncytial virus infection sensitizes cells to apoptosis mediated by tumor necrosis factor-related apoptosis-inducing ligand. J. Virol. 77:9156-9172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kyriakis, J. M., P. Banerjee, E. Nikolakaki, T. Dai, E. A. Rubie, M. F. Ahmad, J. Avruch, and J. R. Woodgett. 1994. The stress-activated protein kinase subfamily of c-Jun kinases. Nature 369:156-160. [DOI] [PubMed] [Google Scholar]
- 24.Lee, J. C., J. T. Laydon, P. C. McDonnell, T. F. Gallagher, S. Kumar, D. Green, D. McNulty, M. J. Blumenthal, J. R. Heys, S. W. Landvatter, et al. 1994. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372:739-746. [DOI] [PubMed] [Google Scholar]
- 25.Lei, K., and R. J. Davis. 2003. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc. Natl. Acad. Sci. USA 100:2432-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lei, K., A. Nimnual, W.-X. Zong, N. J. Kennedy, R. A. Flavell, C. B. Thompson, D. Bar-Sagi, and R. J. Davis. 2002. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH2-terminal kinase. Mol. Cell. Biol. 22:4929-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oberhaus, S. M., R. L. Smith, G. H. Clayton, T. S. Dermody, and K. L. Tyler. 1997. Reovirus infection and tissue injury in the mouse central nervous system are associated with apoptosis. J. Virol. 71:2100-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park, J. S., L. Qiao, Z. Z. Su, D. Hinman, K. Willoughby, R. McKinstry, A. Yacoub, G. J. Duigou, C. S. Young, S. Grant, M. P. Hagan, E. Ellis, P. B. Fisher, and P. Dent. 2001. Ionizing radiation modulates vascular endothelial growth factor (VEGF) expression through multiple mitogen activated protein kinase dependent pathways. Oncogene 20:3266-3280. [DOI] [PubMed] [Google Scholar]
- 29.Parquet, M. C., A. Kumatori, F. Hasebe, K. Morita, and A. Igarashi. 2001. West Nile virus-induced bax-dependent apoptosis. FEBS Lett. 500:17-24. [DOI] [PubMed] [Google Scholar]
- 30.Putcha, G. V., K. L. Moulder, J. P. Golden, P. Bouillet, J. A. Adams, A. Strasser, and E. M. Johnson. 2001. Induction of BIM, a proapoptotic BH3-only BCL-2 family member, is critical for neuronal apoptosis. Neuron 29:615-628. [DOI] [PubMed] [Google Scholar]
- 31.Razvi, E. S., and R. M. Welsh. 1995. Apoptosis in viral infections. Adv. Virus Res. 45:1-60. [DOI] [PubMed] [Google Scholar]
- 32.Richardson-Burns, S. M., D. J. Kominsky, and K. L. Tyler. 2002. Reovirus-induced neuronal apoptosis is mediated by caspase 3 and is associated with the activation of death receptors. J. Neurovirol. 8:365-380. [DOI] [PubMed] [Google Scholar]
- 33.Rodgers, S. E., E. S. Barton, S. M. Oberhaus, B. Pike, C. A. Gibson, K. L. Tyler, and T. S. Dermody. 1997. Reovirus-induced apoptosis of MDCK cells is not linked to viral yield and is blocked by Bcl-2. J. Virol. 71:2540-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rouse, J., P. Cohen, S. Trigon, M. Morange, A. Alonso-Llamazares, D. Zamanillo, T. Hunt, and A. R. Nebreda. 1994. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell 78:1027-1037. [DOI] [PubMed] [Google Scholar]
- 35.Sharp, T. V., H. W. Wang, A. Koumi, D. Hollyman, Y. Endo, H. Ye, M. Q. Du, and C. Boshoff. 2002. K15 protein of Kaposi's sarcoma-associated herpesvirus is latently expressed and binds to HAX-1, a protein with antiapoptotic function. J. Virol. 76:802-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen, Y., and T. E. Shenk. 1995. Viruses and apoptosis. Curr. Opin. Genet. Dev. 5:105-111. [DOI] [PubMed] [Google Scholar]
- 37.Teodoro, J. G., and P. E. Branton. 1997. Regulation of apoptosis by viral gene products. J. Virol. 71:1739-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terradillos, O., A. de La Coste, T. Pollicino, C. Neuveut, D. Sitterlin, H. Lecoeur, M. L. Gougeon, A. Kahn, and M. A. Buendia. 2002. The hepatitis B virus X protein abrogates Bcl-2-mediated protection against Fas apoptosis in the liver. Oncogene 21:377-386. [DOI] [PubMed] [Google Scholar]
- 39.Tournier, C., P. Hess, D. D. Yang, J. Xu, T. K. Turner, A. Nimnual, D. Bar-Sagi, S. N. Jones, R. A. Flavell, and R. J. Davis. 2000. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science 288:870-874. [DOI] [PubMed] [Google Scholar]
- 40.Tyler, K. L., M. K. Squier, A. L. Brown, B. Pike, D. Willis, S. M. Oberhaus, T. S. Dermody, and J. J. Cohen. 1996. Linkage between reovirus-induced apoptosis and inhibition of cellular DNA synthesis: role of the S1 and M2 genes. J. Virol. 70:7984-7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tyler, K. L., M. K. Squier, S. E. Rodgers, B. E. Schneider, S. M. Oberhaus, T. A. Grdina, J. J. Cohen, and T. S. Dermody. 1995. Differences in the capacity of reovirus strains to induce apoptosis are determined by the viral attachment protein sigma 1. J. Virol. 69:6972-6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitfield, J., S. J. Neame, L. Paquet, O. Bernard, and J. Ham. 2001. Dominant-negative c-Jun promotes neuronal survival by reducing BIM expression and inhibiting mitochondrial cytochrome c release. Neuron 29:629-643. [DOI] [PubMed] [Google Scholar]
- 43.Yujiri, T., M. Ware, C. Widmann, R. Oyer, D. Russell, E. Chan, Y. Zaitsu, P. Clarke, K. Tyler, Y. Oka, G. R. Fanger, P. Henson, and G. L. Johnson. 2000. MEK kinase 1 gene disruption alters cell migration and c-Jun NH2-terminal kinase regulation but does not cause a measurable defect in NF-kappa B activation. Proc. Natl. Acad. Sci. USA 97:7272-7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou, G., and B. Roizman. 2000. Wild-type herpes simplex virus 1 blocks programmed cell death and release of cytochrome c but not the translocation of mitochondrial apoptosis-inducing factor to the nuclei of human embryonic lung fibroblasts. J. Virol. 74:9048-9053. [DOI] [PMC free article] [PubMed] [Google Scholar]