Abstract
Murine gammaherpesvirus 68 (MHV-68) glycoprotein B (gB) was identified in purified virions by immunoblotting, immunoprecipitation, and immunoelectron microscopy. It was synthesized as a 120-kDa precursor in infected cells and cleaved into 65-kDa and 55-kDa disulfide-linked subunits close to the time of virion release. The N-linked glycans on the cleaved, virion gB remained partially endoglycosidase H sensitive. The processing of MHV-68 gB therefore appears similar to that of Kaposi's sarcoma-associated herpesvirus gB and human cytomegalovirus gB.
All herpesviruses express glycoproteins that attach virions to cells and fuse virion and cellular membranes (22). Such functions represent major therapeutic targets. The human gammaherpesviruses Epstein-Barr virus and Kaposi's sarcoma-associated herpesvirus, show only very limited infection of nonprimates, so murine gammaherpesvirus 68 (MHV-68), a close relative of Kaposi's sarcoma-associated herpesvirus (9, 31), has proved useful in identifying how gammaherpesvirus genes contribute to host colonization. MHV-68 has a broad cellular tropism that encompasses epithelial cells, B cells, macrophages, and dendritic cells (10, 26, 34). A major task in understanding how gammaherpesvirus glycoproteins influence pathogenesis is therefore to identify the glycoprotein functions of MHV-68.
Glycoprotein B (gB) plays an important role in the entry of both Epstein-Barr virus and Kaposi's sarcoma-associated herpesvirus virions into cells. Kaposi's sarcoma-associated herpesvirus gB binds to heparan sulfate (1) and α3β1 integrin; the latter interaction makes a major contribution to infection (2). The major Epstein-Barr virus receptor binding proteins are gp350/220 (28) and gp42 (15), but Epstein-Barr virus gB is an essential protein (12) and high levels of gB in virions correlate with enhanced infectivity (19). MHV-68 lacks an obvious homologue of gp42, and its positional homologue of gp350/220, gp150, promotes virion release rather than binding (8). MHV-68 gB is therefore likely to play a major role in virion entry even though it lacks the RGD integrin binding motif (32) of Kaposi's sarcoma-associated herpesvirus gB.
MHV-68 gB was originally described as a 110-kDa glycoprotein product of open reading frame 8 that was absent from virions and remained confined to the endoplasmic reticulum during lytic infection (24). A subsequent report identified gB-derived peptides in an 88-kDa virion protein, with the discrepancy attributed to different methods of virion purification (7). Clearly, it is important in defining the molecular basis of MHV-68 tropism to determine whether gB is consistently found in virions, as it is in Kaposi's sarcoma-associated herpesvirus (1, 4) and bovine herpesvirus 4 (16). Our data demonstrate that gB is readily detectable in virions, mainly as 65-kDa and 55-kDa cleavage products of a full-length 120-kDa protein. Cleavage was associated with gB incorporation into virions. The MHV-68 gB is therefore a virion component that is processed very similarly to gB of Kaposi's sarcoma-associated herpesvirus (1, 4).
Identification of gB on the surface of MHV-68-infected cells.
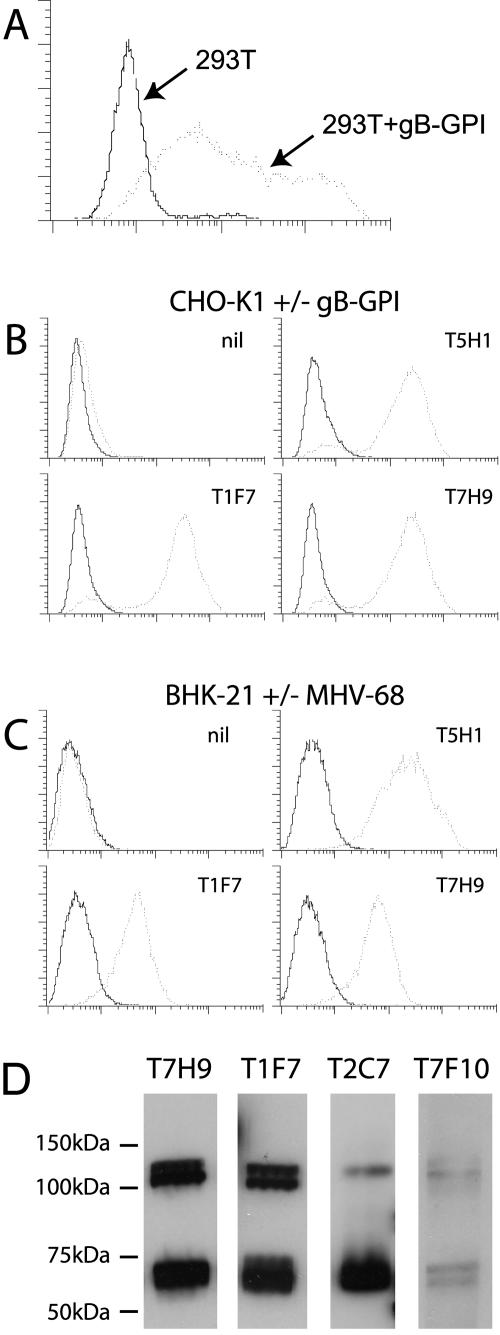
Our first aim was to generate gB-specific monoclonal antibodies. As in other herpesviruses, MHV-68 gB has multiple cytoplasmic endocytosis motifs (for example: YPSV, LL, and YSQL), and full-length gB was poorly expressed at the surface of transfected cells (data not shown). We therefore expressed gB at the cell surface by fusing its extracellular domain to a glycosylphosphatidylinositol (GPI) attachment sequence. Cell surface-expressed gB-GPI was readily detectable with an MHV-68-reactive rabbit serum (Fig. 1A). We then used cells expressing gB-GPI to identify gB reactivity in a large panel of hybridomas derived from MHV-68-immune mice (Fig. 1B). The monoclonal antibodies that recognized gB-GPI also stained intact, MHV-68-infected cells (Fig. 1C), indicating that here, full-length gB reached the cell surface, presumably on virion envelopes. None of seven gB-specific monoclonal antibodies significantly inhibited MHV-68 infection of fibroblasts (data not shown).
FIG. 1.
Identification of gB-specific monoclonal antibodies. A: The extracellular domain of gB (genomic coordinates 16526 to 18617) was amplified by PCR from MHV-68 DNA, including AvrII and NotI restriction sites in the respective 5′ and 3′ primers, and linked to the GPI attachment signal of human decay-accelerating factor in the pBRAD mammalian expression vector (23). 293T cells were transfected with gB-GPI (dotted line) or with empty vector (solid line) with Fugene-6 (Roche Diagnostics, Ltd.) and after 2 days stained with rabbit anti-MHV-68 polyclonal antibody (27) followed by fluorescein isothiocyanate-conjugated swine anti-rabbit immunoglobulin polyclonal antibody (Dako Ltd). B: BALB/c mice were infected intranasally with open reading frame 73-deficient MHV-68 (11). After 3 months, these mice were boosted by intraperitoneal infection with the same virus. After a further 3 days, spleens were harvested and disrupted into single-cell suspensions. These were then fused with NS0 cells and selected with hypoxanthine and azaserine according to established protocols (14). gB-specific monoclonal antibodies were identified on the basis of reactivity against CHO-K1 cells stably transfected with gB-GPI (dotted lines) but not against CHO-K1 cells transfectedwith the empty vector (solid lines). Three examples of gB-specific monoclonal antibodies (T5H1, T1F7, and T7H9) are shown. nil, secondary antibody only. C: The same monoclonal antibodies were used to stain BHK-21 cells that had either been left uninfected (solid lines) or infected overnight with MHV-68 (dotted lines). Dead cells were excluded by propidium iodide staining. D: Four of seven gB-specific monoclonal antibodies identified by reactivity against CHO-gB-GPI cells also detected protein in virus-infected cell lysates by immunoblotting. Supernatants from MHV-68-infected BHK-21 cells were pelleted by ultracentrifugation (35,000 × g, 1 h), boiled in Laemmli buffer, separated by SDS-PAGE, blotted onto polyvinylidene difluoride membranes, and probed with gB-specific monoclonal antibodies as shown, followed by horseradish peroxidase-coupled rabbit anti-mouse immunoglobulin G polyclonal antibody (Dako Ltd). Blots were developed with the ECL detection reagent (Amersham-Pharmacia Biotech).
Using several different gB-specific monoclonal antibodies, we could consistently immunoblot two major gene products in virus-infected cell lysates (Fig. 1D), one of 120 kDa and one of 65 kDa. gB mRNA is not spliced (24), so the simplest explanation for two bands with common epitopes was that the smaller form of gB is cleaved from the larger, as in human cytomegalovirus (21) and Kaposi's sarcoma-associated herpesvirus (1, 4).
Identification of gB as a disulfide-linked virion protein.
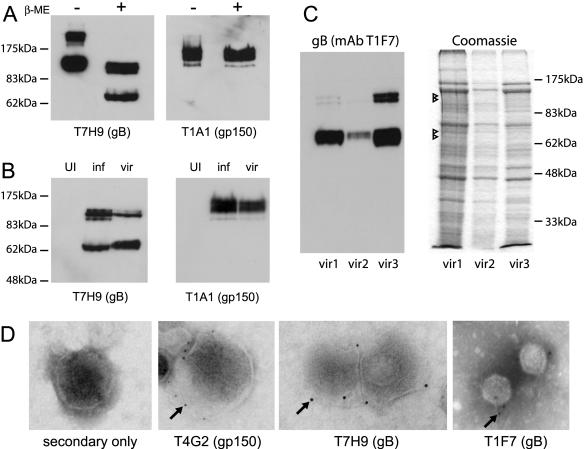
The gB open reading frame encodes an 819-amino-acid mature protein, equivalent to a molecular weight of 93 kDa. Thus, some 20% by mass of full-length gB is likely to be carbohydrate; there are 14 potential attachment sites for O-linked glycans and nine for N-linked glycans. Each major form of gB included at least two distinct bands (Fig. 1D), suggestive of different glycoforms. Neither 120-kDa nor 65-kDa gB was seen in unreduced infected cell lysates (Fig. 2A). Instead there was a major band larger than both, consistent with each having a disulfide-linked partner. The 130-kDa major unreduced band was presumably the 65-kDa gB cleavage product disulfide linked either to the remainder of gB or to itself as a homodimer.
FIG. 2.
MHV-68 gB is a disulfide-linked virion protein. A: Monoclonal antibody T7H9 was used to detect gB in either reduced (with β-mercaptoethanol) or unreduced (without β- mercaptoethanol) infected cell lysates. Monoclonal antibody T1A1 recognizes gp150 (8). B: Uninfected cells (UI), ultracentrifuged infected cell cultures (inf), and purified virions (vir) were immunoblotted for gB (T7H9) or gp150 (T1A1) as indicated. To prepare MHV-68 virions from infected BHK-21 cells, cell debris was pelleted by centrifugation (1,000 × g, 10 min). Virus was then pelleted from the supernatant (30,000 × g, 90 min). The supernatant virus was resuspended in phosphate-buffered saline, sonicated, layered over 5 to 15% Ficoll gradients, and centrifuged again (30,000 × g, 90 min). Virions were recovered as a distinct band, resuspended in phosphate-buffered saline, pelleted (30,000 × g, 90 min), sonicated, aliquoted, and stored at −70°C. The presence of infectious virus in virion preparations was confirmed by plaque assay. C: Three independent virion preparations, from infected BHK-21 cells (vir1) or murine embryonic fibroblasts (vir2 and vir3) were analyzed in parallel by immunoblotting with gB-specific monoclonal antibody T1F7 or by Coomassie staining of all protein. The Coomassie-stained bands corresponding to those detected by monoclonal antibody T1F7 are indicated by arrowheads. Those corresponding to uncleaved gB were difficult to discern. D: Virions purified on Ficoll density gradients were adsorbed to carbon-coated grids and then stained with secondary antibody alone or specifically for gp150 (monoclonal antibody T4G2) or gB (monoclonal antibodies T7H9 and T1F7). Bound antibody was detected with gold-labeled goat anti-mouse immunoglobulin G. Grids were washed in phosphate-buffered saline, rinsed briefly in water, and counterstained with uranyl acetate. The images shown are each representative of at least 50 virions analyzed. Arrows indicate examples of gold particles.
We could readily detect gB by immunoblotting MHV-68 virions (Fig. 2B); the ratio of gB signal between infected cell lysates and purified virions was similar to that of gp150, a known virion protein (25). In virions, the 65-kDa form of gB was relatively abundant compared to the full-length form (Fig. 2B and 2C). We identified the virion bands corresponding to gB by parallel immunoblotting and Coomassie staining of three independent virion preparations (Fig. 2C). Full-length gB was hard to discern as a Coomassie-stained band, consistent with cleaved gB being the major form in virions. Our data differed from those of Bortz et al. (7) in that we could not detect an 88-kDa form of gB.
Immunoelectron microscopy provided further evidence that gB is a virion protein (Fig. 2D). Only small numbers of gold particles were detected, but these were found consistently, and the staining for gB was comparable to that achieved with a gp150-specific monoclonal antibody (T4G2).
Immature N-linked glycans on MHV-68 gB.
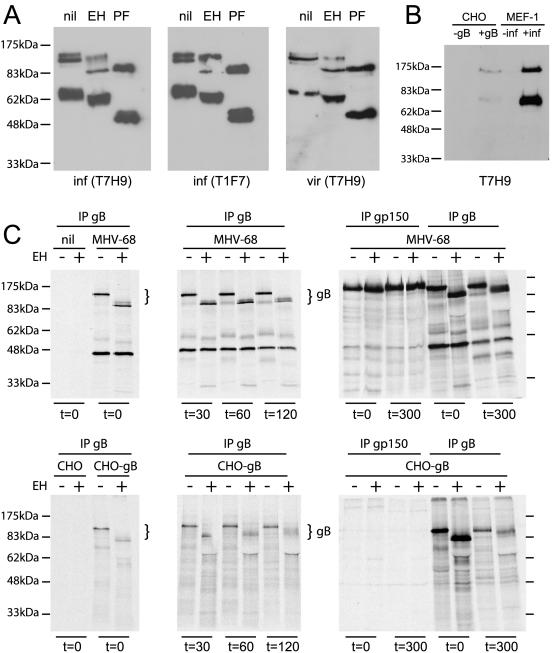
One criterion used by Stewart et al. to argue that gB is retained in the endoplasmic reticulum was that it retained endoglycosidase H-sensitive N-linked glycans (24). However, even virion gB, which must have left the endoplasmic reticulum, retained at least some sensitivity to endoglycosidase H digestion (Fig. 3A). This was not complete sensitivity: peptide N-glycosidase F digestion removes approximately 0.2 kDa more glycan mass than endoglycosidase H (one N-acetylglucosamine residue) from high-mannose or hybrid N-linked glycans, so if all nine N-linked glycans of gB were endoglycosidase H sensitive, peptide N-glycosidase F should have removed only 2 kDa more than endoglycosidase H. Instead, peptide N-glycosidase F removed approximately 12 kDa more glycan from gB than endoglycosidase H did (Fig. 3A), so not all the gB glycans were endoglycosidase H sensitive.
FIG. 3.
MHV-68 gB retains immature N-linked glycans. A: Lysates of infected cells (inf) or purified virions (vir) were treated with endoglycosidase H (EH) or peptide N-glycosidase F (PF) according to the manufacturer's instructions (New England Biolabs) or left untreated (nil). After separation by SDS-PAGE, samples were blotted onto polyvinylidene difluoride membranes and probed for gB with monoclonal antibody T1F7 or monoclonal antibody T7H9 as indicated. B: CHO cell, CHO-gB cells, uninfected MEF-1 cells, and MHV-68-infected MEF-1 cell lysates were immunoblotted for gB with monoclonal antibody T7H9. C: BHK-21 cells were infected overnight (3 PFU/cell) and then labeled with [35S]cysteine-methionine (30 min), followed by a variable chase in the presence of excess unlabeled cysteine-methionine (6). CHO-gB-GPI cells were labeled and chased in parallel. All samples were then lysed on ice for 30 min in 1% Triton X-100-50 mM Tris-Cl (pH 7.4)-150 mM NaCl-5 mM EDTA-1 mM phenylmethylsulfonyl fluoride, with complete protease inhibitors (Roche Diagnostics Ltd.). Lysates were cleared by centrifugation (13,000 × g, 15 min) and incubation (2 h, 4°C) with protein A-Sepharose (Sigma Chemical Co.). For immunoprecipitation, we added T1A1 (anti-gp150) or T7H9 (anti-gB) hybridoma supernatant to the cleared supernatants at a final concentration of 10 μg/ml (1 h, 4°C), followed by protein A-Sepharose (2 h, 4°C). Control lanes show immunoprecipitates of uninfected BHK-21 cells and untransfected CHO cells. All immunoprecipitates were left undigested or digested with endoglycosidase H (EH) before SDS-PAGE and exposure to X-ray film. The prominent 45-kDa band coprecipitating with gB from infected cells, presumably a component of the viral tegument, has been observed previously (24). Similar data were obtained with radioimmunoprecipitation assay buffer lysates (1% Triton X-100, 1% deoxycholate, 0.1% SDS) (data not shown).
We looked for evidence of partial glycan maturation by pulse-chase metabolic labeling and gB immunoprecipitation with monoclonal antibody T7H9, comparing virus-infected cells with CHO-K1 cells expressing gB-GPI. Immunoblotting confirmed that gB-GPI could be processed into 120-kDa and 65-kDa forms (the cytoplasmic tail contributes only a small fraction of the total mass) (Fig. 3B). The 65-kDa form of gB was hard to discern in immunoprecipitates, so our analysis focused on the 120-kDa form (Fig. 3C). There was little sign of gB glycan maturation over a 2-h chase, but after a 5-h chase, there was evidence of limited glycan maturation, with endoglycosidase H digestion giving a less uniform size shift (Fig. 2C, t = 300 min). gp150 in virus-infected cells became completely endoglycosidase H resistant over the same time.
Several 35S-labeled proteins coprecipitated with gB from MHV-68-infected cells (Fig. 3C). Sensitivity to endoglycosidase H digestion suggested that the bands at 55 kDa and 42 kDa were glycoproteins. The 55-kDa band seen here was unlikely to be a gB cleavage product, since we could precipitate 120-kDa but not 65-kDa gB. Further analysis with additional MHV-68 glycoprotein-specific monoclonal antibodies established that the 55-kDa band was a product of viral open reading frame 4 and the 42-kDa band was a product of viral open reading frame 27 (data not shown). These glycoproteins matured to endoglycosidase H resistance over a 5-h chase, whereas the coprecipitating gB did not. GPI-linked gB, which is abundant on the cell surface (Fig. 1), also acquired only partial endoglycosidase H resistance over a 5-h chase. Thus, although gB was apparently passing through the trans-Golgi network, some of its N-linked glycans remained inaccessible to modifying enzymes. Consequently, endoglycosidase H sensitivity did not provide a reliable indicator of gB location within the secretory pathway.
High-mannose glycans are not uncommon on viral glycoproteins (5, 13, 18) and provide a potential target for virion neutralization (3). Whereas macrophages are nonpermissive cells, macrophage lectin binding to viral mannose residues can also limit infection (20). However, lectins represent a possible route to productive infection for a macrophage-tropic virus such as MHV-68. Human immunodeficiency virus gp120 exploits terminal mannose residues to bind to dendritic cells via dendritic cell-specific ICAM-grabbing nonintegrin (31). A similar pathway may allow MHV-68 virions to infect dendritic cells and macrophages.
Cleavage of gB is associated with virion release.
Since 65-kDa gB was generally predominant over full-length gB in virions, we specifically addressed why it was hard to discern in immunoprecipitates from infected cells. The 65-kDa form would have been less strongly labeled due to fewer cysteine and methionine residues, and unlabeled immunoglobulin G heavy chains distorted the immunoprecipitation gels somewhat at 50 to 60 kDa. However, neither consideration seemed sufficient for the lack of a 65-kDa gB signal in virus-infected cells. It seemed more likely that gB cleavage occurred close to the time when virions were released from the 35S-labeled cells. These cells were washed prior to lysis and so would have contained relatively few mature virions, whereas the infected cell preparations used for immunoblotting were ultracentrifuged prior to lysis and so would have contained both cells and virions.
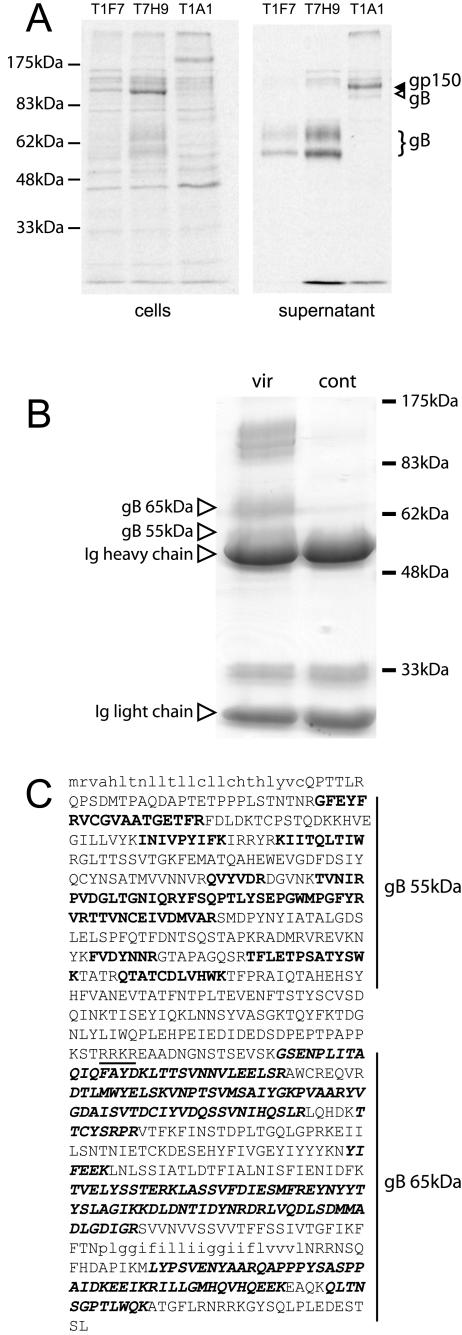
To directly compare the gB in virions and cells, we pulse-labeled infected cells 6 h postinfection, chased the label overnight, and recovered gB separately from the cell and supernatant fractions (Fig. 4A). The supernatant gB was entirely in the smaller, cleaved form, whereas the cell-associated gB was predominantly uncleaved. The cleavage of gB was therefore associated with its incorporation into virions. Labeled gp150 was largely in the supernatant fraction but retained its full-length form.
FIG. 4.
Analysis of gB cleavage products. A: BHK-21 cells were infected for 6 h with MHV-68 (3 PFU/cell), labeled for 30 min with [35S]cysteine-methionine, and chased overnight with an excess of unlabeled cysteine-methionine. Virions were recovered from cell supernatants by ultracentrifugation. Cells and virions were then separately lysed and immunoprecipitated for gB (monoclonal antibody T1F7 or monoclonal antibody T7H9) or gp150 (monoclonal antibody T1A1). B: gB was immunoprecipitated from ultracentrifuged virions plus infected cells (vir) with monoclonal antibody T7H9. Samples were then reduced, denatured, and separated by SDS-PAGE. A representative Coomassie-stained gel of immunoprecipitates is shown. The control lane (cont) is immunoprecipitated antibody only. The higher-molecular-weight, virus-specific bands were not analyzed but presumably represent uncleaved gB. C: The two lower-molecular-weight forms of gB indicated in B were excised and analyzed by matrix-assisted laser desorption ionization-time of flight fingerprinting. The gB amino acid sequence is shown, with the predicted signal sequence and transmembrane domain in lowercase type. The consensus furin cleavage site is underlined. Peptides identified in the 55-kDa gB band are shown in bold type; peptides identified in the 65-kDa band are in bold italic type.
The 55-kDa band that coprecipitated with the 65-kDa form of gB in virions (Fig. 4A) was presumably the second gB cleavage product. In order to identify these products more definitively, we immunoprecipitated gB from MHV-68 virus stocks, reduced and denatured the immunoprecipitated samples, and separated them by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) (Fig. 4B). We then excised Coomassie-stained protein bands for peptide identification by mass spectrometry (Fig. 4C). This established that the 65-kDa gB band was a C-terminal cleavage product, and the coprecipitating 55-kDa band was the corresponding N-terminal cleavage product. Since these cleavage products could be coprecipitated with monoclonal antibodies under nonreducing conditions and separated under reducing conditions, they were presumably disulfide-linked to each other.
Human cytomegalovirus gB is cleaved by furin in the trans-Golgi network (30), close to the site of virus assembly. MHV-68 gB has a furin consensus cleavage sequence at amino acids 424 to 427 (RRKR), which would fit well with the size of the smaller gB products and the peptides derived from them. Rapid virion shedding probably removed the 65-kDa and 55-kDa forms of gB from infected cells soon after cleavage. The full-length gB that was retained in cells (Fig. 4A) may represent a distinct pool, for example, one targeted to the inner nuclear membrane (17). The fact that the bulk of the MHV-68 gB was processed and incorporated into virions very similarly to the gBs of Kaposi's sarcoma-associated herpesvirus (2) and human cytomegalovirus (33) suggests that is likely to have a similar role in cell binding and entry.
Acknowledgments
We thank Jeremy Skepper (Wellcome Trust Multi-imaging Centre) for assistance with immunoelectron microscopy, Brad Spiller for pBRAD, Len Packman (University of Cambridge Department of Biochemistry) for mass spectrometry, and Colin Crump for helpful discussion.
F.B.L. was the recipient of a Leonardo da Vinci scholarship. P.G.S. is an MRC/Academy of Medical Sciences Clinician Scientist (G108/462). This work was supported by an MRC cooperative group grant (G9800903).
REFERENCES
- 1.Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2001. Human herpesvirus 8 envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology 284:235-249. [DOI] [PubMed] [Google Scholar]
- 2.Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407-419. [DOI] [PubMed] [Google Scholar]
- 3.Anders, E. M., C. A. Hartley, and D. C. Jackson. 1990. Bovine and mouse serum beta inhibitors of influenza A viruses are mannose-binding lectins. Proc. Natl. Acad. Sci. USA 87:4485-4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baghian, A., M. Luftig, J. B. Black, Y. X. Meng, C. P. Pau, T. Voss, P. E. Pellett, and K. G. Kousoulas. 2000. Glycoprotein B of human herpesvirus 8 is a component of the virion in a cleaved form composed of amino- and carboxyl-terminal fragments. Virology 269:18-25. [DOI] [PubMed] [Google Scholar]
- 5.Benko, D. M., and W. Gibson. 1986. Primate cytomegalovirus glycoproteins: lectin-binding properties and sensitivities to glycosidases. J. Virol. 59:703-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boname, J. M., and P. G. Stevenson. 2001. MHC class I ubiquitination by a viral PHD/LAP finger protein. Immunity 15:627-636. [DOI] [PubMed] [Google Scholar]
- 7.Bortz, E., J. P. Whitelegge, Q. Jia, Z. H. Zhou, J. P. Stewart, T. T. Wu, and R. Sun. 2003. Identification of proteins associated with murine gammaherpesvirus 68 virions. J. Virol. 77:13425-13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Lima, B. D., J. S. May, and P. G. Stevenson. 2004. Murine γ-herpesvirus-68 lacking gp150 shows defective virion release but establishes normal latency in vivo. J. Virol. 78:5103-5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Efstathiou, S., Y. M. Ho, and A. C. Minson. 1990. Cloning and molecular characterization of the murine herpesvirus 68 genome. J. Gen. Virol. 71:1355-1364. [DOI] [PubMed] [Google Scholar]
- 10.Flano, E., S. M. Husain, J. T. Sample, D. L. Woodland, and M. A. Blackman. 2000. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J. Immunol. 165:1074-1081. [DOI] [PubMed] [Google Scholar]
- 11.Fowler, P., S. Marques, J. P. Simas, and S. Efstathiou. 2003. ORF73 of murine herpesvirus-68 is critical for the establishment and maintenance of latency. J. Gen. Virol. 84: 3405-3416. [DOI] [PubMed] [Google Scholar]
- 12.Herrold, R. E., A. Marchini, S. Fruehling, and R. Longnecker. 1996. Glycoprotein 110, the Epstein-Barr virus homolog of herpes simplex virus glycoprotein B, is essential for Epstein-Barr virus replication in vivo. J. Virol. 70:2049-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klupp, B. G., N. Visser, and T. C. Mettenleiter. 1992. Identification and characterization of pseudorabies virus glycoprotein H. J. Virol. 66:3048-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Köhler, G., and C. Milstein. 1975. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495-497. [DOI] [PubMed] [Google Scholar]
- 15.Li, Q., S. M. Turk, and L. M. Hutt-Fletcher. 1995. The Epstein-Barr virus (EBV) BZLF2 gene product associates with the gH and gL homologs of EBV and carries an epitope critical to infection of B cells but not of epithelial cells. J. Virol. 69:3987-3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lomonte, P., P. Filee, J. R. Lyaku, M. Bublot, P. P. Pastoret, and E. Thiry.1997. Glycoprotein B of bovine herpesvirus 4 is a major component of the virion, unlike that of two other gammaherpesviruses, Epstein-Barr virus and murine gammaherpesvirus 68. J. Virol. 71:3332-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer, G. A., and K. D. Radsak. 2000. Identification of a novel signal sequence that targets transmembrane proteins to the nuclear envelope inner membrane. J. Biol. Chem. 275:3857-3866. [DOI] [PubMed] [Google Scholar]
- 18.Mori, Y., P. Akkapaiboon, X. Yang, and K. Yamanishi. 2003. The human herpesvirus 6 U100 gene product is the third component of the gH-gL glycoprotein complex on the viral envelope. J. Virol. 77:2452-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neuhierl, B., R. Feederle, W. Hammerschmidt, and H. J. Delecluse. 2002. Glycoprotein gp110 of Epstein-Barr virus determines viral tropism and efficiency of infection. Proc. Natl. Acad. Sci. USA 99:15036-15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reading, P. C., J. L. Miller, and E. M. Anders. 2000. Involvement of the mannose receptor in infection of macrophages by influenza virus. J. Virol. 74:5190-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spaete, R. R., R. M. Thayer, W. S. Probert, et al. 1988. Hum. cytomegalovirus strain Towne glycoprotein B is processed by proteolytic cleavage. Virology 167:207-225. [DOI] [PubMed] [Google Scholar]
- 22.Spear, P. G., and R. Longnecker. 2003. Herpesvirus Entry: an Update J. Virol. 77:10179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiller, O. B., C. L. Harris, and B. P. Morgan. 1999. Efficient generation of monoclonal antibodies against surface-expressed proteins by hyperexpression in rodent cells. J. Immunol. Methods 224:51-60. [DOI] [PubMed] [Google Scholar]
- 24.Stewart, J. P., N. J. Janjua, Sunil-Chandra, N. P., A. A. Nash, and J. R. Arrand. 1994. Characterization of murine gammaherpesvirus 68 glycoprotein B (gB) homolog: similarity to Epstein-Barr virus gB (gp110). J. Virol. 68:6496-6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stewart, J. P., N. J. Janjua, S. D. Pepper, G. Bennion, M. Mackett, T. Allen, A. A. Nash, and J. R. Arrand. 1996. Identification and characterization of murine gammaherpesvirus 68 gp150: a virion membrane glycoprotein. J. Virol. 70:3528-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sunil-Chandra, N. P., S. Efstathiou, and A. A. Nash. 1992. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J. Gen. Virol. 73:3275-3279. [DOI] [PubMed] [Google Scholar]
- 27.Sunil-Chandra, N. P., S. Efstathiou, J. Arno, and A. A. Nash. 1992. Virological and pathological features of mice infected with murine gamma-herpesvirus 68. J. Gen. Virol. 73:2347-2356. [DOI] [PubMed] [Google Scholar]
- 28.Tanner, J., J. Weis, D. Fearon, Y. Whang, and E. Kieff. 1987. Epstein-Barr virus gp350/220 binding to the B lymphocyte C3d receptor mediates adsorption, capping, and endocytosis. Cell 50:203-213. [DOI] [PubMed] [Google Scholar]
- 29.van Kooyk, Y., and T. B. Geijtenbeek. 2003. DC-SIGN: escape mechanism for pathogens. Nat. Rev. Immunol. 3:697-709. [DOI] [PubMed] [Google Scholar]
- 30.Vey, M., W. Schafer, B. Reis, et al. 1995. Proteolytic processing of human cytomegalovirus glycoprotein B (gpUL55) is mediated by the human endoprotease furin. Virology 206:746-749. [DOI] [PubMed] [Google Scholar]
- 31.Virgin, H. W., P. Latreille, P. Wamsley, K. Hallsworth, K. E. Weck, A. J. Dal Canto, and S. H. Speck. 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 71:5894-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang, F. Z., S. M. Akula, Sharma-Walia, N., L. Zeng, and B. Chandran. 2003. Hum. herpesvirus 8 envelope glycoprotein B mediates cell adhesion via its RGD sequence. J. Virol. 77:3131-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, X., S. M. Huong, M. L. Chiu, Raab- N. Traub, and E. S. Huang. 2003. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 424:456-461. [DOI] [PubMed] [Google Scholar]
- 34.Weck, K. E., S. S. Kim, H. W. Virgin, and S. H. Speck. 1999. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J. Virol. 73:3273-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]