Abstract
The core of the gp120 glycoprotein from human immunodeficiency virus type 1 (HIV-1) is comprised of three major structural domains: the outer domain, the inner domain, and the bridging sheet. The outer domain is exposed on the HIV-1 envelope glycoprotein trimer and contains binding surfaces for neutralizing antibodies such as 2G12, immunoglobulin G1b12, and anti-V3 antibodies. We expressed the outer domain of HIV-1YU2 gp120 as an independent protein, termed OD1. OD1 efficiently bound 2G12 and a large number of anti-V3 antibodies, indicating its structural integrity. Immunochemical studies with OD1 indicated that antibody responses against the outer domain of the HIV-1 gp120 envelope glycoprotein are rare in HIV-1-infected human sera that potently neutralize the virus. Surprisingly, such outer-domain-directed antibody responses are commonly elicited by immunization with recombinant monomeric gp120. Immunization with soluble, stabilized HIV-1 envelope glycoprotein trimers elicited antibody responses that more closely resembled those in the sera of HIV-1-infected individuals. These results underscore the qualitatively different humoral immune responses elicited during natural infection and after gp120 vaccination and help to explain the failure of gp120 as an effective vaccine.
The envelope glycoprotein of human immunodeficiency virus 1 (HIV-1) is expressed as a gp160 precursor, which is then proteolytically cleaved into two mature glycoproteins, gp120 and gp41, in the Golgi apparatus (2, 18, 19, 53, 67, 73). On the virion surface, HIV-1 envelope glycoprotein spikes function as a trimer of gp120/gp41 heterodimers (13, 20, 38, 52, 61, 69, 75). Because the envelope glycoproteins are the only viral component exposed to the external environment, they represent the sole legitimate target for neutralizing antibodies. In the context of a trimer, the gp120 subunits make up the main exposed surface of an HIV-1 envelope spike and shield most of the gp41 subunit; thus, the gp120 glycoprotein has been a focus for vaccine development (9, 35, 36, 72, 73). Recombinant, soluble monomeric gp120 has been used as the first generation of candidate immunogens for a prophylactic vaccine against HIV-1 infection (3, 5, 6, 15, 25, 27, 39, 54, 65, 71; VaxGen, unpublished data). Although gp120 induces a high level of antibody responses in animals and humans, these antibodies typically exhibit only low ability to neutralize HIV-1. Sera from gp120-immunized subjects sometimes can neutralize a limited scope of T-cell-adapted strains of HIV-1, which are more susceptible to neutralization than primary HIV-1 isolates. Therefore, such vaccine candidates are of limited practical use in preventing HIV-1 infection, as exemplified by the poor protective efficacy observed in clinical trials of gp120 (VaxGen, unpublished). The deficiency of gp120 in eliciting neutralizing antibodies has several explanations, and studies aimed at understanding these gp120 properties may be useful for the future design of useful vaccines.
Many efforts have been made to improve the immunogenicity of HIV-1 envelope glycoproteins. Soluble trimers of HIV-1 envelope glycoproteins have been designed to better mimic the native envelope spikes of HIV-1 (12, 59, 74-76, 78, 79). Homogeneous and stable soluble trimers, including the ectodomains of gp120 and gp41, can be expressed as gp140 fusion proteins by disrupting the proteolytic cleavage site between gp120 and gp41 and by fusion with a trimerization domain from other proteins, e.g., human GCN4 or the T4 bacteriophage fibritin. In mice, such trimeric envelope proteins elicit better neutralizing antibodies against primary isolates of HIV-1 than monomeric gp120 (78). However, the improvement in breadth and titer of the neutralizing antibodies elicited by the soluble trimers is still very limited. The exact nature of antibody responses to immunization with these envelope glycoprotein trimers is not well characterized.
Many monoclonal antibodies (MAbs) to the HIV-1 envelope glycoproteins have been derived from naturally infected humans, as well as from animals immunized with viral envelope glycoproteins (3, 5, 15, 17, 27, 29, 39, 51, 54, 64, 65, 71). Generally, they can be divided into four categories (43). First, some MAbs (immunoglobulin G1b12 [IgG1b12], 2G12, and 2F5) can potently neutralize diverse primary HIV-1 isolates (10, 46, 63). These MAbs are derived from HIV-1-infected individuals. Second, antibodies targeting the gp120 variable regions, mainly the V3 and V2 loops, can effectively neutralize only selected viral strains containing the cognate immunoepitopes (4, 8, 22-24, 45, 48, 58, 66). These neutralizing antibodies are commonly induced during HIV-1 infections and animal immunizations, but because of their narrow breadth, they have limited value for vaccine development. Third, some antibodies directed against the CD4-binding site or CD4-induced gp120 epitopes neutralize primary HIV-1 strains only weakly but are capable of neutralizing a range of laboratory-adapted HIV-1 isolates (50, 62, 80). Finally, a large number of MAbs cannot neutralize HIV-1 even at high concentrations but nevertheless are able to bind to at least some preparations of the HIV-1 envelope glycoproteins in vitro (42, 44). Presumably, these antibodies recognize HIV-1 envelope glycoprotein epitopes not well exposed on the functional, assembled envelope glycoprotein trimers.
The humoral immune response to the envelope glycoproteins during natural HIV-1 infection normally results in high titers of antibodies that are capable of binding the envelope glycoproteins in vitro. However, antisera from HIV-1-infected persons are rarely potent in neutralizing primary HIV-1 isolates in vitro (15, 40, 51). Thus, many antibodies naturally elicited by HIV-1 must have minimal neutralizing ability. Antisera from some rare HIV-1-infected individuals potently neutralize a wide spectrum of HIV-1 strains; examples of such sera include anti-HIV-1#1 and anti-HIV-1#2 (68). Ig preparations (e.g., HIV-Ig) made from the pooled sera of HIV-1-infected humans have been shown to neutralize HIV-1 effectively (16a, 47b). The antibody species that render these antisera potently neutralizing are not well understood.
The primary peptide sequences of the HIV-1 gp120 envelope glycoprotein can be divided into five conserved and five variable regions (31, 41, 47, 60, 70). Elements of the five conserved regions compose the structural “core” of gp120. The five variable regions are highly glycosylated and form surface-exposed loops, protecting the core from potentially neutralizing antibodies (72). Antibody elicitation by the gp120 variable loops may dominate the immune responses against gp120 during natural HIV-1 infection and in the context of vaccination. Although antibodies to the V1/V2 and V3 loops can be neutralizing, their ability to block HIV-1 infection is strain restricted and is therefore of limited value in a prophylactic context (4, 8, 22-24, 45, 48, 58, 66).
The X-ray crystal structure has been obtained for the gp120 core from two HIV-1 strains, HXBc2 and YU2 (34, 35). The envelope glycoproteins of HIV-1HXBc2 and HIV-1YU-2 are vastly different with respect to passage history, coreceptor usage, and sensitivity to neutralization by antibodies (37, 81). Nonetheless, the core structures of these two gp120 glycoproteins are almost identical (34). The gp120 core is composed of an inner domain (ID), an outer domain, and a bridging sheet (35). The outer domain contains several important immunoepitopes on gp120, including those recognized by the 2G12, IgG1b12, and anti-V3 neutralizing antibodies (72). Unfortunately, most of the exposed surface on the outer domain is densely covered by glycans and thus poorly immunogenic (73). All three gp120 core domains contribute to the immunoepitopes near the CD4-binding site; features of gp120 that are thought to contribute to the poor immunogenicity of these structures are discussed below (62, 72). The ID, in conjunction with the N and C termini of gp120, functionally interacts with the gp41 ectodomain, thus maintaining the trimeric structure of the HIV-1 envelope spike (7, 72, 77). In a native trimer of HIV-1 envelope glycoproteins, the ID is thought to be buried inside the spike complex with little potential exposure to antibodies (36). There are several hypothetical reasons why the presence of the ID in a gp120 monomer might be detrimental to the elicitation of neutralizing antibodies. First, relative to the outer domain, the ID is well exposed and has little coverage by glycan; therefore, antibodies to the ID with no neutralizing potential may dominate the immune response (73). Indeed, nonneutralizing antibodies against epitopes located within the ID are among those commonly elicited in HIV-1-infected persons (14, 32). Second, the gp120 core exhibits conformational flexibility and is thought to undergo unfavorable decreases in entropy upon binding antibodies directed against the gp120 regions that interact with the viral receptors. The observed relationship between the binding entropy and neutralizing potency of anti-gp120 antibodies suggests that such interdomain flexibility represents a viral mechanism that has evolved to minimize the production of antibodies to the conserved, exposed gp120 epitopes (33). Finally, the inner and outer domains in a free gp120 monomer may assume conformations that differ from those found in the native envelope glycoprotein spikes, possibly leading to the elicitation of weakly neutralizing or nonneutralizing antibodies.
Here, we express the outer domain of HIV-1 gp120 as an independent protein and study the antigenicity and immunogenicity of this protein.
MATERIALS AND METHODS
Plasmids.
The soluble gp140(-/FT) glycoprotein consists of a human CD5 signal peptide, the HIV-1YU2 gp120 with an altered site for proteolytic cleavage, the gp41 exterior domain, and a trimeric motif from T4 bacteriophage fibritin (76). The sequences encoding this protein and the HIV-1YU2 gp120 glycoprotein were codon optimized for expression in mammalian cells and cloned into pcDNA3.1(Zeo/-) (Invitrogen).
To construct the OD1 expression vector, the coding sequence for amino acids 252 to 482 of the HIV-1YU2 envelope glycoproteins (numbered as in the prototypical HIV-1HXBc2 gp160 sequence) was PCR amplified from the codon-optimized gp140(-/FT) expression vector (30). The amplified fragment was inserted back into the same vector so that the open reading frame read as follows: MPMGSLAPLATLYLLGMLVASVLA-R252PVVST… DNWRS482-Stop. The open reading frame for the OD1-PADRE protein expression ends as “… DNW480GGAKFVAAGHHHHHH-Stop.” The OD1-ΔV3-PADRE expression construct was made by replacing the sequence encoding “…PNNNTRKSINIGPGRALYTTGEIIGDIRQ…” in the V3 loop with a sequence encoding a GAG linker in the OD1-PADRE expression construct. The ID construct was made by deleting amino acids 252 to 482 and inserting a GAG linker as a replacement in the codon-optimized gp140(-/FT) expression vector by using a Quik-Change protocol (Stratagene). The entire coding regions of the above constructs were sequenced to verify the construction.
Transfection and immunoprecipitation.
The env-expressing plasmid was transfected into 293T cells by using Lipofectamine reagent (Invitrogen) according to the manufacturer's recommendations. Beginning at ca. 24 h after transfection, the cells were labeled with 200 μCi of [35S]methionine-cysteine each in 5 ml of methionine-cysteine-free medium overnight. The culture medium was then harvested after centrifugation at 2,500 rpm for 10 min.
For immunoprecipitations, 400 μl of medium was incubated overnight at 4°C with 3 μl of pooled sera from HIV-1-infected persons or 1 μg of a purified MAb and 50 μl of protein A-Sepharose (10% [wt/vol] in phosphate-buffered saline [PBS]; Pharmacia) that had been preincubated with 5% bovine serum albumin in PBS. After three washes with 1× PBS, the beads were boiled for 5 min in 1× sodium dodecyl sulfate (SDS) sample buffer with 2% β-mercaptoethanol. The protein samples were then analyzed on SDS-10% polyacrylamide gels.
Expression of recombinant proteins and immunization of rabbits.
The OD1 protein and derivatives were expressed in the S2 Drosophila cells after stable transfection, as previously described (16). The proteins were secreted into the cell culture media and then purified by using the C-terminal His6 tags. Briefly, the protein was first loaded onto a column of the Ni-NTA Superflow gel (Qiagen, Inc.). The column was washed with 20 mM imidazole in a buffer of 20 mM Tris-HCl (pH 7.4)-500 mM NaCl. The bound protein was then eluted with 250 mM imidazole in a buffer of 20 mM Tris-HCl (pH 7.4)-150 mM NaCl. The protein was concentrated by using Centriprep-10 (Amicon) and quantified by measurements of optical density at 280 nm (OD280). The final products were visualized by Coomassie blue staining of an SDS-10% polyacrylamide gel. The purified proteins were then snap-frozen in a dry ice-ethanol bath and stored at −80°C.
The HIV-1YU2 gp120 and gp140(-/FT) glycoproteins were stably expressed in CHO-K1 cells. The proteins were secreted into protein-free medium (ProCHO5-CDM; BioWhittaker, Inc.) in suspension cultures. After concentration with Pellicon-2-100K microfilters (Millipore, Inc.), the protein was sequentially loaded onto 15 ml of Ni-NTA Superflow gel four times. The resulting gel batches were combined and loaded into a column and washed with 20 mM imidazole in a buffer of 20 mM Tris-HCl (pH 7.4)-500 mM NaCl. The bound protein was then eluted with 250 mM imidazole in a buffer of 20 mM Tris-HCl (pH 7.4)-150 mM NaCl. The protein was then concentrated to 2 to 4 ml by using Centriprep-30 (Amicon) and loaded on a Superose-6 column (26/100; Pharmacia) and eluted with 20 mM Tris-HCl (pH 7.4)-150 mM NaCl. The proteins at apparent molecular masses of ∼178 kDa for the gp120 monomer and ∼630 kDa for the gp140(-/FT) trimer were harvested. After snap-freezing on dry ice-ethanol and storage at −80°C, the proteins were visualized by Coomassie blue staining of a SDS-10% polyacrylamide gel.
Four New Zealand White rabbits in a group were immunized with each of the purified proteins described above. One week prior to immunization, serum samples were harvested from each rabbit to serve as baseline controls. Each rabbit was injected with 120 μg of protein in 1 ml of 1× Ribi adjuvant (MPL+TDM+CWS; Sigma) subcutaneously at 10 sites three times at 3-week intervals. Two weeks after the third injection, serum samples were again harvested, processed, and stored in the same fashion as the preimmunization samples.
ELISA for anti-V3 antibody binding to OD1.
Binding of human monoclonal anti-V3 antibodies to the OD1 protein was determined by enzyme-linked immunosorbent assay (ELISA) (22). Briefly, the OD1 protein at a concentration of 1 μg/ml was coated onto ELISA plates overnight at 4°C, blocked for 1 h at 37°C with 2% bovine serum albumin in PBS and then washed with 0.05% Tween 20-PBS. The MAbs were added at 1:10 serial dilutions starting at 10 μg/ml, followed by incubation for 1.5 h at 37°C. Plates were washed and, for the detection of bound antibodies, alkaline phosphatase-conjugated goat anti-human IgG (Zymed, San Francisco) was added for 1.5 h at 37°C. After a washing step, the substrate p-nitrophenyl phosphate in 10% diethanolamine was added for 30 min to develop color, and plates were read at 410 nm.
ELISA for measuring antibody responses in sera from immunized rabbits or HIV-1-infected humans.
HIV-Ig (NABI Biopharmaceuticals, Boca Raton, Fla.) (16a) was kindly provided by the AIDS Research and Reference Reagent Program as lyophilized powder and reconstituted in 1× PBS to 50 mg of protein/ml. The anti-HIV-1#1 and anti-HIV-1#2 sera were provided by the AIDS Research and Reference Reagent Program in a frozen state.
To measure the overall antibody responses in the sera of immunized rabbits or HIV-1-infected humans, a standard ELISA was performed as previously described (78). Briefly, 96-well EIA/RIA plates (Costar) were coated with 2 μg of either the OD1 protein, the monomeric gp120 protein, or the trimeric gp140(-/FT) glycoprotein/ml in 1× PBS at 4°C overnight. After four washes with 0.2% Tween 20-PBS, the plates were blocked with 1× SuperBlocker (Pierce) at room temperature for 90 min. The antisera were first diluted in 1× PBS, and 1:5 serial dilutions were then prepared. After four washes of the plates, 100 μl of the diluted sera was added to each well, followed by incubation at room temperature for 1 h. The detecting antibody was anti-rabbit IgG conjugated with horseradish peroxidase (Sigma) or anti-human-Fc-horseradish peroxidase (Sigma) at 1:2,000 dilutions. The bound signal was quantified with a TMB substrate kit (Bio-Rad). The OD450 values were measured, and the mean OD450 value obtained from four wells without the antigen coating was subtracted as background. To perform the antibody adsorption by the designated proteins, the diluted sera were first incubated with 100 μg of the OD1 or gp120 protein/ml at 37°C for 1 h. and then the antibody-protein mixtures were assayed by the above-described ELISA.
Neutralization in a single-round infection assay.
Recombinant HIV-1 encoding luciferase and pseudotyped with the HIV-1 envelope glycoproteins was produced as previously described (28). Briefly, 293T cells in 100-mm tissue culture dishes were cotransfected by the Lipofectamine reagents with 2 μg of pSVIIIenv plasmid expressing the HIV-1 envelope glycoprotein variants, 2 μg of the pCMV-PACK plasmid expressing the Gag/Pol and Tat proteins of HIV-1, and 6 μg of pHIV-1-Luc, which expresses an HIV-1 vector with a firefly luciferase reporter gene. Two days after transfection, the recombinant virions in the cell supernatants were harvested and quantitated by measuring the reverse transcriptase activity by [3H]TTP incorporation. Approximately 6 × 103 Cf2Th-CD4/CCR5 cells were seeded into each well of a 96-well luciferase assay plate (EG&G Wallac) and then cultured overnight at 37°C with 5% CO2. For each assay, 300 reverse transcriptase units of the virus stock were added to each well of the target cells, and the cells were cultured for another 48 h and then lysed and used for measurement of luciferase activity. For neutralization assays, the antisera or their dilutions were first mixed with 300 reverse transcriptase units of the virus stock and incubated at 37°C with 5% CO2 for 4 h before being added to the target cells. Pilot studies indicated that a 4-h incubation of sera and viruses resulted in optimal sensitivity for the detection of neutralizing activity (data not shown). The neutralizing activity was judged by comparing the residual infectivity of the target viruses incubated with a given antiserum with that of viruses incubated with the preimmune serum from the same individual rabbit. A 50% reduction of residual infectivity by an antiserum compared to its preimmune serum control was considered significant.
RESULTS
Expression of the outer domain of gp120 as an independent protein.
Two antiparallel strands composed of gp120 amino acid residues 251 to 260 (…251IRPVVSTQLL260…) and 470 to 474 (…470GGGD474…) connect the inner and outer domains (34). The outer domain constructed in the present study includes the gp120 sequence from “252RPVVS…” to “…DNWRS482” fused to the carboxyl terminus of the signal sequence of human CD5. This protein is designated outer domain 1 (OD1). The open reading frame encoding OD1 was expressed under the control of a CMV-IE promoter in the pcDNA3.1(Zeo/-) expression vector (Invitrogen).
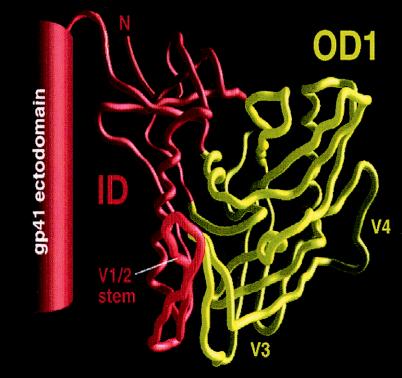
We also attempted to express the complementary portion of the HIV-1 envelope glycoproteins that remains after the removal of the outer domain. This construct, the ID, was made in the context of a stabilized, soluble gp140 trimer, gp140(-/FT) (76). Stable, soluble gp140 trimers result from alteration of the proteolytic cleavage site between gp120 and gp41 and carboxy-terminal fusion of the ectodomain of gp41 with a trimeric motif from T4 bacteriophage fibritin. The ID consists of gp140(-/FT) from which amino acids 252 to 482 were deleted. The boundaries of the OD1 and ID constructs are depicted in Fig. 1.
FIG. 1.
Schematic illustration of the OD1 and ID proteins. The Cα tracing of the gp120 core of HIV-1YU2 is derived from the structure of the CD4-gp120 core-17b Fab complex (35). The gp120 core structures comprising the OD1 protein are colored yellow; the hypothetical location of the V3 loop, which is missing from the gp120 core, is shown. The ID protein (red) includes the ID of the gp120 core and the gp120 V1/V2 loops (not shown), the C1 and C5 regions that are not in the gp120 core, and the gp41 ectodomain.
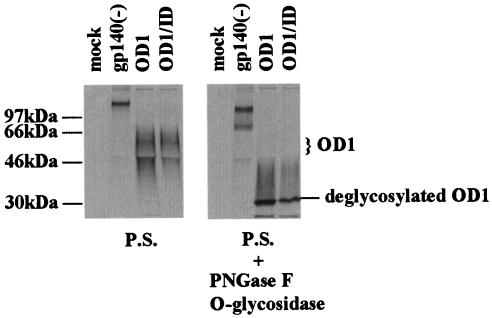
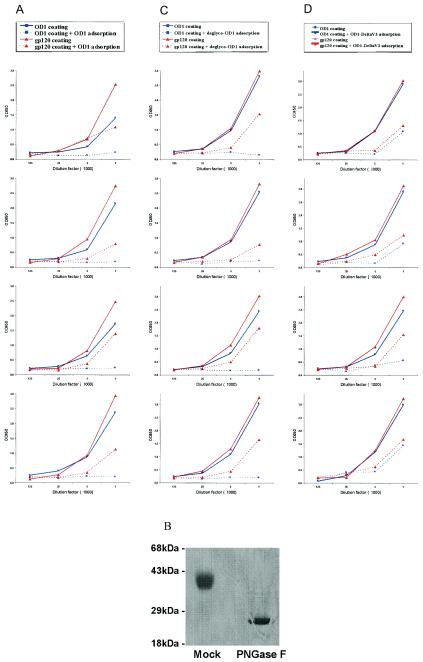
OD1 was transiently expressed in 293T cells and immunoprecipitated with pooled sera from HIV-1-infected persons. OD1 appeared as a 55-kDa band and a broad smear secreted into the cell culture medium. These forms of OD1 could be efficiently deglycosylated by a mixture of endoglycosidases (Fig. 2, right panel). Therefore, the various species of OD1 observed represent glycosylation variants of the protein.
FIG. 2.
Expression of OD1 proteins in 293T cells. 293T cells transfected with a total of 10 μg of envelope glycoprotein expression plasmids were radiolabeled with [35S]cysteine-methionine, and the supernatants were incubated with a mixture of sera from HIV-1-infected persons (P.S.). The precipitates were analyzed on SDS-10% polyacrylamide gels. For OD1 and ID coexpression, 5 μg of each expressor plasmid DNA was transfected. Different species of the OD1 proteins are labeled on the right, and the molecular mass markers are indicated on the left. For deglycosylation, the protein-antibody-Protein A-agarose beads were boiled for 5 min in 1× sample buffer without β-mercaptoethanol. After incubation on ice for a few minutes, 500 U of PNGase F (New England Biolabs) and 0.5 mU of O-glycosidase (Boehringer Mannheim) were added to each sample, followed by incubation for 30 min in a 37°C water bath. The sample was boiled for 5 min before loading on a SDS-10% polyacrylamide gel.
When coexpressed with the ID, the faster-migrating, presumably less-glycosylated, forms of OD1 were less abundant than when OD1 was transfected alone (Fig. 2, left panel). The ID protein itself was expressed at very low level and was detectable after a much longer exposure of the SDS-polyacrylamide gel electrophoresis gel described above (data not shown). Because the immunoprecipitation was performed with a mixture of different antisera, allowing detection of all species of HIV-1 envelope glycoproteins, these results suggest that interactions between the OD1 and ID proteins resulted in more complete glycosylation of the OD1 protein.
We made a construct that expressed the ID protein with a wild-type gp120/gp41 cleavage site and the transmembrane domain and the first 7 amino acids from the gp41 cytoplasmic tail, termed IDΔCT. Although, like the ID protein, IDΔCT facilitated OD1 glycosylation, it did not cooperate with OD1 to mediate viral entry of a pseudotyped luciferase reporter HIV-1 or envelope-mediated cell-cell fusion (data not shown).
Antigenic composition of the OD1 protein.
We evaluated the integrity of conformation-dependent gp120 epitopes on the OD1 protein by immunoprecipitation with MAbs. The 2G12 antibody, which recognizes a carbohydrate epitope on the gp120 outer domain, immunoprecipitated the OD1 proteins very efficiently (Fig. 3) (63). The 39F MAb (kindly donated by J. Robinson, Tulane University), which binds the gp120 V3 loop, preferentially precipitated the more heavily glycosylated form of OD1. Although the major binding surface for the IgG1b12 MAb has been mapped to the outer domain (49, 56), IgG1b12 did not detectably precipitate the OD1 protein (Fig. 3). The OD1 protein did not bind to the CD4 receptor, as judged by coprecipitation with a soluble CD4-Ig fusion protein (data not shown). In addition, the MAbs F105 and F91 directed against the CD4-binding site, the 17b and 48d MAbs directed against the CCR5-binding site, the A32 MAb directed against C1/C4 regions, and the C11 MAb directed against the C1/C5 regions did not precipitate OD1 (data not shown).
FIG. 3.
Binding of MAbs to OD1. Immunoprecipitations of radiolabeled supernatants of transfected 293T cells were performed as in the Fig. 2 legend with either 3 μl of pooled sera from HIV-1-infected persons (P.S.) or 1 μg of purified MAbs.
To evaluate the structural integrity of the V3 loop on the OD1 protein further, the OD1 protein was expressed in Drosophila S2 cells and purified by using a His6 tag at its carboxyl terminus. The OD1 protein was then tested for the ability to bind various anti-V3 antibodies in an ELISA format. These anti-V3 antibodies had been selected by using intact HIV-1SF162 gp120 or synthetic V3 peptides and had been previously characterized for ability to neutralize viral infectivity (21, 22). The OD1 protein bound most of the V3 MAbs selected with native gp120 (Fig. 4A); these MAbs generally exhibit more potent HIV-1-neutralizing activity (21). Interestingly, OD1 also bound MAbs that were selected by synthetic V3 peptides and had some neutralizing capability (Fig. 4B, red curves). In contrast, the binding of OD1 to MAbs that were similarly selected but lacked neutralizing ability was generally weaker (Fig. 4B, blue curves). The binding pattern observed for OD1 was almost identical to that of intact HIV-1SF162 gp120 (21), indicating that the V3 loop of OD1 structurally resembles that of the native gp120 glycoprotein.
FIG. 4.
Binding of human anti-V3 MAbs to the OD1 protein. (A and B) The human MAbs tested were originally selected either with a V3JR-CSF-fusion protein (V3-FP) or gp120LAI (MAbs 694/98 and 1334) (A) or with V3 peptides representing the strains HIV-1MN and HIV-1RF (B). The curves in red represent antibodies with 50% neutralizing activity against HIV-1SF162, and those in blue represent antibodies that achieved <50% neutralization of the same isolate (21). The curves for the negative control antibody, which is directed against the parvovirus B19 protein, are shown in green.
The OD1 protein induced antibody responses in rabbits.
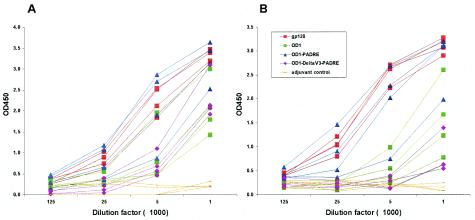
The immunogenicity of the purified OD1 protein in rabbits was compared to that of the full-length HIV-1YU2 gp120 glycoprotein. Previous studies indicated that, as an immunogen, the HIV-1YU2 gp120 core is deficient in Th2-helper epitopes. Fusion of a Pan-reactive epitope for HLA-DR (PADRE) sequence (1) with the gp120 core has been shown to increase its immunogenicity (C. Grundner et al., unpublished results). Thus, to attempt to enhance OD1's immunogenicity, a PADRE sequence was introduced between the C terminus of OD1 and the His6 tag. Furthermore, to evaluate the contribution of V3 to OD1's immunogenicity, the V3 loop was deleted from the OD1-PADRE construct and replaced with a glycine-alanine-glycine linker sequence. All of the proteins were expressed in Drosophila S2 cells and purified by using the His6 tag (Fig. 5). Although complex carbohydrates are not added to proteins synthesized in insect cells, the glycosylation of the OD1 protein produced in the Drosophila cells allowed native folding and efficient recognition by the 2G12 antibody (data not shown). Each protein was mixed with 1× Ribi adjuvant (Sigma) and inoculated into four rabbits. The reactivities of the elicited antibody response to OD1 and to the HIV-1 gp120 glycoprotein were assessed by an ELISA. When OD1 itself was used as the detecting antigen in the assay, all three forms of OD1 could be shown to induce significant antibody responses in the immunized rabbits (Fig. 6A). Notably, the OD1-PADRE protein induced antibody responses to levels close to those of the gp120 controls, whereas the OD1 and OD1-ΔV3-PADRE proteins induced lower levels of antibody responses in rabbits. The titers of antibodies in the sera that could bind to the full-length HIV-1YU2 gp120 glycoprotein were also measured. Sera from rabbits immunized with the OD1-PADRE had titers of antibodies that were capable of binding gp120 at levels close to those of rabbits immunized with the monomeric gp120 protein (Fig. 6B). By comparison, the antibody responses to the full-length gp120 glycoprotein in the animals immunized with the OD1 and OD1-ΔV3-PADRE proteins were less impressive.
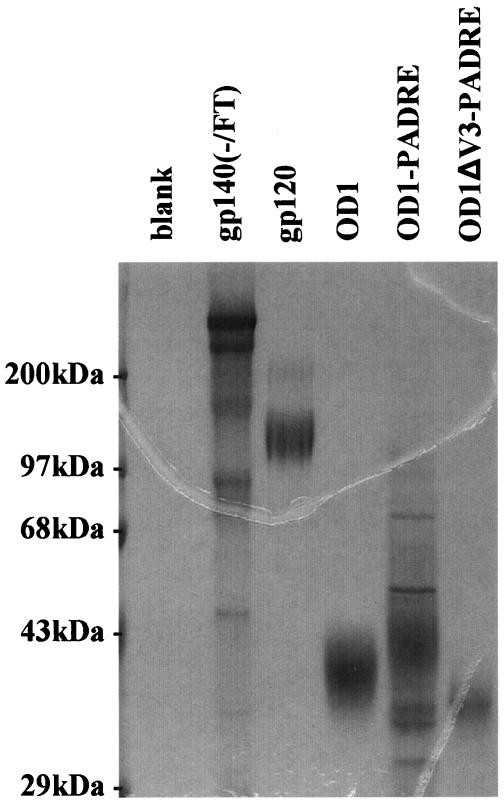
FIG. 5.
Expression and purification of recombinant envelope glycoproteins. The OD1, OD1-PADRE, and OD1-ΔV3-PADRE proteins were stably expressed in Drosophila S2 cells and purified by using the C-terminal His6 tags. The gp120 and gp140(-/FT) glycoproteins were expressed in CHO-K1 cell clones selected for high-level expression. The proteins were first purified by a nickel affinity column and then by size exclusion chromatography. The purified proteins were quantified by OD280 measurements and snap-frozen on dry ice-ethanol. The proteins were added to 1× SDS sample buffer without boiling, run on a 10% SDS-polyacrylamide gel, and visualized by Coomassie blue staining. The OD1, OD1-PADRE, and OD1-ΔV3-PADRE proteins exhibited the same pattern of migration after being boiled in sample buffer with 2% β-mercaptoethanol (data not shown).
FIG. 6.
Binding of immunized rabbit sera to the OD1 protein or the HIV-1YU2 gp120 glycoprotein. Four New Zealand White rabbits in each group were immunized with the purified proteins indicated in the legend. Rabbits were immunized with 120 μg of protein in 1× Ribi adjuvant (Sigma) three times, and serum samples were harvested at 2 weeks after the last injection. Serial dilution of the antisera is indicated on the x axis. The captured antigen on the ELISA plates was either the purified OD1 glycoprotein (A) or the purified HIV-1YU2 gp120 glycoprotein (B).
The sera described above were first heat inactivated and then tested for HIV-1-neutralizing activity. To measure neutralizing ability, 1:10 and 1:40 serum dilutions were incubated with luciferase reporter viruses containing HIV-1KB9 and HIV-1YU2 envelope glycoproteins at 37°C for 4 h. The residual viral infectivities were then measured in a single-round entry assay. As a control, preimmune serum of each individual rabbit was tested in parallel against each virus. Similar to gp120, all three forms of OD1-based immunogens did not induce detectable levels of neutralizing antibodies to either HIV-1KB9 or HIV-1YU2 (data not shown). To test whether OD1 proteins might have utility in priming effective neutralizing antibody responses, two groups of rabbits were injected with either OD1-PADRE or OD1-ΔV3-PADRE three times, followed by a booster injection of the soluble gp140(-/FT) trimer of HIV-1JRFL. The sera from these animals did not demonstrate significant neutralizing activity against HIV-1KB9 or HIV-1YU2 either (data not shown).
Strong antibody responses against the outer domain in rabbits immunized with monomeric gp120.
Monomeric gp120 has been shown to elicit high titers of antibodies that are capable of binding the gp120 glycoprotein and are able to neutralize some selected T-cell-line-adapted HIV-1 strains in vitro (3, 5, 6, 15, 25, 27, 39, 54, 65, 71; VaxGen, unpublished). Such antibody responses are generally not able to neutralize primary HIV-1 isolates, which are the target for vaccines effective against HIV-1 infection in the field. Our experience with gp120 as an immunogen in rabbits is consistent with these properties (26). To better understand the gp120-induced antibody responses qualitatively and quantitatively, we used a competitive ELISA to measure the OD1-reactive antibodies in the sera of gp120-immunized rabbits. Serial dilutions of these sera were incubated with 100 μg of the OD1 protein/ml at 37°C for 1 h prior to ELISA measurements. The ELISA plates were coated with either OD1 or gp120. As a positive control, the OD1-specific antibodies from the target sera were effectively competed by the addition of 100 μg of the OD1 protein/ml (Fig. 7A, solid and broken blue lines). About 80% of the gp120-reactive antibodies in the sera from three of the four gp120-immunized rabbits could be adsorbed by the OD1 protein. The OD1 protein adsorbed a substantial amount of the gp120-reactive antibodies in the serum from the fourth animal. Most of the OD1-specific antibody responses in these rabbits were against the protein elements of gp120, because incubation with the OD1 protein that was deglycosylated by treatment with PNGase F (New England Biolabs) (Fig. 7B) was also able to compete the OD1-specific antibodies from the sera of the four gp120-immunized rabbits (Fig. 7C). Surprisingly, the OD1-specific antibodies in the gp120-immunized rabbit sera were largely directed against elements other than the V3 loop; this was indirectly demonstrated by the efficient adsorption of the gp120-binding antibodies by the OD1-ΔV3-PADRE protein (Fig. 7D). Of note, the V3 loop in a percentage of the gp120 immunogen used in the present study was cleaved at the …NIGPGR314/ALYT… sequence, as determined by mass spectrometry and peptide sequencing, during protein expression in CHO-K1 cells (data not shown). This might reduce the immunogenicity of the V3 loop in such preparations of the monomeric gp120 immunogen.
FIG. 7.
Profiles of antibody responses in rabbits immunized with monomeric gp120 glycoprotein. Four rabbits were immunized with 120 μg of the purified HIV-1YU2 gp120 glycoprotein three times, and serum samples were harvested 2 weeks after the last injection. The antibodies in immunized rabbit sera were detected by an ELISA with the OD1 protein (blue lines) or the gp120 glycoprotein (red lines) as detecting antigen. In one set of experiments, the rabbit antisera were first incubated with 100 μg of the OD1 protein (A), the deglycosylated OD1 proteins (C), or the OD1-ΔV3-PADRE protein (D)/ml, and the glycoprotein-serum mixture was tested in the ELISA by using either the OD1 or gp120 protein as the detecting antigen. (B) To deglycosylate the OD1 protein, 600 μg of the OD1 protein was incubated with 5 μl of PNGase F (New England Biolabs) in 1× G7 buffer at 37°C overnight. Then, 5 μg of the deglycosylated OD1 protein was run on a SDS-14% polyacrylamide gel and visualized after Coomassie blue staining. The experiments shown in panel C, in which the deglycosylated OD1 was added to sera, were conducted on ice to inhibit PNGase F activity.
Profile of antibody responses to soluble gp140(-/FT) trimers.
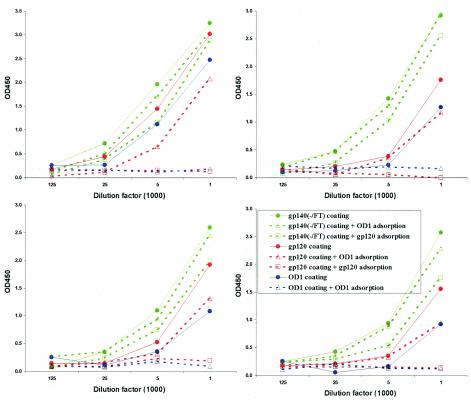
Fusion with trimeric motifs of GCN4 or T4 bacteriophage fibritin has been shown to improve the stability of cleavage-defective HIV-1 envelope glycoprotein trimers (74, 76). These trimeric preparations can induce neutralizing antibodies against primary HIV-1 isolates more efficiently than monomeric gp120 immunogens (78). In the present study, we expressed the fibritin-stabilized soluble trimers of the HIV-1YU2 envelope glycoproteins, gp140(-/FT), in CHO-K1 suspension cells and purified the trimers by using a His6 tag at the carboxyl terminus of the envelope glycoprotein. The resulting protein was further purified by size-exclusion chromatography. When analyzed by Coomassie blue staining of an SDS-10% polyacrylamide gel, the majority of the gp140(-/FT) protein migrated as a 410-kDa band, a finding consistent with the expected behavior of a trimer (Fig. 5). A minor portion of this protein had an apparent molecular mass of ∼360 kDa, a finding consistent with a trimer made of underglycosylated gp140 envelope glycoproteins. A small fraction of this protein exhibited cleavage in the V3 loop (data not shown). Four rabbits were immunized with this preparation of the HIV-1YU2 gp140(-/FT) trimers. The sera from these immunized rabbits demonstrated little capability to neutralize HIV-1YU2 or HIV-1KB9, which are very neutralization-resistant primary isolates, in a single-round entry assay (data not shown). Nonetheless, we sought to examine the immune response to the envelope glycoprotein components of the soluble trimeric preparations. We used the competitive ELISA described above to measure the antibody responses against the gp120 subunit and the gp120 outer domain in the rabbits immunized with the gp140(-/FT) glycoprotein. As a control, incubation with the OD1 protein effectively competed for the OD1-specific antibodies in these serum samples (Fig. 8, solid versus broken blue lines). Similarly, incubation with the monomeric gp120 protein effectively competed for the gp120-specific antibodies in the same sera (Fig. 8). Surprisingly, incubation with the gp120 or OD1 proteins failed to adsorb significant levels of antibodies that were capable of binding the trimeric gp140(-/FT) glycoprotein in the ELISA format (Fig. 8, solid versus broken green lines). Interestingly, preincubation with the OD1 protein had only a mild effect on the gp120-binding antibodies in three of the four sera and a moderate reduction of that in the fourth sample (Fig. 8). These results suggest that a large portion of the antibodies elicited by soluble, stabilized gp140(-/FT) trimers are not able to recognize either monomeric gp120 or OD1.
FIG. 8.
Profiles of antibody responses in rabbits immunized with the trimeric gp140(-/FT) glycoprotein. Four rabbits were immunized with 120 μg of the purified gp140(-/FT) glycoprotein three times and serum samples were harvested 2 weeks after the last injection. The serum antibodies were measured by an ELISA with the OD1 protein (blue lines), the gp120 glycoprotein (red lines), or the gp140(-/FT) glycoprotein (green lines) as detecting antigens. In some experiments, the antisera were incubated with 100 μg of the OD1 protein (▵) or the purified gp120 protein (□)/ml at 37°C for 1 h, and the glycoprotein-antiserum mixture was then tested in the ELISA (dashed lines).
Characterization of antibody responses in potently neutralizing sera from HIV-1-infected individuals.
We wanted to examine the antibody responses to the HIV-1 envelope glycoproteins in the sera of HIV-1-infected individuals that generated potent neutralizing antibodies. HIV-Ig has been prepared from the plasma of HIV-1-infected individuals (NABI Biopharmaceuticals) (16a). HIV-1-neutralizing sera 1 and 2 (anti-HIV-1#1 and anti-HIV-1#2, respectively) were pooled from separate bleeds of individual HIV-1-infected people (16). All three reagents have been shown to neutralize several primary HIV-1 strains. In a pilot experiment, HIV-Ig and anti-HIV-1#2 effectively neutralized HIV-1YU2, whereas all three reagents efficiently neutralized HIV-1HXBc2 in a single-round entry assay (data not shown). Neutralizing concentrations of the three reagents (1:50, 1:200, and 1:800 dilutions of HIV-Ig, anti-HIV-1#1, and anti-HIV-1#2, respectively) were then incubated with 100 μg of the OD1 protein/ml at 37°C for 1 h and tested for the ability to neutralize the HIV-1YU2 and HIV-1HXBC2 luciferase reporter viruses. Preincubation with OD1 had no detrimental effect on the neutralizing potency of these three reagents against HIV-1 (data not shown).
To evaluate the antibody responses against the HIV-1 envelope glycoproteins in the HIV-Ig and anti-HIV-1#1 and anti-HIV-1#2 sera, we measured the antibody titers specific for the outer domain in comparison with the total antibody titers to an intact gp120 monomer or a trimeric gp140(-/FT) glycoprotein. Antibodies specific for OD1 were detected in all three reagents but at relatively low titers. Furthermore, preincubation with a high concentration (100 μg/ml) of the OD1 protein did not significantly compete for antibodies capable of binding the gp140(-/FT) glycoprotein (Fig. 9, solid green lines and circles versus broken green lines and triangles). As a control, incubation with the OD1 protein completely adsorbed the OD1-specific antibodies in the same three reagents (Fig. 9, solid and dashed blue lines). Strikingly, preincubation with 100 μg of the OD1 protein/ml exerted minimal effects on the binding of antibodies to captured monomeric gp120 (Fig. 9, solid and dashed red lines). Apparently, antibodies specific for the gp120 outer domain were poorly represented in these potently neutralizing reagents. Interestingly, preincubation with 100 μg of the monomeric gp120/ml only slightly reduced the reactivity of HIV-Ig, anti-HIV-1#1, and anti-HIV-1#2 with the gp140(-/FT) envelope glycoprotein trimer. As a control, incubation with gp120 completely adsorbed the gp120-specific antibodies from the same reagents (Fig. 9).
FIG. 9.
Profiles of antibodies in HIV-Ig and potently neutralizing sera from HIV-1-infected persons. Antibodies in HIV-Ig and sera (anti-HIV-1#1 and anti-HIV-1#2) from HIV-1-infected persons reactive with the OD1 protein (blue lines), the gp120 monomer (red lines), and the gp140(-/FT) trimer (green lines) were measured in an ELISA. In some experiments, 100 μg of the OD1 protein/ml was used to adsorb the OD1-specific antibodies from the antibodies reactive with the gp120 or gp140(-/FT) proteins (dashed lines with “▵”). Similarly, 100 μg of the purified gp120 protein/ml was used to adsorb antibodies from the pool of gp140(-/FT)-reactive specific antibodies (dashed lines with “□”). The experiments were done twice, and similar results were achieved; the data shown are from a single set of experiments.
DISCUSSION
The outer domain of the HIV-1YU2 gp120 glycoprotein was expressed as an independent protein, OD1. The OD1 protein was precipitated by the 2G12 MAb, which recognizes an array of carbohydrates on the gp120 outer domain (11, 55, 57, 63). The OD1 protein was also recognized by a large number of antibodies directed against the gp120 V3 loop; many of these antibodies have been shown to recognize conformation-dependent V3 loop epitopes (21). The pattern of binding of this panel of anti-V3 antibodies to OD1 closely resembled that seen for the native gp120 glycoprotein. These observations suggest that the structure of the OD1 protein closely mimics that of the outer domain in the native gp120 glycoprotein. Thus, the recombinant OD1 protein should be useful in structural studies designed to characterize the 2G12 epitope and the conformation of the V3 loop.
The OD1 protein and its derivatives failed to induce antibodies capable of neutralizing primary HIV-1 isolates, such as HIV-1KB9 and HIV-1YU2. The OD1 protein did not adsorb the HIV-1-neutralizing antibodies from HIV-Ig or from two well-characterized sera from HIV-1-infected individuals (68). Moreover, the titer of antibodies specific for the outer domain was relatively low in these reagents, even when only the anti-gp120 antibodies were examined. These observations support the notion that the heavily glycosylated portion of the gp120 outer domain, which is potentially accessible to antibodies on the native HIV-1 envelope glycoprotein trimer, is poorly immunogenic.
Interestingly, high titers of antibodies specific for the gp120 outer domain were observed in sera from rabbits immunized with a monomeric gp120 glycoprotein, in stark contrast to the low abundance of antibodies specific for the gp120 outer domain in HIV-Ig, anti-HIV-1#1 and anti-HIV-1#2. A significant portion of the gp120-reactive antibodies in the sera of gp120-immunized rabbits could be adsorbed by the OD1 protein. These observations suggest that immunization with gp120 results in the elicitation of outer-domain-reactive antibodies. Adsorption experiments with deglycosylated or V3 loop-deleted versions of OD1 suggest that many of these antibodies are directed against protein elements of the outer domain other than the V3 loop. Since the HIV-1-neutralizing activity in the sera of animals immunized with gp120 and OD1 is poor, it appears that most of the elicited outer-domain-reactive antibodies are undesirable. The strong correlation between antibody binding to the HIV-1 envelope glycoprotein trimers on the virion surface and neutralization raises the possibility that the elicited antibodies are directed to outer domain elements not accessible on the assembled trimer. The extreme conformational flexibility of the free gp120 glycoprotein (33, 47a) could result in the exposure of such elements; thus, interdomain movement may cause gp120 to resemble its disassembled domains as an immunogen. The resulting misdirection of antibody responses to outer domain elements could contribute to the ineffectiveness of monomeric gp120 as a vaccine.
Soluble, stabilized trimeric gp140 glycoproteins elicit HIV-1-neutralizing antibodies more efficiently than monomeric gp120 glycoproteins (78). In contrast to the antibodies elicited in rabbits by gp120, the antibody response to the soluble gp140(-/FT) protein in rabbits exhibited less outer domain reactivity. The OD1 protein adsorbed little of the gp140-reactive or gp120-reactive antibodies elicited by the soluble gp140(-/FT) trimers. Of note, even the complete gp120 glycoprotein competed only poorly for the gp140-reactive antibodies elicited by these trimeric protein preparations. The antibody profiles elicited in response to soluble gp140(-/FT) resembled those in HIV-Ig and the potently neutralizing sera from HIV-1-infected individuals. The gp140-reactive antibodies elicited by soluble, stabilized trimers and during natural infection may be directed against gp120 epitopes that preferentially exist in envelope glycoprotein trimers or, alternatively or in addition, against gp41 epitopes. Since the neutralizing antibody response to soluble gp140(-/FT) is weaker than those in the potently neutralizing human serum preparations, some of the antibodies elicited by these soluble trimers may be directed against epitopes not well represented on the virion-associated envelope glycoproteins. Nonetheless, the resemblance of the antibody reactivity patterns in soluble gp140(-/FT)-immunized animals and HIV-1-infected humans hints that the expression of soluble, stabilized trimers is a step in the right direction toward a vaccine that can elicit effective neutralizing antibodies.
Acknowledgments
We acknowledge the valuable assistance of the AIDS Research and Reference Reagent Program and thank L. Vujcic and G. Quinnan, the original suppliers of the anti-HIV-1#1 and anti-HIV-1#2 sera. We thank Yvette McLaughlin and Sheri Farnum for assistance with manuscript preparation.
This study was supported by NIH grants (AI24755, AI31783, AI39420, and AI40895), by a Center for AIDS Research grant (AI42848), by an unrestricted research grant from the Bristol-Myers Squibb Foundation, by a gift from late William F. McCarty-Cooper, and by funds from the International AIDS Vaccine Initiative.
REFERENCES
- 1.Alexander, J., J. Sidney, S. Southwood, J. Ruppert, C. Oseroff, A. Maewal, K. Snoke, H. M. Serra, R. T. Kubo, A. Sette, et al. 1994. Development of high potency universal DR-restricted helper epitopes by modification of high-affinity DR-blocking peptides. Immunity 1:751-761. [DOI] [PubMed] [Google Scholar]
- 2.Allan, J. S., J. E. Coligan, F. Barin, M. F. McLane, J. G. Sodroski, C. A. Rosen, W. A. Haseltine, T. H. Lee, and M. Essex. 1985. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV-III. Science 228:1091-1094. [DOI] [PubMed] [Google Scholar]
- 3.Barnett, S. W., S. Rajasekar, H. Legg, B. Doe, D. H. Fuller, J. R. Haynes, C. M. Walker, and K. S. Steimer. 1997. Vaccination with HIV-1 gp120 DNA induces immune responses that are boosted by a recombinant gp120 protein subunit. Vaccine 15:869-873. [DOI] [PubMed] [Google Scholar]
- 4.Beddows, S., S. Louisirirotchanakul, R. Cheingsong-Popov, P. J. Easterbrook, P. Simmonds, and J. Weber. 1998. Neutralization of primary and T-cell line adapted isolates of human immunodeficiency virus type 1: role of V3-specific antibodies. J. Gen. Virol. 79:77-82. [DOI] [PubMed] [Google Scholar]
- 5.Belshe, R. B., G. J. Gorse, M. J. Mulligan, T. G. Evans, M. C. Keefer, J. L. Excler, A. M. Duliege, J. Tartaglia, W. I. Cox, J. McNamara, K. L. Hwang, A. Bradney, D. Montefiori, K. J. Weinhold, et al. 1998. Induction of immune responses to HIV-1 by canarypox virus (ALVAC) HIV-1 and gp120 SF-2 recombinant vaccines in uninfected volunteers. AIDS 12:2407-2415. [DOI] [PubMed] [Google Scholar]
- 6.Berman, P. W., T. J. Gregory, L. Riddle, G. R. Nakamura, M. A. Champe, J. P. Porter, F. M. Wurm, R. D. Hershberg, E. K. Cobb, and J. W. Eichberg. 1990. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature 345:622-625. [DOI] [PubMed] [Google Scholar]
- 7.Binley, J. M., R. W. Sanders, B. Clas, N. Schuelke, A. Master, Y. Guo, F. Kajumo, D. J. Anselma, P. J. Maddon, W. C. Olson, and J. P. Moore. 2000. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J. Virol. 74:627-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broliden, P. A., A. von Gegerfelt, P. Clapham, J. Rosen, E. M. Fenyo, B. Wahren, and K. Broliden. 1992. Identification of human neutralization-inducing regions of the human immunodeficiency virus type 1 envelope glycoproteins. Proc. Natl. Acad. Sci. USA 89:461-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burton, D. R., R. C. Desrosiers, R. W. Doms, W. C. Koff, P. D. Kwong, J. P. Moore, G. J. Nabel, J. Sodroski, I. A. Wilson, and R. T. Wyatt. 2004. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5:233-236. [DOI] [PubMed] [Google Scholar]
- 10.Burton, D. R., J. Pyati, R. Koduri, S. J. Sharp, G. B. Thornton, P. W. Parren, L. S. Sawyer, R. M. Hendry, N. Dunlop, P. L. Nara, et. al. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024-1027. [DOI] [PubMed] [Google Scholar]
- 11.Calarese, D. A., C. N. Scanlan, M. B. Zwick, S. Deechongkit, Y. Mimura, R. Kunert, P. Zhu, M. R. Wormald, R. L. Stanfield, K. H. Roux, J. W. Kelly, P. M. Rudd, R. A. Dwek, H. Katinger, D. R. Burton, and I. A. Wilson. 2003. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science 300:2065-2071. [DOI] [PubMed] [Google Scholar]
- 12.Center, R. J., J. Lebowitz, R. D. Leapman, and B. Moss. 2004. Promoting trimerization of soluble human immunodeficiency virus type 1 (HIV-1) Env through the use of HIV-1/simian immunodeficiency virus chimeras. J. Virol. 78:2265-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan, D. C., D. Fass, J. M. Berger, and P. S. Kim. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263-273. [DOI] [PubMed] [Google Scholar]
- 14.Chien, P. C., Jr., S. Cohen, C. Kleeberger, J. Giorgi, J. Phair, S. Zolla-Pazner, and C. E. Hioe. 2002. High levels of antibodies to the CD4 binding domain of human immunodeficiency virus type 1 glycoprotein 120 are associated with faster disease progression. J. Infect. Dis. 186:205-213. [DOI] [PubMed] [Google Scholar]
- 15.Connor, R. I., B. T. Korber, B. S. Graham, B. H. Hahn, D. D. Ho, B. D. Walker, A. U. Neumann, S. H. Vermund, J. Mestecky, S. Jackson, E. Fenamore, Y. Cao, F. Gao, S. Kalams, K. J. Kunstman, D. McDonald, N. McWilliams, A. Trkola, J. P. Moore, and S. M. Wolinsky. 1998. Immunological and virological analyses of persons infected by human immunodeficiency virus type 1 while participating in trials of recombinant gp120 subunit vaccines. J. Virol. 72:1552-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Culp, J. S., H. Johansen, B. Hellmig, J. Beck, T. J. Matthews, A. Delers, and M. Rosenberg. 1991. Regulated expression allows high level production and secretion of HIV-1 gp120 envelope glycoprotein in Drosophila Schneider cells. Biotechnology 9:173-177. [DOI] [PubMed] [Google Scholar]
- 16a.Cummins, L. M., K. J. Weinhold, T. J. Matthews, A. Langlois, C. F. Perno, R. M. Condie, and J. P. Allain. 1991. Preparation and characterization of an intravenous solution of IgG from human immunodeficiency virus-seropositive donors. Blood 77:1111-1117. [PubMed] [Google Scholar]
- 17.Earl, P. L., C. C. Broder, D. Long, S. A. Lee, J. Peterson, S. Chakrabarti, R. W. Doms, and B. Moss. 1994. Native oligomeric human immunodeficiency virus type 1 envelope glycoprotein elicits diverse monoclonal antibody reactivities. J. Virol. 68:3015-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Earl, P. L., R. W. Doms, and B. Moss. 1990. Oligomeric structure of the human immunodeficiency virus type 1 envelope glycoprotein. Proc. Natl. Acad. Sci. USA 87:648-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Earl, P. L., B. Moss, and R. W. Doms. 1991. Folding, interaction with GRP78-BiP, assembly, and transport of the human immunodeficiency virus type 1 envelope protein. J. Virol. 65:2047-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farzan, M., H. Choe, E. Desjardins, Y. Sun, J. Kuhn, J. Cao, D. Archambault, P. Kolchinsky, M. Koch, R. Wyatt, and J. Sodroski. 1998. Stabilization of human immunodeficiency virus type 1 envelope glycoprotein trimers by disulfide bonds introduced into the gp41 glycoprotein ectodomain. J. Virol. 72:7620-7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorny, M. K., K. Revesz, C. Williams, B. Volsky, M. K. Louder, C. A. Anyangwe, C. Krachmarov, S. C. Kayman, A. Pinter, A. Nadas, P. N. Nyambi, J. R. Mascola, and S. Zolla-Pazner. 2004. The v3 loop is accessible on the surface of most human immunodeficiency virus type 1 primary isolates and serves as a neutralization epitope. J. Virol. 78:2394-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorny, M. K., T. C. VanCott, C. Hioe, Z. R. Israel, N. L. Michael, A. J. Conley, C. Williams, J. A. Kessler, 2nd, P. Chigurupati, S. Burda, and S. Zolla-Pazner. 1997. Human monoclonal antibodies to the V3 loop of HIV-1 with intra- and interclade cross-reactivity. J. Immunol. 159:5114-5122. [PubMed] [Google Scholar]
- 23.Gorny, M. K., J. Y. Xu, S. Karwowska, A. Buchbinder, and S. Zolla-Pazner. 1993. Repertoire of neutralizing human monoclonal antibodies specific for the V3 domain of HIV-1 gp120. J. Immunol. 150:635-643. [PubMed] [Google Scholar]
- 24.Goudsmit, J., C. Debouck, R. H. Meloen, L. Smit, M. Bakker, D. M. Asher, A. V. Wolff, C. J. Gibbs, Jr., and D. C. Gajdusek. 1988. Human immunodeficiency virus type 1 neutralization epitope with conserved architecture elicits early type-specific antibodies in experimentally infected chimpanzees. Proc. Natl. Acad. Sci. USA 85:4478-4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graham, B. S., M. C. Keefer, M. J. McElrath, G. J. Gorse, D. H. Schwartz, K. Weinhold, T. J. Matthews, J. R. Esterlitz, F. Sinangil, P. E. Fast, et al. 1996. Safety and immunogenicity of a candidate HIV-1 vaccine in healthy adults: recombinant glycoprotein (rgp) 120—a randomized, double-blind trial. Ann. Intern. Med. 125:270-279. [DOI] [PubMed] [Google Scholar]
- 26.Grundner, C., T. Mirzabekov, J. Sodroski, and R. Wyatt. 2002. Solid-phase proteoliposomes containing human immunodeficiency virus envelope glycoproteins. J. Virol. 76:3511-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haigwood, N. L., P. L. Nara, E. Brooks, G. A. Van Nest, G. Ott, K. W. Higgins, N. Dunlop, C. J. Scandella, J. W. Eichberg, and K. S. Steimer. 1992. Native but not denatured recombinant human immunodeficiency virus type 1 gp120 generates broad-spectrum neutralizing antibodies in baboons. J. Virol. 66:172-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofmann, W., D. Schubert, J. LaBonte, L. Munson, S. Gibson, J. Scammell, P. Ferrigno, and J. Sodroski. 1999. Species-specific, postentry barriers to primate immunodeficiency virus infection. J. Virol. 73:10020-10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klaniecki, J., T. Dykers, B. Travis, R. Schmitt, M. Wain, A. Watson, P. Sridhar, J. McClure, B. Morein, J. T. Ulrich, et al. 1991. Cross-neutralizing antibodies in rabbits immunized with HIV-1 gp160 purified from simian cells infected with a recombinant vaccinia virus. AIDS Res. Hum. Retrovir. 7:791-798. [DOI] [PubMed] [Google Scholar]
- 30.Korber, B. F., F. Kuiken, C. Pillai, and J. Sodroski. 1998. Numbering positions in HIV relative to HXBc2, p. II-A-54-II-A-69. In B. K. Korber, C. Foley, F. Hahn, B. McCutchan, F. Mellor, and J. Sodroski (ed.), Human retroviruses and AIDS. Los Alamos National Laboratories, Los Alamos, N.Mex.
- 31.Kuiken, C., B. Korber, and R. W. Shafer. 2003. HIV sequence databases. AIDS Rev. 5:52-61. [PMC free article] [PubMed] [Google Scholar]
- 32.Kusk, P., K. Holmback, B. O. Lindhardt, E. F. Hulgaard, and T. H. Bugge. 1992. Mapping of two new human B-cell epitopes on HIV-1 gp120. AIDS 6:1451-1456. [DOI] [PubMed] [Google Scholar]
- 33.Kwong, P. D., M. L. Doyle, D. J. Casper, C. Cicala, S. A. Leavitt, S. Majeed, T. D. Steenbeke, M. Venturi, I. Chaiken, M. Fung, H. Katinger, P. W. Parren, J. Robinson, D. Van Ryk, L. Wang, D. R. Burton, E. Freire, R. Wyatt, J. Sodroski, W. A. Hendrickson, and J. Arthos. 2002. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature 420:678-682. [DOI] [PubMed] [Google Scholar]
- 34.Kwong, P. D., R. Wyatt, S. Majeed, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 2000. Structures of HIV-1 gp120 envelope glycoproteins from laboratory-adapted and primary isolates. Struct. Fold. Des. 8:1329-1339. [DOI] [PubMed] [Google Scholar]
- 35.Kwong, P. D., R. Wyatt, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwong, P. D., R. Wyatt, Q. J. Sattentau, J. Sodroski, and W. A. Hendrickson. 2000. Oligomeric modeling and electrostatic analysis of the gp120 envelope glycoprotein of human immunodeficiency virus. J. Virol. 74:1961-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li, Y., J. C. Kappes, J. A. Conway, R. W. Price, G. M. Shaw, and B. H. Hahn. 1991. Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured human brain tissue: identification of replication-competent and -defective viral genomes. J. Virol. 65:3973-3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu, M., S. C. Blacklow, and P. S. Kim. 1995. A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat. Struct. Biol. 2:1075-1082. [DOI] [PubMed] [Google Scholar]
- 39.Mascola, J. R., S. W. Snyder, O. S. Weislow, S. M. Belay, R. B. Belshe, D. H. Schwartz, M. L. Clements, R. Dolin, B. S. Graham, G. J. Gorse, M. C. Keefer, M. J. McElrath, M. C. Walker, K. F. Wagner, J. G. McNeil, F. E. McCutchan, D. S. Burke, et al. 1996. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. J. Infect. Dis. 173:340-348. [DOI] [PubMed] [Google Scholar]
- 40.McDougall, B., M. H. Nymark, G. Landucci, D. Forthal, and W. E. Robinson, Jr. 1997. Predominance of detrimental humoral immune responses to HIV-1 in AIDS patients with CD4 lymphocyte counts less than 400/mm3. Scand. J. Immunol. 45:103-111. [DOI] [PubMed] [Google Scholar]
- 41.Modrow, S., B. H. Hahn, G. M. Shaw, R. C. Gallo, F. Wong-Staal, and H. Wolf. 1987. Computer-assisted analysis of envelope protein sequences of seven human immunodeficiency virus isolates: prediction of antigenic epitopes in conserved and variable regions. J. Virol. 61:570-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore, J., and A. Trkola. 1997. HIV type 1 coreceptors, neutralization serotypes, and vaccine development. AIDS Res. Hum. Retrovir. 13:733-736. [DOI] [PubMed] [Google Scholar]
- 43.Moore, J. P., and D. R. Burton. 1999. HIV-1 neutralizing antibodies: how full is the bottle? Nat. Med. 5:142-144. [DOI] [PubMed] [Google Scholar]
- 44.Moore, J. P., and J. Sodroski. 1996. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J. Virol. 70:1863-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore, J. P., A. Trkola, B. Korber, L. J. Boots, J. A. Kessler, 2nd, F. E. McCutchan, J. Mascola, D. D. Ho, J. Robinson, and A. J. Conley. 1995. A human monoclonal antibody to a complex epitope in the V3 region of gp120 of human immunodeficiency virus type 1 has broad reactivity within and outside clade B. J. Virol. 69:122-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muster, T., F. Steindl, M. Purtscher, A. Trkola, A. Klima, G. Himmler, F. Ruker, and H. Katinger. 1993. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 67:6642-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Myers, G., B. Berzofsky, B. T. Korber, R. Smith, and G. Pavlakis. 1992. Human retroviruses and AIDS: a compilation and analysis of nucleic and amino acid sequences. Los Alamos National Laboratories, Los Alamos, N.Mex.
- 47a.Myszka, D. G., R. W. Sweet, P. Hensley, M. Brigham-Burke, P. D. Kwong, W. A. Henrickson, R. Wyatt, J. Sodroski, and M. L. Doyle. 2000. Energetics of the HIV gp120-CD4 binding reaction. Proc. Natl. Acad. Sci. USA 97:9026-9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47b.Nichols, C. N., I. Bernal, A. M. Prince, and L. Andrus. 2002. Comparison of two different preparations of HIV immune globulin for efficiency of neutralization of HIV type 1 primary isolates. AIDS Res. Hum. Retrovir. 18:49-56. [DOI] [PubMed] [Google Scholar]
- 48.Ohno, T., M. Terada, Y. Yoneda, K. W. Shea, R. F. Chambers, D. M. Stroka, M. Nakamura, and D. W. Kufe. 1991. A broadly neutralizing monoclonal antibody that recognizes the V3 region of human immunodeficiency virus type 1 glycoprotein gp120. Proc. Natl. Acad. Sci. USA 88:10726-10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pantophlet, R., E. Ollmann-Saphire, P. Poignard, P. W. Parren, I. A. Wilson, and D. R. Burton. 2003. Fine mapping of the interaction of neutralizing and nonneutralizing monoclonal antibodies with the CD4 binding site of human immunodeficiency virus type 1 gp120. J. Virol. 77:642-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Posner, M. R., L. A. Cavacini, C. L. Emes, J. Power, and R. Byrn. 1993. Neutralization of HIV-1 by F105, a human monoclonal antibody to the CD4 binding site of gp120. J. Acquir. Immune Defic. Syndr. 6:7-14. [PubMed] [Google Scholar]
- 51.Profy, A. T., P. A. Salinas, L. I. Eckler, N. M. Dunlop, P. L. Nara, and S. D. Putney. 1990. Epitopes recognized by the neutralizing antibodies of an HIV-1-infected individual. J. Immunol. 144:4641-4647. [PubMed] [Google Scholar]
- 52.Rao, Z., A. S. Belyaev, E. Fry, P. Roy, I. M. Jones, and D. I. Stuart. 1995. Crystal structure of SIV matrix antigen and implications for virus assembly. Nature 378:743-747. [DOI] [PubMed] [Google Scholar]
- 53.Robey, W. G., B. Safai, S. Oroszlan, L. O. Arthur, M. A. Gonda, R. C. Gallo, and P. J. Fischinger. 1985. Characterization of envelope and core structural gene products of HTLV-III with sera from AIDS patients. Science 228:593-595. [DOI] [PubMed] [Google Scholar]
- 54.Rusche, J. R., D. L. Lynn, M. Robert-Guroff, A. J. Langlois, H. K. Lyerly, H. Carson, K. Krohn, A. Ranki, R. C. Gallo, D. P. Bolognesi, et al. 1987. Humoral immune response to the entire human immunodeficiency virus envelope glycoprotein made in insect cells. Proc. Natl. Acad. Sci. USA 84:6924-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanders, R. W., M. Venturi, L. Schiffner, R. Kalyanaraman, H. Katinger, K. O. Lloyd, P. D. Kwong, and J. P. Moore. 2002. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J. Virol. 76:7293-7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saphire, E. O., P. W. Parren, R. Pantophlet, M. B. Zwick, G. M. Morris, P. M. Rudd, R. A. Dwek, R. L. Stanfield, D. R. Burton, and I. A. Wilson. 2001. Crystal structure of a neutralizing human IGG against HIV-1: a template for vaccine design. Science 293:1155-1159. [DOI] [PubMed] [Google Scholar]
- 57.Scanlan, C. N., R. Pantophlet, M. R. Wormald, E. Ollmann Saphire, R. Stanfield, I. A. Wilson, H. Katinger, R. A. Dwek, P. M. Rudd, and D. R. Burton. 2002. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of α1→2 mannose residues on the outer face of gp120. J. Virol. 76:7306-7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scott, C. F., Jr., S. Silver, A. T. Profy, S. D. Putney, A. Langlois, K. Weinhold, and J. E. Robinson. 1990. Human monoclonal antibody that recognizes the V3 region of human immunodeficiency virus gp120 and neutralizes the human T-lymphotropic virus type IIIMN strain. Proc. Natl. Acad. Sci. USA 87:8597-8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Srivastava, I. K., L. Stamatatos, H. Legg, E. Kan, A. Fong, S. R. Coates, L. Leung, M. Wininger, J. J. Donnelly, J. B. Ulmer, and S. W. Barnett. 2002. Purification and characterization of oligomeric envelope glycoprotein from a primary R5 subtype B human immunodeficiency virus. J. Virol. 76:2835-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Starcich, B. R., B. H. Hahn, G. M. Shaw, P. D. McNeely, S. Modrow, H. Wolf, E. S. Parks, W. P. Parks, S. F. Josephs, R. C. Gallo, et al. 1986. Identification and characterization of conserved and variable regions in the envelope gene of HTLV-III/LAV, the retrovirus of AIDS. Cell 45:637-648. [DOI] [PubMed] [Google Scholar]
- 61.Tan, K., J. Liu, J. Wang, S. Shen, and M. Lu. 1997. Atomic structure of a thermostable subdomain of HIV-1 gp41. Proc. Natl. Acad. Sci. USA 94:12303-12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thali, M., U. Olshevsky, C. Furman, D. Gabuzda, M. Posner, and J. Sodroski. 1991. Characterization of a discontinuous human immunodeficiency virus type 1 gp120 epitope recognized by a broadly reactive neutralizing human monoclonal antibody. J. Virol. 65:6188-6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trkola, A., M. Purtscher, T. Muster, C. Ballaun, A. Buchacher, N. Sullivan, K. Srinivasan, J. Sodroski, J. P. Moore, and H. Katinger. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 70:1100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.VanCott, T. C., J. R. Mascola, R. W. Kaminski, V. Kalyanaraman, P. L. Hallberg, P. R. Burnett, J. T. Ulrich, D. J. Rechtman, and D. L. Birx. 1997. Antibodies with specificity to native gp120 and neutralization activity against primary human immunodeficiency virus type 1 isolates elicited by immunization with oligomeric gp160. J. Virol. 71:4319-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.VanCott, T. C., J. R. Mascola, L. D. Loomis-Price, F. Sinangil, N. Zitomersky, J. McNeil, M. L. Robb, D. L. Birx, and S. Barnett. 1999. Cross-subtype neutralizing antibodies induced in baboons by a subtype E gp120 immunogen based on an R5 primary human immunodeficiency virus type 1 envelope. J. Virol. 73:4640-4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.VanCott, T. C., V. R. Polonis, L. D. Loomis, N. L. Michael, P. L. Nara, and D. L. Birx. 1995. Differential role of V3-specific antibodies in neutralization assays involving primary and laboratory-adapted isolates of HIV type 1. AIDS Res. Hum. Retrovir. 11:1379-1391. [DOI] [PubMed] [Google Scholar]
- 67.Veronese, F. D., A. L. DeVico, T. D. Copeland, S. Oroszlan, R. C. Gallo, and M. G. Sarngadharan. 1985. Characterization of gp41 as the transmembrane protein coded by the HTLV-III/LAV envelope gene. Science 229:1402-1405. [DOI] [PubMed] [Google Scholar]
- 68.Vujcic, L. K., and G. V. Quinnan, Jr. 1995. Preparation and characterization of human HIV type 1 neutralizing reference sera. AIDS Res. Hum. Retrovir. 11:783-787. [DOI] [PubMed] [Google Scholar]
- 69.Weissenhorn, W., A. Dessen, S. C. Harrison, J. J. Skehel, and D. C. Wiley. 1997. Atomic structure of the ectodomain from HIV-1 gp41. Nature 387:426-430. [DOI] [PubMed] [Google Scholar]
- 70.Willey, R. L., R. A. Rutledge, S. Dias, T. Folks, T. Theodore, C. E. Buckler, and M. A. Martin. 1986. Identification of conserved and divergent domains within the envelope gene of the acquired immunodeficiency syndrome retrovirus. Proc. Natl. Acad. Sci. USA 83:5038-5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wrin, T., and J. H. Nunberg. 1994. HIV-1MN recombinant gp120 vaccine serum, which fails to neutralize primary isolates of HIV-1, does not antagonize neutralization by antibodies from infected individuals. AIDS 8:1622-1623. [DOI] [PubMed] [Google Scholar]
- 72.Wyatt, R., P. D. Kwong, E. Desjardins, R. W. Sweet, J. Robinson, W. A. Hendrickson, and J. G. Sodroski. 1998. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393:705-711. [DOI] [PubMed] [Google Scholar]
- 73.Wyatt, R., and J. Sodroski. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280:1884-1888. [DOI] [PubMed] [Google Scholar]
- 74.Yang, X., M. Farzan, R. Wyatt, and J. Sodroski. 2000. Characterization of stable, soluble trimers containing complete ectodomains of human immunodeficiency virus type 1 envelope glycoproteins. J. Virol. 74:5716-5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang, X., L. Florin, M. Farzan, P. Kolchinsky, P. D. Kwong, J. Sodroski, and R. Wyatt. 2000. Modifications that stabilize human immunodeficiency virus envelope glycoprotein trimers in solution. J. Virol. 74:4746-4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang, X., J. Lee, E. M. Mahony, P. D. Kwong, R. Wyatt, and J. Sodroski. 2002. Highly stable trimers formed by human immunodeficiency virus type 1 envelope glycoproteins fused with the trimeric motif of T4 bacteriophage fibritin. J. Virol. 76:4634-4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang, X., E. Mahony, G. H. Holm, A. Kassa, and J. Sodroski. 2003. Role of the gp120 inner domain beta-sandwich in the interaction between the human immunodeficiency virus envelope glycoprotein subunits. Virology 313:117-125. [DOI] [PubMed] [Google Scholar]
- 78.Yang, X., R. Wyatt, and J. Sodroski. 2001. Improved elicitation of neutralizing antibodies against primary human immunodeficiency viruses by soluble stabilized envelope glycoprotein trimers. J. Virol. 75:1165-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang, C. W., Y. Chishti, R. E. Hussey, and E. L. Reinherz. 2001. Expression, purification, and characterization of recombinant HIV gp140. The gp41 ectodomain of HIV or simian immunodeficiency virus is sufficient to maintain the retroviral envelope glycoprotein as a trimer. J. Biol. Chem. 276:39577-39585. [DOI] [PubMed] [Google Scholar]
- 80.Zhang, W., A. P. Godillot, R. Wyatt, J. Sodroski, and I. Chaiken. 2001. Antibody 17b binding at the coreceptor site weakens the kinetics of the interaction of envelope glycoprotein gp120 with CD4. Biochemistry 40:1662-1670. [DOI] [PubMed] [Google Scholar]
- 81.Zhang, Y. J., R. Fredriksson, J. A. McKeating, and E. M. Fenyo. 1997. Passage of HIV-1 molecular clones into different cell lines confers differential sensitivity to neutralization. Virology 238:254-264. [DOI] [PubMed] [Google Scholar]