Abstract
Functional activities that have been ascribed to the nef gene product of simian immunodeficiency virus (SIV) and human immunodeficiency virus (HIV) include CD4 downregulation, major histocompatibility complex (MHC) class I downregulation, downregulation of other plasma membrane proteins, and lymphocyte activation. Monkeys were infected experimentally with SIV containing difficult-to-revert mutations in nef that selectively eliminated MHC downregulation but not these other activities. Monkeys infected with these mutant forms of SIV exhibited higher levels of CD8+ T-cell responses 4 to 16 weeks postinfection than seen in monkeys infected with the parental wild-type virus. Furthermore, unusual compensatory mutations appeared by 16 to 32 weeks postinfection which restored some or all of the MHC-downregulating activity. These results indicate that nef does serve to limit the virus-specific CD8 cellular response of the host and that the ability to downregulate MHC class I contributes importantly to the totality of nef function.
nef is one of six auxiliary genes found in simian immunodeficiency virus (SIV) and human immunodeficiency virus (HIV). Unlike the gag, pol, and env genes, nef is not essential for the ability of virus to replicate. However, nef does contribute to the levels of persisting viral replication observed in infected monkeys and humans and to the propensity to cause disease. In contrast to infection by parental nef+ SIV, rhesus monkeys infected with nef-deleted forms of SIV typically maintain low or unmeasureable levels of postacute viremia, typically <200 copies of viral RNA per ml of plasma (4, 30). A minority of monkeys develop moderate postacute viral loads, and some progress to disease even in the absence of a nef gene (4, 8, 16). Studies in humans have been facilitated by the discovery of rare cases of infection by nef-deleted forms of HIV-1 (15, 31). Most of these humans have exhibited viral loads at or below the limits of detection for prolonged periods. However, some have developed moderate viral loads and some have exhibited declining CD4+ lymphocyte counts (22, 35). Thus, the relative importance of nef and the phenotypic effects of infection with nef-deleted virus appear to be similar in the SIV/rhesus monkey and HIV-1/human systems.
What is the function of Nef that is responsible for these phenotypic effects? The answer has unfortunately been clouded by the demonstration of a diverse array of activities associated with expression of this protein in vitro. These include downregulation of the CD4 receptor for virus entry (1, 18), downregulation of major histocompatibility complex (MHC) class I molecules from the surface of cells (47), downregulation of other surface transmembrane proteins (10, 55), and lymphocyte activation to facilitate viral replication (3, 9, 17, 49, 51). The Nef proteins of both SIV and HIV exhibit these properties. Downregulation of CD4 could minimize CD4's ability to interfere with the appearance or function of envelope on virions (34, 43); it could also help to prevent toxic effects associated with superinfection.
Downregulation of MHC class I molecules can make infected cells less susceptible to lysis by cytotoxic T lymphocytes, and it has been proposed that Nef-mediated downregulation of MHC class I is an important immune evasion mechanism employed by HIV and SIV (13, 62). However, because downregulation of MHC class I in vitro typically requires relatively high levels of Nef expression, some have questioned the physiologic relevance of the phenomenon (28, 62).
The ability of Nef to interact with the zeta chain of the T-cell receptor (10, 24) is responsible in whole or in part for Nef-mediated downregulation of CD3 (53). Concerted downregulation of CD3, CD4, and CD28 (55) as well as the ability of Nef to interact with cellular kinases (44, 45, 50) is probably related to alterations in a number of lymphocyte signaling pathways, T-cell receptor signaling in particular (3, 20, 46, 48, 55, 56, 58, 60). The effects of Nef on CD28, CD4, CD3, and MHC class I expression are all genetically separable by mutations (55).
Because replication of both SIV and HIV occurs maximally in lymphocytes that are activated, because most lymphocytes in the body are resting or minimally activated, and because Nef is an early gene product, early expression of Nef in a minimally activated cell in vivo may help to maximize the amounts of virus production by increasing the state of cellular activation. The recent discovery that SIVΔnef replication in lymphoid tissues is restricted to regions of highly activated T lymphocytes (52) is consistent with this logic. The interleukin-2-dependent 221 cell line has been used to reflect these lymphoid cell-activating properties of Nef because viral replication in these cells is heavily dependent upon Nef in the absence of interleukin-2 (3).
Nef has also been reported to induce Fas ligand, and this could serve to limit immune-mediated killing of infected cells by the induction of apoptosis in reactive cells responding to the sites of infection (6, 60, 61). Other activities have also been described for Nef (39, 56, 57, 59).
Here, we show that Nef does serve to limit the CD8+ cellular response to SIV and that the ability to downregulate MHC contributes importantly to the totality of Nef function.
MATERIALS AND METHODS
Site-specific mutagenesis.
Mutations were engineered with a splice overlap extension technique (23). PCR fragments containing the desired mutations were inserted into the pSIV-3′ vector (containing SIV nef sequences) with SacI and EcoRI restriction enzymes (New England Biolabs, Beverly, Mass.). Thorough DNA sequences of the resultant recombinant vectors verified the proper sequences for all mutants; recombinant clones selected for study contained the exactly desired sequence.
Virus stocks and monkey infection.
Stocks of SIV were prepared by transfection of CEMX174 cells with cloned DNA and harvest of the cell-free supernatant at or near the peak of virus production 6 to 10 days after transfection as previously described (27). Monkeys were infected by intravenous inoculation of SIV diluted in RPMI medium without serum to contain 50 ng of p27 per dose. The concentrations of p27 were determined by antigen capture with the Coulter kit according to the manufacturer's recommendations. Animals were screened for the presence of the Mamu-A*01 allele with a PCR single-site protocol as previously described (32).
Rhesus macaques (Macaca mulatta) were housed at the New England Primate Research Center in a centralized animal biolevel 3 containment facility in accordance with the standards of the Association for Assessment and Accreditation of Laboratory Animal Care and Harvard Medical School's Animal Care and Use Committee. Animals were tested and found free of simian retrovirus type D, simian T-lymphotropic virus 1, and herpes B virus before assignment to the experimental protocol. All procedures were conducted under ketamine anesthesia, and euthanasia of animals was performed when deemed necessary by the veterinary staff.
Viral loads.
Plasma viral load was determined with a real time reverse transcription-PCR procedure, essentially as described (36). As used in the current studies, the assay has a threshold of 100 copy equivalents/ml, with an interassay coefficient of variation of <25%.
Immunofluorescent staining and flow cytometry.
Monomers of biotinylated Mamu-A*01 class I MHC molecules complexed with the SIV Gag peptide CTPYDINQM (Gag181-189) were generated as previously described (5). Tetramers were prepared from purified monomers by the gradual addition over 24 h of allophycocyanin-streptavidin (Molecular Probes) to a 4:1 final molar ratio. Whole blood or peripheral blood mononuclear cells isolated by Ficoll-sodium diatrizoate centrifugation (Ficoll 1077; Sigma) were washed with phosphate-buffered saline containing 2% fetal bovine serum and incubated with conjugated antibodies and the Mamu-A*01/Gag181-189 tetramer for 30 min at 22°C. After staining, the cells were washed and resuspended in phosphate-buffered saline-2% fetal bovine serum. Antibodies used included fluorescein isothiocyanate-conjugated anti-human CD3 (SP34, Pharmingen, San Diego, Calif.), and peridinin chlorophyll protein-conjugated anti-human CD8 (BD Biosciences, Mountain View, Calif.). Simultest reagents were used as isotype controls (BD Biosciences). Analysis was performed with a FACSCalibur flow cytometer (Becton Dickinson). In general, a total of 200,000 events were acquired, and analysis of tetramer-staining cells was carried out on CD3+ CD8+ gated lymphocytes. Levels of Mamu-A*01/Gag181-189 staining in controls consisting of either Mamu-A*01-negative, SIV-infected animals or Mamu-A*01-positive, SIV-uninfected animals consistently yielded levels of background staining of 0.05% or less.
Isolation of genomic (cellular) DNA.
CEMx174 cells (5 × 106) infected with SIV recovered at weeks 16 and 32 postinfection were lysed, and the DNA was isolated according to the recommendations of the Qiagen QIAamp DNA blood minikit (Qiagen, Inc., Valencia, Calif.).
PCR amplification of nef sequences.
A total of 20 μl of each DNA preparation served as the template for PCR amplification. PCR primers flanking the nef locus of SIVmac239 were chosen for a first round of amplification: F13 (9161 to 9174), 5′-TATATTCATTTCCTGATCCGCCAAC-3′, and R16 (10151 to 10171), 5′-CCCCAGTACCTCCCCGTAACA-3′. The 100-μl volume reactions contained 10 μl of TaqPlus Long 10x high-salt buffer (Stratagene, La Jolla, Calif.), 5 μl of 4 mM deoxynucleoside triphosphate mix (Stratagene), 2 μl of each primer (100 pmol/μl), 1 μl of TaqPlus Long polymerase (5 U/μl) (Stratagene), and 60 μl of distilled sterile water. The PCR samples were heated at 95°C for 5 min, followed by 30 cycles of PCR amplification in a thermal cycler (MJ Research, Watertown, Mass.). Each cycle consisted of a 94°C denaturation step for 1 min, a 58°C annealing step for 1 min, and a 72°C polymerization and elongation step for 3 min, followed by a final elongation step at 72°C for 10 min.
A second round of PCR was employed in order to introduce Xbal and MluI restriction sites to facilitate cloning into the pCG7CG expression vector. The primers used to introduce the restriction sites were mac239Nef Xbal-5′ (5′-GCTCTAGACTAAAGATGGGTGGAGCTATTT CCATGA-3′) and mac239NefMluI-3′ (5′-ACGCGTCAGCGAGTTTCCTTCTTGTCA-3′). The conditions for the PCR were the same except that 1 μl of the first-round PCR product was used as a template. Additionally, the PCR protocol was adjusted to 68°C for 1 min for the annealing step.
Cloning.
PCR products were gel purified with the Qiagen QIAquick gel extraction kit (Qiagen, Inc.) and then cloned with a TA cloning kit (Invitrogen, Carlsbad, Calif.). Ligated products were transformed into Escherichia coli XL-2 Blue ultracompetent cells (Stratagene) and plated onto Luria-Bertani agar plates containing ampicillin (100 μg/ml), 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (40 μl of a 40-mg/ml stock solution), and isopropylthiogalactopyranoside (IPTG) (40 μl of a 100 mM stock solution).
Sequencing of nef clones.
White colonies were chosen and miniprep DNA was prepared with the Promega Wizard Plus Miniprep DNA purification system (Promega, Madison, Wis.). Individual clones containing nef inserts were sequenced across the entire insert on a Beckman Coulter CEQ 8000 genetic analysis system with the dye-labeled dideoxy terminator cycle sequencing kit as specified by the manufacturer (Beckman Coulter, Fullerton, Calif.). In addition to individual clone sequencing, the PCR product from each animal was sequenced with the recommendations of the dye-labeled dideoxy terminator cycle sequencing kit for sequencing PCR products (Beckman Coulter). All sequences were analyzed with Sequencher 4.1 software (GeneCodes Corp., Ann Arbor, Mich.).
Transient assays of receptor downregulation by Nef.
Open reading frames encoding mutant and variant Nef proteins were subcloned into the pCGCG bicistronic expression vector containing green fluorescent protein (GFP) under the translational control of the internal ribosome entry site (37). Jurkat T cells expressing high levels of human CD4 were maintained and transfected by electroporation as described previously (21, 37). After overnight culture, aliquots of 2 × 105 cells were reacted with saturating amounts of phycoerythrin-conjugated monoclonal antibody HIT3A (Becton Dickinson) specific for CD3-ɛ subunit, phycoerythrin-conjugated monoclonal antibody Leu-3A (Becton Dickinson) specific for CD4, phycoerythrin-conjugated monoclonal antibody W6/32 (Immunotech) specific for class I MHC complex, or phycoerythrin-conjugated monoclonal antibody CD28.2 (Pharmingen), specific for human CD28. Flow cytometry analyses for GFP and surface expression of CD4, CD3, CD28, and class I MHC were performed on Epics Elite or FACSCalibur flow cytometer as described previously (37, 54).
RESULTS
Properties of MHC-selective mutants.
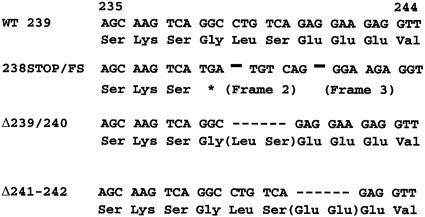
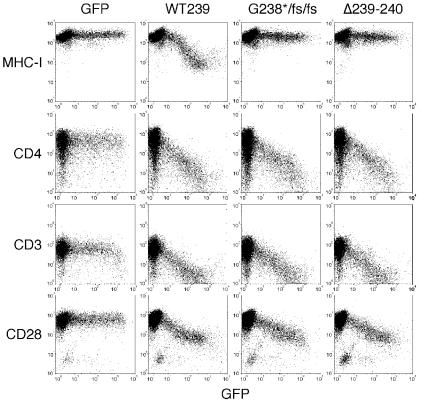
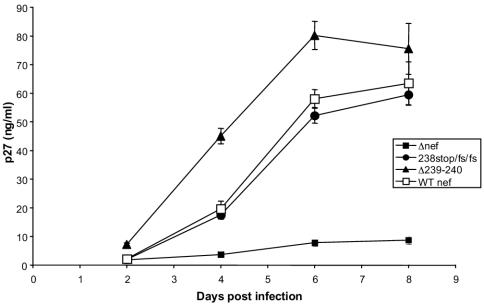
The sequences in SIV Nef that are required for MHC downregulation have been mapped to the C-terminal region (54). For the purpose of experimental monkey infection, we set out to make mutations that would be difficult to revert and that selectively affected MHC downregulation. Mutations that resulted in two-amino-acid deletions within SIV239 Nef residues 238 to 242 selectively affected the ability to downregulate MHC class I (Fig. 1 and 2). The mutant with deletion of Nef residues 239 and 240 (Δ239-240) was selected for testing in rhesus monkeys. NefΔ239-240 behaved like the wild-type parental Nef in the downregulation of CD4, CD3, and CD28 but was negative for any detectable downregulation of MHC class I (Fig. 2). SIV239 NefΔ239-240 also behaved like the parental SIV239 in its ability to replicate in 221 cells in the absence of interleukin-2 (Fig. 3). The mutant form of SIV239 Nef with a stop signal at codon 238 followed by two frameshift (fs) mutations (Fig. 1) behaved like NefΔ239-240 in the in vitro assays (Fig. 2 and 3). We therefore forwarded one deletion mutant (SIV239 NefΔ239-240) and the truncation mutant (SIV239 Nef238stop/fs/fs) for testing in rhesus monkeys.
FIG. 1.
Difficult-to-revert mutations in nef that selectively eliminate ability to downregulate MHC class I molecules. WT 239 refers to the nef sequence present in the wild-type parental SIV239 between Nef amino acid residues 235 and 244. Δ239-240 and Δ241-242 are Nef variants deleted at residues 239 to 240 and 241 to 242, respectively. 238stop/fs/fs is a Nef variant with a stop codon introduced at residue 238 followed by two downstream frameshift mutations.
FIG. 2.
Selective disruption of class I MHC downregulation by difficult-to-revert mutations in SIVmac239 Nef. Jurkat T cells were transiently transfected with pCGCG bicistronic vectors expressing the green fluorescent protein (GFP) marker alone or coexpressing green fluorescent protein and wild-type (wt 239) or mutant Nef proteins (238stop/fs/fs, Δ239-240, and Δ239-241). CD28, CD4, CD3-ɛ, and CD28 class I MHC cell surface expression and green fluorescent protein fluorescence were quantitated simultaneously by flow cytometry.
FIG. 3.
SIV239 with MHC-selective mutations replicates like wild-type parental SIV239 in 221 cells in the absence of interleukin-2. SIV containing 10 ng of p27 was used to infect 221 cells in the presence of 5% serum without interleukin-2 as previously described (4). Virus production in the cell-free supernatant was monitored on the indicated days by assay of levels of p27 antigen. Error bars are based on quadruplicate measurements.
Phenotype of rhesus monkey infection.
Two rhesus monkeys were inoculated with normalized doses of SIV239 NefΔ239-240 and two were inoculated with SIV239 Nef238stop/fs/fs. As controls, two monkeys were similarly inoculated at the same time with the parental SIV239 wild type and two were inoculated with SIV239Δnef. SIV239Δnef has a 181-bp deletion in nef beginning at nucleotide 175 of the nef coding sequence that abrogates all functional activities of Nef. To facilitate quantitation of CD8+ T-cell responses with MHC tetramers, all eight of these monkeys expressed the Mamu A*01 MHC class I allele.
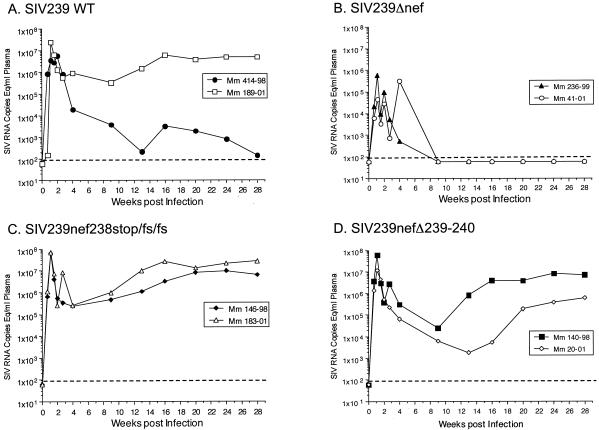
The levels of virion-associated SIV RNA in plasma were monitored in blood samples taken at various times after the inoculations (Fig. 4). In the monkeys that received wild-type SIV239, plasma RNA levels peaked at day 14 (5,600,000 per ml in animal 414-98) and at day 8 (24,000,000 per ml in animal 189-01). The average of 14.8 × 106 RNA copies per ml at peak in these two animals is quite close to the average seen previously in 10 animals (40 × 106 RNA copies per ml) (29). One of the control animals infected with wild-type SIV239 (189-01) exhibited postacute viral loads (≈3 × 106 copies per ml) at 12 to 30 weeks postinfection that were very similar to what we typically observed previously (29). However, the other monkey that received wild-type SIV239 (414-98) was unusual in that it exhibited postacute viral loads in the range of 300 to 3,000 copies per ml (Fig. 4). The two monkeys that received SIV239Δnef were similar to other such animals that we have studied previously in that viral loads at peak height (day 8) were on average 50-fold lower than those seen in animals inoculated with wild-type SIV239. Viral loads at set point in the two monkeys infected with SIV239Δnef were also typical in that they were near or below the limit of detection (Fig. 4).
FIG. 4.
Viral loads in monkeys infected with wild-type (WT) SIV239 or SIV239 mutated in Nef. Dashed lines indicate the threshold sensitivity of the assay (100 copies/ml). Mm, M. mulatta.
Viral loads at peak height in the four animals that received the MHC-selective mutants were indistinguishable from those seen in the wild-type SIV239-infected animals (Fig. 4). They all peaked at day 8 with an average of 52 × 106 copies/ml (range, 12× 106 to 70 × 106 copies per ml). Thus, there appeared to be no inherent defect in the ability of these mutated strains to replicate in monkeys. The four monkeys that received the MHC-selective nef mutants appeared to exhibit lower viral loads 4 to 14 weeks postinfection than the viral loads seen in SIV239-infected control monkey 189-01 over this time range. However, the differences were not statistically significant when compared to the two Mamu A*01-positive control monkeys used in this study. When viral loads from four additional wild-type-infected Mamu A*01+ monkeys from previous studies were included in the analysis, statistically significant differences in viral loads were again not observed at any time point compared to the viral loads in the four monkeys that received the MHC-selective nef mutants.
Immune responses in infected rhesus monkeys.
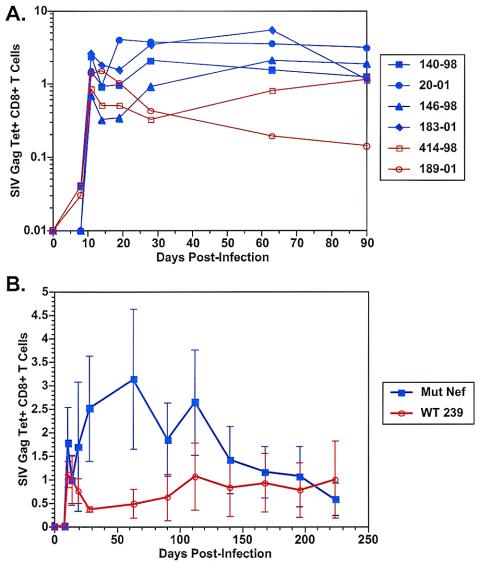
The ability of CD8+ T lymphocytes from infected monkeys to react with antigenic peptide and MHC I tetramer corresponding to the Mamu-A*01-restricted Gag181-189 epitope CTPYDINQM was monitored. The four monkeys infected with the SIV strains carrying the MHC-selective nef mutations exhibited higher levels of CD8+ cellular responses than the two monkeys infected with the parental wild-type SIV239 at 4 to 14 weeks after infection (Fig. 5A and B) (P = 0.06 by Mann-Whitney U test at weeks 4 and 9). Differences became less discernible by 16 to 20 weeks postinfection. To increase the power of the analyses, we separately included tetramer data from two other Mamu-A*01+, SIV239-infected monkeys from another study for which such data were available. Again, all four monkeys infected with the SIV strains with MHC-selective nef mutations made Gag tetramer responses 4 to 14 weeks after infection that were consistently higher than those seen in the four wild-type SIV239-infected monkeys. These differences were statistically significant (P = 0.02, 0.04, and 0.02 at weeks 4, 8 to 9, and 13 to 14 after infection, respectively, by Mann-Whitney U test).
FIG. 5.
Viral Gag-specific CD8+ cellular responses measured by Gag tetramer staining. (A) Results for individual animals. (B) Averages from the four monkeys infected with MHC-selective SIV mutants versus averages from the two control monkeys infected with parental SIV239. Numbers refer to the percentage of CD3+ CD8+ Gag181-189 tetramer-positive cells.
Sequence evolution.
We next charted the evolution of sequence changes in nef over time. nef sequences present at 16 and 32 weeks postinfection were amplified by PCR of DNA from cells infected with recovered virus. Direct sequencing of the amplified products gave a sense of the predominant amino acid present at each location, and sequencing of individual clones gave a sense of the range of variation within each population at those times. Little variation was observed in the week 16 samples from the two control animals infected with wild-type virus; two positions of amino acid variation were observed in the bulk population in monkey 414-98 at that time, and none were observed in monkey 189-01.
In contrast, greater levels of variation were observed in the monkeys infected with the MHC-selective mutants at 16 weeks. All four of these monkeys retained their original NefΔ239-240 or Nef238stop/fs/fs mutation, which is not surprising given the expected difficulty in generating revertants of these mutations. However, these four monkeys exhibited three to six predicted amino acid variations per nef gene in the sequence of the bulk population and three to eleven predicted amino acid variations in individual clones. Sequence changes noted in the bulk population usually appeared in the individual clones. Examples of predicted amino acid sequence changes in individual clones from the four animals are shown in Fig. 6. The two monkeys infected with SIV239 NefΔ239-240 (140-98 and 20-01) showed a clustering of amino acid changes within the region spanning residues 11 to 36, and the two monkeys infected with SIV239 Nef238stop/fs/fs (146-98 and 183-01) showed a clustering of amino acid changes within the region spanning residues 101 to 112 at week 16 (Fig. 6).
FIG. 6.
Sequence changes in Nef 16 and 32 weeks after infection. Circles identify the locations of amino acids that correspond to the HIV-1 consensus amino acid.
One of the striking features of the nef sequence variation in the four animals infected with the MHC-selective mutants was that many of the variations changed the amino acid to that observed in the consensus sequence for the Nef protein of HIV-1. More than 50% of the amino acid changes in Nef observed at 16 weeks changed the amino acid to the HIV-1 Nef consensus at this residue. These included E36K, S101P, S112T, S161P, E190K, and Y229H. The S101P change created an additional PXXP motif, as is present in most HIV-1 Nef sequences and absent in all SIVmac and SIVsm sequences (33).
By 32 weeks, the nef sequences had evolved even further in the monkeys infected with the MHC-selective mutants. One monkey, 140-98, had to be sacrificed because of deteriorating health prior to 32 weeks, but nef sequences were obtained from recovered SIV from the other three animals at 32 weeks. Again, all clones retained the original NefΔ239-240 or Nef238stop/fs/fs mutation from the appropriate animals. Week 32 clones exhibited 7 to 12 amino acid changes per nef gene in various clones. Certain signature changes present at both weeks 16 and 32 in the same animal confirmed the validity of the sequence determinations. For example, S161P was observed in the majority of clones at weeks 16 and 32 in monkey 146-98 but was not observed in other animals. E36K was observed with reasonable frequency in both week 16 and week 32 clones from monkey 20-01, but this change was not observed in other animals. S112T was observed in the majority of clones at weeks 16 and 32 in monkey 183-01, was observed in animal 146-98 only at week 32, and was not observed in the other animals. K105R and Y229H predominated in monkeys 146-98 and 183-01 at both 16 and 32 weeks but did not appear in monkey 20-01 until 32 weeks.
Thus, a pattern of sequence changes was observed that showed some commonality among all animals infected with the MHC-selective nef mutants but even more striking commonality based on what the individual animal had received for the SIV inoculum. Thus, common changes such as S101P and Y229H occurred in all four animals infected with the MHC-selective mutants, and other signature changes arose specifically with NefΔ239-240 or with Nef238stop/fs/fs. Amino acid substitutions in Nef in these four animals accumulated at an astonishing rate of about 7% per year, an extremely unusual high rate (12, 26).
Sequence changes restore ability to downregulate MHC class I.
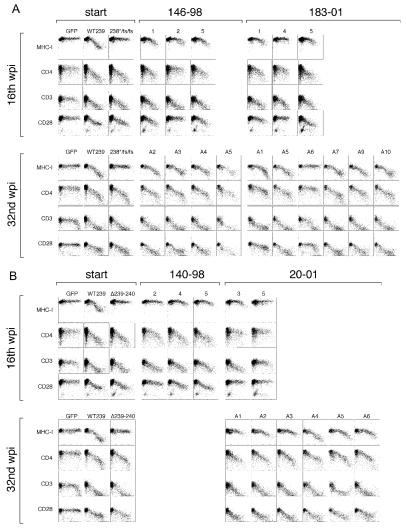
Full-length nef clones obtained at 16 and 32 weeks were inserted into the expression vector and used in downregulation assays. Of 11 nef clones obtained at week 16 from the four monkeys, 8 showed various levels of MHC-downregulating activity (Fig. 7). None of these eight showed MHC-downregulating activity as strong as that of the parental SIV239 Nef. The five nef clones obtained at week 16 from the two monkeys infected with SIV239 Nef238stop/fs/fs (monkeys 146-98 and 183-01) in particular showed a consistent, intermediate gain-of-function phenotype. Of 16 nef clones analyzed from week 32, 15 showed readily detectable MHC-downregulating activity (Fig. 7). There was a clear progression from week 16 to week 32 in the ability to restore MHC-downregulating activity. Eight of the 16 nef clones from week 32 showed MHC-downregulating activity that was indistinguishable from or only slightly less than that observed with the parental SIV239 Nef. Four of the ten nef clones obtained at week 16 showed loss of CD28 downregulating activity, but by week 32 14 of 16 clones analyzed exhibited CD28-downregulating activity that was comparable to that of the parental SIV239 Nef. The vast majority of clones at both 16 and 32 weeks exhibited CD4- and CD3-downregulating activity that was indistinguishable from or only slightly less than that observed with the parental SIV239 Nef.
FIG. 7.
Variant Nef sequences at 16 and 32 weeks postinfection exhibit restoration or partial restoration of the ability to downregulate MHC class I. Analysis of sequences recovered from monkeys that received SIVmac239 carrying the Nef238stop/fs/fs (A) or the NefΔ239-240 (B) mutant. Analysis was performed as described in the legend to Fig. 2.
DISCUSSION
A surprising number of functional activities have been observed for Nef. Lymphoid cell activation and downregulation of CD4, CD3, CD28, and MHC class I have been assayed in our current experiments. In addition, infectivity enhancement and induction of Fas ligand have also been described as functional activities (40, 61). Nef has also been reported to induce lymphocyte chemotaxis toward infected macrophages (57) and to inhibit proapoptotic signaling in infected cells (19). Infectivity enhancement may relate in whole or in part to CD4 downregulation (34, 43) and increased cellular activation (3, 20, 46). Induction of Fas ligand has been suggested to increase apoptotic death of reactive cells trying to respond to infection (60).
The goal of our current studies was to obtain evidence for the contribution of one activity of this multitude, MHC class I downregulation, to the ability of SIV to replicate in rhesus monkeys. Although the MHC-selective NefΔ239-240 and Nef238stop/fs/fs mutations had no effect on lymphoid cell activation in the 221 cell assay or on downregulation of CD4, CD3, or CD28, we do not know whether these mutations affected other known or unknown activities. Nonetheless, the stronger CD8 responses in mutant-infected animals and the strong selection for unusual compensatory sequence changes that restored MHC-downregulating activity provide convincing evidence for a contribution of this activity to the persistent replication of SIV in rhesus monkeys. Although a number of other viruses encode gene products that interfere with MHC expression (7, 11, 14, 25, 42, 63), we are aware of only one other analogous report in which a functional role in immune evasion has been validated in an animal system as we have done here. Stevenson et al. (51a) knocked out the class I MHC-downregulating K3 gene from murine gammaherpesvirus 68 and found stronger virus-specific CD8+ T-cell responses and lower viral loads in mice.
Mutations became fixed in the population of Nef sequences in monkeys infected with the MHC-selective mutants at an astonishing rate, resulting in about 7% amino acid changes per year. Even the highly variable gp120 envelope protein accumulates sequence changes at a rate of only about 2% per year (12, 26), more than three times less than the rate seen in Nef in the monkeys infected with the MHC-selective mutants in the current study. This suggests strong selective pressure for the appearance of sequence variants and strong selective advantage of the mutant forms that became fixed in the population. In a previous study (41), a simple point mutation that eliminated MHC-downregulating activity reverted within a few weeks of monkey infection. In all clones analyzed at 16 and 32 weeks after infection in our current study, the difficult-to-revert NefΔ239-240 and Nef 238stop/fs/fs mutations were retained, forcing the virus to try to compensate by changing residues elsewhere in the Nef protein.
The changes in Nef progressively increased the ability to downregulate MHC from weeks 0 to 16 to 32 such that by week 32 the ability to downregulate MHC class I was similar to that of the parental SIV239 Nef. Based on the large number of sequence changes and the time required to restore full MHC-downregulating activity, the virus was clearly under strong selective pressure over a prolonged period to restore the activity; this provides unambiguous evidence for the contribution of MHC downregulation to the ability of SIV to replicate in monkeys. The ability to restore the activity by sequence changes quite distant in the linear sequence is testimony to the remarkable flexibility and multiplicity built into the Nef protein structure.
Surprisingly, despite similar functional activities and about 30% sequence identity at the amino acid level, HIV-1 nef differs from SIVmac nef in the location of mutations that will selectively knock out the ability to downregulate MHC class I (54). Several groups have shown that mutation of HIV-1 nef in the vicinity of the coding sequences for PXXPXXPXXP, corresponding to residues 101 to 110 in Fig. 6, will selectively knock out the ability to downregulate MHC class I (21, 38, 54). In contrast, Swigut et al. (56) and our data here show that MHC-selective mutations map to the C-terminal region of SIV239 Nef. Minimal amino acid changes that restored MHC-downregulating activity to NefΔ239-240 and Nef238stop/fs/fs are interestingly located in the same general vicinity in the Nef linear sequence as the MHC-selective mutations in HIV-1 Nef. In fact, the S101P change seen in SIV Nef in all four animals in our current study creates a PXXPXXP sequence that in HIV-1 is essential for HIV-1 Nef's ability to downregulate MHC class I. Amazingly, more than 50% of the amino acid changes in SIV Nef in the monkeys infected with SIV239 NefΔ239-240 and SIV239 Nef238stop/fs/fs resulted in the consensus HIV-1 amino acid at that location. This could be viewed as an accelerated, partial recapitulation of the evolution that occurred naturally over millennia in the emergence of HIV-1. A converse evolution of HIV-1 to SIV-like nef sequences was observed previously in monkeys infected with a recombinant SIV containing an HIV-1 nef gene (SHIVnef) (2).
CD8+ T-cell responses were significantly higher in monkeys that received the MHC-selective mutant SIVs than in monkeys that received parental SIV239, from 4 to 14 weeks postinfection. It seems likely that the evolution of sequence variants with restored or partially restored MHC-downregulating activity was responsible for decreased ability to discern effects on the number of virus-specific CD8 cells at later time points. Stronger CD8+ antiviral responses would be expected to translate into lower viral loads, but we were unable to demonstrate any statistically significant differences in viral loads among the groups, excluding of course the SIVΔnef animals. It seems likely that the evolution of restored MHC-downregulating activity minimized the magnitude and duration of any effects of the mutations on viral load. Other factors may also have contributed. The number of monkeys employed was necessarily limited, and significant differences may have been observed with much larger numbers of monkeys.
A controlling immune response is likely to consist of multifactorial components; the effects of the MHC activity of Nef influenced the levels of only one of these multifactorial components, virus-specific CD8+ cellular responses. SIV destruction of virus-specific CD4+ helper cells may have limited the effectiveness of increased numbers of virus-specific CD8+ cells that resulted from the loss of MHC-downregulating activity. Nonetheless, although we were not able to demonstrate significant differences in viral load, the selective pressure for sequence change to restore MHC-downregulating activity clearly indicates that the absence of this activity did serve to limit the replication of the virus at least somewhat.
A reasonable portion of the nef clones at 16 weeks lost the ability to downregulate CD28, but by 32 weeks the vast majority of clones behaved like the wild type for both CD28 and MHC downregulation. This result suggests that some of the sequence evolution between 16 and 32 weeks resulted in restoration of CD28-downregulating activity and further suggests that downregulation of CD28 is also a biologically relevant functional activity of Nef.
The large number of functional activities assigned to Nef has raised doubts about whether all are relevant. Our results validate MHC downregulation as a biologically relevant functional activity of Nef. It should be possible to use the SIV/rhesus monkey system as we have done here to validate the relative importance of other functional activities of Nef. Also, vaccine strategies that employ Nef expression as one component of the immunogen should modify the nef gene in such a way as to eliminate the ability to downregulate MHC class I.
Acknowledgments
We thank Hannah Sanford and Jackie Gillis for technical assistance and John Altman for providing the SIV Gag181-189 Mamu A*01 tetramers.
This work was supported by PHS grants RR00168 (NEPRC), AI25328 (R.C.D.), AI35365 (R.C.D.), AI45314 (R.P.J.) and AI42561 (J.S.). This work was also funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract NO1-CO-124000 (J.L.). T.S. is a Charles H. Revson Fellow.
REFERENCES
- 1.Aiken, C., J. Konner, N. Landau, M. E. Lenburg, and D. Trono. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76:853-864. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, L., Z. Du, A. Y. Howe, S. Czajak, and R. C. Desrosiers. 1999. Induction of AIDS in rhesus monkeys by a recombinant simian immunodeficiency virus expressing nef of human immunodeficiency virus type 1. J. Virol. 73:5814-5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander, L., Z. Du, M. Rosenzweig, J. U. Jung, and R. C. Desrosiers. 1997. A role for natural simian immunodeficiency virus and human immunodeficiency virus type 1 nef alleles in lymphocyte activation. J. Virol. 71:6094-6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander, L., P. O. Illyinskii, S. M. Lang, R. E. Means, J. Lifson, K. Mansfield, and R. C. Desrosiers. 2003. Determinants of increased replicative capacity of serially passaged simian immunodeficiency virus with nef deleted in rhesus monkeys. J. Virol. 77:6823-6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen, T. M., T. U. Vogel, D. H. Fuller, B. R. Mothe, S. Steffen, J. E. Boyson, T. Shipley, J. Fuller, T. Hanke, A. Sette, J. D. Altman, B. Moss, A. J. McMichael, and D. I. Watkins. 2000. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J. Immunol. 164:4968-4978. [DOI] [PubMed] [Google Scholar]
- 6.Ameisen, J. C. 2001. Apoptosis subversion: HIV-Nef provides both armor and sword. Nat. Med. 7:1181-1182. [DOI] [PubMed] [Google Scholar]
- 7.Andersson, M., S. Paabo, T. Nilsson, and P. A. Peterson. 1985. Impaired intracellular transport of class 1 MHC antigens as a possible means for adenoviruses to evade immune surveillance. Cell 43:215-222. [DOI] [PubMed] [Google Scholar]
- 8.Baba, T. W., V. Liska, A. H. Khimani, N. B. Ray, P. J. Dailey, D. Penninck, R. Bronson, M. F. Greene, H. M. McClure, L. N. Martin, and R. M. Ruprecht. 1999. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat. Med. 5:194-203. [DOI] [PubMed] [Google Scholar]
- 9.Baur, A. S., E. T. Sawai, P. Dazin, W. J. Fanti, C. Cheng-Mayer, and B. M. Peterlin. 1994. HIV-1 nef leads to inhibition or activation of T cells depending on its intracellular localization. Immunity 1:373-384. [DOI] [PubMed] [Google Scholar]
- 10.Bell, I., C. Ashman, J. Maughan, E. Hooker, F. Cook, and T. A. Reinhart. 1998. Association of simian immunodeficiency virus Nef with the T-cell receptor (TCR) zeta chain leads to TCR down-modulation. J. Gen. Virol. 72:2717-2727. [DOI] [PubMed] [Google Scholar]
- 11.Burgert, H.-G., and S. Kvist. 1985. An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class 1 antigens. Cell 41:987-997. [DOI] [PubMed] [Google Scholar]
- 12.Burns, D. P. W., and R. C. Desrosiers. 1991. Selection of genetic variants of simian immunodeficiency virus in persistently infected rhesus monkeys. J. Virol. 65:1843-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins, K. L., B. K. Chen, S. A. Kalams, B. D. Walker, and D. Baltimore. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391:397-401. [DOI] [PubMed] [Google Scholar]
- 14.Coscoy, L., and D. Ganem. 2000. Kaposi's sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc. Natl. Acad. Sci. USA 97:8051-8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. McPhee, A. L. Greenway, A. Ellett, C. Chatfield, V. A. Lawson, S. Crowe, A. Maerz, S. Sonza, J. Learmont, J. S. Sullivan, A. Cunningham, D. Dwyer, D. Downton, and J. Mills. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270:988-991. [DOI] [PubMed] [Google Scholar]
- 16.Desrosiers, R. C. 1994. Safety issues facing development of a live-attenuated, multiply deleted HIV-1 vaccine. AIDS Res. Hum. Retroviruses 10:331-332. [DOI] [PubMed] [Google Scholar]
- 17.Du, Z., S. M. Lang, V. G. Sasseville, A. A. Lackner, P. O. Ilyinskii, M. D. Daniel, J. U. Jung, and R. C. Desrosiers. 1995. Identification of a nef allele that causes lymphocyte activation and acute disease in macaque monkeys. Cell 82:665-674. [DOI] [PubMed] [Google Scholar]
- 18.Garcia, J. V., and A. D. Miller. 1991. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature 350:508-511. [DOI] [PubMed] [Google Scholar]
- 19.Geleziunas, R., W. Xu, K. Takeda, H. Ichijo, and W. C. Greene. 2001. HIV-1 Nef inhibits ASK1-dependent death signalling providing a potential mechanism for protecting the infected host cell. Nature 410:834-838. [DOI] [PubMed] [Google Scholar]
- 20.Glushakova, S., J. C. Grivel, K. Suryanarayana, P. Meylan, J. D. Lifson, R. Desrosiers, and L. Margolis. 1999. Nef enhances human immunodeficiency virus replication and responsiveness to interleukin-2 in human lymphoid tissue ex vivo. J. Virol. 73:3968-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenberg, M. E., A. J. Iafrate, and J. Skowronski. 1998. The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J. 17:2777-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenough, T. C., J. L. Sullivan, and R. C. Desrosiers. 1999. Declining CD4 T-cell counts in a person infected with nef-deleted HIV-1. N. Engl. J. Med. 340:236-237. [DOI] [PubMed] [Google Scholar]
- 23.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51-59. [DOI] [PubMed] [Google Scholar]
- 24.Howe, A. Y. M., J. U. Jung, and R. C. Desrosiers. 1998. Zeta chain of the T-cell receptor interacts with Nef of simian immunodeficiency virus and human Immunodeficiency virus type 2. J. Virol. 72:9827-9834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishido, S., C. Wang, B. S. Lee, G. B. Cohen, and J. U. Jung. 2000. Downregulation of major histocompatibility complex class I molecules by Kaposi's sarcoma-associated herpesvirus K3 and K5 proteins. J. Virol. 74:5300-5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson, P. R., T. E. Hamm, S. Goldstein, S. Kitov, and V. M. Hirsch. 1991. The genetic fate of molecularly cloned simian immunodeficiency virus in experimentally infected macaques. Virology 185:217-228. [DOI] [PubMed] [Google Scholar]
- 27.Johnson, R. P., J. D. Lifson, S. C. Czajak, K. S. Cole, K. H. Manson, R. Glickman, J. Yang, D. C. Montefiori, R. Montelaro, M. Wyand, and R. C. Desrosiers. 1999. Highly attenuated vaccine strains of simian immunodeficiency virus protect against vaginal challenge: inverse relation of degree of protection with level of attenuation. J. Virol. 73:4952-4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson, W. E., and R. C. Desrosiers. 2002. Viral persistance: HIV's strategies of immune system evasion. Annu. Rev. Med. 53:499-518. [DOI] [PubMed] [Google Scholar]
- 29.Johnson, W. E., J. D. Lifson, S. M. Lang, R. P. Johnson, and R. C. Desrosiers. 2003. Importance of B-cell responses for immunological control of variant strains of simian immunodeficiency virus. J. Virol. 77:375-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for the development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 31.Kirchhoff, F., T. C. Greenough, D. B. Brettler, J. L. Sullivan, and R. C. Desrosiers. 1995. Absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 332:228-232. [DOI] [PubMed] [Google Scholar]
- 32.Knapp, L. A., E. Lehmann, M. S. Piekarczyk, J. A. Urvater, and D. I. Watkins. 1997. A high frequency of Mamu-A*01 in the rhesus macaque detected by polymerase chain reaction with sequence-specific primers and direct sequencing. Tissue Antigens 50:657-661. [DOI] [PubMed] [Google Scholar]
- 33.Kuiken, C., B. Foley, B. Hahn, P. Marx, F. McCutchan, J. Mellors, S. Wolinsky, and B. Korber. 2001. HIV sequence compendium. Los Alamos National Laboratory, Los Alamos, N.Mex.
- 34.Lama, J., A. Mangasarian, and D. Trono. 1999. Cell-surface expression of CD4 reduces HIV-1 infectivity by blocking Env incorporation in a Nef- and Vpu-inhibitable manner. Curr. Biol. 9:622-631. [DOI] [PubMed] [Google Scholar]
- 35.Learmont, J. C., A. F. Geczy, J. Mills, L. J. Ashton, C. H. Raynes-Greenow, R. J. Garsia, W. B. Dyer, L. McIntyre, R. B. Oelrichs, D. I. Rhodes, N. J. Deacon, and J. S. Sullivan. 1999. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1. A report from the Sydney Blood Bank Cohort. N. Engl. J. Med. 340:1715-1722. [DOI] [PubMed] [Google Scholar]
- 36.Lifson, J. D., J. L. Rossio, M. Piatak, Jr., T. Parks, L. Li, R. Kiser, V. Coalter, B. Fisher, B. M. Flynn, S. Czajak, V. M. Hirsch, K. A. Reimann, J. E. Schmitz, J. Ghrayeb, N. Bischofberger, M. A. Nowak, R. C. Desrosiers, and D. Wodarz. 2001. Role of CD8+ lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J. Virol. 75:10187-10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lock, M., M. E. Greenberg, A. J. Iafrate, T. Swigut, J. Muench, F. Kirchhoff, N. Shohdy, and J. Skowronski. 1999. Two elements target SIV Nef to the AP-2 clathrin adaptor complex, but only one is required for the induction of CD4 endocytosis. EMBO J. 18:2722-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mangasarian, A., V. Piguet, J. K. Wang, Y. L. Chen, and D. Trono. 1999. Nef-induced CD4 and major histocompatibility complex class I (MHC-I) down-regulation are governed by distinct determinants: N-terminal alpha helix and proline repeat of Nef selectively regulate MHC-I trafficking. J. Virol. 73:1964-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Messmer, D., J. Bromberg, G. Devgan, J. M. Jacque, A. Granelli-Piperno, and M. Pope. 2002. Hum. immunodeficiency virus type 1 Nef mediates activation of STAT3 in immature dendritic cells. AIDS Res. Hum. Retroviruses 18:1043-1050. [DOI] [PubMed] [Google Scholar]
- 40.Miller, M. D., M. T. Warmerdam, K. A. Page, M. B. Feinberg, and W. C. Greene. 1995. Expression of the human immunodeficiency virus type 1 (HIV-1) nef gene during HIV-1 production increases progeny particle infectivity independently of gp160 or viral entry. J. Virol. 69:579-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munch, J., N. Stolte, D. Fuchs, C. Stahl-Hennig, and F. Kirchhoff. 2001. Efficient class I major histocompatibility complex down-regulation by simian immunodeficiency virus Nef is associated with a strong selective advantage in infected rhesus macaques. J. Virol. 75:10532-10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ploegh, H. L. 1998. Viral strategies of immune evasion. Science 280:248-253. [DOI] [PubMed] [Google Scholar]
- 43.Ross, T. M., A. E. Oran, and B. R. Cullen. 1999. Inhibition of HIV-1 progeny virion release by cell-surface CD4 is relieved by expression of the viral Nef protein. Curr. Biol. 9:613-621. [DOI] [PubMed] [Google Scholar]
- 44.Saksela, K., G. Cheng, and D. Baltimore. 1995. Proline-rich (PxxP) motifs in HIV-1 nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of nef+ viruses but not for down-regulation of CD4. EMBO J. 14:484-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sawai, E. T., A. Baur, H. Struble, B. M. Peterlin, J. A. Levy, and C. Cheng-Mayer. 1994. Human immunodeficiency virus type 1 Nef associates with a cellular serine kinase in T lymphocytes. Proc. Natl. Acad. Sci. USA 91:1539-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schrager, J. A., and J. W. Marsh. 1999. HIV-1 Nef increases T-cell activation in a stimulus-dependent manner. Proc. Natl. Acad. Sci. USA 96:8167-8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwartz, O., V. Marechal, S. LeGall, F. Lemonnier, and J.-M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 nef protein. Nat. Med. 2:338-342. [DOI] [PubMed] [Google Scholar]
- 48.Simmons, A., V. Aluvihare, and A. McMichael. 2001. Nef triggers a transcriptional program in T cells imitating single-signal T-cell activation and inducing HIV virulence mediators. Immunity 14:763-777. [DOI] [PubMed] [Google Scholar]
- 49.Skowronski, J., D. Parks, and R. Mariani. 1993. Altered T-cell activation and development in transgenic mice expressing the HIV-1 nef gene. EMBO J. 12:703-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith, B. L., B. W. Krushelnycky, D. Mochly-Rosen, and P. Berg. 1996. The HIV nef protein associates with protein kinase C theta. J. Biol. Chem. 271:16753-16757. [DOI] [PubMed] [Google Scholar]
- 51.Spina, C. A., T. J. Kwoh, M. Y. Chowers, J. C. Guatelli, and D. D. Richman. 1994. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J. Exp. Med. 179:115-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51a.Stevenson, P. G., J. S. May, X. G. Smith, S. Marques, H. Adler, U. H. Koszinowski, J. P. Simas, and S. Efstathiou. 2003. K3-mediated evasion of CD8(+) T cells aids amplification of a latent gamma-herpesvirus. Nat. Immunol. 3:733-740. [DOI] [PubMed] [Google Scholar]
- 52.Sugimoto, C., K. Tadakuma, I. Otani, T. Moritoyo, H. Akari, F. Ono, Y. Yoshikawa, T. Sata, S. Izumo, and K. Mori. 2003. nef gene is required for robust productive infection by simian immunodeficiency virus of T-cell-rich paracortex in lymph nodes. J. Virol. 77:4169-4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swigut, T., M. Greenberg, and J. Skowronski. 2003. Cooperative interactions of simian immunodeficiency virus Nef, AP-2, and CD3-zeta mediate the selective induction of T-cell receptor-CD3 endocytosis. J. Virol. 77:8116-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swigut, T., A. J. Iafrate, J. Muench, F. Kirchhoff, and J. Skowronski. 2000. Simian and human immunodeficiency virus Nef proteins use different surfaces to downregulate class I major histocompatibility complex antigen expression. J. Virol. 74:5691-5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swigut, T., N. Shohdy, and J. Skowronski. 2001. Mechanism for down-regulation of CD28 by Nef. EMBO J. 20:1593-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Swingler, S., B. Brichacek, J. M. Jacque, C. Ulich, J. Zhou, and M. Stevenson. 2003. HIV-1 Nef intersects the macrophage CD40L signalling pathway to promote resting-cell infection. Nature 424:213-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swingler, S., A. Mann, J. Jacque, B. Brichacek, V. G. Sasseville, K. Williams, A. A. Lackner, E. N. Janoff, R. Wang, D. Fisher, and M. Stevenson. 1999. HIV-1 Nef mediates lymphocyte chemotaxis and activation by infected macrophages. Nat. Med. 5:997-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang, J. K., E. Kiyokawa, E. Verdin, and D. Trono. 2000. The Nef protein of HIV-1 associates with rafts and primes T cells for activation. Proc. Natl. Acad. Sci. USA 97:394-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolf, D., V. Witte, B. Laffert, K. Blume, E. Stromer, S. Trapp, P. d'Aloja, A. Schurmann, and A. S. Baur. 2001. HIV-1 Nef associated PAK and PI3-kinases stimulate Akt-independent Bad-phosphorylation to induce anti-apoptotic signals. Nat. Med. 7:1217-1224. [DOI] [PubMed] [Google Scholar]
- 60.Xu, X. N., B. Laffert, G. R. Screaton, M. Kraft, D. Wolf, W. Kolanus, J. Mongkolsapay, A. J. McMichael, and A. S. Baur. 1999. Induction of Fas ligand expression by HIV involves the interaction of Nef with the T-cell receptor zeta chain. J. Exp. Med. 189:1489-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu, X. N., G. R. Screaton, F. M. Gotch, T. Dong, R. Tan, N. Almond, B. Walker, R. Stebbings, K. Kent, S. Nagata, J. E. Stott, and A. J. McMichael. 1997. Evasion of cytotoxic T lymphocyte (CTL) responses by Nef-dependent induction of Fas ligand (CD95L) expression on simian immunodeficiency virus-infected cells. J. Exp Med. 186:7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang, O. O., P. T. Nguyen, S. A. Kalams, T. Dorfman, H. G. Gottlinger, S. Stewart, I. S. Chen, S. Threlkeld, and B. D. Walker. 2002. Nef-mediated resistance of human immunodeficiency virus type 1 to antiviral cytotoxic T lymphocytes. J. Virol. 76:1626-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.York, I. A., C. Roop, D. W. Andrews, S. R. Riddell, F. L. Graham, and D. C. Johnson. 1994. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell 77:525-535. [DOI] [PubMed] [Google Scholar]