Abstract
Breastfeeding plays a substantial role in mother-to-child transmission of human immunodeficiency virus type 1 (HIV-1). Mammary epithelial cells, as well as macrophages and lymphocytes, are thought to serve as sources of the virus in breast milk. Soluble factors in breast milk exert various biological functions, including immune tolerance or immune modulation, and may influence milk-borne infection with HIV-1. In this study we show that transforming growth factor β (TGF-β), a major cytokine in breast milk, inhibited HIV-1 infection of mammary epithelial MCF-7 cells but enhanced that of macrophages. TGF-β downregulated the HIV-1 long terminal repeat (LTR) promoter in MCF-7 cells but upregulated it in macrophages. Stimulation with TGF-β suppressed NF-κB binding to the HIV-1 LTR in MCF-7 cells, at least in part by downregulating induced IκB kinase expression. Cell type-dependent effects of TGF-β on HIV-1 expression may play a role in milk-borne infection with HIV-1.
Mother-to-child transmission accounts for the majority of human immunodeficiency virus type 1 (HIV-1) infections of children. In the developing countries where exclusive bottle-feeding is not feasible, up to 14 to 24% of babies born from HIV-1-infected mothers will be infected through breastfeeding (6, 7, 25). Although 75% of all milk-borne transmission appears to occur by 6 months of age, transmission continues throughout the lactation period (6, 7, 25).
HIV-1 strains are classified according to their coreceptor usage, and R5-tropic virus that utilizes CCR5 as an entry coreceptor is almost always a transmitting virus (31, 33). Breast milk contains both HIV-1-infected cells and cell-free virions, both of which appear to be contagious. The cell composition of breast milk changes throughout lactation period. Colostrum and early (<2 weeks after delivery) milk are rich in cells, such as macrophages (50 to 75%), neutrophils (20 to 40%) and lymphocytes (3 to 10%) (8), and macrophages appear to be the principal cellular carriers of HIV-1 in colostrum and early milk (28). Macrophages are susceptible to R5-tropic HIV-1 but much less to X4-tropic virus (1, 3) which utilizes CXCR4 as an entry coreceptor. Although the major source of HIV-1 in mature (>2 weeks after delivery) milk remains unknown, mammary epithelial cells, the predominant cell type in mature milk (8), have been shown to be susceptible to certain (mostly X4-tropic) HIV-1 strains (18, 28, 30).
Breast milk also contains a number of soluble factors that exert various functions, including antimicrobial activities and induction of immune tolerance or immune modulation. Certain milk whey components such as lactoferrin (24) and prostaglandin E (5, 29) have been shown to inhibit HIV-1 replication. Transforming growth factor-β (TGF-β) is a major cytokine in breast milk. Among the three isoforms of TGF-β, TGF-β1, expressed in endothelial, hematopoietic, and connective tissue cells, and TGF-β2, expressed in epithelial and neuronal cells, are contained in human milk. Colostrum contains 140 pg (67 to 186 pg) of TGF-β1 and 3,325 pg (1,376 to 5,394 pg) of TGF-β2 per ml, while mature milk contains 83 pg (17 to 114 pg) of TGF-β1 and 1,644 pg (592 to 2,697 pg) of TGF-β2 per ml (9).
TGF-β acts as an anti-HIV-1 or pro-HIV-1 factor, depending on cell type, virus strain, timing of treatment, or combination of other factors (13, 15, 17, 27); however, no substantial differences in effects on HIV infection among the TGF-β isoforms could be found (13). Here we studied whether TGF-β influences HIV-1 expression in major breast milk cells such as mammary epithelial cells and macrophages. Our data implicate bifunctional activity of TGF-β in mother-to-child transmission of HIV-1.
MATERIALS AND METHODS
Cells.
Human mammary epithelial MCF-7 cells were obtained from the American Type Culture Collection and propagated in RPMI 1640 medium supplemented with 10% fetal calf serum (Gibco-BRL, Gaithersburg, Md.), 100 U of penicillin per ml, and 100 μg streptomycin per ml. Monocyte-derived macrophages were propagated from healthy donors' peripheral blood mononuclear cells by sorting CD14-positive cell fraction with AutoMACS (Miltenyi Biotec, Auburn, Calif.) according to the manufacturer's instructions and propagated in Dulbecco's minimal essential medium supplemented with 10% human male AB serum (Sigma Chemical Co., St. Louis, Mo.), penicillin and streptomycin. Purity of CD14-positive cells was more than 96% by flow cytometric analysis (data not shown). CD14-negative peripheral blood mononuclear cells, designated peripheral blood lymphocytes, contained less than 0.3% CD14-positive cells (data not shown).
Viruses and single- or multiple-round viral replication assays.
For single-round viral replication assays, replication-incompetent luciferase reporter molecular clone pNL43-luc-R−E− was complemented with Env glycoprotein from ADA (R5 HIV-1), JR-FL (R5 HIV-1), 89.6 (R5X4 HIV-1), ELI1 (primary X4 HIV-1), HXB2 (X4 HIV-1) or amphotropic murine leukemia virus by transfecting 293T cells with plasmids encoding them, as described previously (23). Single-round viral replication assays were performed as described previously (23).
Multiple-round viral replication assays were performed with HIV-1 89.6 that was prepared by 293T cell transfection with the respective molecular clones, as described previously (21). Viral replication was assessed by reverse transcriptase assays for cell-free supernatants, as described previously (21).
Plasmids and transfection.
Plasmid pGL-HIV-1-LTR contains the HIV-1 long terminal repeat (LTR) from strain HXB2 in pGL2-basic (Promega, Madison, Wis.) backbone. LTR sequence was truncated at −116 relative to transcription start site in pGL-HIV-1-LTR(−116), while NF-κB and SP1 binding sites on HIV-1 LTR were deleted in plasmids pGL-HIV-1-LTRΔNF-κB and pGL-HIV-1-LTRΔSP1, respectively (16). Plasmids pCMV-HA/Smad2 and pCMV-Flag/Smad3 were kindly provided by J. L. Wrana (Mount Sinai Hospital, Toronto, Canada) (11), and pCMV-hFAST1 was a gift of B. Vogelstein (Johns Hopkins Oncology Center, Baltimore, Md.) (32). Plasmid pHTLV-luc contains the human T-cell leukemia virus type I (HTLV-I) LTR promoter followed by the luciferase gene (10). One-day-old MCF-7 cell monolayer or 5-day-old monocyte-derived macrophages monolayer were transfected with modified calcium phosphate method, and luciferase assays were performed, as described previously (20, 22).
Reagents.
Human lactoferrin were purchased from Sigma Chemical Co., and recombinant human TGF-β2 and polyclonal neutralizing anti-TGF-β antibody were purchased from R&D Systems (Minneapolis, Minn.). Early (<2weeks after delivery) and mature (>4 weeks after delivery) breast milk samples were donated from three healthy breastfeeding mothers. After centrifugation of the samples at 14,000 × g for 15 min, milk whey was separated from solid precipitate and stored at −80°C before use.
Nuclear extracts and gel mobility shift assays.
Nuclear extracts were prepared from MCF-7 cells or monocyte-derived macrophages that had been untreated or treated with TGF-β (200 pg/ml), as described previously (19). Gel mobility shift assays were performed as described previously (19), with oligonucleotides corresponding to sequences spanning HIV-1 LTR NF-κB binding sites or SP1 binding sites as primers. Antibodies to NF-κB p50 or p65 were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.).
Real-time RT-PCR.
MCF-7 cells untreated or treated with TGF-β or milk whey were harvested, total RNA was extracted with a QIAamp RNA kit (Qiagen, Tokyo, Japan) and measurement of induced IκB kinase mRNA levels was performed by real-time RT-PCR with the iCycler iQ real-time detection system (Bio-Rad Laboratories, Inc., Hercules, Calif.) and QuantiTect SYBR Green RT-PCR kit (Qiagen). Primers were 5′-CCCAGCCCTACACGAAAGGACCTGCTTCTC-3′ and 5′-TCAGACATCAGGAGGTGCTGGGACTCTAT-3′. Reactions were incubated at 50°C for 30 min and then 95°C for 15 min, followed by 50 cycles of 95°C for 15 s and 62°C for 60 s. IκB kinase mRNA levels were normalized to tubulin mRNA by calculating the IκB kinase and tubulin ratio for each sample.
RESULTS
MCF-7 cells are susceptible to HIV-1 infection.
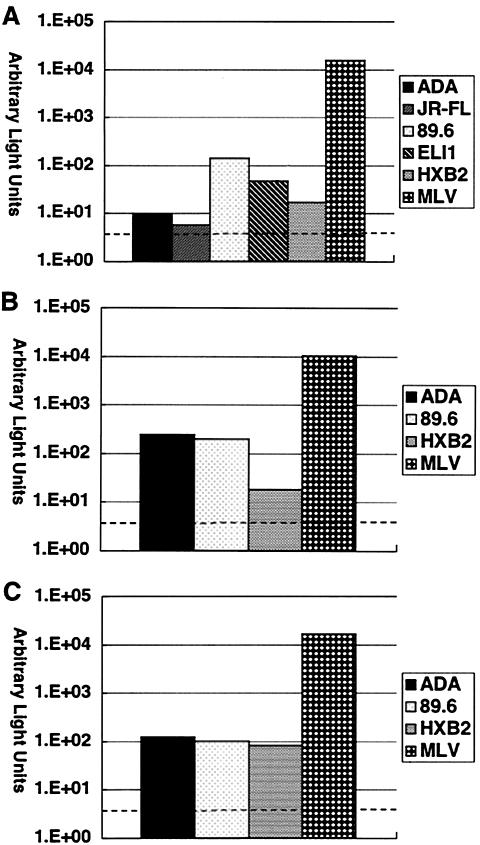
It has been shown previously that primary mammary epithelial cells as well as certain breast cancer cell lines are susceptible to HIV-1. Therefore, we first tested whether MCF-7 cells are capable of supporting HIV-1 infection. To avoid any soluble factor derived from lymphocytes or macrophages and to precisely delineate susceptibility to the HIV-1 phenotypes, we performed single-round viral replication assays with HIV-1 molecular clone stocks prepared from 293T cell culture supernatants. As shown in Fig. 1A, MCF-7 cells supported infection with certain HIV-1 strains, although infection efficiency was far less than that of amphotropic murine leukemia virus. Compared to monocyte-derived macrophages that were more susceptible to R5 HIV-1 than to X4 HIV-1 (Fig. 1B) or peripheral blood lymphocytes that were susceptible comparably to R5 and X4 HIV-1 (Fig. 1C), MCF-7 cells apparently were more susceptible to dual (R5X4) or X4 HIV-1 than to R5 HIV-1. Since HIV-1 89.6 replicated comparably in all cell types tested, the following experiments were performed exclusively with this virus.
FIG. 1.
Susceptibility of breast milk cell analogues to HIV-1 infection. In single-round viral replication assays, MCF-7 cells (A), monocyte-derived macrophages (B), and peripheral blood lymphocytes (C) were infected with NL4-3-Luc-R−E− (3 × 105 cpm of reverse transcriptase activity each) supplemented with Env glycoprotein derived from the indicated strains, and the infected cell lysates were subjected to luciferase assays. A broken line indicates background luciferase activity. Representative results from three independent experiments are shown.
TGF-β inhibits HIV-1 infection of MCF-7 cells but enhances that of monocyte-derived macrophages.
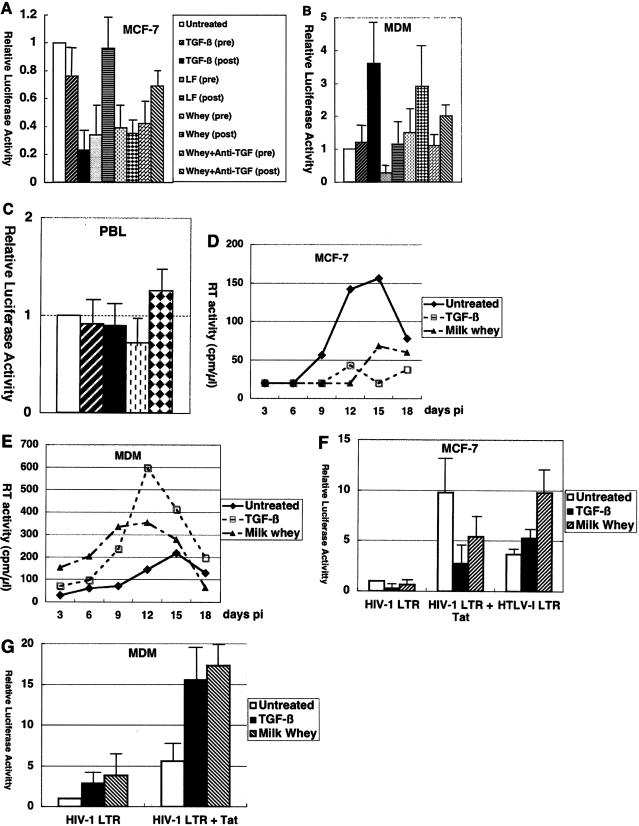
Next, we investigated whether exposure to milk whey or its components can influence the susceptibility of mammary epithelial MCF-7 cells to HIV-1. While treatment of cells with crude milk whey or lactoferrin, a known anti-HIV-1 factor, suppressed HIV-1 infection, TGF-β had a minimal effect when added to MCF-7 cells prior to infection. In contrast, posttreatment with TGF-β as well as crude milk whey suppressed HIV-1 infection (Fig. 2A), suggesting that TGF-β has inhibitory effects on a postentry step(s) of the HIV-1 replicative cycle in MCF-7 cells. The inhibitory effect of crude milk whey posttreatment was partially restored by neutralizing anti-TGF-β antibody, suggesting that TGF-β played a role in that effect.
FIG. 2.
Cell type-dependent effects of TGF-β on HIV-1 infection. (A, B, and C) In single-round viral replication assays, TGF-β inhibited HIV-1 infection of MCF-7 cells, enhanced HIV-1 infection of monocyte-derived macrophages at a postentry step(s), and had a minimal effect on HIV-1 infection of peripheral blood lymphocytes. MCF-7 cells (A), monocyte-derived macrophages (B), and peripheral blood lymphocytes (C) were either untreated or treated for 16 h before infection (pre) or treated after infection (post) with NL4-3-Luc-R−E− supplemented with Envglycoprotein from 89.6 with TGF-β (200 pg/ml), human lactoferrin (LF) (100 ng/ml), or crude milk whey (10%) with and without anti-TGF-β antibody (10 μg/ml). Cells were harvested 48 h after infection, and cell lysates were subjected to luciferase assays. Arbitrary light units are shown. Error bars represent standard deviations from duplicate experiments. (D, E) In multiple-round viral replication assays, TGF-β inhibited HIV-1 infection of MCF-7 cells and enhanced HIV-1 infection of monocyte-derived macrophages. Approximately 2 × 105 MCF-7 cells (D) or 4 × 105 monocyte-derived macrophages (E) were untreated or treated with TGF-β (200 pg/ml) or crude milk whey (10%) for 16 h before and continuously after infection with HIV-1 89.6. The experiments were repeated twice with similar results. Another experiment in which monocyte-derived macrophages were also infected with ELI1 also gave similar results (data not shown). (F, G) Cell type-dependent effects of TGF-β on HIV-1 LTR activity. MCF-7 cells (F) and monocyte-derived macrophages (G) were transfected with pGL-HIV-1-LTR and pSV2-CAT, pGL-HIV-1-LTR and pSV2-Tat, or pHTLV-I-luc and either untreated or treated with TGF-β (200 pg/ml) or crude milk whey (10%). Results are shown as luciferase activity relative to that of pGL-HIV-1-LTR and pSV2-CAT when untreated. Error bars represent standard deviations from triplicate experiments.
We also investigated the effects of milk whey or TGF-β on HIV-1 infection of macrophages, another major source of HIV-1 in breast milk. In striking contrast to HIV-1 infection of MCF-7 cells, posttreatment of monocyte-derived macrophages with crude milk whey or TGF-β enhanced HIV-1 infection (Fig. 2B). The effect mediated by posttreatment with crude milk whey was reduced to some extent by neutralizing anti-TGF-β antibody, suggesting that TGF-β contributed to that effect. Pretreatment of monocyte-derived macrophages with either reagent had a minimal effect.
TGF-β as well as crude milk whey had a minimal effect on HIV-1 infection of peripheral blood lymphocytes (Fig. 2C) at the concentration used (200 pg of TGF-β per ml and 10% crude milk whey).
Since crude milk whey or TGF-β appears to have dichotomous effects on HIV-1 infection, depending on cell types and timing of treatment, we tested their effects on multiple rounds of HIV-1 replication. Cells were treated with the reagents for 16 h before infection and throughout the experiments. As shown in Fig. 2D and 2E, TGF-β suppressed HIV-1 replication in MCF-7 cells but enhanced that in monocyte-derived macrophages.
Crude milk whey samples derived from three different donors had similar effects, and results obtained with early and mature milk were comparable (data not shown). Our early and mature milk whey samples contained 1.5 to 2.5 ng and 1.0 to 2.0 ng of TGF-β2 per ml, respectively (data not shown). Since 10% milk whey was used in our experiments, the final concentration of TGF-β would be approximately 200 pg/ml. Therefore, although breast milk must be diluted in baby's gastrointestinal tract, we consider that concentrations of milk whey (10%) and TGF-β (200 pg/ml) were physiologically relevant.
Thus, the net effects of crude milk whey and TGF-β on HIV-1 infection may be negative in MCF-7 cells and positive in monocyte-derived macrophages.
TGF-β downregulates HIV-1 LTR activity in MCF-7 cells but upregulates it in monocyte-derived macrophages.
Previous studies have demonstrated that TGF-β upregulates HIV-1 LTR activity in mesangial cells and murine B cell lines. However, since TGF-β plays pleiotropic roles, depending on the cell types involved and combinations with other factors, we tested its effect on HIV-1 LTR activity in MCF-7 cells. As shown in Fig. 2F, HIV-1 LTR activity, either basal and Tat induced, was downregulated by TGF-β in MCF-7 cells. That effect was specific, because TGF-β did not have inhibitory effect on an irrelevant promoter like the HTLV-I LTR at this concentration.
Macrophages are another major cellular components in breast milk and probably serve as an important source of HIV-1 in this body fluid. In similar experiments, TGF-β upregulated basal and Tat-induced HIV-1 LTR activity in monocyte-derived macrophages (Fig. 2G).
Overexpression of FAST-1 and Smad2 but not Smad3 augmented TGF-β-mediated effects.
TGF-β conveys its signal from membrane receptors into the nucleus by specific mediators called SMADs (reviewed in 11). SMADs activation can lead to transcriptional activation through direct binding to specific DNA sequences and association with another transcription factor such as FAST-1 (32). To further investigate mechanisms whereby TGF-β exerts its effects on HIV-1 LTR, those mediators were overexpressed in transient expression assays.
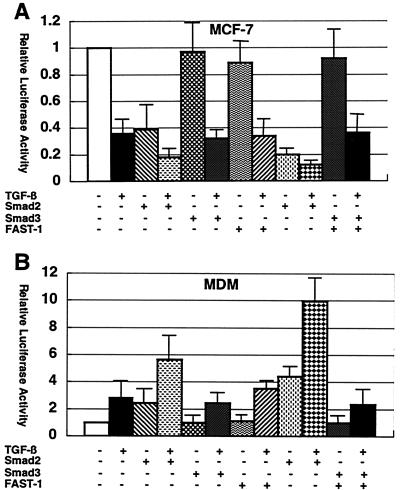
Cotransfection of Smad2, FAST-1, or both with pGL-HIV-1-LTR augmented TGF-β-mediated effects, downregulation in MCF-7 cells, and upregulation in monocyte-derived macrophages (Fig. 3A and 3B). However, Smad3 alone and in combination with FAST-1 barely influenced HIV-1 LTR activity. Thus, Smad2-FAST-1 association apparently plays a role in TGF-β-mediated effects on HIV-1 LTR. It has been shown that hFAST-1 is expressed in all normal human tissues including breast tissue and mediates responses to TGF-β by interacting with Smad2 and Smad4 (32).
FIG. 3.
Augmentation of TGF-β-mediated effects by FAST-1 and Smad2. MCF-7 cells (A) and monocyte-derived macrophages (B) were transfected with pGL-HIV-1-LTR along with pcDNA3.1 or expression vector of the indicated factor and either untreated or treated with TGF-β (200 pg/ml). Results are shown as luciferase activity relative to that of pGL-HIV-1-LTR and pcDNA3.1 when untreated. Error bars represent standard deviations from triplicate experiments.
Next, we investigated whether Smad2-FAST-1 complex can bind to HIV-1 LTR; however, extensive search with gel mobility shift assay using a panel of oligonucleotides spanning HIV-1 LTR sequence failed to identify a Smad2-FAST-1 binding site(s) (data not shown). Therefore, TGF-β/Smad2/FAST-1 may mediate its effects on HIV-1 LTR indirectly, possibly through transcriptional regulation of other gene(s). Alternatively, TGF-β may mediate SMAD-independent signaling pathway (reviewed in 4).
NF-κB binding sites are critical for TGF-β-mediated effect on HIV-1 LTR.
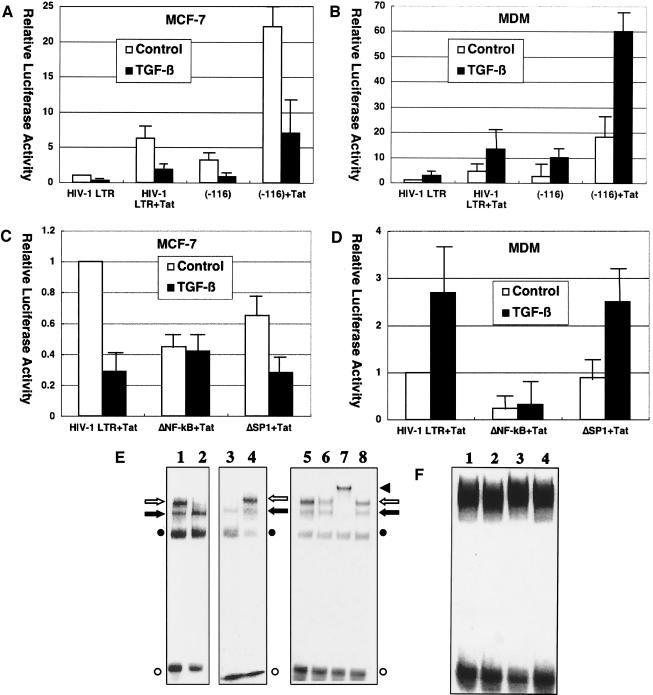
As mentioned above, TGF-β-mediated effects on HIV-1 LTR may be independent of direct binding of SMADs/FAST-1 to HIV-1 LTR and TGF-β signaling may exert its effects on HIV-1 LTR through other cis-acting element(s). To demonstrate which cis-acting element(s) are involved in TGF-β-mediated effects, a panel of HIV-1 LTR-reporter constructs were tested in transient expression assays. Since a 5′-truncation to −116 relative to transcription start site had little effect on TGF-β-mediated activity (Fig. 4A and 4B), we focused on NF-κB binding sites and SP1 binding sites, major elements located downstream of −116. Deletion of SP1 binding sites had little effect, while deletion of NF-κB binding sites almost abrogated TGF-β-mediated activity in both MCF-7 cells and monocyte-derived macrophages (Fig. 4C and 4D).
FIG. 4.
TGF-β effects on HIV-1 infection are mediated by NF-κB activity. (A to D) Critical role of NF-κB binding sites in TGF-β-mediated effects on HIV-1 LTR. MCF-7 cells (A) or monocyte-derived macrophages (B) were transfected with pGL-HIV-1-LTR or pGL-HIV-1-LTR(−116) along with pSV2-Tat and either untreated or treated with TGF-β (200 pg/ml). Also, MCF-7 cells (C) or monocyte-derived macrophages (D) were transfected with pGL-HIV-1-LTR, pGL-HIV-1-LTRΔNF-κB, or pGL-HIV-1-LTRΔSP1 along with pSV2-Tat and either untreated or treated with TGF-β (200 pg/ml). Error bars represent standard deviations from triplicate experiments. (E, F) TGF-β effects on NF-κB binding to HIV-1 LTR. (E) 32P-labeled oligonucleotides corresponding to sequences spanning the HIV-1 LTR NF-κB binding sites were incubated with nuclear extracts (15 μg of protein) from MCF-7 cells (lanes 1, 2, and 5 to 8) or monocyte-derived macrophages (lanes 3 and 4) and analyzed by gel mobility shift assays. Those cells were either untreated (lanes 1 and 3) or treated (lanes 2 and 4 to 8) with TGF-β (200 pg/ml) for 10 min before harvest. In addition, anti-p65 antibody (lane 6), anti-p50 antibody (lane 7), or control serum (lane 8) was added to the reaction. An open arrow and a solid arrow indicate the p50/p65 heterodimer (NF-κB) and p50 homodimer, respectively. An open circle and a solid circle indicate free probe and nonspecific complex, respectively. A solid triangle indicates a complex supershifted by anti-p50 antibody. (F) 32P-labeled oligonucleotides corresponding to sequences spanning the HIV-1 LTR SP1 binding sites were incubated with nuclear extracts from MCF-7 cells (lanes 1 and 2) or monocyte-derived macrophages (lanes 3 and 4) and analyzed by gel mobility shift assays. Those cells were either untreated (lanes 1 and 3) or treated with TGF-β (200 pg/ml) for 10 min before harvest (lanes 2 and 4).
To confirm the aforementioned results obtained from transient expression assays, we performed gel mobility shift assays using nuclear extracts from MCF-7 cells that had been untreated and treated with TGF-β. Untreated MCF-7 cells had weak but detectable NF-κB activity. TGF-β treatment of MCF-7 cells abrogated NF-κB activity on HIV-1 LTR, but had little effect on SP1 activity (Fig. 4E and 4F). On the contrary, NF-κB activity in nuclear extracts from untreated monocyte-derived macrophages was enhanced in those from TGF-β-treated monocyte-derived macrophages (Fig. 4E and 4F). It is noteworthy that TGF-β stimulation of HIV-1 LTR through NF-κB activity has been shown in HaCat cells (human keratinocytes) and 300.19 (mouse pre-B) cells (14). These results indicate that TGF-β mediates both inhibitory and stimulatory effects on HIV-1 LTR, at least in part, through the NF-κB pathway.
TGF-β downregulates expression of IκB kinase.
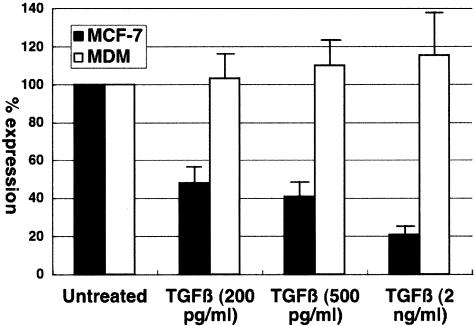
In a recent study, TGF-β has been shown to downregulate expression of IκB kinase in mouse mammary epithelial cells. IκB kinase is induced upon various stimuli, degrades IκB, and induces NF-κB activity. To demonstrate whether TGF-β is capable of downregulating IκB kinase expression in MCF-7 cells, real-time RT-PCR was performed. As shown in Fig. 5, TGF-β treatment reduced IκB kinase mRNA levels in MCF-7 cells in a dose-dependent manner but had minimal effect in monocyte-derived macrophages. Thus, IκB kinase appears to be one of the targets of TGF-β action on mammary epithelial cells.
FIG. 5.
Regulation of IκB kinase gene expression by TGF-β. MCF-7 cells (solid bars) or monocyte-derived macrophages (open bars) were either untreated or treated with the indicated amount of TGF-β for 60 min, and IκB kinase mRNA levels were determined by real-time RT-PCR. In untreated MCF-7 cells and monocyte-derived macrophages, IκB kinase mRNA levels were approximately 0.1% and 0.3%, respectively, of that of the housekeeping gene tubulin mRNA. The change in expression is shown as expression relative to that in untreated cells. Error bars represent standard deviations from triplicate experiments.
DISCUSSION
Although milk-borne infection accounts for a considerable number of mother-to-child transmissions of HIV-1, the precise molecular and cellular mechanisms by which maternal cell-derived virus is transmitted through the gastrointestinal mucosa to infants remain obscure. In addition to macrophages and lymphocytes, mammary epithelial cells are thought to be the sources of HIV-1 in breast milk. Interestingly, the susceptibility of those cells to HIV-1 strains differs: macrophages support replication of R5-tropic HIV-1 much better than that of X4-tropic HIV-1, lymphocytes support replication of R5- and X4-tropic HIV-1 equally well, and mammary epithelial cells support replication of X4-tropic rather than R5-tropic HIV-1 (18, 28, 30). It is, however, unclear which cell type plays a major role in mother-to-child transmission.
A recent study has shown a differential distribution of HIV-1 variants between breast milk and peripheral blood: the major variant in one compartment corresponded to a minor variant in the other compartment (2). Such observations suggest that a host factor(s) in breast milk can modulate HIV-1 infection. Interestingly, while peripheral blood often harbored both R5- and X4-tropic viruses, HIV-1 variants in breast milk were always R5 tropic (2). Therefore, a breast milk-derived host factor(s) may favor replication of R5-tropic HIV-1 but not X4-tropic HIV-1.
In this study, we identified TGF-β, a major cytokine in breast milk, as a bifunctional modulator of HIV-1 infection. TGF-β favored HIV-1 infection of macrophages but suppressed that of mammary epithelial MCF-7 cells. The pro-HIV-1 effect of TGF-β on macrophages was already reported (13), but it has not been demonstrated how TGF-β influences HIV-1 infection of mammary epithelial cells. TGF-β appears to have dichotomous effects on HIV-1 infection of lymphocytes: it enhanced HIV-1 infection at 10 ng/ml or lower, but suppressed it at 1 ng/ml or higher (26). Peripheral blood lymphocytes were relatively refractory to TGF-β at the concentration (200 pg/ml) used in this study. Since the TGF-β concentration in breast milk is as high as 1 to 2 ng/ml, HIV-1 infection of lymphocytes may be inhibited in this setting.
Thus, the milk-borne cytokine TGF-β may be most beneficial to HIV-1 infection of macrophages, and therefore R5-tropic HIV-1 predominance in breast milk and preferential mother-to-child transmission of R5-tropic HIV-1. However, breast milk contains a number of host factors that variably modulate HIV-1 infection. Lactoferrin inhibits replication of both R5- and X4-tropic HIV-1 in various cell types (24). Prostaglandin E2 inhibits or enhances HIV-1 infection, depending on the virus strains, cell types infected, and the timing of treatment (5, 29). Interestingly, an identified factor in breast milk that is sensitive to cathepsin D has been shown to enhance X4 HIV-1 infection of MCF-7 cells (18). Thus, it appears that the net effect of breast milk on HIV-1 infection depends on the balance of those pro- and anti-HIV-1 factors. Further extensive studies are required for a better understanding of the interaction between HIV-1 and milk-derived host factors.
Acknowledgments
We thank N. Landau, J. Sodroski, M. Martin, K. Peden, J. L. Wrana, B. Vogelstein, and K. T. Jeang for reagents, S. Chiyoda, H. Okuda, and K. Deguchi (Nagasaki Red Cross Blood Center) for blood samples, and M. Yokoyama for excellent technical assistance and graphic work.
This work was supported in part by a grant provided by a Research for the Future Program (JSPS-RFTF97L00705) of the Japan Society for the Promotion of Science and by a Grant-in-Aid from the Ministry of Education, Science, Sports and Culture, Japan.
REFERENCES
- 1.Asjφ, B., J. Albert, A. Karlsson, L. Morfeldt-Månson, G. Biberfeld, K. Lidman, and E. M. Fenyφ. 1986. Replicative properties of human immunodeficiency virus from patients with varing severity of HIV infection. Lancet ii:660-662. [PubMed] [Google Scholar]
- 2.Becquart, P., N. Chomont, P. Roques, A. Ayouba, M. D. Kazatchkine, L. Bélec, and H. Hocini. 2002. Compartmentalization of HIV-1 between breast milk and blood of HIV-infected mothers. Virology 300:109-117. [DOI] [PubMed] [Google Scholar]
- 3.Cheng-Mayer, C., D. Seto, M. Tateno, and J. A. Levy. 1988. Biological features of HIV-1 that correlate with virulence in the host. Science 240:80-82. [DOI] [PubMed] [Google Scholar]
- 4.Derynck, R., and Y. E. Zhang. 2003. Smad-dependent and Smad-independent pathways in TGF-β family signaling. Nature 425:577-584. [DOI] [PubMed] [Google Scholar]
- 5.Dumais, N., S. Bounou, M. Olivier, and M. J. Tremblay. 2002. Prostaglandin E2-mediated activation of HIV-1 long terminal repeat transcription in human T cells necessitates CCAAT/Enhancer Binding protein (C/EBP) binding sited in addition to cooperative interactions between C/EBPβ and cyclic adenosine 5′-monophosphate response element binding protein. J. Immunol. 168:274-282. [DOI] [PubMed] [Google Scholar]
- 6.Dunn, D. T., M. L. Newell, A. E. Ades, and C. S. Peckham. 1992. Risk of human immunodeficiency virus type 1 transmission through breastfeeding. Lancet 340:585-588. [DOI] [PubMed] [Google Scholar]
- 7.Ekpini, E. R., S. Z. Wiktor, G. A. Satten, G. T. Adjorlolo-Johnson, T. S. Sibailly, C.-Y. Ou, J. M. Karron, K. Brattegaard, J. P. Whitaker, E. Gnaore, K. M. De Cock, and A. E. Greenberg. 1997. late postnatal mother-to-child transmission of HIV-1 in Abidjan, Côte d'Ivoire. Lancet 349:1054-1059. [DOI] [PubMed] [Google Scholar]
- 8.Ho, F. C. S., R. L. C. Wong, and J. W. M. Lawton. 1979. Human colostral and breast milk cells. A light and electron microscopic study. Acta Paediatr. Scand. 68:389-396. [DOI] [PubMed] [Google Scholar]
- 9.Kalliomäki, M., A. Ouwehand, H. Arvilommi, P. Kero, and E. Isolauri. 1999. Transformaing grownth factor-β in breast milk: a potential regulator of atopic disease at an early age. J. Allergy Clin. Immunol. 104:1251-1257. [DOI] [PubMed] [Google Scholar]
- 10.Kibler, K. V., and K. T. Jeang. 2001. CREB/ATF-dependent repression of cyclin A by human T-cell leukemia virus type 1 Tax protein. J. Virol. 75:2161-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kretzschmar, M., and J. Massagué. 1998. SMADs: mediators and regulators of TGF-β signaling. Curr. Opinion Genet. Develop. 8:103-111. [DOI] [PubMed] [Google Scholar]
- 12.Labbé, E., C. Silvesttri, P. A. Hoodless, J. L. Wrana, and L. Attisano. 1998. Smad2 and Smad3 positively and negatively regulate TGFβ-dependent transcription through the forkhead DNA-binding protein FAST2. Mol. Cell 2:109-120. [DOI] [PubMed] [Google Scholar]
- 13.Lazdins, J. K., T. Klimkait, K. Woods-Cook, M. Walker, E. Alteri, D. Cox, N. Cerletti, R. Shipman, G. Bilbe, and G. McMaster. 1991. In vitro effect of transforming growth factor-β on progression of HIV-1 infection in primary mononuclear phagocytes. J. Immunol. 147:1201-1207. [PubMed] [Google Scholar]
- 14.Li, J. M., X. Shen, P. P.-C. Hu, and X.-F. Wang. 1998. Transforming growth factor β stimulates the human immunodeficiency virus type 1 enhancer and requires NF-κB activity. Mol. Cell. Biol. 18:110-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lotz, M., and P. Seth. 1993. TGF-β and HIV infection. Ann. N. Y. Acad. Sci. 685:501-511. [DOI] [PubMed] [Google Scholar]
- 16.Margolis, D. M., A. B. Rabson, S. E. Straus, and J. M. Ostrove. 1992. Transactivation of the HIV-1 LTR by HSV-1 immediate-early genes. Virology 186:788-791. [DOI] [PubMed] [Google Scholar]
- 17.McKiel, V., Z. Gu, M. A. Wainberg, and J. Hiscott. 1995. Inhibition of human immunodeficiency virus type 1 multiplication by transforming growth factor beta 1 and AZT in HIV-1-infected myeloid cells. J. Interferon Cytokine Res. 15:849-855. [DOI] [PubMed] [Google Scholar]
- 18.Messaoudi, K. E., L. F. Thiry, C. Liesnard, N. Van Tieghem, A. Bollen, and N. Moguilevsky. 2000. A human milk factor susceptible to cathepsin D inhibitors enhances human immunodeficiency virus type 1 infectivity and allows virus entry into a mammary epithelial cell line. J. Virol. 74:1004-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moriuchi, H., M. Moriuchi, and J. I. Cohen. 1995. Proteins and cis-acting elements associated with transactivation of the varicella-zoster virus (VZV) immediate-early gene 62 promoter by VZV open reading frame 10 protein. J. Virol. 69:4693-4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moriuichi, H., M. Moriuchi, and A. S. Fauci. 1997. NF-κB potently upregulates expression of RANTES, an anti-HIV chemokine. J. Immunol. 158:3483-3491. [PubMed] [Google Scholar]
- 21.Moriuchi, H., M. Moriuchi, J. Arthos, J. Hoxie, and A. S. Fauci. 1997. Promonocytic U937 subclones expressing CD4 and CXCR4 are resistant to infection with and cell-to-cell fusion by T-cell-tropic human immunodeficiency virus type 1. J. Virol. 71:9664-9671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moriuchi, M., H. Moriuchi, S. E. Straus, and J. I. Cohen. 1994. Varicella-zoster virus (VZV) virion-associated transactivator open reading frame 62 protein enhances the infectivity of VZV DNA. Virology 200:297-300. [DOI] [PubMed] [Google Scholar]
- 23.Moriuchi, M., H. Moriuchi, W. Turner, and A. S. Fauci. 1998. Exposure to bacterial products renders macrophages highly susceptible to T-tropic human immunodeficiency virus type 1: implications for in vivo coinfections. J. Clin. Investig. 102:1540-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moriuchi, M., and H. Moriuchi. 2001. A milk protein lactoferrin enhances human T cell leukemia virus type I and suppresses HIV-1 infection. J. Immunol. 166:4231-4236. [DOI] [PubMed] [Google Scholar]
- 25.Nduati, R., G. John, D. Mbori-Ngacha, B. Richardson, J. Overbaugh, A. Mwatha, J. Ndinya-Achola, J. Bwayo, F. E. Onyango, J. Hughes, and J. Kreiss. 2000. Effect of breastfeeding and formula feeding on transmission of HIV-1. A randomized clinical trial. JAMA 283:1167-1174. [DOI] [PubMed] [Google Scholar]
- 26.Peterson, P. K., G. Gekker, C. C. Chao, R. Schut, T. W. Molitor, and H. H. Balfour, Jr. 1991. Cocaine potentiates HIV-1 replication in human peripheral blood mononuclear cell cocultures. Involvement of transforming growth factor-β. J. Immunol. 146:81-84. [PubMed] [Google Scholar]
- 27.Poli, G., A. L. Kinter, J. S. Justement, P. Bressler, J. H. Kehrl, and A. S. Fauci. 1991. Transforming growth factor β suppresses human immunodeficiency virus expression and replication in infected cells of the monocyte/macrophage lineage. J. Exp. Med. 173:589-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Southern, S. O. 1998. Milk-borne transmission of HIV. Characterization of productively infected cells in breast milk and interactions between milk and saliva. J. Hum. Virol. 1:328-337. [PubMed] [Google Scholar]
- 29.Thivierge, M., C. Le Gouill, M. J. Tremblay, J. Stañková, and M. Rola-Pleszczynski. 1998. Prostaglandin E2 induces resistance to human immunodeficiency virus-1infection in monocyte-derived macrophages; downregulation of CCR5 expression by cyclic adenosine monophosphate. Blood 92:40-45. [PubMed] [Google Scholar]
- 30.Toniolo, A., C. Serra, P. G. Conalde, F. Basolo, V. Falcone, and A. Dolei. 1995. Productive HIV-1 infection of normal human mammary epithelial cells. AIDS 9:859-866. [DOI] [PubMed] [Google Scholar]
- 31.Van't Wout, A. B., N. A. Kootstra, G. A. Mulder-Kampinga, N. Albrect-van Lent, H. J. Scherpbier, J. Veenstra, K. Boer, R. A. Couttinho, and F. Miedema. 1994. Macrophage-tropic variants initiate human immunodeficiency virus type 1 infection after sexual, parental, and vertical transmission. J. Clin. Investig. 94:2060-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou, S., L. Zawel, C. Lengauer, K. W. Kinzler, and B. Vogelstein. 1998. Characteriztion of human FAST-1, a TGFβ and activin signal transducer. Mol. Cell 2:121-127. [DOI] [PubMed] [Google Scholar]
- 33.Zhu, T., H. Mo, N. Wang, D. S. Nam, Y. Cao, R. A. Koup, and D. D. Ho. 1993. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science 261:1179-1181. [DOI] [PubMed] [Google Scholar]