Abstract
The peptidyl-prolyl isomerase cyclophilin A (CypA) increases the kinetics by which human immunodeficiency virus type 1 (HIV-1) spreads in tissue culture. This was conclusively demonstrated by gene targeting in human CD4+ T cells, but the role of CypA in HIV-1 replication remains unknown. Though CypA binds to mature HIV-1 capsid protein (CA), it is also incorporated into nascent HIV-1 virions via interaction with the CA domain of the Gag polyprotein. These findings raised the possibility that CypA might act at multiple steps of the retroviral life cycle. Disruption of the CA-CypA interaction, either by the competitive inhibitor cyclosporine (CsA) or by mutation of CA residue G89 or P90, suggested that producer cell CypA was required for full virion infectivity. However, recent studies indicate that CypA within the target cell regulates HIV-1 infectivity by modulating Ref1- or Lv1-mediated restriction. To examine the relative contribution to HIV-1 replication of producer cell CypA and target cell CypA, we exploited multiple tools that disrupt the HIV-1 CA-CypA interaction. These tools included the drugs CsA, MeIle4-CsA, and Sanglifehrin; CA mutants exhibiting decreased affinity for CypA or altered CypA dependence; HeLa cells with CypA knockdown by RNA interference; and Jurkat T cells homozygous for a deletion of the gene encoding CypA. Our results clearly demonstrate that target cell CypA, and not producer cell CypA, is important for HIV-1 CA-mediated function. Inhibition of HIV-1 infectivity resulting from virion production in the presence of CsA occurs independently of the CA-CypA interaction or even of CypA.
Human immunodeficiency virus type 1 (HIV-1) gag encodes a precursor polyprotein (Pr55gag) that is necessary for virion particle formation and egress from the host cell (20, 25). Concurrent with virion release from the host cell, the precursor protein is cleaved by the viral protease to produce mature proteins, including the viral capsid protein (CA), which are required for infection of susceptible target cells.
With the use of a yeast two-hybrid screen, it was discovered that Pr55gag binds to cyclophilin A (CypA), a ubiquitously expressed protein that belongs to a large family of peptidyl-prolyl isomerases (37). The interaction was shown to be direct by using recombinant protein in vitro, and confirmed in vivo, as purified HIV-1 virions specifically incorporate CypA (24, 48). The CypA binding site mapped to the CA domain within Pr55gag (37). CA residues A88, G89, and P90, and residues in the hydrophobic pocket of CypA, were found to be crucial for interaction in vitro and in the context of virions (12, 13, 18, 21, 24, 37, 48, 55). X-ray and nuclear magnetic resonance analysis of CypA in complex with various Gag fragments confirmed that these residues participate in direct protein-protein interactions (27, 40, 47, 51, 57).
The importance of CypA for efficient spread of HIV-1 in tissue culture was demonstrated by disrupting the interaction between CypA and CA by using any of several approaches. These included replication assays with virus bearing CA mutations disruptive of CypA binding (13, 24, 48) and replication of HIV-1 in human T-cell lines with targeted disruption of the CypA gene (15). The CA-CypA interaction is disrupted by cyclosporine (CsA) (37), a competitive inhibitor that forms a high-affinity complex with CypA (30). CsA and related compounds also inhibit replication of HIV-1 but not of related viruses such as simian immunodeficiency virus MAC239 (SIVMAC239) (14, 23, 48) that do not bear a CypA binding site on CA (14, 24, 37, 48).
Examination of CypA-deficient virions failed to detect significant abnormalities in virion protein content, viral precursor protein processing kinetics, viral genomic RNA packaging, endogenous reverse transcriptase (RT) activity, or virion ultrastructure (13, 15, 24, 32, 48, 53). Nonetheless, disruption of CypA incorporation into virions by any of the approaches mentioned above decreased virion infectivity with a block detected early after infection, after membrane fusion at the stage of viral cDNA synthesis (13, 15, 48). These results were consistent with the hypothesis that CA-associated, intravirion CypA renders HIV-1 virions fully infectious.
Recent studies linked the HIV-1 CA-CypA interaction to the function of the antiviral restriction factors Lv1 and Ref1. Disruption of the CA-CypA interaction frees HIV-1 from Lv1 restriction in macaque and owl monkey cells (7, 33, 50) and renders HIV-1 susceptible to Ref1 restriction in human cells (43, 50). In contrast to what was reported with the previous studies, virion-associated CypA was not important for these effects, suggesting that only target cell CypA is relevant for the CA-specific effects on HIV-1 infectivity.
By perturbing single-cycle HIV-1 replication assays with several tools that disrupt the CA-CypA interaction, we examined the relative importance of producer cell CypA and target cell CypA for HIV-1 replication. We conclude that HIV-1 CA binding to target cell CypA is relevant only during viral entry and that disruption of the CA-CypA interaction during virion assembly does not alter virion titer. Virions produced in the presence of CsA and other cyclophilin binding drugs exhibit reduced infectivity due to mechanisms that are independent of CA and CypA.
MATERIALS AND METHODS
Plasmids.
pNL4-3 contains a full infectious provirus, HIV-1NL4-3 (3). pNL4-3/G89V carries a mutation in CA that disrupts the CypA binding site. It was created by replacing the BssHII-SpeI fragment in pNL4-3 with a fragment that contained the G89V mutation (50). p8.9NΔSB is a construct that expresses HIV-1 gag and pol from the cytomegalovirus immediate-early promoter and has a deletion in the psi region (7). NL4-3/A92E and NL4-3/G89V are p8.9NΔSB with A92E or G89V mutations in CA, respectively, as previously described (7). NL4-3/CA9 and NL4-3/MVP5180 are p8.9NΔSB in which the CypA binding region has been replaced with the CypA binding regions of HIV-1 group O isolate CA9 or MVP5180, respectively. These versions of p8.9ΔSB were generated by PCR with the following oligonucleotides: 5′-GCAGATTGGGATAGAACTCATCCACCAGCTGTGGGGCCGTTACCACCAGGGCAGATAAGGG-3′ and 5′-CCCTTATCTGCCCTGGTGGTAACGGCCCCACAGCTGGTGGATGAGTTCTATCCCAATCTGC-3′ for CA9 and 5′-GGGATAGAACTCATCCACCAGCCATGGGGCCGTTACCACCAGGGCAGATAAGGG-3′ and 5′-CCCTTATCTGCCCTGGTGGTAACGGCCCCATGGCTGGTGGATGAGTTCTATCCC-3′ for MVP5180. The introduced mutations were verified by sequencing. CSGW is an HIV-1 vector expressing enhanced green fluorescent protein (GFP) under the control of the spleen focus-forming virus long terminal repeat (9). The pMDG plasmid encodes a vesicular stomatitis virus G (VSV-G) protein (8). pCIG3 N and B are murine leukemia virus (MLV)-derived constructs expressing N- and B-tropic CA (8). pCL-Eco contains psi-minus Moloney MLV expressed from the cytomegalovirus immediate-early promoter (38).
To express short interfering RNA (siRNA) from a plasmid, we engineered pSUPER as described by others (17). pSUPER encodes a short hairpin RNA (shRNA) that is processed in the cell to generate siRNAs. The following oligonucleotides were used to generate a pSUPER plasmid for CypA protein knockdown: 5′-GATCCCCGGGTTCCTGCTTTCACAGATTCAAGAGATCTGTGAAAGCAGGAACCCTTTTTGGAAA-3′ and 5′-AGCTTTTCCAAAAAGGGTTCCTGCTTTCACAGATCTCTTGAATCTGTGAAAGCAGGAACCCGGG-3′. pMH-CypA147 is a virus-based shRNA vector that targets CypA expression. To generate it, the H1 promoter and the hairpin cassette were cut from pSUPER with EcoRI and SalI and subcloned into pMSCV-ΔU3 (6).
To restore CypA synthesis in HeLa CypA knockdown (KD) cells, a CypA cDNA bearing silent mutations that render it nontargetable by the CypA147 shRNA (44) was subcloned into CSGW with BamHI and NotI sites to generate CSW-ntCypA. An otherwise isogenic vector (CSW-ntCypA/R55A) carrying the R55A mutation in the hydrophobic pocket of CypA that abolishes interaction with HIV-1 CA (12) was also engineered.
Viruses.
HIV-1 viruses were produced by transfecting 106 293T or HeLa cells/well in six-well plates with 4 μg of pNL4-3 or pNL4-3/G89V with Lipofectamine 2000. HIV-1 vectors were produced the same way but using 2 μg of CSGW, 1.7 μg of wild-type or mutant p8.9ΔSB, and 0.3 μg of pMDG. To produce shRNA transducing vector targeting CypA, 293T cells were cotransfected with 1.7 μg of pCL-Eco, 2 μg of pMH-CypA147, and 0.3 μg of pMDG. To produce CypA-transducing vectors, 293T cells were cotransfected with 2 μg of CSW-ntCypA or CSW-ntCypA/R55A, 1.7 μg of p8.9ΔSB, and 0.3 μg of pMDG. For production of VLPs, 2.5 × 106 293T cells/60-mm-diameter plate were cotransfected with 7 μg of pCIG3 N or B and 1 μg of pMDG. The medium was changed after 6 h. Virion-containing supernatant was harvested 24, 48, and 72 h posttransfection. Supernatants from the three time points were pooled, centrifuged at 300 × g for 5 min to remove cell debris, and filtered through an 0.45-μm-pore-size filter (Pall Acrodisc). If drugs were used during virion production, the clarified supernatant was layered onto a 25% sucrose cushion and accelerated at 100,000 × g in an SW41 rotor. The pelleted virions were resuspended in RPMI-10% fetal bovine serum and stored at −80°C. Virions were normalized prior to infection by measuring RT activity as previously described (28).
Infection.
Adherent cells were plated in 24-well plates (4 × 104 cells/well) and infected with virus stocks. Cells in suspension were seeded in 24-well plates at 105 cells/well. Virion stocks were added to the cells in a final volume of 500 μl/well. For infections with full-length virus, dextran sulfate was added 16 h postinfection (100 μg/ml) to preclude spread of the virus. At 48 h postinfection, cells were fixed in 4% formaldehyde-phosphate-buffered saline and assayed for intracellular p24 by using KC57, an RD1-conjugated anti-p24 antibody (Coulter Immunology). GFP expression in infected cells was analyzed 48 h postinfection by flow cytometry.
To generate HeLa cells with stable expression from pMH-CypA147, cells were seeded into 24-well plates at 3 × 104 cells/well, covered with 500 μl of clarified 293T supernatant, and spinoculated for 70 min at 1,200 × g. Single-cell clones were screened by Western blotting for CypA expression.
To restore CypA expression in HeLa CypA KD cells, 3 × 104 cells/well in 24-well plates were infected four times with 250 μl of clarified 293T supernatant containing CSW-ntCypA or CSW-ntCypA/R55A transducing vector. CypA expression was confirmed by Western blotting.
Flow cytometry.
GFP fluorescence was recorded using the FL1 channel. For analysis of cells that were immunostained with the RD1-conjugated antibody, cells were analyzed using FL2. Flow cytometry was done using a FACSCalibur instrument and Cellquest software (Becton Dickinson). Ten thousand live cells were analyzed per sample.
Cells and drugs.
Adherent cells were maintained in Dulbecco modified Eagle medium, and suspension cells were maintained in RPMI, each supplemented with 10% fetal bovine serum and antibiotics. CsA (Bedford Laboratories) was prepared in dimethyl sulfoxide at 10 mg/ml and diluted in tissue culture medium for each experiment to 2.5 μM. MeIle4-CsA and Sanglifehrin (gifts from Novartis, Basel, Switzerland) were prepared in dimethyl sulfoxide at 10 mg/ml and 10 mM, respectively, and diluted further in tissue culture medium to 2.5 μM for each experiment. As2O3 (Sigma) was prepared as described previously (8) and diluted further in phosphate-buffered saline to the indicated concentrations prior to use. Dextran sulfate (5 mg/ml; Sigma) was prepared in H2O and was used at 100 μg/ml as described previously (8).
Western blotting.
HeLa clones with normal CypA expression after pMH-CypA147 transduction (control), HeLa CypA knockdown (CypA KD) cells, and HeLa CypA KD cells with restored wild-type or R55A mutant CypA expression were normalized by cell number, and lysates were subjected to Western blotting with antibodies to CypA (rabbit polyclonal anti-CypA; Biomol) and β-actin (horseradish peroxidase-conjugated polyclonal antiactin; Santa Cruz Biotechnology). Coomassie blue staining of the membranes confirmed that loading of samples had been properly normalized. Virions produced as described above were pelleted through a sucrose cushion, resuspended in 2× sodium dodecyl sulfate loading buffer, and subjected to Western blotting with antibodies to CypA and p24 (anti-p24 monoclonal 183-H12-5C; National Institutes of Health AIDS Research and Reference Reagent Program).
RESULTS
CsA administration at different times in the virus life cycle has additive inhibitory effects on HIV-1 infectivity.
CsA competes with HIV-1 CA for binding to CypA (37) and inhibits HIV-1 replication (14, 23, 48). Single-cycle replication assays were performed with full-length infectious HIV-1 to determine which step in the retrovirus life cycle is disrupted by CsA. Wild-type HIV-1NL4-3 virions were produced by transfection of 293T cells and used to infect human CD4+ T cells. As previously described, dextran sulfate was added to the infected cells 16 h postinfection to block virus spread and ensure only one round of infection and the percentage of infected cells was determined by flow cytometry after staining with anti-CA antibody (8).
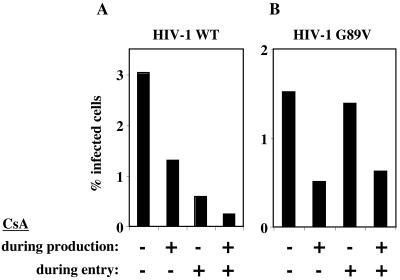
When 2.5 μM CsA was present during HIV-1 virion production, the infectivity of wild-type virions was reduced approximately twofold (Fig. 1A) and the CypA content of purified virions was reduced (Fig. 2). When Jurkat target cells were infected in the presence of CsA, a sixfold decrease in infectivity was observed (Fig. 1A). If CsA was present during both assembly and entry, there was a 12-fold reduction in titer (Fig. 1A), indicating that the effects of the drug during production and entry were additive.
FIG. 1.
Inhibition of HIV-1 infectivity by CsA is CA dependent if drug is administered to target cells during virus entry but independent of the CA-CypA interaction if drug is present during virion production. HIV-1NL4-3 virions, either wild type (A) or CA mutant G89V (B), were produced by transfection of 293T cells. The CA/G89V mutation abolishes interaction with CypA. Virions were purified by filtration and centrifugation through a 25% sucrose cushion, normalized by RT activity, and used to infect Jurkat cells. The percentage of cells infected was determined by flow cytometry after immunostaining for CA. CsA (2.5 μM) was added either during virion production or during infection, or both, as indicated. Shown are representative results of a single experiment. Identical results were obtained on three separate occasions with independently produced viral stocks of various multiplicities of infection.
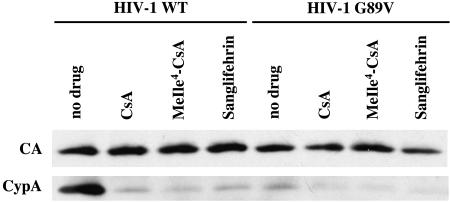
FIG. 2.
Disruption of CypA incorporation into virions by competitive inhibitors of CypA-CA interaction. HIV-1NL4-3 virions, either wild type or G89V mutant, were produced and purified as for Fig. 1. Where indicated, 2.5 μM CsA, MeIle4-CsA, or Sanglifehrin was added during virion production. Purified virions were subjected to Western blotting with antibodies to CypA and CA.
Inhibition of infectious HIV-1 virion production by CsA is independent of the CA-CypA interaction.
Interaction with CypA is disrupted by mutations in the proline-rich loop connecting HIV-1 CA helices IV and V, in particular CA mutant G89V (55). NL4-3/G89V mutant virions were produced by transfection of 293T cells and used to infect Jurkat cells. CypA incorporation into NL4-3/G89V virions was significantly decreased (Fig. 2). It was therefore expected that treatment of target cells with CsA would not decrease the titer of this virus. Indeed, CsA addition to target cells during infection with NL4-3/G89V virus did not decrease viral infectivity (Fig. 1B). However, the infectivity of NL4-3/G89V virus produced in the presence of CsA was reduced to the same extent as with the wild-type virus (compare Fig. 1A and B). If both producer and target cells were treated with CsA, the reduction in NL4-3/G89V infectivity was identical to drug-free infection with NL4-3/G89V produced in the presence of drug (Fig. 1B). These results indicate that CsA inhibition of infectious virion production does not involve the CA-CypA interaction.
MeIle4-CsA and Sanglifehrin inhibit infectious HIV-1 virion production via a CA-independent mechanism.
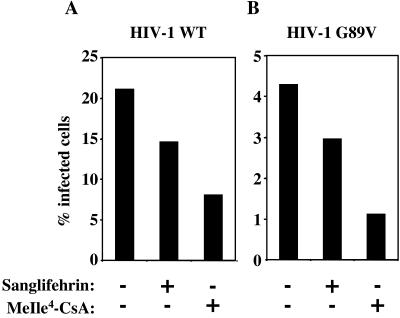
The experiments above indicate that the CA-CypA interaction is uniquely important during virion entry. To clarify the mechanism by which CsA inhibits the production of infectious virions, we examined the effect of two additional drugs. As a complex with CypA, CsA binds and inhibits the calcium-dependent phosphatase calcineurin (26, 35). MeIle4-CsA is an analogue of CsA that binds CypA as tightly as the parent compound does but is unable to form a complex with calcineurin (23, 48). Sanglifehrin is structurally unrelated to CsA, but it also binds to cyclophilin (22, 42) and might be expected to disrupt the CypA-CA interaction. In fact, both MeIle4-CsA and Sanglifehrin blocked the incorporation of CypA into nascent virion particles (Fig. 2). MeIle4-CsA or Sanglifehrin treatment of producer cells reduced infectivity of wild-type virions by about 2.5- or 1.5-fold, respectively, compared to virions produced with no drug (Fig. 3A). The effect of the two drugs on the titer of nascent NL4-3/G89V virions was identical to the effect of the two drugs on the wild-type virus (Fig. 3B), confirming that the mechanism of inhibition was independent of the CA-CypA interaction.
FIG. 3.
MeIle4-CsA or Sanglifehrin inhibits infectious HIV-1 virion production independently of the CA-CypA interaction. Wild-type (A) or CA/G89V (B) virions were produced and assayed for infectivity as for Fig. 1. Where indicated, 2.5 μM MeIle4-CsA or Sanglifehrin was added during virion production.
Producer cell CypA is not required for production of fully infectious HIV-1 virions.
To determine if producer cell CypA is necessary to render HIV-1 virions fully infectious, siRNA was used to downregulate CypA expression. HeLa cells were transduced with a retrovirus expressing an shRNA (17) specific for CypA cDNA. Alignment of the sequence against the human genome (http://www.ncbi.nlm.nih.gov/genome/seq/HsBlast.html) revealed no homologous sequences other than CypA. The 15 known human cyclophilin family members (15) each possess at least five nucleotide mismatches with respect to the shRNA (data not shown). Single-cell clones of the transduced HeLa cells were screened by Western blotting for CypA expression (Fig. 4A), and clones with wild-type levels of expression (control) or undetectable expression (CypA KD) were selected for further analysis.
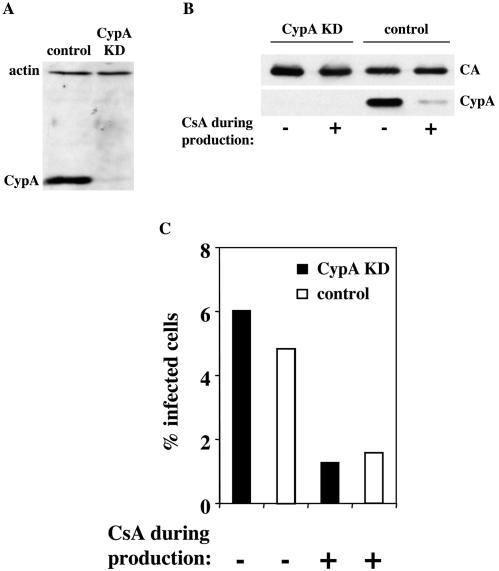
FIG. 4.
Producer cell CypA has no effect on HIV-1 virion infectivity. HeLa cells were cloned after transduction with an MLV-based vector delivering an shRNA expression construct specific for CypA mRNA. After normalization by cell number and screening by immunoblotting for CypA and β-actin expression, clones with knockdown of CypA protein (CypA KD) or with the original level of CypA protein (control) were selected for further analysis (A). Virions were produced by transfection of CypA KD or control HeLa cells with pNL4-3 in the presence of 2.5 μM CsA, as indicated (B). Virions were purified as for Fig. 1, normalized by RT activity, probed in a Western blot assay with antibodies to CypA and CA (B), and used to infect Jurkat cells in drug-free medium (C); the percentage of Jurkat cells infected was determined by flow cytometry after immunostaining for CA (C).
The effect of producer cell CypA on HIV-1 virion infectivity was assessed by transfecting CypA KD and control HeLa cells with pNL4-3 to produce HIV-1 virions. Both transfected CypA KD and control virion producer cells were treated with CsA to determine if the inhibitory effect of the drug was dependent upon the presence of CypA. Virions were purified from the culture supernatant, and Western blotting confirmed the absence of CypA in association with virions produced by the CypA KD cells or from the control cells treated with CsA (Fig. 4B). Virions produced by CypA KD cells were as infectious in Jurkat target cells as were virions produced by control HeLa cells (Fig. 4C). Additionally, the reduction in virion infectivity due to CsA was of equal magnitude when the drug was added to CypA KD and when it was added to control cells during virus production (Fig. 4C). These results indicate that producer cell CypA plays no role in HIV-1 virion infectivity and that the inhibitory effects of CsA during virion production are CypA independent.
The target cell determines cell-line-specific effects of CsA on the replication of HIV-1 CA variants.
Though spreading infection of most HIV-1 isolates is inhibited by CsA, some group O isolates, HIV-1MVP5180 and HIV-1CA9, are CsA resistant in Jurkat cells or peripheral blood mononuclear cells (14). CA mutant A92E confers CsA resistance (or CypA independence) to spreading HIV-1 infection in Jurkat cells but CsA dependence (or paradoxical sensitivity to CypA) in HeLa or H9 cells (1, 11, 15, 54). Since these cell-type-specific phenotypes are determined by CypA, we next assessed the relative contributions of target cell CypA and producer cell CypA to the phenotypes of these CA variants.
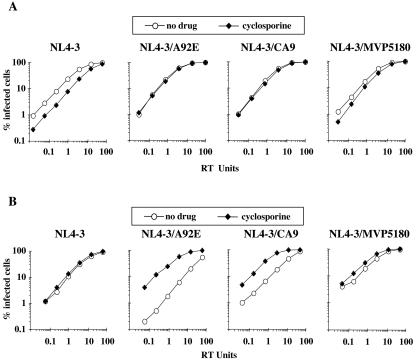
HIV-1 vectors were engineered that encode either the CA/A92E mutant or NL4-3 chimeric CAs bearing the CypA binding regions of the group O isolates HIV-1MVP5180 and HIV-1CA9 (Fig. 5). VSV-G-pseudotyped virions produced by transfection of 293T cells with the engineered HIV-1 CA variants, as well as the wild type, were used to infect Jurkat cells. When CsA was present during the infection, the titer of the wild-type vector was reduced fivefold (Fig. 6A). The A92E mutant, the NL4-3/CA9 chimera, and the NL4-3/MVP5180 chimera each exhibited relative resistance to CsA (Fig. 6A). Identical results were obtained if, rather than Jurkat cells, 293T cells were used as target cells (data not shown).
FIG. 5.
Amino acid sequence alignment of the CypA binding regions from wild-type HIV-1NL4-3, the A92E mutant, the NL4-3/CA9 chimera, and the NL4-3/MVP5180 chimera. Residues constituting the CypA binding loop and alpha-helices 4 and 5 are indicated. Dashed lines indicate residues identical to HIV-1NL4-3. Residues that differ from HIV-1NL4-3 are indicated with lowercase letters. Note that in all three variants the A92 residue is altered.
FIG. 6.
Cell-type-specific effects of CsA on HIV-1 replication are determined by the target cell. VSV-G-pseudotyped HIV-1-GFP vectors, either wild type, CA mutant A92E, CA chimera NL4-3/CA9, or CA chimera NL4-3/MVP5180, were produced by transfection of 293T cells, normalized by RT activity, and used to infect Jurkat cells (A) or HeLa cells (B). Where indicated, 2.5 μM CsA was added to the medium during infection.
When the same virus stocks produced in 293T cells were used to infect HeLa cells, very different results were obtained. The A92E mutant and the CA chimeras showed reduced titers compared to the wild type (Fig. 6B). CsA administration during infection of the HeLa cells had no effect on the titer of the wild type, but the titers of the CA variants were stimulated by as much as 20-fold (Fig. 6B).
All vectors used in Fig. 6 were produced by transfection of 293T cells. Identical results were obtained if the vectors were produced in HeLa cells, no matter which target cell was used (data not shown). Also, addition of CsA to the producer cells had no effect on the phenotype of these viruses (data not shown). Taken together, these results indicate that these CypA-dependent, CA-specific, and cell-type-specific phenotypes are completely determined by the target cell, not by the producer cell.
CsA phenotypes of HIV-1 CA variants result from effects of the drug on target cell CypA.
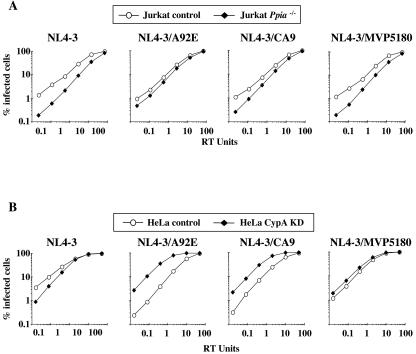
The human genome encodes at least 15 human cyclophilins, each of which is a potential CsA ligand (15). We therefore tested the hypothesis that the CsA resistance in Jurkat cells, or CsA dependence in HeLa cells, exhibited by the CA variants, is due to effects of the drug on CypA, as opposed to effects on other cyclophilin family members. VSV-G-pseudotyped HIV-1 vectors were used to infect Ppia−/− Jurkat cells (15) or CypA KD HeLa cells (Fig. 4A). In Jurkat cells, the effect of CypA gene disruption mirrored the effect of CsA in wild-type Jurkat cells. In particular, replication of A92E and NL4-3/CA9 was relatively resistant to the CypA-deficient condition (Fig. 7A). Similarly, the effect of CypA KD in HeLa cells mirrored the effect of CsA in control HeLa cells: replication of the CA variants was stimulated by the knockdown of CypA expression (Fig. 7B), as it was by CsA (Fig. 6B). Consistent with this observation, CsA did not stimulate A92E infectivity in the CypA KD HeLa cells (Fig. 8).
FIG. 7.
Target cell CypA regulates the cell-type-specific effects on HIV-1 replication. HIV-1-GFP transducing vectors, either wild type, CA mutant A92E, CA chimera NL4-3/CA9, or CA chimera NL4-3/MVP5180, were produced by transfection of 293T cells, normalized by RT activity, and used to infect Jurkat cells (A) or HeLa cells (B). The Jurkat cells were wild type (control) or homozygous for a deletion of the gene encoding CypA (Ppia−/−), as indicated (A). HeLa cells with wild-type levels of CypA (control) or with knocked-down CypA expression (CypA KD) were used, as indicated (B).
FIG. 8.
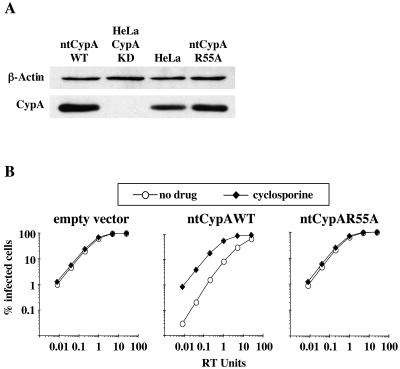
CypA binding to HIV-1 CA is required for HeLa cell resistance to HIV-1 A92E. HeLa CypA KD cells were retrovirally transduced with CypA cDNAs bearing silent mutations (ntCypA) that confer resistance to the RNAi in these cells. (A) Lysates from CypA KD cells transduced with empty vector, ntCypA wild type, or ntCypA bearing the active-site mutant R55A, were probed in a Western blot assay with anti-CypA or antiactin antibodies. Lysate from wild-type HeLa is shown as a control. (B) The transduced cell populations in panel A were challenged with VSV-G-pseudotyped HIV-1-GFP vector bearing CA mutant A92E. Where indicated, 2.5 μM CsA was added to the medium during infection.
To demonstrate that the effect of RNAi transduction on A92E replication in CypA KD HeLa cells was indeed due to disruption of CypA protein, CypA protein was restored by transduction of nontargetable CypA cDNA (ntCypA) bearing silent mutations that render it resistant to the interfering RNA (RNAi) construct used here. When CypA protein was restored (Fig. 8A), A92E infection was again inhibited (Fig. 8B). In addition, the replication efficiency of A92E was rescued by CsA (Fig. 8B). Transduction of ntCypA cDNA encoding the active-site mutant R55A, which does not bind HIV-1 CA (12), failed to restore the A92E phenotype (Fig. 8). These studies indicate that the cell-type-dependent effects of CsA are dependent upon the CA-CypA interaction.
The CsA dependence of HIV-1 CA variants in HeLa cells is not explained by Ref1-mediated restriction.
Recent evidence suggests that CypA binding to wild-type HIV-1 CA protects the virus from Ref1 (43, 50), an antiviral activity that also restricts N-tropic MLV (49). Paradoxically, CsA protects HIV-1 from a similarly acting antiviral activity in nonhuman primate cells called Lv1 (50), which now appears to be due to TRIM5-alpha (46). We therefore tested the hypothesis that CsA dependence of CA/A92E might result from altered Ref1 activity in HeLa cells.
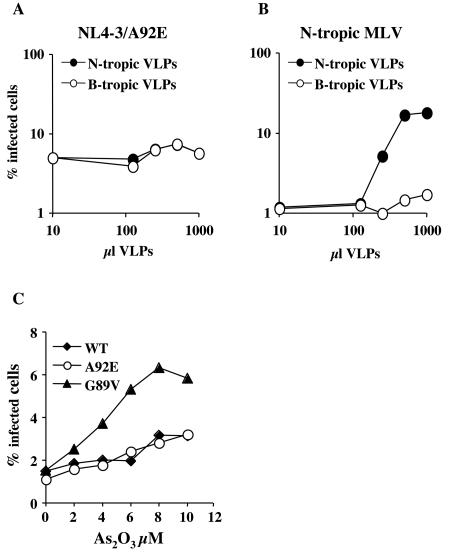
Both Lv1 and Ref1 can be saturated by loading cells with VLPs, as long as the VLPs bear CAs from susceptible viruses. Specifically, HIV-1 CA/G89V VLPs will boost the titer of N-tropic MLV vectors, (50). Wild-type HIV-1 is not susceptible to Ref1, and wild-type HIV-1 VLPs have no effect on the titer of N-tropic MLV (50). Despite multiple attempts, we were unable to detect any effect of HIV-1 CA/A92E VLPs on the titer of HIV-1 CA/A92E (data not shown). Similarly, neither B- nor N-tropic VLPs were able to increase the titer of the NL4-3/A92E mutant (Fig. 9A), despite the fact that N-tropic MLV titer was increased significantly by titrating N-tropic VLPs (Fig. 9B).
FIG. 9.
Ref1 activity does not explain the phenotype of the CA/A92E mutant in HeLa cells. (A and B) HeLa cells were transduced by VSV-G-pseudotyped HIV-1-GFP vector bearing the CA/A92E mutation (A) or by VSV-G-pseudotyped N-tropic MLV-GFP vector (B), in the presence of the indicated amounts of N-tropic or B-tropic MLV VLPs. The percentage of GFP-positive (infected) cells, as determined by flow cytometry, is plotted as a function of the VLP dose. Shown are results from a representative experiment. Identical results were obtained on three occasions with a wide range of multiplicities of infection. (C) VSV-G-pseudotyped HIV-1-GFP vectors, wild type or bearing either CA mutation, A92E or G89V, were used to infect HeLa cells in the presence of As2O3. The percentage of GFP-positive cells is plotted as a function of As2O3 concentration.
Ref1 activity targeting N-tropic MLV can also be overcome by As2O3 (8). To test if the CsA dependence of NL4-3/A92E reflects sensitivity to Ref1, HeLa cells were infected with HIV-1 vectors in the presence of As2O3 over a range of drug concentrations. Infectivity of G89V, a mutant which is targeted by Ref1 (43, 50), was increased by fourfold (Fig. 9B). In contrast, the drug had no effect on either NL4-3/A92E or wild-type NL4-3 (Fig. 9B), indicating that CA/A92E does not render HIV-1 sensitive to Ref1.
DISCUSSION
Though CypA binds mature CA, interaction was first discovered with the Gag polyprotein (37), and the findings of most subsequent studies were consistent with the hypothesis that producer cell CypA is important for HIV-1 replication. CypA binds the Gag polyprotein more strongly than it binds mature CA (16, 19). CypA is incorporated into HIV-1 virions or Gag particles with a fixed stoichiometry, and disruption of CypA incorporation into virions by gag mutants correlated with decreased infectivity (24, 39, 48). The hypothesis seemed especially secure when it was found that, like the gag mutants, competitive inhibitors that block CypA incorporation also inhibited virion infectivity (14, 23, 48). These drugs had no effect on the infectivity of related viruses such as HIV-2 and SIVMAC239 (14, 23, 48), viruses that do not incorporate CypA into particles.
The experiments presented here render the original hypothesis unlikely and show that target cell CypA interaction with HIV-1 CA is important for viral infectivity. We were, in fact, unable to detect any contribution to the infectivity of HIV-1 virions by producer cell CypA. There are perhaps several technical reasons why this result was not apparent before. The simultaneous analysis of CypA function with the large number of experimental tools that have accumulated over the years, including CA mutants with altered CypA affinity or CypA dependence, three different competitive inhibitors of the CA-CypA interaction, and cell lines in which CypA expression was disrupted by two different genetic methods, permitted us to detect effects that were not apparent previously. Jurkat cells engineered to be CypA deficient by gene targeting were valuable to prove the importance of CypA for spreading infection (15), but these cells are difficult to transfect or otherwise manipulate to produce virus for single-cycle assays. The recent development of RNAi in mammalian cells permitted us to disrupt CypA expression in transfectable cell lines and to more readily assess the role of CypA. Finally, more sensitive and accurate assays for quantitating HIV-1 infectivity in single-cycle assays are now available. This last point is critical since the effects of CypA on HIV-1 replication in human cells are modest in magnitude and dependent on the multiplicity of infection (50).
These new conclusions are consistent with most reports in the literature. Aside from minimal effects on the kinetics of gag processing or virion release (45, 53), disruption of CypA incorporation into virions has no effect on biochemical or ultrastructural characteristics of HIV-1 virions, including endogenous reverse transcription (13, 15, 24, 32, 48, 53). We had proposed that virion-associated CypA might promote virion disassembly (36), but the stability of virion cores is not detectably altered by CypA disruption (53), and structural models place CypA on the outside of the core (29, 34), where it would seem unlikely to disrupt CA-CA interactions.
The results reported here are also in agreement with recent experiments from our lab, and others, indicating that target cell CypA modulates HIV-1 sensitivity to CA-specific restriction factors (33, 50). The mechanism by which target cell CypA modulates HIV-1 restriction remains to be determined. CypA catalyzes the rate of cis-trans interconversion of the peptide bond connecting HIV-1 CA residues G89 and P90 (10): perhaps HIV-1 susceptibility to restriction factors is conformation dependent. Alternatively, as a component of a putative restriction factor complex, CypA might determine if incoming virus is recognized or somehow regulate the antiviral activity of the complex itself.
We also provided evidence that CsA inhibits infectious HIV-1 virion production and entry via independent mechanisms. The magnitude of inhibition was greater if CsA was administered during entry than if it was administered during production, and the effects were additive if the drug was present at both times (Fig. 1). Consistent with the fact that the G89V mutation already abolishes CypA binding to CA, infectivity of the HIV-1NL4-3 G89V mutant was not decreased further if CsA was added during infection of target cells. However, CsA added during virion production decreased infectivity of the HIV-1NL4-3 G89V mutant to the same degree as for the wild-type virus. Others have reported similar findings (4). These results indicate that the inhibitory effect of CsA during virion production is independent of the CA interaction with cyclophilin. An additional indication that CsA has CA-independent effects was provided by the finding that VSV-G pseudotyping suppresses the effects of CsA on virion production but not the effects of CA mutants (5). Here these findings were extended further by showing that the inhibitory effect of CsA was of equal magnitude when virions were produced from cells with CypA knockdown by RNAi (Fig. 4). Thus, the inhibitory effect of CsA on virion assembly is independent of CypA.
MeIle4-CsA had the same effect as CsA did (Fig. 3), indicating that inhibition of calcineurin phosphatase activity (41) is not required for the antiviral effect. Sanglifehrin also has the same effect (Fig. 3). The only property that Sanglifehrin shares with the other drugs is the ability to bind to cyclophilin family members (42, 56). This suggests, then, that inhibition by CsA involves targeting of one of the cyclophilin family members. Our knockdown data rule out CypA (Fig. 4), but there are 14 more cyclophilins to test (15). Consistent with the CypA independence of the drug effect, there was no correlation between the ability of the drugs to disrupt CypA binding to CA and their effectiveness at inhibiting virion infectivity. For example, Sanglifehrin was a less potent inhibitor of infectivity, but all three drugs eliminated CypA incorporation into virions with comparable efficiency (Fig. 2).
How does CsA disrupt the production of infectious virions? Suppression of the drug effect by VSV-G pseudotyping (5) suggests that CsA might block Env function. VSV-G pseudotyping also eliminates the requirement for Nef (4), and CsA has minimal effect on nef mutant virus (4), suggesting that CsA inhibits Nef function, though not all investigators have reported the same result (31). Interestingly, SIV Nef or HIV-2 Nef suppresses the inhibitory effect of CsA on HIV-1 virions (31). Further work will be required to determine which cyclophilin family member and which viral protein are targeted by CsA during virion production.
Previous experiments suggested that the CsA dependence of the A92E mutant in H9 cells results from elevated levels of CypA in H9 cells, compared to Jurkat cells (2, 54). This hypothesis seems not to hold up in that CsA dependence of A92E is more pronounced in HeLa than in H9 cells (data not shown), yet the levels of CypA expression in HeLa cells are not significantly elevated compared to those in 293T cells, a cell line which exhibits the same phenotype as Jurkat cells (data not shown).
The phenotype of the A92E mutant in HeLa cells resembles a phenomenon termed Lv1 restriction. In nonhuman primate cells, HIV-1 replication is inhibited at an early postentry step of infection and can be rescued by CsA (50). In human cells, a similar activity called Ref1 specifically restricts N-tropic MLV and HIV-1 if the CypA-CA interaction is eliminated during infection by genetic or pharmacological means (43, 50). In both cases, target cell CypA plays a role during infection: its interaction with HIV-1 CA protects the virus from Ref1-mediated restriction in human cells and renders it sensitive to Lv1-mediated restriction in monkey cells.
We used two different approaches, saturation by N-tropic VLPs and suppression by As2O3, to address the question whether the CsA-dependent phenotype of HIV-1 CA variants in HeLa cells results from altered Ref1 activity in these cells. Both experiments indicated that Ref1 is not restricting NL4-3/A92E (Fig. 9). Additionally, we attempted to saturate the putative restriction factor by using HIV-1 VLPs bearing the A92E mutation. Evidence of a saturable restriction factor was not obtained (data not shown).
The group O viruses HIV-1CA9 and HIV-1MVP5180 are resistant to CsA in Jurkat cells or peripheral blood mononuclear cells (14, 52). Based on the similarity in phenotype with the A92E mutant, we hypothesized that the group O viruses would behave similarly in single-cycle assays. Vectors bearing the CypA binding loop from these viruses (Fig. 5) indeed exhibited the same cell-type-specific response to CsA (Fig. 6 and 7). Thus, viruses bearing CAs that confer this phenotype occur in infected patients. Also, the dramatically different phenotypes of different human cell lines raise the possibility that such variations might occur among people and might render individuals differentially susceptible to HIV-1 infection.
Acknowledgments
We thank Young-Nam Lee and Alexander Lesokhin for technical assistance.
This work was supported by National Institutes of Health grant RO1AI36199 to J.L. and made use of core facilities provided by the Columbia Rockefeller Center for AIDS Research.
REFERENCES
- 1.Aberham, C., S. Weber, and W. Phares. 1996. Spontaneous mutations in the human immunodeficiency virus type 1 gag gene that affect viral replication in the presence of cyclosporins. J. Virol. 70:3536-3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackerson, B., O. Rey, J. Canon, and P. Krogstad. 1998. Cells with high cyclophilin A content support replication of human immunodeficiency virus type 1 Gag mutants with decreased ability to incorporate cyclophilin A. J. Virol. 72:303-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aiken, C. 1998. Mechanistic independence of Nef and cyclophilin A enhancement of human immunodeficiency virus type 1 infectivity. Virology 248:139-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aiken, C. 1997. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 71:5871-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asmal, M., J. Colgan, F. Naef, B. Yu, Y. Lee, M. Magnasco, and J. Luban. 2003. Production of ribosome components in effector CD4+ T cells is accelerated by TCR stimulation and coordinated by ERK-MAPK. Immunity 19:535-548. [DOI] [PubMed] [Google Scholar]
- 7.Berthoux, L., S. Sebastian, E. Sokolskaja, and J. Luban. 2004. Lv1 inhibition of human immunodeficiency virus type 1 is counteracted by factors that stimulate synthesis or nuclear translocation of viral cDNA. J. Virol. 78:11739-11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berthoux, L., G. J. Towers, C. Gurer, P. Salomoni, P. P. Pandolfi, and J. Luban. 2003. As2O3 enhances retroviral reverse transcription and counteracts Ref1 antiviral activity. J. Virol. 77:3167-3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Besnier, C., Y. Takeuchi, and G. Towers. 2002. Restriction of lentivirus in monkeys. Proc. Natl. Acad. Sci. USA 99:11920-11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosco, D. A., E. Z. Eisenmesser, S. Pochapsky, W. I. Sundquist, and D. Kern. 2002. Catalysis of cis/trans isomerization in native HIV-1 capsid by human cyclophilin A. Proc. Natl. Acad. Sci. USA 99:5247-5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braaten, D., C. Aberham, E. K. Franke, L. Yin, W. Phares, and J. Luban. 1996. Cyclosporine A-resistant human immunodeficiency virus type 1 mutants demonstrate that Gag encodes the functional target of cyclophilin A. J. Virol. 70:5170-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braaten, D., H. Ansari, and J. Luban. 1997. The hydrophobic pocket of cyclophilin is the binding site for the human immunodeficiency virus type 1 Gag polyprotein. J. Virol. 71:2107-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braaten, D., E. K. Franke, and J. Luban. 1996. Cyclophilin A is required for an early step in the life cycle of human immunodeficiency virus type 1 before the initiation of reverse transcription. J. Virol. 70:3551-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braaten, D., E. K. Franke, and J. Luban. 1996. Cyclophilin A is required for the replication of group M human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus SIVCPZGAB but not group O HIV-1 or other primate immunodeficiency viruses. J. Virol. 70:4220-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braaten, D., and J. Luban. 2001. Cyclophilin A regulates HIV-1 infectivity, as demonstrated by gene targeting in human T cells. EMBO J. 20:1300-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bristow, R., J. Byrne, J. Squirell, H. Trencher, T. Carter, B. Rodgers, E. Saman, and J. Duncan. 1999. Human cyclophilin has a significantly higher affinity for HIV-1 recombinant p55 than p24. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 20:334-336. [DOI] [PubMed] [Google Scholar]
- 17.Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550-553. [DOI] [PubMed] [Google Scholar]
- 18.Bukovsky, A. A., A. Weimann, M. A. Accola, and H. G. Gottlinger. 1997. Transfer of the HIV-1 cyclophilin-binding site to simian immunodeficiency virus from Macaca mulatta can confer both cyclosporin sensitivity and cyclosporin dependence. Proc. Natl. Acad. Sci. USA 94:10943-10948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colgan, J., H. E. Yuan, E. K. Franke, and J. Luban. 1996. Binding of the human immunodeficiency virus type 1 Gag polyprotein to cyclophilin A is mediated by the central region of capsid and requires Gag dimerization. J. Virol. 70:4299-4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Craven, R. C., R. P. Bennett, and J. W. Wills. 1991. Role of the avian retroviral protease in the activation of reverse transcriptase during virion assembly. J. Virol. 65:6205-6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorfman, T., A. Weimann, A. Borsetti, C. T. Walsh, and H. G. Gottlinger. 1997. Active-site residues of cyclophilin A are crucial for its incorporation into human immunodeficiency virus type 1 virions. J. Virol. 71:7110-7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fehr, T., J. Kallen, L. Oberer, J. J. Sanglier, and W. Schilling. 1999. Sanglifehrins A, B, C and D, novel cyclophilin-binding compounds isolated from Streptomyces sp. A92-308110. II. Structure elucidation, stereochemistry and physico-chemical properties. J. Antibiot. (Tokyo) 52:474-479. [DOI] [PubMed] [Google Scholar]
- 23.Franke, E. K., and J. Luban. 1996. Inhibition of HIV-1 replication by cyclosporine A or related compounds correlates with the ability to disrupt the Gag-cyclophilin A interaction. Virology 222:279-282. [DOI] [PubMed] [Google Scholar]
- 24.Franke, E. K., H. E. Yuan, and J. Luban. 1994. Specific incorporation of cyclophilin A into HIV-1 virions. Nature 372:359-362. [DOI] [PubMed] [Google Scholar]
- 25.Freed, E. O. 1998. HIV-1 gag proteins: diverse functions in the virus life cycle. Virology 251:1-15. [DOI] [PubMed] [Google Scholar]
- 26.Friedman, J., and I. Weissman. 1991. Two cytoplasmic candidates for immunophilin action are revealed by affinity for a new cyclophilin: one in the presence and one in the absence of CsA. Cell 66:799-806. [DOI] [PubMed] [Google Scholar]
- 27.Gamble, T. R., F. F. Vajdos, S. Yoo, D. K. Worthylake, M. Houseweart, W. I. Sundquist, and C. P. Hill. 1996. Crystal structure of human cyclophilin A bound to the amino-terminal domain of HIV-1 capsid. Cell 87:1285-1294. [DOI] [PubMed] [Google Scholar]
- 28.Goff, S., P. Traktman, and D. Baltimore. 1981. Isolation and properties of Moloney murine leukemia virus mutants: use of a rapid assay for release of virion reverse transcriptase. J. Virol. 38:239-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grattinger, M., H. Hohenberg, D. Thomas, T. Wilk, B. Muller, and H. G. Krausslich. 1999. In vitro assembly properties of wild-type and cyclophilin-binding defective human immunodeficiency virus capsid proteins in the presence and absence of cyclophilin A. Virology 257:247-260. [DOI] [PubMed] [Google Scholar]
- 30.Handschumacher, R. E., M. W. Harding, J. Rice, R. J. Drugge, and D. W. Speicher. 1984. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science 226:544-547. [DOI] [PubMed] [Google Scholar]
- 31.Khan, M., L. Jin, M. B. Huang, L. Miles, V. C. Bond, and M. D. Powell. 2004. Chimeric human immunodeficiency virus type 1 (HIV-1) virions containing HIV-2 or simian immunodeficiency virus Nef are resistant to cyclosporine treatment. J. Virol. 78:1843-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kong, L. B., D. An, B. Ackerson, J. Canon, O. Rey, I. S. Chen, P. Krogstad, and P. L. Stewart. 1998. Cryoelectron microscopic examination of human immunodeficiency virus type 1 virions with mutations in the cyclophilin A binding loop. J. Virol. 72:4403-4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kootstra, N. A., C. Munk, N. Tonnu, N. R. Landau, and I. M. Verma. 2003. Abrogation of postentry restriction of HIV-1-based lentiviral vector transduction in simian cells. Proc. Natl. Acad. Sci. USA 100:1298-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li, S., C. P. Hill, W. I. Sundquist, and J. T. Finch. 2000. Image reconstructions of helical assemblies of the HIV-1 CA protein. Nature 407:409-413. [DOI] [PubMed] [Google Scholar]
- 35.Liu, J., J. D. Farmer, Jr., W. S. Lane, J. Friedman, I. Weissman, and S. L. Schreiber. 1991. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66:807-815. [DOI] [PubMed] [Google Scholar]
- 36.Luban, J. 1996. Absconding with the chaperone: essential cyclophilin-Gag interaction in HIV-1 virions. Cell 87:1157-1159. [DOI] [PubMed] [Google Scholar]
- 37.Luban, J., K. L. Bossolt, E. K. Franke, G. V. Kalpana, and S. P. Goff. 1993. Human immunodeficiency virus type 1 Gag protein binds to cyclophilins A and B. Cell 73:1067-1078. [DOI] [PubMed] [Google Scholar]
- 38.Naviaux, R. K., E. Costanzi, M. Haas, and I. M. Verma. 1996. The pCL vector system: rapid production of helper-free, high-titer, recombinant retroviruses. J. Virol. 70:5701-5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ott, D. E., L. V. Coren, D. G. Johnson, R. C. Sowder II, L. O. Arthur, and L. E. Henderson. 1995. Analysis and localization of cyclophilin A found in the virions of human immunodeficiency virus type 1 MN strain. AIDS Res. Hum. Retrovir. 11:1003-1006. [DOI] [PubMed] [Google Scholar]
- 40.Reimer, U., M. Drewello, M. Jakob, G. Fischer, and M. Schutkowski. 1997. Conformational state of a 25-mer peptide from the cyclophilin-binding loop of the HIV type 1 capsid protein. Biochem. J. 326:181-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenwirth, B., A. Billich, R. Datema, P. Donatsch, F. Hammerschmid, R. Harrison, P. Hiestand, H. Jaksche, P. Mayer, P. Peichl, V. Quesniaux, F. Schatz, H.-J. Schuurman, R. Traber, R. Wenger, B. Wolff, G. Zenke, and M. Zurini. 1994. Inhibition of human immunodeficiency virus type 1 replication by SDZ NIM 811, a nonimmunosuppressive cyclosporine analog. Antimicrob. Agents Chemother. 38:1763-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanglier, J. J., V. Quesniaux, T. Fehr, H. Hofmann, M. Mahnke, K. Memmert, W. Schuler, G. Zenke, L. Gschwind, C. Maurer, and W. Schilling. 1999. Sanglifehrins A, B, C and D, novel cyclophilin-binding compounds isolated from Streptomyces sp. A92-308110. I. Taxonomy, fermentation, isolation and biological activity. J. Antibiot. (Tokyo) 52:466-473. [DOI] [PubMed] [Google Scholar]
- 43.Sayah, D. M., and J. Luban. 2004. Selection for loss of Ref1 activity in human cells releases human immunodeficiency virus type 1 from cyclophilin A dependence during infection. J. Virol. 78:12066-12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sayah, D. M., E. Sokolskaja, L. Berthoux, and J. Luban. 2004. Cyclophilin A retrotransposition into TRIM5 explains owl monkey resistance to HIV-1. Nature 430:569-573. [DOI] [PubMed] [Google Scholar]
- 45.Streblow, D. N., M. Kitabwalla, M. Malkovsky, and C. D. Pauza. 1998. Cyclophilin A modulates processing of human immunodeficiency virus type 1 p55Gag: mechanism for antiviral effects of cyclosporin A. Virology 245:197-202. [DOI] [PubMed] [Google Scholar]
- 46.Stremlau, M., C. M. Owens, M. J. Perron, M. Kiessling, P. Autissier, and J. Sodroski. 2004. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 427:848-853. [DOI] [PubMed] [Google Scholar]
- 47.Tang, C., Y. Ndassa, and M. F. Summers. 2002. Structure of the N-terminal 283-residue fragment of the immature HIV-1 Gag polyprotein. Nat. Struct. Biol. 9:537-543. [DOI] [PubMed] [Google Scholar]
- 48.Thali, M., A. Bukovsky, E. Kondo, B. Rosenwirth, C. T. Walsh, J. Sodroski, and H. G. Gottlinger. 1994. Functional association of cyclophilin A with HIV-1 virions. Nature 372:363-365. [DOI] [PubMed] [Google Scholar]
- 49.Towers, G., M. Bock, S. Martin, Y. Takeuchi, J. P. Stoye, and O. Danos. 2000. A conserved mechanism of retrovirus restriction in mammals. Proc. Natl. Acad. Sci. USA 97:12295-12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Towers, G. J., T. Hatziioannou, S. Cowan, S. P. Goff, J. Luban, and P. D. Bieniasz. 2003. Cyclophilin A modulates the sensitivity of HIV-1 to host restriction factors. Nat. Med. 9:1138-1143. [DOI] [PubMed] [Google Scholar]
- 51.Vajdos, F. F., S. Yoo, M. Houseweart, W. I. Sundquist, and C. P. Hill. 1997. Crystal structure of cyclophilin A complexed with a binding site peptide from the HIV-1 capsid protein. Protein Sci. 6:2297-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiegers, K., and H. G. Krausslich. 2002. Differential dependence of the infectivity of HIV-1 group O isolates on the cellular protein cyclophilin A. Virology 294:289-295. [DOI] [PubMed] [Google Scholar]
- 53.Wiegers, K., G. Rutter, U. Schubert, M. Grattinger, and H. G. Krausslich. 1999. Cyclophilin A incorporation is not required for human immunodeficiency virus type 1 particle maturation and does not destabilize the mature capsid. Virology 257:261-274. [DOI] [PubMed] [Google Scholar]
- 54.Yin, L., D. Braaten, and J. Luban. 1998. Human immunodeficiency virus type 1 replication is modulated by host cyclophilin A expression levels. J. Virol. 72:6430-6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoo, S., D. G. Myszka, C. Yeh, M. McMurray, C. P. Hill, and W. I. Sundquist. 1997. Molecular recognition in the HIV-1 capsid/cyclophilin A complex. J. Mol. Biol. 269:780-795. [DOI] [PubMed] [Google Scholar]
- 56.Zenke, G., U. Strittmatter, S. Fuchs, V. F. Quesniaux, V. Brinkmann, W. Schuler, M. Zurini, A. Enz, A. Billich, J. J. Sanglier, and T. Fehr. 2001. Sanglifehrin A, a novel cyclophilin-binding compound showing immunosuppressive activity with a new mechanism of action. J. Immunol. 166:7165-7171. [DOI] [PubMed] [Google Scholar]
- 57.Zhao, Y., Y. Chen, M. Schutkowski, G. Fischer, and H. Ke. 1997. Cyclophilin A complexed with a fragment of HIV-1 gag protein: insights into HIV-1 infectious activity. Structure 5:139-146. [DOI] [PubMed] [Google Scholar]