Abstract
Cervical cancer results from cervical infection by human papillomaviruses (HPVs), especially HPV16. An effective vaccine against these HPVs is expected to have a dramatic impact on the incidence of this cancer and its precursor lesions. The leading candidate, a subunit prophylactic HPV virus-like particle (VLP) vaccine, can protect women from HPV infection. An alternative improved vaccine that avoids parenteral injection, that is efficient with a single dose, and that induces mucosal immunity might greatly facilitate vaccine implementation in different settings. In this study, we have constructed a new generation of recombinant Salmonella organisms that assemble HPV16 VLPs and induce high titers of neutralizing antibodies in mice after a single nasal or oral immunization with live bacteria. This was achieved through the expression of a HPV16 L1 capsid gene whose codon usage was optimized to fit with the most frequently used codons in Salmonella. Interestingly, the high immunogenicity of the new recombinant bacteria did not correlate with an increased expression of L1 VLPs but with a greater stability of the L1-expressing plasmid in vitro and in vivo in absence of antibiotic selection. Anti-HPV16 humoral and neutralizing responses were also observed with different Salmonella enterica serovar Typhimurium strains whose attenuating deletions have already been shown to be safe after oral vaccination of humans. Thus, our findings are a promising improvement toward a vaccine strain that could be tested in human volunteers.
Cervical cancer is the second leading cause of cancer deaths in women worldwide, and virtually all of these tumors are attributable to infection with a subset of human papillomaviruses (HPVs), of which HPV16 is found most frequently (6, 42). An effective vaccine against these HPVs would, therefore, be expected to have a dramatic impact on the incidence of this cancer and its precursor lesions, as well as on the less common tumors attributable to these viruses. The leading candidate is a prophylactic subunit HPV virus-like particle (VLP) vaccine (reviewed in references 36 and24). A proof of principal efficacy trial showed that women vaccinated with HPV16 VLPs were highly protected against genital mucosal infection by this viral type (19). However, the requirement for multiple injections for a vaccine whose anticipated target population will be older than the population that receives childhood vaccines may represent a substantial hurdle for widespread implementation. This is particularly true in the developing world, which accounts for more than three-quarters of the worldwide cases of cervical cancer (6). Recombinant attenuated Salmonella strains that are attenuated yet invasive have been widely used as mucosal vaccine vectors to deliver pathogen-specific protective epitopes into the mucosal-associated lymphoid tissues. Via this route, both mucosal and systemic immune responses against the carrier and the foreign antigens may be obtained (reviewed in references 11, 22, and 37). We have shown that nasal vaccination of mice with Salmonella organisms expressing the HPV16 major capsid protein L1, which self-assembles into VLPs, induces anti-HPV16 conformational and neutralizing antibodies in serum and genital secretions, provided the attenuated Salmonella enterica serovar Typhimurium strains exhibit the PhoPc phenotype (3, 4, 31). However, even with the original PhoPc strain, a double nasal immunization was required to induce high anti-HPV16 VLP antibody titers, while oral immunization was inefficient (31). The observations of low levels of L1 expression together with a high instability of the L1-encoding plasmids in the absence of antibiotic selection strongly suggested that either the L1 protein or the L1 gene could be toxic to the bacteria. As the viral L1 gene exhibits a highly unfavorable codon usage for expression in Salmonella, we designed and tested herein a synthetic nucleotide sequence (referred to as L1S hereafter) encoding the L1 protein and containing the most frequently used codons in Salmonella. Our data show that anti-HVP16 VLP humoral and neutralizing responses after either nasal or oral immunization with the new recombinant strains were highly increased. Interestingly, this was not associated with an increased L1 expression but with a remarkable stability of the L1S-expressing plasmid in vitro and in vivo. In addition, immunogenicity was not restricted to PhoPc, as shown with other S. enterica serovar Typhimurium strains whose attenuating deletions are suitable for human use.
MATERIALS AND METHODS
Plasmid constructions and bacterial strains used.
The L1S gene was synthesized by Microsynth, Buchs, Switzerland. The open reading frame (ORF) was flanked in 5′ with a NcoI restriction site and in 3′ with a HindIII restriction site. The L1S NcoI-HindIII fragment was inserted in place of the original L1 NcoI-HindIII fragment in the plasmid pFS14nsd HPV16-L1 (31). The resulting plasmid, pFS14nsd HPV16-L1S, was introduced by electroporation (38) into the attenuated S. enterica serovar Typhimurium strains PhoPc (CS022 [27]) and PhoP− (CS015 [26]), both a kind gift from John Mekalanos, Boston, Mass., and strains χ4989 (Δcya Δcrp [4]), χ4990 (Δcya Δcrp-cdt [4]), and ΔaroA (SL7207 [16]), a kind gift from Irene Corthésy-Theulaz, Lausanne, Switzerland.
HPV16 L1 and VLP analysis.
Expression of L1 in Salmonella lysates was analyzed by Western blotting as previously described (31) by using the anti-HPV16 L1 monoclonal antibody, CAMVIR-1 (Anawa). Data were normalized to the content in bacteria as measured by the optical density at 600 nm of the cultures. The HPV16 VLP content was measured by a sandwich enzyme-linked immunosorbent assay (ELISA) as previously described (4) by using two monoclonal antibodies that recognize conformational epitopes on HPV16 VLPs, H16E70, and H16 V5, kindly provided by N. D. Christensen, Hershey, Pa. (9).
Immunization of mice, analysis of anti-HPV16 VLP antibodies, and recovery of S. enterica serovar Typhimurium.
Six-week-old female BALB/c mice from Iffa Credo, France, were used in all experiments. Twenty microliters of bacterial inoculum was administered orally (108 to 109 CFU) or intranasally (106 to 107 CFU) under anesthesia as previously described (17, 31). Sampling of blood and vaginal washes as well as determination of anti-HPV16 VLP antibody titers by ELISA were performed as reported earlier (17, 31). Recovery of S. enterica serovar Typhimurium was determined in organs from euthanized mice as previously described (31).
Neutralization assays.
Neutralizations assays were performed with secreted alkaline phosphatase (SEAP) HPV16 pseudoviruses as described in detail by Pastrana et al. (34). Briefly, OptiPrep-purified SEAP HPV16 pseudoviruses diluted 2,000-fold were incubated on ice for 1 h with twofold serial serum dilutions, and the pseudovirus-antibody mixtures were used to infect 293TT cells for 3 days. The SEAP content in 10 μl of clarified cell supernatant was determined by using a Great EscAPe SEAP chemiluminescence detection kit (BD Biosciences Clontech). Neutralization titers were defined as the reciprocal of the highest serum dilution that caused at least a 50% reduction in SEAP activity (with 100% SEAP activity ranging from 50 to 100 relative light units).
RESULTS
Design of an HPV16 L1 nucleotide sequence with most frequently used codons in Salmonella.
The codons used for translation of major endogenous proteins in S. enterica serovar Typhimurium (7, 14) were considered to design an optimized L1 ORF. From the 506 codons of the original HPV16 L1 sequence (HPV16 114/B [18]), 163 were modified in codons most frequently used in Salmonella (Fig. 1). This included all the codons of the original L1 sequence which are rarely found in Salmonella (136) and some (27 of 72) of the less frequently used codons. The L1 ORF was then replaced in plasmid pFS14nsd-HPV16 L1 (31) by the new L1S ORF, yielding pFS14nsd-HPV16 L1S. The new plasmid was first introduced in the attenuated S. enterica serovar Typhimurium strain PhoPc (27) to generate the recombinant strain, called PhoPc L1S hereafter. Four others L1S recombinant attenuated Salmonella strains were subsequently produced (see below); Table 1 summarizes the different strains and abbreviations used in this study.
FIG. 1.
Codon-optimized HPV16 L1S ORF. The nucleotide sequence of L1S is shown with the modified codons underlined; modified nucleotides are in bold.
TABLE 1.
Salmonella strains used in this study
| Strain (attenuation) | Plasmid electroporated | Abbreviation | Reference |
|---|---|---|---|
| CS022 (PhoPc, pho-42) | PhoPc | 26 | |
| pFS14nsd-HPV16 L1 | PhoPc L1 | 31 | |
| pFS14nsd-HPV16 L1S | PhoPc L1S | This work | |
| χ4989 (Δcya Δcrp) | 4 | ||
| pFS14nsd-HPV16 L1 | χ4989 L1 | 4 | |
| pFS14nsd-HPV16 L1S | χ4989 L1S | This work | |
| χ4990 (Δcya Δcrp-cdf) | 4 | ||
| pFS14nsd-HPV16 L1 | χ4990 L1 | 4 | |
| pFS14nsd-HPV16 L1S | χ4990 L1S | This work | |
| CS015 (PhoP−, ΔphoPQ) | 26 | ||
| pFS14nsd-HPV16 L1 | PhoP− L1 | 4 | |
| pFS14nsd-HPV16 L1S | PhoP− L1S | This work | |
| SL7207 (ΔaroA) | 16 | ||
| pFS14nsd-HPV16 L1 | AroA L1 | This work | |
| pFS14nsd-HPV16 L1S | AroA L1S | This work |
HPV16 L1 and VLP expression.
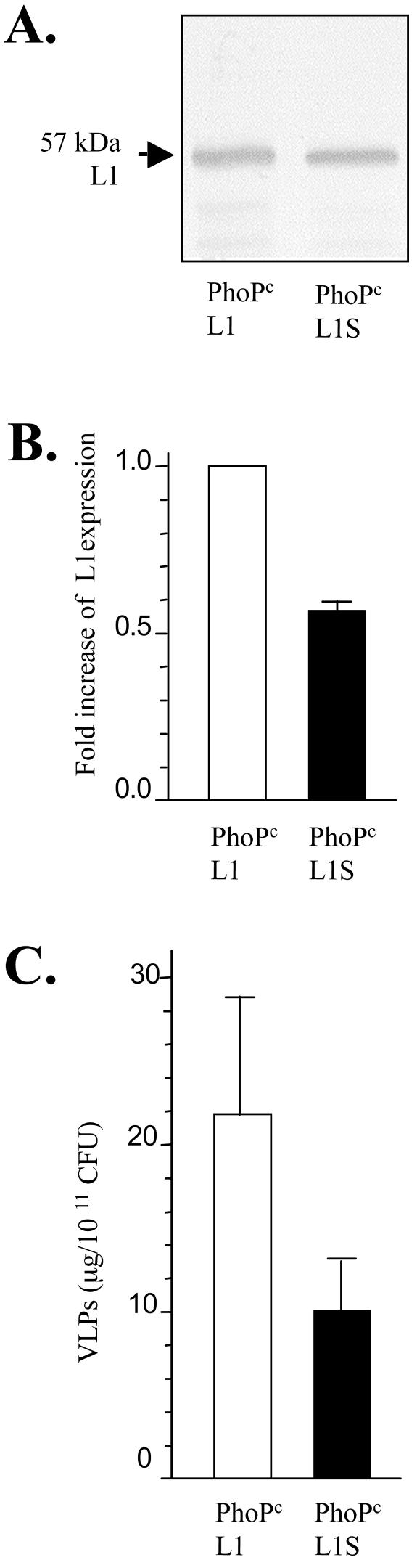
The expression of the L1 protein in the lysates of exponential cultures of PhoPc L1 and PhoPc L1S were compared by Western blotting (Fig. 2A). Surprisingly, expression of L1 in the bacterial cultures was not improved with the new L1S sequence but rather decreased by twofold (Fig. 2B). This finding was confirmed when the amounts of VLPs produced in the two recombinant strains were compared by sandwich ELISA (Fig. 2C). A striking difference in the growth rate of the two strains was noticed when the time to reach mid-log phase after inoculation of 50 ml of Luria-Bertani (LB) broth with a single colony was compared (ca. 7 h for PhoPc L1S and ca. 15 h for PhoPc L1). This may suggest that the optimized codon usage of L1 with respect to the corresponding cognate tRNAs maximized the growth rate without a concomitant increase in L1S translation.
FIG. 2.
HPV16L1 expression in PhoPc L1 and PhoPc L1S recombinant strains. An immunoblot of bacterial lysates with anti-HVP16 L1 monoclonal antibody is shown, and the 57-kDa protein band identified as L1 is indicated by an arrow (A). Scanning of the L1 protein bands obtained after immunoblotting of the bacterial lysates from the two recombinant strains (three independent experiments) was performed by using National Institutes of Health Image software. The results are shown as the means of pixel densities of the L1 protein bands normalized to the content in bacteria and are expressed as increases in expression (n-fold) in comparison to PhoPc L1 (B). The amounts of VLPs determined by sandwich ELISA are shown in micrograms per 1011 CFU (C).
Stability of the L1S-encoding plasmid in vitro and in vivo.
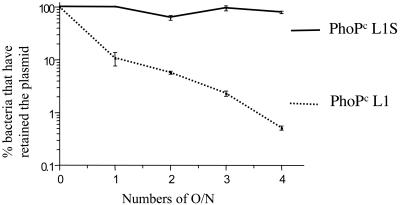
We have previously reported that the original L1-encoding plasmid was rapidly lost by plasmid segregation in Salmonella in the absence of antibiotic selection in vivo (4, 31). The stability of the L1S- and L1-encoding plasmids was first compared in vitro. For this purpose the percentages of bacteria still harboring the L1- or L1S-encoding plasmids were compared during four successive overnight cultures in the absence of antibiotic selection (Fig. 3). As expected, the L1-encoding plasmid was rapidly lost. In contrast, the L1S-encoding plasmid was recovered in most of the bacteria after ca. 50 generation times in the absence of antibiotic selection. The stability of the L1S-encoding plasmid was further examined in vivo after nasal and oral immunization of mice (Table 2). In contrast to the original L1-encoding plasmid (4), the L1S-encoding plasmid was completely stable for at least 2 weeks in the organs close to the sites of infection or entry. Some instability of the L1S plasmid was, however, observed in more distant organs such as the spleen, where ca. 10% of the bacteria were still harboring the L1S plasmid but no bacteria harboring the L1 plasmid were detected. We should also note that there is no evidence of a higher invasiveness or persistence of the L1S-harboring bacteria, despite the faster growing capacity of these bacteria observed in vitro.
FIG. 3.
L1 and L1S plasmid stability in vitro. The number of successive overnight cultures at 1/1000 dilution performed in LB broth without antibiotic is indicated on the horizontal axis. Each morning, bacteria were plated on LB agar in the presence or absence of antibiotic. The vertical axis represents the percentage of bacteria that have retained the plasmid. Error bars indicate the standard errors of the means. O/N, overnight cultures.
TABLE 2.
Recovery of Salmonella PhoPc carrying L1- or L1S-encoding plasmids 2 weeks after nasal or oral immunization
| Route of immunization | Organ(s) analyzed | Means of total Salmonella recovereda
|
% of Salmonella bearing the plasmid:
|
||
|---|---|---|---|---|---|
| PhoPc L1b | PhoPc L1S | PhoPc L1 | PhoPc L1S | ||
| Nasal | Lung | 5.30 ± 0.02 | 4.14 ± 0.21 | 3.2 | 100 |
| Cervical lymph nodes | 3.39 ± 0.09 | 2.95 ± 0.09 | 10 | 100 | |
| Peyer's patches | 2.51 ± 0.34 | 2.17 ± 0.42 | NDc | 100 | |
| Spleen | 3.76 ± 0.13 | 2.88 ± 0.14 | ND | 8 | |
| Oral | Peyer's patches | 2.02 ± 0.29 | 2.38 ± 0.29 | ND | 100 |
| Mesenteric lymph nodes | 1.70 ± 0.75 | 2.67 ± 0.15 | ND | 27 | |
| Spleen | 2.07 ± 0.87 | 2.46 ± 0.12 | ND | 16 | |
Means (log10) of CFU/organ ± standard error of the means.
Data after nasal immunization with PhoPc L1 are taken from Benyacoub et al. (4).
Not detectable.
Anti-HPV16 VLP antibody and HPV16 neutralization titers induced by PhoPc L1S or PhoPc L1.
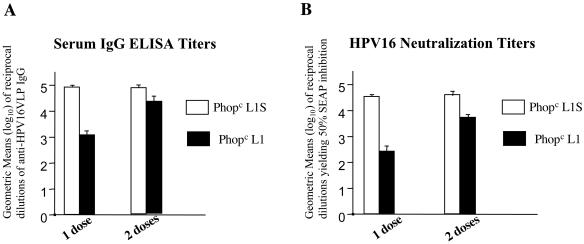
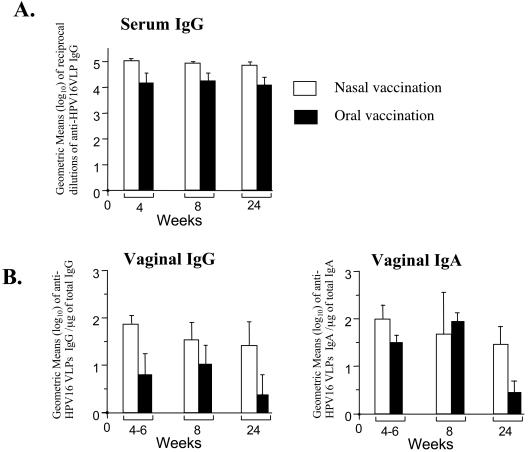
Our final aim was to test whether expression of the HPV16 L1S gene would improve the immunogenicity of the HPV16 VLP antigen in S. enterica serovar Typhimurium. Direct comparisons of the serum immune responses induced after nasal immunization of female BALB/c mice with PhoPc harboring either the original L1 sequence or the codon-optimized L1S sequence are shown in Fig. 4. In addition to anti-HPV16 VLP conformational antibody titers (Fig. 4A), HPV16 neutralization titers are shown in Fig. 4B. We used the SEAP HPV16 pseudovirus assay (34) to determine the endpoint neutralization titers. These neutralization titers are only slightly lower than the VLP ELISA titers and confirm the prophylactic potential of a Salmonella-based vaccine. Comparison of single nasal immunizations shows that a major improvement with the L1S strain is that HPV16 ELISA and neutralization titers are two orders of magnitude higher than those achieved with the original PhoPc L1 strain (Fig. 4 and references 4 and 31). The anti-VLP antibody titers measured in serum and vaginal secretions of the mice at 4 to 6, 8, and 24 weeks after a single immunization with PhoPc L1S are shown in Fig. 5. A single nasal vaccination induced high and long-lasting anti-HPV16 VLP immunoglobulin G (IgG) titers in serum, as well as specific IgG and IgA titers in vaginal washes. These antibody titers are similar to those induced after a double nasal vaccination with the original PhoPc L1 strain (4, 31). Interestingly, oral vaccination with PhoPc L1S was also highly immunogenic, although less than nasal vaccination, while even a double oral vaccination with the original PhoPc strain was inefficient (31). Administration of two nasal (Fig. 4) or three oral doses of PhoPc L1S (data not shown) did not increase the immune responses.
FIG. 4.
Comparison of serum anti-HPV16 VLP antibody and HPV16 neutralization titers after nasal vaccination with PhoPc L1S or PhoPc L1. Groups of five BALB/c mice were intranasally vaccinated with 106 to 107 CFU of PhoPc L1S or PhoPc L1 as a single dose or as two doses at week 0 and week 2. Serum was sampled 4 weeks after the last immunization, and HPV16 VLP-specific IgG (A) and HPV16 neutralization (B) titers are indicated. Data are expressed as the geometric means (log10) of the reciprocal serum dilutions of specific IgG (A) or reciprocal serum dilutions yielding 50% SEAP inhibition (B) from individual mice. Error bars indicate the standard errors of the means.
FIG. 5.
Anti-HPV16 VLP systemic (A) and vaginal (B) antibody titers after nasal and oral vaccination with PhoPc L1S. Groups of five to eight BALB/c mice were immunized with 106 to 107 CFU by the nasal route or 108 to 109 CFU by the oral route. Serum and vaginal washes were sampled at the indicated weeks after immunization. Data are expressed as the geometric means (log10) of the reciprocal dilutions of specific IgG from individual mice in serum (A) and specific IgG and IgA per microgram of total IgG and IgA, respectively, in secretions (B). Error bars indicate the standard errors of the means.
Serum anti-HPV16 VLP antibody and HPV16 neutralization titers following nasal or oral vaccination with differently attenuated S. enterica serovar Typhimurium strains expressing the codon-optimized or the original L1 gene.
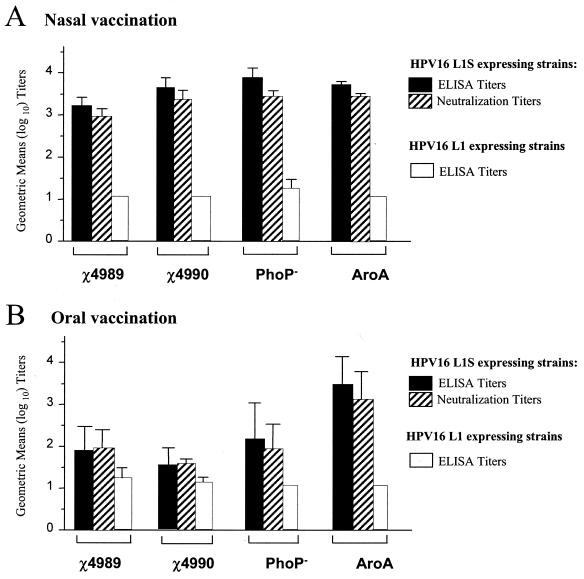
We have previously shown that nasal vaccination of mice with differently attenuated S. enterica serovar Typhimurium strains expressing the original L1-encoding plasmid induced only low levels of or no anti-HPV16 VLPs antibodies (4). Given the high immunogenicity observed with the PhoPc strain expressing the L1S encoding plasmid, we further introduced this plasmid in different strains including χ4989, χ4990, PhoP−, and AroA (Table 1 gives precise attenuations, abbreviations, and references). The serum anti-HVP16 VLP IgG and neutralizing titers measured in mice 6 to 7 weeks after a single nasal or oral vaccination with these new recombinant strains are shown in Fig. 6. In contrast to the strains expressing the original L1 gene, all the new recombinant strains induced consistent anti-HPV16 VLP humoral and HPV16 neutralizing responses after a single nasal vaccination, although the titers are about one order of magnitude lower than those achieved with the PhoPc L1S strain (Fig. 4 and 5). As expected, oral vaccination was less immunogenic, with the exception of the AroA L1S strain, which induced similar anti-HPV16 VLP IgG and HPV16 neutralizing titers after both routes of vaccination.
FIG. 6.
Serum anti-HPV16 VLP IgG and HPV16-neutralization titers after nasal or oral vaccination with χ4989, χ4990, PhoP−, and AroA expressing L1S or L1. Groups of four to seven BALB/c mice were immunized with ca. 106 to 107 CFU by the nasal route (A) or ca. 108 to 109 CFU by the oral route (B) with the indicated recombinant strains expressing L1S or L1. Serum was sampled 6 to 7 weeks after immunization, and HPV16 VLP-specific IgG (plain bars) or HPV16 neutralization (striped bars) titers are shown. Data are expressed as the geometric means (log10) of the reciprocal serum dilutions of specific IgG (A) or reciprocal serum dilutions yielding 50% SEAP inhibition (B) from individual mice. Error bars indicate the standard errors of the means.
DISCUSSION
The development of a Salmonella-based vaccine against HPV infection and associated lesions would be of great value for worldwide implementation with the theoretical advantage of inducing long-lasting systemic and mucosal immunity with a single oral vaccination. However, although we showed the feasibility of such a strategy in mice (31), several drawbacks had to be addressed before a Salmonella-based vaccine could be safely tested in women. The drawbacks included the requirement of a particular Salmonella phenotype (PhoPc [3, 4]) and the use of the nasal route of immunization to efficiently induce neutralizing antibody responses, as well as the observation that the L1-encoding plasmid was unstable without antibiotic selection (31, 4) or poorly expressed when stabilized with a semilethal complementation system (3). Here we report that most of these problems are solved by using a codon optimization strategy for the expression of the HPV16 L1 capsid gene (HPV16 L1S). Indeed, expression of the synthetic L1S gene is stable in Salmonella and results in higher immunogenicity when differently attenuated bacteria are delivered by either the nasal or oral route. Immunogenicity strongly correlated with HPV16 neutralization as assayed with the SEAP HPV16 pseudovirus assay. This further demonstrates the great potential of Salmonella-based vaccines to prevent HPV16 infections.
Expression of native papillomavirus capsid genes is limited in mammalian cells, but the resulting lack of immunogenicity of HPV DNA vaccines could be relieved by codon optimization (20, 23, 43). The influence of codon usage on immunogenicity has been recognized for other DNA vaccines (1, 12, 32, 39), where higher expression of the heterologous genes resulted in higher immunogenicity. As the codon usage of the original HPV16 capsid gene is also suboptimal for translation in Salmonella, we anticipated that expression of a codon-optimized L1S gene would result in higher VLP expression and, consequently, higher immunogenicity of the recombinant Salmonella. To our surprise, the higher immunogenicity of the differently attenuated L1S recombinant Salmonella does not correlate with higher amounts of L1 or VLPs produced in these bacteria. In fact, the opposite is true, and lower amounts of HPV16 VLPs were produced when the L1S gene was expressed (ranging from ca. 3 μg/1011 CFU for the AroA L1S strain to 23 μg/1011 CFU for χ4989 L1S) compared to the expression of the original L1 sequence (VLP amounts between 20 and 60 μg/1011 CFU[4]). This is in contrast to the >104 increase in L1 expression obtained in mammalian cells with a human-optimized HPV16 L1 gene (20). We should note, however, that we cannot exclude the possibility that the amounts of VLPs expressed in the bacteria may vary when the Salmonella are invading the mouse tissues, where the metabolic constraints are different. Unfortunately, we are unable to measure VLP expression in vivo, given the relatively low number of bacteria recovered (103 to 104 CFU/organ) and the low VLP expression achieved (<1 fg/bacteria).
Another notable feature associated with the expression of the codon-optimized L1S sequence is the improved stability of the L1S-expressing plasmid in vitro and in vivo in the absence of antibiotic selection. This may contribute to the higher immunogenicity of the recombinant Salmonella, as it results in a longer persistence of the VLP antigen carried by the bacteria. Such an explanation is in agreement with the idea that a longer persistence of antigens in the mucosa-associated lymphoid tissues is a key mechanism that underlies the immune responses elicited by Salmonella vaccine strains (35) and contrasts with the other suggestion that the initial amount of antigen that primes the mucosal lymphoid tissue is the critical point for inducing efficient immune responses (8, 10). Different approaches have been used to improve plasmid stability in bacterial carriers (reviewed in references 13 and 25). These approaches include the use of in vivo inducible promoters or balanced lethal plasmid stabilization systems, but to our knowledge codon optimization of heterologous antigens was not previously reported to induce plasmid stabilization.
Interestingly, plasmid stability and lower VLP expression were associated with a faster growth rate of the L1S-expressing bacteria in vitro. It is assumed that the investment in the translation system is optimized to provide a maximal growth rate of bacteria, and this is achieved by an adequate balance between the different tRNAs and their cognate codons (5). Our observations suggest that optimizing the codon usage of the heterologous L1 gene released the tRNA pool, allowing translation of endogenous bacterial protein and thereby increasing the growth rate to the detriment of L1 or VLP expression. This increased growth rate in vitro did not correlate with an increased invasion and/or persistence of the bacteria in vivo, and, therefore, we do not anticipate that L1S expression may affect the safety of a Salmonella vaccine strain.
The immunogenicity of PhoPc L1S in mice is really improved and compares well with that induced with purified HPV16 VLPs, the leading prototype prophylactic subunit vaccine now in phase III clinical trials (reviewed in reference 24). A single nasal immunization with PhoPc L1S induced serum and vaginal anti-HVP16 VLPs IgG titers that were similar to results with three subcutaneous injections with 1 μg of purified HPV16 VLPs or three nasal/aerosol immunizations with 5 μg of VLP doses together with the mucosal adjuvant cholera toxin, including induction of specific IgA in vaginal washes for the mucosal protocols (2, 29). Although we have shown that nasal vaccination with recombinant Salmonella can be highly efficient at low doses and without concomitant lung inflammation (28), there are still safety concerns for using such a route of immunization in humans. Here we report that the safer oral route can be used since a single oral vaccination with PhoPc L1S was immunogenic, and though the VLP-specific titers are lower than following nasal immunization, they are similar to those induced after three nasal or aerosol doses of 5 μg of VLP without adjuvant (2).
One of the major limitations for testing an HPV16 Salmonella-based vaccine in humans was the reported reversibility of the PhoPc strain, which harbors a single attenuating mutation (PhoQ24 [27]), and the necessity of this phenotype for inducing efficient anti-VLP responses in mice (3, 4). Here we show that other S. enterica serovar Typhimurium strains (χ4990, PhoP−, and aroA) whose attenuating mutations have been tested in S. enterica serovar Typhi and have been shown to be safe in humans (χ4632 [30, 40], Ty800 [15], and CVD908-htrA [41]) can induce anti-VLP and HPV16-neutralizing responses in mice after nasal vaccination. The titers are, however, one or two orders of magnitude lower than those induced by the PhoPc strain, which is in agreement with previous findings (3, 4). Whether expression of PhoQ24 (3) may enhance the immunogenicity of the new L1S recombinant strains remains to be tested. Although oral immunization was less efficient than nasal immunization, the immunogenicity of AroA L1S was less affected by immunization by the oral route. This result is highly encouraging as the S. enterica serovar Typhi vaccine strains that harbor Aro deletions (CVD908 htrA) has the best record of safety and immunogenicity in humans (reviewed in reference 13, 21, and 33). Thus, a recombinant CVD908 htrA L1S strain may represent the best candidate oral live vaccine to test in human volunteers for the prophylaxis of HPV16 infections and associated lesions.
Acknowledgments
We thank Véronique Revaz and Susanne Wirth for their critical reading of the manuscript, Neil Christensen for providing monoclonal antibodies H16.E70 and H16.V5, and John Schiller for providing the SEAP HPV16 pseudovirus assay.
This work was supported by the Fonds de Service of the Department of Gynecology and by grants from the Leenaards Foundation (to D.N.-H.) and Swiss National Science Foundation (number 631-057969.99 to D.N.-H. and number 32-63021.00 to D.B.).
REFERENCES
- 1.Andre, S., B. Seed, J. Eberle, W. Schraut, A. Bultmann, and J. Haas. 1998. Increased immune response elicited by DNA vaccination with a synthetic gp120 sequence with optimized codon usage. J. Virol. 72:1497-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balmelli, C., R. Roden, A. Potts, J. Schiller, P. De Grandi, and D. Nardelli-Haefliger. 1998. Nasal immunization of mice with human papillomavirus type 16 virus-like particles elicits neutralizing antibodies in mucosal secretions. J. Virol. 72:8220-8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baud, D., J. Benyacoub, V. Revaz, M. Kok, F. Ponci, M. Bobst, R. Curtiss III, P. De Grandi, and D. Nardelli-Haefliger. 2004. Immunogenicity against human papillomavirus type 16 virus-like particles is strongly enhanced by the PhoPc phenotype in Salmonella enterica serovar Typhimurium. Infect. Immun. 72:750-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benyacoub, J., S. Hopkins, A. Potts, S. Kelly, J.-P. Kraehenbuhl, R. Curtiss III, P. De Grandi, and D. Nardelli-Haefliger. 1999. The nature of the attenuation of Salmonella typhimurium strains expressing human papillomavirus type 16 virus-like particles determines the specific antibody responses in nasally immunized mice. Infect. Immun. 67:3674-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg, O. G., and C. G. Kurland. 1997. Growth rate-optimised tRNA abundance and codon usage. J. Mol. Biol. 270:544-550. [DOI] [PubMed] [Google Scholar]
- 6.Bosch, F. X., A. Lorincz, N. Munoz, C. J. Meijer, and K. V. Shah. 2002. The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 55:244-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns, D. M., and I. R. Beacham. 1985. Rare codons in E. coli and S. typhimurium signal sequences. FEBS Lett. 189:318-324. [DOI] [PubMed] [Google Scholar]
- 8.Cardenas, L., U. Dasgupta, and J. D. Clements. 1994. Influence of strain viability and antigen dose on the use of attenuated mutants of Salmonella as vaccine carriers. Vaccine 12:833-840. [DOI] [PubMed] [Google Scholar]
- 9.Christensen, N. D., J. Dillner, C. Eklund, J. J. Carter, G. C. Wipf, C. A. Reed, N. M. Cladel, and D. A. Galloway. 1996. Surface conformational and linear epitopes on HPV-16 and HPV-18 L1 virus-like particles as defined by monoclonal antibodies. Virology 223:174-184. [DOI] [PubMed] [Google Scholar]
- 10.Covone, M. G., M. Brocchi, E. Palla, W. D. da Silveira, R. Rappuoli, and C. L. Galeotti. 1998. Levels of expression and immunogenicity of attenuated Salmonella enterica serovar Typhimurium strains expressing Escherichia coli mutant heat-labile enterotoxin. Infect. Immun. 66:224-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtiss, R., J. O. Hassan, J. Herr, S. M. Kelly, M. Levine, G. G. Mahairas, D. Milich, D. Peterson, F. Schödel, J. Srinivasan, C. Tacket, S. A. Tinge, and R. Wright. 1994. Nonrecombinant and recombinant avirulent Salmonella vaccines, p. 340-351. In G. P. Talwar (ed.), Recombinant and synthetic vaccines. Narosa Publishing House, New Delhi, India.
- 12.Demi, L., A. Bojak, S. Steck, M. Graf, J. Wild, R. Schirmbeck, H. Wolf, and R. Wagner. 2001. Multiple effects of codon usage optimization on expression and immunogenicity of DNA candidate vaccines encoding the human immunodeficiency virus type 1 Gag protein. J. Virol. 75:10991-11001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garmory, H., K. A. Brown, and R. W. Titball. 2002. Salmonella vaccines for use in humans: present and future perspectives. FEMS Microbiol. Rev. 26:339-353. [DOI] [PubMed] [Google Scholar]
- 14.Grosjean, H., and W. Fiers. 1982. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Proc. Natl. Acad. Sci. USA 86:7077-7081. [DOI] [PubMed] [Google Scholar]
- 15.Hohmann, E. L., C. A. Oletta, K. P. Killeen, and S. I. Miller. 1996. phoP/phoQ-deleted Salmonella typhi (Ty800) is a safe and immunogenic single-dose typhoid fever vaccine in volunteers. J. Infect. Dis. 173:1408-1414. [DOI] [PubMed] [Google Scholar]
- 16.Hoiseth, S. K., and B. A. D. Stocker. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291:238-239. [DOI] [PubMed] [Google Scholar]
- 17.Hopkins, S., J.-P. Kraehenbuhel, F. Schödel, A. Potts, D. Peterson, P. De Grandi, and D. Nardelli-Haefliger. 1995. A recombinant Salmonella typhimurium vaccine induces local immunity by four different routes of immunization. Infect. Immun. 63:3279-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirnbauer, R., J. Taub, H. Greenstone, R. Roden, M. Dürst, L. Gissmann, D. R. Lowy, and J. T. Schiller. 1993. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J. Virol. 67:6929-6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koutsky, L. A., D. A. Ault, C. M. Wheeler, D. R. Brown, E. Barr, F. B. Alvarez, L. Chiacchierini, and K. Jansen. 2002. A controlled trial of a human papillomavirus type 16 vaccine. N. Engl. J. Med. 347:1645-1651. [DOI] [PubMed] [Google Scholar]
- 20.Leder, C., J. A. Kleinschmidt, C. Wiethe, and M. Muller. 2001. Enhancement of capsid gene expression: preparing the human papillomavirus type 16 major structural gene L1 for DNA vaccination purposes. J. Virol. 75:9201-9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine, M. M., C. O. Tacket, and M. B. Sztein. 2001. Host-Salmonella interaction: human trials. Microbes Infect. 3:1271-1279. [DOI] [PubMed] [Google Scholar]
- 22.Levine, M. M., G. C. Woodrow, J. B. Kaper, and G. S. Cobon. 1997. Attenuated Salmonella as a live vector for expression of foreign antigens. Dekker, New York, N.Y.
- 23.Liu, W. J., K. N. Zhao, F. G. Gao, G. R. Leggatt, G. J. Fernando, and I. H. Frazer. 2002. Polynucleotide viral vaccines: codon optimisation and ubiquitin conjugation enhances prophylactic and therapeutic efficacy. Vaccine 20:862-869. [DOI] [PubMed] [Google Scholar]
- 24.Lowy, D. R., and I. H. Frazer. 2003. Chapter 16: prophylactic human papillomavirus vaccines. J. Natl. Cancer Inst. Monogr. 31:111-116. [DOI] [PubMed] [Google Scholar]
- 25.Mastroeni, P., J. A. Chabalgoity, S. J. Dunstan, D. J. Maskell, and G. Dougan. 2001. Salmonella: immune responses and vaccines. Vet. J. 161:132-164. [DOI] [PubMed] [Google Scholar]
- 26.Miller, S. I., A. M. Kukral, and J. J. Mekalanos. 1989. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc. Natl. Acad. Sci. USA 86:5054-5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller, S. I., and J. J. Mekalanos. 1990. Constitutive expression of the phoP regulon attenuates Salmonella virulence and survival within macrophages. J. Bacteriol. 172:2485-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nardelli-Haefliger, D., J. Benyacoub, R. Lemoine, S. Hopkins-Donaldson, A. Potts, F. Hartmann, J.-P. Kraehenbuhl, and P. De Grandi. 2001. Nasal vaccination with attenuated Salmonella typhimurium strains expressing the hepatitis B nucleocapsid: dose response analysis. Vaccine 19:2854-2861. [DOI] [PubMed] [Google Scholar]
- 29.Nardelli-Haefliger, D., R. Roden, C. Balmelli, A. Potts, J. Schiller, and P. De Grandi. 1999. Mucosal but not parenteral immunization with purified human papillomavirus type 16 virus-like particles induces neutralizing titers of antibodies throughout the estrous cycle of mice. J. Virol. 74:9609-9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nardelli-haefliger, D., J. P. Kraehenbuhl, R. Curtiss III, F. Schodel, A. Potts, S. Kelly, and P. De Grandi. 1996. Oral and rectal immunization of adult female volunteers with a recombinant attenuated Salmonella typhi vaccine strain. Infect. Immun. 64:5219-5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nardelli-haefliger, D., R. Roden, J. Benyacoub, R. Sahli, J. P. Kraehenbuhl, J. T. Schiller, P. Lachat, A. Potts, and P. Degrandi. 1997. Human papillomavirus type 16 virus-like particles expressed in attenuated Salmonella typhimurium elicit mucosal and systemic neutralizing antibodies in mice. Infect. Immun. 65:3328-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narum, D. L., S. N. Kumar, W. O. Rogers, S. R. Fuhrmann, H. Liang, M. Oakley, A. Taye, B. K. Sim, and W. L. Hoffman. 2001. Codon optimization of gene fragments encoding Plasmodium falciparum merzoite proteins enhances DNA vaccine protein expression and immunogenicity in mice. Infect. Immun. 69:7250-7253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasetti, M. F., M. M. Levine, and M. B. Sztein. 2003. Animal models paving the way for clinical trials of attenuated Salmonella enterica serovar Typhi live oral vaccines and live vectors. Vaccine 21:401-418. [DOI] [PubMed] [Google Scholar]
- 34.Pastrana, D. V., C. B. Buck, Y. Y. Pang, C. D. Thompson, P. E. Castle, P. C. FitzGerald, S. Kruger Kjaer, D. R. Lowy, and J. T. Schiller. 2004. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology 321:205-216. [DOI] [PubMed] [Google Scholar]
- 35.Roberts, M., S. N. Chatfield, and G. Dougan. 1994. Salmonella as carriers of heterologous antigens, p. 27-58. In D. T. O'Hagan (ed.), Novel delivery systems for oral vaccines. CRC Press, Inc., Boca Raton, Fla.
- 36.Schiller, J. T., and A. Hidesheim. 2000. Developing HPV virus-like particle vaccines to prevent cervical cancer: a progress report. J. Clinical Virol. 19:67-74. [DOI] [PubMed] [Google Scholar]
- 37.Schödel, F. 1992. Prospects for oral vaccination using recombinant bacteria expressing viral epitopes. Adv. Virus Res. 41:409-446. [DOI] [PubMed] [Google Scholar]
- 38.Schödel, F., G. Enders, M.-C. Jung, and H. Will. 1990b. Recognition of a hepatitis B virus nucleocapsid T-cell epitope expressed as a fusion protein with the subunit B of Escherichia coli heat labile enterotoxin in attenuated salmonellae. Vaccine 8:569-572. [DOI] [PubMed] [Google Scholar]
- 39.Stradford, R., G. Douce, L. Zhang-Barber, N. Fairweather, J. Eskola, and G. Dougan. 2000. Influence of codon usage on the immunogenicity of a DNA vaccine against tetanus. Vaccine 19:810-815. [DOI] [PubMed] [Google Scholar]
- 40.Tacket, C. O., S. M. Kelly, F. Schodel, G. Losonsky, J. P. Nataro, R. Edelman, M. M. Levine, and R. Curtiss III. 1997. Safety and immunogenicity in humans of an attenuated Salmonella typhi vaccine vector strain expressing plasmid-encoded hepatitis b antigens stabilized by the Asd-balanced lethal vector system. Infect. Immun. 65:3381-3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tacket, C. O., M. B. Sztein, G. A. Losonsky, S. S. Wasserman, J. P. Nataro, R. Edelman, D. Pickard, G. Dougan, S. N. Chatfield, and M. M. Levine. 1997. Safety of live oral Salmonella typhi vaccine strains with deletions in htrA and aroC aroD and immune response in humans. Infect. Immun. 65:452-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walboomers, J. M., M. V. Jacobs, M. M. Manos, F. X. Bosch, J. A. Kummer, K. V. Shah, P. J. Snijders, J. Peto, C. J. Meijer, and N. Munoz. 1999. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 189:12-19. [DOI] [PubMed] [Google Scholar]
- 43.Zhou, J., W. J. Liu, S. W. Peng, X. Y. Sun, and I. Frazer. 1999. Papillomavirus capsid protein expression level depends on the match between codon usage and tRNA availability. J. Virol. 73:4972-4982. [DOI] [PMC free article] [PubMed] [Google Scholar]