Abstract
We have recently shown that cyclooxygenase-2 (COX-2) transcription is markedly induced after herpes simplex virus type 1 and pseudorabies virus (PRV) infections of rat embryonic fibroblast (REF) cells (N. Ray and L. W. Enquist, J. Virol. 78:3489-3501, 2004). For this study, we investigated the role of cyclooxygenase induction in the replication and growth of PRV. We demonstrate here a concordant increase in COX-2 mRNA and protein levels after the infection of REF cells. Inhibitors blocking the activity of cyclooxygenases caused a dramatic reduction in PRV growth. Viral growth could be restored if prostaglandin E2, the final product of COX-2 activity, was added simultaneously with the COX inhibitors. Immediate-early protein IE180, major capsid protein VP5, and glycoprotein expression were slightly reduced in the presence of COX-2 inhibitors, but expression of the early protein EP0 was not affected by COX inhibition. Viral DNA replication was marginally reduced in the presence of a COX-1/2 inhibitor, but there was no defect in viral DNA cleavage. Electron microscopy analysis revealed an increased number of unusual empty capsid structures in the nuclei of cells infected with PRV in the presence of a COX-1/2 inhibitor. These capsid structures shared some characteristics with procapsids but had a novel appearance by negative staining. Our data establish a role for COX-1 and COX-2 in facilitating the efficient growth and replication of PRV in primary cells.
Pseudorabies virus (PRV) is a neurotropic alphaherpesvirus that establishes a latent, or quiescent, state of infection in the nervous system of its natural host, the adult pig. In young piglets and secondary hosts such as rats, dogs, cats, cattle, sheep, and goats, PRV infection is lethal. In these non-natural hosts, PRV is capable of spreading through the nervous system via synaptically connected neurons. Accordingly, some attenuated strains of PRV are used as tracers by neuroanatomists for mapping networks of synaptically connected neurons in rodents and other species (reviewed in reference 12).
We have recently shown that PRV and herpes simplex virus type 1 (HSV-1) infections of cultured rat embryonic fibroblasts induce changes in the transcription of numerous cellular mRNAs (39). The majority of virus-induced changes in cellular gene expression occur late in infection, and the affected genes belong to diverse functional classes and pathways. One of the most highly induced genes encodes COX-2, a key enzyme in arachidonic acid metabolism and prostaglandin (PG) synthesis.
Phospholipid hydrolysis by phospholipases A2 and C releases arachidonic acid, which is then metabolized either by cyclooxygenases into PGs, prostacyclins, and thromboxanes or by lipoxygenases into leukotrienes. Cyclooxygenases convert arachidonic acid into PGH2, which is the precursor for all other PGs. COX-1 and COX-2 are the two primary isoforms of the enzyme. COX-1 is constitutively expressed in many tissues and is involved in a variety of functions, such as cytoprotection of the gastric mucosa, the regulation of renal blood flow, bone metabolism, nerve growth and development, wound healing, and platelet aggregation (9, 10, 14, 28). Although COX-2 is constitutively expressed in the brain, kidney, and testes, in most other tissues its expression is induced by pro-inflammatory or mitogenic agents, including cytokines, tumor promoters, endotoxins, and mitogens (5).
Infections by several viruses, including many herpesviruses, such as HSV, human cytomegalovirus (HCMV), Epstein-Barr virus, and murine gammaherpesvirus 54, have been reported to alter COX-2 expression (4, 7, 8, 22, 23, 29, 30, 35, 39, 41, 47, 48, 52, 53). In fact, rhesus cytomegalovirus even encodes a COX-2 homolog in its genome, emphasizing the importance of this enzyme (16). In addition, many studies have examined the regulation of COX-2 expression and PGE2 production during viral infection as well as the effect of PGE2 production on viral replication and virulence (reviewed in reference 46).
Prostaglandins are potent mediators of many critical physiological and inflammatory responses, and they modulate the host defense against various pathogens. They suppress some innate immune factors, including nitric oxide (NO) production, and have effects on the acquired immune response, specifically by suppressing the TH1 response. For instance, PGE2 can inhibit the production of gamma interferon by activated human T cells in vitro (45) and that of TH1 cytokines such as interleukin-12 in vivo (25, 32). In addition to inhibiting the production of TH1 cytokines, PGE2 switches the immune response toward a TH2 response, which is less effective in mounting an antiviral response (3, 25). PGE2 is one of the most potent and abundant PGs present during inflammatory reactions (1, 13, 17).
The very early host responses to viral infections are usually nonspecific and include the induction of cytokines such as interferons and tumor necrosis factor alpha. Nitric oxide synthase (NOS) is an interferon-inducible protein that is activated during innate immune responses (reviewed in reference 40). When present at high concentrations after expression of the inducible isoform of NOS (iNOS), NO functions as a cytotoxic molecule, reacting with proteins or H2O2 to form a highly toxic compound called peroxynitrite (ONOO−) (19, 31, 40). Nitric oxide is also thought to participate in the antiviral response to infection by attenuating the replication of both DNA and RNA viruses (40). The products of COX and NOS enzymes, PGs and NO, have been shown to share an antagonistic relationship with one another. The inhibition of COX activity in vesicular stomatitis virus (VSV)-infected cells causes a reduction in VSV propagation and a concordant increase in extracellular NO levels. Treatment with an iNOS inhibitor, L-NAME, or exogenous PGE2 in the presence of COX inhibitors can restore VSV growth and decrease NO production, underscoring a role for PGs in counteracting the antiviral effects of NO (6).
Besides their role in immunomodulation and counteraction of the antiviral effects of NO, PGs have been shown to be involved in modulating transcription from viral promoters. The human immunodeficiency virus type 1 (HIV-1) long terminal repeat (LTR) contains sequences that are important for DNA integration, as well as signals, such as an internal polymerase II promoter, that are necessary for transcription of the integrated retroviral DNA. PGE2 can increase transcription driven by the HIV-1 LTR in T lymphocytes (11). Transcription of one of the immediate-early genes (IE2) of HCMV was reduced in cells that were treated with COX-2 inhibitors. Therefore, a potential role for COX induction in the context of a virus infection is the activation of transcription from viral promoters via PGs.
To better understand the role of COX induction and prostaglandin production in herpesvirus replication and pathogenesis, we analyzed the effects of specific and nonspecific COX inhibitors on PRV replication. The inhibition of either COX-1 or COX-2 by use of an isoform-specific inhibitor caused a moderate inhibition of PRV growth (25- to 30-fold). However, when both COX isoforms were inhibited simultaneously, either with a nonspecific COX inhibitor or with a combination of specific COX-1 and COX-2 inhibitors, PRV infectious yields were dramatically reduced (>200,000-fold). We performed ultrastructural studies to determine the effects of COX inhibitors on capsid and virion maturation. COX inhibition during PRV infection led to an accumulation of unusual empty capsid-like structures in the nuclei of infected cells. Our data establish a role for COX-1 and COX-2 in facilitating the efficient growth and replication of PRV in primary cells.
MATERIALS AND METHODS
Cells, viruses, and reagents.
Rat embryonic fibroblasts (REFs) were isolated, and their growth was arrested as described previously (39). Passage 12 REF cells were used for all studies. Cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and infected in DMEM supplemented with 2% FBS. PRV strain Becker was used for all experiments unless otherwise specified. The cytotoxicities of drugs were determined by the use of Cell Titer Aqueous One solution (Promega) according to the manufacturer's directions. Indomethacin (Sigma Chemicals) was dissolved in dimethyl sulfoxide (DMSO) to a stock solution of 200 mM and was used in most experiments at a concentration of 500 μM. NS398 (Tocris) was dissolved in DMSO to a stock solution of 100 mM and was used in most experiments at a concentration of 250 μM. FR122047 (Tocris) was dissolved in water to a stock solution of 10 mM and was used in most experiments at a concentration of 50 μM. A solvent control of 0.25% DMSO was used in experiments whenever indomethacin or NS398 was used. Prostaglandin E2 (Sigma Chemicals) was dissolved in ethanol to 1 mg/ml and further diluted to 50 μg/ml (140 μM) with DMEM plus 2% FBS. The 140 μM stock was stored at −20°C in 1-ml aliquots for up to 1 month.
Real-time PCR.
REF cells were infected with PRV Becker at a multiplicity of infection (MOI) of 5 PFU/cell. At the indicated times postinfection, cells were harvested in Trizol reagent (Invitrogen). Total RNAs were isolated from Trizol lysates according to the manufacturer's instructions. cDNAs were prepared from 20-μg samples of total RNA, and serial 10-fold dilutions were used for real-time PCR analysis. Primers were designed with PrimerExpress 2.0 software (Applied Biosystems [ABI]). Reactions were set up in 25-μl volumes with 10-fold dilutions of cDNA, PCR primers (100 nM [each]), and SYBR Green PCR master mix (ABI). An ABI PRISM 7900 sequence detection system was used to monitor the level of SYBR green fluorescence over 40 cycles of PCR. At the end of the cycling phase, a dissociation curve was produced by slow denaturation of the PCR end products to ensure the specificity of amplification. For each sample, the quantity of cDNA corresponding to the gene of interest was normalized to the quantity of 18S rRNA. Relative expression levels were calculated by the 2−ΔΔCt method (26) if the primer pair for the gene of interest passed a validation test described in ABI user bulletin 2 (http://docs.appliedbiosystems.com/pebiodocs/04303859.pdf). If the primer pair failed validation, the standard curve method described in the same publication was used.
Western blot analysis.
REF cells were infected with PRV Becker at an MOI of 5 PFU/cell. At the indicated times postinfection, the cells were washed with phosphate-buffered saline (PBS), collected in PBS containing Complete Mini protease inhibitor cocktail (Roche Biochemicals), and stored at −80°C. The cells were thawed on ice and boiled in sodium dodecyl sulfate (SDS) loading buffer for 5 min, and DNAs were sheared by use of a 26-gauge needle prior to separation by SDS-polyacrylamide gel electrophoresis. SeeBlue Plus2 prestained protein standards (Invitrogen) were included as molecular weight markers. Proteins were electrotransferred to nitrocellulose membranes and blocked in PBS with 0.1% Tween 20 and 5% nonfat milk. Rabbit polyclonal COX-1 and COX-2 antisera (Cayman Chemicals) were used at dilutions of 1:500 and 1:1,000, respectively. A monoclonal PRV-IE180 antiserum was a gift from T. J. Chang and was used at a dilution of 1:100. A polyclonal PRV-EP0 antiserum was a gift from E. Ono and was used at a dilution of 1:5,000. As a loading control, membranes were incubated with a monoclonal antibody (clone AC-40) to actin (Sigma Chemicals) at a 1:200 dilution. The proteins were visualized by use of an ECL chemiluminescent detection system (Amersham Pharmacia). Protein bands were quantitated with Image J software (http://rsb.info.nih.gov/ij/).
End-point virus yield assay.
Monolayers of REF cells were grown in six-well dishes and infected with various PRV strains in the presence or absence of COX-1/2 inhibitors at an MOI of 1 PFU/cell. After a 1-h incubation, the inocula were removed and replaced with 1.5 ml of fresh medium, with or without inhibitors. At 24 h postinfection (hpi), the cells and supernatants were collected and frozen at −80°C. After three cycles of freezing and thawing, the samples were titrated on Vero cells to determine the end-point virus yields. In all cases, the total number of PFU present in the 1.5-ml cultures was plotted.
Transmission electron microscopy.
REF cells were infected with PRV Becker, with or without COX-1/2 inhibitors, at an MOI of 5 PFU/cell. At 12 hpi, the cells were washed twice in PBS and processed as described previously (44). Briefly, the cells were fixed with 3% glutaraldehyde in 100 mM sodium cacodylate (pH 7.4) for 1 h, the fixative was replaced with PBS containing 1% bovine serum albumin, and the cells were scraped into an Eppendorf tube and pelleted. The pellets were washed three times with 200 mM sodium cacodylate, postfixed for 1 h on ice in 2% osmium tetroxide in 100 mM sodium cacodylate, and further washed in water and then 50 mM sodium maleate (pH 5.2) three times each. En bloc staining was performed with 1% uranyl acetate in 50 mM sodium maleate (pH 5.2). The pellets were again washed in water three times, dehydrated by three washes each in 70, 95, and 100% ethanol, incubated with propylene oxide for 20 min, and then incubated with a 1:1 mixture of propylene oxide and resin overnight. The next morning, fresh resin was added for 4 h, and the samples were transferred to Beem capsules and polymerized overnight in a 60°C oven. The samples were then sectioned, stained, and observed by use of a LEO 912 AB transmission electron microscope.
Capsid purification. (i) Standard procedure.
Virus capsids were purified according to a protocol adapted from the work of Thomsen et al. (50). Briefly, about 2 × 107 to 3 × 107 REF cells were infected with PRV Becker at an MOI of 5 PFU/cell in the presence of 0.25% DMSO or 500 μM indomethacin and were harvested at 12 hpi. The cell pellets were resuspended in 0.5 ml of capsid lysis buffer (1 M NaCl, 40 mM Tris-HCl [pH 7.5], 2 mM EDTA, 2% Triton X-100), freeze-thawed three times, sonicated, and centrifuged for 5 min at 3,600 × g. The cleared extract was layered on a 20 to 65% linear sucrose gradient and centrifuged at 24,000 rpm in an SW41 rotor for 60 min at 4°C.
(ii) Room temperature procedure.
REF cells (3 × 107) were infected at an MOI of 5 PFU/cell in the presence of 0.25% DMSO or 500 μM indomethacin and were harvested at 12 hpi. All subsequent steps were performed at room temperature to avoid the destabilization of procapsids. The cells were resuspended in 0.5 ml of PBS, the cells and nuclei were lysed by sonication (Branson Sonifier 250; setting 3 for two cycles of 5 s each), and 50 μl of Complete Mini protease inhibitor stock (1 tablet in 1 ml of PBS; Roche Biochemicals) was added to the lysed cells. The samples were clarified by spinning at 16,000 × g for 5 min, and the supernatant was layered on top of a 20 to 50% linear sucrose gradient containing protease inhibitors. The gradient was centrifuged in an SW41 rotor at 24,000 rpm for 60 min.
Negative staining.
Samples were negatively stained by applying a small drop of the capsid suspension for 2 min onto a glow-discharged copper grid bearing a nitrocellulose substrate covered with a thin layer of evaporated carbon. The grid was then washed two times with distilled water, stained for 45 s with 2% aqueous uranyl acetate, and then wicked off with filter paper and allowed to dry. Negatively stained samples were also observed by use of a LEO 912 AB transmission electron microscope.
Total DNA isolation.
Monolayers of REF cells were infected with PRV Becker in the presence or absence of 500 μM indomethacin. At the indicated times postinfection, cells were harvested in PBS and pelleted in a microcentrifuge for 5 min at 3,000 rpm. The pellets were flash frozen in liquid nitrogen and stored at −80°C until all samples had been collected. The frozen cell pellets were thawed on ice, resuspended in 0.5 ml of lysis buffer (100 mM NaCl, 10 mM Tris [pH 8.0], 25 mM EDTA, 0.5% SDS, 0.1 mg of proteinase K/ml), and incubated at 55°C overnight. The lysates were then subjected to two phenol-chloroform-isoamyl alcohol extractions and one chloroform-isoamyl alcohol extraction before precipitation with a 1/2 volume of 8 M ammonium acetate and 2 volumes of 95% ethanol. The precipitated DNA pellets were washed twice with 70% ethanol and resuspended in 200 μl of distilled water. These DNA samples were used in the slot blot and Southern blots assays described below.
Slot blotting.
Total DNAs (250-ng samples) extracted from infected cells were denatured and applied to a nylon membrane by use of a slot blot apparatus (Schleicher and Schuell). After UV cross-linking, the membrane was probed with digoxigenin (DIG)-labeled DNAs generated by random priming of the entire PRV genome with a hexanucleotide primer mix and Klenow DNA polymerase by use of a DIG High Prime DNA labeling kit (Roche Molecular Biochemicals).
Southern blotting.
Six-microgram samples of total DNA from infected cells were digested with BamHI, separated in a 1% agarose gel, transferred to a nylon membrane, and probed with a DIG-labeled PRV DNA containing the long terminal BamHI fragment of the genome. The 255-bp probe was prepared by a PCR containing a pCH100 template, the primers oCH99 (CTCACTAAGAATTGGCAAGGTGC) and oCH100 (ATCGCCATCCACAACCTCCTG), deoxynucleoside triphosphates, DIG-dUTP, and Taq DNA polymerase (C. Hengartner and L. W. Enquist, unpublished data). The pCH100 plasmid had been previously constructed by cloning a 255-bp insert that was PCR amplified from PRV Becker (nucleotides 99 to 353 of the genome sequence; GenBank accession no. BK001744) by the use of primers oCH99 and oCH100 into the T-tail vector pGEM-T Easy (Hengartner and Enquist, unpublished data).
RESULTS
COX-2 mRNA and protein levels increase upon PRV Becker infection.
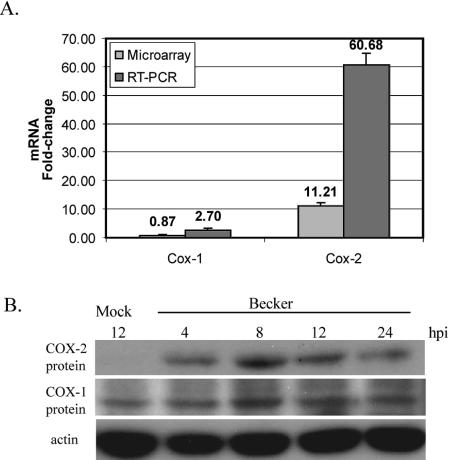
With a microarray-based study aimed at profiling host cell gene expression changes elicited by PRV and HSV-1 infections, we found that the mRNA encoding the inducible isoform of cyclooxygenase, COX-2, was highly induced by PRV infection (39). The transcript levels were >11-fold higher in PRV-infected cells late in infection (8 hpi) than in mock-infected cells. We examined the induction of cyclooxygenase mRNA further by performing real-time PCR with cDNAs obtained from PRV- or mock-infected cells at 8 hpi. COX-2 mRNA levels were increased 60-fold in infected cells (Fig. 1A). The transcript level of COX-1, the constitutive isoform, was practically unchanged at 8 hpi according to our microarray data, although real-time PCR indicated a slight (less than threefold) induction of this transcript upon PRV infection. The synthesis of COX-2 protein also increased following PRV infection. However, COX-1 protein levels remained unchanged after PRV Becker infection (Fig. 1B). The COX-2 protein persisted at elevated levels in infected cells for up to 24 hpi. The COX-2 mRNA induction was more transient and was reduced to 3.5-fold above the level in mock-infected cells at 12 hpi (39).
FIG. 1.
PRV infection induces COX-2 mRNA and protein expression. (A) Real-time PCRs were performed on total RNAs from PRV-infected REF cells (MOI = 5) at 8 hpi with COX-1- or COX-2-specific primers. The data were compared to previously published microarray data (39) for COX-1 and COX-2 mRNA levels. (B) RIPA lysates from PRV-infected REF cells (MOI of 5 PFU/cell) were collected at the indicated times postinfection and analyzed by Western blotting with rabbit polyclonal antibodies against COX-1 and COX-2 (top two panels). The membrane was probed with an anti-actin antibody to provide a loading control (bottom panel).
Simultaneous inhibition of both COX isoforms causes a dramatic reduction in PRV yield that can be rescued by PGE2.
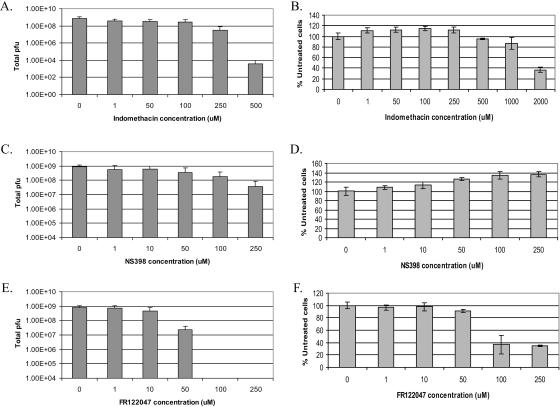
We used the following three COX inhibitors in our studies: indomethacin, an inhibitor of both cyclooxygenase isoforms; NS398, a COX-2-specific inhibitor; and FR122047, a COX-1-specific inhibitor. We performed end-point virus yield assays (as described in Materials and Methods) and tested increasing concentrations of each inhibitor for their effects on the virus yield at 24 hpi in PRV Becker-infected cells (Fig. 2A, C, and E). We also tested the cytotoxicities of various concentrations of inhibitors on uninfected cells by measuring the percentages of live cells after 24 h relative to those of cells that were treated with DMSO (Fig. 2B, D, and F). For each inhibitor, we determined the optimal drug concentration to achieve the largest reduction in titer with the least cytotoxicity. These optimal concentrations were 500 μM for indomethacin, 250 μM for NS398, and 50 μM for FR122047. To obtain reliable estimates of the reductions in viral titer caused by these inhibitors, we performed the end-point virus yield assays at least three times with the optimal drug concentrations. We found that 500 μM indomethacin caused a 246,000-fold drop in the virus yield with only about 5% toxicity, 250 μM NS398 reduced the virus yield 23-fold, and 50 μM FR122047 reduced the virus yield about 32-fold, with both NS398 and FR122047 having minimal toxicities. Furthermore, we infected cells in the presence of 250 μM NS398 in combination with 50 μM FR122047 and found that virus growth was dramatically reduced, similar to the effect of indomethacin treatment on infected cells (data not shown).
FIG. 2.
COX inhibitors cause a dramatic reduction in PRV infectious yield. REF cells were infected with PRV (MOI = 1) in the presence of increasing concentrations of indomethacin (A), NS398 (C), or FR122047 (E). At 24 hpi, the cells and supernatants were collected, freeze-thawed three times, and titrated on Vero cells. For determinations of the cytotoxicities of the COX inhibitors, uninfected REF cells grown in 96-well dishes were treated with increasing concentrations of indomethacin (B), NS398 (D), or FR122047 (F) for 24 h. Cell Titer Aqueous One solution was used to determine the percentages of metabolically active cells relative to cells that were not treated with a drug. Error bars represent the standard deviations of at least three experiments.
PGE2 is one of the major products of the COX-2-regulated PG synthesis pathway. Since the inhibition of COX-2 activity resulted in reduced virus growth, we asked if the addition of exogenous PGE2 could restore virus growth in the presence of a COX-2 inhibitor. Increasing concentrations of PGE2 were applied to cells that were infected in the presence of DMSO or NS398. As shown in Fig. 3, the virus yield was restored by the addition of 8 μM PGE2 in the presence of 250 μM NS398. In additional experiments, a maximal restoration of virus growth was observed at 4 or 6 μM PGE2 (data not shown), suggesting that a range of PGE2 concentrations (4 to 8 μM) can be effective in restoring virus growth in the presence of NS398.
FIG. 3.
Prostaglandin E2 can compensate for NS398-induced inhibition of infectious PRV production. REF cells were infected with PRV (MOI = 1) in the presence of 250 μM NS398 and increasing concentrations of PGE2. At 24 hpi, the cells and supernatants were collected, freeze-thawed three times, and titrated on Vero cells.
Indomethacin-induced reduction in PRV yield is reversible.
Cells were infected in the presence of 0.25% DMSO or 500 μM indomethacin. At the indicated times postinfection, the DMSO- or indomethacin-containing medium was replaced with DMEM supplemented with 2% FBS. All samples were collected at 24 hpi and titrated on Vero cells. As shown in Fig. 4, even up to 12 hpi, the inhibitory effects of indomethacin on PRV growth were reversible.
FIG. 4.
The inhibitory effects of indomethacin on PRV growth are reversible. REF cells were infected with PRV (MOI = 1) in the presence of 500 μΜ indomethacin or a DMSO solvent control. At the indicated times postinfection, the DMSO- or indomethacin-containing medium was removed and replaced with DMEM supplemented with 2% FBS. At 24 h postinfection, the cells and supernatants were collected, freeze-thawed three times, and titrated on Vero cells.
Effects of COX inhibitors on viral protein expression and synthesis of viral DNA.
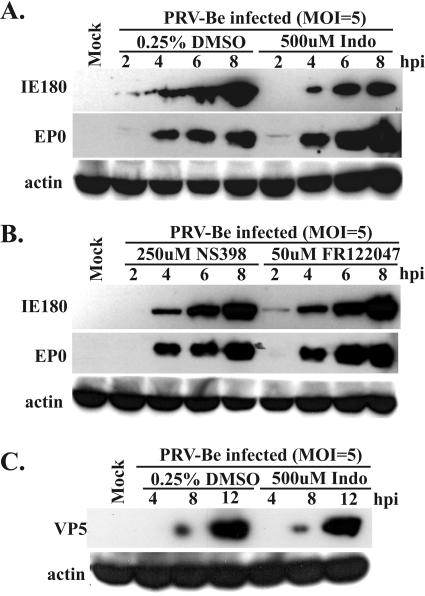
To determine the step(s) in the viral life cycle that was blocked by COX inhibitors, we examined the expression of alpha-, beta-, and gammaviral genes as well as the synthesis of viral DNA in the presence of COX inhibitors. Lysates from cells infected in the presence of 0.25% DMSO, 500 μM indomethacin, 250 μM NS398, or 50 μM FR122047 were separated in SDS-polyacrylamide gels and processed for Western blots with antibodies against IE180 (α gene), EP0 (β gene), and the major capsid protein, VP5 (γ gene). At 8 hpi, expression of the IE180 protein was reduced by 67% in the presence of indomethacin, 16% in the presence of NS398, and 13% in the presence of FR122047 (Fig. 5A and B). Similarly, the expression of the late protein VP5 and the glycoproteins gB and gC was marginally reduced in the presence of COX inhibitors (Fig. 5C and data not shown). However, none of the inhibitors had any significant effect on the levels of the early protein EP0 (Fig. 5A and B).
FIG. 5.
Immediate-early, early, and late protein synthesis in the presence of COX inhibitors. REF cells were infected with PRV (MOI = 5) in the presence of 0.25% DMSO (A and C), 500 μM indomethacin (A and C), 250 μM NS398 (B), or 50 μM FR122047 (B). Whole-cell lysates were collected at the indicated times postinfection and analyzed by Western blotting with antibodies raised against the immediate-early protein IE180, the early protein EP0, and the late protein VP5. The blots were also probed with an anti-actin antibody to provide a loading control.
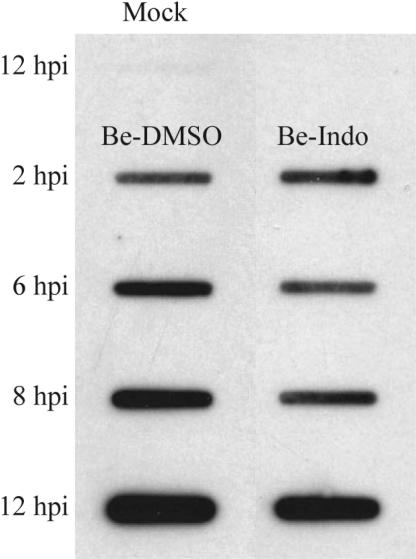
We next investigated the effect of indomethacin on viral DNA synthesis. The total DNA from cells infected in the presence of 0.25% DMSO or 500 μM indomethacin was isolated and then probed with random-primed DIG-labeled PRV DNA. The accumulation of viral DNA in indomethacin-treated cells was marginally lower than that in DMSO-treated cells (Fig. 6). At 6 hpi, there was a 47% reduction in viral DNA accumulation in indomethacin-treated cells relative to the DMSO control cells.
FIG. 6.
PRV DNA replication is reduced in the presence of indomethacin. REF cells were infected with PRV (MOI = 5) in the presence of 0.25% DMSO or 500 μM indomethacin. At the indicated times postinfection, samples were collected for total DNA isolation and were analyzed by slot blotting with a random-primed DIG-labeled probe for the entire PRV genome. A mock-infected sample was included to assess the level of nonspecific hybridization to contaminating host DNA.
COX inhibitors cause the accumulation of empty capsids in nuclei of infected cells.
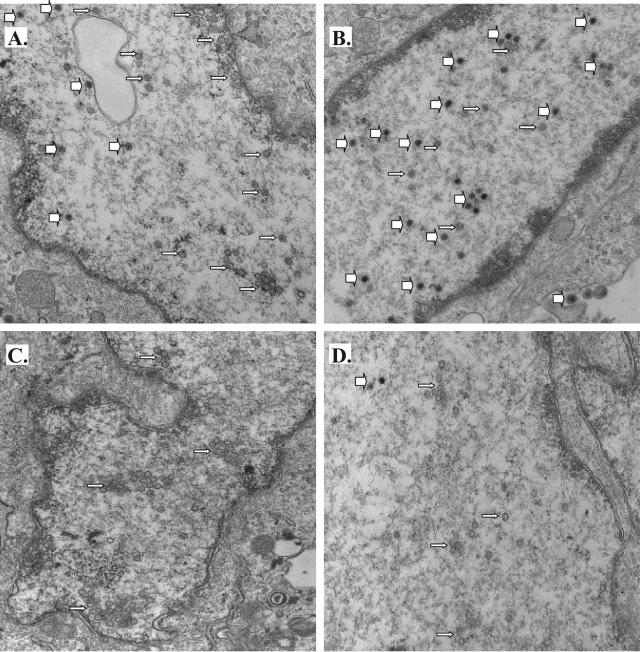
Since the treatment with COX inhibitors dramatically reduced PRV growth without dramatic changes in viral protein expression or the synthesis of viral DNA, we used transmission electron microscopy to look for obvious blocks in virus assembly in infected, indomethacin-treated cells. Cells infected in the absence of any inhibitors displayed the typical hallmarks of a productive PRV infection at 12 hpi (Fig. 7A and B). Full capsids (thick white arrows) and empty capsids (thin white arrows) were found in their nuclei, full capsids as well as doubly enveloped virions were seen in the cytoplasm, and finally, many virions were visible on their plasma membranes, presumably engaged in final egress from the cell. On the other hand, in cells infected in the presence of indomethacin, the picture was remarkably different. In that case, a predominance of empty capsids was obvious in the nucleus, and very few, if any, capsids filled with DNA were visible in the nucleus or cytoplasm (Fig. 7C and D). Moreover, NS398-treated cells infected with PRV Becker looked very much like the indomethacin-treated cells (data not shown). Similar trends were observed for two independent experiments. For both the control and indomethacin-treated cells, we captured 25 to 30 images and determined the median number of full capsids seen in the images for each treatment. Images that are representative of the median number of full capsids seen in a field are shown in Fig. 7A and C. In addition, to provide an idea of the range of phenotypes observed, we also show images with the maximum number of full capsids per frame observed for each treatment (Fig. 7B and D).
FIG. 7.
COX inhibitors cause an accumulation of empty capsids in the nuclei of PRV-infected cells. REF cells were infected with PRV Becker at an MOI of 5 PFU/cell in the presence of no drug (A and B) or 500 μM indomethacin (C and D). At 12 h postinfection, the cells were fixed and processed for transmission electron microscopy. The numbers of full capsids in 25 to 30 images were counted for each treatment to determine the median number of full capsids per frame. Representative images showing the median numbers of full capsids in control or indomethacin-treated cells are shown (A and C). In addition, the frames with the maximum numbers of full capsids per frame for each treatment are shown (B and D) to provide an idea of the range of observations.
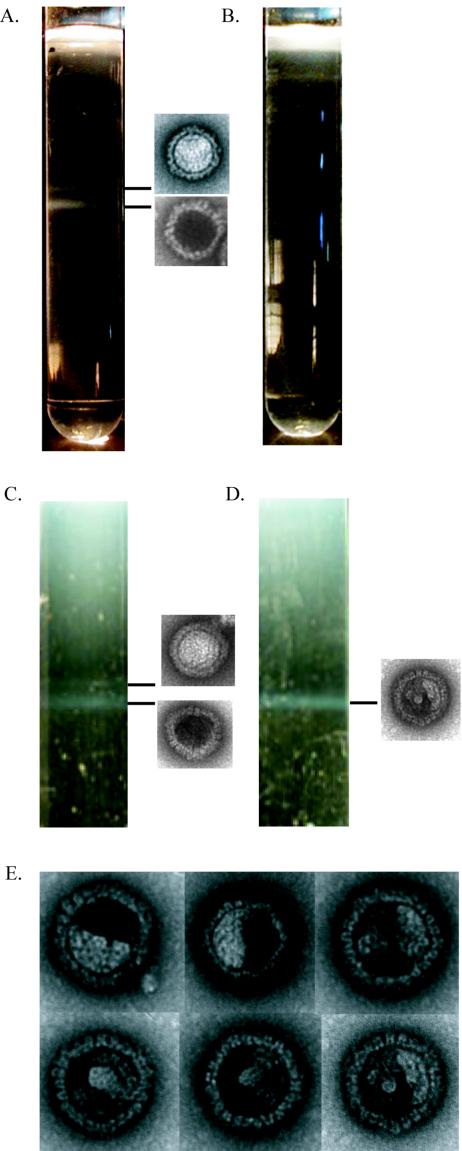
We attempted to confirm this result by purifying capsids from cells infected in the presence of 0.25% DMSO or 500 μM indomethacin. Three distinct types of herpesvirus capsids, called A, B, and C capsids, have been previously described (15). C capsids contain viral DNA and mature into infectious virions (36). B capsids are filled with scaffolding protein but lack viral DNA, whereas A capsids lack both protein and DNA (33, 43). Using sucrose gradients, we were able to visualize B- and C-capsid bands (top and bottom bands, respectively) from DMSO-treated but not indomethacin-treated cells (Fig. 8A and B). When we infected three times as many cells, we were able to detect a faint C-capsid but no B-capsid band in the indomethacin-treated sample (data not shown). Negative staining and electron microscopy confirmed the sucrose gradient band identities as DNA-filled or empty capsids.
FIG.8.
Capsids produced in indomethacin-treated cells are unstable for purification at 4°C. The capsid purification protocols are described in Materials and Methods. Sucrose gradients were used to purify capsids at 4°C (A and B) or 21°C (C and D) from REF cells infected with PRV (MOI = 5) in the presence of 0.25% DMSO (A and C) or 500 μM indomethacin (B and D). The identity of each capsid band was determined by negative staining and transmission electron microscopy, as shown in the pictures adjacent to the capsid bands. (E) Collage of the unusual capsid structures observed by EM analysis of the band obtained from indomethacin-treated cells by the room temperature procedure (D).
Despite repeated attempts, we were unable to isolate the empty capsids seen by electron microscopy (EM) from infected indomethacin-treated cells, suggesting that these capsid-like structures are unstable under the current purification conditions. The empty capsid-like structures may be procapsids, capsid precursors that are unstable on ice (34). To examine this possibility, we infected cells in the presence of DMSO or indomethacin and, at 12 hpi, incubated them on ice for 1 h. The cells were then fixed, processed, and visualized by electron microscopy. For the indomethacin-treated cells that were subjected to ice treatment prior to fixation, we observed a subtle reduction in the number of empty capsids in infected nuclei compared to that for indomethacin-treated control cells (no ice) (data not shown). These results are consistent with the hypothesis that the empty capsids seen accumulating in the nuclei of indomethacin-treated infected cells may in fact be procapsids and can therefore not be purified by using standard capsid purification protocols.
We next attempted a direct biochemical isolation of procapsids from infected REF cells by using a protocol adapted from one described by Newcomb et al. (34). Procapsids are unstable at 4°C, so the isolation was performed at room temperature (21 to 25°C) in the presence of ample protease inhibitors to inhibit proteolytic activity, which would otherwise be enhanced by the elevated temperature. Under these conditions, we were able to visualize a prominent capsid band from DMSO- or indomethacin-treated infected cells in sucrose gradients similar to those used in the previous capsid purification (Fig. 8C and D). In the DMSO-treated sample, we also observed a faint lighter band. We examined the contents of these bands by negative staining followed by EM and found that the top and bottom bands seen in the DMSO-treated sample were comprised of empty- and DNA-filled capsids, respectively. The band seen in the indomethacin-treated sample was comprised of polyhedral capsid structures filled with a patchy electron-dense material different from either of the capsid structures seen in the DMSO sample (Fig. 8E).
Concatameric viral DNA is cleaved in the presence of COX inhibitors.
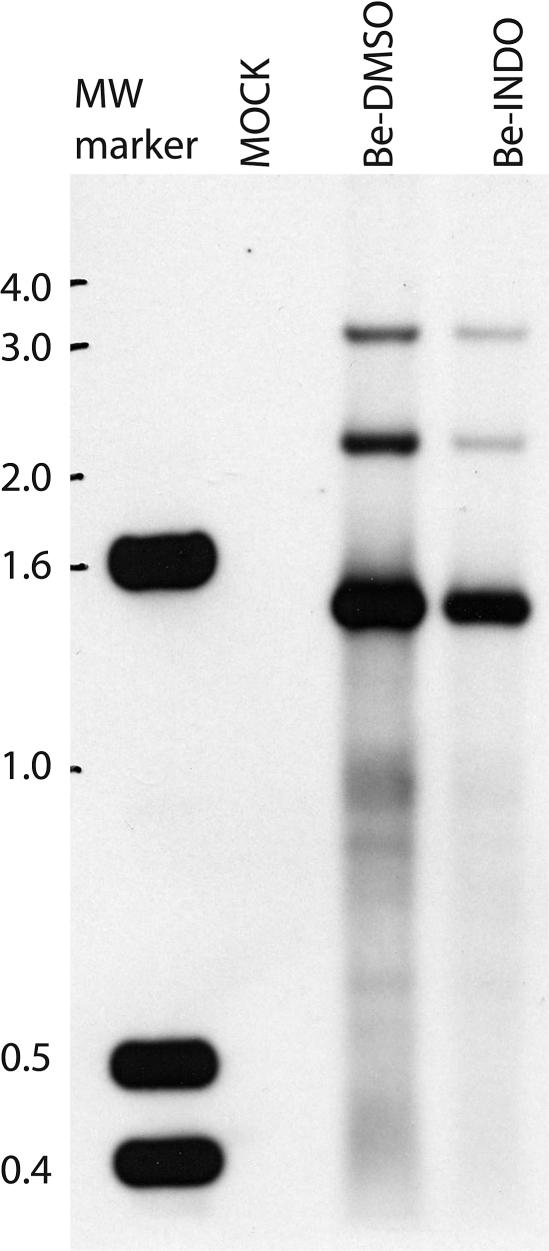
Since the level of PRV DNA accumulation was only marginally reduced by indomethacin treatment, we speculated that the increased number of empty capsids seen in the nuclei of indomethacin-treated infected cells might reflect a defect in viral DNA cleavage. To test this hypothesis, we prepared a labeled probe from a viral DNA fragment within 500 bp of the linear end of the PRV genome (24). In a Southern blot with DNAs isolated from PRV-infected cells, this probe detected a 1.5-kb BamHI fragment derived from cleaved viral DNA and a 3-kb BamHI fragment derived from concatameric DNA (Fig. 9).
FIG. 9.
Cleavage of viral DNA proceeds normally in indomethacin-treated PRV-infected cells. REF cells were mock infected or infected with PRV (MOI = 5) in the presence of 0.25% DMSO (Be-DMSO) or 500 μM indomethacin (Be-INDO). At 8 h postinfection, samples were collected for total DNA isolation, digested with BamHI, electrophoresed in an agarose gel, and analyzed by Southern blotting with the probe described in Materials and Methods.
We reasoned that if indomethacin treatment affected the cleavage of viral DNA, the ratio of the 3-kb band to the 1.5-kb band would be higher for the indomethacin-treated sample than for the DMSO-treated sample. Indomethacin treatment was found to have no apparent effect on viral DNA cleavage, as the relative intensities of the two bands remained unchanged by indomethacin treatment (Fig. 9). Since the 3-kb band in both samples was much fainter than the 1.5-kb band, we further deduced that the majority of the viral DNA was cleaved into unit-length genomes by 8 hpi. We also observed a 2.2-kb band which was not predicted by our current BamHI restriction map of the PRV genome. This band was probably an internal viral BamHI DNA fragment that cross-hybridized with the probe. Finally, we also observed an overall reduction in the intensities of the bands detected in the indomethacin-treated sample, which was consistent with the reduction in the synthesis of viral DNA seen earlier (Fig. 6).
DISCUSSION
The activities of both isoforms of the cyclooxygenase enzyme (COX-1 and COX-2) are required for the efficient viral replication of PRV. COX-2 mRNA was induced starting at 4 hpi and was maximally expressed by 8 hpi upon PRV infection, and elevated levels of COX-2 protein were detected up to 24 hpi. In contrast, the levels of the COX-1 protein, the constitutive isoform, remained mostly constant upon infection with PRV. Similar results for COX-1 and COX-2 mRNA levels were obtained after HSV-1 infection (reference 39 and data not shown). Interestingly, infection by HCMV, which has a much slower life cycle, also induces COX-2 mRNA and protein expression between 4 and 8 hpi (53). This time frame is very early in the growth cycle of HCMV (immediate-early proteins are first detected at about 6 hpi) but is late in the PRV growth cycle (late protein expression begins as early as 4 hpi). For HCMV, it has been proposed that the induction of COX-2 early in the virus life cycle may be a response to a component of the entering virion, such as glycoprotein B. However, our observation of a temporal conservation in COX-2 induction after infection by members of two different herpesvirus subfamilies further suggests the presence of a conserved host response to herpesvirus infections. At this time, however, the specific molecular players and events involved in such a host response remain unclear.
COX-2 expression is induced after infection by a variety of DNA and RNA viruses. PRV infection causes a robust increase in COX-2 mRNA and protein expression. The effects of COX-1- and COX-2-specific inhibitors on the virus yield (32- and 23-fold reduction, respectively) were far less dramatic than that of the nonspecific inhibitor indomethacin (246,000-fold reduction) or the combined effect of the two specific inhibitors. The effects seen with the individual application of specific inhibitors suggest that viral replication is quite sensitive to the total level of cyclooxygenase activity in the cell. The dramatic reduction seen with the nonspecific inhibitor further suggests that cyclooxygenase activity is critical to the viral replication cycle. The inhibition of COX-1 by selective inhibitors has been shown to cause an increased expression of COX-2 (49). It is conceivable that the selective inhibition of COX-2 may also effect a compensatory increase in COX-1 protein expression and/or enzyme activity. These facts may shed light on our observations and explain the relatively modest effects of the selective inhibitors despite the dramatic reduction in viral titers due to the inhibition of both COX isoforms by indomethacin or by a combination of COX-1- and COX-2-specific inhibitors.
The reduction in virus yield caused by COX inhibitors was reversible and was not cell type dependent. Treatment with indomethacin had similar effects on PRV yield whether pig kidney (PK15) or Vero cells were infected (2). The addition of exogenous PGE2, the final product of COX-1/2 activity, to cells infected in the presence of NS398 restored the virus yield to levels obtained in control cells. The fact that the rescue was partial may have been due to the involvement of other prostaglandins. It may also be that the functional local PGE2 concentration in the cytoplasm exceeded that achieved by our exogenous supplementation due to limitations of solubility and efficient uptake.
Recently, PGE2 has been reported to play a role in mediating pain by inhibiting the transmission of nociceptive inputs via the glycine receptor alpha 3 (GlyR α3) (18). PRV Becker causes intense pain and scratching in a variety of animal models, whereas the attenuated strain PRV Bartha does not. Interestingly, infection by PRV Becker increases the expression of GlyR α3 mRNA 3.4-fold at 5 hpi (39). The pain caused by a wild-type PRV infection may be a result of the increased expression of GlyR α3 after PRV infection. Moreover, higher PGE2 levels resulting from increased COX-2 expression after infection may play a role in mediating pain sensitization by counteracting the activity of GlyR α3. In agreement with this hypothesis, the infection of REF cells by the attenuated strain PRV Bartha resulted in a reduced induction of COX-2 and no induction of GlyR α3 mRNA (38).
Despite numerous studies demonstrating their importance, there is no clear understanding of the molecular function of cyclooxygenases in viral growth. Some reports suggest that the anti-inflammatory properties of COX-2 are important for controlling viral pathogenesis. However, the requirement of COX enzyme activity for viral growth in cultured cells in vitro implies that one or more basic steps of the viral replication cycle require the active enzymes. Several possible functions of COX-2 can be considered. Firstly, the COX-2 enzyme affects tumorigenesis and causes increased adhesion to extracellular matrix proteins, the inhibition of butyrate-induced apoptosis, decreased expression of E-cadherin and the transforming growth factor β2 receptor, and the stimulation of Bcl-2 protein expression (51). The overexpression of COX-2 either in epithelial cells or in PC12 cells leads to resistance to apoptosis (27, 51). Therefore, the induction of cyclooxygenases may eliminate the apoptosis of infected cells, resulting in efficient growth and a higher infectious yield.
Secondly, a VSV infection induces COX-2 in vivo, causing an increased production of PGs, which can counteract the antiviral effects of nitric oxide (6). PRV infection also increases the expression of nitric oxide synthase in the rat central nervous system (42). The observed increase in COX expression after PRV infection may reflect a response to modulate the antiviral effects of nitric oxide.
Finally, in HIV-1-infected monocyte-derived macrophages and human brain microvascular endothelial cells, COX-2 expression and PGE2 production are induced (37). In turn, PGE2 can activate transcription from the HIV-1 LTR via both NF-κB-dependent and -independent signaling pathways (11). It is possible that COX-2 expression facilitates transcription from PRV viral promoters such as the IE180 (the only known PRV immediate-early gene) promoter. Consistent with this hypothesis, we did observe a modest reduction in the level of the IE180 protein in cells that were treated with indomethacin (Fig. 5A and B). Moreover, we have isolated a mutant with a reduced sensitivity to indomethacin by serial passages of PRV Becker in the presence of increasing concentrations of the drug (2). This mutant shows an increased expression of IE180 relative to that in strain Becker in the presence of indomethacin.
The COX-2 enzyme was maximally induced 8 h after infection by PRV, whereas the transcription of IE180 began very early after infection. Therefore, if the role of COX-2 in PRV infection is indeed the activation of IE180-induced transcription of viral genes, it seems likely that the initial activity of IE180 is independent of COX-2 expression. This explains our observation of the lack of any effect on EP0 protein expression after treatment with COX inhibitors (Fig. 5A and B). The continued presence of IE180 is essential to the maintenance of the normal transcriptional program of PRV (21). Consequently, COX-2 expression may be required for sustained expression of the IE180 protein at late times postinfection, thereby allowing the normal transcription of several late viral genes. We observed a rather modest diminution of late protein expression (VP5, gB, and gC) (Fig. 5C and data not shown) after indomethacin treatment. Low rates of protein turnover may explain the apparent resistance of late protein levels to treatment by COX inhibitors. In addition, we observed a moderate reduction in viral DNA synthesis in the presence of indomethacin (Fig. 6), which may also be explained as a downstream effect of diminished IE180 protein expression.
We investigated the effects of COX inhibition in a mouse flank model of PRV infection developed by E. Brittle, A. Reynolds, and L. Enquist (unpublished data). We infected mice by scratching 106 PFU of PRV Becker onto their flanks and administered 14 mg of indomethacin or vehicle/kg of body weight/day orally starting 6 h prior to infection. Animals that were not treated with the drug developed symptoms of infection (scratching) by 40 hpi and had severe erythematous lesions spanning the entire infected dermatome. For animals that were treated with indomethacin, we observed a delay in the onset of symptoms of infection, and the severity of the dermatome lesions was reduced. However, owing to the high toxicity of indomethacin, we observed no statistically significant increase in the mean time to death for drug-treated animals. We next infected wild-type and COX-2−/− mice with PRV Becker in a similar manner and observed a very marginal delay in the onset of symptoms and time to death. These results are consistent with our tissue culture experiments, which suggested that the inhibition of both isoforms of the cyclooxygenase enzyme is required for a dramatic reduction in PRV growth.
By far the clearest effect of indomethacin (or NS398) treatment on PRV-infected cells was the accumulation of large numbers of empty capsids in the nuclei and, more strikingly, a dramatic reduction in the number of filled capsids in the nuclei and the cytoplasm, as seen by transmission electron microscopy. The capsid-like structures observed by EM in thin sections of indomethacin-treated infected cells were unstable during purification at 4°C. However, we were successful in isolating them from cells at room temperature. The purified capsid structures resembled neither the empty nor the filled capsids seen in the DMSO-treated sample. Although they shared an instability at 4°C, the indomethacin-induced capsids seemed to be distinct from procapsids since their appearance was polyhedral and not spherical, as might be expected for procapsids (20). At this point in our studies, the identity and composition of these novel capsid structures remain unclear. Further study of these “defective” capsids may help us to elucidate the mechanism of herpesvirus capsid assembly and maturation.
In summary, we have demonstrated a requirement for both COX-1 and COX-2 in PRV growth and replication. To our knowledge, the effects of COX-1-specific inhibitors on virus growth have not been studied thus far for any other virus. The concurrent inhibition of both isoforms leads to a dramatic reduction in the infectious virus yield, and the antiviral effects of these inhibitors are reversible. We found that COX-1/2 inhibitors have a modest effect on IE180 expression and viral DNA replication. However, our most striking observation was that the inhibition of COX activity in the context of PRV infection leads to an apparent defect in capsid assembly or maturation, as evidenced by the unusual accumulation of a novel form of capsid in the nuclei of infected cells. Like procapsids, the structural integrity of these novel capsids is cold sensitive, but they are polyhedral in shape and their content is not known. The role of the COX enzymes in assembly is not known, as these enzymes are involved in many cellular processes. Since COX inhibitors block PRV and HSV growth in vitro, they may have an ameliorating effect in the treatment of natural infections or viral reactivation.
Acknowledgments
We thank T. J. Chang and E. Ono for their generous gifts of IE180 and EP0 antisera, E. Brittle and A. Reynolds for technical assistance with mouse flank infection experiments, and C. Hengartner for valuable technical guidance and critical reading of the manuscript. F. Homa and J. Brown provided advice on capsid and procapsid purification.
This work was supported by NIH grant 5P01 CA87661 to L. W. Enquist.
REFERENCES
- 1.Appleton, I., A. Tomlinson, and D. A. Willoughby. 1996. Induction of cyclo-oxygenase and nitric oxide synthase in inflammation. Adv. Pharmacol. 35:27-78. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett, E. K. 2004. Isolation and characterization of an indomethacin resistant pseudorabies virus mutant. Senior thesis. Princeton University, Princeton, N.J.
- 3.Betz, M., and B. S. Fox. 1991. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J. Immunol. 146:108-113. [PubMed] [Google Scholar]
- 4.Bratcher, D. F., C. J. Harrison, N. Bourne, L. R. Stanberry, and D. I. Bernstein. 1993. Effect of indomethacin on ultraviolet radiation-induced recurrent herpes simplex virus disease in guinea-pigs. J. Gen. Virol. 74:1951-1954. [DOI] [PubMed] [Google Scholar]
- 5.Chen, N., and C. S. Reis. 2002. Distinct roles of eicosanoids in the immune response to viral encephalitis: or why you should take NSAIDS. Viral Immunol. 15:133-146. [DOI] [PubMed] [Google Scholar]
- 6.Chen, N., J. L. Warner, and C. S. Reiss. 2000. NSAID treatment suppresses VSV propagation in mouse CNS. Virology 276:44-51. [DOI] [PubMed] [Google Scholar]
- 7.Cheng, A. S., H. L. Chan, N. W. Leung, C. T. Liew, K. F. To, P. B. Lai, and J. J. Sung. 2002. Expression of cyclooxygenase-2 in chronic hepatitis B and the effects of anti-viral therapy. Aliment. Pharmacol. Ther. 16:251-260. [DOI] [PubMed] [Google Scholar]
- 8.Corasaniti, M. T., C. Bellizzi, R. Russo, C. Colica, D. Amantea, and G. Di Renzo. 2003. Caspase-1 inhibitors abolish deleterious enhancement of COX-2 expression induced by HIV-1 gp120 in human neuroblastoma cells. Toxicol. Lett. 139:213-219. [DOI] [PubMed] [Google Scholar]
- 9.DeWitt, D. L., and W. L. Smith. 1990. Cloning of sheep and mouse prostaglandin endoperoxide synthases. Methods Enzymol. 187:469-479. [DOI] [PubMed] [Google Scholar]
- 10.DeWitt, D. L., and W. L. Smith. 1988. Primary structure of prostaglandin G/H synthase from sheep vesicular gland determined from the complementary DNA sequence. Proc. Natl. Acad. Sci. USA 85:1412-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dumais, N., B. Barbeau, M. Olivier, and M. J. Tremblay. 1998. Prostaglandin E2 up-regulates HIV-1 long terminal repeat-driven gene activity in T cells via NF-kappaB-dependent and -independent signaling pathways. J. Biol. Chem. 273:27306-27314. [DOI] [PubMed] [Google Scholar]
- 12.Enquist, L. W., P. J. Husak, B. W. Banfield, and G. A. Smith. 1998. Infection and spread of alphaherpesviruses in the nervous system. Adv. Virus Res. 51:237-347. [DOI] [PubMed] [Google Scholar]
- 13.Fontana, A., F. Kristensen, R. Dubs, D. Gemsa, and E. Weber. 1982. Production of prostaglandin E and an interleukin-1 like factor by cultured astrocytes and C6 glioma cells. J. Immunol. 129:2413-2419. [PubMed] [Google Scholar]
- 14.Funk, C. D., L. B. Funk, M. E. Kennedy, A. S. Pong, and G. A. Fitzgerald. 1991. Human platelet/erythroleukemia cell prostaglandin G/H synthase: cDNA cloning, expression, and gene chromosomal assignment. FASEB J. 5:2304-2312. [PubMed] [Google Scholar]
- 15.Gibson, W., and B. Roizman. 1972. Proteins specified by herpes simplex virus 8: characterization and composition of multiple capsid forms of subtypes 1 and 2. J. Virol. 10:1044-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen, S. G., L. I. Strelow, D. C. Franchi, D. G. Anders, and S. W. Wong. 2003. Complete sequence and genomic analysis of rhesus cytomegalovirus. J. Virol. 77:6620-6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartung, H. P., and K. V. Toyka. 1987. Phorbol diester TPA elicits prostaglandin E release from cultured rat astrocytes. Brain Res. 417:347-349. [DOI] [PubMed] [Google Scholar]
- 18.Harvey, R. J., U. B. Depner, H. Wassle, S. Ahmadi, C. Heindl, H. Reinold, T. G. Smart, K. Harvey, B. Schutz, O. M. Abo-Salem, A. Zimmer, P. Poisbeau, H. Welzl, D. P. Wolfer, H. Betz, H. U. Zeilhofer, and U. Muller. 2004. GlyR alpha3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science 304:884-887. [DOI] [PubMed] [Google Scholar]
- 19.Hibbs, J. B., Jr. 1991. Synthesis of nitric oxide from l-arginine: a recently discovered pathway induced by cytokines with antitumour and antimicrobial activity. Res. Immunol. 142:565-569. [DOI] [PubMed] [Google Scholar]
- 20.Homa, F. L., and J. C. Brown. 1997. Capsid assembly and DNA packaging in herpes simplex virus. Rev. Med. Virol. 7:107-122. [DOI] [PubMed] [Google Scholar]
- 21.Ihara, S., L. Feldman, S. Watanabe, and T. Ben-Porat. 1983. Characterization of the immediate-early functions of pseudorabies virus. Virology 131:437-454. [DOI] [PubMed] [Google Scholar]
- 22.Janelle, M. E., A. Gravel, J. Gosselin, M. J. Tremblay, and L. Flamand. 2002. Activation of monocyte cyclooxygenase-2 gene expression by human herpesvirus 6. Role for cyclic AMP-responsive element-binding protein and activator protein-1. J. Biol. Chem. 277:30665-30674. [DOI] [PubMed] [Google Scholar]
- 23.Khyatti, M., and J. Menezes. 1990. The effect of indomethacin, prostaglandin E2 and interferon on the multiplication of herpes simplex virus type 1 in human lymphoid cells. Antiviral Res. 14:161-172. [DOI] [PubMed] [Google Scholar]
- 24.Klupp, B. G., C. J. Hengartner, T. C. Mettenleiter, and L. W. Enquist. 2004. Complete, annotated sequence of the pseudorabies virus genome. J. Virol. 78:1683-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuroda, E., T. Sugiura, K. Zeki, Y. Yoshida, and U. Yamashita. 2000. Sensitivity difference to the suppressive effect of prostaglandin E2 among mouse strains: a possible mechanism to polarize Th2 type response in BALB/c mice. J. Immunol. 164:2386-2395. [DOI] [PubMed] [Google Scholar]
- 26.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 27.McGinty, A., Y. W. Chang, A. Sorokin, D. Bokemeyer, and M. J. Dunn. 2000. Cyclooxygenase-2 expression inhibits trophic withdrawal apoptosis in nerve growth factor-differentiated PC12 cells. J. Biol. Chem. 275:12095-12101. [DOI] [PubMed] [Google Scholar]
- 28.Merlie, J. P., D. Fagan, J. Mudd, and P. Needleman. 1988. Isolation and characterization of the complementary DNA for sheep seminal vesicle prostaglandin endoperoxide synthase (cyclooxygenase). J. Biol. Chem. 263:3550-3553. [PubMed] [Google Scholar]
- 29.Mori, N., H. Inoue, T. Yoshida, T. Tanabe, and N. Yamamoto. 2001. Constitutive expression of the cyclooxygenase-2 gene in T-cell lines infected with human T cell leukemia virus type I. Int. J. Cancer 94:813-819. [DOI] [PubMed] [Google Scholar]
- 30.Murono, S., H. Inoue, T. Tanabe, I. Joab, T. Yoshizaki, M. Furukawa, and J. S. Pagano. 2001. Induction of cyclooxygenase-2 by Epstein-Barr virus latent membrane protein 1 is involved in vascular endothelial growth factor production in nasopharyngeal carcinoma cells. Proc. Natl. Acad. Sci. USA 98:6905-6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nathan, C. 1992. Nitric oxide as a secretory product of mammalian cells. FASEB J. 6:3051-3064. [PubMed] [Google Scholar]
- 32.Newberry, R. D., W. F. Stenson, and R. G. Lorenz. 1999. Cyclooxygenase-2-dependent arachidonic acid metabolites are essential modulators of the intestinal immune response to dietary antigen. Nat. Med. 5:900-906. [DOI] [PubMed] [Google Scholar]
- 33.Newcomb, W. W., and J. C. Brown. 1991. Structure of the herpes simplex virus capsid: effects of extraction with guanidine hydrochloride and partial reconstitution of extracted capsids. J. Virol. 65:613-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newcomb, W. W., B. L. Trus, N. Cheng, A. C. Steven, A. K. Sheaffer, D. J. Tenney, S. K. Weller, and J. C. Brown. 2000. Isolation of herpes simplex virus procapsids from cells infected with a protease-deficient mutant virus. J. Virol. 74:1663-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palumbo, G. J., W. C. Glasgow, and R. M. Buller. 1993. Poxvirus-induced alteration of arachidonate metabolism. Proc. Natl. Acad. Sci. USA 90:2020-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perdue, M. L., J. C. Cohen, C. C. Randall, and D. J. O'Callaghan. 1976. Biochemical studies of the maturation of herpesvirus nucleocapsid species. Virology 74:unknown. [PubMed] [Google Scholar]
- 37.Pereira, C. F., L. A. Boven, J. Middel, J. Verhoef, and H. S. Nottet. 2000. Induction of cyclooxygenase-2 expression during HIV-1-infected monocyte-derived macrophage and human brain microvascular endothelial cell interactions. J. Leukoc. Biol. 68:423-428. [PubMed] [Google Scholar]
- 38.Ray, N. 2004. Host transcriptional response to alphaherpesvirus infections: implications of COX-2 induction on virus growth. Ph.D. thesis. Princeton University, Princeton, N.J.
- 39.Ray, N., and L. W. Enquist. 2004. Transcriptional response of a common permissive cell type to infection by two diverse alphaherpesviruses. J. Virol. 78:3489-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reiss, C. S., and T. Komatsu. 1998. Does nitric oxide play a critical role in viral infections? J. Virol. 72:4547-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savard, M., C. Belanger, M. J. Tremblay, N. Dumais, L. Flamand, P. Borgeat, and J. Gosselin. 2000. EBV suppresses prostaglandin E2 biosynthesis in human monocytes. J. Immunol. 164:6467-6473. [DOI] [PubMed] [Google Scholar]
- 42.Serrano, F., L. W. Enquist, and J. P. Card. 2002. Pseudorabies virus-induced expression of nitric oxide synthase isoforms. Physiol. Behav. 77:557-563. [DOI] [PubMed] [Google Scholar]
- 43.Sherman, G., and S. L. Bachenheimer. 1988. Characterization of intranuclear capsids made by ts morphogenic mutants of HSV-1. Virology 163:471-480. [DOI] [PubMed] [Google Scholar]
- 44.Smith-Somerville, H. 15February2001, posting date. Resin embedding: basic processing protocol for tissue culture cells grown in plastic dishes. [Online.] http://www.hei.org/research/depts/aemi/resem.htm.
- 45.Snijdewint, F. G., P. Kalinski, E. A. Wierenga, J. D. Bos, and M. L. Kapsenberg. 1993. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J. Immunol. 150:5321-5329. [PubMed] [Google Scholar]
- 46.Steer, S. A., and J. A. Corbett. 2003. The role and regulation of COX-2 during viral infection. Viral Immunol. 16:447-460. [DOI] [PubMed] [Google Scholar]
- 47.Steer, S. A., J. M. Moran, L. B. Maggi, Jr., R. M. Buller, H. Perlman, and J. A. Corbett. 2003. Regulation of cyclooxygenase-2 expression by macrophages in response to double-stranded RNA and viral infection. J. Immunol. 170:1070-1076. [DOI] [PubMed] [Google Scholar]
- 48.Symensma, T. L., D. Martinez-Guzman, Q. Jia, E. Bortz, T. T. Wu, N. Rudra-Ganguly, S. Cole, H. Herschman, and R. Sun. 2003. COX-2 induction during murine gammaherpesvirus 68 infection leads to enhancement of viral gene expression. J. Virol. 77:12753-12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanaka, A., S. Hase, T. Miyazawa, and K. Takeuchi. 2002. Up-regulation of cyclooxygenase-2 by inhibition of cyclooxygenase-1: a key to nonsteroidal anti-inflammatory drug-induced intestinal damage. J. Pharmacol. Exp. Ther. 300:754-761. [DOI] [PubMed] [Google Scholar]
- 50.Thomsen, D. R., W. W. Newcomb, J. C. Brown, and F. L. Homa. 1995. Assembly of the herpes simplex virus capsid: requirement for the carboxyl-terminal twenty-five amino acids of the proteins encoded by the UL26 and UL26.5 genes. J. Virol. 69:3690-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsujii, M., and R. N. DuBois. 1995. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell 83:493-501. [DOI] [PubMed] [Google Scholar]
- 52.Wachsman, M., L. Aurelian, and J. W. Burnett. 1990. The prophylactic use of cyclooxygenase inhibitors in recurrent herpes simplex infections. Br. J. Dermatol. 123:375-380. [DOI] [PubMed] [Google Scholar]
- 53.Zhu, H., J. P. Cong, D. Yu, W. A. Bresnahan, and T. E. Shenk. 2002. Inhibition of cyclooxygenase 2 blocks human cytomegalovirus replication. Proc. Natl. Acad. Sci. USA 99:3932-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]