Abstract
Here we report that the N-pyridinylmethyl cyclam analog AMD3451 has antiviral activity against a wide variety of R5, R5/X4, and X4 strains of human immunodeficiency virus type 1 (HIV-1) and HIV-2 (50% inhibitory concentration [IC50] ranging from 1.2 to 26.5 μM) in various T-cell lines, CCR5- or CXCR4-transfected cells, peripheral blood mononuclear cells (PBMCs), and monocytes/macrophages. AMD3451 also inhibited R5, R5/X4, and X4 HIV-1 primary clinical isolates in PBMCs (IC50, 1.8 to 7.3 μM). A PCR-based viral entry assay revealed that AMD3451 blocks R5 and X4 HIV-1 infection at the virus entry stage. AMD3451 dose-dependently inhibited the intracellular Ca2+ signaling induced by the CXCR4 ligand CXCL12 in T-lymphocytic cells and in CXCR4-transfected cells, as well as the Ca2+ flux induced by the CCR5 ligands CCL5, CCL3, and CCL4 in CCR5-transfected cells. The compound did not interfere with chemokine-induced Ca2+ signaling through CCR1, CCR2, CCR3, CCR4, CCR6, CCR9, or CXCR3 and did not induce intracellular Ca2+ signaling by itself at concentrations up to 400 μM. In freshly isolated monocytes, AMD3451 inhibited the Ca2+ flux induced by CXCL12 and CCL4 but not that induced by CCL2, CCL3, CCL5, and CCL7. The CXCL12- and CCL3-induced chemotaxis was also dose-dependently inhibited by AMD3451. Furthermore, AMD3451 inhibited CXCL12- and CCL3L1-induced endocytosis in CXCR4- and CCR5-transfected cells. AMD3451, in contrast to the specific CXCR4 antagonist AMD3100, did not inhibit but enhanced the binding of several anti-CXCR4 monoclonal antibodies (such as clone 12G5) at the cell surface, pointing to a different interaction with CXCR4. AMD3451 is the first low-molecular-weight anti-HIV agent with selective HIV coreceptor, CCR5 and CXCR4, interaction.
Numerous publications over the last years have demonstrated the importance of chemokine receptors for human immunodeficiency virus (HIV) entry. Chemokines are chemotactic cytokines, which are classified as CC or CXC, depending on the positioning of the conserved cysteine residues. The first discovered HIV coreceptor, fusin/LESTR, now designated CXC-chemokine receptor 4 (CXCR4), mediates entry of X4, previously called T-tropic, or syncytium-inducing viruses (7, 30). These viruses can be inhibited by the only known natural ligand of CXCR4, the CXC-chemokine CXCL12 (stromal cell-derived factor 1, or SDF-1) (8, 47), which is a highly efficacious lymphocyte chemoattractant (9). Following CXCR4, the CC-chemokine receptor CCR5 was discovered to mediate the entry of R5, previously called M-tropic, or non-syncytium-inducing viruses (1, 13, 23, 27, 28). The CC-chemokines, CCL3 (macrophage inflammatory protein 1α, or MIP-1α), CCL4 (MIP-1β), and CCL5 (regulated on activation normal T-cell expressed and secreted, or RANTES), all natural ligands of CCR5, were shown to inhibit the replication of R5 viruses (15).
Several years ago, a class of chemical compounds, the bicyclams, were described as potent anti-HIV agents that were active against a broad range of HIV type 1 (HIV-1) and HIV-2 strains but not against simian immunodeficiency virus (SIV) or any other RNA or DNA viruses (11, 21, 22). The compounds failed to inhibit virus-cell binding and were ineffective in inhibiting the viral reverse transcriptase or protease (22). It was suggested that the bicyclams interfered with a postbinding event coinciding with the virus fusion-uncoating process (21). The precise mechanism of action of the bicyclams remained unknown until, following the discovery of the chemokine receptors as cofactors for HIV entry, it was demonstrated that they specifically interact with CXCR4 (25, 56). The prototype compound of the bicyclam class, AMD3100, is active against a broad range of X4 HIV strains and clinical isolates (50% inhibitory concentration [IC50], 1 to 10 nM) but not against R5 HIV-1 strains such as BaL or ADA (56). The pharmacokinetics, safety, and antiviral efficacy of AMD3100 were evaluated in phase I and II clinical trials (38, 54).
Other CXCR4 antagonists described following our reports on the interaction of AMD3100 with CXCR4 were ALX-40-4C, a polycationic nonapeptide solely consisting of arginine residues (26), and T22, a synthetic derivative of basic oligopeptides isolated from horseshoe crabs (46). These agents also inhibited X4 HIV-1 strains and CXCL12-induced intracellular Ca2+ signaling, and none of these molecules were capable of interacting with the chemokine receptor CCR5 (26, 46). Furthermore, a polycationic peptoid, CGP64222, which was published as the first Tat/TAR antagonist (35), was later shown to owe its anti-HIV activity to interaction with CXCR4 (19).
The fact that some healthy individuals, who were repeatedly exposed to HIV infection but remained uninfected, were found to be homozygous for a 32-bp deletion in the CCR5 gene (20, 42, 52) triggered the search for CCR5 antagonists as potential anti-HIV agents. Derivatives of the CC-chemokine CCL5 have been described as CCR5 antagonists with activity against R5 HIV-1 strains (3, 60), and the first nonpeptide CCR5 antagonist described was TAK-779 (4). This compound inhibited 125I-CCL5 binding to CCR5-transfected cells with an IC50 of 1.4 nM. The compound did not inhibit the binding of CCL5, CCL11 (eotaxin), CCL17 (thymus and activation-regulated chemokine, or TARC), and CXCL12 to CCR1-, CCR3-, CCR4-, or CXCR4-transfected cells, respectively. However, TAK-779 inhibited the binding of CCL2 (MCP-1) to CCR2b-transfected cells with an IC50 of 27 nM. Thus, TAK-779 is active against R5 viruses (IC50, 2 to 4 nM) and not against X4 viruses (4). The first orally available CCR5 antagonistic compound with potent anti-HIV activity (IC50, 0.4 to 9 nM) described is an oxime-piperidine compound called SCH-C (SCH351125) (61), and it showed antiviral efficacy in a clinical phase II study (51).
Although rare, HIV-1 infection in the Δ32-CCR5 individuals can occur (31), and a recent study of a Δ32-CCR5 person infected with HIV-1 demonstrated that the viral isolates from this patient exclusively used CXCR4 for target cell entry (45). These data demonstrate that the viral infection can be mediated and persistently maintained by viruses that exclusively utilize CXCR4. Also, Δ32-CCR5 HIV-1-infected persons show a relatively rapid CD4+-T-cell decline (31), suggesting the predominant presence of X4 viruses in these persons.
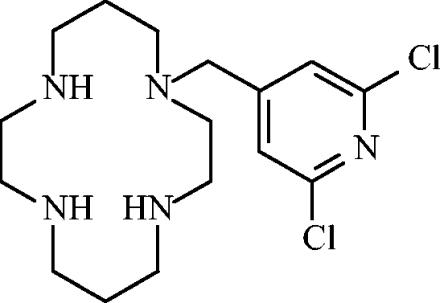
Although at least a dozen potential coreceptors have been described (for a review, see reference 6), CXCR4 and CCR5 are the two most relevant coreceptors for HIV entry (14, 65). Even the use of two coreceptors in vitro may add up to the use of only one coreceptor when examined in human lymphoid tissue ex vivo (33). As R5 viruses are predominant during the transmission of the virus and in the asymptomatic stage of infection, CCR5 antagonists may be useful during the early phases of HIV infection. The X4 viruses emerge later in the course of HIV infection, and the conversion of R5 to X4 strains often correlates with a drop in CD4+ T cells and is associated with a rapid progression to AIDS (17, 57). Therefore, CXCR4 antagonists may be useful in delaying the onset of disease. Thus, a therapeutic intervention targeting both CCR5 and CXCR4 may be highly valuable for inhibiting viral transmission and replication in addition to diminishing the pathogenic effects of late-stage X4 viruses. Moreover, an increasing number of reports have shown that HIV gp120 can trigger signaling and chemotactic events specifically through CCR5 and CXCR4 and in a CD4-independent manner (41). Such effects induced by the viral envelope would also be effectively blocked by CCR5/CXCR4 antagonists. Here, we describe the first specific dual CCR5/CXCR4 antagonist, AMD3451 (Fig. 1), with antiviral activity against a broad range of HIV strains and primary clinical isolates in different cell types.
FIG. 1.
Chemical structure of AMD3451.
MATERIALS AND METHODS
Viruses, cells, cell lines, and cell culture.
The HIV-1 IIIB strain and the HIV-2 ROD strain and the HIV-1 strains BaL and ADA were obtained through the MRC (Centralised Facility for AIDS Reagents, Potters Bar, Hertfordshire, United Kingdom). The HIV-1 T-tropic molecular clone NL4.3 was obtained from the National Institute of Allergy and Infectious Disease AIDS Reagent Program. The HIV-1 HE strain was isolated from a Belgian AIDS patient. The HIV-1 NL4.3 strains were made resistant to AMD3100 (24) or to CXCL12 (55) in MT-4 cells as described previously. For the isolation of primary HIV-1 isolates, blood was collected from HIV-1-infected patients, isolated by Ficoll gradient, and mixed with 3-day-old phytohemagglutinin (PHA)-stimulated peripheral blood mononuclear cells (PBMCs) from a healthy donor. Cultures were maintained for 14 to 18 days, with the addition of fresh medium and interleukin-2 (IL-2) every 3 to 4 days. The supernatant was spun down and stored at −80°C in 0.5-ml aliquots. The coreceptor use of these clinical isolates was determined on the astroglioma U87.CD4 cell line transfected with either CCR1, CCR2b, CCR3, CCR5, or CXCR4, which were kindly provided by D. R. Littman. The CD4+ lymphocytic SUP-T1 and Molt-4 cell lines and the CD4+ monocytic THP-1 cell line were obtained from the American Type Culture Collection. Human osteosarcoma HOS.CD4 cells that express human CD4 and the chemokine receptor CCR1, CCR2b, CCR3, CCR4, CCR5, or CXCR4 (17) were obtained through the National Institute of Allergy and Infectious Disease AIDS Reagent Program. The transformed MT-4 T-cell line (36) has been described elsewhere. PBMCs from healthy donors were isolated by density gradient centrifugation and stimulated with PHA at 1 μg/ml (Sigma, Bornem, Belgium) for 3 days at 37°C. The PHA-stimulated blasts were washed twice with phosphate-buffered saline (PBS) and exposed to the viral stocks (1 ng of p24 antigen [Ag] per 106 cells) at 37°C in culture medium (RPMI 1640 medium with 10% fetal calf serum). After 1 h, the cells were washed three times with PBS. The cells were seeded at 2 × 105 cells per well into a 96-well plate and cultured in medium containing IL-2 (25 U/ml) and various concentrations of AMD3451, the bicyclam AMD3100, CXCL12, or CCL5. The cell supernatant was collected at day 12, and HIV-1 core Ag in the culture supernatant was analyzed by a p24 Ag enzyme-linked immunosorbent assay (ELISA) kit (NEN Life Science Products Inc., Boston, Mass.). For HIV-2 p27 Ag detection, the INNOTEST from Innogenetics (Temse, Belgium) was used. Human primary monocytes/macrophages (M/M) were obtained by previously published procedures (2, 48). Briefly, PBMCs were separated by Ficoll-Hypaque gradient and seeded in plastic 48-well plates (Costar, Cambridge, Mass.) at 1.8 × 106 cells/ml in RPMI 1640 (Gibco Labs, Gaithersburg, Md.) with the addition of 50 U of penicillin/ml, 50 μg of streptomycin/ml, 2 mM l-glutamine, and 20% heat-inactivated, mycoplasma-free, and endotoxin-free fetal calf serum (HyClone, Logan, Utah). On the fifth day of culture, nonadherent cells were removed by repeated gentle washing with warm medium. Adherent cells obtained with this technique consisted of >98% differentiated M/M. M/M were exposed to various concentrations of AMD3451 for 10 min; then they were challenged with 300 CCID50 (50% cell culture infective doses) per ml of HIV-1 BaL. After 2 h of incubation, M/M were extensively washed with warm medium to remove the excess virus and then cultured in the presence of drug under the same conditions as before. M/M were washed and fed every 5 days with fresh medium and replenished with compound where required. At established time points, supernatants were collected and virus production was determined by p24 Ag ELISA. For the assessment of cytotoxicity, mock-infected M/M were treated with various concentrations of the test compound. Assessment of the cytotoxic effect was performed twice weekly by visual inspection and then by counting cells and trypan blue dye exclusion at day 14 after the beginning of treatment.
Chemokines and MAbs.
Recombinant human chemokines CXCL10 (IP-10), CXCL12 (SDF-1), CCL2 (MCP-1), CCL3 (MIP-1α), CCL3L1 (LD78β), CCL11 (eotaxin), CCL20 (MIP-3α), CCL22 (MDC), and CCL25 (TECK) were purchased from PeproTech (London, United Kingdom), and human CCL3 (MIP-1α), CCL4 (MIP-1β), and CCL5 (RANTES) were purchased from R&D Systems (Abingdon, United Kingdom). CCL7 (MCP-3) was chemically synthesized according to the published protein sequence (64). The anti-CXCR4 monoclonal antibodies (MAbs), clone 12G5 (no. 170), no. 171, no. 172, and no. 173, reacting specifically with the human CXCR4, and the anti-CCR5 MAbs (clones 2D7, 45502, 45523, 45531, and 45549), reacting specifically with human CCR5, were all purchased from R&D Systems.
Analysis of CXCR4 expression.
SUP-T1 cells were incubated with AMD3100 or CXCL12 (at different concentrations) or PBS for different time periods (1 or 15 min) and at different temperatures (on ice or at room temperature), and the cells were washed once with PBS. The anti-CXCR4 MAb (10 μg/ml) was then added for 30 min at room temperature. The cells were washed twice in PBS and then incubated with fluorescein isothiocyanate-conjugated goat anti-mouse antibody (Caltag Laboratories, Burlingame, Calif.) for 30 min at room temperature and washed twice in PBS. Cells were analyzed by a FACScan or a FACScalibur (Becton Dickinson, San Jose, Calif.) flow cytometer. The percentage of positive cells and the mean fluorescence intensity (MFI) values are indicated on each histogram. The region for positivity was defined by using a control isotype MAb (Becton Dickinson). The percent inhibition of MAb binding in the presence of different concentrations of compound was calculated by using the MFI values, as described previously (56).
HIV entry PCR.
CXCR4- or CCR5-transfected cells were seeded in 24-well plates at 106 cells per well. Virus stocks (diluted to a p24 titer of 10,000 pg/ml) were treated with 500 U of RNase-free DNase (Roche Molecular Biochemicals)/ml for 1 h at room temperature. Then the cells in each well were infected with 1,000 pg of p24. After incubation at 37°C for 2 h, the cells were washed twice with PBS and total DNA was extracted from the infected cells by using the QIAamp DNA mini kit (QIAGEN, Hilden, Germany). The DNA was eluted from the QIAamp spin columns in a final volume of 200 μl of elution buffer. Then 10 μl of each DNA sample was subjected to 38 cycles of HIV-1 long terminal repeat (LTR) R/U5-specific and 28 cycles of β-actin-specific PCR on a Biometra T3 thermocycler. Each cycle comprised a 45-s denaturation step at 95°C, a 45-s annealing step at 61°C, and a 45-s extension step at 72°C. The primers used were as follows: LTR R/U5 sense primer, 5′-GGCTAACTAGGGAACCCACTG-3′ (nucleotides 496 to 516, according to the HIV-1 HXB-2 DNA sequence; see reference 50); LTR R/U5 antisense primer, 5′-CTGCTAGAGATTTTCCACACTGAC-3′ (nucleotides 612 to 635); β-actin sense primer, 5′-TCTGGCGGCACCACCATGTACC-3′ (nucleotides 2658 to 2679); β-actin antisense primer, 5′-CGATGGAGGGGCCGGACTCG-3′ (nucleotides 2961 to 2980). The reaction mixtures contained PCR buffer (supplied with the enzyme), 200 μM (each) dATP, dGTP, dCTP, and dTTP (Life Technologies), 0.4 μM (each) forward and reverse primers, and 0.5 U of SuperTaq DNA polymerase (HT Biotechnology, Cambridge, England) in a total volume of 25 μl. After gel electrophoresis through a 2% agarose gel, the amplified DNA fragments were visualized by ethidium bromide. In preliminary experiments, the exponential range of the PCR amplification curve was determined for both the HIV-1 LTR R/U5 and the β-actin PCRs by varying the amount of input DNA and the number of PCR cycles. Based on these experiments, appropriate conditions were chosen to perform the PCRs (37).
Measurement of intracellular calcium concentrations.
Ca2+ mobilization assays were performed by the use of a fluorometric imaging plate reader (FLIPR) (Molecular Devices, Sunnyvale, Calif.) as described previously (49). Briefly, the cells were loaded with the fluorescent calcium indicator Fluo-3 acetoxymethyl (Molecular Probes, Leiden, The Netherlands) in the appropriate culture medium for 45 min at 37°C, after which the cells were washed three times in Hanks balanced salt solution buffer containing 20 mM HEPES and 0.2% bovine serum albumin (pH 7.4). Chemokine-induced Ca2+ flux was then simultaneously measured in all 96 wells in a black-wall microtiter plate and in real time with a FLIPR, and data were expressed as fluorescence units versus time.
Chemotaxis assay.
For the chemotaxis assay, CCR5-transfected Jurkat cells (a CD4+-T-cell line with endogenous CXCR4 and stably transfected with human CCR5 [MRC, Centralised Facility for AIDS Reagents]) were preincubated for 10 min with AMD3451 at the indicated concentrations. Then 5-μm-pore-size Transwell filter membranes (Costar) were loaded with 106 cells and transferred to a 24-well plate containing 100 ng of CXCL12/ml or 500 ng of CCL4/ml in 600 μl of buffer. The plate was then incubated at 37°C and 5% CO2 for 4 h, after which the filter inserts were carefully removed and the migrated cells were collected from the wells and fixed with 1% paraformaldehyde. Then each sample was counted for 2 min in a FACSCalibur flow cytometer, and viable cells were analyzed by the conventional forward and side scatter gating. A serial of standards (1/2 dilutions of 106 cells to 98 cells) was used to calibrate the exact amount of cells that were in the samples by linear regression. To calculate the percentage of migrated cells, the numbers of migrated cells in the compound-exposed samples were compared with the number of migrated cells in the untreated positive control (no pretreatment with AMD3451).
Receptor internalization assay.
U87.CD4 cells stably transfected with green fluorescent protein (GFP)-coupled CXCR4 (U87.CD4.CXCR4-GFP) were seeded in 0.001% poly-d-lysine-coated eight-well Lab-Tek chamber slides (Nalge Nunc International, Naperville, Ill.) at 4 × 104 cells per well. The next day, the cells were preincubated in cell culture medium with or without 400 μM AMD3451 for 15 min at room temperature. Then CXCL12 was added at a final concentration of 1 μg/ml. After incubation at 37°C for 45 min, the chamber slides were placed on ice and the cells were washed once with ice-cold PBS, fixed with 1% paraformaldehyde in PBS for 5 min on ice, and washed three times with ice-cold PBS. The chambers were removed from the glass slides, and a coverslip was placed on the cells. On the other hand, CEM cells stably transfected with GFP-coupled CCR5 were washed once with calcium flux assay buffer and preincubated with or without AMD3451 at 400 μM for 15 min at room temperature. After 30 min of incubation at 37°C with CCL3L1, added at a final concentration of 100 ng/ml, cells were placed on glass slides and a coverslip was fixed on the slide with nail polish. For both cell lines, cell-associated fluorescence was examined by a Nikon fluorescence microscope (Tokyo, Japan).
Site-directed mutagenesis and expression of mutant receptors.
Point mutations were introduced in the CXCR4 receptor by oligonucleotide-directed mutagenesis, and wild-type and mutant receptors were expressed in COS-7 cells as described previously (32, 37). The His residues, His113, His203, and His281, located in the extracellular loops or in the transmembrane domains, were individually mutated to Ala residues. In addition, four Asp residues (Asp171 [located in transmembrane domain IV {TM-IV}], Asp182 and Asp193 [located in extracellular loop 2], and Asp262 [located in TM-VI]) were mutated to Asn residues (32).
Receptor binding assays.
The human chemokine Met-CXCL12 was kindly provided by Michael A. Luther (Glaxo Wellcome). This CXCL12 contains an additional NH2-terminal methionine; however, the protein shows the same binding properties as natural ligand CXCL12 (18, 58). 125I-labeled Met-CXCL12 was prepared by oxidative iodination with IODO-GEN (Pierce), followed by high-pressure liquid chromatography purification to separate unlabeled and labeled compound. The MAb 12G5 was kindly provided by Jim Hoxie (University of Pennsylvania, Philadelphia). 12G5 was 125I-labeled by using Bolton-Hunter reagent (Amersham Pharmacia Biotech) as described previously (59). The transfected COS-7 cells were transferred to culture plates 1 day after transfection. The number of cells seeded per well was determined by the apparent expression efficiency of the individual clones, and the number of cells per well was adjusted, aiming at 5 to 10% binding of the added radioligand. Two days after transfection, cells were assayed by competition binding performed on whole cells for 3 h at 4°C with 12 pM 125I-Met-CXCL12 or 32 pM 125I-12G5 plus variable amounts of unlabeled peptide or nonpeptide compounds in 400 μl of 50 mM HEPES (pH 7.7) supplemented with 1 mM CaCl2, 5 mM MgCl2, and 0.5% (wt/vol) bovine serum albumin (Sigma). After incubation, cells were washed quickly four times in 4°C binding buffer supplemented with 0.5 M NaCl. Determinations were made in duplicate.
RESULTS
Antiretroviral activity profile of AMD3451.
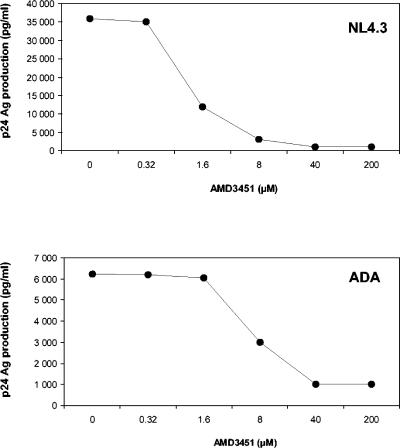
AMD3451 was active in MT-4 cells against laboratory X4 HIV-1 strains such as IIIB and NL4.3, which exclusively use CXCR4 to enter the cells. The IC50 was 1.2 μM for the IIIB strain and 1.8 μM for the NL4.3 strain (Table 1). The IIIB strain and the NL4.3 strain were equally well inhibited by AMD3451 in the CXCR4-transfected HOS.CD4 cells (Table 1). Surprisingly, AMD3451 was also active against the HIV-1 strains BaL and ADA, which use CCR5 as the main coreceptor to enter the cells (1, 28) in CCR5-transfected cell lines and in PBMCs (Table 1). AMD3451 also inhibited HIV-1 BaL replication in purified M/M, with an IC50 of 23.9 μM, and no cellular toxicity was observed in M/M at 400 μM. As described, AMD3100 was completely inactive against these R5 virus strains (56). The virus p24 Ag production profile of HIV-1 NL4.3 (X4 virus) and HIV-1 ADA (R5 virus) in PBMCs (from the same donor) in the presence of increasing concentrations of AMD3451 is shown in Fig. 2. Moreover, AMD3451 also proved active against virus strains such as HIV-1 HE and HIV-2 ROD, which can use both CXCR4 and CCR5 (and CCR3) to enter the target cells. However, AMD3451 was not active against these viruses when evaluated in CCR3-transfected U87.CD4 cells (Table 1). Thus, AMD3451 inhibited viral replication in CCR5- and CXCR4-transfected cells but not in CCR3-transfected cells.
TABLE 1.
Antiviral activity of AMD3451 against HIV strains in different cell linesa
| Strain | Phenotype (coreceptor use) | Cell type | AMD3451 IC50 (μM) |
|---|---|---|---|
| HIV-1 ADA | R5 | PBMC | 6.0 |
| HIV-1 BaL | R5 | HOS.CD4.CCR5 | 8.3 |
| U87.CD4.CCR5 | 17.6 | ||
| M/M | 23.9 | ||
| HIV-1 IIIB | X4 | MT-4 | 1.2 |
| HOS.CD4.CXCR4 | 3.2 | ||
| HIV-1 NL4.3 | X4 | MT-4 | 1.8 |
| HOS.CD4.CXCR4 | 6.9 | ||
| U87.CD4.CXCR4 | 17.6 | ||
| PBMC | 3.0 | ||
| HIV-1 | |||
| NL4.3AMD3100res | X4 | MT-4 | 9.7 |
| HIV-1 NL4.3CXCL12res | X4 | MT-4 | 6.1 |
| HIV-1 HE | R3, R5, X4 | MT-4 | 26.5 |
| HOS.CD4.CXCR4 | 7.9 | ||
| HOS.CD4.CCR5 | 6.1 | ||
| U87.CD4.CCR3 | >40 | ||
| HIV-2 ROD | R3, R5, X4 | MT-4 | 9.0 |
| U87.CD4.CCR3 | >40 |
Effect of AMD3451 on the replication of HIV strains in different cell lines, PHA-stimulated PBMCs, and M/M. The virus yield was monitored in the cell-free supernatant 4 to 14 days after infection by viral p24 HIV-1 Ag or p27 HIV-2 Ag ELISA. Results represent mean values of the results from at least three separate experiments. The phenotypes (coreceptor use) of the viruses were determined for their ability to replicate in U87.CD4 cells transfected with CCR3, CCR5, or CXCR4. The data shown are the mean IC50 of the results from at least three separate experiments.
FIG. 2.
Dose-dependent inhibition of HIV-1 NL4.3 (X4 virus) and HIV-1 ADA (R5 virus) replication in PBMCs by AMD3451. The data shown are the mean IC50s of the results from four experiments.
The development of an AMD3100-resistant NL4.3 strain (NL4.3AMD3100res virus) in MT-4 cells by culture of the virus in the presence of progressively increasing concentrations of AMD3100 was previously reported (24). AMD3451 retained its antiviral activity against this strain (IC50, 9.7 μM compared to 1.8 μM for the wild-type NL4.3 virus) (Table 1). More recently, an NL4.3 virus resistant to CXCL12 was also generated in the same T-cell line (55). It took about 3 months to obtain a highly CXCL12-resistant virus (NL4.3CXCL12res virus) in MT-4 cells (IC50 of CXCL12 >2,000 ng/ml compared to 130 ng/ml for the parental NL4.3 strain). Again, AMD3451 remained active against the NL4.3CXCL12res virus (IC50, 6.1 μM) (Table 1). However, AMD3451 completely inhibited the NL4.3 and NL4.3CXCL12res strains at 8 μM, whereas the NL4.3AMD3100res virus became completely suppressed at a dose of 200 μM.
When AMD3451 was tested against four different R5 clinical isolates, its IC50 only ranged between 2.8 and 7.3 μM. For two R5/X4 clinical isolates, the IC50s were 1.8 and 3.6 μM, and for two X4 clinical isolates, the IC50s were 2.6 and 5.9 μM (Table 2). CCL5 at 500 ng/ml inhibited all four R5 isolates by >95% and inhibited the two X4/R5 isolates by 34 and 59%, respectively, but as expected, it did not inhibit the two X4 isolates (data not shown). CXCL12 at 500 ng/ml did not inhibit the four R5 isolates and one of the X4/R5 isolates, but it inhibited the other X4/R5 isolate by 11% and the two X4 isolates by 67 and 11%, respectively (data not shown). The antiviral activity of the CCR5 antagonist TAK-779 against the four clinical R5 isolates was very variable (IC50s ranging from 18 ng/ml for isolate 19 to >5,000 ng/ml for isolate 15) (data not shown). The variable inhibitory effects of these agents on HIV-1 replication have also been described by other groups (39, 53, 62). Thus, although AMD3451 does not seem to be a highly potent antiviral agent, the data demonstrate its remarkable consistency in viral inhibition. The CCID50 of AMD3451 in all of these different cell types ranged from 80 μM (e.g., MT-4 cells) to 400 μM (e.g., PBMCs).
TABLE 2.
Antiviral activity of AMD3451 against HIV-1 clinical isolates in PBMCsa
| Clinical HIV-1 isolate no. | Phenotype (coreceptor use) | AMD3451 IC50 (μM) |
|---|---|---|
| 6 | R5 | 7.3 |
| 14 | R5 | 2.8 |
| 15 | R5 | 5.7 |
| 19 | R5 | 4.7 |
| 16 | R5, X4 | 1.8 |
| 18 | R5, X4 | 3.6 |
| 10 | X4 | 2.6 |
| 17 | X4 | 5.9 |
Effect of AMD3451 on the replication of primary HIV-1 isolates in PHA-stimulated PBMCs. The virus yield was monitored in the cell-free supernatant 10 to 12 days after infection by viral p24 Ag ELISA. The phenotypes (coreceptor use) of the clinical isolates were determined by their ability to replicate in U87.CD4 cells transfected with CCR5 or CXCR4. The data shown are the mean IC50 of the results from at least three different experiments.
AMD3451 inhibits viral entry.
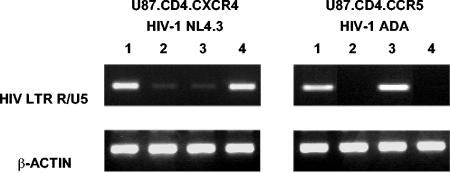
A PCR-based HIV-1 entry assay was set up to verify that AMD3451 efficiently blocks HIV infection at a very early step. A semiquantitative HIV-1 LTR R/U5-specific PCR was performed on total DNA isolated from U87.CD4.CXCR4 and U87.CD4.CCR5 cells at 2 h after infection with the X4 HIV-1 NL4.3 strain and the R5 HIV-1 ADA strain, respectively. Synthesis of the LTR R/U5 DNA transcript, also known as strong-stop DNA, is the first step of the reverse transcription process and is accomplished at a very early stage of the infection, immediately after viral entry and uncoating. When the cells were preincubated with AMD3451 at 200 μM, viral entry was strongly inhibited in both transfected cell lines (Fig. 3). As a control for the viral entry assays, AMD3100 (1 μM) (but not TAK-779 [Takeda, Osaka, Japan]) inhibited the entry of the X4 virus, whereas TAK-779 (2 μM) (but not AMD3100) inhibited the entry of R5 virus (Fig. 3). Thus, AMD3451 inhibits both X4 and R5 HIV-1 infection at the level of virus entry into the cells.
FIG. 3.
PCR-based detection of virus (HIV-1 NL4.3 and HIV-1 ADA) entry into U87.CD4.CXCR4 and U87.CD4.CCR5 cells. Two hours after infection with HIV-1, total DNA was extracted from the cells and analyzed by PCR with HIV-1-specific primers from the LTR R/U5 region. Lane 1, positive control; lane 2, 200 μM AMD3451; lane 3, 1 μM AMD3100; lane 4, 2 μM TAK-779. DNA recovery was controlled by PCR with β-actin-specific primers. The data shown are from one representative experiment out of two.
Ca2+ mobilization studies.
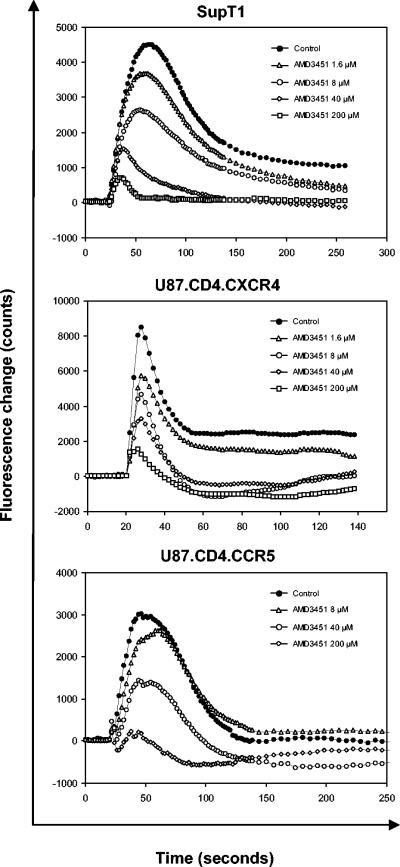
The specific and potent inhibitory effect of AMD3451 on the different HIV strains and isolates suggested a dual interaction of AMD3451 with both HIV-1 coreceptors CXCR4 and CCR5. Therefore, the effect of AMD3451 on chemokine-induced calcium mobilization was examined in the lymphocytic SUP-T1 and monocytic THP-1 cell lines, in chemokine receptor-transfected HOS, U87, and HEK293 cell lines, in PHA/IL-2-stimulated PBMCs, and in purified monocytes. Figure 4 shows the dose-dependent inhibitory effect of AMD3451 on the Ca2+ flux induced by CXCL12 in SUPT-1 cells and in U87.CD4.CXCR4 cells and on the Ca2+ flux induced by CCL4 in U87.CD4.CCR5 cells. Similar observations were made in CXCR4- and CCR5-transfected HOS.CD4 cells. AMD3451 at 200 μM completely blocked the Ca2+ signaling induced by 30 ng of CXCL12/ml in HOS.CD4.CXCR4 cells. At 60, 20, and 6 μM, AMD3451 inhibited the CXCL12-induced Ca2+ flux by 78, 40, and 18%, respectively, in these cells (data not shown). In HOS.CD4.CCR5 cells, a complete inhibition of the Ca2+ flux induced by CCL5 was observed when the cells were preincubated with AMD3451 at 200 μM. AMD3451 at 60 and 20 μM inhibited the CCL5-induced Ca2+ flux by 45 and 19%, respectively. Comparable results were obtained in the CCR5-transfected HEK293 cells (data not shown).
FIG. 4.
Dose-dependent inhibitory effect of AMD3451 on CXCL12-induced Ca2+ flux in SUP-T1 cells (top) and U87.CD4.CXCR4 cells (middle) and on CCL4-induced Ca2+ flux in U87.CD4.CCR5 cells (bottom). After loading with the fluorescent calcium indicator Fluo-3, the cells were preincubated for 10 min with AMD3451 at the indicated concentrations and then stimulated with chemokine (10-ng/ml CXCL12 or 5-ng/ml CCL4). The transient increase in intracellular calcium concentration was recorded by monitoring the change in fluorescence of the cells as a function of time by using the FLIPR. Each data point represents the average fluorescence of four identical microplate wells. The data shown are from one representative experiment out of three.
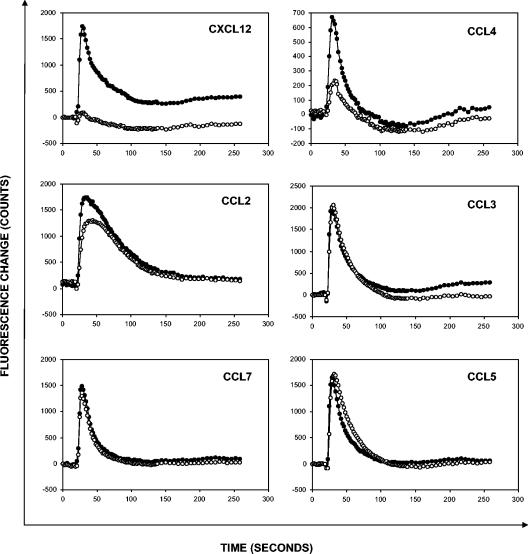
AMD3451, at 200 μM, did not inhibit the Ca2+ flux induced by CCL3 in HOS.CD4.CCR1 cells, the Ca2+ flux induced by CCL11 in U87.CD4.CCR3 cells, or the Ca2+ flux induced by CCL22 in HOS.CD4.CCR4 cells. Furthermore, the compound (200 μM) was unable to inhibit the Ca2+ flux induced by CCL2 and CCL7 (ligands for CCR2 and CCR1/CCR2/CCR3, respectively) in the monocytic THP-1 cell line (data not shown). AMD3451 also failed to block the calcium response elicited by the CCR9 ligand CCL25 (TECK) in T-lymphoid Molt-4 cells and the calcium fluxes induced by the CCR6 ligand CCL20 and the CXCR3 ligand CXCL10 (IP-10) in PHA/IL-2-stimulated PBMCs (data not shown). In freshly isolated monocytes, AMD3451 at a concentration of 200 μM inhibited the Ca2+ flux induced by the CXCR4-specific chemokine CXCL12 and the CCR5-specific chemokine CCL4, but not that of CCL5 (ligand for CCR5, but also for CCR1 and CCR3), CCL3 (ligand for CCR5, but also for CCR1), CCL2 (CCR2 ligand), or CCL7 (CCR1/CCR2/CCR3 ligand) (Fig. 5). Thus, AMD3451 clearly interacts with CXCR4 and CCR5, but no interaction could be observed with CCR1, CCR2, CCR3, CCR4, CCR6, CCR9, or CXCR3. Importantly, AMD3451 by itself did not induce Ca2+ flux in the HOS.CD4 transfected cells or THP-1 cells at concentrations up to 400 μM.
FIG. 5.
Effects of AMD3451 on the Ca2+ flux induced by CXCL12, CCL2, CCL3, CCL4, CCL5, and CCL7 in freshly isolated human monocytes. After loading with the fluorescent calcium indicator Fluo-3, the cells were preincubated for 10 min with (open symbols) or without (closed symbols) compound and then stained with chemokine (50 ng/ml). The transient increase in intracellular calcium concentration was recorded by monitoring the change in fluorescence of the cells as a function of time by using the FLIPR. Each data point represents the average fluorescence of four identical microplate wells. The data shown are from one representative experiment out of three.
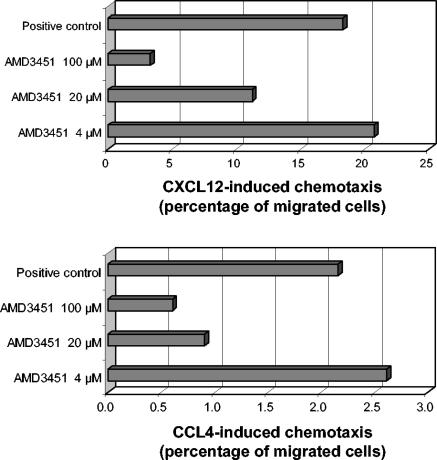
AMD3451 inhibits CXCL12- and CCL4-induced chemotaxis.
The capacity of AMD3451 to inhibit CXCL12- or CCL4-induced migration of CCR5-transfected CXCR4+ Jurkat cells was also investigated (Fig. 6). CXCL12 elicited a strong chemotactic response in these cells, and the percentage of cells that migrated towards CXCL12 gradually decreased when the cells were preincubated with AMD3451 at increasing concentrations. In addition, AMD3451 also dose-dependently inhibited the CCL4-induced migration of the cells. A 50% inhibition of both chemotactic responses was obtained with AMD3451 at a concentration of ∼40 μM.
FIG. 6.
Concentration-dependent inhibitory effect of AMD3451 on CXCL12- and CCL4-induced chemotaxis of CCR5-transfected Jurkat cells. The cells were preincubated for 10 min with AMD3451 at the indicated concentrations. Then 5-μm-pore-size filter inserts were loaded with 106 cells and transferred to a 24-well plate containing 100-ng/ml CXCL12 or 500-ng/ml CCL4 in 600 μl of buffer. Transmigration of the cells from the insert to the lower compartment, containing the chemokine, was monitored microscopically. After 4 h of incubation, the membrane inserts were removed and the cells in the wells were collected and counted by flow cytometry. The percentage of migrated cells matches the ratio of the numbers of migrated cells in the lower wells to the number of migrated cells in the positive control (no pretreatment with AMD3451). The exact number of cells in the lower wells was calculated by linear regression with a standard curve. The data shown are from one representative experiment out of two.
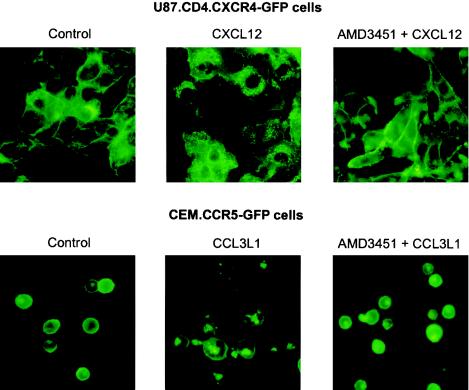
AMD3451 blocks CXCL12- and CCL3L1-induced internalization of CXCR4 and CCR5.
By means of fluorescence microscopy, we also demonstrated the inhibitory effect of AMD3451 on the chemokine-induced endocytosis of CXCR4 and CCR5 in stably transfected U87.CD4 cells expressing GFP-coupled CXCR4 and CEM cells expressing GFP-coupled CCR5. As shown in Fig. 7 (left panel), the fluorescent chemokine receptors were mainly concentrated on the cell membrane in unstimulated cells. Upon stimulation with 1 μg of CXCL12/ml and 100 ng of CCL3L1/ml, the chemokine receptors CXCR4 and CCR5, respectively, became sequestered in endosomal vesicles, which were visible as bright spots accumulating near the nucleus (Fig. 7, middle panel). When cells were first preincubated with AMD3451 at a final concentration of 400 μM, no internalization of both CXCR4 and CCR5 could be observed (Fig. 7, right panel)
FIG. 7.
Inhibitory effect of AMD3451 on CXCL12- and CCL3L1-induced endocytosis of CXCR4 (top) and CCR5 (bottom), respectively. U87.CD4.CXCR4-GFP and CEM.CCR5-GFP cells were preincubated for 15 min in the absence (left and middle panels) or presence (right panels) of 200 μM AMD3451. Then the cells were stimulated, except there was no stimulus under the control conditions (left panels). U87.CD4.CXCR4-GFP cells were stimulated with 1-μg/ml CXCL12 (top), and CEM.CCR5 cells were stimulated with 100-ng/ml CCL3L1 (bottom). After incubation at 37°C, the subcellular localization of the fluorescently labeled CXCR4 and CCR5 proteins was examined in the different cell samples by fluorescence microscopy. The pictures were obtained from one representative experiment which was repeated twice with comparable results.
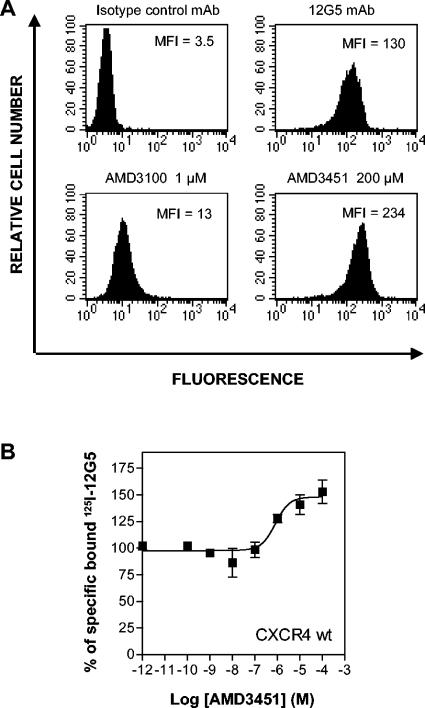
AMD3451 does not inhibit the binding of CXCR4- or CCR5-specific MAbs.
The MAb 12G5 specifically reacts with the second extracellular loop of human CXCR4 and recognizes this receptor on many T-cell lines (44). To learn more about the site of interaction of AMD3451 with the CXCR4 protein, we compared the effect of AMD3451 on MAb 12G5 binding with that of the specific CXCR4 antagonist AMD3100 in Molt-4 cells (Fig. 8A). As previously reported, AMD3100 interfered in a dose-dependent manner with the cell surface binding of MAb 12G5 (56). At 1 μM, AMD3100 showed a marked inhibition (92%) of the binding of MAb 12G5 to CXCR4 in Molt-4 cells (Fig. 8A). However, with AMD3451 at a concentration of 200 μM, no inhibition of MAb 12G5 binding was observed; on the contrary, an enhancement of the binding of the MAb occured (Fig. 8A). Irrespective of whether the preincubation of the cells with the compound was performed for 15 or 60 min, on ice, at room temperature, or at 37°C, similar results were obtained. This also demonstrates that AMD3451 is unable to induce the internalization of CXCR4. Comparable results were obtained with the other anti-CXCR4 MAbs, no. 171, 172, and 173, which all recognize the second extracellular loop of CXCR4. In addition, as demonstrated in Fig. 8B, binding studies with 125I-12G5 in wild-type CXCR4-transfected cells show that the binding of the CXCR4-specific antibody is enhanced for 50% at a concentration of 0.67 μM. This increase was also observed in earlier studies for the monocyclam AMD3389 (32). We also determined the effect of AMD3451 on the binding of different CCR5-specific antibodies. AMD3451 did not interfere with the binding of any of the MAbs (clones 2D7, 45502, 45523, 45531, and 45549) binding to CCR5 in U87.CD4.CCR5 or HOS.CD4.CCR5 cells (data not shown).
FIG. 8.
(A) Opposite effects of AMD3451 and AMD3100 on the binding of anti-CXCR4 (12G5) MAb to Molt-4 cells. After preincubation for 30 min on ice with or without the compounds at the indicated concentrations, the cells were stained with phycoerythrin-conjugated 12G5 MAb and analyzed by flow cytometry. The upper panel shows the aspecific background fluorescence of Molt-4 cells, as determined by an isotype control MAb. The second panel shows Molt-4 cells stained with the antibody in the absence of any compound. The MFI of the cell population is indicated in each histogram. The data shown are from one representative experiment out of four. (B) Effect of AMD3451 on competition binding of anti-CXCR4 antibody 12G5. The binding assay was performed with COS-7 cells transfected with wild-type (wt) CXCR4 with 125I-12G5 as a radioligand. The data are shown as means ± standard errors of the means (n = 3).
Effect of different CXCR4 mutants on binding of AMD3451.
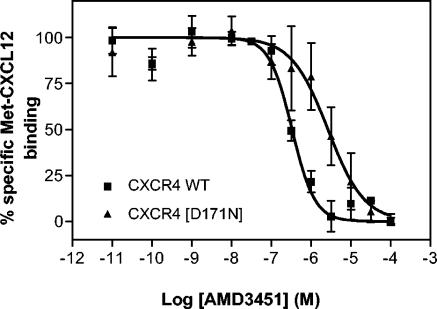
Our previous studies revealed that both Asp171 (in TM-IV) and Asp262 (in TM-VI), located at each end of the main ligand-binding crevice of CXCR4, are essential for the binding of AMD3100 to the receptor and for the ability of the compound to inhibit the signaling induced by the chemokine ligand CXCL12 (32, 37). As shown in Table 3, all of the single mutations were expressed well in the COS-7 cells, and all bound the chemokine ligand Met-CXCL12 with an affinity similar to that of the wild-type CXCR4 receptor (as represented by maximum specific binding). The affinity of AMD3451, as determined in competition for 125I-Met-CXCL12 binding, was also unaffected by all of these mutations, except for the Asn substitution of Asp171 located in the extracellular part of TM-IV, which impaired cyclam binding 12-fold (Fig. 9 and Table 3). The IC50s for AMD3451 against CXCL12 binding were 0.42 and 5.3 μM in the wild-type CXCR4- and [D171N] CXCR4-transfected cells, respectively (Table 3).
TABLE 3.
Affinity of Met-CXCL12 and AMD3451 for the wild-type CXCR4 receptor and His- and Asp-substituted CXCR4 receptor mutantsa
| CXCR4 form | AMD3451
|
Met-CXCL12
|
Bmax ± SEM | ||||
|---|---|---|---|---|---|---|---|
| Ki ± SEM | IC50 (μM) | n | Kd ± SEM | IC50 (nM) | n | ||
| Wild type | −6.37 ± 0.09 | 0.42 | 5 | −9.26 ± 0.10 | 0.55 | 17 | 32 ± 5 |
| H113A | −5.70 ± 0.25 | 2.02 | 2 | −9.47 ± 0.16 | 0.34 | 9 | 13 ± 4.7 |
| D171N | −5.28 ± 0.38 | 5.30 | 5 | −9.13 ± 0.28 | 0.74 | 8 | 14 ± 4 |
| D182N | −6.25 ± 0.21 | 0.56 | 3 | −8.98 ± 0.20 | 1.04 | 8 | 113 ± 50 |
| D193N | −6.47 ± 0.15 | 0.34 | 3 | −8.89 ± 0.26 | 1.28 | 6 | 109 ± 45 |
| H203A | −5.61 ± 0.29 | 2.47 | 3 | −9.11 ± 0.25 | 0.77 | 6 | 21 ± 14 |
| H281A | −5.56 ± 0.15 | 2.77 | 4 | −9.32 ± 0.15 | 0.48 | 11 | 42 ± 25 |
| D262N | −6.24 ± 0.12 | 0.57 | 3 | −8.86 ± 0.17 | 1.38 | 9 | 28 ± 11 |
The data were obtained from competition binding on COS-7 cells expressing the wild-type and mutant CXCR4 receptors by using 125I-Met-CXCL12 as a radioligand. Bmax, maximum specific binding (fmol/105 cells).
FIG. 9.
Effect on AMD3451 competition for CXCL12 binding of the Asp-to-Asn substitution at position 171 in the CXCR4 receptor. The binding assay was performed with COS-7 cells transfected with wild-type (WT) CXCR4 or CXCR4 [D171N] with 125I-Met-CXCL12 as a radioligand. The data are shown as means ± standard errors of the means (n = 3).
DISCUSSION
CCR5 and CXCR4 are the principal chemokine receptors used in association with CD4 by HIV to enter its target cells (6, 28, 30). The coreceptors are important determinants of viral tropism and pathogenesis and are obvious targets for antiviral drug development. CXCR4 is the coreceptor used by X4 HIV strains to enter its target cells (7, 30), whereas CCR5 is the main coreceptor for R5 HIV strains (1, 13, 15, 23, 27, 28). CXCL12, the natural ligand for CXCR4, has been shown to inhibit X4 virus strains and primary isolates through CXCR4 blockage (8, 47). However, variability in its antiviral activity has been observed (43), and CXCL12 has been found to stimulate the replication of certain R5 HIV isolates (43). Also, the CCR5 ligand CCL5, which can inhibit R5 viral replication, in certain conditions does not inhibit but even enhances the replication of certain HIV strains (34).
AMD3451 is consistently active against a broad range of X4 HIV-1 and HIV-2 strains, R5 HIV-1 strains, R5/X4 HIV strains, and primary isolates in different cell lines, PBMCs, and M/M (Tables 1 and 2; Fig. 2 and 3). We have never observed an enhancement of viral replication in the presence of AMD3451. In addition, AMD3451 is active against several SIV strains, such as SIVagm, which also uses CCR5 as the main coreceptor to enter human T cells (data not shown).
The antiviral activity profile of AMD3451, together with our finding that AMD3451 acts at the level of virus entry into the cells, suggests that the compound may interact with both CXCR4 and CCR5, the most important coreceptors for HIV entry. We first provided evidence to support this hypothesis at the level of chemokine signaling through CCR5 and CXCR4. AMD3451 inhibited CXCL12-induced intracellular Ca2+ signaling in a dose-dependent fashion in different cell types. Also, AMD3451 dose-dependently inhibited the Ca2+ flux induced by the CC-chemokines CCL5 or CCL4 in CCR5-transfected cells. The IC50 of AMD3451 required to inhibit the CXCL12-induced and CCL5- or CCL4-induced Ca2+ flux was between 10 to 40 μM, which was somewhat higher than the average IC50 of the compound to inhibit HIV infection. Besides CXCR4 and CCR5, AMD3451 did not interact with any other chemokine receptor (CCR1, CCR2, CCR3, CCR4, CCR6, CCR9, or CXCR3) that was evaluated in this study. By itself, AMD3451 did not induce an intracellular Ca2+ response at concentrations up to 400 μM, indicating that the compound is devoid of agonistic activity. Chemotaxis provided additional evidence for the dual CXCR4/CCR5 antagonism of AMD3451. Both CXCL12- and CCL4-induced migration of CXCR4+ CCR5+ cells was concentration-dependently blocked by AMD3451, although again at relatively higher concentrations than those for antiviral activity. However, for the specific CXCR4 antagonist AMD3100 as well, the CXCL12-induced chemotactic response was inhibited at about 1 to 10 μM, which is ∼1,000-fold higher than its active antiviral concentration range (IC50, 1 to 10 nM) (56). Remarkably, there seemed to be a slight enhancement of the chemokine-induced migration of cells that were preincubated with low concentrations of compound (i.e., concentrations of AMD3451 too low to inhibit chemotaxis). This phenomenon can certainly not be assigned to any stimulating (agonistic) effect of the compound itself. In addition, the compound is not present in the lower well during the chemotaxis assay, as is the case for the chemokine, so it cannot function as an attractant. The exact reason for this weak enhancement of chemotactic activity of the cells in the presence of low compound concentrations is not yet known, but it is probably just caused by variability within the experiment. In addition, AMD3451 at 400 μM could completely block the CXCL12- and CCL3L1-induced internalization of CXCR4 and CCR5. Moreover, AMD3451 did not inhibit, but rather enhanced, the binding of the CXCR4-specific MAb 12G5 at the cell surface. This is in sharp contrast with the observations made for the bicyclams. Here, a close correlation between anti-HIV activity and inhibition of MAb 12G5 binding was found for more than 20 different bicyclam derivatives (29). Compounds with the highest MAb 12G5 binding inhibitory capacity exhibited the most potent anti-HIV activity (10, 29). The second extracellular loop of CXCR4 offers the main epitope for MAb 12G5 (40, 44), although other regions of the receptor may also influence its binding affinity. This MAb can neutralize HIV-1 and HIV-2 infections, even if CD4 independent (44), but its antiviral activity is variable, depending on the cell line and virus strain used (9). The enhancement of MAb 12G5 binding by AMD3451 may indicate that the interaction of AMD3451 with CXCR4 may influence extracellular loop 2, which holds important epitopes for 12G5 (5), in a way favorable for antibody recognition. This phenomenon was also observed for the monocyclam AMD3389 (32). Also, substitutions in a homologous region of extracellular loop 2 of CXCR4 and CCR5 are described to alter coreceptor activities for HIV-1 membrane fusion and virus entry (12). Another anti-CXCR4 MAb, recognizing the amino-terminal domain of the receptor (2B11), was not significantly influenced in its binding to CXCR4 in the presence of AMD3451.
Although sometimes the concentrations of AMD3451 used in the assays to evaluate its antagonistic properties are as high as the CCID50 obtained in the antiviral assays, it is important to mention that in these assays the compound is in contact with the cells for only a few hours, and in these assays, no toxicity of the compound at the highest concentration (400 μM) was observed whatsoever.
The dual specificity of AMD3451 towards CXCR4 and CCR5 is both functionally and structurally interesting. Earlier studies by Labrosse et al. demonstrated that substitution of any of the four aspartic acids of the second loop of CXCR4 resulted in a marked resistance to AMD3100 (40), which could be expected if AMD3100 directly interacts with these amino acids. More-recent studies identified Asp171 and Asp262, located at each end of the main ligand-binding crevice of the CXCR4 receptor, as being the molecular anchor points for the two cyclam moieties of AMD3100 (32, 37). Here, we demonstrate that AMD3451 inhibition of Met-CXCL12 binding is dependent on Asp171 but independent of Asp262. These data are in line with those observed for other monocylam compounds, such as AMD3389 (32). Our finding that AMD3100 and AMD3451 have opposite effects on anti-CXCR4 MAb binding at the cell surface (i.e., inhibition by AMD3100 versus enhancement by AMD3451) also points to a different mode of interaction of both compounds with the receptor protein. This is further supported by the observation that the AMD3100-resistant HIV-1 NL4.3 strain remains sensitive to AMD3451.
Focusing on CCR5, there is no aspartic acid residue corresponding to either Asp171 or Asp262 of CXCR4. Moreover, the lower net positive charge of the V3 domain of HIV-1 gp120 of R5 HIV-1 strains compared to that of X4 HIV-1 strains (16) and, subsequently, the different gp120-coreceptor interaction also points to a slightly different interaction mechanism of AMD3451 with CXCR4 and CCR5. In addition, we compared the effect of AMD3451 on binding with the chemokine receptor CCR5 with that of the recently described CCR5-antagonist SCH-C (63). Unlike SCH-C, AMD3451 did not interfere with the binding of the anti-CCR5 MAb clones 45523 and 45531, and both compounds showed no hindrance of the binding of the anti-CCR5 MAb clones 2D7, 45502, and 45549. Thus, the exact interaction site(s) of AMD3451 with CCR5 and CXCR4 is still unclear and subject to further studies.
It may be possible to delay or prevent the progression of AIDS by pharmacological blockade of the HIV coreceptors. Although the HIV coreceptor repertoire is still expanding, CXCR4 and CCR5 are the two coreceptors that are the most relevant for HIV infection in vivo. Thus, because of its dual interaction with both CXCR4 and CCR5 and, consequently, its potential to block cellular infection of X4, R5, and R5/X4 viruses, AMD3451 can be considered an important antiviral lead compound for the further development of an effective microbicide.
Acknowledgments
We thank Sandra Claes and Erik Fonteyn for excellent technical assistance and Sofie Struyf and Jo Van Damme for help in the initial Ca2+ flux signaling experiments.
K.P. has an IWT fellowship from the Vlaams Instituut voor Innovatie door Wetenschap en Technologie in Vlaanderen. S.H. is a Postdoctoral Research Assistant of the Fonds voor Wetenschappelijk Onderzoek (FWO)-Vlaanderen, and K.V. is a postdoctoral researcher of the Onderzoeksfonds K. U. Leuven. This work was supported by grants from the FWO Vlaanderen (Krediet no. G.0267.04), the Geconcerteerde Onderzoeksacties (Vlaamse Gemeenschap) (Krediet 00/12), and the Danish Medical Research Council.
REFERENCES
- 1.Alkhatib, G., C. Combadiere, C. C. Broder, Y. Feng, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1996. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955-1958. [DOI] [PubMed] [Google Scholar]
- 2.Aquaro, S., O. Wedgwood, C. Yarnold, D. Cahard, R. Pathinara, C. McGuigan, R. Calio, E. De Clercq, J. Balzarini, and C. F. Perno. 2000. Activities of masked 2′,3′-dideoxynucleoside monophosphate derivatives against human immunodeficiency virus in resting macrophages. Antimicrob. Agents Chemother. 44:173-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arenzana-Seisdedos, F., J. L. Virelizier, D. Rousset, I. Clark-Lewis, P. Loetscher, B. Moser, and M. Baggiolini. 1996. HIV blocked by chemokine antagonist. Nature 383:400. [DOI] [PubMed] [Google Scholar]
- 4.Baba, M., O. Nishimura, N. Kanzaki, M. Okamoto, H. Sawada, Y. Iizawa, M. Shiraishi, Y. Aramaki, K. Okonogi, Y. Ogawa, K. Meguro, and M. Fujino. 1999. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 96:5698-5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baribaud, F., T. G. Edwards, M. Sharron, A. Brelot, N. Heveker, K. Price, F. Mortari, M. Alizon, M. Tsang, and R. W. Doms. 2001. Antigenically distinct conformations of CXCR4. J. Virol. 75:8957-8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. [DOI] [PubMed] [Google Scholar]
- 7.Berson, J. F., D. Long, B. J. Doranz, J. Rucker, F. R. Jirik, and R. W. Doms. 1996. A seven-transmembrane domain receptor involved in fusion and entry of T-cell-tropic human immunodeficiency virus type 1 strains. J. Virol. 70:6288-6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bleul, C. C., M. Farzan, H. Choe, C. Parolin, I. Clark-Lewis, J. Sodroski, and T. A. Springer. 1996. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 382:829-833. [DOI] [PubMed] [Google Scholar]
- 9.Bleul, C. C., R. C. Fuhlbrigge, J. M. Casasnovas, A. Aiuti, and T. A. Springer. 1996. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J. Exp. Med. 184:1101-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bridger, G. J., R. T. Skerlj, S. Padmanabhan, S. A. Martellucci, G. W. Henson, S. Struyf, M. Witvrouw, D. Schols, and E. De Clercq. 1999. Synthesis and structure-activity relationships of phenylenebis(methylene)-linked bis-azamacrocycles that inhibit HIV-1 and HIV-2 replication by antagonism of the chemokine receptor CXCR4. J. Med. Chem. 42:3971-3981. [DOI] [PubMed] [Google Scholar]
- 11.Bridger, G. J., R. T. Skerlj, D. Thornton, S. Padmanabhan, S. A. Martellucci, G. W. Henson, M. J. Abrams, N. Yamamoto, K. De Vreese, R. Pauwels, and E. De Clercq. 1995. Synthesis and structure-activity relationships of phenylenebis(methylene)-linked bis-tetraazamacrocycles that inhibit HIV replication. Effects of macrocyclic ring size and substituents on the aromatic linker. J. Med. Chem. 38:366-378. [DOI] [PubMed] [Google Scholar]
- 12.Chabot, D. J., and C. C. Broder. 2000. Substitutions in a homologous region of extracellular loop 2 of CXCR4 and CCR5 alter coreceptor activities for HIV-1 membrane fusion and virus entry. J. Biol. Chem. 275:23774-23782. [DOI] [PubMed] [Google Scholar]
- 13.Choe, H., M. Farzan, Y. Sun, N. Sullivan, B. Rollins, P. D. Ponath, L. Wu, C. R. Mackay, G. LaRosa, W. Newman, N. Gerard, C. Gerard, and J. Sodroski. 1996. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 85:1135-1148. [DOI] [PubMed] [Google Scholar]
- 14.Cilliers, T., J. Nhlapo, M. Coetzer, D. Orlovic, T. Ketas, W. C. Olson, J. P. Moore, A. Trkola, and L. Morris. 2003. The CCR5 and CXCR4 coreceptors are both used by human immunodeficiency virus type 1 primary isolates from subtype C. J. Virol. 77:4449-4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cocchi, F., A. L. DeVico, A. Garzino-Demo, S. K. Arya, R. C. Gallo, and P. Lusso. 1995. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science 270:1811-1815. [DOI] [PubMed] [Google Scholar]
- 16.Cocchi, F., A. L. DeVico, A. Garzino-Demo, A. Cara, R. C. Gallo, and P. Lusso. 1996. The V3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection. Nat. Med. 2:1244-1247. [DOI] [PubMed] [Google Scholar]
- 17.Connor, R. I., K. E. Sheridan, D. Ceradini, S. Choe, and N. R. Landau. 1997. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J. Exp. Med. 185:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crump, M. P., J. H. Gong, P. Loetscher, K. Rajarathnam, A. Amara, F. Arenzana-Seisdedos, J. L. Virelizier, M. Baggiolini, B. D. Sykes, and I. Clark-Lewis. 1997. Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. EMBO J. 16:6996-7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daelemans, D., D. Schols, M. Witvrouw, C. Pannecouque, S. Hatse, S. van Dooren, F. Hamy, T. Klimkait, E. De Clercq, and A. M. VanDamme. 2000. A second target for the peptoid Tat/transactivation response element inhibitor CGP64222: inhibition of human immunodeficiency virus replication by blocking CXC-chemokine receptor 4-mediated virus entry. Mol. Pharmacol. 57:116-124. [PubMed] [Google Scholar]
- 20.Dean, M., M. Carrington, C. Winkler, G. A. Huttley, M. W. Smith, R. Allikmets, J. J. Goedert, S. P. Buchbinder, E. Vittinghoff, E. Gomperts, S. Donfield, D. Vlahov, R. Kaslow, A. Saah, C. Rinaldo, R. Detels, and S. J. O'Brien. 1996. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science 273:1856-1862. [DOI] [PubMed] [Google Scholar]
- 21.De Clercq, E., N. Yamamoto, R. Pauwels, M. Baba, D. Schols, H. Nakashima, J. Balzarini, Z. Debyser, B. A. Murrer, and D. Schwartz. 1992. Potent and selective inhibition of human immunodeficiency virus (HIV)-1 and HIV-2 replication by a class of bicyclams interacting with a viral uncoating event. Proc. Natl. Acad. Sci. USA 89:5286-5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Clercq, E., N. Yamamoto, R. Pauwels, J. Balzarini, M. Witvrouw, K. De Vreese, Z. Debyser, B. Rosenwirth, P. Peichl, and R. Datema. 1994. Highly potent and selective inhibition of human immunodeficiency virus by the bicyclam derivative JM3100. Antimicrob. Agents Chemother. 38:668-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng, H., R. Liu, W. Ellmeier, S. Choe, D. Unutmaz, M. Burkhart, P. Di Marzio, S. Marmon, R. E. Sutton, C. M. Hill, C. B. Davis, S. C. Peiper, T. J. Schall, D. R. Littman, and N. R. Landau. 1996. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381:661-666. [DOI] [PubMed] [Google Scholar]
- 24.De Vreese, K., V. Kofler-Mongold, C. Leutgeb, V. Weber, K. Vermeire, S. Schacht, J. Anne, E. De Clercq, R. Datema, and G. Werner. 1996. The molecular target of bicyclams, potent inhibitors of human immunodeficiency virus replication. J. Virol. 70:689-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donzella, G. A., D. Schols, S. W. Lin, J. A. Este, K. A. Nagashima, P. J. Maddon, G. P. Allaway, T. P. Sakmar, G. Henson, E. De Clercq, and J. P. Moore. 1998. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat. Med. 4:72-77. [DOI] [PubMed] [Google Scholar]
- 26.Doranz, B. J., K. Grovit-Ferbas, M. P. Sharron, S. H. Mao, M. B. Goetz, E. S. Daar, R. W. Doms, and W. A. O'Brien. 1997. A small-molecule inhibitor directed against the chemokine receptor CXCR4 prevents its use as an HIV-1 coreceptor. J. Exp. Med. 186:1395-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doranz, B. J., J. Rucker, Y. Yi, R. J. Smyth, M. Samson, S. C. Peiper, M. Parmentier, R. G. Collman, and R. W. Doms. 1996. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell 85:1149-1158. [DOI] [PubMed] [Google Scholar]
- 28.Dragic, T., V. Litwin, G. P. Allaway, S. R. Martin, Y. Huang, K. A. Nagashima, C. Cayanan, P. J. Maddon, R. A. Koup, J. P. Moore, and W. A. Paxton. 1996. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667-673. [DOI] [PubMed] [Google Scholar]
- 29.Este, J. A., C. Cabrera, E. De Clercq, S. Struyf, J. Van Damme, G. Bridger, R. T. Skerlj, M. J. Abrams, G. Henson, A. Gutierrez, B. Clotet, and D. Schols. 1999. Activity of different bicyclam derivatives against human immunodeficiency virus depends on their interaction with the CXCR4 chemokine receptor. Mol. Pharmacol. 55:67-73. [DOI] [PubMed] [Google Scholar]
- 30.Feng, Y., C. C. Broder, P. E. Kennedy, and E. A. Berger. 1996. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 272:872-877. [DOI] [PubMed] [Google Scholar]
- 31.Garred, P., J. Eugen-Olsen, A. K. Iversen, T. L. Benfield, A. Svejgaard, and B. Hofmann. 1997. Dual effect of CCR5 Δ32 gene deletion in HIV-1-infected patients. Copenhagen AIDS Study Group. Lancet 349:1884. [DOI] [PubMed] [Google Scholar]
- 32.Gerlach, L. O., R. T. Skerlj, G. J. Bridger, and T. W. Schwartz. 2001. Molecular interactions of cyclam and bicyclam non-peptide antagonists with the CXCR4 chemokine receptor. J. Biol. Chem. 276:14153-14160. [DOI] [PubMed] [Google Scholar]
- 33.Glushakova, S., Y. Yi, J. C. Grivel, A. Singh, D. Schols, E. De Clercq, R. G. Collman, and L. Margolis. 1999. Preferential coreceptor utilization and cytopathicity by dual-tropic HIV-1 in human lymphoid tissue ex vivo. J. Clin. Investig. 104:R7-R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordon, C. J., M. A. Muesing, A. E. Proudfoot, C. A. Power, J. P. Moore, and A. Trkola. 1999. Enhancement of human immunodeficiency virus type 1 infection by the CC-chemokine RANTES is independent of the mechanism of virus-cell fusion. J. Virol. 73:684-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamy, F., E. R. Felder, G. Heizmann, J. Lazdins, F. Aboul-ela, G. Varani, J. Karn, and T. Klimkait. 1997. An inhibitor of the Tat/TAR RNA interaction that effectively suppresses HIV-1 replication. Proc. Natl. Acad. Sci. USA 94:3548-3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harada, S., Y. Koyanagi, and N. Yamamoto. 1985. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science 229:563-566. [DOI] [PubMed] [Google Scholar]
- 37.Hatse, S., K. Princen, L. O. Gerlach, G. Bridger, G. Henson, E. De Clercq, T. W. Schwartz, and D. Schols. 2001. Mutation of Asp171 and Asp262 of the chemokine receptor CXCR4 impairs its coreceptor function for human immunodeficiency virus-1 entry and abrogates the antagonistic activity of AMD3100. Mol. Pharmacol. 60:164-173. [DOI] [PubMed] [Google Scholar]
- 38.Hendrix, C. W., C. Flexner, R. T. MacFarland, C. Giandomenico, E. J. Fuchs, E. Redpath, G. Bridger, and G. W. Henson. 2000. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob. Agents Chemother. 44:1667-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kinter, A., A. Catanzaro, J. Monaco, M. Ruiz, J. Justement, S. Moir, J. Arthos, A. Oliva, L. Ehler, S. Mizell, R. Jackson, M. Ostrowski, J. Hoxie, R. Offord, and A. S. Fauci. 1998. CC-chemokines enhance the replication of T-tropic strains of HIV-1 in CD4(+) T cells: role of signal transduction. Proc. Natl. Acad. Sci. USA 95:11880-11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Labrosse, B., A. Brelot, N. Heveker, N. Sol, D. Schols, E. De Clercq, and M. Alizon. 1998. Determinants for sensitivity of human immunodeficiency virus coreceptor CXCR4 to the bicyclam AMD3100. J. Virol. 72:6381-6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu, Q. H., D. A. Williams, C. McManus, F. Baribaud, R. W. Doms, D. Schols, E. De Clercq, M. I. Kotlikoff, R. G. Collman, and B. D. Freedman. 2000. HIV-1 gp120 and chemokines activate ion channels in primary macrophages through CCR5 and CXCR4 stimulation. Proc. Natl. Acad. Sci. USA 97:4832-4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu, R., W. A. Paxton, S. Choe, D. Ceradini, S. R. Martin, R. Horuk, M. E. MacDonald, H. Stuhlmann, R. A. Koup, and N. R. Landau. 1996. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86:367-377. [DOI] [PubMed] [Google Scholar]
- 43.Marechal, V., F. Arenzana-Seisdedos, J. M. Heard, and O. Schwartz. 1999. Opposite effects of SDF-1 on human immunodeficiency virus type 1 replication. J. Virol. 73:3608-3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKnight, A., D. Wilkinson, G. Simmons, S. Talbot, L. Picard, M. Ahuja, M. Marsh, J. A. Hoxie, and P. R. Clapham. 1997. Inhibition of human immunodeficiency virus fusion by a monoclonal antibody to a coreceptor (CXCR4) is both cell type and virus strain dependent. J. Virol. 71:1692-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michael, N. L., J. A. Nelson, V. N. KewalRamani, G. Chang, S. J. O'Brien, J. R. Mascola, B. Volsky, M. Louder, G. C. White, D. R. Littman, R. Swanstrom, and T. R. O'Brien. 1998. Exclusive and persistent use of the entry coreceptor CXCR4 by human immunodeficiency virus type 1 from a subject homozygous for CCR5 Δ32. J. Virol. 72:6040-6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murakami, T., T. Nakajima, Y. Koyanagi, K. Tachibana, N. Fujii, H. Tamamura, N. Yoshida, M. Waki, A. Matsumoto, O. Yoshie, T. Kishimoto, N. Yamamoto, and T. Nagasawa. 1997. A small molecule CXCR4 inhibitor that blocks T cell line-tropic HIV-1 infection. J. Exp. Med. 186:1389-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oberlin, E., A. Amara, F. Bachelerie, C. Bessia, J. L. Virelizier, F. Arenzana-Seisdedos, O. Schwartz, J. M. Heard, I. Clark-Lewis, D. F. Legler, M. Loetscher, M. Baggiolini, and B. Moser. 1996. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature 382:833-835. [DOI] [PubMed] [Google Scholar]
- 48.Perno, C. F., and R. Yarchoan. 1993. Culture of HIV in monocytes and macrophages, p. 12.4.1-12.4.11. In J. E. Coligan, A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, and W. Strober (ed.), Current protocols in immunology. J. Wiley & Sons, New York, N.Y.
- 49.Princen, K., S. Hatse, K. Vermeire, E. De Clercq, and D. Schols. 2003. Evaluation of SDF-1/CXCR4-induced Ca2+ signaling by fluorometric imaging plate reader (FLIPR) and flow cytometry. Cytometry 51A:35-45. [DOI] [PubMed] [Google Scholar]
- 50.Ratner, L., W. Haseltine, R. Patarca, K. J. Livak, B. Starcich, S. F. Josephs, E. R. Doran, J. A. Rafalski, E. A. Whitehorn, and K. Baumeister. 1985. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature 313:277-284. [DOI] [PubMed] [Google Scholar]
- 51.Reynes, J., R. Rouzier, T. Kanouni, V. Baillat, B. Baroudy, A. Keung, C. Hogan, M. Markowitz, and M. Laughlin. 2002. SCH C: safety and antiviral effects of a CCR5 receptor antagonist in HIV-1 infected subjects, p. 53. Program Abstr. 9th Conf. Retrovir. Opportunistic Infect., Seattle, Wash.
- 52.Samson, M., F. Libert, B. J. Doranz, J. Rucker, C. Liesnard, C. M. Farber, S. Saragosti, C. Lapoumeroulie, J. Cognaux, C. Forceille, G. Muyldermans, C. Verhofstede, G. Burtonboy, M. Georges, T. Imai, S. Rana, Y. Yi, R. J. Smyth, R. G. Collman, R. W. Doms, G. Vassart, and M. Parmentier. 1996. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382:722-725. [DOI] [PubMed] [Google Scholar]
- 53.Schmidtmayerova, H., B. Sherry, and M. Bukrinsky. 1996. Chemokines and HIV replication. Nature 382:767. [DOI] [PubMed] [Google Scholar]
- 54.Schols, D., S. Claes, E. De Clercq, C. Hendrix, G. Bridger, G. Calandra, G. Henson, S. Fransen, W. Huang, J. Whitcomb, and C. Petropoulos. 2002. AMD-3100, a CXCR4 antagonist, reduced HIV viral load and X4 virus levels in humans, p. 53. Program Abstr. 9th Conf. Retrovir. Opportunistic Infect., Seattle, Wash.
- 55.Schols, D., J. A. Este, C. Cabrera, and E. De Clercq. 1998. T-cell-line-tropic human immunodeficiency virus type 1 that is made resistant to stromal cell-derived factor 1α contains mutations in the envelope gp120 but does not show a switch in coreceptor use. J. Virol. 72:4032-4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schols, D., S. Struyf, J. Van Damme, J. A. Este, G. Henson, and E. De Clercq. 1997. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J. Exp. Med. 186:1383-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schuitemaker, H., M. Koot, N. A. Kootstra, M. W. Dercksen, R. E. de Goede, R. P. van Steenwijk, J. M. Lange, J. K. Schattenkerk, F. Miedema, and M. Tersmette. 1992. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J. Virol. 66:1354-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Signoret, N., J. Oldridge, A. Pelchen-Matthews, P. J. Klasse, T. Tran, L. F. Brass, M. M. Rosenkilde, T. W. Schwartz, W. Holmes, W. Dallas, M. A. Luther, T. N. Wells, J. A. Hoxie, and M. Marsh. 1997. Phorbol esters and SDF-1 induce rapid endocytosis and down modulation of the chemokine receptor CXCR4. J. Cell Biol. 139:651-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Signoret, N., M. M. Rosenkilde, P. J. Klasse, T. W. Schwartz, M. H. Malim, J. A. Hoxie, and M. Marsh. 1998. Differential regulation of CXCR4 and CCR5 endocytosis. J. Cell Sci. 111:2819-2830. [DOI] [PubMed] [Google Scholar]
- 60.Simmons, G., P. R. Clapham, L. Picard, R. E. Offord, M. M. Rosenkilde, T. W. Schwartz, R. Buser, T. N. Wells, and A. E. Proudfoot. 1997. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science 276:276-279. [DOI] [PubMed] [Google Scholar]
- 61.Strizki, J. M., S. Xu, N. E. Wagner, L. Wojcik, J. Liu, Y. Hou, M. Endres, A. Palani, S. Shapiro, J. W. Clader, W. J. Greenlee, J. R. Tagat, S. McCombie, K. Cox, A. B. Fawzi, C. C. Chou, C. Pugliese-Sivo, L. Davies, M. E. Moreno, D. D. Ho, A. Trkola, C. A. Stoddart, J. P. Moore, G. R. Reyes, and B. M. Baroudy. 2001. SCH-C (SCH 351125), an orally bioavailable, small molecule antagonist of the chemokine receptor CCR5, is a potent inhibitor of HIV-1 infection in vitro and in vivo. Proc. Natl. Acad. Sci. USA 98:12718-12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trkola, A., C. Gordon, J. Matthews, E. Maxwell, T. Ketas, L. Czaplewski, A. E. Proudfoot, and J. P. Moore. 1999. The CC-chemokine RANTES increases the attachment of human immunodeficiency virus type 1 to target cells via glycosaminoglycans and also activates a signal transduction pathway that enhances viral infectivity. J. Virol. 73:6370-6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsamis, F., S. Gavrilov, F. Kajumo, C. Seibert, S. Kuhmann, T. Ketas, A. Trkola, A. Palani, J. W. Clader, J. R. Tagat, S. McCombie, B. Baroudy, J. P. Moore, T. P. Sakmar, and T. Dragic. 2003. Analysis of the mechanism by which the small-molecule CCR5 antagonists SCH-351125 and SCH-350581 inhibit human immunodeficiency virus type 1 entry. J. Virol. 77:5201-5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Damme, J., P. Proost, J. P. Lenaerts, and G. Opdenakker. 1992. Structural and functional identification of two human, tumor-derived monocyte chemotactic proteins (MCP-2 and MCP-3) belonging to the chemokine family. J. Exp. Med. 176:59-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang, Y. J., and J. P. Moore. 1999. Will multiple coreceptors need to be targeted by inhibitors of human immunodeficiency virus type 1 entry? J. Virol. 73:3443-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]