Abstract
The protein product of varicella-zoster virus (VZV) ORF47 is a serine/threonine protein kinase and tegument component. Evaluation of two recombinants of the Oka strain, rOka47ΔC, with a C-terminal truncation of ORF47, and rOka47D-N, with a point mutation in the conserved kinase motif, showed that ORF47 kinase function was necessary for optimal VZV replication in human skin xenografts in SCID mice but not in cultured cells. We now demonstrate that rOka47ΔC and rOka47D-N mutants do not infect human T-cell xenografts. Differences in the growth of kinase-defective ORF47 mutants allowed an examination of requirements for VZV pathogenesis in skin and T cells in vivo. Although virion assembly was reduced and no virion transport to cell surfaces was observed, epidermal cell fusion persisted, and VZV polykaryocytes were generated by rOka47ΔC and rOka47D-N in skin. Virion assembly was also impaired in vitro, but VZV-induced cell fusion continued to cause syncytia in cultured cells infected with rOka47ΔC or rOka47D-N. Intracellular trafficking of envelope glycoprotein E and the ORF47 and IE62 proteins, components of the tegument, was aberrant without ORF47 kinase activity. In summary, normal VZV virion assembly appears to require ORF47 kinase function. Cell fusion was induced by ORF47 mutants in skin, and cell-cell spread occurred even though virion formation was deficient. VZV-infected T cells do not undergo cell fusion, and impaired virion assembly by ORF47 mutants was associated with a complete elimination of T-cell infectivity. These observations suggest a differential requirement for cell fusion and virion formation in the pathogenesis of VZV infection in skin and T cells.
Varicella-zoster virus (VZV) is a ubiquitous human herpes virus and the causative agent of varicella (chicken pox) and zoster (shingles) (1, 8). Varicella is characterized by viremia and skin lesions. VZV infects T cells, which may be a mechanism for its transport from respiratory epithelial sites of inoculation to dermal and epidermal cells (1, 19, 20, 38). Thus, T cells as well as skin are critical targets for VZV pathogenesis. VZV establishes latency in sensory ganglia and causes zoster upon reactivation. Two major advances have provided new opportunities for understanding the molecular mechanisms of VZV pathogenesis. First, VZV cosmids permit the construction of VZV recombinant viruses with targeted genetic mutations (4, 15, 22). Second, the SCIDhu mouse model, in which skin and T-cell xenografts are infected in vivo, makes it possible to define the effects of genetic mutations in VZV on virulence for differentiated human cells within their unique tissue microenvironments (2, 3, 13, 24-28, 31, 34, 35, 37).
VZV virion production begins with the assembly of capsids, which appear to undergo initial envelopment at the nuclear membrane and de-envelopment in the cytoplasm. Final envelopment in the trans-Golgi network (TGN) (7) is followed by virion transport to cell surfaces. Syncytium formation, a hallmark of VZV infection in cultured cells, reflects cell fusion mediated by combinations of glycoproteins gB, gE, gH, and gL (5). VZV skin lesions contain many multinucleated giant cells. Although defective particles predominate in vitro, VZV virions generated in skin xenografts resemble those in varicella or zoster lesions (27).
VZV ORF47 protein is a serine/threonine kinase related to herpes simplex virus UL13 (33, 36). The ORF47 protein autophosphorylates and phosphorylates the major immediate-early transactivator, IE62 protein (1, 18, 30), IE63 protein (17), gE (16, 29), and ORF32 protein (32). VZV gE is essential for replication (23) and requires ORF47 phosphorylation to mediate cell fusion and TGN trafficking for virion assembly (16). The ORF47 protein, while dispensable in cultured cells, proved to be required for skin and T-cell infection (12, 28). However, the mutants expressing kinase-defective ORF47 protein retained some capacity to infect skin (3). Infectious virus was recovered from 32% of implants inoculated with rOka47ΔC (a recombinant of the Oka strain with a C-terminal truncation of ORF47) and from 22% of those inoculated with rOka47D-N (a recombinant with a point mutation in the conserved kinase motif), while virus was recovered from 82% of rOka-infected skin xenografts. By days 21 and 28, rOka47ΔC and rOka47D-N titers were ≤10% of the titers in skin xenografts inoculated with rOka. ORF47 kinase function mapped to the C terminus, and specifically to the conserved DYS motif (residues 282 to 84), which is involved in phosphate transfer, as shown with rOka47ΔC and rOka47D-N mutants (3, 10, 11). ORF47-IE62 protein binding was preserved, but eliminating ORF47 kinase activity caused nuclear retention of these proteins (3). Nevertheless, like the ROKA47S null mutant, the plaque phenotypes and growth kinetics of rOka47ΔC and rOka47D-N resembled those of rOka in vitro (3, 12).
In these experiments, we examined replication of the kinase-defective ORF47 mutants in human T-cell xenografts, further characterized their infectivity in skin, examined ORF47 protein interactions with gE, and evaluated the effects on VZV virion formation of blocking ORF47 kinase. Taken together, the analysis of kinase-defective ORF47 mutants suggested a differential requirement for cell fusion and virion formation in VZV infection of skin and T cells.
MATERIALS AND METHODS
Recombinant viruses and animals.
Recombinant viruses were constructed as described previously (3). The rOka47ΔC strain has a truncated ORF47 that terminates at the start of the sequence for the kinase domain in the 3′ half of ORF47; the rOka47D-N strain has rOka with an intact open reading frame for ORF47 and a point mutation at nucleotide 847, which replaced aspartic acid with asparagine in the conserved DYS kinase motif. ROKA47S, an ORF47 null mutant, was kindly provided by Jeffrey Cohen, National Institutes of Health (12). Recombinant viruses were isolated by the transfection of human melanoma cells with the mutated cosmid and the four intact cosmids (15, 31). Melanoma cells were maintained in tissue culture medium (Mediatech, Washington, D.C.) supplemented with 10% fetal calf serum (Tissue Culture Biologicals, Tulare, Calif.), nonessential amino acids, and antibiotics. The VZV recombinants, rOka47ΔC, rOka47D-N, and rOka, were generated by using cosmids pSpe5, pSpe21, and pFsp4 of vaccine Oka origin; pAvr102 and pAfl17 were of parent Oka origin. Viruses were passaged to human embryonic lung fibroblasts and stored at −80°C in fetal calf serum with 10% dimethyl sulfoxide for SCIDhu experiments.
Thymus or liver (T-cell) and skin xenografts were prepared in male homozygous C.B-17 scid/scid mice (3, 26, 27). Human fetal tissues were obtained with informed consent according to federal and state regulations; animals were cared for according to the Animal Welfare Act PL 94-279. T-cell xenografts were harvested at 14 and 21 days after inoculation or mock infection; tissues were analyzed by infectious focus assay, hematoxylin and eosin staining, and in situ hybridization (26, 27). For in situ hybridization, xenograft sections were probed with a 12.9-kb biotinylated plasmid, pVZV-C, that consists of the HindIII fragment C of VZV genomic DNA in pBR322. The negative control was the pBR322 vector alone. Hybridization was detected with a streptavidin-alkaline phosphatase conjugate and visualized with nitroblue tetrazolium salt and 5-bromo-4-chloro-3-indoyl phosphate p-toluidine salt.
Electron microscopy.
For scanning electron microscopy (SEM), samples were fixed and stored in 4% paraformaldehyde. Samples were washed three times with phosphate-buffered saline (PBS) for 5-min intervals and fixed with 1% glutaraldehyde in sodium cacodylate buffer for 2 h on ice. The fixed samples were washed three times with PBS and fixed with 1% osmium tetroxide for 2 h on ice. After five rinses with double-distilled water, the samples were dehydrated in ethanol of 60, 70, 80, 90, 95, 99, and 100% twice for 20 min each. The dehydrated samples were dried by critical point dryer, mounted on aluminum stubs by silver colloid, sputter coated with a gold palladium alloy, and viewed with a Hitachi S-4000 scanning electron microscope (Hitachi Instruments, Inc., Mito City, Japan).
For transmission electron microscopy (TEM), samples were fixed for 20 min with 4% paraformaldehyde and 0.05% glutaraldehyde in 0.1% sodium cacodylate buffer (pH 7.6) at 4°C; the sample was stored in 1% paraformaldehyde in sodium cacodylate buffer at 4°C. The samples were incubated in 0.1% Triton X-100 in PBS for 10 min, washed three times with 0.1 M sodium phosphate buffer (pH 7.4) (PB) for 5 min, and incubated with freshly prepared 0.05% sodium borohydride and 0.1% glycine in PB for 10 min. The samples were rinsed in PB, fixed in 2.5% glutaraldehyde in PB for 10 min at room temperature, and rinsed three times with PB. The sample was fixed in 0.5% osmium tetroxide in PB for 2 h at 4°C and rinsed three times in PB for 20 min and three times in double-distilled water for 10 min. After a 20-min incubation with 2.5% uranyl acetate in double-distilled water, the sample was dehydrated in ethanol. The samples were incubated with ethanol and Epon at a ratio of 2:1 for 1 h, ethanol and Epon at a ratio of 1:2 for 1 h, in 100% Epon for 2 h, and in 100% Epon overnight. The samples were embedded in fresh Epon and placed in a 60°C oven for 24 h. The embedded samples were thin sectioned with a Reichert Ultracut E ultramicrotome (Reichert-Jung, Vienna, Austria), placed on copper grids, stained with uranyl acetate and Reynold's lead citrate, and viewed with a Hitachi H-7000 transmission electron microscope (Hitachi Instruments, Inc.).
Immunoprecipitation and Western blotting.
For immunoprecipitation experiments, melanoma cells infected with rOka, rOka47ΔC, rOka47D-N, or ROKA47S were lysed with radioimmunoprecipitation assay buffer (50 mM Tris [pH 8], 150 mM NaCl, 1% Igepal CA-360 [Sigma], 0.1% sodium dodecyl sulfate [SDS; Bio-Rad], 0.5% deoxycholic acid [Sigma]) containing a Complete Mini tablet (Roche, Inc.) in one 1-ml volume per T75 flask. The infected cell lysates were precleared with rabbit preimmune serum (4 μl) and protein A Sepharose CL-4B beads (36 μl) (Pharmacia, Inc.) for 30 min at 4°C. The supernatant was incubated overnight with rabbit anti-ORF47 antiserum (4 μl) and protein A Sepharose (36 μl). Samples were washed three times with HNTG buffer (20 mM HEPES, 150 mM NaCl, 0.1% Triton X-100, 5% glycerol), boiled in sample buffer, and separated by SDS-polyacrylamide gel electrophoresis in 7.5% gels. Proteins were transferred to Immobilon membranes. gE was detected with a rabbit polyclonal anti-gE antiserum generated by immunization with a glutathione transferase construct containing the complete 1.8-kb ORF68, which encodes gE.
Immunofluorescence and immunochemistry.
Infected melanoma cells were fixed in 2% formaldehyde with 0.1% Triton for 1 h after 24 and 30 h of infection. Cells were washed five times in PBS for 5 min, blocked with 5% donkey serum in PBS for 30 min, and incubated overnight with either rabbit anti-gE polyclonal antiserum and murine anti-p230 trans-Golgi antibody (BD Biosciences Pharmingen) or with rabbit anti-gI polyclonal antiserum (generous gift from Saul Silverstein, Columbia University, New York, N.Y.) (21). After five washes with PBS, the secondary antibodies, Texas Red-coupled anti-mouse and fluorescein isothiocyanate (FITC)-coupled anti-rabbit antibodies (Jackson ImmunoResearch, Inc.), were added for 1 h and shielded from light. After five PBS washes, coverslips were mounted with Vectashield (Vector Laboratories, Inc., Burlingame, Calif.) and stored in the dark. Imaging was performed at the Cell Sciences Imaging Facility, Stanford University, with a MultiProbe 2010 laser confocal microscope (Molecular Dynamics, Sunnyvale, Calif.).
For immunofluorescence, skin implants were harvested from SCIDhu mice, stored overnight in 4% paraformaldehyde in 1× PBS, and transferred to 30% sucrose (30% sucrose in 0.1 M PB) for 48 h. Skin implants were cut into sections 50 μm thick and collected sequentially in a cryoprotectant solution (250 ml of glycerol, 300 ml of ethylene glycol, 450 ml of Tris-buffered saline [TBS; pH 6.7]) for storage at −20°C. Sections were transferred sequentially to wells in 24-well plates containing TBS for 5 min, blocking solution (10% goat serum, 0.3% Triton in TBS) for overnight incubation at 4°C, and primary antibody solution (rabbit anti-ORF47 [1:250], mouse anti-gE [1:750], 5% goat serum, 0.3% Triton in TBS) for overnight incubation at 4°C, followed by three washes in TBS (two times for 10 min and one time for 30 min), incubation in secondary antibody solution (Texas Red-coupled anti-mouse and FITC-coupled anti-rabbit antibodies [1:50; Jackson ImmunoResearch, Inc.], 5% goat serum, 0.3% Triton in TBS) overnight at 4°C and shielded from light, and three washes in TBS as above. Sections were transferred to slides, and coverslips were mounted with Vectashield H-1200 (Vector Laboratories, Inc.) and stored in the dark. Imaging was performed at the Cell Sciences Imaging Facility, Stanford University, with a MultiProbe 2010 laser confocal microscope (Molecular Dynamics). Immunohistochemistry was performed as described previously (27). Sections were incubated with monoclonal gE antibody (Chemicon) at a 1:1,000 dilution; mouse immunoglobulin G (IgG) (Vector) at the same dilution was used as a control.
RESULTS
ORF47 kinase function in VZV replication in human T-cell xenografts.
In order to compare the role of ORF47 kinase activity as a determinant of virulence in T cells with our observation that kinase-defective ORF47 mutants replicate, albeit to a limited extent, in skin, we inoculated T-cell xenografts with rOka, rOka47ΔC, or rOka47D-N. All three viruses yielded similar titers in fibroblasts infected in vitro as determined by infectious center assays. The titers of the infected fibroblasts that were used to inoculate the T-cell xenografts were as follows: rOka, 2.3 × 103; rOka47ΔC, 4.3 ×103; and rOka47D-N, 5.7 × 103 (Fig. 1, y axis). VZV rOka replicated to ∼3 ×105 PFU per implant at day 14, increasing to 2.5 × 106 per xenograft at day 21 after inoculation (Fig. 1). No infectious virus was recovered from any xenografts harvested at day 14 or day 21 after inoculation with the ORF47 kinase-defective mutants, rOka47ΔC or rOka47D-N.
FIG. 1.
Replication of VZV rOka and kinase-defective ORF47 mutants in T-cell xenografts in vivo. T-cell xenografts were infected with rOka, rOka47ΔC, or rOka47D-N grown in human embryonic lung fibroblasts and harvested after 14 and 21 days. The titers of the infected fibroblasts that were used to inoculate the T-cell xenografts are shown on the y axis. Bars represent mean numbers of plaques (± standard errors of the means) by infectious center assay of 4 to 6 implants. Xenografts that did not contain infectious virus were excluded from the averages.
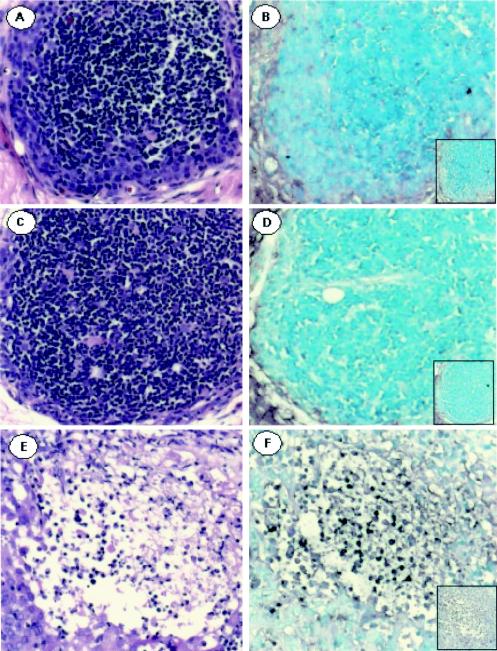
In order to determine whether abortive replication might occur in T-cell xenografts, sections obtained at day 14 or day 21 after inoculation were probed for VZV DNA by in situ hybridization. VZV DNA was not detected in T-cell xenografts inoculated with rOka47ΔC or rOka47D-N (Fig. 2A and C). However, VZV-infected T cells were detected throughout xenografts infected with rOka (Fig. 2E). rOka-infected T cells showed no evidence of cell fusion. No cell lysis or altered tissue architecture was apparent after inoculation of T-cell xenografts with rOka47ΔC (Fig. 2B) or rOka47D-N (Fig. 2D). In contrast, xenografts infected with rOka showed extensive tissue disruption and cell loss (Fig. 2F).
FIG. 2.
Analysis of T-cell xenografts infected with rOka or ORF47 kinase-defective mutants by in situ hybridization for VZV DNA. T-cell xenograft sections were stained with hematoxylin and eosin after inoculation with rOka47ΔC (B), rOka47D-N (D), or rOka (F). Panels A, C, and E show in situ hybridization with a HindIII VZV DNA probe after inoculation with rOka47ΔC (B), rOka47D-N (D), or rOka (F). Insets in panels B, D, and F are controls with the pBR322 vector alone.
Virion formation in skin xenografts and melanoma cells infected with rOka, rOka47ΔC, or rOka47D-N.
Although rOka47ΔC and rOka47D-N mutants were indistinguishable from rOka by plaque morphology and growth kinetics in the infectious center assay, these kinase-defective ORF47 mutants had restricted replication in skin (3). To investigate this difference, we analyzed infected melanoma cells and skin xenografts by SEM and TEM. By TEM, complete enveloped virions, ∼200 nm in diameter, were easily visualized in cytoplasmic vacuoles within melanoma cells infected with rOka (Fig. 3A and B). The virions had a phenotype characterized by a capsid with a dark core (DNA genome) surrounded by a tegument, which, in turn, was covered by a dark glycoprotein-laden envelope (arrow). The enveloped virions were enclosed within a cytoplasmic vacuole. The rOka virions emerged onto the cell surface in typical viral highways (Fig. 3C). Melanoma cells infected with recombinant viruses, rOka47D-N (Fig. 3G to I) and rOka47ΔC (Fig. 3J to L), contained far fewer viral particles, which were also smaller in size, in the cytoplasm. For example, the rOka47D-N particle (Fig. 3H) measured 125 nm in diameter, whereas the prototype particle (Fig. 3A) measured 170 nm; rOka47D-N particles were on average about 30% smaller and had ill-defined envelopes (Fig. 3G and H). The number of capsids detected in nuclei was also decreased to 3 to 5 in rOka47ΔC- and rOka47D-N-infected cells, while at least 20 capsids were detected in the nuclei of rOka-infected cells (data not shown). Surfaces of rOka47D-N-infected cells had almost no detectable viral particles, although surface filaments were prominent (Fig. 3I). These surface filaments, which were 200 to 330 nm, were much larger than the surface viral particles, which were 100 to 180 nm in diameter (Fig. 3C). In rOka47ΔC-infected cells, the cytoplasmic vacuoles were large but often contained only a single pleomorphic viral particle (Fig. 3J). Although a few viral particles were seen on the surfaces of cells infected with rOka47ΔC (Fig. 3L), the number of surface particles was reduced markedly in these cells and cells infected with rOka47ΔD-N and in comparison to cells infected with rOka, as illustrated in Fig. 3 (panel I and L versus panel C).
FIG. 3.
Imaging of melanoma cells infected with rOka and kinase-defective ORF47 mutants by TEM and SEM. Cells were examined by TEM (A, B, G, H, J, and K) or SEM (C, I, and L). Cells infected with rOka are shown in panels A to C. Schematic representations of panels A to C are shown in panels D to F. Cells infected with rOka47D-N are shown in panels G to I. Cells infected with rOkaΔC are shown in panels J to L. The prototype structures for virions inside cytoplasmic vacuoles and on the cell surface are illustrated in panels A to C, while aberrant viral particles are shown in panels G, H, J, and K.
When skin xenografts infected with rOka were examined for surface virions by SEM, virions of 150 to 200 nm in diameter were detected (Fig. 4A and B). At higher magnification, some virions showed indentations of about 30 nm in diameter within the envelope. This aberrant surface structure has been observed previously in VZV virions from cultured cells (14). Despite an extensive search of multiple fields, no virions were identified by SEM in skin sections from xenografts infected with rOka47D-N (Fig. 4C and D). A few spherical forms were observed, but they lacked the characteristic surface structure of viral particles. Similarly, SEM analysis of skin sections obtained after rOka47ΔC infection or mock infection of xenografts revealed no virions (data not shown). The analysis by SEM at low magnification (×2,000) of skin xenografts infected with rOka showed at least one area containing viral particles in each field. In contrast, no surface virions were detected when many fields of skin sections infected with rOkaORF47ΔC or rOkaORF47D-N were examined by two independent observers.
FIG. 4.
TEM and SEM of skin xenografts infected with rOka or rOka47D-N. Skin sections were examined by TEM (A to D) or SEM (E and F). Skin xenografts infected with rOka showed viral particles on the surface of infected skin cells (A and B). No viral particles but a few spherical structures were observed (arrows) on skin cells infected with rOka47D-N (C and D). TEM showed viral particles in rOka-infected skin cells located in the cytoplasm (E). Viral particles were smaller and less frequent in rOka47D-N-infected skin cells (F).
Analysis by TEM showed that VZV virions were abundant in skin sections infected with rOka (Fig. 4E). The virions were located in clusters near the outer plasma membrane and were 150 to 200 nm in diameter. Most virions were complete, having both capsid and envelope structures (Fig. 4E, arrows). In contrast, very few cells contained virions in skin xenografts infected with rOka47D-N (Fig. 4F, arrows). Further, when virions were found, they were fewer in number than those observed in rOka-infected skin cells. For example, the number of rOka47D-N particles was 45, whereas 240 rOka particles were detected per micrograph (60 μm2). In addition, most rOka47D-N virions were slightly smaller compared to rOka virions. The difference in virion size appeared to be due to incomplete envelopment of rOka47D-N virions. No virions were identified in skin sections from rOka47ΔC-infected or mock-infected xenografts evaluated by TEM (data not shown).
Effects of blocking ORF47 kinase activity on gE and gI in vitro.
Our previous experiments showed that ORF47 protein and kinase-defective ORF47 proteins bind to the major transactivator, the IE62 protein, and that ORF47-ORF47 binding persists in the absence of autophosphorylation (3). In order to further investigate the consequences of blocking ORF47 kinase activity, we examined the effects on gE. Melanoma cells were infected with rOka, rOka47ΔC, or rOka47D-N; cell lysates were prepared, and ORF47 protein and associated proteins were precipitated with ORF47 antiserum. Complex formation between ORF47 and gE proteins was detected by Western blotting in cells infected with rOka, rOka47ΔC, and rOka47D-N (Fig. 5). No complex formation occurred in cells infected with ROKA47S, which expresses only the first 165 amino acids of ORF47 protein due to the insertion of a stop codon (12). As in the case of ORF47-IE62 protein interactions, ORF47 protein binding to gE was mediated by the N-terminal portion of the protein and was independent of ORF47 kinase activity.
FIG. 5.
Immunoprecipitation of gE protein with ORF47 antiserum from melanoma cells infected with rOka or kinase-defective ORF47 mutants. Melanoma cells were infected with rOka, rOka47D-N (DN), rOka47ΔC (ΔC), or ROKA47S (47S). Infected cell lysates were incubated with ORF47 antiserum, subjected to SDS-polyacrylamide gel electrophoresis, and probed with polyclonal anti-gE antibody by Western blotting. ROKA47S was kindly provided by J. Cohen, National Institute of Allergy and Infectious Diseases.
gE was detected in plasma membranes and in the TGN in melanoma cells infected with rOka (Fig. 6A and C). However, in cells infected with rOka47D-N (Fig. 6D and F) or rOka47ΔC (Fig. 6G and I), the membrane localization of gE was reduced markedly, and gE was detected instead in a punctate distribution in apparent intracellular vesicles. gE localization was much more prominent in the region of the TGN which accumulates in the center of VZV polykaryocytes, with a surrounding ring of nuclei. The colocalization of gE to the TGN was demonstrated by staining with the TGN marker, p230 (Fig. 6B, C, E, F, H, and I). Patterns of gE distribution were the same in cells infected with rOka47D-N and rOka47ΔC.
FIG. 6.
Expression and intracellular localization of gE in melanoma cells infected with rOka or kinase-defective ORF47 mutants. Melanoma cells were infected with rOka (A, B, and C), rOka47D-N (D, E, and F), or rOka47ΔC (G, H, and I) for 30 h and examined for gE (A, D, and G) and TGN localization (B, E, and H) by confocal microscopy. gE was detected with FITC-labeled anti-rabbit IgG (green). The TGN marker p230 was detected with mouse anti-p230 and secondary Texas Red-conjugated antibody (red). Merged images are shown in panels C, F, and I.
VZV gI trafficking was also altered by a lack of ORF47 kinase activity. In melanoma cells infected with rOka, gI was expressed predominantly on plasma membranes (Fig. 7A and C), but gI was redirected to or retained in the cytoplasm in cells infected with rOka47D-N (Fig. 7D and F) or rOka47ΔC (Fig. 7G and I). gE showed the same limited membrane expression (Fig. 7E and H) as observed in Fig. 6. Colocalization of gI with gE suggests that more gI is directed to the TGN without ORF47 kinase activity.
FIG. 7.
Expression and intracellular localization of gI in melanoma cells infected with rOka or kinase-defective ORF47 mutants. Melanoma cells were infected with rOka (A, B, and C), rOka47D-N (D, E, and F), or rOka47ΔC (G, H, and I) for 30 h and examined for gI (A, D, and G) and gE (B, E, and H) by confocal microscopy. gI was detected with gI polyclonal antiserum and Texas Red-labeled anti-rabbit IgG (red). gE was detected with mouse monoclonal anti-gE antibody and secondary FITC-conjugated antibody (green). Merged images are shown in panels C, F, and I. Anti-gI antiserum was kindly provided by S. Silverstein, Columbia University.
ORF47 kinase function as a determinant of the localization of gE and ORF47 protein in infected skin cells in vivo.
Given the effects of disrupting ORF47 kinase activity on gE in cultured cells, gE localization, along with ORF47 protein distribution, was examined in skin xenografts infected with rOka or rOka47ΔC. Xenografts were harvested 21 days after inoculation. By confocal microscopy, gE expression in rOka-infected skin was observed on plasma membranes throughout cutaneous VZV lesions (Fig. 8A and E). Plasma membrane expression of gE was evident in cells near the epidermal surface which were infected initially, as shown in the upper right areas of panel A, as well as cells within newly infected areas below the initial lesion, which appear towards the lower left region of the representative section. In contrast to infection with rOka, sections of skin xenografts that were infected with rOka47ΔC displayed a reduced and disrupted expression of gE on plasma membranes, which was associated with a significant increase in the level of cytoplasmic localization of gE (Fig. 8B and F). The pattern of gE expression in skin xenografts infected with rOka and the ORF47 kinase-defective mutant resembled the patterns of gE expression in melanoma cells infected with rOka, rOka47ΔC, or rOka47D-N in vitro (Fig. 6).
FIG. 8.
Confocal analysis of skin xenografts infected with rOka or rOka47ΔC. Skin sections that were infected with rOka (A, C, and E) or rOka47ΔC (B, D, and F) were incubated with anti-gE monoclonal antibody followed by anti-mouse antibody labeled with Texas Red (A, B, E, and F) and ORF47 antiserum followed by anti-rabbit FITC-conjugated antibody (C, D, E, and F). DAPI (4′,6′-diamidino-2-phenylindole) counterstain (A, B, C, and D) was used to demonstrate the contrast between the small, fragmented nuclei (A, arrow) in advanced skin lesions and intact, larger nuclei (A, arrowhead) in areas of newly infected epidermal cells. Enlargements (E and F) demonstrate gE membrane localization and nuclear as well as cytoplasmic localization of ORF47 protein in rOka-infected skin cells (E) and increased cytoplasmic gE and ORF47 protein in rOka47ΔC-infected xenografts (F). Controls are shown in insets: panel A, uninfected skin tissue incubated with anti-gE antibody and ORF47 antiserum; panel E, skin tissue infected with rOka, incubated with mouse IgG and rabbit preimmune serum; panel F, skin tissue infected with rOka47ΔC, incubated with mouse IgG and rabbit preimmune serum.
ORF47 protein expression was cytoplasmic and nuclear within areas of rOka-infected skin sections that contained newly infected cells with intact nuclei and in which syncytium formation remained limited (Fig. 8C and E). In areas where cellular disruption had already occurred, ORF47 protein appeared to be associated with membranes of the disintegrating cells. Skin xenografts infected with rOka47ΔC showed very little expression of ORF47 protein. ORF47 protein was detected only in regions where cellular breakdown was evident and appeared to be cytoplasmic and rarely nuclear (Fig. 8D and F).
Patterns of gE localization in skin infected with rOka and examined by immunohistochemistry were as observed with immunofluorescence staining (Fig. 9). gE was localized predominantly to plasma membranes, and polykaryocyte formation was extensive in rOka-infected epidermal cells (Fig. 9A and B). Skin xenografts infected with rOka47D-N (Fig. 9C and D) and rOka47ΔC (Fig. 9E and F) confirmed that gE expression was characterized by reduced membrane localization and increased intracellular accumulation, but polykaryocyte formation persisted (Fig. 9B, D and F). Thus, cell fusion proceeded despite the marked disruption of gE trafficking as well as reduced virion formation.
FIG. 9.
Immunohistochemical analysis of skin xenografts infected with rOka, rOka47ΔC, or rOka47D-N. Sections were prepared from skin implants harvested at day 21 after infection with rOka (A and B), rOka47D-N (C and D), or rOka47ΔC (E and F), stained with monoclonal antibody to gE and counterstained with hematoxylin. Sections in panels A, C, and E are at a magnification of ×10; panels B, D and F are at amagnification of ×63 and are enlargements of the areas indicated with arrows in panels A, C, and E, respectively. Panels G, H, I, and J are controls. Panel G, uninfected skin xenografts, incubated with gE antibody(magnification, ×10); panel H, rOka-infected skin xenografts, incubated with mouse IgG (magnification, ×10); panel I, rOka47D-N-infected skin xenografts, incubated with mouse IgG (magnification, ×63); panel J, rOka47ΔC-infected skin xenografts, incubated with mouse IgG (magnification, ×10).
DISCUSSION
The pathogenesis of primary VZV infection appears to depend upon its tropism for migratory T cells, which may transport the virus from mucosal sites of inoculation, and on its capacity to form skin lesions containing high titers of cell-free infectious virus, which can be transmitted to other susceptible individuals in the population (1, 15, 19, 20, 38). This evaluation of the VZV mutant viruses rOka47ΔC and rOka47D-N, expressing kinase-defective forms of ORF47 protein, in SCIDhu mice with T-cell xenografts demonstrated that ORF47 kinase function was essential for VZV infection of human T cells within their differentiated microenvironment in vivo. In contrast, kinase-defective ORF47 mutants retained some capacity, albeit substantially diminished, for replication in human skin xenografts in SCID mice (3). The absence of ORF47 kinase function also resulted in disrupted gE localization, characterized by reduced plasma membrane expression, in epidermal cells in skin xenografts. Binding of ORF47 protein to gE was mediated by its N terminus and persisted in the absence of ORF47 kinase function. IE62 protein binding to ORF47 protein was also preserved in rOka47ΔC and rOka47D-N mutants, but IE62 exhibited aberrant nuclear localization at late times after infection (3).
The mislocalization of gE, as well as the ORF47 and IE62 proteins, in cells infected with rOka47ΔC and rOka47D-N suggested that eliminating ORF47 kinase function might interfere with the production of complete VZV virions (3). However, plaque morphology and growth kinetics, evaluated by infectious center assays, were indistinguishable in melanoma cells and fibroblasts infected with rOka, rOkaORF47ΔC, or rOkaORF47D-N. Despite the apparent preservation of infectivity in cultured cells, blocking ORF47 kinase activity reduced replication in skin and eliminated VZV replication in T cells in vivo. Therefore, to further analyze these differences, we examined VZV virion production and envelopment by SEM and TEM. These investigations revealed a marked decrease in VZV virion formation, envelopment, and transport to cell surfaces in skin xenografts in vivo. Furthermore, even though plaque morphology was intact and growth of the mutants was equivalent to rOka by infectious center assay, assembly of rOkaORF47ΔC or rOkaORF47D-N virions was impaired in vitro. Thus, ORF47 kinase function appears to be essential for normal VZV virion formation. However, the continued formation of syncytia in cultured cells infected with the kinase-defective ORF47 mutants indicated that VZV-induced cell fusion events allow incomplete virions to be transferred from cell to cell in vitro. Similarly, polykaryocytes were generated, and some infectious virus progeny was produced in differentiated epidermal cells in vivo. In contrast to cultured cells and skin, the impact of blocking ORF47 kinase function on VZV virion formation was the complete elimination of infectivity in T cells in vivo. VZV infection does not trigger the fusion of T cells (16). Although other mechanisms could be involved, interference with the production of VZV virions and their release for entry into uninfected T cells appears to be sufficient to explain the failure of rOka47ΔC and rOka47D-N to infect T cells in vivo.
The localization of gE within VZV-infected cells is important for cell-cell fusion and virion envelopment and appears to be controlled by differential phosphorylation of critical clusters of acidic residues containing serines and threonines by ORF47 protein and casein kinase II (9, 16, 41). Our experiments with rOkaORF47ΔC and rOkaORF47D-N showed that inactivating the ORF47 kinase function, while leaving the binding of gE to ORF47 protein intact, reduced the expression of gE on plasma membranes and caused a retention or redirection of more gE to cytoplasmic vesicles and to the TGN. VZV gE usually traffics to plasma membranes and to the TGN, where it is needed for association with tegument proteins and final envelopment (6, 7, 14, 16, 39). Increased gE localization to the TGN in the absence of ORF47 kinase function could result from interference with casein kinase II-mediated phosphorylation through binding of the kinase-defective ORF47 protein to gE.
gE, along with its heterodimer partner, gI, contributes to creating the characteristic VZV syncytia in melanoma cells and fibroblasts (5, 22, 24, 40). In our experiments, cell fusion persisted despite decreased gE expression on plasma membranes in cells infected with rOka47ΔC or rOka47D-N in vitro and in vivo. These observations suggest either that a relatively small concentration of gE in plasma membranes is sufficient for its fusion functions or that gE is less important than the other VZV fusogens, gB and gH (reviewed in reference 5), in facilitating cell-cell spread. As noted, the differential impact of blocking ORF47 kinase activity on VZV-induced cell fusion and virion production was also apparent in skin xenografts infected with rOka47ΔC or rOka47D-N. Fused epidermal cells containing incomplete VZV virions were present in VZV lesions generated by these kinase-defective ORF47 mutants in vivo. Nevertheless, VZV spread in skin was impaired substantially when ORF47 kinase activity was eliminated, whereas plaque formation was not disrupted in vitro. Since many viral proteins are phosphorylated by ORF 47 kinase, the limited growth of the mutants in skin cells may reflect lack of ORF47-mediated posttranslational modification of viral protein targets in addition to gE. We have demonstrated that the cell-cell spread of VZV is tightly controlled by innate responses of epidermal cells, especially by alpha interferon (IFN-α), which is expressed constitutively in vivo but is not a factor in melanoma cells in vitro (20). Kinase-defective ORF47 mutants may not down-regulate IFN-α. Intact VZV down-regulated IFN-α in vivo, whereas kinase-defective ORF47 mutants may be less effective in blocking the antiviral mechanisms of epidermal cells. Alternatively, cellular kinases may substitute for ORF47 protein in cultured cells but not in epidermal cells in vivo (3).
In summary, the phosphorylating functions of ORF47 protein determined the intracellular localization of critical VZV protein substrates, including gE, ORF47 protein, and IE62 protein. Eliminating ORF47 kinase activity impaired virion production and transfer to cell surfaces, reduced VZV virulence in skin, and blocked VZV infectivity for T cells. Experiments with kinase-defective ORF47 mutants provided evidence that VZV-induced cell fusion is critical for skin infection and allows some replication even if virion formation is compromised substantially. In contrast, kinase-defective ORF47 mutants did not replicate in T-cell xenografts in vivo, which supports the concept that the T-cell tropism of VZV depends upon assembly and release of complete virions.
Acknowledgments
This work was supported by grants from the National Institute of Allergy and Infectious Diseases (AI20459 and AI053846 to A.M.A.) and a Developmental and Neonatal Biology Training Grant (T32 HD07249 to J.B and AI22795 to C.G.).
We thank Hideki Ito and Chia-Chi Ku for technical assistance.
REFERENCES
- 1.Arvin, A. M. 2001. Varicella-zoster virus, p. 2731-2767. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 2.Baiker, A., C. Bagowski, H. Ito, M. Sommer, L. Zerboni, K. Fabel, J. Hay, W. Ruyechan, and A. M. Arvin. 2004. The immediate-early 63 protein of varicella-zoster virus: analysis of functional domains required for replication in vitro and for T-cell and skin tropism in the SCIDhu model in vivo. J. Virol. 78:1181-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besser, J., M. H. Sommer, L. Zerboni, C. P. Bagowski, H. Ito, J. Moffat, C. C. Ku, and A. M. Arvin. 2003. Differentiation of varicella-zoster virus ORF47 protein kinase and IE62 protein binding domains and their contributions to replication in human skin xenografts in the SCID-hu mouse. J. Virol. 77:5964-5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen, J. I., and K. E. Seidel. 1993. Generation of varicella-zoster virus (VZV) and viral mutants from cosmid DNAs: VZV thymidylate synthetase is not essential for replication in vitro. Proc. Natl. Acad. Sci. USA 90:7376-7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole, N. L., and C. Grose. 2003. Membrane fusion mediated by herpesvirus glycoproteins: the paradigm of varicella-zoster virus. Rev. Med. Virol. 13:207-222. [DOI] [PubMed] [Google Scholar]
- 6.Crump, C. M., Y. Xiang, L. Thomas, F. Gu, C. Austin, S. A. Tooze, and G. Thomas. 2001. PACS-1 binding to adaptors is required for acidic cluster motif-mediated protein traffic. EMBO J. 20:2191-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gershon, A. A., D. L. Sherman, Z. Zhu, C. A. Gabel, R. T. Ambron, and M. D. Gershon. 1994. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J. Virol. 68:6372-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grose, C. 1981. Variation on a theme by Fenner: the pathogenesis of chickenpox. Pediatrics 68:735-737. [PubMed] [Google Scholar]
- 9.Grose, C., W. Jackson, and J. A. Traugh. 1989. Phosphorylation of varicella-zoster virus glycoprotein gpI by mammalian casein kinase II and casein kinase I. J. Virol. 63:3912-3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanks, S. K., and T. Hunter. 1995. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 9:576-596. [PubMed] [Google Scholar]
- 11.Hanks, S. K., A. M. Quinn, and T. Hunter. 1988. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241:42-52. [DOI] [PubMed] [Google Scholar]
- 12.Heineman, T. C., and J. I. Cohen. 1995. The varicella-zoster virus (VZV) open reading frame 47 (ORF47) protein kinase is dispensable for viral replication and is not required for phosphorylation of ORF63 protein, the VZV homolog of herpes simplex virus ICP22. J. Virol. 69:7367-7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito, H., M. H. Sommer, L. Zerboni, H. He, D. Boucaud, J. Hay, W. Ruyechan, and A. M. Arvin. 2003. Promoter sequences of varicella-zoster virus glycoprotein I targeted by cellular transcription factors Sp1 and USF determine virulence for human skin and T cells in SCIDhu mice in vivo. J. Virol. 77:489-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones, F., and C. Grose. 1988. Role of cytoplasmic vacuoles in varicella-zoster virus glycoprotein trafficking and virion envelopment. J. Virol. 62:2701-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kemble, G. W., P. Annunziato, O. Lungu, R. E. Winter, T.-A. Cha, S. J. Silverstein, and R. R. Spaete. 2000. Open reading frame S/L of varicella-zoster virus encodes a cytoplasmic protein expressed in infected cells. J. Virol. 74:11311-11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenyon, T. K., J. I. Cohen, and C. Grose. 2002. Phosphorylation by the varicella-zoster virus ORF47 protein serine kinase determines whether endocytosed viral gE traffics to the trans-Golgi network or recycles to the cell membrane. J. Virol. 76:10980-10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenyon, T. K., J. Lynch, J. Hay, W. Ruyechan, and C. Grose. 2001. Varicella-zoster virus ORF47 protein serine kinase: characterization of a cloned, biologically active phosphotransferase and two viral substrates, ORF62 and ORF63. J. Virol. 75:8854-8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinchington, P. R., J. K. Hougland, A. M. Arvin, W. T. Ruyechan, and J. Hay. 1992. The varicella-zoster virus immediate-early protein IE62 is a major component of virus particles. J. Virol. 66:359-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ku, C. C., J. A. Padilla, C. Grose, E. C. Butcher, and A. M. Arvin. 2002. Tropism of varicella-zoster virus for human tonsillar CD4+ T lymphocytes that express activation, memory, and skin homing markers. J. Virol. 76:11425-11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ku, C. C., L. Zerboni, H. Ito, M. Wallace, and A. M. Arvin. Submitted for publication.
- 21.Lungu, O., C. A. Panagiotidis, P. W. Annunziato, A. A. Gershon, and S. J. Silverstein. 1998. Aberrant intracellular localization of varicella-zoster virus regulatory proteins during latency. Proc. Natl. Acad. Sci. USA 95:7080-7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mallory, S., M. Sommer, and A. M. Arvin. 1997. Mutational analysis of the role of glycoprotein I in varicella-zoster virus replication and its effects on glycoprotein conformation and trafficking. J. Virol. 71:8279-8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mo, C., J. Lee, M. Sommer, C. Grose, and A. M. Arvin. 2002. The requirement of varicella-zoster virus glycoprotein E for viral replication and the effects of glycoprotein I on gE in melanoma cells. Virology 304:176-186. [DOI] [PubMed] [Google Scholar]
- 24.Moffat, J., C. Mo, J. Cheng, M. Sommer, L. Zerboni, S. Stamatis, and A. M. Arvin. 2004. Functions of the C-terminal domain of varicella-zoster virus glycoprotein E in viral replication in vitro and skin and T-cell tropism in vivo. J. Virol. 78:12406-12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moffat, J., H. Ito, M. Sommer, S. Taylor, and A. M. Arvin. 2002. Glycoprotein I of varicella-zoster virus is required for viral replication in skin and T cells. J. Virol. 76:8468-8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moffat, J. F., M. D. Stein, H. Kaneshima, and A. M. Arvin. 1995. Tropism of varicella-zoster virus for human CD4+ and CD8+ T lymphocytes and epidermal cells in SCID-hu mice. J. Virol. 69:5236-5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moffat, J. F., L. Zerboni, P. R. Kinchington, C. Grose, H. Kaneshima, and A. M. Arvin. 1998. Attenuation of the vaccine Oka strain of varicella-zoster virus and role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mouse. J. Virol. 72:965-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moffat, J. F., L. Zerboni, M. H. Sommer, T. C. Heineman, J. I. Cohen, H. Kaneshima, and A. M. Arvin. 1998. The ORF47 and ORF66 putative protein kinases of varicella-zoster virus determine tropism for human T cells and skin in the SCID-hu mouse. Proc. Natl. Acad. Sci. USA 95:11969-11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montalvo, E. A., and C. Grose. 1986. Varicella-zoster virus glycoprotein gpI is selectively phosphorylated by a virus-induced protein kinase. Proc. Natl. Acad. Sci. USA 83:8967-8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng, T. I., L. Keenan, P. R. Kinchington, and C. Grose. 1994. Phosphorylation of varicella-zoster virus open reading frame (ORF) 62 regulatory product by viral ORF 47-associated protein kinase. J. Virol. 68:1350-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niizuma, T., L. Zerboni, M. H. Sommer, H. Ito, S. Hinchliffe, and A. M. Arvin. 2003. Construction of varicella-zoster virus recombinants from parent Oka cosmids and demonstration that ORF65 protein is dispensable for infection of human skin and T cells in the SCID-hu mouse model. J. Virol. 77:6062-6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy, S. M., E. Cox, I. Iofin, W. Soong, and J. I. Cohen. 1998. Varicella-zoster virus (VZV) ORF32 encodes a phosphoprotein that is posttranslationally modified by the VZV ORF47 protein kinase. J. Virol. 72:8083-8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 34.Santos, R., C. C. Hatfield, B. P. Faga, J. A. Padilla, N. L. Cole, J. F. Moffat, A. M. Arvin, W. T. Ruyechan, J. Hay, and C. Grose. 2000. Varicella-zoster virus gE escape mutant VZV-MSP exhibits an accelerated cell-to-cell spread phenotype in both infected cell cultures and SCID-hu mice. Virology 275:306-317. [DOI] [PubMed] [Google Scholar]
- 35.Sato, B., H. Ito, S. Hinchliffe, M. H. Sommer, L. Zerboni, and A. M. Arvin. 2003. Mutational analysis of open reading frames 62 and 71, encoding the varicella-zoster virus immediate-early transactivating protein, IE62, and effects on replication in vitro and in skin xenografts in the SCID-hu mouse. J. Virol. 77:5607-5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith, R. F., and T. F. Smith. 1989. Identification of new protein kinase-related genes in three herpesviruses, herpes simplex virus, varicella-zoster virus, and Epstein-Barr virus. J. Virol. 63:450-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sommer, M. H., E. Zagha, O. K. Serrano, C. C. Ku, L. Zerboni, A. Baiker, R. Santos, M. Spengler, J. Lynch, C. Grose, W. Ruyechan, J. Hay, and A. M. Arvin. 2001. Mutational analysis of the repeated open reading frames, ORFs 63 and 70 and ORFs 64 and 69, of varicella-zoster virus. J. Virol. 75:8224-8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soong, W., J. C. Schultz, A. C. Patera, M. H. Sommer, and J. I. Cohen. 2000. Infection of human T lymphocytes with varicella-zoster virus: an analysis with viral mutants and clinical isolates. J. Virol. 74:1864-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wan, L., S. S. Molloy, L. Thomas, G. Liu, Y. Xiang, S. L. Rybak, and G. Thomas. 1998. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell 94:205-216. [DOI] [PubMed] [Google Scholar]
- 40.Wang, Z.-H., M. D. Gershon, O. Lungu, Z. Zhu, S. Mallory, A. M. Arvin, and A. A. Gershon. 2001. Essential role played by the C-terminal domain of glycoprotein I in envelopment of varicella-zoster virus in the trans-Golgi network: interactions of glycoproteins with tegument. J. Virol. 75:323-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao, Z., W. Jackson, and C. Grose. 1993. Identification of the phosphorylation sequence in the cytoplasmic tail of the varicella-zoster virus Fc receptor glycoprotein gpI. J. Virol. 67:4464-4473. [DOI] [PMC free article] [PubMed] [Google Scholar]