Abstract
We have previously reported that a pseudotype virus generated by reconstitution of hepatitis C virus (HCV) chimeric envelope glycoprotein E1-G or E2-G on the surface of a temperature-sensitive mutant of vesicular stomatitis virus (VSVts045) interacts independently with mammalian cells to initiate infection. Here, we examined whether coexpression of both of the envelope glycoproteins on pseudotype particles would augment virus infectivity and/or alter the functional properties of the individual subunits. Stable transfectants of baby hamster kidney (BHK) epithelial cells expressing either one or both of the chimeric envelope glycoproteins of HCV on the cell surface were generated. The infectious titer of the VSV pseudotype, derived from a stable cell line incorporating both of the chimeric glycoproteins of HCV, was ∼4- to 5-fold higher than that of a pseudotype bearing E1-G alone or ∼25- to 30-fold higher than that of E2-G alone when assayed with a number of mammalian cell lines. Further studies suggested that that the E1-G/E2-G or E2-G pseudotype was more sensitive to the inhibitory effect of heparin than the E1-G pseudotype. Treatment of the E1-G/E2-G pseudotype with a negatively charged sulfated sialyl lipid (NMSO3) displayed a ∼4-fold-higher sensitivity to neutralization than pseudotypes with either of the two individual glycoproteins. In contrast, VSVts045, used as a backbone for the generation of pseudotypes, displayed at least 20-fold-higher sensitivity to NMSO3-mediated inhibition of virus plaque formation. The effect of low-density lipoprotein on the E1-G pseudotype was greater than that apparent for the E1-G/E2-G pseudotype. The treatment of cells with monoclonal antibodies to CD81 displayed an inhibitory effect upon the pseudotype with E1-G/E2-G or with E2-G alone. Taken together, our results indicate that the HCV E1 and E2 glycoproteins have separable functional properties and that the presence of these two envelope glycoproteins on VSV/HCV pseudotype particles increases infectious titer.
Hepatitis C virus (HCV) is a major causative agent of parentally transmitted hepatitis (12) and is associated with liver cirrhosis and hepatocellular carcinoma (2). Approximately 25% of infected individuals appear to clear viremia without therapeutic intervention (3, 30), and the mechanism leading to this natural resolution of HCV infection is not completely understood. The majority of HCV-infected individuals do not resolve infection and may eventually develop chronic hepatitis. The study of HCV is challenging due to its inefficient replication in cell culture and the lack of a small-animal model.
The HCV genome is a linear, positive-sense, single-stranded RNA molecule of ∼9,500 nucleotides. It encodes a polyprotein precursor of ∼3,000 amino acids (13) which is cleaved by both host and viral proteases to generate several distinct polypeptides (24, 26). The structural proteins, core, E1, and E2, of HCV physically interact and may have a role in virus assembly (8, 15, 34). The glycosylated polypeptides (E1 and E2) are most likely anchored onto the envelope lipid bilayer of the virus and facilitate virus entry by interaction with the host cell surface. E1 has two hydrophobic domains located at an internal position between amino acid residues 261 and 291 and in the C-terminal region between amino acid residues 329 and 383 (48). The C-terminal domain has the retention signal for the endoplasmic reticulum (ER) membrane, while the internal domain is involved in binding with capsid protein (17, 35) and may have membrane-active properties (14). Proteomic computational analyses suggested that E1 is a truncated class II fusion protein, and similarities exist between E2 and the receptor-binding portion of the E protein of tick-borne encephalitis virus (22). In vitro expression studies have suggested that the glycoproteins of HCV associate to form a heterodimer stabilized by noncovalent interactions and a high-molecular-weight disulfide-linked aggregate. Both types of complexes accumulate in the ER, a proposed site for HCV assembly and budding, and the transmembrane domains of E1 and E2 play a major role in ER retention of the E1E2 complex (40). However, the functional significance of these complexes is not clear at this time. E2 exhibits the highest degree of genetic heterogeneity, especially in the hypervariable region 1 (HVR1) located at the N terminus (50). Takikawa et al. (49) have suggested fusion activity in cocultured cells expressing the HCV chimeric envelope glycoproteins consisting of the ectodomains of E1 and E2. The induction of cell fusion was shown to require both of the chimeric E1 and E2 proteins in a low-pH-dependent environment.
The lack of an efficient in vitro system for the propagation of HCV makes it difficult to identify cell surface attachment factors or virus receptors. Lagging and coworkers were the first researchers to report the use of vesicular stomatitis virus (VSV)/HCV pseudotype virus in understanding the role of the individual envelope glycoproteins in the initiation of viral infection (32). Pseudotypes expressing either chimeric E1-G or E2-G glycoprotein displayed a distinct pattern of infectivity, suggesting an individual role for each glycoprotein in cell surface interaction, and sera derived from chimpanzees immunized with homologous HCV glycoproteins neutralized virus infectivity for both pseudotypes. A different study (36) suggested that both glycoproteins of HCV are needed for maximal infectivity. HCV pseudotype virus was also used as a surrogate model in determining virus neutralization activity in patient sera (33). Pseudotype-neutralizing activity of patient sera did not exhibit a correlation with the genotype of the infecting HCV strain, suggesting that a common neutralizing epitope(s) is present among HCV genotypes.
Recently, murine leukemia virus (MuLV) and lentivirus pseudotypes have been generated by expression of an unmodified HCV envelope genomic region in 293T cells (5, 20, 27). However, the differences in susceptibilities of the cell types to MuLV or lentivirus pseudotype (5, 52) and VSV pseudotype infectivity (32, 7, 9, 37) and the reason for the lack of infectivity of lentivirus pseudotypes bearing chimeric HCV glycoproteins (27) remain unclear at this time. In the present study, we have investigated the role of the ectodomains from both E1 and E2 chimeric glycoproteins in mediating virus infectivity. Results from this study suggested that the incorporation of both E1 and E2 chimeric glycoproteins into pseudotypes allowed for a significantly enhanced pseudotype titer in a number of mammalian cells with functional activities that are both shared and distinct from those of pseudotypes expressing single HCV glycoproteins.
MATERIALS AND METHODS
Cell lines and plasmids.
Baby hamster kidney cells (BHK-21), human breast cancer cells (MCF-7), and human hepatoma cells (Huh-7, Hep3B, and HepG2) were grown and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum and antibiotics (100 U of penicillin and 100 μg of streptomycin per ml). The plasmids expressing chimeric E1-G and E2-G from HCV genotype 1a (GenBank accession number M62321 [13]) under the control of a cytomegalovirus (CMV) or MuLV promoter have been described previously (7, 32, 38). BHK stable transfectants were generated first by the introduction of E1-G under the control of a CMV promoter. Cells resistant to treatment with G418 (800 μg/ml) were selected and transfected with E2-G under the control of an MuLV promoter. Stable transfectants expressing E2-G were selected by treatment with puromycin (2 μg/ml). Pooled cells expressing both E1-G and E2-G were maintained at lower concentrations of G418 (400 μg/ml) and puromycin (1 μg/ml).
Antibodies.
Mouse monoclonal antibody (MAb) 3D5-C3 (anti-E1), and MAb 3E5-1 (anti-E2) were kindly provided by Michael Houghton (Chiron Corporation, Emeryville, Calif.). A rabbit antiserum to E2 glycoprotein was kindly provided by Arvind Patel (Institute of Virology, Glasgow, United Kingdom). An anti-mouse immunoglobulin (Ig)-fluorescein isothiocyanate (FITC) conjugate and an anti-rabbit Ig-tetramethyl rhodamine isothiocyanate conjugate were purchased (Molecular Probes, Eugene, Oreg.).
FACS analysis.
BHK cells stably transfected with E1-G and/or E2-G plasmids were treated with anti-E1 or anti-E2 MAb or isotype-specific unrelated MAb as a negative control. Cells were stained with FITC-conjugated anti-mouse Ig for fluorescence-activated cell sorter (FACS) analysis (9). Nonspecific background staining was determined with mock-transfected cells treated separately with E1- or E2-specific MAb, or isotype-matched unrelated MAbs, and FITC-conjugated anti-mouse Ig for comparison. FITC-positive cells were detected by FACScan (Becton Dickinson), and results were analyzed with Cell Quest version 3.2 software. Ten thousand cells were analyzed for each sample, and a gate was set on the basis of a dot plot for 90° light scatter versus forward-angle light scatter to exclude dead cells and debris from analysis.
Pseudotype generation from stable transfectants of cells expressing E1-G and/or E2-G.
The incorporation of HCV E1-G or E2-G chimeric glycoprotein into a temperature-sensitive mutant of VSV (VSVts045) has been previously described (32, 38). VSVts045 has a G protein with a single amino acid change in the ectodomain and a thermoreversible folding phenotype (19). At the nonpermissive temperature (40.5°C), G protein of VSVts045 (VSV-G) is synthesized and core glycosylated normally but does not fold correctly and fails to translocate to the infected cell surface. However, the temperature-sensitive mutant virus does not always tightly regulate this process; as a result, leakage of G cannot be ruled out. To safeguard against this possibility, we have used a stock of VSVts045 selected after four rounds of plaque-to-plaque purification, which did not exhibit background virus leakage at 40.5°C. This virus stock was used for the generation of VSV/HCV pseudotype by infecting BHK stable transfectants expressing E1-G and/or E2-G. Cells transfected with an empty vector or VSV-G were similarly treated with VSVts045 as a negative and positive control, respectively. The culture fluid was flash frozen in aliquots, and each aliquot was thawed only once for use in a single round of VSVts045 replication by plaque assay at a permissive temperature (32°C). Treatment of pseudotype virus with an antiserum to VSV-G did not alter virus titer, suggesting the absence of revertant VSV-G in the virus preparation. On the other hand, treatment of positive control virus, which incorporates native VSV-G, exhibited pseudotype neutralization as previously reported (32). Together, the technical optimizations by (i) a careful selection of stock VSVts045 mutant virus and (ii) a rigid temperature control during the entire experimental procedure were keys to successful generation of the pseudotypes.
Chemicals.
Heparin purified from porcine intestine (Sigma, St. Louis, Mo.) was procured. NMSO3, sodium [2,2-bis(docosyl-oxymethyl)propyl-5-acetoamido-3,5-dideoxyl-4,7,8,9-tetra-O-(sodium-oxy sulfonyl)-d-glycero-α-d-galacto-2-nonulopyranosid]onate (molecular weight, 1,458.7), is a sulfated sialyl lipid (31, 47) and was produced by GLSynthesis at greater than 98% purity (Microbiotix, Worcester, Mass.). NMSO3 was dissolved in sterile distilled water.
Western blot analysis.
The culture fluid containing pseudotype virus was clarified by low-speed centrifugation, filtered through a 0.45-μm-pore filter (Millipore Corp., Bedford, Mass.), and pelleted by ultracentrifugation with an SW41 rotor at 25,000 rpm for 90 min. Culture fluid from mock-transfected cells was similarly treated for use as a negative control. Proteins from pelleted virus were separated by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis (SDS-12% PAGE) and transferred onto nitrocellulose membranes. Affinity-purified HCV E1/E2 (kindly provided by Michael Houghton) was used as the authentic envelope glycoprotein markers in SDS-PAGE. HCV E1 or E2 protein was detected by Western blot using specific mouse MAbs and anti-mouse immunoglobulin conjugated to peroxidase. The peroxidase signal was visualized by chemiluminescence. A rabbit antiserum to E2 protein (kindly provided by Arvind Patel) was also used for the detection of HCV E2 glycoprotein. An anti-rabbit immunoglobulin conjugated to peroxidase was used for detection of the viral protein band by chemiluminescence.
Assay for inhibition of pseudotype virus plaque formation.
Heparin or NMSO3 at various concentrations was added to a predetermined amount of VSV/HCV pseudotype virus and incubated at 37°C for 1 h. The virus-inhibitor mixture was added to the cell monolayer in a 6-well plate for plaque assay at 32°C. Untreated virus was used similarly for comparison.
Cell surface blocking assay.
Low-density lipoprotein (LDL) was used to block the respective cell surface receptor activity. Cells in 6-well plates were cooled to 4°C, and unbound cells were removed by rinsing with ice-cold DMEM. LDL (CalBiochem, San Diego, Calif.) was added to each well at various concentrations in cold medium, and cells were incubated for 30 min on ice. A predetermined titer of pseudotype virus inoculum was added to each well and incubated at 4°C for 30 min with intermittent tilting. Unbound ligands from each well were removed by rinsing three times with ice-cold DMEM. Cells were then incubated at 32°C for 30 min before the addition of an agar overlay for plaque formation.
Monoclonal antibodies to human CD81, JS81 (Pharmingen, San Diego, Calif.), and 5A6 (kindly provided by Shoshana Levy, Stanford University School of Medicine) were used for CD81 blocking. Cells were incubated with the specific MAb at 32°C for 1 h. Cells were washed and incubated with a predetermined titer of pseudotype virus for plaque assay.
RESULTS
Coexpression of HCV chimeric envelope glycoproteins on pseudotype particles.
Recombinant vaccinia virus expressing the T7 polymerase (vvT7)-based transient expression system has been previously utilized for generation of pseudotype virus (33, 37). To avoid vaccinia virus and to improve pseudotype preparation, we have generated stable transfectants of BHK cells expressing either one or both of the chimeric envelope glycoproteins of HCV on the cell surface. These stable transfectants were examined for the expression of HCV envelope glycoproteins prior to the generation of VSV/HCV pseudotype virus. Cell surface expression of the E1-G and/or E2-G glycoprotein on stable transfectants of BHK was quantitated by FACS analysis. Envelope glycoproteins were recognized by MAb 3D5-C3 (anti-E1) and MAb 3E5-1 (anti-E2), both targeting linear epitopes. Our results suggested a similar level of cell surface expression for both E1-G (50%) and E2-G (56%) proteins on BHK cells stably transfected with both gene constructs (Fig. 1A). Approximately 44% of E1-G-transfected cells displayed surface expression of E1 chimeric glycoprotein by FACS, and a similar analysis suggested ∼61% cell surface expression of E2 chimeric glycoprotein. The lack of expression of the envelope glycoproteins in a higher number of transfected cells could be due to the use of a pooled cell population that would have a varied or limited level of gene expression. Mock-transfected cells were treated in parallel with the respective MAbs (Fig. 1A) or an unrelated MAb of the same isotype as the negative controls (data not shown).
FIG. 1.
(A) FACS analysis for cell surface expression of chimeric E1-G and E2-G on stable transfectants of BHK cells. Cells were treated with E1-specific MAb 3D5-C3 or E2-specific MAb 3E5-1(white peak) or mock-transfected negative control (grey peak) and stained with FITC-conjugated anti-mouse Ig for FACS analysis. Mock-transfected cells were also treated separately with isotype-specific unrelated MAb and FITC-conjugated anti-mouse Ig for comparison, and similar results were obtained (data not shown). The percentage of FITC-positive cells is indicated in each histogram. (B) incorporation of chimeric E1-G and E2-G glycoproteins onto VSV-HCV pseudotype particles. Pelleted pseudotype viruses were lysed by gel loading buffer and separated by SDS-10% PAGE under reducing conditions, and proteins were transferred onto a nitrocellulose membrane for Western blot analysis. E1 protein was detected by using MAb 3D5-C3, and E2 was detected by a specific rabbit antiserum followed by anti-mouse or anti-rabbit immunoglobulin peroxidase conjugate. The peroxidase signal was visualized by chemiluminescence. Affinity-purified recombinant E1/E2 (rE1 and rE2) were also run as authentic protein markers and detected by using E1- and E2-specific MAbs. Positions of E1-G, E2-G, and high-molecular-weight aggregates (marked by an asterisk) are marked with arrows. Molecular weights of these protein bands were also authenticated from the positions of protein molecular weight markers (data not shown).
Pelleted pseudotype virus, generated from stable transfectants of BHK cells, was subjected to SDS-PAGE under reducing conditions followed by Western blot analysis (Fig. 1B). Immunoblotting with an anti-E1 or anti-E2 antibody demonstrated that the pseudotype preparation contained both E1-G and E2-G and a high-molecular-weight aggregate of the glycoproteins probably representing a non-disulfide-linked E1-G/E2-G homo- or heteromeric complex. A major portion of the aggregate was observed with pseudotype virus on the top of the gel when antiserum to E2 was used in the immunoblot. However, this aggregate could not be detected with E1- or E2-specific MAb, probably due to a masking of the antigenic sites in the aggregated complex. Previous studies from other investigators also suggested the presence of both noncovalently associated E1 and E2 and their disulfide-linked aggregates in HCV-like particles derived from insect cells and lentivirus-derived HCV pseudotype (42, 43). Results from this set of experiments indicated that HCV chimeric envelope glycoproteins are associated with the VSV pseudotype virus.
Coexpression of E1-G/E2-G on VSV pseudotype increases infectious titer.
VSV pseudotype virus entry into cells is likely initiated by specific interactions between the ectodomains of HCV E1 and E2 glycoproteins and host cell surface molecules. To understand the contribution of both of the HCV envelope glycoproteins in virus entry, and for the nature of mammalian cells recognized by the virus glycoproteins, infectivity of pseudotype virus bearing E1-G and/or E2-G was determined by plaque assay with BHK-21, MCF-7, HepG2, Hep3B, and Huh-7 cells. Inoculum from each pseudotype virus was titrated by serial dilution, with VSVts045 (backbone for pseudotype virus) used for comparison, to determine the comparative plaquing efficiency of the pseudotypes in cell lines of diverse origin. Pseudotypes bearing E1-G or E2-G displayed plaque numbers in human cells (MCF-7, HepG2, and Hep3B) which were significantly higher (Table 1) than those apparent in cells of nonhuman origin (BHK). Furthermore, Huh-7 cells displayed the highest titer for each of the three pseudotyped viruses by approximately threefold compared to other human cell lines. A similar analysis with VSVts045 suggested titers that were fivefold lower in HepG2 cells than in BHK cells, with little additional variation between cell lines with respect to the overall titer. Analysis determined that coexpression of E1-G and E2-G led to a significant increase in the overall titer achieved. The titers of the E1-G/E2-G pseudotype proved to be an average of at least 4- to 5-fold higher than those produced with the incorporation of E1-G alone and 25- to 30-fold higher than that which is seen with E2-G alone. The differences in plaque numbers between pseudotypes bearing E1-G and/or E2-G and VSVts045 in human and nonhuman cells may be related to the nature of the cell surface molecules or receptors interacting with the ectodomains of E1 or E2 glycoproteins.
TABLE 1.
Comparative pseudotype titers of different cell types
| Cell type | Pseudotype titer (PFU/ml)a
|
||||
|---|---|---|---|---|---|
| Vector control | E1-G | E2-G | E1-G/E2-G | VSVts045 control | |
| BHK-21 | 1.0 × 102 | 5.2 × 103 | 1.3 × 103 | 2.8 × 104 | 1.0 × 108 |
| MCF-7 | 1.2 × 102 | 1.7 × 104 | 3.7 × 103 | 7.7 × 104 | 1.2 × 108 |
| HepG2 | 10-20 | 1.6 × 104 | 2.9 × 103 | 7.8 × 104 | 2.0 × 107 |
| Hep3B | ND | 1.5 × 104 | 2.9 × 103 | 6.5 × 104 | 1.1 × 108 |
| Huh-7 | 1.6 × 102 | 6.2 × 104 | 1.3 × 104 | 3.7 × 105 | 1.8 × 108 |
Results are presented as the average from six different batches of pseudotype virus preparations. E1-G or E1-G/E2-G pseudotype titer variations were within ± 20% of the average virus titer, while variations of E2-G were ± 10%. However, VSVts045 titers varied by ± 5% in repeated experiments. ND, not determined.
GAGs facilitate infection of pseudotype virus.
To infect a host, a virus must attach itself to the surface of a cell. The molecules to which a virus binds constitute a diverse collection of cellular proteins, carbohydrates, and lipids. By using a pseudotype that incorporates HCV E2 glycoprotein, we have shown that E2 interacts with cell surface glycosaminoglycans (GAGs). Here, we examined the interaction of E1-G/E2-G together with cell surface GAGs compared to either of these subunits alone on a pseudotype virus. Prior treatment of HepG2 cells with heparinase showed a reduction in overall virus titer for the E1-G/E2-G pseudotype that was approximately one-half of that seen with a pseudotype bearing E2-G alone at each of the heparinase concentrations analyzed (2, 4, 8, and 16 U/ml), while E1-G pseudotype infectivity was not inhibited upon heparinase treatment of cells (data not shown). We further examined HCV glycoprotein interaction with the cell surface by using heparin. E1-G/E2-G pseudotype virus was previously incubated with various concentrations of heparin at 32°C and subsequently added onto the cell surface for adsorption. An inhibition of virus infectivity (∼70%) at ∼12.5 μg of heparin/ml for E1-G/E2-G was observed in BHK (Fig. 2) and HepG2 cells (data not shown), and virus infectivity did not significantly decrease with a further increase in the concentration of heparin. On the other hand, an ∼25% reduction in E1-G and an ∼80% reduction of E2-G pseudotype titers were observed with 12.5 μg of heparin/ml. Interestingly, when Sindbis virus (Sin Toto 1101) was used as an unrelated positive control, a gradual plaque reduction of up to ∼90% was apparent over a range of 12.5 to 100 μg of heparin/ml in BHK cells. On the other hand, VSVts045 control did not display a significant reduction in plaque numbers at 12.5 or 25 μg of heparin/ml and was only reduced as the heparin concentration was further increased. Results from these experiments suggested that pseudotype virus generated from E2-G and E1-G/E2-G are almost equally sensitive to heparin as an inhibitor of plaque formation, and the ectodomain of E2 interacts with cell surface GAG. Our results also suggested that the E1 chimeric protein is able to interact with the cell surface for pseudotype infectivity in a manner which is independent of heparin-containing molecules. On the other hand, the E2 glycoprotein present in the E1-G/E2-G pseudotype may be able to better utilize alternate coreceptor functions of GAGs for virus uptake, and as discussed previously, this attachment is specific and depends on the degree of sulfation. Our data also suggest that the ectodomains of E1 and E2 glycoproteins interact independently, as evident from the results of heparinase treatment.
FIG. 2.
Dose-dependent inhibitory role of heparin in pseudotype virus plaque formation. Virus of a known titer was incubated with the indicated doses of heparin for 1 h and added onto a BHK cell monolayer for adsorption. VSVts045 was similarly treated with heparin and included as a control in this assay. Cells were washed and overlaid with agar for plaque formation. The results are presented as the means of three independent experiments together with standard deviations.
Effect of sulfated sialyl lipid on pseudotype virus infectivity.
In general, negatively charged polysaccharides inhibit the adsorption of viruses on the cell membrane by interference of static electric binding between the viral envelope and the cell membrane. The sulfated sialyl lipid (NMSO3) has four sulfate residues in one molecule, is negatively charged, and may inhibit the binding of virus to the cell membrane. Antiviral activity of NMSO3, both in vitro and in vivo, has been reported previously (28, 29, 31, 47); however, the exact mechanism of antiviral activity of NMSO3 remains unknown at this time. Here, we examined the effect of NMSO3 on HCV pseudotype virus infectivity. We incubated pseudotype virus of a known titer with different concentrations of NMSO3. This mixture was added to mammalian cells for virus adsorption and plaque formation. Untreated virus was used as a control. Pseudotypes incorporating either E1-G or E2-G displayed a ∼50% reduction of virus titer at ∼40 μg of NMSO3/ml, while pseudotypes bearing both E1-G and E2-G displayed a 50% reduction of virus titer at 10 μg of NMSO3/ml (Fig. 3). On the other hand, VSVts045 displayed a very high sensitivity, as 0.5 μg of NMSO3/ml reduced infectivity by >50% under similar experimental conditions. Together, these results suggested that pseudotypes with E1-G or E2-G alone were ∼4-fold less sensitive to NMSO3-mediated inhibition of infectivity than pseudotypes with E1-G/E2-G together. We do not rule out the possibility that NMSO3 may mediate an inhibitory effect on viruses other than through negative charge. NMSO3 has been shown to act as a specific inhibitor for P-selectin, a carbohydrate-binding cell adhesion molecule (45). The inhibitory effect of NMSO3 on pseudotype plaque formation appeared to interfere with virus attachment to the cells, and this effect was significantly higher (>20-fold) on the VSVts045 backbone used for the generation of the pseudotypes than on the E1-G/E2-G pseudotype. We do not rule out the possibility of NMSO3 engaging the cellular coreceptor or receptor for inhibition of virus infectivity, and this possibility would require further investigation to understand the mechanism of action.
FIG. 3.
Dose-dependent effect of NMSO3 on pseudotype virus plaque formation. Pseudotype virus of known titer or VSVts045 (control) was treated with different concentrations of NMSO3 before addition to BHK cells. The results are shown as the mean plaque numbers with standard deviations from three different experiments.
Engagement of the LDL-R inhibits pseudotype virus infectivity.
The LDL receptor (LDL-R) is the patriarch of a family of cell surface receptors that transport macromolecules into cells by receptor-mediated endocytosis in clathrin-coated pits (10). A continuous uptake of LDL occurs due to a recycling of the LDL-R to the surface after its dissociation from LDL within the cell. Since the LDL-R has been suggested to be a candidate receptor for HCV entry (1, 39), we examined whether excess human LDL may act as a ligand to block the LDL-R for HCV pseudotype entry into mammalian cells. Huh-7 cells were incubated with LDL at 4°C for 30 min prior to the addition of the pseudotype virus. LDL had a ∼46% inhibitory effect on E1-G/E2-G pseudotype virus titer at 150 μg/∼106 cells. On the other hand, LDL reduced E1-G pseudotype virus titer by 60% at 75 μg/∼106 cells (Fig. 4). However, LDL failed to significantly reduce the plaque number of the E2-G pseudotype under these experimental conditions, indicating a possible role for LDL-R in E1-G-related pseudotype virus entry. The nature of the interaction between HCV E1 and the LDL-R family is not well understood at this time and will require additional studies.
FIG. 4.
Role of LDL-R in pseudotype virus infectivity. Prechilled Huh-7 cells were incubated with different concentrations of LDL for 30 min at 4°C. Pseudotype virus of known titer was added to the cells and incubated for an additional 30 min. The cell monolayer was washed with ice-cold DMEM, overlaid with agar, and transferred to 32°C for plaque formation. The mean values of the percent plaque reduction with standard deviations from three different experiments are shown.
CD81 inhibits pseudotype virus infectivity.
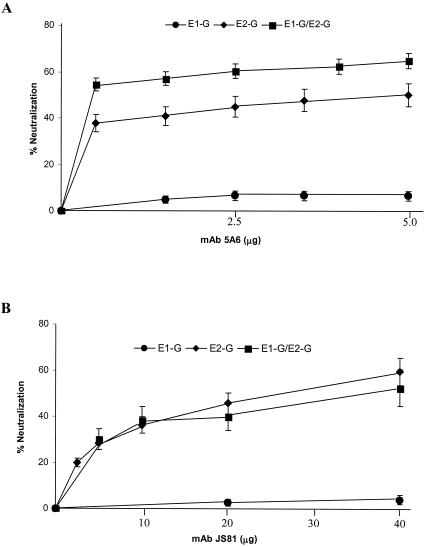
To determine the role of the cell surface CD81 molecule in virus infectivity, a monoclonal antibody (JS81) binding to the extracellular domain of CD81 and a different anti-CD81 MAb (5A6) were used in a pseudotype virus neutralization assay. MCF-7 or Huh-7 cells were incubated with serial dilutions of the monoclonal antibody at 32°C for 30 min. Antibody was removed, and cells were extensively washed. Pseudotype virus generated from E1-G and/or E2-G or the VSVts045 control of a predetermined titer was added onto antibody-adsorbed Huh-7 cells to assess virus infectivity. A monoclonal antibody of the same isotype (IgG1) as that of an unrelated human parainfluenza virus was used as a negative control under identical conditions. Virus titers were determined by plaque assay as described above. Incubation of Huh-7 cells with the monoclonal antibody 5A6 to human CD81 prior to pseudotype adsorption decreased E2-G and E1-G/E2-G pseudotype plaque numbers by ∼60% at a concentration of <2.5 μg/106 cells (Fig. 5). A further increase in the concentration of antibody to CD81 did not appreciably decrease pseudotype plaque numbers. On the other hand, a higher concentration of JS81 (∼40 μg/106 cells) was required to inhibit E2-G or E1-G/E2-G pseudotype virus titer to ∼60%. The difference in the concentrations of these two CD81-specific antibodies for an inhibitory role on virus infectivity appears to be due to the intrinsic nature of the antibodies. Neither of these two MAbs displayed any significant inhibitory effect on E1-G pseudotype virus. These results further suggested that both E1 and E2 retain their individual properties upon reconstitution into pseudotypes, and binding of E2 with CD81 may facilitate pseudotype entry into mammalian cells.
FIG. 5.
Blocking CD81 inhibits pseudotype plaque formation. MAb 5A6 or JS81 was used to block CD81 activity on Huh-7 cells. Cells were separately incubated with CD81-specific antibodies at 32°C for 1 h, washed, and treated with a predetermined titer of pseudotype virus for plaque assay. Results are shown as the mean percent neutralization with standard deviations from three different experiments.
DISCUSSION
In this study, we have used stable transfectants of BHK cells expressing both of the chimeric envelope glycoproteins of HCV onto the cell surface for generation of VSV/HCV pseudotype by using VSVts045 as a backbone and observed that the incorporation of E1 and E2 increases infectious titer of the pseudotype in a number of mammalian cells. A significant increase in titer of the pseudotype bearing both E1-G and E2-G was consistently observed compared with pseudotype bearing either of these glycoproteins alone. The increase in pseudotype titer for coexpression of both of the glycoproteins on the pseudotype surface may be due to an additive effect in recognizing multiple receptor molecules on the cell surface by the individual HCV glycoproteins. Alternatively, an association between the ectodomains of the two envelope glycoproteins of HCV may create conformationally altered domains leading to stronger ligand recognition by the host cell surface. However, recent results from a study on lentivirus pseudotype reactivity with MAbs directed against conformation-dependent epitopes (42) suggest that coexpression of E1 and E2 on the pseudotype surface may not alter the conformation of the ectodomains of HCV envelope glycoproteins. In the present study, we have examined five different mammalian cell lines for infectivity, and the pseudotypes exhibited a maximal plaque titer in Huh-7 cells among the hepatocyte cell lines (Huh-7, Hep3B, and HepG2). The G glycoprotein of the backbone VSVts045 used in pseudotype preparation does not appear to compromise HCV-specific infectivity, as we consistently observed a distinct functional role for HCV envelope glycoproteins located on pseudotype particles and their role in infectivity. Each of the pseudotype viruses have exhibited differential sensitivity to a number of ligands in comparison to the parental VSV (7, 37). Recently, MuLV and lentivirus pseudotypes have been generated from 293T cells by expression of an unmodified HCV envelope genomic region (5, 20, 27). Overexpression of the unmodified E1 and E2 may result in a loss of ER retention, and a portion of these recombinant viral glycoproteins were translocated onto the cell surface. However, the reported differences in susceptibilities of the cell types to MuLV or lentivirus pseudotype (5, 52) and VSV pseudotype infectivity and the role of individual virus glycoproteins in pseudotype infectivity (7, 32, 36, 37) remain unclear at this time. The cell surface expression levels of virus envelope glycoproteins significantly contribute to the pseudotype titer (11). It was previously reported that the ectodomains of HCV E1 and E2, when fused to the transmembrane domain and cytoplasmic tail of VSV G glycoprotein, are efficiently expressed on the cell surface (32). This contrasts with the expression system used for the generation of human immunodeficiency virus-derived HCV pseudotype associated with 293 T cells (5, 27). The production of pseudotype virus in this manner (without modification for cell surface expression) is perhaps the first such occurrence reported in the literature. The modest efficiency of MuLV or lentivirus pseudotypes derived from expression of unmodified E1-E2 of HCV may not have precluded their use in infectivity studies due to the high sensitivity of the green fluorescent protein or luciferase reporter system. However, the lack of infectivity from HCV E1 glycoprotein using a lentivirus pseudotype system (27) is surprising, considering other studies where MuLV/HCV E1 displayed a modest level of infectivity (5) and upon experimental observations from our laboratory and others (32, 36, 37).
Proteoglycans are present abundantly in the extracellular matrices or cell surfaces and mediate many fundamental cellular processes (25). A proteoglycan is formed by the linkage of GAGs such as heparan sulfate or chondroitin sulfate to a protein core. GAGs provide a mechanism of binding and adherence for several human pathogens (46). Heparin-binding proteins are known to interact with heparin via electrostatic charge interactions generated between the negatively charged sulfate groups on heparin and the positively charged amino acids within the protein's heparin-binding domain. This event is followed by an initial virus-cell contact that facilitates an interaction between the viral attachment protein and the receptor molecule. The pseudotype generated from the expression of both E1-G and E2-G was sensitive to the use of heparin as an inhibitor of plaque formation in a manner similar to that of the pseudotype generated with E2-G alone. The HVR1 of E2 may bind with heparan sulfate to provide additional means to facilitate virus attachment to host cells (4, 7). Further examination with a negatively charged sulfated sialyl lipid, NMSO3, has suggested inhibition of VSV/HCV pseudotype virus infectivity which was significantly less than that of the parental VSVts045. Pseudotypes sensitive to the inhibitory activity of NMSO3 are likely to be affected at the first step of interaction with the host cell surface. A ∼4-fold-lower dose of NMSO3 was required to inhibit one-half of the pseudotype virus bearing both E1 and E2 compared to those pseudotypes bearing a single HCV chimeric glycoprotein. These observations cannot explain the antiviral mechanism of NMSO3 based solely on charged residues. Further studies will help in advancing our understanding of the mechanism of NMSO3-mediated inhibition of virus infectivity.
The binding of HCV E2 to the major extracellular loop of CD81, a tetraspanin expressed on various cell types, may play a role in HCV infectivity (44). Our earlier study indicated that anti-CD81 antibody or a soluble form of the CD81 ligand partially blocks E2-G pseudotype infectivity (37). Other investigators have previously suggested that E2 binding of CD81 is conformation dependent (21) and that E1E2 complexes bind more efficiently to CD81 than truncated E2 (18). However, in the present study, the use of anti-CD81 antibodies displayed a similar effect in blocking the pseudotypes bearing both E1-G and E2-G or E2-G alone, with only a slightly greater inhibition displayed on the E1-G/E2-G pseudotype by MAb 5A6. The E2 glycoprotein or its truncated form (E2661) binds specifically to CD81 (44). However, the considerable variance which exists in the ability of the E2 protein from different HCV isolates to interact with CD81 suggests that additional factors other than CD81 may mediate viral recognition by liver cells. This is also apparent from the infectivity of HepG2 cells to pseudotypes in the absence of CD81 on the cell surface. However, a human hepatocyte cell line, Huh-7, which has been observed to express CD81, displayed a significantly higher titer to VSV/HCV pseudotype, indicating a potential role for CD81 in virus binding. A similar study that utilized an excess of LDL to competitively occupy the LDL-R revealed that the E1-G pseudotype appeared to have a more specific reliance upon the LDL-R. A similar experiment with E1-G/E2-G suggested a lower inhibitory effect upon competitive engagement of the LDL-R. A partial inhibition of MuLV- or lentivirus-HCV pseudotype infectivity by a polypeptide of the human CD81 large extracellular loop or anti-CD81 antibody and a weak inhibition by competitive engagement of LDL-R (5, 27, 52) further indicated that HCV pseudotype virus infection may require the expression of both CD81 and LDL-R (5).
Several lines of evidence suggest some common functional properties of HCV envelope glycoproteins when reconstituted onto surfaces of pseudotypes generated from VSV, MuLV, and lentivirus packaging systems or from baculovirus-derived HCV-like particles, which exhibit a complementary nature between these studies. These lines of evidence are as follows: (i) an initial contact of HCV partly depends on sulfated polysaccharides present on mammalian cells, and this contact appeared to be stronger with the E2 glycoprotein of HCV (4, 7, 36, 37, 49); (ii) HVR1 of E2 contains a neutralizing epitope along with other antigenic sites inducing neutralizing antibodies in patient sera (6, 33, 38); (iii) CD81 may have a role through interaction with E2 for virus infectivity (5, 23, 37), while virus titer decreases to some extent upon interruption of LDL-R activity, and this may be mediated via an E1-specific interaction (5, 37); (iv) pseudotype virus generated from E1 and/or E2 requires low pH for infectivity (27, 37); (v) the titers of neutralizing antibodies were distributed among sera from patients infected with other genotypes of HCV (33), and similar observations were also made with pseudotyped HCV E1/E2 on retroviral core-packaging components with green fluorescent protein integration signals (6); and (vi) human monoclonal antibodies directed to E2 exhibit only partial neutralization (∼50 to 60%) of E2 or E1-E2 pseudotype virus (38, 42), while higher plaque reduction (80 to 90%) of the E2 pseudotype was observed with an HVR1 mimotope-specific rabbit antiserum or other antibodies (6, 27, 38).
The major functional difference observed between the MuLV or lentivirus pseudotype and the VSV/HCV pseudotype was the restricted cell tropism (5, 27), and the reason for this difference is not clear at present. Differences in titer were also observed between Huh-7 and Hep3B cells by pseudotypes generated from MuLV and lentivirus (5, 52). We generated the VSV/HCV pseudotype where the machinery and cellular requirements of VSVts045 was used for virus plaque formation in mammalian cells as a readout for infectivity titer. Even a low number of pseudotypes from this system can generate distinct and visible plaques from replication of the parental VSVts045 genome at a permissive temperature (32°C) in a number of mammalian cell lines. The HCV envelope glycoprotein density may be significantly reduced in MuLV or lentivirus particles, as these pseudotypes are generated from natural ER resident viral glycoproteins. This may result in a low level of ligand incorporation into the lentivirus/HCV pseudotype and might be responsible for an inability to infect those cell lines which display lower receptor availability or susceptibility to infection. The CMV promoter driving a reporter gene incorporated into the MuLV or lentivirus pseudotype may also not accurately reflect the infectivity titer after normalization with the level of p24 as the input virus, and a known high background of p24 antigen concentration is often observed from both enveloped particles and naked cores. A previous report has suggested that the production of functional MuLV/visna virus pseudotypes requires efficient incorporation of the visna virus envelope constructs into MuLV cores (11). The pseudotype preparation may contain both enveloped particles and naked cores, and both particle types will be quantitated in the reverse transcriptase assay. MuLV/VSV-G pseudotypes were shown to have higher specific infectivity compared to the MuLV/visna virus pseudotypes. However, in repeated experiments, MuLV/VSV-G pseudotypes had lower reverse transcriptase activity but always had the highest specific infectivity. This finding suggests that the incorporation of MuLV cores into VSV-G-containing envelope is more efficient than the incorporation of cores into the visna virus envelopes or that the VSV-G envelope is more fusogenic or functional than the visna virus envelope glycoprotein when incorporated into MuLV cores, resulting in a higher specific infectivity.
In summary, we have observed a significantly higher VSV/HCV pseudotype titer generated from cell lines coexpressing both E1-G and E2-G in comparison to either of these glycoproteins alone on pseudotype particles. Transmembrane regions of E1 and E2 are suggested to be involved in the folding of the glycoproteins (16, 41, 43), and previous studies have also suggested that the ectodomains of E1 and E2 may participate in this process as well (51) and may be sufficient for heterodimer formation (49). Our results clearly indicate that the presence of the ectodomains alone from both E1 and E2 of HCV significantly increases infectious pseudotype titer. Based on our results, future studies are necessary to further understand the nature of interactions between the ectodomains of HCV envelope glycoproteins and their interplay with mammalian cells in facilitating virus attachment and entry into susceptible host cells.
Acknowledgments
We thank Ilya Frolov, Michael Houghton, Shoshana Levy, and Arvind Patel for providing the necessary reagents for this study and Lin Cowick for preparation of the manuscript.
This research was supported by grant DK58023 from the National Institutes of Health.
REFERENCES
- 1.Agnello, V., G. Abel, M. Elfahal, G. B. Knight, and Q.-X. Zhang. 1999. Hepatitis C virus and other Flaviviridae viruses enter cells via low density lipoprotein receptor. Proc. Natl. Acad. Sci. USA 96:12766-12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alter, H. J., R. H. Purcell, J. W. Shih, J. C. Melpolder, M. Houghton, Q. L. Choo, and G. Kuo. 1989. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N. Engl. J. Med. 321:1494-1500. [DOI] [PubMed] [Google Scholar]
- 3.Alter, M. J., D. Kruszon-Moran, O. V. Nainan, G. M. McQuillan, F. Gao, L. A. Moyer, R. A. Kaslow, and H. S. Margolis. 1999. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N. Engl. J. Med. 341:556-562. [DOI] [PubMed] [Google Scholar]
- 4.Barth, H., C. Schafer, M. I. Adah, F. Zhang, R. J. Linhardt, H. Toyoda, A. Kinoshita-Toyoda, T. Toida, T. H. Van Kuppevelt, E. Depla, F. Von Weizsacker, H. E. Blum, and T. F. Baumert. 2003. Cellular binding of hepatitis C virus envelope glycoprotein E2 requires cell surface heparan sulfate. J. Biol. Chem. 278:41003-41012. [DOI] [PubMed] [Google Scholar]
- 5.Bartosch, B., J. Dubuisson, and F. L. Cosset. 2003. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J. Exp. Med. 197:633-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartosch, B., J. Bukh, J. C. Meunier, C. Granier, R. E. Engle, W. C. Blackwelder, S. U. Emerson, F. L. Cosset, and R. H. Purcell. 2003. In vitro assay for neutralizing antibody to hepatitis C virus: evidence for broadly conserved neutralization epitopes. Proc. Natl. Acad. Sci. USA 100:4199-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basu, A., A. Beyene, K. Meyer, and R. Ray. 2004. The hypervariable region 1 of the E2 glycoprotein of hepatitis C virus binds to glycosaminoglycans, but this binding does not lead to infection in a pseudotype system. J. Virol. 78:4478-4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumert, T. F., S. Ito, D. T. Wong, and T. J. Liang. 1998. Hepatitis C virus structural proteins assemble into viruslike particles in insect cells. J. Virol. 72:3827-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beyene, A., A. Basu, K. Meyer, and R. Ray. 2004. Influence of N-linked glycans on intracellular transport of hepatitis C virus E1 chimeric glycoprotein and its role in pseudotype virus infectivity. Virology 324:273-285. [DOI] [PubMed] [Google Scholar]
- 10.Brown, M. S., J. Herz, and J. L. Goldstein. 1997. LDL-receptor structure. calcium cages, acid baths and recycling receptors. Nature 338:629-630. [DOI] [PubMed] [Google Scholar]
- 11.Bruett, L., and J. E. Clements. 2001. Functional murine leukemia virus vectors pseudotyped with the visna virus envelope show expanded visna virus cell tropism. J. Virol. 75:11464-11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choo, Q. L., G. Kuo, A. J. Weiner, L. R. Overby, D. W. Bradley, and M. Houghton. 1989. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244:359-362. [DOI] [PubMed] [Google Scholar]
- 13.Choo, Q. L., K. H. Richman, J. H. Han, K. Berger, C. Lee, C. Dong, C. Gallegos, D. Coit, R. Medina-Selby, P. J. Barr, A. J. Weiner, D. W. Bradley, G. Kuo, and M. Houghton. 1991. Genetic organization and diversity of the hepatitis C virus. Proc. Natl. Acad. Sci. USA 88:2451-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciccaglione, A. R., A. Costantino, C. Marcantonio, M. Equestre, A. Geraci, and M. Rapicetta. 2001. Mutagenesis of hepatitis C virus E1 protein affects its membrane-permeabilizing activity. J. Gen. Virol. 82:2243-2250. [DOI] [PubMed] [Google Scholar]
- 15.Clayton, R. F., A. Owsianka, J. Aitken, S. Graham, D. Bhella, and A. H. Patel. 2002. Analysis of antigenicity and topology of E2 glycoprotein present on recombinant hepatitis C virus-like particles. J. Virol. 76:7672-7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cocquerel, L., J. C. Meunier, A. Pillez, C. Wychowski, and J. Dubuisson. 1998. A retention signal necessary and sufficient for endoplasmic reticulum localization maps to the transmembrane domain of hepatitis C virus glycoprotein E2. J. Virol. 72:2183-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cocquerel, L., S. Duvet, J. C. Meunier, A. Pillez, R. Cacan, C. Wychowski, and J. Dubuisson. 1999. The transmembrane domain of hepatitis C virus glycoprotein E1 is a signal for static retention in the endoplasmic reticulum. J. Virol. 73:2641-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cocquerel, L., C. C. Kuo, J. Dubuisson, and S. Levy. 2003. CD81-dependent binding of hepatitis C virus E1E2 heterodimers. J. Virol. 77:10677-10683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doms, R. W., R. A. Lamb, J. K. Rose, and A. Helenius. 1993. Folding and assembly of viral membrane proteins. Virology 193:545-562. [DOI] [PubMed] [Google Scholar]
- 20.Drummer, H. E., A. Maerz, and P. Poumbourios. 2003. Cell surface expression of functional hepatitis C virus E1 and E2 glycoproteins. FEBS Lett. 546:385-390. [DOI] [PubMed] [Google Scholar]
- 21.Flint, M., C. Maidens, L. D. Loomis-Price, C. Shotton, J. Dubuisson, P. Monk, A. Higginbottom, S. Levy, and J. A. McKeating. 1999. Characterization of hepatitis C virus E2 glycoprotein interaction with a putative cellular receptor, CD81. J. Virol. 73:6235-6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garry, R. F., and S. Dash. 2003. Proteomics computational analyses suggest that hepatitis C virus E1 and pestivirus E2 envelope glycoproteins are truncated class II fusion proteins. Virology 307:255-265. [DOI] [PubMed] [Google Scholar]
- 23.Germi, R., J.-M. Crance, D. Garin, J. Guimet, H. Lortat-Jacob, R. W. H. Ruigrok, J.-P. Zarski, and E. Drouet. 2002. Cellular glycosaminoglycans and low density lipoprotein receptor are involved in hepatitis C virus adsorption. J. Med. Virol. 68:206-215. [DOI] [PubMed] [Google Scholar]
- 24.Grakoui, A., C. Wychowski, C. Lin, S. M. Feinstone, and C. M. Rice. 1993. Expression and identification of hepatitis C virus polyprotein cleavage products. J. Virol. 67:1385-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassell, J. R., J. H. Kimura, and V. C. Hascall. 1986. Proteoglycan core protein families. Annu. Rev. Biochem. 55:539-567. [DOI] [PubMed] [Google Scholar]
- 26.Hijikata, M., N. Kato, Y. Ootsuyama, M. Nakagawa, and K. Shimotohno. 1991. Gene mapping of the putative structural region of the hepatitis C virus genome by in vitro processing analysis. Proc. Natl. Acad. Sci. USA 88:5547-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu, M., J. Zhang, M. Flint, C. Logvinoff, C. Cheng-Mayer, C. M. Rice, and J. A. McKeating. 2003. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc. Natl. Acad. Sci. USA 100:7271-7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaneko, H., K. Kato, S. Mori, and S. Shigeta. 2001. Antiviral activity of NMSO3 against adenovirus in vitro. Antivir. Res. 52:281-288. [DOI] [PubMed] [Google Scholar]
- 29.Kaneko, H., S. Mori, S. Shigeta, S. Ohno, and K. Aoki. 2003. Antiviral effect of sulfated sialyl lipid against a clinical strain of adenovirus. Nippon Ganka Gakkai Zasshi 107:196-201. (In Japanese.) [PubMed] [Google Scholar]
- 30.Kenny-Walsh, E., et al. 1999. Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin. N. Engl. J. Med. 340:1228-1233. [DOI] [PubMed] [Google Scholar]
- 31.Kimura, K., S. Mori, K. Tomita, K. Ohno, K. Takahashi, S. Shigeta, and M. Terada. 2000. Antiviral activity of NMSO3 against respiratory syncytial virus infection in vitro and in vivo. Antivir. Res. 47:41-51. [DOI] [PubMed] [Google Scholar]
- 32.Lagging, L. M., K. Meyer, R. J. Owens, and R. Ray. 1998. Functional role of hepatitis C virus chimeric glycoproteins in the infectivity of pseudotyped virus. J. Virol. 72:3539-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lagging, L. M., K. Meyer, J. Westin, R. Wejstal, G. Norkrans, M. Lindh, and R. Ray. 2002. Neutralization of pseudotyped vesicular stomatitis virus expressing hepatitis C virus envelope glycoprotein 1 or 2 by serum from patients. J. Infect. Dis. 185:1165-1169. [DOI] [PubMed] [Google Scholar]
- 34.Lo, S. Y., M. J. Selby, and J. H. Ou. 1996. Interaction between hepatitis C virus core protein and E1 envelope protein. J. Virol. 70:5177-5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma, H. C., C. H. Ke, T.Y. Hsieh, and S. Y. Lo. 2002. The first hydrophobic domain of the hepatitis C virus E1 protein is important for interaction with the capsid protein. J. Gen. Virol. 83:3085-3092. [DOI] [PubMed] [Google Scholar]
- 36.Matsuura, Y., H. Tani, K. Suzuki, T. Kimura-Someya, R. Suzuki, H. Aizaki, K. Ishii, K. Moriishi, C. S. Robinson, M. A. Whitt, and T. Miyamura. 2001. Characterization of pseudotype VSV possessing HCV envelope proteins. Virology 286:263-275. [DOI] [PubMed] [Google Scholar]
- 37.Meyer, K., A. Basu, and R. Ray. 2000. Functional features of hepatitis C virus glycoproteins for pseudotype virus entry into mammalian cells. Virology 276:214-226. [DOI] [PubMed] [Google Scholar]
- 38.Meyer, K., A. Basu, C. T. Przysiecki, L. M. Lagging, A. D. M. Di Bisceglie, A. J. Conley, and R. Ray. 2002. Complement-mediated enhancement of antibody function for neutralization of pseudotype virus containing hepatitis C virus E2 chimeric glycoprotein. J. Virol. 76:2150-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monazahian, M., I. Bohme, S. Bonk, A. Koch, C. Scholz, S. Grethe, and R. Thomssen. 1999. Low density lipoprotein receptor as a candidate receptor for hepatitis C virus. J. Med. Virol. 57:223-229. [DOI] [PubMed] [Google Scholar]
- 40.Op De Beeck, A., L. Cocquerel, and J. Dubuisson. 2001. Biogenesis of hepatitis C virus envelope glycoproteins. J. Gen. Virol. 82:2589-2595. [DOI] [PubMed] [Google Scholar]
- 41.Op De Beeck, A., and J. Dubuisson. 2003. Topology of hepatitis C virus envelope glycoproteins. Rev. Med. Virol. 13:233-241. [DOI] [PubMed] [Google Scholar]
- 42.Op De Beeck, A., C. Voisset, B. Bartosch, Y. Ciczora, L. Cocquerel, Z. Keck, S. Foung, F.-L. Cosset, and J. Dubuisson. 2004. Characterization of functional hepatitis C virus envelope glycoproteins. J. Virol. 78:2994-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel, J., A. H. Patel, and J. McLauchlan. 2001. The transmembrane domain of the hepatitis C virus E2 glycoprotein is required for correct folding of the E1 glycoprotein and native complex formation. Virology 279:58-68. [DOI] [PubMed] [Google Scholar]
- 44.Pileri, P., Y. Uematsu, S. Campagnoli, G. Galli, F. Falugi, R. Petracca, A. J. Weiner, M. Houghton, D. Rosa, G. Grandi, and S. Abrignani. 1998. Binding of hepatitis C virus to CD81. Science 282:938-941. [DOI] [PubMed] [Google Scholar]
- 45.Shodai, T., J. Suzuki, S. Kudo, S. Itoh, M. Terada, S. Fujita, H. Shimazu, and T. Tsuji. 2003. Inhibition of P-selectin-mediated cell adhesion by a sulfated derivative of sialic acid. Biochem. Biophys. Res. Commun. 312:787-793. [DOI] [PubMed] [Google Scholar]
- 46.Shukla, D., and P. G. Spear. 2001. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J. Clin. Investig. 108:503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takahashi, K., K. Ohashi, Y. Abe, S. Mori, K. Taniguchi, T. Ebina, O. Nakagomi, M. Terada, and S. Shigeta. 2002. Protective efficacy of a sulfated sialyl lipid (NMSO3) against human rotavirus-induced diarrhea in a mouse model. Antimicrob. Agents Chemother. 46:420-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takamizawa, A., C. Mori, I. Fuke, S. Manabe, S. Murakami, J. Fujita, E. Onishi, T. Andoh, I. Yoshida, and H. Okayama. 1991. Structure and organization of the hepatitis C virus genome isolated from human carriers. J. Virol. 65:1105-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takikawa, S., K. Ishii, H. Aizaki, T. Suzuki, H. Asakura, Y. Matsuura, and T. Miyamura. 2000. Cell fusion activity of hepatitis C virus envelope proteins. J. Virol. 74:5066-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiner, A. J., H. M. Geysen, C. Christopherson, J. E. Hall, T. J. Mason, G. Saracco, F. Bonino, K. Crawford, C. D. Marion, K. A. Crawford, M. Brunetto, P. J. Barr, T. Miyamura, J. McHutchinson, and M. Houghton. 1992. Evidence for immune selection of hepatitis C virus (HCV) putative envelope glycoprotein variants: potential role in chronic HCV infections. Proc. Natl. Acad. Sci. USA 89:3468-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yi, M., Y. Nakamoto, S. Kaneko, T. Yamashita, and S. Murakami. 1997. Delineation of regions important for heteromeric association of hepatitis C virus E1 and E2. Virology 231:119-129. [DOI] [PubMed] [Google Scholar]
- 52.Zhang, J., G. Randall, A. Higginbottom, P. Monk, C. M. Rice, and J. A. McKeating. 2004. CD81 is required for hepatitis C virus glycoprotein-mediated viral infection. J. Virol. 78:1448-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]