Abstract
Hepatitis delta virus (HDV) contains a circular RNA which encodes a single protein, hepatitis delta antigen (HDAg). HDAg exists in two forms, a small form (S-HDAg) and a large form (L-HDAg). S-HDAg can transactivate HDV RNA replication. Recent studies have shown that posttranslational modifications, such as phosphorylation and acetylation, of S-HDAg can modulate HDV RNA replication. Here we show that S-HDAg can be methylated by protein arginine methyltransferase (PRMT1) in vitro and in vivo. The major methylation site is at arginine-13 (R13), which is in the RGGR motif of an RNA-binding domain. The methylation of S-HDAg is essential for HDV RNA replication, especially for replication of the antigenomic RNA strand to form the genomic RNA strand. An R13A mutation in S-HDAg inhibited HDV RNA replication. The presence of a methylation inhibitor, S-adenosyl-homocysteine, also inhibited HDV RNA replication. We further found that the methylation of S-HDAg affected its subcellular localization. Methylation-defective HDAg lost the ability to form a speckled structure in the nucleus and also permeated into the cytoplasm. These results thus revealed a novel posttranslational modification of HDAg and indicated its importance for HDV RNA replication. This and other results further showed that, unlike replication of the HDV genomic RNA strand, replication of the antigenomic RNA strand requires multiple types of posttranslational modification, including the phosphorylation and methylation of HDAg.
Hepatitis delta virus (HDV) is a satellite virus and requires the presence of hepatitis B virus (HBV) for virus assembly and production (42). Its genome is a single-stranded, circular RNA of 1.7 kb. Unlike other satellite viruses, HDV can replicate independently of its helper virus, HBV, and does not share sequence homology with HBV. The virus contains a single protein species, hepatitis delta antigen (HDAg), which exists in two forms, including a small form of 195 amino acids (S-HDAg) and a large form of 214 amino acids (L-HDAg) (16). HDV RNA replication occurs in the nucleus via host RNA polymerases (11, 30, 33). The viral genomic RNA replicates by a rolling-circle mechanism into a full-length antigenomic RNA and also transcribes an antigenomic-strand mRNA (0.8 kb) which contains the HDAg open reading frame (ORF). The full-length antigenomic RNA, in turn, is replicated into the genomic-strand RNA by another round of rolling-circle replication. The replication of genomic and antigenomic RNAs and transcription of the HDAg-encoding mRNA are carried out by independent mechanisms, probably by different polymerases (30, 31, 33). The 0.8-kb mRNA is translated into S-HDAg, which is required for continuous HDV RNA replication (21). In the later stages of viral replication, an RNA editing event occurs at the amber termination codon of the S-HDAg ORF to allow the synthesis of L-HDAg (40, 41), which is required for virus assembly (2, 24). L-HDAg was also thought to inhibit HDV RNA replication when expressed artificially early in viral replication (4, 24), but a recent study showed that L-HDAg does not play a significant role in regulating viral RNA levels in cells (29).
Posttranslational modifications of HDAg include isoprenylation, phosphorylation, and acetylation. Isoprenylation occurs at the C-terminal end of L-HDAg and has been demonstrated to mediate protein-protein interactions between L-HDAg and the HBV surface antigen (18), which is indispensable for HDV virus assembly (13), and to enhance the transdominant inhibitory function of L-HDAg (17). The phosphorylation of HDAg occurs at multiple residues, with Serine-177 (S-177) being the major phosphorylation site (3, 35). S-177 phosphorylation is required for efficient viral RNA replication, particularly for replication of the antigenomic RNA strand (6, 35). Recently, the acetylation of HDAg was reported (36), and this process is also involved in the replication of HDV RNA and may regulate the subcellular localization of HDAg. The acetylation of Lysine-72 (K-72) is necessary for the nuclear transport of HDAg. A K72A substitution reduced viral RNA accumulation and resulted in the earlier appearance of L-HDAg, indicating that the posttranslational modification of K-72 affects the regulation of RNA editing. Since HDAg has multiple features of a cellular transcription factor and participates in HDV RNA replication, we further explored other possible posttranslational modifications of HDAg. Here we report the methylation of HDAg.
It has been reported that the phosphorylation, acetylation, and methylation of histone proteins are involved in the regulation of chromatin activation (10, 12, 19, 27, 54). Histone methylation occurs at arginine and lysine residues and is catalyzed by two families of proteins, the protein arginine methyltransferase (PRMT) family (8, 26, 47, 49) and the SET-domain-containing methyltransferase family (23, 38, 48). At least three types of PRMT activities that transfer methyl groups from S-adenosyl-l-methionine (AdoMet) to the guanidine group of arginine residues have been reported (25). Type I PRMT enzymes catalyze the formation of ω-monomethylarginine and asymmetric ω-NG,NG-dimethylarginine, whereas type II enzymes catalyze the formation of ω-monomethylarginine and symmetric ω-NG,N′G-dimethylarginine. Type III enzymes catalyze monomethylation and have been found only in Saccharomyces cerevisiae (55). The substrates of type I PRMT include many RNA-binding proteins (43), transporting proteins (37, 39), transcription factors (34), nuclear matrix proteins (53), and cytokines. The functions of protein arginine methylation include the regulation of transcription (8, 10), modulation of the affinity of nucleic acid-binding proteins, interactions with other proteins (22), the regulation of interferon signaling pathways (34), and the targeting of nuclear proteins (10, 14, 43). The following four type I PRMTs have been reported: PRMT1 (49), PRMT3 (50), coactivator-associated arginine methyltransferase (CARM1) (7) from mammalian cells, and arginine methyltransferase I (RMT1) from yeast cells (53).
S-HDAg is an RNA-binding protein and localizes predominantly to the nucleus (9, 52). A previous study showed that S-HDAg and HDV RNA form speckled structures, which are presumed to be the HDV RNA replication complexes in the nucleus (1). Although significant efforts have been focused on understanding the biological functions of S-HDAg, its mechanism of involvement in HDV replication remains largely unknown. In this paper, we present biochemical evidence that S-HDAg is subject to protein arginine methylation in vitro and in vivo and show that the methylation of S-HDAg is required for HDV RNA replication.
MATERIALS AND METHODS
Plasmid construction.
Plasmids pBSδ1.2G and pBSδ1.2AG (templates for in vitro transcription of the 1.2× genome-length genomic and antigenomic HDV RNAs, respectively) have been described elsewhere (30). Plasmid pET-Sm was used for expression of the wild-type small delta antigen in Escherichia coli (44). The mutant pET-Sm-R10A plasmid was constructed by PCR-based site-directed mutagenesis, for which a pair of divergent 5′-phosphorylated primers, 5′-GCCGGGGGGAGGGAAGACATCCTCGAG-3′ and 5′-GTCTTTCCTTCTTTCGGACCGGCT-3′, were used in a 50-μl reaction mixture that contained 50 ng of pET-Sm, a 0.32 μM concentration of each primer, a 0.2 mM concentration of each deoxynucleoside triphosphate, and 2.5 U of Pfu polymerase. The PCR was cycled as follows: 95°C for 5 min and then 28 cycles of 94°C for 1 min, 60°C for 1 min, and 72°C for 12 min, followed by a final step at 72°C for 15 min. The pET-Sm-R13A plasmid was also constructed by PCR-based site-directed mutagenesis using the primer pair 5′-GCCGAAGACATCCTCGAGCAGTGGGTG-3′ and 5′-CCCCCCGCGGTCTTTCCTTCTTTCTTC-3′. The same PCR conditions were used. The mutants were sequenced for at least 300 nucleotides across the mutation sites to confirm the presence of the mutations and to ensure that no other mutations were introduced. The plasmid pCD3.1-WT was constructed by use of a pCDNA3.1/V5-His TOPO TA expression kit (Invitrogen). The inserted fragment was the ORF of S-HDAg, which was amplified by a PCR with the primer pair 5′-GAATTCATGAGCCGGCCCGAAGGAAGGAAAAACCGC-3′ and 5′-ATAAGAATGCGGCCGCCTATGGGAATCCCT GGCTTCCCCTTATGTC-3′, with plasmid pET-Sm as the template. The PCR was cycled as follows: 95°C for 5 min and then 28 cycles of 94°C for 1 min, 60°C for 1 min, and 72°C for 5 min, followed by a final step at 72°C for 15 min. The construction of plasmids pCD3.1-R10A and pCD3.1-R13A followed the same procedure as described above, with pET-Sm-R10A and pET-Sm-R13A, respectively, as DNA templates.
Cell culture, transfection, and drug treatments.
A human hepatoma cell line cell was cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and incubated at 37°C in 5% CO2. The cells were seeded overnight in either six-well plates or 60-mm-diameter petri dishes prior to transfection. Transfections with pCD3.1-WT, pCD3.1-R10A, and pCD3.1-R13A DNAs were performed by use of the Fugene 6 reagent (Roche) according to the manufacturer's directions.
The 1.2× genome-length HDV RNAs were transcribed from plasmids pBSδ1.2G and pBSδ1.2AG by the use of T7 Megascript kits (Ambion) after linearization with the restriction enzyme NotI. A fluorescein-labeled genomic or antigenomic RNA was also transcribed from the linearized pBSδ1.2G or pBSδ1.2AG plasmid in a 20-μl transcription reaction mixture containing a 0.5 mM concentration (each) of ATP, GTP, and CTP, 0.3 mM UTP, 0.2 mM fluorescein-12-UTP (Boehringer Mannheim), 40 mM Tris-HCl (pH 8.0), 6 mM MgCl2, 10 mM dithiothreitol, 2 mM spermidine, 10 mM NaCl, 1 U of RNasin, 10 U of T7 RNA polymerase (Ambion), and 1 μg of linearized plasmid DNA at 37°C for 2 h. After the reaction took place, the DNA template was digested with 2 U of DNase I (RNase-free) at 37°C for 15 min. For the removal of unincorporated nucleotides, RNAs were precipitated with either LiCl or ammonium acetate-ethanol. The capped mRNA for HDAg was transcribed from plasmid pX9-I/II after linearization with the restriction enzyme HindIII by use of a T7 m-Message m-Machine kit (Ambion) (31). All RNA transfection experiments were performed by use of the DMRIE-C reagent (Gibco BRL) according to the manufacturer's directions.
For the transfection of RNA-protein complexes, 0.5 μg of partially purified wild-type or R10A or R13A mutant S-HDAg was mixed with 3 μg of in vitro-transcribed HDV RNA in a final volume of 25 μl in 10 mM HEPES (pH 7.4) at room temperature for 10 min. The protein-RNA transfections were mediated by the use of DOTAP (Boehringer Mannheim) according to the manufacturer's directions.
To study the effects of S-adenosyl-homocystine (AdoHcy) on HDAg localization, we seeded Huh7 cells overnight in either six-well plates or eight-well chamber slides. Before transfection, the cells were pretreated with 0, 2, or 8 mM AdoHcy for 2 h and subsequently transfected with DNA or RNA as described above. After transfection, the medium was changed, replenished with new medium containing AdoHcy, and incubated for another 48 h.
Northern blot hybridization analysis.
RNAs were extracted from cells by the use of Tri-Reagent (Molecular Research Center, Inc.) according to the manufacturer's protocol. RNA samples were separated by electrophoresis through 1.2% morpholinepropanesulfonic acid (MOPS)-formaldehyde-containing agarose gels, transferred to membranes, hybridized, and washed as described previously (30). The detection of genomic and antigenomic HDV RNAs was performed by using in vitro-transcribed 32P-labeled probes generated from pTMδSalB and pBSδHX, respectively (30). The washed membranes were exposed to Biomax MR or MS X-ray film (Kodak).
Purification of HDV small delta antigen.
To express and purify recombinant S-HDAg from E. coli, we incubated an overnight culture of BL21(DE3) cells harboring the pET-Sm plasmid (44) in 500 ml of Luria-Bertani-ampicillin medium at 37°C. After the cell density at 600 nm reached 0.5, 0.2 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added to the medium to induce S-HDAg expression. The culture was then incubated at 28°C for 18 h. E. coli cells were harvested by centrifugation at 4°C and then resuspended in 25 ml of ice-cold sonication buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.1% Tween 20, 5 mM β-mercaptoethanol [β-ME], protease inhibitor cocktail [Roche], 1 mM phenylmethylsulfonyl fluoride [PMSF]). The cell suspension was sonicated on ice 10 times (10 s each time) and centrifuged at 9,000 × g for 20 min. The supernatant was used for further purification.
A heparin column (5 ml) (Amersham) was equilibrated with sonication buffer before use. The supernatant mentioned above was applied (at a flow rate of 1 ml/min) onto the pre-equilibrated heparin column. After the sample was loaded, the column was washed with 50 ml of washing buffer 1 (50 mM Tris-HCl [pH 8.0], 300 mM NaCl, 0.5% Tween 20, 5 mM β-ME, protease inhibitor cocktail [Roche], 1 mM PMSF), followed by washing with 50 ml of washing buffer 2 (50 mM Tris-HCl [pH 8.0], 650 mM NaCl, 5 mM β-ME, protease inhibitors cocktail [Roche], 1 mM PMSF). S-HDAg was eluted with 20 ml of elution buffer (50 mM Tris-HCl [pH 8.0], 1 M NaCl, 5 mM β-ME, protease inhibitor cocktail [Roche], 1 mM PMSF), and 1-ml fractions were collected. Aliquots of each fraction were analyzed by sodium dodecyl sulfate-12.5% polyacrylamide gel electrophoresis (SDS-12.5% PAGE). Proteins were visualized by staining with Coomassie blue. Fractions containing S-HDAg were pooled and dialyzed against 1 liter of dialysis buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 5 mM β-ME, protease inhibitor cocktail [Roche], 1 mM PMSF).
Immunolocalization.
For an evaluation of the subcellular distribution of S-HDAg by immunocytochemistry, cells were grown on eight-well chamber slides and transfected with plasmid DNAs encoding various HDAgs. After 24 to 48 h in the presence or absence of the methylation inhibitors, cells were fixed with paraformaldehyde and permeabilized with 0.1% Triton X-100. An indirect immunofluorescence analysis of HDAg was performed with the anti-HDAg monoclonal antibody 3G3 (15) and a goat anti-mouse fluorescein isothiocyanate-conjugated antibody. Stained cells were mounted and imaged on a Bio-Rad MRC 1000 argon/krypton laser confocal system.
In vitro methylation assay.
The methyltransferase PRMT1 was prepared as a recombinant glutathione S-transferase (GST) fusion protein and eluted from glutathione-agarose beads (Sigma) with 20 mM glutathione (26). PRMT3 and CARM1 were prepared as described previously (26). Approximately 0.5 μg of commercially obtained histone H4 (Sigma) or recombinant S-HDAg was incubated with 1 μg of methyltransferase in the presence of 6 μM S-adenosyl-[methyl-3H]methionine ([3H]AdoMet) (14.7 Ci/mmol; Perkin Elmer Life Science) in 35 μl of reaction buffer (20 mM Tris-HCl [pH 8.0], 200 mM NaCl, 0.4 mM EDTA) at 30°C for 1 h (26). The reactions were stopped by the addition of SDS (final concentration, 2%), followed by boiling for 3 min. The samples were analyzed by SDS-PAGE with 12.5% polyacrylamide gels and visualized by fluorography.
RESULTS
The recombinant HDV small delta antigen can be specifically methylated by PRMT1 in vitro.
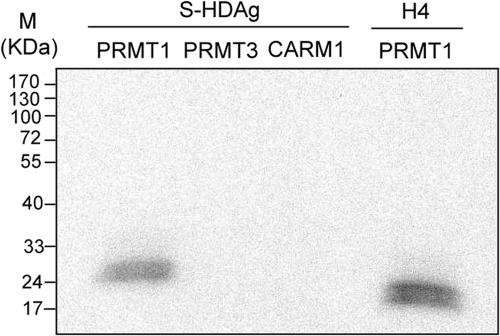
To explore all of the potential posttranslational modifications of HDAg, we set out to examine whether S-HDAg was methylated. An amino acid sequence analysis showed that S-HDAg (5) has two RGG motifs that resemble other known sites of PRMT-mediated methylation (49). We used partially purified GST-fused PRMTs to perform in vitro methylation experiments. The results showed that S-HDAg was methylated by PRMT1 in vitro (Fig. 1). Other type I protein PRMTs, including PRMT3 and CARM1, could not methylate the protein (Fig. 1). Histone H4 was used as a positive control which could be methylated by PRMT1. Therefore, S-HDAg is specifically methylated by PRMT1, which is a predominantly nuclear protein that exists in a large complex of 300 to 400 kDa and methylates arginine residues in RGG and RXR motifs of many proteins, including RNA-binding proteins (49). These results show that HDAg can be methylated.
FIG. 1.
Methylation of S-HDAg by PRMTs in vitro. Partially purified recombinant S-HDAg (0.5 μg) was incubated with GST-fused PRMT1 (1 μg), PRMT3, or CARM1 and [3H]AdoMet under the conditions described in Materials and Methods. The products were analyzed by SDS-PAGE and fluorography. The histone H4 protein served as a positive control for PRMT1 methylation.
Arginine-13 is essential for methylation in vitro.
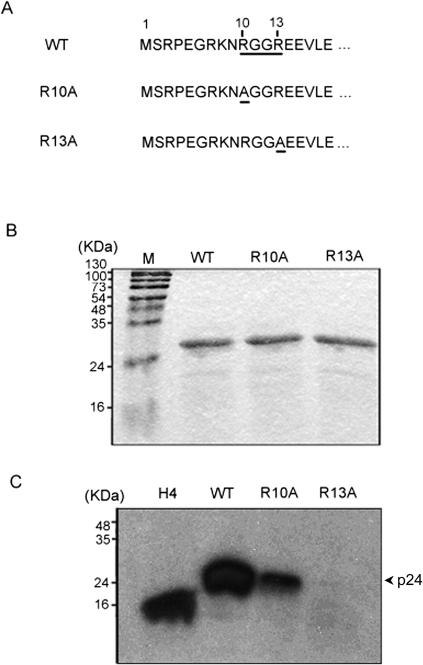
The amino acid sequence analysis of S-HDAg showed that there are two RGG motifs in the protein. The first motif, RGGR, is located at the N terminus, at amino acid residues 10 to 13, and the other motif, RGG, is found at residues 91 to 93. We first examined the role of the RGGR motif in HDAg methylation by site-directed mutagenesis of the arginine residues (Fig. 2A). Wild-type or mutant proteins were expressed in E. coli, partially purified, quantified, and used for in vitro methylation by PRMT1. Methylation of the R13A mutant was almost completely abolished, while that of the R10A mutant decreased substantially compared with that of wild-type HDAg (Fig. 2). We concluded that both Arg-13 and Arg-10 contribute to the methylation of HDAg; however, it appears that Arg-13 has to be methylated first before Arg-10 can be methylated since the R13A mutant was completely devoid of methylation. This effect can be explained by the possible conformational changes induced by R13 methylation.
FIG. 2.
Identification of methylation site of S-HDAg. (A) N-terminal amino acid sequence of HDAg. The RGGR motif is underlined. The mutations in R10A and R13A are also underlined. (B) Protein purification of wild-type and R10A and R13A mutant S-HDAg. The recombinant proteins were purified from E. coli, separated by SDS-PAGE with a 12.5% acrylamide gel, and stained with Coomassie blue. (C) In vitro methylation assay with PRMT1. The same amounts of proteins as those use for panel B were incubated with [3H]AdoMet and PRMT1 as described in Materials and Methods. The methylation products were monitored by SDS-PAGE and fluorography. The position of the 24-kDa S-HDAg protein is indicated. The histone H4 protein served as a positive control.
We also examined the possible methylation of the 91-RGG-93 motif by site-directed mutagenesis of the arginine residue. However, the R91A mutant could still be methylated by PRMT1 in vitro, suggesting that this RGG motif is not important for the methylation of HDAg (data not shown). Therefore, we focused on examining the 10-RGGR-13 motif in this work.
The methylated small delta antigen can facilitate HDV antigenomic RNA replication via protein-RNA complex transfection.
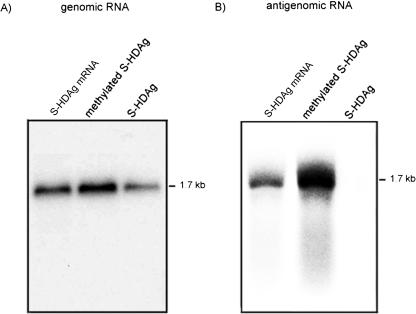
As a previous study (44) indicated, when a mixture of the recombinant S-HDAg derived from E. coli and an in vitro-transcribed genomic or antigenomic HDV RNA was used for the transfection of Huh7 cells, the HDV genomic RNA could replicate while the antigenomic RNA could not. In contrast, when HDV RNAs and an mRNA encoding S-HDAg were combined for RNA transfection, both genomic and antigenomic RNAs could replicate (31). One of the possible explanations of these results is that a certain posttranslational modification of S-HDAg is required for the replication of HDV antigenomic-strand RNA, since the E. coli-derived HDAg is likely not posttranslationally modified. Thus, we were interested in examining whether in vitro-methylated S-HDAg could facilitate HDV antigenomic RNA replication. We first methylated the purified recombinant S-HDAg protein derived from E. coli by using PRMT1 in vitro; the methylated S-HDAg was mixed with an HDV genomic RNA or antigenomic RNA to form protein-RNA complexes. The protein-RNA complexes were transfected into Huh7 cells via liposomes. The replicated HDV RNA (i.e., the RNA of the opposite sense) was detected by Northern blotting on day 3 posttransfection. The results showed that in the presence of unmethylated S-HDAg, HDV genomic, but not antigenomic, RNA could replicate, in agreement with previous results (44) (Fig. 3). Interestingly, the methylated S-HDAg protein enabled the replication of both genomic and antigenomic RNAs (Fig. 3). To examine the possible mechanism for the effects of methylation, we used a UV cross-linking method to compare the RNA-binding activities of methylated and unmethylated HDAg. The results showed that there was no significant difference (data not shown). Therefore, the difference in the replication efficiencies of methylated and unmethylated S-HDAg was not due to a difference in protein-RNA complex-forming abilities. We concluded that the methylation of S-HDAg plays an important role in the replication of antigenomic RNA to form genomic RNA.
FIG. 3.
Role of methylation of S-HDAg in HDV RNA replication. The HDV genomic (A) or antigenomic (B) RNA was transfected together with S-HDAg mRNA, in vitro-methylated S-HDAg, or unmethylated S-HDAg into Huh7 cells. At 3 days posttransfection, total cellular RNAs were extracted, and antigenomic RNA (A) or genomic RNA (B) was detected by Northern blotting.
Methylation of the small delta antigen is required for HDV RNA replication in vivo following RNA transfection.
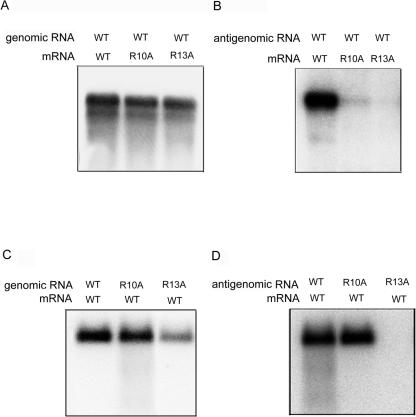
To further establish the importance of HDAg methylation in HDV RNA replication, we examined the ability of the HDAg methylation mutants to promote RNA replication by using the established RNA transfection method, in which HDV genomic or antigenomic RNA was cotransfected with a wild-type or mutant S-HDAg-encoding mRNA (31). When genomic RNA was used for transfection, the replication of HDV RNA was not affected by either R10A or R13A mutation (Fig. 4A). In contrast, when HDV antigenomic RNA was used, the mutant R13A mRNA did not enable RNA replication, whereas the mutant R10A mRNA enabled a very low level of HDV RNA replication (Fig. 4B). This result indicates that methylated HDAg is required for the initiation of RNA replication from the antigenomic strand, whereas unmethylated HDAg can support only genomic-strand RNA replication.
FIG. 4.
Role of methylation of S-HDAg in the initiation and maintenance of HDV RNA replication. The wild-type HDV genomic RNA (A) or antigenomic RNA (B) was transfected together with mRNAs encoding the wild type, the R10A mutant, or the R13A mutant into Huh7 cells. Conversely, genomic RNA (C) or antigenomic RNA (D) encoding wild-type, R10A, or R13A HDAg was transfected together with wild-type S-HDAg mRNA into Huh7 cells. At day 3 posttransfection, total RNAs were extracted, and the replicated RNA (RNA of the opposite sense) was detected by Northern blotting with an antigenomic RNA probe (A and C) or a genomic RNA probe (B and D).
We also introduced the same mutations into the HDV genomic or antigenomic RNA and cotransfected these mutant RNAs with the wild-type mRNA. In this experiment, wild-type S-HDAg encoded by the transfected mRNA will initiate the replication of genomic and antigenomic RNAs; however, the mutant S-HDAg encoded from the replicated genomic-length RNA will predominate in the later stages of the HDV replication cycle, once the transfected mRNA is degraded. Thus, this experiment examined whether the methylation of S-HDAg is required for continuous HDV RNA replication after RNA replication has been initiated. The results showed that R13A mutant antigenomic RNA could not replicate at all, while the corresponding genomic RNA replicated to only a small degree (Fig. 4C and D). Replication of the R10A mutant genomic or antigenomic RNA was largely unaffected. From these results, we conclude that the methylation of S-HDAg is important for both the initiation and maintenance of HDV RNA replication throughout the viral replication cycle.
We also used the methylation inhibitor S-adenosyl-homocysteine (AdoHcy) to verify the importance of methylation in HDV RNA replication. Huh7 cells were pretreated with different concentrations of AdoHcy for 2 h and then transfected with HDV RNA. AdoHcy was present throughout the experiment, and the replicated HDV RNA (the RNA of the opposite sense) was detected by Northern blotting after 2 days of drug treatment. We found that HDV RNA replication was reduced when concentrations of 2 mM or higher of AdoHcy were used (Fig. 5). We also used another methylation inhibitor, adenosine dialdehyde (AdOx), at a concentration of 20 μM to repeat this experiment; HDV RNA replication was also significantly inhibited by this compound (data not shown). Taken together, these results show that the methylation of S-HDAg is necessary for HDV RNA replication, in particular for the replication of the antigenomic strand to form the genomic strand.
FIG. 5.
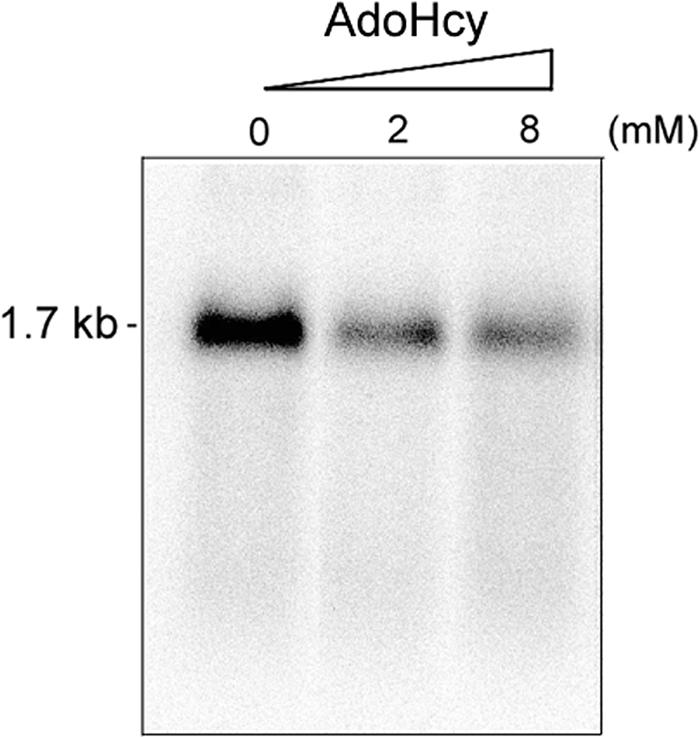
Effects of the methylation inhibitor AdoHcy on HDV RNA replication. Huh7 cells were pretreated with 0, 2, or 8 mM AdoHcy for 2 h and then cotransfected with HDV genomic RNA and wild-type S-HDAg mRNA. At day 2 posttransfection, the total RNAs were extracted, and the replicated RNA was detected by Northern blotting with an antigenomic RNA probe. AdoHcy was present throughout the experiment.
Methylation of the RGG motif affects the subcellular localization of HDAg.
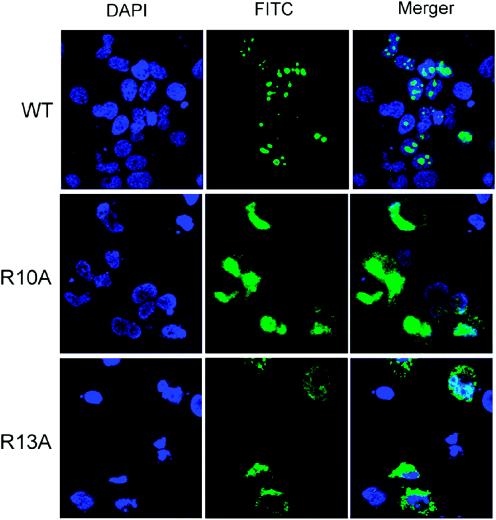
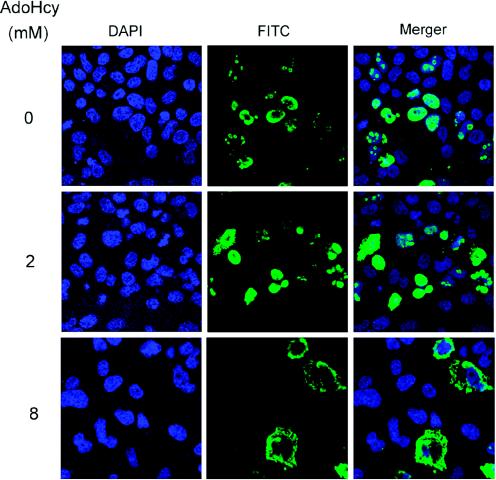
We next attempted to address how methylated S-HDAg participates in HDV RNA replication. Previous studies have shown that the methylation of nuclear proteins reduces their nucleic acid-binding activities due to the reduction of hydrophilic interactions (45). As stated earlier, we compared the RNA-binding activities of in vitro-methylated and unmethylated S-HDAg by using UV cross-linking or gel mobility shift assays; no significant difference was detected (data not shown). From the gel shifting assay data, we also excluded the possibility that methylation induced an RNA-protein conformational change. Recently, it was shown that the methylation of hnRNP A2 affects the subcellular localization of the protein (37). We therefore compared the subcellular localization of the wild-type and mutant S-HDAg proteins by immunocytochemistry. In agreement with earlier studies (1, 9, 52), wild-type S-HDAg was localized to the nucleus and formed speckles in the nucleus (Fig. 6). The R10A mutant protein was also localized mainly to the nucleus; however, it could not form speckled structures (Fig. 6). In comparison, the R13A mutant protein was found in both the cytoplasm and the nucleus, but it was more pronounced in the cytoplasm (Fig. 6). We also treated the HDAg-expressing cells with the methylation inhibitor AdoHcy prior to and after the expression of wild-type HDAg. At the highest concentration used (8 mM), S-HDAg was localized almost exclusively to the cytoplasm. With 2 mM AdoHcy, HDAg remained in the nucleus, but it had a substantially reduced ability to form speckled structures (Fig. 7). Correspondingly, HDV RNA replication was significantly inhibited at a 2 mM or higher concentration of AdoHcy (Fig. 5). From these results, we conclude that the methylation of S-HDAg affects the subcellular localization of HDAg, which is important for HDV RNA replication.
FIG. 6.
Subcellular localization of wild-type and mutant S-HDAgs. Huh7 cells were transfected with pCD3.1-WT, pCD3.1-R10A, and pCD3.1-R13A, which encode wild-type, R10A, and R13A HDAg, respectively. At 48 h posttransfection, the cells were fixed, permeabilized, and then probed with a monoclonal antibody against S-HDAg and a goat anti-mouse antibody conjugated with fluorescein isothiocyanate (green). The nuclei were stained with DAPI (4′,6′-diamidino-2-phenylindole) (blue).
FIG. 7.
The methylation inhibitor AdoHcy affects the subcellular localization of S-HDAg. Huh7 cells were plated on chamber slides and transfected with pCD3.1-WT DNA. Throughout the experiment, AdoHcy at 0, 2, or 8 mM was present in the medium as indicated. At 48 h posttransfection, the cells were stained as described in the legend to Fig. 6.
The methylation of S-HDAg is involved in the transportation of antigenomic RNA, but not genomic RNA.
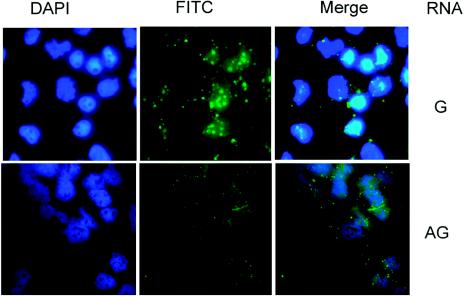
To further examine the importance of the methylation of S-HDAg in HDV RNA transportation and replication, we introduced fluorescein-labeled HDV genomic or antigenomic RNA together with E. coli-expressed S-HDAg into Huh7 cells according to previously described procedures (9, 44). HDV RNA was detected directly by fluorescence microscopy 1 h later. This experiment could reveal the ability of unmethylated S-HDAg to transport HDV RNA into the nucleus, since recombinant S-HDAg from E. coli is not methylated. We found that HDV genomic RNA was transported into the nucleus, forming speckled structures (Fig. 8) similar to those of S-HDAg (Fig. 6). We concluded that the HDV genomic RNA can be transported into the nucleus by unmethylated S-HDAg and is associated with the replication complex. In contrast, very little antigenomic RNA could be detected in the nuclei when it was introduced by the same procedure into the cells (the cytoplasmic RNA would be washed away in this procedure) (9). Since E. coli-derived HDAg is not methylated, this result suggests that the methylation of S-HDAg is important for nuclear transport of the HDV antigenomic RNA. We could not directly test the effects of in vitro-methylated HDAg because the efficiency of in vitro methylation was not high enough.
FIG. 8.
Transportation of genomic RNA, but not antigenomic RNA, is mediated by unmethylated wild-type S-HDAg. Fluorescein-labeled genomic or antigenomic RNA was incubated with E. coli-derived wild-type S-HDAg, and then the RNA-protein complex was transfected into Huh7 cells. One hour later, the cells were washed, fixed, and examined by fluorescence microscopy.
DISCUSSION
In this report, we demonstrated a novel posttranslational modification, namely, methylation, of the delta antigen of HDV and showed that the methylation is accomplished by PRMT1. PRMT1 methylates arginine residues in RGG and RXR motifs of RNA-binding proteins (49). S-HDAg is an RNA-binding protein that is predominantly located in the nucleus and that has two RGG motifs. By site-directed mutagenesis, we identified Arginine-13 as the main methylation site in S-HDAg for PRMT1. We also used antibodies that recognize monomethylated and dimethylated arginine or lysine residues to demonstrate the methylation of HDAg in vivo. We could detect HDAg with either of the two antibodies, which suggests that both arginine and lysine residues of HDAg are methylated (data not shown). However, because of the high background level, we could not demonstrate unequivocally the methylation of HDAg in vivo. We did not further pursue lysine methylation in this study.
We found that the methylation of S-HDAg is required for HDV RNA replication. This conclusion was reached by performing transfections of in vitro-methylated recombinant S-HDAg together with HDV genomic or antigenomic RNA. The replication of antigenomic RNA was supported by methylated S-HDAg but not by unmethylated S-HDAg. Furthermore, the mutant HDAgs that were defective in methylation could not support HDV RNA replication in the HDV RNA-HDAg mRNA transfection studies. The inhibition of cellular methylation by methylation inhibitors also decreased the replication of HDV RNA; however, we could not rule out the possibility that these inhibitors inhibited HDV RNA replication indirectly through the inhibition of cellular factors. Nevertheless, these results combined support the conclusion that methylation is essential for HDV RNA replication. Previously, it was demonstrated that the phosphorylation and acetylation of HDAg are essential for HDV RNA replication (6, 35, 36). Thus, multiple types of posttranslational modifications of S-HDAg are required for HDV RNA replication. It is interesting that the phosphorylation of S-177 affects only antigenomic, not genomic, RNA replication (35), similar to the effects of R13 methylation. Thus, antigenomic RNA replication appears to require a methylated and phosphorylated S-HDAg, whereas genomic RNA replication does not require HDAg modification. This finding adds to the growing list of the differences between the replication mechanisms of genomic and antigenomic RNA strands. For example, replication of the genomic RNA strand, but not the antigenomic strand, is inhibited by L-HDAg at the beginning of the RNA replication cycle (32). Also, the replication mechanisms of these two strands have different sensitivities to α-amanitin (30, 33). These findings are adding weight to the proposal that the replication of these two strands is carried out by different cellular machineries (30, 33).
The mechanism by which the methylation of HDAg is involved in HDV RNA replication is not yet clear. The first possibility is that methylation affects the conformation of the HDV RNA-HDAg complex. This possibility is similar to the functions of histone proteins in regulating DNA-dependent transcription. For example, the arginine methyltransferase CARM1 (also known as PRMT4) interacts with GRIP1, a coactivator of nuclear hormone receptors (7); as a result, CARM1/PRMT4 acts as a coactivator of nuclear receptor activity through an arginine methylation domain, which is capable of methylating histone H3. Arginine methylation is thus a histone modification that correlates with the active state of transcription, much like acetylation. Interestingly, nuclear receptors can recruit CARM1/PRMT4 methyltransferase and p300 acetylase to stimulate transcription (46), raising the possibility of cross talk between methylation and acetylation. Similarly, PRMT1 has been shown to bind to the p160 coactivators (20). A previous study (51) showed that the methylation of Arg-3 of H4 augments the subsequent acetylation of H4, indicating once again the cross talk between the two stimulating modifications. Histone methylation may cooperate with histone acetylation, phosphorylation, and other types of modification to modulate chromatin structure in a way that promotes transcriptional activation. The methylation of histone proteins may affect the conformation of chromatin (12, 19). We tried to determine whether S-HDAg methylation affects the HDAg-RNA structure by using a gel mobility shift assay. However, we did not find any significant difference between the methylated and the unmethylated protein-RNA complexes; nevertheless, this possibility could not be completely ruled out because the efficiency of in vitro methylation may be too low to show a difference.
The second possibility is that methylation affects the RNA-binding activity and subcellular localization of HDAg; the latter possibility is frequently observed for the methylation of other RNA-binding proteins (14, 37). We did not find any change in the RNA-binding properties of HDAg by methylation. However, we demonstrated that the localization of S-HDAg was affected by methylation. S-HDAg was exported to or retained in the cytoplasm after the addition of the methylation inhibitor AdoHcy. The methylation-defective mutants of S-HDAg, R13A and R10A, had altered subcellular localization and distribution patterns. In particular, these mutants failed to form speckled structures in the nucleus, which may be associated with the RNA replication machinery (1). Furthermore, we showed directly that unmethylated HDAg could not transport antigenomic RNA into the nucleus, while it could transport genomic RNA. These results combined suggest that the methylation of HDAg is involved in HDV replication partly through affecting the subcellular localization of S-HDAg. Since HDV RNA replication takes place in the nucleus (9, 28, 30), the retention of HDAg and antigenomic RNA in the cytoplasm would inhibit HDV RNA replication.
The third possibility is that HDAg may directly serve as a transcription factor and that the methylation of HDAg may activate its activity in transcription. Previous studies (30, 33) have suggested that HDV genomic and antigenomic RNAs may be replicated by two different mechanisms. The replication of antigenomic RNA to form genomic RNA may be accomplished by RNA polymerase II, whereas the replication of genomic RNA to form antigenomic RNA may be mediated by a polymerase I-like enzyme. Our finding here that the methylation of S-HDAg is required for the replication of antigenomic to genomic RNA and for the formation of the speckled structures in the nucleus raised the possibility that methylation may enable S-HDAg to interact with the RNA polymerase II transcription complex but is not necessary for interaction with the polymerase I transcription complex. The methylation and demethylation of proteins have been suggested to play regulatory functions in cells. We hypothesize that the regulation of the methylation of S-HDAg may control the replication of genomic RNA and antigenomic RNA through interactions with different components of the RNA replication machineries.
Acknowledgments
We thank D. Gerke for the preparation of GST-fused PRMT1, CARM1, and PRMT3.
This work was partially supported by NIH research grants AI47348 and DK55274.
REFERENCES
- 1.Bichko, V. V., and J. M. Taylor. 1996. Redistribution of the delta antigens in cells replicating the genome of hepatitis delta virus. J. Virol. 70:8064-8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang, F.-L., P.-J. Chen, S.-J. Tu, C.-J. Wang, and D.-S. Chen. 1991. The large form of hepatitis D antigen is crucial for assembly of hepatitis D virus. Proc. Natl. Acad. Sci. USA 88:8490-8494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang, M. F., S. C. Baker, L. H. Soe, T. Kamahora, J. G. Keck, S. Makino, S. Govindarajan, and M. M. Lai. 1988. Human hepatitis delta antigen is a nuclear phosphoprotein with RNA-binding activity. J. Virol. 62:2403-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chao, M., S.-Y. Hsieh, and J. Taylor. 1990. Role of two forms of hepatitis delta virus antigen: evidence for a mechanism of self-limiting genome replication. J. Virol. 64:5066-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chao, Y. C., C. M. Lee, H. S. Tang, S. Govindarajan, and M. M. Lai. 1991. Molecular cloning and characterization of an isolate of hepatitis delta virus from Taiwan. Hepatology 13:345-352. [PubMed] [Google Scholar]
- 6.Chen, C. W., Y. G. Tsay, H. L. Wu, C. H. Lee, D. S. Chen, and P. J. Chen. 2002. The double-stranded RNA-activated kinase, PKR, can phosphorylate hepatitis D virus small delta antigen at functional serine and threonine residues. J. Biol. Chem. 277:33058-33067. [DOI] [PubMed] [Google Scholar]
- 7.Chen, D., H. Ma, H. Hong, S. S. Koh, S. M. Huang, B. T. Schurter, D. W. Aswad, and M. R. Stallcup. 1999. Regulation of transcription by a protein methyltransferase. Science 284:2174-2177. [DOI] [PubMed] [Google Scholar]
- 8.Chevillard-Briet, M., D. Trouche, and L. Vandel. 2002. Control of CBP co-activating activity by arginine methylation. EMBO J. 21:5457-5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou, H. C., T. Y. Hsieh, G. T. Sheu, and M. M. Lai. 1998. Hepatitis delta antigen mediates the nuclear import of hepatitis delta virus RNA. J. Virol. 72:3684-3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davie, J. K., and S. Y. Dent. 2002. Transcriptional control: an activating role for arginine methylation. Curr. Biol. 12:R159-R161. [DOI] [PubMed] [Google Scholar]
- 11.Filipovska, J., and M. M. Konarska. 2000. Specific HDV RNA-templated transcription by Pol II in vitro. RNA 6:41-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischle, W., Y. Wang, and C. D. Allis. 2003. Histone and chromatin cross-talk. Curr. Opin. Cell Biol. 15:172-183. [DOI] [PubMed] [Google Scholar]
- 13.Glenn, J. S., J. A. Watson, C. M. Havel, and J. M. White. 1992. Identification of a prenylation site in delta virus large antigen. Science 256:1331-1333. [DOI] [PubMed] [Google Scholar]
- 14.Green, D. M., K. A. Marfatia, E. B. Crafton, X. Zhang, X. Cheng, and A. H. Corbett. 2002. Nab2p is required for poly(A) RNA export in Saccharomyces cerevisiae and is regulated by arginine methylation via Hmt1p. J. Biol. Chem. 277:7752-7760. [DOI] [PubMed] [Google Scholar]
- 15.Hwang, S. B., and M. M. C. Lai. 1993. A unique conformation at the carboxyl terminus of the small hepatitis delta antigen revealed by a specific monoclonal antibody. Virology 193:924-931. [DOI] [PubMed] [Google Scholar]
- 16.Hwang, S. B., K. S. Jeng, and M. M. C. Lai. 1995. Studies of functional roles of hepatitis delta antigen in delta virus RNA replication, p. 95-109. In G. Dinter-Gottleib (ed.), The unique hepatitis delta virus. R. G. Landes Company, Austin, Tex.
- 17.Hwang, S. B., and M. M. Lai. 1994. Isoprenylation masks a conformational epitope and enhances trans-dominant inhibitory function of the large hepatitis delta antigen. J. Virol. 68:2958-2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang, S. B., and M. M. Lai. 1993. Isoprenylation mediates direct protein-protein interactions between hepatitis large delta antigen and hepatitis B virus surface antigen. J. Virol. 67:7659-7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imhof, A. 2003. Histone modifications: an assembly line for active chromatin? Curr. Biol. 13:R22-R24. [DOI] [PubMed] [Google Scholar]
- 20.Koh, S. S., D. Chen, Y. H. Lee, and M. R. Stallcup. 2001. Synergistic enhancement of nuclear receptor function by p160 coactivators and two coactivators with protein methyltransferase activities. J. Biol. Chem. 276:1089-1098. [DOI] [PubMed] [Google Scholar]
- 21.Kuo, M. Y., M. Chao, and J. Taylor. 1989. Initiation of replication of the human hepatitis delta virus genome from cloned DNA: role of delta antigen. J. Virol. 63:1945-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwak, Y. T., J. Guo, S. Prajapati, K.-J. Park, R. M. Surabhi, B. Miller, P. Gehrig, and R. B. Gaynor. 2003. Methylation of SPT5 regulates its interaction with RNA polymerase II and transcriptional elongation properties. Mol. Cell 11:1055-1066. [DOI] [PubMed] [Google Scholar]
- 23.Landry, J., A. Sutton, T. Hesman, J. Min, R. M. Xu, M. Johnston, and R. Sternglanz. 2003. Set2-catalyzed methylation of histone H3 represses basal expression of GAL4 in Saccharomyces cerevisiae. Mol. Cell. Biol. 23:5972-5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, C. Z., P. J. Chen, and D. S. Chen. 1995. Large hepatitis delta antigen in packaging and replication inhibition: role of the carboxyl-terminal 19 amino acids and amino-terminal sequences. J. Virol. 69:5332-5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee, H. W., S. Kim, and W. K. Paik. 1977. S-Adenosylmethionine: protein-arginine methyltransferase. Purification and mechanism of the enzyme. Biochemistry 16:78-85. [DOI] [PubMed] [Google Scholar]
- 26.Li, H., S. Park, B. Kilburn, M. A. Jelinek, A. Henschen-Edman, D. W. Aswad, M. R. Stallcup, and I. A. Laird-Offringa. 2002. Lipopolysaccharide-induced methylation of HuR, an mRNA-stabilizing protein, by CARM1. Coactivator-associated arginine methyltransferase. J. Biol. Chem. 277:44623-44630. [DOI] [PubMed] [Google Scholar]
- 27.Li, J., Q. Lin, H. G. Yoon, Z. Q. Huang, B. D. Strahl, C. D. Allis, and J. Wong. 2002. Involvement of histone methylation and phosphorylation in regulation of transcription by thyroid hormone receptor. Mol. Cell. Biol. 22:5688-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macnaughton, T. B., and M. M. Lai. 2002. Genomic but not antigenomic hepatitis delta virus RNA is preferentially exported from the nucleus immediately after synthesis and processing. J. Virol. 76:3928-3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macnaughton, T. B., and M. M. Lai. 2002. Large hepatitis delta antigen is not a suppressor of hepatitis delta virus RNA synthesis once RNA replication is established. J. Virol. 76:9910-9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macnaughton, T. B., S. T. Shi, L. E. Modahl, and M. M. C. Lai. 2002. Rolling circle replication of hepatitis delta virus RNA is carried out by two different cellular RNA polymerases. J. Virol. 76:3920-3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Modahl, L. E., and M. M. C. Lai. 1998. Transcription of hepatitis delta antigen mRNA continues throughout hepatitis delta virus (HDV) replication: a new model of HDV RNA transcription and replication. J. Virol. 72:5449-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Modahl, L. E., and M. M. Lai. 2000. The large delta antigen of hepatitis delta virus potently inhibits genomic but not antigenomic RNA synthesis: a mechanism enabling initiation of viral replication. J. Virol. 74:7375-7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Modahl, L. E., T. B. Macnaughton, N. Zhu, D. L. Johnson, and M. M. Lai. 2000. RNA-dependent replication and transcription of hepatitis delta virus RNA involve distinct cellular RNA polymerases. Mol. Cell. Biol. 20:6030-6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mowen, K. A., J. Tang, W. Zhu, B. T. Schurter, K. Shuai, H. R. Herschman, and M. David. 2001. Arginine methylation of STAT1 modulates IFNalpha/beta-induced transcription. Cell 104:731-741. [DOI] [PubMed] [Google Scholar]
- 35.Mu, J.-J., H.-L. Wu, B.-L. Chiang, R.-P. Chang, D.-S. Chen, and P.-J. Chen. 1999. Characterization of the phosphorylated forms and the phosphorylated residues of hepatitis delta virus delta antigens. J. Virol. 73:10540-10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mu, J. J., Y. G. Tsay, L. J. Juan, T. F. Fu, W. H. Huang, D. S. Chen, and P. J. Chen. 2004. The small delta antigen of hepatitis delta virus is an acetylated protein and acetylation of lysine 72 may influence its cellular localization and viral RNA synthesis. Virology 319:60-70. [DOI] [PubMed] [Google Scholar]
- 37.Nichols, R. C., X. W. Wang, J. Tang, B. J. Hamilton, F. A. High, H. R. Herschman, and W. F Rigby. 2000. The RGG domain in hnRNP A2 affects subcellular localization. Exp. Cell. Res. 256:522-532. [DOI] [PubMed] [Google Scholar]
- 38.Nishioka, K., S. Chuikov, K. Sarma, H. Erdjument-Bromage, C. D. Allis, P. Tempst, and D. Reinberg. 2002. Set9, a novel histone H3 methyltransferase that facilitates transcription by precluding histone tail modifications required for heterochromatin formation. Genes Dev. 16:479-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pawlak, M. R., S. Banik-Maiti, J. A. Pietenpol, and H. E. Ruley. 2002. Protein arginine methyltransferase I: substrate specificity and role in hnRNP assembly. J. Cell Biochem. 87:394-407. [DOI] [PubMed] [Google Scholar]
- 40.Polson, A. G., B. L. Bass, and J. L. Casey. 1996. RNA editing of hepatitis delta virus antigenome by dsRNA-adenosine deaminase. Nature 380:454-456. [DOI] [PubMed] [Google Scholar]
- 41.Polson, A. G., H. L. Ley, B. L. Bass, and J. L. Casey. 1998. Hepatitis delta virus RNA editing is highly specific for the amber/W site and is suppressed by hepatitis delta antigen. Mol. Cell. Biol. 18:1919-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rizzetto, M., B. Hoyer, M. G. Canese, J. W.-K. Shih, R. H. Purcell, and J. L. Gerin. 1980. Delta agent: association of d antigen with hepatitis B surface antigen and RNA in serum of d-infected chimpanzees. Proc. Natl. Acad. Sci. USA 77:6124-6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen, E. C., M. F. Henry, V. H. Weiss, S. R. Valentini, P. A. Silver, and M. S. Lee. 1998. Arginine methylation facilitates the nuclear export of hnRNP proteins. Genes Dev. 12:679-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheu, G.-T., and M. M. C. Lai. 2000. Recombinant hepatitis delta antigen from E. coli promotes hepatitis delta virus RNA replication only from the genomic strand but not the antigenomic strand. Virology 278:578-586. [DOI] [PubMed] [Google Scholar]
- 45.Smith, J. J., K. P. Rucknagel, A. Schierhorn, J. Tang, A. Nemeth, M. Linder, H. R. Herschman, and E. Wahle. 1999. Unusual sites of arginine methylation in poly(A)-binding protein II and in vitro methylation by protein arginine methyltransferases PRMT1 and PRMT3. J. Biol. Chem. 274:13229-13234. [DOI] [PubMed] [Google Scholar]
- 46.Stallcup, M. R., D. Chen, S. S. Koh, H. Ma, Y. H. Lee, H. Li, B. T. Schurter, and D. W. Aswad. 2000. Co-operation between protein-acetylating and protein-methylating co-activators in transcriptional activation. Biochem. Soc. Trans. 28:415-418. [PubMed] [Google Scholar]
- 47.Strahl, B. D., S. D. Briggs, C. J. Brame, J. A. Caldwell, S. S. Koh, H. Ma, R. G. Cook, J. Shabanowitz, D. F. Hunt, M. R. Stallcup, and C. D. Allis. 2001. Methylation of histone H4 at arginine 3 occurs in vivo and is mediated by the nuclear receptor coactivator PRMT1. Curr. Biol. 11:996-1000. [DOI] [PubMed] [Google Scholar]
- 48.Strahl, B. D., P. A. Grant, S. D. Briggs, Z. W. Sun, J. R. Bone, J. A. Caldwell, S. Mollah, R. G. Cook, J. Shabanowitz, D. F. Hunt, and C. D. Allis. 2002. Set2 is a nucleosomal histone H3-selective methyltransferase that mediates transcriptional repression. Mol. Cell. Biol. 22:1298-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang, J., A. Frankel, P. J. Cook, S. Kim, W. K. Paik, K. R. Williams, S. Clarke, and H. R. Herschman. 2000. PRMT1 is the predominant type I protein arginine methyltransferase in mammalian cells. J. Biol. Chem. 275:7723-7730. [DOI] [PubMed] [Google Scholar]
- 50.Tang, J., J. D. Gary, S. Clarke, and H. R. Herschman. 1998. PRMT 3, a type I protein arginine N-methyltransferase that differs from PRMT1 in its oligomerization, subcellular localization, substrate specificity, and regulation. J. Biol. Chem. 273:16935-16945. [DOI] [PubMed] [Google Scholar]
- 51.Wang, H., Z. Q. Huang, L. Xia, Q. Feng, H. Erdjument-Bromage, B. D. Strahl, S. D. Briggs, C. D. Allis, J. Wong, P. Tempst, and Y. Zhang. 2001. Methylation of histone H4 at arginine 3 facilitating transcriptional activation by nuclear hormone receptor. Science 293:853-857. [DOI] [PubMed] [Google Scholar]
- 52.Xia, Y. P., C. T. Yeh, J. H. Ou, and M. M. Lai. 1992. Characterization of nuclear targeting signal of hepatitis delta antigen: nuclear transport as a protein complex. J. Virol. 66:914-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu, C., P. A. Henry, A. Setya, and M. F. Henry. 2003. In vivo analysis of nucleolar proteins modified by the yeast arginine methyltransferase Hmt1/Rmt1p. RNA 9:946-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu, W., H. Cho, and R. M. Evans. 2003. Acetylation and methylation in nuclear receptor gene activation. Methods Enzymol. 364:205-223. [DOI] [PubMed] [Google Scholar]
- 55.Zobel-Thropp, P., J. D. Gary, and S. Clarke. 1998. delta-N-methylarginine is a novel posttranslational modification of arginine residues in yeast proteins. J. Biol. Chem. 273:29283-29286. [DOI] [PubMed] [Google Scholar]