Abstract
Marek's disease virus (MDV) is an oncogenic alphaherpesvirus that induces T-cell lymphomas in poultry. We report the construction of bacterial artificial chromosome (BAC) clones of the highly oncogenic RB-1B strain by inserting mini-F vector sequences into the US2 locus. MDV reconstituted from two BAC clones induced rapid-onset lymphomas similar to those induced by the wild-type virus. Virus reconstituted from another BAC clone that showed a 7.7-kbp deletion in the internal and terminal unique long repeat regions was nononcogenic, suggesting that the deleted region may be associated with oncogenicity. The generation of the oncogenic BAC clones of MDV is a significant step in unraveling the oncogenic determinants of this virus.
Bacterial artificial chromosomes (BAC) containing full-length genomes of several herpesviruses have enabled the application of rapid mutagenesis strategies to identify functions of individual genes and determinants of pathogenicity (1). Marek's disease (MD) is a contagious lymphoproliferative disease of poultry caused by the highly oncogenic alphaherpesvirus MD virus (MDV). The application of BAC mutagenesis strategies to study MDV oncogenicity has been hampered by the nonavailability of BAC clones of oncogenic strains, as two of the previously reported BAC clones derived from attenuated MDV strains were unable to induce tumors (4, 7). The RB-1B strain of MDV isolated in the early 1980s is a highly oncogenic strain (5, 6) that consistently induces a high incidence of MD with rapid-onset tumors in visceral organs. We have previously shown that RB-1B infection in 1-week-old Rhode Island red chickens caused a 100% incidence of MD with tumors in visceral organs 6 to 7 weeks after infection (4). Taking advantage of this high oncogenic potential, we have chosen the RB-1B strain for the construction of BAC clones to identify oncogenic determinants of MDV.
A clone-purified fourth-passage stock of the RB-1B strain of MDV (5), tested free of avian leukosis, reticuloendotheliosis, and chicken infectious anemia viruses, was used for the preparation of viral DNA for the construction of RB-1B BAC clones. Viral DNA was prepared from chicken embryo fibroblast (CEF) cultures by sodium dodecyl sulfate-proteinase K extraction (7). RB-1B BAC construction was carried out by insertion of the mini-F plasmid pHA1 into the US2 gene of MDV essentially as previously described (7). Briefly, secondary CEF cultures were cotransfected with RB-1B virus-infected genomic DNA and plasmid pDS-pHA1 (7) and passaged five or six times on primary CEF in medium containing 250 μg of mycophenolic acid, 50 μg of xanthine, and 100 μg of hypoxanthine per ml at 4-day intervals. DNA from these cells was electroporated into Escherichia coli DH10B cells and plated on Luria-Bertani plates containing 30 μg of chloramphenicol per ml. Transfection of high-molecular-weight extrachromosomal BAC DNA from three single colonies (designated pRB-1B-1, pRB-1B-2, and pRB-1B-5) into primary CEF produced MDV-specific plaques in about 4 to 5 days after transfection.
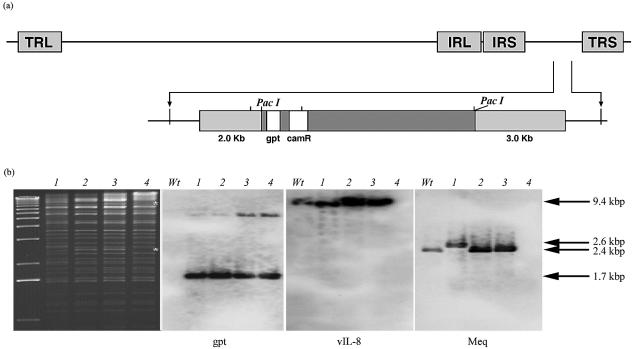
EcoRI digestion of the pRB-1B BAC clones showed a restriction enzyme pattern similar to that of previously reported MDV BAC pCVI988 (4). However, the restriction profile of pRB-1B-2 DNA was distinct by the absence of two fragments of approximately 8 and 2.5 kbp (Fig. 1b, asterisks). Southern blot hybridization of the EcoRI-digested DNA with the digoxigenin-labeled gpt probe detected a major 1.7-kbp fragment in all three pRB-1B clones, indicating that the BAC vector was correctly inserted in the US2 region (Fig. 1a). The vIL-8 probe detected a single 9.4-kbp EcoRI fragment in pRB-1B-1 and pRB-1B-5 DNA, identical to that seen in wild-type RB-1B and the previously reported pCVI988 clone (4). However, the pRB-1B-2 clone did not hybridize with the vIL-8 and meq probes (Fig. 1b) while pRB-1B clones 1 and 5 and wild-type RB-1B showed a single 2.4-kbp band of the meq gene.
FIG. 1.
Genome structure of the pRB-1B clones. (a) Schematic structure of the pRB-1B BAC showing the insertion of the pDS-pHA1 vector in the US2 locus. (Top) MDV genome structure with TRL, IRL, internal unique short repeat (IRS), and terminal unique short repeat (TRS) regions. (Bottom) Structure of the 7.2-kbp PacI mini-F vector (dark gray box) containing the gpt and chloramphenicol resistance (camR) genes flanked by the 2.0- and 3.0-kb regions (light gray boxes) bordering the US2 locus. (b) Digitally scanned images of ethidium bromide-stained agarose gel of EcoRI-digested BAC DNA. Lanes: 1, pCVI988-10 (4); 2, pRB-1B-1; 3, pRB-1B-5; 4, pRB-1B-2. The unmarked lane on the left contains a 1-kb molecular weight marker. Asterisks indicate the missing 8.0- and 2.5-kbp fragments in the pRB-1B-2 clone. Also shown are Southern blots of separate agarose gels of EcoRI-digested wild-type RB1B (Wt) and the BAC clones probed with digoxigenin-labeled gpt, vIL-8, and meq probes. The sizes of the bands detected by the probes are shown on the right.
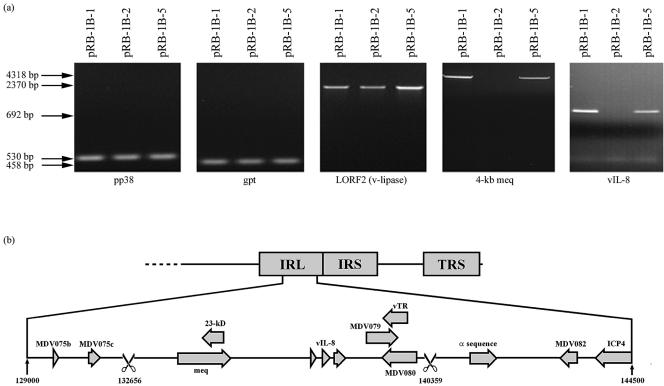
The pRB-1B BAC clones were also analyzed by PCR tests with primers (Table 1) specific for the gpt, pp38, LORF-2, and vIL-8 genes or a 4-kbp meq region. PCR tests for the pp38, gpt, and LORF-2 genes gave bands of the expected sizes on the DNAs of all three pRB-1B BAC clones, demonstrating that the three clones have identical genome structures in these regions. However, pRB-1B-2 DNA was negative in PCR tests for the meq and vIL-8 genes (Fig. 2a), demonstrating that pRB-1B-2 has a deletion in both copies of the meq and vIL-8 regions. Further PCR tests with the BAC2-for and BAC2-rev primers designed from the flanking regions of the predicted deletion gave a much shorter product of approximately 6.5 kbp on pRB-1B-2 DNA (data not shown) instead of a product with the expected size of 14 kbp. Sequence analysis of this product showed that a 7,701-bp region, corresponding to positions 1262 to 8963 and 132657 to 140358 (in the terminal unique long repeat [TRL] and internal unique long repeat [IRL] regions, respectively, of the published Md5 sequence; accession number AF243438) was deleted in the pRB-1B-2 clone. Deletion of this region results in the complete loss of MDV005/076 (meq), MDV004/077 (23-kDa protein gene), MDV003/078 (vIL-8), MDV002/079 (ICP0), MDV001/080, and the MDV-encoded RNA subunit of the telomerase (vTR) gene (2) from pRB-1B-2 (Fig. 2b). It is not clear whether the deletion was generated during the construction of the BAC or whether it represented a naturally occurring deletion mutant present in the wild-type RB-1B virus stocks used to generate the BAC clones.
TABLE 1.
Primers used to amplify the different regions examined in this study
| Primer | Sequence (5′-3′) | Md5 sequence positiona | Description |
|---|---|---|---|
| gpt for | ATGAGCGAAAAATACATCGTC | gpt probe | |
| gpt rev | TTAGCGACCGGAGATTGGCGG | ||
| Lorf2 for | GATCGTCTATGTACAATGCC | 14520-14539 | LORF2 |
| Lorf2 rev | TATTATCGAATCGATTCATA | 16890-16871 | |
| pp38 for | TTGTCTTTCTGCCCGCACCG | 127848-127829 | pp38 |
| pp38 rev | TCGCACTGCTGGGCAAGCTC | 127318-127337 | |
| Meq4Kb for | GAATGAGCTCGTTCGGCCAATCGAATTTCT | 133022-133041 | 4-kb meq region |
| Meq4Kb rev | GGCGGCATGCTCCGTCAACAAACGCACTAA | 137340-137321 | |
| vIL-8 for | GCAACCATGGAGGCGTTGTTG | 138069-138079 | vIL-8 probe |
| vIL-8 rev | AGCTCTAGATCAAAGACAGAT | 138743-138731 | |
| Meq for | GCACTCTAGAGGTGTAAAGAGATGTCTCAG | 134855-134875 | meq probe |
| Meq rev | TAACTCGAGGAGAAGAAACATGGGGCATAG | 135941-135920 | |
| BAC2-for | ACGGATGAGCTCCTTCTGTAAACGATTGCGGA | 132005-132024 | IRL-TRL |
| BAC2-rev | GAAATGCCGTATTCTCCTCGTCTC | 146279-146256 | |
| MDV gB for | GGTTCAACCGTGATCCGTCTA | 63493-63473 | gB Taqman probe |
| MDV gB rev | CGATTCCTTCACCCCACT | 63415-63430 | |
| MDV gB FAM | ACCGCCGCGAAAATGTCCCG | 63451-63470 | |
| MDV pp38 for | GAAAACAGAAGCGGAATGCG | 13946-13965 | pp38 Taqman probe |
| MDV pp38 rev | CGATCCAAAGCGCTCATCTC | 13995-14014 | |
| MDV pp38 FAM | CCCCGCATTTCTCGCCGTCCTC | 13970-13989 |
GenBank accession number AF243438.
FIG. 2.
Genomic structure of pRB-1B BAC. (a) Ethidium bromide-stained agarose gels showing PCR products of pp38, gpt, LORF2 (v-lipase), the 4-kb region harboring the meq gene, and vIL-8 from pRB-1B BAC clones amplified with the specific primers listed in Table 1. The sizes of the PCR products are shown on the left. (b) Schematic diagram of the pRB-1B-2 IRL region with the deletion (scissors) and the genes in the region. The identical structure of the TRL region is not shown.
In vitro replication of the wild-type virus and the three BAC-derived RB-1B viruses was analyzed by infecting freshly seeded CEF with virus stocks. At 24-h intervals for 5 days, DNA was extracted from infected cells and viral copy numbers were estimated by quantitative PCR (qPCR) tests (3) with primers specific for the pp38 gene. PCR results indicated that all four of the viruses replicated in culture in vitro, although there were differences in their growth curves. The wild-type and pRB-1B-5 viruses showed similar replication curves, while the copy numbers of the pRB-1B-1 and pRB-1B-2 viruses were approximately 10-fold lower at various time points (data not shown).
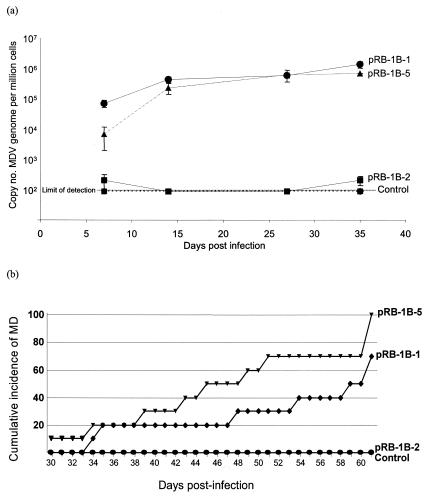
For analysis of the in vivo replication of the BAC-derived viruses, groups of 10 1-day-old specific-pathogen-free HPRS Rhode Island red chickens were infected with 4 × 103 PFU of each of the virus stocks by the intra-abdominal route. A larger virus challenge dose was used deliberately to examine whether the deletion mutant derived from the pRB-1B-2 clone would be oncogenic even with this large dose. Virus replication in vivo was measured with a glycoprotein B-specific real-time qPCR on DNA samples from peripheral blood leukocytes collected from five birds from each group at 7, 14, 27, and 35 days postinfection. The virus genome copy numbers in the DNA samples from birds infected with pRB-1B-1 and -5 showed a steady increase, indicating viral replication with very similar copy numbers (close to 106 copies per million cells) at 27 and 35 days postinfection (Fig. 3a). However, qPCR on DNA samples from peripheral blood leukocytes of birds infected with pRB-1B-2 were very low at all four time points and were close to that of the DNA from the negative control group. This was also confirmed by a separate qPCR test with pp38-specific primers, which gave identical results (data not shown). These results confirmed that the pRB-1B-2 virus replicated in vivo at low levels very close to the detection limit of the threshold cycle values of qPCR tests. However, the pRB-1B-2 virus was able to persist in the infected birds as we were successful in isolating the virus from the spleens of 80% of the birds at the end of the experiment.
FIG. 3.
(a) In vivo replication of the pRB-1B-derived viruses. Real-time qPCR showing the copy numbers of the MDV genome in sequential samples of DNA from peripheral blood leukocytes from birds infected with pRB-1B-derived viruses. (b) Cumulative incidence of MD in birds infected with viruses derived from the pRB-1B clones. Virus stocks (4 × 103 PFU) derived from the pRB-1B clones were inoculated by the intra-abdominal route into groups (n = 10) of 1-day old specific-pathogen-free line P birds. The cumulative incidence of the disease in the 61-day experimental period is expressed as a percentage.
The pathogenicity of the viruses reconstituted from the three pRB-1B clones, monitored from clinical signs, as well as by gross pathological and histopathological lesions, appeared to be related to the rates of in vivo replication. Birds infected with the pRB-1B-1 and -5 viruses, both of which replicated at high titers as shown by high genome copy numbers in qPCR assays, induced high levels of MD tumors from 4 weeks after infection (Fig. 3b). On the other hand, none of the birds infected with the pRB-1B-2 virus, which failed to show in vivo replication by qPCR test, developed any clinical disease or showed any evidence of gross or histopathological lesions, although viruses could be isolated from the spleens of 8 of 10 birds at the end of the experiment. MDV derived from pRB-1B-5 was highly oncogenic as it induced MD in 100% of the birds with a mean time to onset of the disease of 47.4 days. MDV derived from pRB-1B-1 induced the disease in 70% of the birds infected during the 61-day experimental period, with a mean time to disease onset of 50.4 days. These differences in oncogenicity between the two viruses were not statistically significant. Nevertheless, such variations may be attributable to secondary mutations elsewhere in their genomes. The kinetics of the disease and the type of lesions caused by the BAC-derived viruses were very similar to those described for the wild-type RB-1B isolate (4), with tumors seen predominantly in the kidneys, gonads, and liver. Although the precise reasons for the poor in vivo replication and lack of oncogenicity of pRB-1B-2 virus is not clear, it is likely to be related to the large deletion in the repeat region. However, confirmation of this can only be obtained by examining revertant viruses in which the deleted region has been replaced.
In summary, we have successfully constructed stable BAC clones of the virulent RB-1B strain of MDV. Viruses reconstituted from two clones were highly oncogenic despite the insertion of the mini-F cassette in the US2 locus. The pRB-1B clones were stable as shown by the presence of BAC sequences in the reisolated viruses. We have also established several transformed T-cell lines from tumors induced by these oncogenic BAC clones. This is the first report of the construction of BAC clones of a highly oncogenic MDV strain and provides the opportunity to exploit the power of bacterial DNA repair and modification machinery to identify molecular determinants of MDV oncogenicity by BAC mutagenesis.
Acknowledgments
We are grateful to Peter Chesters for assisting in the cloning of the deleted region and Mick Gill for assistance with digital imaging.
This project is funded partly by the Biotechnology and Biological Sciences Research Council, United Kingdom.
REFERENCES
- 1.Adler, H., M. Messerle, and U. H. Koszinowski. 2003. Cloning of herpesviral genomes as bacterial artificial chromosomes. Rev. Med. Virol. 13:111-121. [DOI] [PubMed] [Google Scholar]
- 2.Fragnet, L., M. A. Blasco, W. Klapper, and D. Rasschaert. 2003. The RNA subunit of telomerase is encoded by Marek's disease virus. J. Virol. 77:5985-5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaiser, P., G. Underwood, and F. Davison. 2003. Differential cytokine responses following Marek's disease virus infection of chickens differing in resistance to Marek's disease. J. Virol. 77:762-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petherbridge, L., K. Howes, S. J. Baigent, M. A. Sacco, S. Evans, N. Osterrieder, and V. Nair. 2003. Replication-competent bacterial artificial chromosomes of Marek's disease virus: novel tools for generation of molecularly defined herpesvirus vaccines. J. Virol. 77:8712-8718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schat, K., B. W. Calnek, and J. Fabricant. 1982. Characterisation of two highly oncogenic strains of Marek's disease virus. Avian Pathol. 11:593-605. [DOI] [PubMed] [Google Scholar]
- 6.Schat, K. A., B. W. Calnek, J. Fabricant, and H. Abplanalp. 1981. Influence of oncogenicity of Marek's disease virus on evaluation of genetic resistance. Poult. Sci. 60:2559-2566. [DOI] [PubMed] [Google Scholar]
- 7.Schumacher, D., B. K. Tischer, W. Fuchs, and N. Osterrieder. 2000. Reconstitution of Marek's disease virus serotype 1 (MDV-1) from DNA cloned as a bacterial artificial chromosome and characterization of a glycoprotein B-negative MDV-1 mutant. J. Virol. 74:11088-11098. [DOI] [PMC free article] [PubMed] [Google Scholar]