Abstract
A novel plasmid-based adenovirus vector system that enables manufacturing of replication-incompetent (ΔE1) adenovirus type 11 (Ad11)-based vectors is described. Ad11 vectors are produced on PER.C6/55K cells yielding high-titer vector batches after purification. Ad11 seroprevalence proves to be significantly lower than that of Ad5, and neutralizing antibody titers against Ad11 are low. Ad11 seroprevalence among human immunodeficiency virus-positive (HIV+) individuals is as low as that among HIV− individuals, independent of the level of immune suppression. The low level of coinciding seroprevalence between Ad11 and Ad35 in addition to a lack of correlation between high neutralizing antibody titers towards either adenovirus strongly suggest that the limited humoral cross-reactive immunity between these two highly related B viruses appears not to preclude the use of both vectors in the same individual. Ad11 transduces primary cells including smooth muscle cells, synoviocytes, and dendritic cells and cardiovascular tissues with higher efficiency than Ad5. Ad11 and Ad35 appear to have a similar tropism as judged by green fluorescent protein expression levels determined by using a panel of cancer cell lines. In addition, Ad5 preimmunization did not significantly affect Ad11-mediated transduction in C57BL/6 mice. We therefore conclude that the Ad11-based vector represents a novel and useful candidate gene transfer vehicle for vaccination and gene therapy.
Adenovirus vectors are being developed for gene therapy purposes with the aim to treat inherited or acquired disease (6, 10, 15, 21) as well as for therapeutic and prophylactic vaccination strategies (5, 32). The use of adenovirus for vaccination has recently been fueled by highly promising results demonstrating protection against viruses and viral diseases in rodents and nonhuman primates (28, 34, 35) as well as induction of T- and B-cell responses in humans in early phase I vaccine trials with healthy volunteers (7). However, high seroprevalence and high neutralizing antibody (NAb) titers against the commonly used vectors, i.e., adenovirus type 5 (Ad5) and Ad2 (39), hamper the application of C group-based vectors, since circulating NAbs efficiently capture administered recombinant vectors obscuring therapeutic effect (19). It has been shown, at least in rodents (1, 42), nonhuman primates (3), and humans in early phase I clinical trials (7), that high levels of NAbs decrease gene transfer efficiency or blunt vaccine potency. Since levels of NAbs vary among individuals, overdosing with recombinant vector in an attempt to overcome the neutralizing activity may result in either excellent clinical results or severe vector-mediated toxicity. Thus, the presence of anti-Ad5 preexisting immunity does not allow accurate dose control and thereby may prevent the universal use of Ad5-based vectors as gene transfer vehicles in humans.
Although many strategies are being pursued to avoid vector neutralization (3, 5, 19, 23, 41, 43), a most viable strategy is the use of rare human adenovirus types (25, 39). Vogels et al. identified subgroup B2 adenoviruses, i.e., Ad35 and Ad11, as types least neutralized by serum from healthy human blood donors and generated an Ad35 vector-manufacturing system (39). Here, we present a novel, Ad11 vector-based manufacturing platform that allows easy vector generation and yields high-titer purified Ad11 vector. We demonstrate that Ad11 is not hampered by preexisting anti-Ad5 neutralizing activity. Moreover, by using the Ad11 vector, we performed extensive serology testing worldwide with the blood of healthy volunteers and immune-compromised individuals demonstrating low seroprevalence corresponding with low titers. Furthermore, we show that the Ad11 vector infects cells and tissues that are considered important targets for gene therapy or vaccination with high efficiency, in contrast to Ad5. Also, we show that despite high genome homology, cross-neutralization at the antibody level does not seem to occur between the two closely related B2 group Ad11 and Ad35 adenoviruses in the human population, which might allow for heterologous subgroup B vector readministration. Therefore, the results presented warrant further investigations with the Ad11-based adenoviral vector system in vaccination and gene therapy studies.
MATERIALS AND METHODS
Ad11 genome sequence.
The human adenovirus Ad11p virus stock was a kind gift from Jan de Jong (University of Rotterdam, Rotterdam, The Netherlands). Wild-type Ad11 virus was propagated on PER.C6 cells and purified as described previously (39). The nucleotide sequence of wild-type Ad11 was determined via shot-gun sequence technology (Lark Technologies Inc.) essentially as described previously (39).
Primary cells and cell lines.
PER.C6 (8) and PER.C6/55K (39) cells were routinely maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum and 10 mM MgCl2. Cell lines A549, K562, MCF-7, SK-N-MC, and HS766T were obtained from the American Type Culture Collection and were cultured according to instructions provided with the cell lines. Human saphenous vein smooth muscle cells (SMCs) and human synoviocytes derived from rheumatoid arthritis patients were isolated, cultured, and transduced as described previously (22, 40). Organ culture experiments with saphenous vein wall segments were performed as described previously (12, 23).
Immature dendritic cells (imDCs) derived from human peripheral blood mononuclear cells were obtained by Ficoll density gradient separation, and monocyte-derived dendritic cells were purified as described previously (39).
Ad11 plasmid system and vector generation.
In accordance with previously reported Ad5 and Ad35 vector plasmid systems, a similar construction strategy has been applied for Ad11. Briefly, the system consists of a plasmid (pAdApt) containing 5 kb of the left end of the Ad11p genome and a cosmid (Pwe.Ad11.dE3) containing the remainder (31 kb) of the Ad11p genome. Plasmid pAdApt11 contains the Ad11 left inverted terminal repeat (ITR), the packaging signal (wild-type Ad11, nucleotides [nt] 1 to 464), and an expression cassette consisting of the cytomegalovirus promoter linked to a multiple cloning site followed by a simian virus 40 poly(A) transcription termination signal and further containing part of the Ad11 genome corresponding to nt 3400 to 4670 of wild-type Ad11. This latter sequence enables homologous recombination with the Ad11 backbone cosmid in complementing cells. To fully utilize the multiple cloning site, an undesired HindIII restriction site, present in the Ad11-overlapping (nt 3400 to 4670) fragment of pAdApt, was removed by partial HindIII digestion, filling in of the protruding DNA ends, and subsequent religation of the linear vector. In the reciprocal fragment present in the cosmid, this HindIII enzyme restriction site was also removed.
To generate cosmid pWE.Ad11, a 26.6-kb NdeI fragment of the Ad11 genome (nt 6539 to 33164) was cloned into cosmid pWE.dNdeI. In order to generate pWE.Ad11.dNdeI, two fragments including either Ad11.pIX (nt 3400 to 6770) or Ad11.right ITR (nt 33095 to 34794) were obtained by PCR amplification introducing NotI and NdeI sites. Both fragments were digested with NotI and NdeI and simultaneously cloned into the NotI site of pWE15 (Clonetech) in a three-point ligation reaction. Cosmid pWE15 derivates were packaged by using λ phage packaging extracts (Stratagene). For vaccination purposes, a pWE.Ad11.dE3 cosmid was constructed by generating two PCR fragments (nt 26669 to 27184 and 30568 to 33177) flanking E3, whereby an SrfI enzyme restriction site was artificially introduced for linking both fragments. PCR fragments were digested with StuI-SfrI or SfrI-NdeI and subsequently ligated into StuI-NdeI-digested pWE.Ad11, thereby deleting 3,384 bp of the E3 region of Ad11 (nt 27184 to 30609). Reconstitution of the full-length Ad11 (ΔE1) genome was achieved by homologues recombination via cotransfection of pAdApt11 and pWe.Ad11 (with E3) or pWE.Ad11.dE3 (without E3) in the PER.C6/55K packaging cell line using Lipofectamine.
Upon transfection and cytopathic effect, recombinant Ad11 viruses were plaque purified and further expanded on adherent PER.C6/55K cells. Purified Ad11 vector batches were obtained by cesium chloride (CsCl) gradient centrifugation, and high-performance liquid chromatography virus particle determinations were performed by using previously reported methods (27).
Ad11 vector tropism studies.
Unless indicated otherwise, 105 cells of diverse primary origin were seeded in 24-well plates (in triplicate) and incubated with Ad11-enhanced green fluorescent protein (eGFP) by using 500, 2,000, and 5,000 virus particles (vp) per cell. Virus exposure was allowed for 2 h at 37°C, upon which nonattached virus particles were removed via medium replacement. After 48 h, cells were harvested, washed with 1% fetal bovine serum, centrifuged, and resuspended in cell fix prior to fluorescence-activated cell sorter (FACS) analyses. The percentage of cells positive for GFP expression and levels of fluorescence per cell were determined by using FACScalibur (Becton Dickinson) and Cell-Quest Pro software.
Human sera and neutralization assay.
Human sera derived from healthy blood donors (ages 20 to 70) from Japan (n = 64), the United States (n = 128), Europe (The Netherlands [n = 55] and the United Kingdom [n = 64]), and sub-Saharan Africa (n = 200) were heat inactivated for 15 min at 56°C. NAb titers against Ad11 and Ad5 were determined by using a recently developed luciferase transgene inhibition detection assay (31). Briefly, 25 μl of heat-inactivated serum was mixed with 75 μl of medium and added to well 1 of a 96-well plate. Subsequently, 50 μl of well 1 was added to well 2, which already contained 50 μl of medium, and so forth until well 11, when 50 μl of well 11 was discarded. Well 12 received medium only to serve as a positive control. To this mixture, 5 × 106 vp of recombinant adenovirus containing the luciferase reporter gene (Ad11 or Ad5) in a volume of 50 μl was added followed by the addition of 100 μl (104) of A549 cells (multiplicity of infection of 500). By doing so, a serum dilution range of 1/16 to 1/16,384 was obtained. African serum samples as well as serum samples (n = 110) obtained from 55 individuals participating in the Amsterdam cohort studies were tested by using the same protocol, starting from a serum dilution of only 1/32. Luciferase reporter gene expression in cells was assessed by using a Trilux luminescence detector and luciferase substrate according to the manual provided by the manufacturer.
Ad11 vector mouse studies.
Groups (n = 5) of 6- to 8-week-old BALB/c mice received either one or two intramuscular injections of 1010 vp of Ad5.Empty or Ad11.Empty. Immunizations were performed at days 0 and 21 to induce high anti-vector immunity when Ad.Empty vector was injected twice. Control groups (n = 5) received either one or two administrations of phosphate-buffered saline. Induction of the anti-vector humoral response was assessed at day 35 post-vector administration by using a recently described luciferase expression inhibition assay (31). Also, naïve and preimmunized animals received an intramuscular injection of 1010 vp of Ad5.Luc or Ad11.Luc 2 weeks after the last Ad.Empty vector administration. Animals were sacrificed 2 days after Ad5.Luc or Ad11.Luc administration, upon which the gastrocnemius muscle of the right leg (injected leg) was homogenized and processed for luciferase luminescence measurements according to manufacturer's instructions (Promega). The gastrocnemius muscle of the left leg served as a negative control.
Nucleotide sequence accession number.
The wild-type Ad11 nucleotide sequence has been deposited in the GenBank database under accession number AY598970.
RESULTS
Ad11 virus generation.
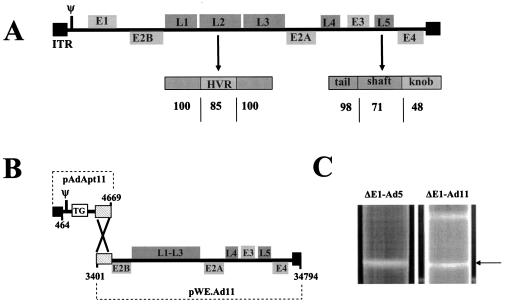
The complete nucleotide sequence of the genome of adenovirus serotype 11p was generated and compared with other human adenoviruses, revealing a high homology (>98% on the DNA level) with Ad35. Major differences were located only in the hypervariable region of the hexon and the fiber knob (Fig. 1A). The obtained serotype Ad11p sequences were compared with published Ad11 sequences (18, 33), revealing a number of discrepancies resulting in single amino acid substitutions. Most importantly, the absence of a nucleotide (guanine) at position 32050 (GenBank accession number NC_004001) was identified, resulting in a frame shift in E4orf6/7 in the published sequence. Next, an Ad11 vector plasmid system that allows easy insertion of heterologous genes through manipulation of the pAdApt11 plasmid was generated (Fig. 1B). As expected, E1-deleted Ad11 vector could not be propagated on Ad5 E1-based packaging cell lines such as PER.C6 cells, indicating that the Ad5 E1 proteins are unable to trans-complement for the deficiency of E1 in Ad11 vectors. Based on the significant amino acid homology within the E1 region of Ad11 and Ad35 (E1B-55K, 99.0%; E1B-19, 99.4%; E1A-11S, 99.1%; E1A-13S, 99.2%), it was expected that E1-deleted Ad11 vectors can be propagated on the PER.C6/55K cell line. The PER.C6/55K cell line has been developed to support the propagation of E1-deleted Ad35 vectors (39). Thus far, many vector batches of E1-deficient and ΔE1/ΔE3 Ad11 vector containing different transgenes have been successfully produced on PER.C6/55K cell cultures. Recombinant Ad11 vectors were purified by using standard CsCl gradient separation (Fig. 1C) resulting in postpurification virus particle yields that are similar to those of Ad5 and Ad35, ranging from 1011 to 1012 vp/ml (data not shown).
FIG. 1.
(A) Schematic representation of the Ad11 genome organization. Shown are the locations of the ITRs, the packaging signal (Ψ), the region encoding proteins required early (E) during infection, and the regions encoding proteins required late (L) during infection. Also shown are the hexon (L2) with the location of the hypervariable region (HVR) and the amino acid homology with Ad35. Likewise, for the adenovirus fiber molecule (L5), position and amino acid homology with Ad35 are shown for fiber tail, fiber shaft, and fiber knob regions. (B) Schematic representation of the Ad11 plasmid system generated to allow easy generation of replication-deficient recombinant Ad11 vector. The pAdApt11 plasmid contains nt 1 to 4669 of the Ad11 genome with an expression cassette instead of E1 sequences. Cosmid pWE.Ad11 contains nt 3401 to 34794 of the Ad11 genome. The two DNAs both have an identical 1,268-bp fragment that mediates homologous recombination in PER.C6/55K packaging cells. (C) Typical banding pattern obtained after CsCl gradient separation. The arrow denotes the position in the gradient of mature Ad5 and Ad11 virions.
Seroprevalence of Ad11.
With the availability of recombinant Ad11 vector, the seroprevalence of serotype Ad11 can be determined with high accuracy and sensitivity by using a recently developed luciferase expression inhibition assay (31). Results of the seroprevalence of Ad11 and Ad5 in healthy blood donors from diverse geographical regions are shown in Fig. 2A. The data demonstrate that Ad11 is less seroprevalent (range, 18 to 31%) than Ad5 (range, 50 to 90%). Subsequent titration of sera that tested positive (Fig. 2B) revealed divergence in serum titers for each location demonstrating lower NAb titers (3- to 10-fold compared to Ad5) against Ad11 in Europe, the United States, and Africa but equal titers for Ad11 and Ad5 in Japanese samples. These experiments confirm the low seroprevalence of Ad11 in serum derived from healthy blood donors as reported previously in studies using a wild-type virus replication inhibition assay (39). Also, these data provide insight into Ad11 seroprevalence in sub-Saharan Africa, an important region in need of vaccines to combat infectious diseases.
FIG. 2.
(A) Human sera were derived from patients in Belgium, the United Kingdom, and The Netherlands (EUR); Stanford, Calif., and New York, N.Y. (USA); many different countries in sub-Saharan Africa (AFRICA); and Japan as the sole representative of Asia. Sera were scored positive when sufficient neutralizing activity (NAbs) was present in serum diluted 1/32 to neutralize Ad5 (black bars) or Ad11 (grey bars) by ≥90% as measured by inhibition of luciferase activity in A549 cells (31). (B) Box-and-whisker plots of Ad-NAb titers against Ad5 (white bars) and Ad11 (grey bars) in positive serum samples. Horizontal bars within the boxed area indicate the median Ad-NAb titer. Serum titers were defined by the dilution at which ≥90% infection inhibition was observed. (C) Percentages of serum samples positive for Ad5 (black bars) or Ad11 (grey bars) at 24 or 72 months after entry into the Amsterdam cohort studies on AIDS initiative when patients were immune competent and asymptomatic (24m) or severely immune compromised and symptomatic (72m). Sera were scored positive when sufficient neutralizing activity was present in serum (NAbs) diluted 1/32 to neutralize Ad5 (black bar) or Ad11 (grey bar) by ≥90% as measured by inhibition of luciferase activity in A549 cells. (D) Box-and-whisker plots of Ad-NAb titers against Ad5 (white bars) and Ad11 (grey bars) in positive serum samples; horizontal bars within the boxed area indicate the median Ad-NAb titer. Serum titers were defined by the dilution at which ≥90% infection inhibition was observed.
Next, heat-inactivated sera from 55 male human immunodeficiency virus-positive (HIV+) volunteers participating in the Amsterdam cohort studies on AIDS were tested. The first serum sample was taken at a median time of 24 months after HIV type 1 seroconversion or HIV+ entry in the cohort, when subjects were immune competent and asymptomatic (median CD4+-T-cell count, >400). A second serum sample was collected at a median of 76 months after study entry or seroconversion, when patients were immune compromised and symptomatic (median CD4+-T-cell count, <50). At both time points, 52% of the sera contained sufficient antibody activity to neutralize Ad5 ≥90% (Fig. 2C). In contrast, Ad11 was neutralized at a much lower rate, i.e., in 18.2% (24 months) to 27.8% (72 months) of the serum samples. The observed increase from 18.2 to 27.8% over time in this population was not significant (P = 0.08 by McNemar test) and is within the same range as seroprevalence data obtained from healthy individuals. Likewise, serum neutralization titers obtained in immune-compromised individuals proved similar to those found in healthy human blood donors (Fig. 2D). Finally, for both Ad5 and Ad11, the seroconversions (those patients who changed from negative to positive) were summarized and divided by the total observation time of those patients with negative serology in order to calculate the incidence. During the study, the incidences per 100 persons per year were 3.3 seroconversions (95% confidence interval, 0.7 to 9.5) for Ad5 and 3.8 seroconversions (95% confidence interval, 1.5 to 7.9) for Ad11.
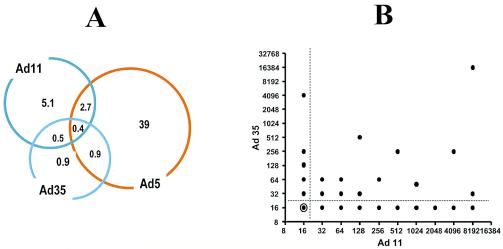
To investigate whether cross-neutralization between Ad11 and Ad35 is likely to occur within the human population, we surveyed Ad35 and Ad11 coincidence within human serum samples. Seroprevalences for Ad5, Ad11, and Ad35 have been determined from serum samples derived from healthy Japanese, European, and American volunteers and proved to be present in decreasing order, ranging from 39 to 5.1 to 0.9% for Ad5, Ad11, and Ad35, respectively. The low level of coincidence (0.5%) between Ad11 and Ad35 (Fig. 3A) indicates an absence of cross-neutralizing antibodies, which is further strengthened by the complete absence of any correlation in Ad35 and Ad11 serum titers in the tested serum panel (Fig. 3B). The difference in adenovirus seroprevalence between data shown in Fig. 2A and 3A is due to the usage of different methods, i.e., a replication inhibition assay (Fig. 3A) versus a more sensitive luciferase marker gene inhibition assay (Fig. 2A).
FIG. 3.
(A) Graphic representation of the adenovirus NAb presence in human serum samples. Circles represent the seroprevalence of Ad5 (39%), Ad35 (0.9%), and Ad11 (5.1%) in 554 serum samples from healthy blood donors in Europe, the United States, and Asia. Samples were tested by using a virus replication inhibition assay as described previously (39). Percentages of samples that tested positive for combinations of two or three adenovirus serotypes are depicted in the overlapping area of the respective circles corresponding to the particular serotypes. (B) Ad35 and Ad11 neutralizing antibodies in African serum samples (n = 200) detected by using a luciferase-transgene inhibition detection assay. Titers were defined as the first serum dilution that yields ≥90% inhibition of luciferase activity. Titers <32 were considered negative. Of the 200 serum samples tested, 116 samples were negative for both Ad35 and Ad11 (white circle with black center). Twenty serum samples contained detectable antibody titers against both Ad11 and Ad35, whereby titers against the two viruses were not matched.
Ad11 vector tropism.
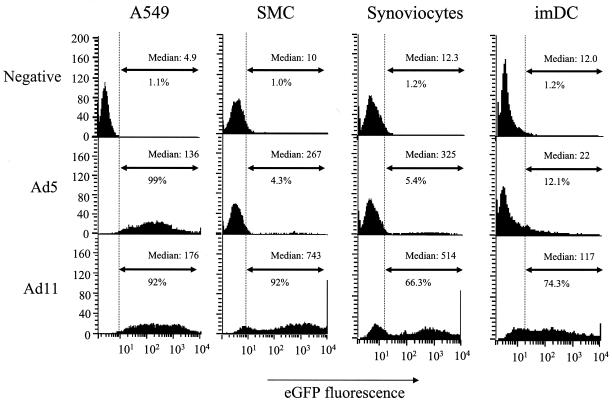
Ad11 and Ad5 viruses equipped with the eGFP reporter gene were used in head-to-head comparison studies involving a set of human primary cells that are considered important target cells for gene therapy or vaccination approaches (Fig. 4). A549 cells were transduced in parallel since this cell line proved equally susceptible for Ad5 and Ad11 and thus allowed testing for the quality of the vector batches used. The results obtained identify Ad11 as being superior to Ad5 for transduction of either saphenous vein SMCs, synoviocytes, or imDCs since both the number of GFP-positive cells as well as the level of GFP fluorescence per cell are significantly increased when Ad11 is used. Next, Ad5 and Ad11 vectors were investigated for their ability to transduce endothelial cells lining the vessel wall in ex vivo human saphenous vein cultures. In contrast to Ad5, recombinant Ad11 transduction resulted in the clear presence of LacZ-positive cells at the luminal site of the vessel (Fig. 5A) that proved to be predominantly endothelial cells (Fig. 5B). Transduction appears to be localized, which is presumably caused by the local loss of the endothelial cell layer which is inherent to mechanical stress during surgical procedures. In the next series of experiments, a panel of human cancer cell lines was used to investigate whether recombinant Ad11 and recombinant Ad35 have similar or distinctive tropisms. The results, summarized in Table 1, indicate that Ad11 and Ad35 are equally capable of transducing these cancer cell types from diverse tissues despite major deviations in the amino acid homology of the fiber knob region of the viruses. A clear correlation between the level of transgene expression and expression of the CD46 molecule could not be established.
FIG. 4.
FACS histograms showing eGFP fluorescence on human A549 cells, saphenous vein SMCs, synoviocytes, and imDCs. Cells were transduced with Ad5 or Ad11 at a multiplicity of infection of 500 vp per cell for 2 h. Samples were analyzed 48 h postinfection. Uninfected cells (negative) were used to set the background gate (dashed line) at approximately 1%. Percentages given below the arrow indicate number of GFP-positive cells. Median fluorescence of eGFP expression per cell is given in the upper-right corner of each histogram.
FIG. 5.
Saphenous vein sections were exposed for 2 h to 2 × 109 and 4 × 108 vp/ml of Ad5 and Ad11 expressing the LacZ marker gene. Sections were washed by medium replacement. β-Galactosidase staining was performed 72 h post-virus exposure. Cross sections were counterstained with nuclear fast red. (A) Macroscopic overview of LacZ staining. (B) Histological cross sections of human saphenous vein stained for LacZ expression demonstrating the presence of LacZ-positive endothelial cells lining the vessel wall.
TABLE 1.
Tropism of Ad11 and Ad35 recombinant vectorsa
| Cell line | Tissue type | % CD46 expression | % eGFP+ cells
|
Median fluorescence
|
||
|---|---|---|---|---|---|---|
| Ad11 | Ad35 | Ad11 | Ad35 | |||
| K562 | Erythroid | 100 | 78.6 | 75.3 | 23.9 | 22.2 |
| SK-N-MC | Neuronal | 0 | 43.2 | 51.4 | 23.3 | 26.7 |
| MCF-7 | Breast | 98 | 99.7 | 98.0 | 4,043.0 | 5,953.0 |
| HS766T | Pancreas | 100 | 99.8 | 99.8 | 9,589.0 | 9,493.0 |
Cells were seeded at 105 cells per ml in 6-well plates and subsequently exposed to 1,000 vp (per cell) of either Ad11 or Ad35 vector carrying eGFP. Forty-eight hours later, cells were harvested, washed, and subjected to FACS analysis. Background fluorescence of each cell population was set at 1% with nontransduced cells. Both the percentage of GFP-positive (GFP+) cells as well as the median fluorescence per cell are shown. Cell membrane expression of the BC-1 isoform of CD46 was determined by using antibody CD46 (WS IV) clone 122.2 at a 1-in-50 dilution (stock concentration, 2.57 mg/ml). As a negative control, secondary antibody fluorescein isothiocyanate- labeled goat anti-mouse immunoglobulin alone was tested at the same concentration.
Bypassing anti-Ad5 preexisting immunity with Ad11 vector.
To analyze Ad11-mediated transduction of a luciferase marker gene in the presence or absence of anti-Ad5 NAb, mice were preimmunized either once or twice by 1010 vp of Ad5.Empty (n = 10). Serum titers against the vectors were determined 2 weeks post-vector immunization (two administrations) or 4 weeks postimmunization (one administration). In vitro determination of anti-vector neutralizing activity demonstrated that anti-vector immunity rose from moderate (geometric mean titer [GMT], 169 and 441 for 1× Ad5.Empty and 1× Ad11.Empty, respectively) to high (GMT, 2,522 and 3,327 for 2× Ad5.Empty and 2× Ad11.Empty, respectively) but clearly remained adenovirus serotype specific (Fig. 6A and B, respectively).
FIG. 6.
Induced anti-Ad5 (black diamonds) and anti-Ad11 (white diamonds) NAb titers plotted per mouse after either one immunization with Ad.Empty vector (A) or two immunizations with Ad.Empty vector (B). Diamonds represent the lowest dilution of individual sera that gives ≥90% inhibition of luciferase activity in the neutralization assay. The dotted line indicates the lowest serum dilution used in the neutralization assay. Geometric mean NAb titers are depicted (black bars). The amount of luciferase activity per microgram of protein obtained in naïve mice following administration of Ad5-Luc or Ad11-Luc served as 100% control and was used to calculate the percentage of remaining luciferase activity in either Ad5- or Ad11-preimmunized mice, respectively. (C) Black bars represent the percentage of remaining luciferase activity using Ad5-luciferase vector obtained in mice preimmunized once with Ad5 (pre-Ad5) or Ad11 (pre-Ad11). White bars represent the percentage of remaining luciferase activity using Ad11-luciferase vector in these mice. (D) Black bars represent the percentage of remaining luciferase activity using Ad5-luciferase vector obtained in mice preimmunized twice with either Ad5 (pre-Ad5) or Ad11 (pre-Ad11). White bars represent the percentage of remaining luciferase activity using Ad11-luciferase vector in these mice. In all cases, values represent the mean ± the standard error of the mean of five mice per group.
Also, 2 weeks after the preimmunization, mice received either Ad5-Luc or Ad11-Luc vectors (1010 vp intramuscularly). Mice (n = 10) injected with phosphate-buffered saline served as naïve controls. Forty-eight hours after Ad5-Luc or Ad11-Luc vector administration, the efficiency of gene transfer was assessed. The median values of the luciferase activity of the individual groups were compared for low and high levels of preexisting immunity and are depicted in Fig. 6C and D, respectively. In the presence of anti-Ad5 neutralizing activity, gene transfer with Ad5.Luc is severely hampered with both high as well as low anti-Ad5 preexisting immunity (0% remaining luciferase activity in both groups). Likewise, in the presence of anti-Ad11 NAbs, gene transfer with Ad11.Luc is severely hampered at both low and high levels of anti-Ad11 NAb (0 and 1.2%, respectively). Thus, full neutralization of the luciferase vector is obtained in the presence of even moderate levels of anti-vector-specific neutralizing activity in homologous administration regimens. In contrast, when Ad5.Luc is administered to Ad11-preimmunized animals or when Ad11-Luc is administered to Ad5-preimmunized animals, a high level of luciferase marker gene activity could still be measured, irrespective of the level of anti-vector immunity. These results thus show that the Ad11 vector has the ability to deliver a transgene to muscle tissue in the presence of either low- or high-level anti-Ad5 immunity.
DISCUSSION
Preexisting immunity towards Ad5 is considered a major hurdle for Ad5-based gene transfer and vaccination strategies. Therefore, several strategies are being employed to circumvent anti-vector immunity, including (i) the physical shielding of the adenovirus coat (2, 4), (ii) the use of nonhuman adenovirus serotypes (14, 16, 41, 43), and (iii) the use of rare human serotypes (25, 39). The shielding of adenoviral capsid proteins is being investigated by encapsulation of the virus capsids with, for instance, poly-lactic-glycolic acid (2) or complexation with poly-l-lysine or lipids (4). Application of nonhuman adenovirus vectors represents a viable strategy since NAbs are not expected to be present in humans, and therefore, this strategy is actively being pursued by using adenovirus derived from diverse species including chimpanzee (41), pig (43), canine (16), and ovine (14). However, current challenges regarding manufacturing capacity and the lack of knowledge regarding nonhuman adenovirus disease association in humans represent parameters that need careful investigation. We have chosen rare human adenovirus types as possible alternatives for Ad5, and it was previously published that Ad35 and Ad11 have low seroprevalence in the human population as determined by use of a replication inhibition assay (39). As such, a vector based on either Ad11 or Ad35 might allow for accurate dose control since one administered dose should always result in a predictable clinical effect. Here, we have shown that we successfully generated an Ad11 vector system that allows generation of recombinant Ad11 vectors on the PER.C6/55K cell line as easily as that of recombinant Ad5 vector, with consistent high yield and with similar purification profiles. By using the recombinant Ad11 vector, serological and epidemiological studies were initiated which led to the conclusions that (i) Ad11 has a low seroprevalence worldwide compared to Ad5; (ii) Ad11 serum titers are up to 10-fold lower than those of Ad5, in accordance with low seroprevalence, except in serum derived from Japan; (iii) Ad11 seroprevalence and serum titer are similar in immune-compromised patients compared to healthy blood donors; (iv) no evidence was found that could indicate that immune-compromised patients are at higher risk of attracting an Ad11 infection compared to healthy individuals; and (v) in sera from healthy volunteers, no correlation was found for Ad11 and Ad35 coincidence (i.e., cross-neutralization on the level of antibodies is not likely to occur between these closely related adenoviruses). Clearly, results obtained in Japan regarding seroprevalence and titers indicate that more investigations into the epidemiology of Ad11 are required to gain better insight into the biology of this virus and its interaction with human immune defense systems. Likewise, it is currently unknown whether B2 group viruses establish infection mainly through opportunistic infection or whether latent infection can also occur and, if so, what cells or tissues in the human body serve as a virus reservoir (14). In this respect, the availability of recombinant Ad11 vector might play an important role in elucidating such scientific questions.
Our studies with recombinant Ad11 vector in primary cells, organ culture, and cancer cell lines demonstrate that the Ad11-based vector infects smooth muscle cells, synoviocytes, and dendritic cells, which are important target cells for strategies aimed at treatment of cardiovascular disease (11, 12) and rheumatoid arthritis (38) or ex vivo vaccination (37), with much higher efficiency than Ad5. Given the high divergence in the knob region between Ad11 and Ad35, a difference in tropism could be expected that perhaps also explains differences between Ad11 and Ad35 in their known disease association; i.e., Ad11 frequently causes keratoconjunctivitis whereas, to the best of our knowledge, this has not been reported for Ad35 (13). Recently, the human membrane cofactor protein CD46 has been identified as a high-affinity receptor for subgroup B viruses including Ad11, Ad35 (9, 24), and Ad3 (29). Four isoforms of CD46 have been described (20, 26), and CD46 is expressed on the surface of all nucleated human cells (17). At present, it is not known whether only a certain CD46 isoform(s) can function as a high-affinity receptor for B2 adenoviruses or if all CD46 molecules can be utilized. Also, which CD46 isoforms are expressed in diverse cell types, be it either primary or cell line, is at present uncharted, as is their relative abundance on the surface of different human cells. Thus, elucidating the exact usage of CD46 isoforms by Ad11 or Ad35 is a prerequisite to map possible differences in the ability of these viruses to target cells or tissues in vivo or in vitro.
The preimmunization studies with Ad5 and Ad11 vectors yielded a model system in mice with anti-vector titers that nicely correlate with the GMT range of neutralizing antibody titers commonly observed in humans.
Although the antibody response proved serotype specific, an inhibitory effect (up to twofold reduction in luciferase activity) was observed in mice treated with Ad11-luciferase following Ad5 preimmunization. This reduction in marker gene expression might be due to either a specific innate immunity via a complement activation mechanism described previously for bacteriophages (30) or, most likely, cross-reactive T cells (36). The apparent lack of cross-neutralization between Ad11 and Ad35 in sera from human individuals may allow Ad11 and Ad35 readministration strategies, although this clearly needs further investigation, especially at the T-cell level.
The homologous neutralization experiments showed that high-level Ad11-specific neutralizing antibodies against Ad11 vector could be obtained in mice. Therefore, the low seroprevalence observed for Ad11 in the human population is likely not caused by an intrinsic ability of Ad11 to evade a host immune system. The latter finding strengthens our present hypothesis that impaired transmission most likely accounts for the observed low seroprevalence of Ad11, although this needs to be investigated further.
In summary, we have generated a manufacturing platform that allows the easy manufacture of replication-deficient Ad11 vector at high titer. The Ad11 vector displays a superior tropism for a panel of cells of interest for both gene therapy strategies as well as vaccination. Moreover, Ad11 vector has a significantly lower seroprevalence than Ad5 in the human population and is not hampered in its ability to transfer a heterologous gene by the presence of anti-Ad5 neutralizing activity. Collectively, these data thus support the further development of Ad11 as a vector for gene transfer either for the treatment of inherited or congenital diseases or for therapeutic and prophylactic vaccination strategies.
Acknowledgments
We thank Germaine Penders and Dennis de Lange for skillful production of virus batches. We thank Gerrit Jan Weverling for statistical analysis and critical review of the manuscript.
REFERENCES
- 1.Barouch, D. H., P. F. McKay, S. M. Sumida, S. Santra, S. S. Jackson, D. A. Gorgone, M. A. Lifton, B. K. Chakrabarti, L. Xu, G. J. Nabel, and N. L. Letvin. 2003. Plasmid chemokines and colony-stimulating factors enhance the immunogenicity of DNA priming-viral vector boosting human immunodeficiency virus type 1 vaccines. J. Virol. 77:8729-8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beer, S. J., C. B. Matthews, C. S. Stein, B. D. Ross, J. M. Hilfinger, and B. L. Davidson. 1998. Poly (lactic-glycolic) acid copolymer encapsulation of recombinant adenovirus reduces immunogenicity in vivo. Gene Ther. 5:740-746. [DOI] [PubMed] [Google Scholar]
- 3.Casimiro, D. R., L. Chen, T.-M. Fu, R. K. Evans, M. J. Caulfield, M.-E. Davies, A. Tang, M. Chen, L. Huang, V. Harris, D. C. Freed, K. A. Wilson, S. Dubey, D.-M. Zhu, D. Nawrocki, H. Mach, R. Troutman, L. Isopi, D. Williams, W. Hurni, Z. Xu, J. G. Smith, S. Wang, X. Liu, L. Guan, R. Long, W. Trigona, G. J. Heidecker, H. C. Perry, N. Persaud, T. J. Toner, Q. Su, X. Liang, R. Youil, M. Chastain, A. J. Bett, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chillon, M., J. H. Lee, A. Fasbender, and M. J. Welsh. 1998. Adenovirus complexed with polyethylene glycol and cationic lipid is shielded from neutralizing antibodies in vitro. Gene Ther. 5:995-1002. [DOI] [PubMed] [Google Scholar]
- 5.Clark, K. R., and P. R. Johnson. 2001. Gene delivery of vaccines for infectious disease. Curr. Opin. Mol. Ther. 3:375-384. [PubMed] [Google Scholar]
- 6.Crystal, R. G., N. G. McElvaney, M. A. Rosenfeld, C. S. Chu, A. Mastrangeli, J. G. Hay, S. L. Brody, H. A. Jaffe, N. T. Eissa, and C. Danel. 1994. Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Nat. Genet. 8:42-51. [DOI] [PubMed] [Google Scholar]
- 7.Emini, E. A. 2003. Status of the Mench HIV-1 vaccine program. Keystone Symposia on HIV-1 Vaccine Development, Banff, Canada.
- 8.Fallaux, F. J., A. Bout, I. van der Velde, D. J. van den Wollenberg, K. M. Hehir, J. Keegan, C. Auger, S. J. Cramer, H. van Ormondt, A. J. van der Eb, D. Valerio, and R. C. Hoeben. 1998. New helper cells and matched early region 1-deleted adenovirus vectors prevent generation of replication-competent adenoviruses. Hum. Gene Ther. 9:1909-1917. [DOI] [PubMed] [Google Scholar]
- 9.Gaggar, A., D. M. Shayakhmetov, and A. Lieber. 2003. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 9:1408-1412. [DOI] [PubMed] [Google Scholar]
- 10.Gahery-Segard, H., V. Molinier-Frenkel, C. Le Boulaire, P. Saulnier, P. Opolon, R. Lengagne, E. Gautier, A. Le Cesne, L. Zitvogel, A. Venet, C. Schatz, M. Courtney, T. Le Chevalier, T. Tursz, J. G. Guillet, and F. Farace. 1997. Phase I trial of recombinant adenovirus gene transfer in lung cancer. Longitudinal study of the immune responses to transgene and viral products. J. Clin. Investig. 100:2218-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gruchal, M., H. Roy, S. Bhardwaj, and S. Yla-Herttuala. 2004. Gene therapy for cardiovascular diseases. Curr. Pharm. Des. 10:407-423. [DOI] [PubMed] [Google Scholar]
- 12.Havenga, M. J., A. A. Lemckert, J. M. Grimbergen, R. Vogels, L. G. Huisman, D. Valerio, A. Bout, and P. H. Quax. 2001. Improved adenovirus vectors for infection of cardiovascular tissues. J. Virol. 75:3335-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hierholzer, J. C. 1992. Adenoviruses in the immunocompromised host. Clin. Microbiol. Rev. 5:262-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofmann, C., P. Loser, G. Cichon, W. Arnold, G. W. Both, and M. Strauss. 1999. Ovine adenovirus vectors overcome preexisting humoral immunity against human adenoviruses in vivo. J. Virol. 73:6930-6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knowles, M. R., K. W. Hohneker, Z. Zhou, J. C. Olsen, T. L. Noah, P. C. Hu, M. W. Leigh, J. F. Engelhardt, L. J. Edwards, K. R. Jones, et al. 1995. A controlled study of adenoviral-vector-mediated gene transfer in the nasal epithelium of patients with cystic fibrosis. N. Engl. J. Med. 333:823-831. [DOI] [PubMed] [Google Scholar]
- 16.Kremer, E. J., S. Boutin, M. Chillon, and O. Danos. 2000. Canine adenovirus vectors: an alternative for adenovirus-mediated gene transfer. J. Virol. 74:505-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liszewski, M. K., T. W. Post, and J. P. Atkinson. 1991. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu. Rev. Immunol. 9:431-455. [DOI] [PubMed] [Google Scholar]
- 18.Mei, Y. F., J. Skog, K. Lindman, and G. Wadell. 2003. Comparative analysis of the genome organization of human adenovirus 11, a member of the human adenovirus species B, and the commonly used human adenovirus 5 vector, a member of species C. J. Gen. Virol. 84:2061-2071. [DOI] [PubMed] [Google Scholar]
- 19.Molnar-Kimber, K. L., D. H. Sterman, M. Chang, E. H. Kang, M. ElBash, M. Lanuti, A. Elshami, K. Gelfand, J. M. Wilson, L. R. Kaiser, and S. M. Albelda. 1998. Impact of preexisting and induced humoral and cellular immune responses in an adenovirus-based gene therapy phase I clinical trial for localized mesothelioma. Hum. Gene Ther. 9:2121-2133. [DOI] [PubMed] [Google Scholar]
- 20.Post, T. W., M. K. Liszewski, E. M. Adams, I. Tedja, E. A. Miller, and J. P. Atkinson. 1991. Membrane cofactor protein of the complement system: alternative splicing of serine/threonine/proline-rich exons and cytoplasmic tails produces multiple isoforms that correlate with protein phenotype. J. Exp. Med. 174:93-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puumalainen, A. M., M. Vapalahti, R. S. Agrawal, M. Kossila, J. Laukkanen, P. Lehtolainen, H. Viita, L. Paljarvi, R. Vanninen, and S. Yla-Herttuala. 1998. Beta-galactosidase gene transfer to human malignant glioma in vivo using replication-deficient retroviruses and adenoviruses. Hum. Gene Ther. 9:1769-1774. [DOI] [PubMed] [Google Scholar]
- 22.Quax, P. H., J. M. Grimbergen, M. Lansink, A. H. Bakker, M. C. Blatter, D. Belin, V. W. van Hinsbergh, and J. H. Verheijen. 1998. Binding of human urokinase-type plasminogen activator to its receptor: residues involved in species specificity and binding. Arterioscler. Thromb. Vasc. Biol. 18:693-701. [DOI] [PubMed] [Google Scholar]
- 23.Quax, P. H., M. L. Lamfers, J. H. Lardenoye, J. M. Grimbergen, M. R. de Vries, J. Slomp, M. C. de Ruiter, M. M. Kockx, J. H. Verheijen, and V. W. van Hinsbergh. 2001. Adenoviral expression of a urokinase receptor-targeted protease inhibitor inhibits neointima formation in murine and human blood vessels. Circulation 103:562-569. [DOI] [PubMed] [Google Scholar]
- 24.Segerman, A., J. P. Atkinson, M. Marttila, V. Dennerquist, G. Wadell, and N. Arnberg. 2003. Adenovirus type 11 uses CD46 as a cellular receptor. J. Virol. 77:9183-9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seshidhar Reddy, P., S. Ganesh, M. P. Limbach, T. Brann, A. Pinkstaff, M. Kaloss, M. Kaleko, and S. Connelly. 2003. Development of adenovirus serotype 35 as a gene transfer vector. Virology 311:384-393. [DOI] [PubMed] [Google Scholar]
- 26.Seya, T., A. Hirano, M. Matsumoto, M. Nomura, and S. Ueda. 1999. Human membrane cofactor protein (MCP, CD46): multiple isoforms and functions. Int. J. Biochem. Cell Biol. 31:1255-1260. [DOI] [PubMed] [Google Scholar]
- 27.Shabram, P. W., D. D. Giroux, A. M. Goudreau, R. J. Gregory, M. T. Horn, B. G. Huyghe, X. Liu, M. H. Nunnally, B. J. Sugarman, and S. Sutjipto. 1997. Analytical anion-exchange HPLC of recombinant type-5 adenoviral particles. Hum. Gene Ther. 8:453-465. [DOI] [PubMed] [Google Scholar]
- 28.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 29.Sirena, D., B. Lilienfeld, M. Eisenhut, S. Kalin, K. Boucke, R. R. Beerli, L. Vogt, C. Ruedl, M. F. Bachmann, U. F. Greber, and S. Hemmi. 2004. The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3. J. Virol. 78:4454-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sokoloff, A. V., I. Bock, G. Zhang, M. G. Sebestyen, and J. A. Wolff. 2000. The interactions of peptides with the innate immune system studied with use of T7 phage peptide display. Mol. Ther. 2:131-139. [DOI] [PubMed] [Google Scholar]
- 31.Sprangers, M. C., W. Lakhai, W. Koudstaal, M. Verhoeven, B. F. Koel, R. Vogels, J. Goudsmit, M. J. Havenga, and S. Kostense. 2003. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors. J. Clin. Microbiol. 41:5046-5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stephenson, J. R. 2001. Genetically modified viruses: vaccines by design. Curr. Pharm. Biotechnol. 2:47-76. [DOI] [PubMed] [Google Scholar]
- 33.Stone, D., A. Furthmann, V. Sandig, and A. Lieber. 2003. The complete nucleotide sequence, genome organization, and origin of human adenovirus type 11. Virology 309:152-165. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan, N. J., T. W. Geisbert, J. B. Geisbert, L. Xu, Z. Y. Yang, M. Roederer, R. A. Koup, P. B. Jahrling, and G. J. Nabel. 2003. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature 424:681-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sullivan, N. J., A. Sanchez, P. E. Rollin, Z. Y. Yang, and G. J. Nabel. 2000. Development of a preventive vaccine for Ebola virus infection in primates. Nature 408:605-609. [DOI] [PubMed] [Google Scholar]
- 36.Sumida, S. M., D. M. Truitt, M. G. Kishko, J. C. Arthur, S. S. Jackson, D. A. Gorgone, M. A. Lifton, W. Koudstaal, M. G. Pau, S. Kostense, M. J. Havenga, J. Goudsmit, N. L. Letvin, and D. H. Barouch. 2004. Neutralizing antibodies and CD8+ T lymphocytes both contribute to immunity to adenovirus serotype 5 vaccine vectors. J. Virol. 78:2666-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tuettenberg, A., H. Jonuleit, T. Tuting, J. Bruck, J. Knop, and A. H. Enk. 2003. Priming of T cells with Ad-transduced DC followed by expansion with peptide-pulsed DC significantly enhances the induction of tumor-specific CD8+ T cells: implications for an efficient vaccination strategy. Gene Ther. 10:243-250. [DOI] [PubMed] [Google Scholar]
- 38.van der Laan, W. H., P. H. Quax, C. A. Seemayer, L. G. Huisman, E. J. Pieterman, J. M. Grimbergen, J. H. Verheijen, F. C. Breedveld, R. E. Gay, S. Gay, T. W. Huizinga, and T. Pap. 2003. Cartilage degradation and invasion by rheumatoid synovial fibroblasts is inhibited by gene transfer of TIMP-1 and TIMP-3. Gene Ther. 10:234-242. [DOI] [PubMed] [Google Scholar]
- 39.Vogels, R., D. Zuijdgeest, R. van Rijnsoever, E. Hartkoorn, I. Damen, M. P. de Bethune, S. Kostense, G. Penders, N. Helmus, W. Koudstaal, M. Cecchini, A. Wetterwald, M. Sprangers, A. Lemckert, O. Ophorst, B. Koel, M. van Meerendonk, P. Quax, L. Panitti, J. Grimbergen, A. Bout, J. Goudsmit, and M. Havenga. 2003. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J. Virol. 77:8263-8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wijnberg, M. J., P. H. Quax, N. M. Nieuwenbroek, and J. H. Verheijen. 1997. The migration of human smooth muscle cells in vitro is mediated by plasminogen activation and can be inhibited by alpha2-macroglobulin receptor associated protein. Thromb. Haemost. 78:880-886. [PubMed] [Google Scholar]
- 41.Xiang, Z., G. Gao, A. Reyes-Sandoval, C. J. Cohen, Y. Li, J. M. Bergelson, J. M. Wilson, and H. C. Ertl. 2002. Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J. Virol. 76:2667-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang, Z. Y., L. S. Wyatt, W. P. Kong, Z. Moodie, B. Moss, and G. J. Nabel. 2003. Overcoming immunity to a viral vaccine by DNA priming before vector boosting. J. Virol. 77:799-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zakhartchouk, A., Y. Zhou, and S. K. Tikoo. 2003. A recombinant E1-deleted porcine adenovirus-3 as an expression vector. Virology 313:377-386. [DOI] [PubMed] [Google Scholar]