Abstract
It has become evident that naturally occurring CD25+ regulatory T cells (Treg cells) not only influence self-antigen specific immune response but also dampen foreign antigen specific immunity. This report extends our previous findings by demonstrating that immunity to certain herpes simplex virus (HSV) vaccines is significantly elevated and more effective if Treg cell response is curtailed during either primary or recall immunization. The data presented here show that removal of CD25+ Treg cells prior to SSIEFARL-CpG or gB-DNA immunization significantly enhanced the resultant CD8+ T-cell response to the immunodominant SSIEFARL peptide. The enhanced CD8+ T-cell reactivity in Treg cell-depleted animals was between two- and threefold and evident in both acute and memory stages. Interestingly, removal of CD25+ Treg cells during the memory recall response to plasmid immunization resulted in a twofold increase in CD8+ T-cell memory pool. Moreover, in the challenge experiments, memory CD8+ T cells generated with plasmid DNA in the absence of Treg cells cleared the virus more effectively compared with control groups. We conclude that CD25+ Treg cells quantitatively as well as qualitatively affect the memory CD8+ T-cell response generated by gB-DNA vaccination against HSV. However, it remains to be seen if all types of vaccines against HSV are similarly affected by CD25+ Treg cells and if it is possible to devise means of limiting Treg cell activity to enhance vaccine efficacy.
It has been clear that the outcome of several in vivo immunological events is influenced by T cells that suppress the function of other cells involved in immunity. Recent focus has been on regulatory T cells (Treg cells) initially recognized to prevent genetically susceptible mice from developing certain autoimmune diseases (2, 19, 20). Subsequently, Treg cells were shown to influence transplantation immunity as well as immune and inflammatory reactions to infectious agents (3, 4, 12, 17, 22). Of particular interest, Treg cells had a pivotal influence on the outcome of chronic parasitic infections (3). Additionally, it was shown that animals depleted of Treg cells showed markedly superior acute and memory CD8+ T-cell responses to infection with herpes simplex virus (HSV) (22). Animals lacking Treg cells in fact generated more effective protective immunity. Subsequently, Treg cell depletion was also shown to result in more severe immunopathological responses to virus infection.
The fact that immunity to HSV was superior and more sustained when infection occurred in Treg cell-depleted animals was considered to impact upon vaccine design. Thus, HSV is one of those clinically important agents for which there is currently no effective vaccine. Conceivably, the inhibiting activity of Treg cells could help explain the difficulty in achieving effective immunity.
In the present report, we measured the influence of Treg cells on the immune response of mice to DNA and peptide vaccine preparations against HSV. Our results show that immunity to DNA and peptide vaccines measured systemically were compromised by the presence of Treg cells. Most strikingly, if Treg cells were inhibited prior to recall in the memory phase, responses to DNA vaccination were elevated twofold and animals showed notably increased resistance to challenge. Our results are discussed in terms of selecting vaccine approaches that are least affected by Treg cell response as well as the need to identify procedures that minimize the Treg cell response during vaccination. We also comment about mechanistic events that could account for Treg cell interference.
MATERIALS AND METHODS
Animals, virus, and DNA vaccine preparation.
Female C57BL/6 mice, 5 to 6 weeks of age, were purchased from Harlan Sprague Dawley (Indianapolis, Ind.). Animals were used in compliance with institutional animal health and care regulations, and all procedures used in the experiments with animals were approved by the local Institutional Animal Care and Use Committee. HSV-1 KOS (American Type Culture Collection, Manasas, Va.) and vaccinia virus encoding glycoprotein B (gB) of HSV-1 were grown and plaque titrated on Vero cells and kept at −80°C until use. Plasmid DNA was prepared as described previously (8, 24).
Depletion of CD4+ CD25+ T cells.
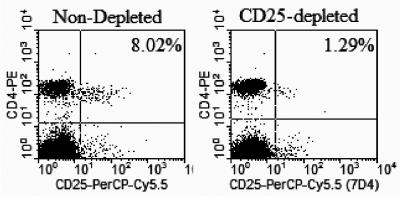
Before immunization, mice were depleted of CD4+ CD25+ regulatory T cells by intraperitoneal administration of anti-CD25 monoclonal antibody clone PC61 (American Type Culture Collection, Manassas, Va.). The antibody was used as the ammonium sulfate precipitate of hybridoma culture supernatant or as ascites produced from PC61 hybridoma in nu/nu mice and purified by Prosep G immunoglobulin purification kit (Millipore, Bedford, Mass.). The depletion capability of these monoclonal antibody preparations did not differ significantly. Depletion efficiency was checked by staining with anti-CD25 antibody clone 7D4 (BD Bioscience, Pharmingen, San Diego, Calif.) and flow cytometry. Results (Fig. 1) show that a high level (≥80%) of depletion was reached by day 4 after intraperitoneal injection of 1.2 mg of depleting anti-CD25 clone PC61.
FIG. 1.
Depletion of CD4+ CD25+ T cells in C57BL/6 mice. Mice were injected intraperitoneally with 1.2 mg of anti-CD25 monoclonal antibody clone PC61. Four or 5 days later, peripheral blood mononuclear cells were stained with CD25+-peridinin chlorophyll protein Cy5.5 clone 7D4 and CD4+-phycoerythrin. Flow cytometry analysis was done in FACScan (Becton Dickinson), and data were analyzed with CellQuest software.
Immunization.
C57BL/6 mice 5 to 6 weeks old were injected with anti-CD25 antibody or normal rat immunoglobulin 4 days earlier to deplete CD4+ CD25+ T cells and then injected with 50 μg of plasmid DNA encoding glycoprotein B of HSV-1 (gB-DNA) intramuscularly or SSIEFARL peptide (HSV gB498-505) combined with bioactive CpG1826 (SS-CpG) (Coley Pharmaceutical Group) in the footpad. Vaccination was repeated after 3 weeks. Another group of mice were also depleted or not and infected with 106 PFU of HSV-1 KOS in the footpad. HSV infection was used here as a positive control. Primary assessment of immune response was performed after 7 days for mice infected with HSV-1 or immunized with SS-CpG and 12 days for mice vaccinated with gB-DNA. Memory responses were assessed at 60 days post-secondary immunization. Control mice for the gB-DNA-immunized group were injected with 50 μg of plasmid DNA encoding β-galactosidase (β-galactosidase DNA), and control groups for SS-CpG were given nonbioactive CpG1982 or CpG2138.
ELISPOT for IFN-γ.
ELISPOT plates (MultiScreen HA sterile plates, Millipore, Bedford, Mass.) were coated with capture anti-gamma interferon (IFN-γ) antibody in carbonate buffer, pH 9.6, overnight (BD Biosciences Pharmingen, San Diego, Calif.). Plates were then blocked with RPMI 1640 (Sigma, St. Louis, Mo.) supplemented with 10% fetal bovine serum. Responder cells from spleens or lymph nodes of immunized and control mice and stimulator cells prepared from naïve mouse spleens pulsed with HSV-gB498-505 peptide and x-irradiated were added to coated plates and incubated at 37°C for 48 to 72 h and thereafter developed, and spots were counted as fully described elsewhere (24).
Intracellular cytokine staining for IFN-γ.
We stimulated 106 spleen or lymph node cells per well with SSIEFARL in the presence of GolgiPlug (BD Biosciences Pharmingen, San Diego, Calif.) and 50 U of interleukin-2 (Hemagen) per ml for 5 h at 37°C. The cells were processed further as described by Kumaraguru and Rouse (11). The fluorescently labeled antibodies used were purchased from BD Biosciences Pharmingen, San Diego, Calif..
CTL assay.
A standard 4-h 51Cr release assay was performed to assess cytolytic activity of the CD8+ T cells isolated from immunized and control mice as described elsewhere (9, 24). Data were corrected by the formula ([experimental release − spontaneous release)/(total release − spontaneous release)] × 100.
Challenge and virus titration.
Mice were challenged at 60 days following initial immunization. A vaccinia virus challenge model, where intraperitoneal injection of vaccinia virus causes initial replication in the ovaries, was used to test efficacy of virus clearance in vaccinated animals. Female C57BL/6 mice were injected intraperitoneally with a recombinant vaccinia virus encoding gB of HSV-1 at a low dose (105 PFU) and a high dose (107 PFU). Ovaries were collected on days 3, 5, and 7, homogenized, clarified in phosphate-buffered saline, and used for virus titration. A conventional viral plaque assay was used.
Statistics.
Where appropriate, significant differences were calculated with Student's t test. P ≤ 0.05 was considered statistically significant.
RESULTS
Effect of CD25+ depletion prior to DNA or SS-CpG vaccination on CD8+ T-cell response.
C57BL/6 mice were either depleted 4 days previously with anti-CD25 antibody clone PC61 or given normal rat immunoglobulin and exposed to infectious HSV, gB-DNA, or SS-CpG vaccine formulations. At 7 or 12 days postimmunization, both spleens and lymph nodes were collected to quantify CD8+ T-cell responses to the immunodominant peptide SSIEFARL by ELISPOT or intracellular cytokine staining for IFN-γ. The results of representative experiments are shown in Fig. 2.
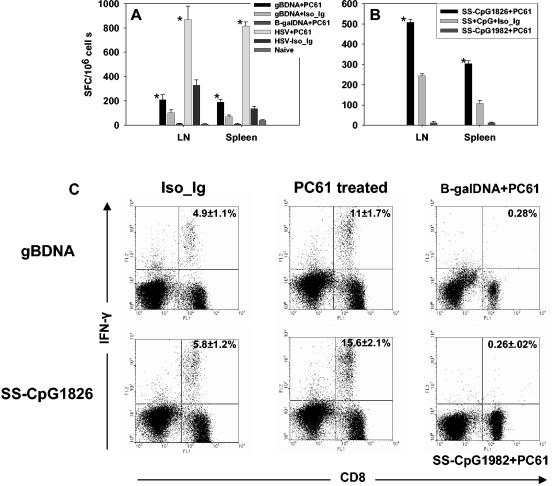
FIG. 2.
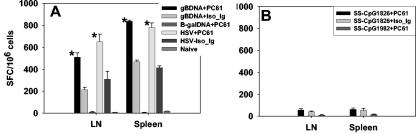
CD8+ T-cell primary response to vaccination after depletion of CD4+ CD25+ T cells. C57BL/6 mice were either depleted of CD25+ T cells or injected with isotype immunoglobulin. Five days later, the animals were vaccinated with gB-DNA intramuscularly or SS-CpG in the footpad. Control mice were injected with either β-galactosidase DNA or nonbioactive CpG1982 or -2138. HSV infection of depleted and nondepleted mice was used as a positive control. Spleen and draining lymph nodes were collected for analysis on either day 7 postimmunization for SS-CpG or day 12 for gB-DNA vaccination. IFN-γ ELISPOT and intracellular staining were performed as described in Materials and Methods. (A) IFN-γ ELISPOT for gB-DNA vaccination and HSV infection, (B) ELISPOT for SS-CpG vaccination, (C) intracellular staining for IFN-γ gB-DNA- and SS-CpG-vaccinated mice. The percentage shown in each cytogram represents the mean of IFN-γ-producing CD8+ T cells obtained from each of four mice per group in three separate experiments. *, statistically significant (P ≤ 0.05) compared to isotype immunoglobulin- and CpG1982-treated groups.
As is evident, these acute-phase responses were elevated in mice that were depleted of CD25+ Treg cells prior to antigen exposure. This supports previous observations that depleted mice exposed to infectious virus showed increase of immune response over nondepleted animals. DNA vaccination of Treg cell-depleted mice showed two- and threefold increase over nondepleted mice in lymph nodes and spleen, respectively. Similarly, vaccination with SS-CpG showed 2- and 2.8-fold increases in depleted versus nondepleted mice in lymph nodes and spleen, respectively.
Thus, the pattern of response observed with the two vaccine preparations in Treg cell-depleted mice, although less in extent, was similar in profile to that of Treg cell-depleted virus-infected mice (Fig. 2A). Taken together, these results indicated that eliminating the influence of the Treg cell population before vaccination with gB-DNA or SS-CpG peptide enhanced the primary CD8+ T-cell response.
Depletion of CD4+ CD25+ T cells prior to primary vaccination improves the memory pool of CD8+ T cells.
We examined the influence of CD4+ CD25+ T-cell depletion on systemic T-cell memory generated with gB-DNA or SS-CpG vaccination. In the first instance, mice were depleted of Treg cells prior to primary vaccination. Restimulation was performed on day 21, and memory responses were measured 60 days later. Figure 3 shows IFN-γ-producing memory CD8+ T cells in a representative experiment. Memory CD8+ T-cell response in gB-DNA- or SS-CpG-immunized mice decreased two- and fourfold when measured at 60 days post-secondary immunization, respectively. Treg cell-depleted animals had approximately twofold higher responses than nondepleted mice with both types of vaccines. Higher responses dominated in the spleens than the lymph nodes. Compared to the two vaccine preparations, HSV infection (Fig. 3A) had more responding CD8+ T cells than gB-DNA or SS-CpG immunization. These results show that removal of Treg cells prior to primary immunization positively influenced the magnitude of the memory CD8+ T cells of animals vaccinated with gB-DNA or SS-CpG.
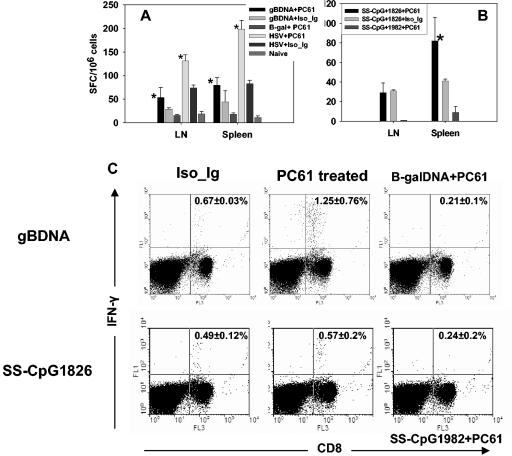
FIG. 3.
CD8+ T-cell memory response to vaccination following depletion of CD4+ CD25+ T cells. C57BL/6 mice were either depleted of CD25+ T cells or injected with isotype immunoglobulin. Five days later the animals were immunized with gB-DNA intramuscularly or SS-CpG in the footpad. Control mice were injected with either β-galactosidase DNA or nonbioactive CpG 1982 or 2138. HSV infection of depleted and nondepleted mice was used as a positive control. Spleen and draining lymph nodes were collected for analysis on day 60 postimmunization. IFN-γ ELISPOT and intracellular staining were performed as described in Materials and Methods. (A) ELISPOT for gB-DNA vaccination and HSV infection, (B) ELISPOT for SS-CpG vaccination, (C) intracellular staining for gB-DNA- and SS-CpG-vaccinated mice. The percentage shown in each cytogram represents the mean of IFN-γ-producing CD8+ T cells obtained from each of four mice per group in two experiments performed. *, statistically significant (P ≤ 0.05) compared to isotype immunoglobulin- and CpG1982-treated groups.
We were curious to know if depletion of Treg cells in the memory phase equally enhanced the T-cell response. Therefore, in the second instance mice were infected with HSV or vaccinated with gB-DNA or SS-CpG. Sixty days later the animals were depleted of CD4+ CD25+ T cells and boosted after 5 days with gB-DNA or SS-CpG. Responses were measured 5 days later. Figures 4A and C show memory recall responses of CD8+ T cells that produced IFN-γ upon restimulation in vitro. An approximately twofold increase in the number of IFN-γ-producing spleen CD8+ T cells was observed between depleted DNA-vaccinated mice and nondepleted mice. HSV-infected mice had a similar pattern of response (Fig. 4A). Finally, depleting Treg cells at both the primary and memory phases did not produce further enhancement of the immune response to gB-DNA immunization or HSV infection (Fig. 5A). There was no statistically significant difference (P ≥ 0.05) between doubly depleted or singly depleted mice. This indicated that reactivation of the residual memory CD8+ T-cell pool was also subject to regulation by Treg cells and was affected by the removal of these cells. These results also indicated that Treg cells control the reactivity of memory T cells and that inhibiting the function of Treg cells even when memory is established allows the memory T cells to rapidly reactivate to a higher frequency.
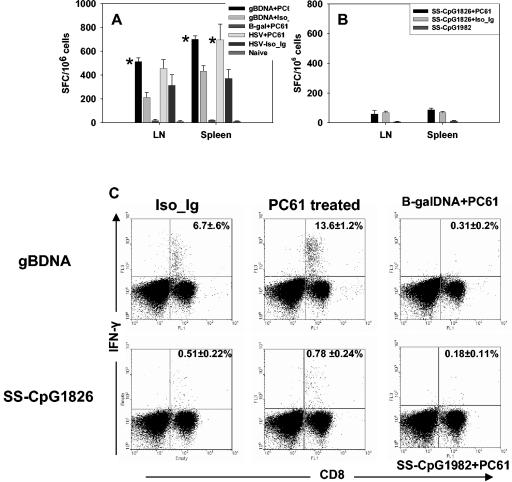
FIG. 4.
Effect of CD4+ CD25+ T-cell depletion on memory recall responses. Mice were immunized without prior depletion of Treg cells. At 60 days after initial immunization, the mice were depleted of Treg cells with anti-CD25 monoclonal antibody and 5 days later boosted with gB-DNA or SS-CpG. Control mice were injected with either β-galactosidase DNA or nonbioactive CpG1982 or -2138. HSV infection of depleted and nondepleted mice was used as a positive control. Responses were analyzed 5 days later with IFN-γ ELISPOT and intracellular assays as described in Materials and Methods. (A) IFN-γ ELISPOT for gB-DNA vaccination and HSV infection, (B) ELISPOT for SS-CpG vaccination, (C) intracellular staining for gB-DNA- and SS-CpG -vaccinated mice. The percentage shown in each cytogram represents the mean of IFN-γ-producing CD8+ T cells obtained from each of four mice per group in two separate experiments. *, statistically significant (P ≤ 0.05) compared to isotype immunoglobulin- and CpG1982-treated groups.
FIG. 5.
Influence of double depletion of CD4+ CD25+ T cells on memory recall responses. Mice were depleted of Treg cells prior to vaccination and depleted again at 60 days post-initial immunization. Control mice were injected with either β-galactosidase DNA or nonbioactive CpG1982 or -2138. ELISPOT and intracellular staining for IFN-γ were performed 5 days boosting with gB-DNA or SS-CpG. (A) ELISPOT for gB-DNA vaccination and HSV infection, (B) ELISPOT for SS-CpG vaccination.
Surprisingly, depletion of Treg cells in the memory phase of SS-CpG-immunized mice did not lead to significant expansion of the responding CD8+ T cells (Fig. 4B and C). Both depleted and nondepleted mice from the SS-CpG-immunized group responded similarly, and the magnitude of response was severalfold lower than that observed in gB-DNA-immunized or HSV-infected mice (Fig. 4A and C). This observation indicates that even though there is enhancement of primary immune response in the absence of Treg cells, the effect had less impact on the memory response if the primary CD8+ T cells were generated in the absence of CD4+ T-cell help. Therefore, depletion of Treg cells before SS-CpG immunization did not contribute to the magnitude of the memory response.
Cytotoxic T lymphocytes generated by vaccination following Treg cell depletion efficiently lyse their targets.
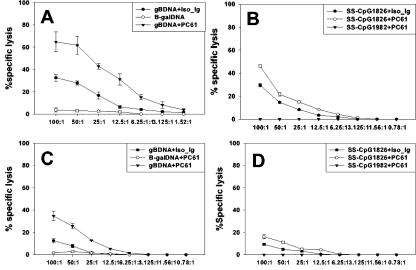
We assessed whether the CD8+ T cells generated in CD4+ CD25+ depleted mice after vaccination were functional CTLs. A standard chromium release assay was performed on splenocytes after expansion for 5 days in vitro. Figures 6A and B show that in the acute phase, incubation of effector T cells from gB-DNA- and SS-CpG-vaccinated Treg cell-depleted mice with target cells showed increased lysis of targets pulsed with gB498-505 peptide. CTL activity of cells isolated from depleted mice was higher than that of nondepleted animals. Similarly, CTL activity of cells from Treg cell-depleted SS-CpG-vaccinated mice was higher than that of nondepleted mice. Memory (Fig. 6C and D) CTL activity of the gB-DNA-vaccinated group showed even larger differences between depleted and nondepleted mice. SS-CpG immunization resulted in a poor memory CTL response, as effector cells from such mice did not lyse the targets efficiently. When these two forms of immunization were compared to Treg cells from depleted virus-infected mice, the latter had more potent CTLs (data not shown) than gB-DNA- and SS-CpG-vaccinated mice at both primary and memory phases.
FIG. 6.
Cytotoxic T lymphocytes generated in the absence of regulatory T cells efficiently lyse peptide-sensitized targets. Splenocytes were isolated and expanded in vitro for 5 days, and CTL activity was determined as described in Materials and Methods. (A and B) CTL activity measured during the acute phase of immunization for gB-DNA and SS-CpG1826, respectively. (C and D) Memory-phase CTL activity for gB-DNA and SS-CpG1826, respectively.
The difference between the depleted and nondepleted groups at the memory phase suggested that removal of Treg cell control allowed generation of a high frequency of CTLs that efficiently lysed their target, which accounted for a better memory response in terms of efficacy as described subsequently. Control lysis assays with major histocompatibility complex class I mismatched target cells or MC38 cells pulsed with an irrelevant peptide from ovalbumin showed that lysis was specific to the HSV antigen-sensitized targets (data not shown).
Treg cell-depleted and vaccinated mice clear challenge virus efficiently.
To show that the enhanced immune response following removal of regulatory T cells affected the outcome of a challenge by virus, we measured the clearance of a recombinant vaccinia virus encoding gB of HSV. This challenge model utilized the fact that vaccinia virus initially replicates in the ovaries of mice, which could provide a good measure of systemic responses against challenge by virus. Separate groups of gB-DNA- or SS-CpG-vaccinated mice were intraperitoneally infected with two different doses of vaccinia virus gB, a low dose, 105 PFU, and high dose, 107 PFU per mouse, and followed for 7 days. Table 1 shows titers of vaccinia virus gB titrated on Vero cells from homogenized ovaries of mice challenged with a low dose of virus. Challenge virus was detected in all groups of mice vaccinated with peptide, either Treg depleted or nondepleted, although titers were only modest. Virus replication could be detected in these mice through day 7 of observation. In contrast, the depleted and nondepleted gB-DNA-vaccinated groups showed that on day 3 replication ensued and an approximately 2 log difference in viral titer was observed between depleted and nondepleted animals. For the depleted group, the virus could only be titrated on day 3 and was not detected on days 5 and 7.
TABLE 1.
Mean titers of virus in the ovaries collected at days 3, 5, and 7 following challenge with 105 PFU/mouse of vaccinia virus gB
| Group | Mean log10 titer ± SDa
|
||
|---|---|---|---|
| Day 3 | Day 5 | Day 7 | |
| HSV + PC61 | 0 | 0 | 0 |
| HSV + isotype Ig | 1.28 ± 0.31 | 0 | 0 |
| Phosphate-buffered saline | 3.08 ± 1.1 | 4.4 ± 0.19 | 3.73 ± 0.19 |
| SS-CpG1826 + PC61 | 1.21 ± 0.40 | 1.11 ± 0.93 | 1.0 ± 0.67 |
| SS-CpG1826 + isotype Ig | 1.92 ± 0.14 | 1.01 ± 0.5 | 1.1 ± 0.41 |
| SS-CpG2138 + PC61 | 4.81 ± 1.3 | 4.01 ± 0.29 | 3.37 ± 0.39 |
| gB-DNA + PC61 | 1.28 ± 0.62 | 0 | 0 |
| gB-DNA + isotype Ig | 3.00 ± 1.78 | 2.51 ± 0.2 | 0 |
| β-Galactosidase DNA | 4.5 ± 0.1 | 4.54 ± 0.68 | 3.98 ± 0.67 |
Values represent means for four mice per group in two separate experiments.
Challenge with high-dose vaccinia virus gB showed that virus replicated in the ovaries of all mice irrespective of depletion status and vaccination (Table 2). All SS-CpG-vaccinated groups were not protected from the high virus dose challenge, and titers reached 4 logs of magnitude and could be detected throughout the observation period. Threefold difference in viral titers was shown between nondepleted and depleted gB-DNA-vaccinated mice on both days 3 and 5, and the virus could still be detected on day 7 in nondepleted gB-DNA-vaccinated group. Control groups, SS-CpG1982- and β-galactosidase-treated mice, had the highest viral titers after both a low dose and a high dose challenge, which shows evidence of virus replication in this challenge model. Moreover, in these control animals the ovaries were hyperemic and largely edematous by day 7.
TABLE 2.
Mean titers of virus in the ovaries collected at days 3, 5, and 7 following challenge with 107 PFU/mouse of vaccinia virus gB
| Group | Mean log10 titer ± SDa
|
||
|---|---|---|---|
| Day 3 | Day 5 | Day 7 | |
| HSV + PC61 | 1.12 ± 0.16 | 0 | 0 |
| HSV + isotype Ig | 2.79 ± 0.21 | 1.10 ± 0.12 | 0 |
| Phosphate buffered saline | 4.11 ± 1.5 | 5.56 ± 0.9 | 3.23 ± 0.32 |
| SS-CpG1826 + PC61 | 4.12 ± 0.16 | 4.0 ± 1.01 | 2.15 ± 0.78 |
| SS-CpG1826 + isotype Ig | 4.790 ± 0.29 | 4.10 ± 0.24 | 2.29 ± 0.65 |
| SS-CpG2138 + PC61 | 4.11 ± 1.5 | 5.56 ± 0.9 | 3.23 ± 0.32 |
| gB-DNA PC61 | 1.21 ± 0.65 | 1.1 ± 0.43 | 0 |
| gB-DNA + isotype Ig | 3.99 ± 1.18 | 3.21 ± 0.23 | 2.31 ± 0.21 |
| β-Galactosidase DNA + PC61 | 4.9 ± 0.13 | 5.3 ± 1.89 | 4.18 ± 0.77 |
Values are means for four mice per group in two separate experiments..
This challenge model indicated that depletion of CD25+ Treg cells led to induction of CTLs or other mechanisms that contributed to efficient virus clearance. Although virus replication still occurred, the time of clearance was reduced to at least 5 days in DNA-vaccinated Treg cell-depleted mice and 3 days in HSV-infected Treg cell-depleted mice at the high virus challenge dose. Overall, depletion of Treg cells influenced the efficacy of DNA vaccination.
DISCUSSION
It is evident now that naturally occurring CD25+ Treg cells not only influence self-antigen specific immune response (15, 18, 20) but also dampen foreign antigen specific immunity (3, 4, 22). Our initial observation with herpes simplex virus infection showed that the magnitude of CD8+ T-cell response was tightly regulated by CD25+ Treg cells. This report extends the previous findings by demonstrating that immunity to certain HSV vaccines is significantly enhanced and more effective if the Treg cell response is curtailed during primary or recall immunization. The data presented here show that removal of CD25+ Treg cells prior to SSIEFARL-CpG or gB-DNA immunization significantly enhanced the resultant CD8+ T-cell response to the immunodominant SSIEFARL peptide. This was shown by different in vitro assays, ELISPOT, CTL assay and intracellular IFN-γ staining that measured the CD8+ T-cell reactivity to SSIEFARL epitope.
The enhanced CD8+ T-cell reactivity in Treg cell-depleted animals was between two- and threefold and was evident in both the acute and memory stages. Interestingly, removal of CD25+ Treg cells during the memory phase prior to plasmid recall immunization resulted in a twofold increase in effector cells, and virus-challenged animals cleared infection more effectively. A boost of such immunity by Treg cell depletion was not noted in CpG peptide-immunized mice. We conclude that CD25+ Treg cells quantitatively as well as qualitatively affect the CD8+ T-cell immune response generated by gB-DNA vaccination against HSV. However, it remains to be seen if all types of vaccines against HSV are similarly affected by CD25+ Treg cells and if it is possible to devise means of limiting Treg cell activity to enhance vaccine efficacy.
The renaissance of Treg cells emphasized their role in limiting the expression of AIDS. More recently, however, it became evident that Treg cells influence the immune response to exogenous antigens, especially those expressed by pathogens. Our observation that the CD8+ and later the CD4+ response to HSV was limited if Treg cells were present during primary infection raised several questions. Among these was whether the response was unique to a replicating virus and if the phenomenon might serve to limit the efficacy of certain vaccines. Our observation that the magnitude of the CD8+ T-cell response to a DNA vaccine as well as an adjuvanted peptide vaccine was elevated approximately the same as the virus when the response of Treg cell-depleted or nondepleted animals were compared was surprising. Accordingly, we had expected that the activation of Treg cells was a combination of recognition by viral antigen-specific Treg cells and activation, perhaps nonspecific, by components of the virus or stress molecules generated by dying infected cells. However, the responses to both the DNA vaccine encoding gB and the CpG peptide vaccine were equally subject to Treg cell control, as was the response to HSV.
CD4+ CD25+ T cells were reported to influence mostly CD4+ cells (1, 14). Here additional evidence shows that murine CD4+ CD25+ T cells can also regulate the responses of CD8+ cells. The fact that there was a marked difference between depleted and nondepleted groups of mice indicated that clonal expansion of CD8+ T cells was inhibited in mice not depleted of Treg cells. The mechanisms involved in the regulation of antigen-specific CD8+ T cells were not directly studied, but a recent report (6) showed substantial inhibition of interleukin-2 transcription and interleukin-2 production which coincided with equally marked inhibition of interleukin-2 receptor α expression. Additionally, the same report suggested that poor performance of the CD8+ T cells under the influence of Treg cells was due to limited transcription and production of IFN-γ and other molecules such as perforin and granzyme B, responsible for the cytolytic activity of CD8+ T cells.
The gB-DNA vaccine could be recognized by CD8+ and CD4+ T cells, including Treg cells, but at present we do not have positive evidence for the latter. The peptide vaccine should only be recognized by CD8+ T cells but was regulated by Treg cells, and hence it needs to be explained how the Treg cell function is expressed in this instance. The observation that there was a regulatory mechanism imposed on CpG peptide vaccination which inhibited the immune responses indicated that the mechanism may involve nonspecific activation of regulatory T cells. Although there is no evidence of nonspecific activation of Treg cells, the use of CpG, a ligand for Toll-like receptor 9 expressed by dendritic cells in the vaccine preparation may have induced a cytokine-chemokine milieu conducive for activation of Treg cells, since there are reports demonstrating that Treg cells are particularly sensitive to inflammatory cytokines/chemokines (5, 10).
Direct interaction of CpG and Treg cells can be ruled out because murine Treg cells do not express Toll-like receptor 9. However, the finding by Caramalho et al. (7) that seven out of nine murine Toll-like receptors are expressed by Treg cells suggests that a rather wide spectrum of inflammation-associated endogenous and pathogen-specific molecules might directly influence their activation. This possible line of evidence for nonspecific Treg cell activation is also seen in the study by Moser et al. (16) in which CpG-treated dendritic cells were first pulsed with OT-I peptide and injected into mice previously adoptively transferred with OT-I cells. Reponses in mice depleted of Treg cells were greatly enhanced compared to nondepleted mice. However, examination of this effect in Toll-like receptor 9-deficient cells and animals might give insight into the mechanism of Treg cell activation in the case of immunization with a major histocompatibility complex class I-restricted peptide and CpG.
When Treg cells in gB-DNA-immunized mice were depleted in the memory phase and boosted with antigen, more CD8+ T cells were recalled and increased twofold in comparison to nondepleted animals. The control of memory T cells by Treg cells has also been reported by Kursar et al. (12). In their studies on Listeria monocytogenes, when DNA-immunized mice were depleted of Treg cells in the memory phase and then later restimulated, a 10-fold increase in the responding CD8+ T cells was observed. In a recent study the same authors showed a similar effect of memory depletion of Treg cells on CD8+ T cells during vaccination with nonviable Listeria monocytogenes (13). Although we used a different antigen-vector combination, we obtained a somewhat inferior response at recall compared to that of Kursar et al. This difference in increase could result from a less restricted activation of Listeria-specific memory T cells (12) compared to HSV-specific cells, especially those generated by DNA encoding gB, which generally gives a weak immune response. What is not known in both cases is whether the Treg cells exert direct control on the memory CD8+ T cells or through other means. It is also clear that Treg cells control the generation of effector CD8+ T cells as well as the expansion of the T-cell memory pool of CD8+ upon reexposure to antigen, but what is not known is whether Treg cells play a role in the contraction and maintenance of CD8+ T-cell memory.
Importantly, depletion of regulatory T cells notably affected the level of memory response generated after gB-DNA vaccination. In contrast, depletion of Treg cells did not improve memory to peptide or CpG immunization. The poor performance of CD8+ T cells generated by peptide vaccination could result from the events occurring at the priming stage. It has been reported earlier that priming CD8+ T-cell response in the absence of helper T cells impairs the memory response of those CD8+ T cells (25). Evidently, removal of Treg did not alter the programming of the CD8+ T cells to mimic that which occurs during priming in the presence of CD4+ T-cell help.
From the immunization standpoint, it is important to understand what other consequences may apply to the Treg cell manipulation approach to vaccination against microbes. Taguchi and Takahashi (23) reported that injection of anti-CD25 antibodies into normal animals induced localized autoimmune disease. However, no such side effect was observed in the present study following administration of the depleting antibody. In Sutmuller's (21) studies on tumor vaccination involving removal of Treg cells, autoimmunity developed only when CTLA-4 was used in combination to exclude the suppressive function of CD25+ Treg cells. Thus, this point is critical in understanding how to carefully manipulate such vital cells so as to benefit vaccination against viral infections. Likely, a vaccination protocol to include manipulation of Treg cells would mean applying a reagent in a single dose followed shortly by the vaccine, since the immune-enhancing effect of depleting CD25+ Treg cells was observed only after a few days of depletion. Such a procedure would eliminate repeated depletion of Treg cells.
In summary, the data reported here suggest that the level of immune response observed in intact animals to DNA vaccination may be a result of a higher threshold of T-cell activation imposed by CD4+ CD25+ Treg cells. Consequently, vaccination against infectious agents may be enhanced by altering the regulatory pathway involving Treg cells, which may improve vaccine efficacy. Indeed, depletion of CD4+ CD25+ T cells improves DNA vaccine efficacy, which implies that the rational design of vaccines against viruses should consider means of circumventing the suppressive function of the regulatory T cells in inducing primary immune response or secondary responses during boosting of existing immunity. However, it remains to precisely define the strategy that could allow achievement of careful and successful manipulation of regulatory T cells, either a low-dose immunologic approach, which is a less likely approach, a chemical approach, or other means yet to be described.
Acknowledgments
This work was supported by grants AI 14981 and AI 46462 from the National Institutes of Health.
REFERENCES
- 1.Alyanakian, M.-A., S. You, D. Damotte, C. Gouarin, A. Esling, C. Garcia, S. Havouis, L. Chatenoud, and J.-F. Bach. 2003. Diversity of regulatory CD4+T cells controlling distinct organ-specific autoimmune diseases. Proc. Natl. Acad. Sci. USA 100:15806-15811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asano, M., M. Toda, N. Sakaguchi, and S. Sakaguchi. 1996. Autoimmune disease as a consequence of developmental abnormality of a T-cell subpopulation. J. Exp Med. 184:387-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belkaid, Y. 2003. The role of CD4+CD25+ regulatory T cells in Leishmania infection. Expert Opin. Biol. Ther. 3:875-885. [DOI] [PubMed] [Google Scholar]
- 4.Belkaid, Y., C. A. Piccirillo, S. Mendez, E. M. Shevach, and D. L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420:502-507. [DOI] [PubMed] [Google Scholar]
- 5.Bystry, R. S., V. Aluvihare, K. A. Welch, M. Kallikourdis, and A. G. Betz. 2001. B cells and professional APCs recruit regulatory T cells via CCL4. Nat. Immunol. 2:1126-1132. [DOI] [PubMed] [Google Scholar]
- 6.Camara, N. O., F. Sebille, and R. I. Lechler. 2003. Human CD4+CD25+ regulatory cells have marked and sustained effects on CD8+ T-cell activation. Eur. J. Immunol. 33:3473-3483. [DOI] [PubMed] [Google Scholar]
- 7.Caramalho, I., T. Lopes-Carvalho, D. Ostler, S. Zelenay, M. Haury, and J. Demengeot. 2003. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J. Exp. Med. 197:403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eo, S. K., M. Gierynska, A. A. Kamar, and B. T. Rouse. 2001. Prime-boost immunization with DNA vaccine: mucosal route of administration changes the rules. J. Immunol. 166:5473-5479. [DOI] [PubMed] [Google Scholar]
- 9.Eo, S. K., U. Kumaraguru, and B. T. Rouse. 2001. Plasmid DNA encoding CCR7 ligands compensate for dysfunctional CD8+ T-cell responses by effects on dendritic cells. J. Immunol. 167:3592-3599. [DOI] [PubMed] [Google Scholar]
- 10.Iellem, A., M. Mariani, R. Lang, H. Recalde, P. Panina-Bordignon, F. Sinigaglia, and D. D'Ambrosio. 2001. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4+CD25+ regulatory T cells. J. Exp. Med. 194:847-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumaraguru, U., and B. T. Rouse. 2000. Application of the intracellular gamma interferon assay to recalculate the potency of CD8+ T-cell responses to herpes simplex virus. J. Virol. 74:5709-5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kursar, M., K. Bonhagen, J. Fensterle, A. Kohler, R. Hurwitz, T. Kamradt, S. H. E. Kaufmann, and H.-W. Mittrucker. 2002. Regulatory CD4+CD25+ T cells restrict memory CD8+ T cell responses. J. Exp. Med. 196:1585-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kursar, M., A. Kohler, S. H. E. Kaufmann, and H.-W. Mittrucker. 2004. Depletion of CD4+ T cells during immunization with nonviable listeria monocytogenes causes enhanced CD8+ T cell-mediated protection against listeriosis. J. Immunol. 172:3167-3172. [DOI] [PubMed] [Google Scholar]
- 14.LeGuern, C. 2003. Regulation of T-cell functions by MHC class II self-presentation. Trends Immunol. 24:633-638. [DOI] [PubMed] [Google Scholar]
- 15.McHugh, R. S., E. M. Shevach, and A. M. Thornton. 2001. Control of organ-specific autoimmunity by immunoregulatory CD4(+)CD25(+) T cells. Microbes Infect. 3:919-927. [DOI] [PubMed] [Google Scholar]
- 16.Oldenhove, G., M. de Heusch, G. Urbain-Vansanten, J. Urbain, C. Maliszewski, O. Leo, and M. Moser. 2003. CD4+ CD25+ regulatory T cells control T helper cell type 1 responses to foreign antigens induced by mature dendritic cells in vivo. J. Exp. Med. 198:259-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roncarolo, M. G., and M. K. Levings. 2000. The role of different subsets of T regulatory cells in controlling autoimmunity. Curr. Opin. Immunol. 12:676-683. [DOI] [PubMed] [Google Scholar]
- 18.Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance maintained by activated T cells expressing interleukin-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151-1164. [PubMed] [Google Scholar]
- 19.Shevach, E. M. 2000. Regulatory T cells in autoimmmunity. Annu. Rev. Immunol. 18:423-449. [DOI] [PubMed] [Google Scholar]
- 20.Suri-Payer, E., A. Z. Amar, A. M. Thornton, and E. M. Shevach. 1998. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J. Immunol. 160:1212-1218. [PubMed] [Google Scholar]
- 21.Sutmuller, R. P. M., L. M. van Duivenvoorde, A. van Elsas, T. N. M. Schumacher, M. E. Wildenberg, J. P. Allison, R. E. M. Toes, R. Offringa, and C. J. M. Melief. 2001. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25+ regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic t lymphocyte responses. J. Exp. Med. 194:823-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suvas, S., U. Kumaraguru, C. D. Pack, S. Lee, and B. T. Rouse. 2003. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J. Exp. Med. 198:889-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taguchi, O., and T. Takahashi. 1996. Administration of anti-interleukin-2 receptor alpha antibody in vivo induces localized autoimmune disease. Eur. J. Immunol. 26:1608-1612. [DOI] [PubMed] [Google Scholar]
- 24.Toka, F. N., M. Gierynska, and B. T. Rouse. 2003. Codelivery of CCR7 ligands as molecular adjuvants enhances the protective immune response against herpes simplex virus type 1. J. Virol. 77:12742-12752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Stipdonk, M. J., G. Hardenberg, M. S. Bijker, E. E. Lemmens, N. M. Droin, D. R. Green, and S. P. Schoenberger. 2003. Dynamic programming of CD8+ T lymphocyte responses. Nat. Immunol. 4:361-365. [DOI] [PubMed] [Google Scholar]