Abstract
Introduction
In osteoarthritis (OA), the subchondral bone undergoes a remodelling process involving several factors synthesized by osteoblasts. In this study, we investigated the expression, production, modulation, and role of PAR-2 in human OA subchondral bone osteoblasts.
Materials and methods
PAR-2 expression and production were determined by real-time PCR and flow cytometry, respectively. PAR-2 modulation was investigated in OA subchondral bone osteoblasts treated with IL-1β (100 pg/ml), TNF-α (5 ng/ml), TGF-β1 (10 ng/ml), PGE2 (500 nM), IL-6 (10 ng/ml) and IL-17 (10 ng/ml). Membranous RANKL protein was assessed by flow cytometry, and OPG, MMP-1, MMP-9, MMP-13, IL-6 and intracellular signalling pathways by specific ELISAs. Bone resorptive activity was measured by using a co-culture model of human PBMC and OA subchondral bone osteoblasts.
Results
PAR-2 expression and production (p<0.05) were markedly increased when human OA subchondral bone osteoblasts were compared to normal. On OA osteoblasts, PAR-2 production was significantly increased by IL-1β, TNF-α and PGE2. Activation of PAR-2 with a specific agonist, SLIGKV-NH2, induced a significant up-regulation of MMP-1, MMP-9, IL-6, and membranous RANKL, but had no effect on MMP-13 or OPG production. Interestingly, bone resorptive activity was also significantly enhanced following PAR-2 activation. The PAR-2 effect was mediated by activation of the MAP kinases Erk1/2 and JNK.
Conclusion
This study is the first to demonstrate that PAR-2 activation plays a role in OA subchondral bone resorption via an up-regulation of major bone remodelling factors. These results shed new light on the potential of PAR-2 as a therapeutic target in OA.
Keywords: Inflammation, Bone, Osteoblasts, Molecular pathways, Modelling and remodelling
Introduction
A family of cell surface membrane receptors, the proteinase-activated receptors (PARs) belonging to the G-protein coupled receptor family has been recently identified. These receptors are activated through the cleavage of their N-terminal domain by serine proteases, which expose a new N-terminal sequence that acts as a tethered ligand, binding and activating the receptor itself [1,2]. To date, four members have been characterized and named PAR-1 to PAR-4. PARs are expressed on a variety of cells such as platelets, endothelial, tumor, inflammatory and articular cells, and their activation elicits numerous physiological and pathological processes. Among the PARs, activation of PAR-2 has been implicated in the inflammatory process and arthritic diseases including osteoarthritis (OA) [3–7]. In this latter disease, findings from our group showed that PAR-2 was involved in cartilage by increasing catabolic and pro-inflammatory pathways [5].
OA is the most common disabling chronic disease in the world. It is characterized by degradation and loss of articular cartilage followed by synovial membrane inflammation. The notion that early OA is characterized only by the degeneration of the articular cartilage has recently been reconsidered, as subchondral bone alterations were also found to be involved in the early phase of the disease process [8–10]. Data even suggest that subchondral bone is a driving force behind the cartilage degradation observed in OA.
Altered subchondral bone remodelling appears to occur during the OA process and involves both bone resorption and formation. Studies performed in animal models of OA have revealed, at an early stage of the disease, a thinning of the subchondral plate indicating bone resorption [11–13], and, at a later stage, a subchondral bone formation process resulting in sclerosis of the tissue [11,13,14]. However, it has recently been suggested that in humans there are different stages of attempts to repair the damaged subchondral bone tissue, which include an increase in bone resorption followed by abnormal bone sclerosis. Hence, the complete characterization of the subchondral bone specific biochemical changes that distinguish the different stages during the OA process still remains to be determined. However, and in accordance with the literature, these changes are associated with local osteoblast metabolism involving abnormal activation of biochemical pathways. Among these, proteases, eicosanoids, and the factors belonging to the TNF family, OPG (osteoprotegerin) and RANKL (receptor activator of nuclear factor kappa B ligand), were found at abnormal levels in human OA subchondral bone osteoblasts [12,15–21].
We hypothesized that PAR-2 is expressed and produced by human OA subchondral bone osteoblasts and that its activation plays an important role in the progression of the disease. We thus investigated the presence and role of PAR-2 in human OA subchondral bone osteoblasts. Data showed that PAR-2 is expressed and produced at elevated levels by these cells, and a specific PAR-2 activation led to an up-regulation of MMP-1, MMP-9, IL-6, and membranous RANKL production and induced bone resorptive activity by these cells. This occurred via the activation of extracellular signal-regulated kinase 1/2 (Erk1/2) and Jun N-terminal kinase (JNK).
Materials and methods
Specimen selection
Normal (control) human subchondral bones were obtained from the femoral condyles of individuals at autopsy within 12 h of death (mean age±SD: 65±16). These individuals had no history of joint disease and died of causes unrelated to arthritic diseases. Moreover, the tissues were examined macroscopically and microscopically (histology) to ensure that only normal tissue was used. Human OA specimens were obtained from femoral condyles of patients undergoing total knee arthroplasty (mean age±SD: 72±8). All patients were evaluated by a certified rheumatologist and diagnosed as having OA according to the American College of Rheumatology clinical criteria [22]. None of the normal individuals or OA patients had received medication that would interfere with bone metabolism. The institutional Ethics Committee Board of the University of Montreal Hospital Centre approved the use of the human articular tissues.
Subchondral bone osteoblast culture
The subchondral bone osteoblast culture was prepared as previously described [15]. Briefly, subchondral bone plate was isolated and cut into small pieces and digested twice for 30 min and once for 4 h with collagenase type I in BGJb medium (both from Sigma-Aldrich Canada, Oakville, ON, Canada) without serum at 37 °C in a humidified atmosphere of 5% CO2/95% air. After this period, the bone pieces were cultured in BGJb medium containing 20% heat-inactivated fetal calf serum (FCS; Gibco-BRL, Burlington, ON, Canada) and an antibiotic mixture (100 U/ml penicillin base and 100 μg/ml streptomycin base) (Gibco-BRL) at 37 °C in the humidified atmosphere. When cells were observed in the culture flask, the culture medium was replaced with fresh medium containing 10% FCS and cells were cultured until confluence. The phenotypic features of osteoblast cultures were determined by incubating the cells in the absence or presence of 1,25(OH)2D3 (50 nM) as previously described [17,23]. Data showed that these cells were mature differentiated cells as they expressed the bone specific markers, alkaline phosphatase and osteocalcin. The osteoblasts were harvested with Cell Dissociation Buffer (Gibco-BRL) which contains no protease. Cells were then used for PAR-2 expression and production determination or seeded and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco-BRL) containing 10% FCS at 37 °C until confluence.
For the experiments, cells were incubated with DMEM containing 0.5% FCS for 24 h before treatment, then treated with IL-1β (100 pg/ml), TNF-α (5 ng/ml), TGF-β1 (10 ng/ml), PGE2 (500 nM), IL-6 (10 ng/ml) and IL-17 (10 ng/ml) (all from R&D Systems, Minneapolis, MN, USA) in DMEM/0.5% FCS for 72 h for PAR-2 protein determination. For the membranous RANKL, OPG, MMP-1, MMP-9, MMP-13 and IL-6 synthesis determinations, cells were treated with the specific synthetic PAR-2-activating peptide (PAR-2-AP, SLIGKV-NH2) (Bachem, Torrance, CA, USA) for 72 h in DMEM/0.5% FCS. The PAR-2-AP concentrations, 100 and 400 μM, were chosen in accordance with our previous study and those of others [5,24,25]. For the signalling pathways investigation, cells were treated with PAR-2-AP for 0–60 min in DMEM/0.5% FCS.
RNA extraction, reverse transcription (RT), and real-time polymerase chain reaction (PCR)
Total RNA from human osteoblasts was extracted with TRIzol® (Invitrogen, Burlington, ON, Canada) as previously described [20] and genomic DNA was removed following the manufacturer’s instructions (Ambion Inc., Austin, TX, USA). The RNA was quantified with the RiboGreen® RNA quantification kit (Molecular Probes, Eugene, OR, USA). Complementary DNA (cDNA) was reverse-transcribed (RT) from 1 μg total RNA purified in a 50 μl reaction mixture containing 1 mM each of deoxynucleotide triphosphates (dNTP) (Invitrogen), 0.4 U/μl RNase inhibitor, 2.5 μM of random hexamer (both from GE Healthcare, Baie d’Urfé, QC, Canada), 2.5 U/μl of reverse-transcriptase (Invitrogen), 5 mM of MgCl2 and 1× of PCR buffer. The reaction mixture was incubated in a DNA thermal cycle at 42 °C for 15 min and then stored at −20 °C before use. Real-time PCR was performed using primers specific for the human PAR-2 and for the human housekeeping gene glyceraldehydes-3-phosphate dehydrogenase (GAPDH). The primers were 5′-GAAGCCTTATTGGTAAGGTTG (sense) and 5′-CAGAGAGGAGGTCAGCCAAG (anti-sense) for PAR-2 and 5′-CAGAACATCATCCCTGCCTCT (sense) and 5′-GCTTGACAAAGTGGTCGTTGAG (anti-sense) for GAPDH. In brief, 10 μl of the cDNA obtained from the RT reactions were amplified in a total volume of 25 μl consisting of 1× Quantitect SYBR Green PCR Master Mix (Qiagen, Mississauga, ON, Canada), 0.5 U/reaction uracil-N-glycosylase (UNG) (Invitrogen), and gene-specific primers which were added at a final concentration of 200 nM. Real-time quantitation mRNA was performed in the Rotor-Gene 6® RG-3000A (Corbett Research, Mortlake, Australia) according to the manufacturer’s instructions. Data were processed with Rotor-Gene version 6 Software and were given as threshold cycle (CT) corresponding to the PCR cycle at which an increase in reporter fluorescence above a baseline signal can first be detected. Plasmid DNAs containing target gene sequences were used to generate the standard curves. The CT was converted to number of molecules and the values for each sample were calculated as the ratio of the number of molecules of the target gene to the number of molecules of GAPDH and expressed as arbitrary unit.
Membranous RANKL and PAR-2 determination
Membranous RANKL and PAR-2 were determined using the flow cytometry methodology as previously described [20]. Briefly, at the end of the incubation period, cells were washed once in PBS, harvested with the Cell Dissociation Buffer at 37 °C, and centrifuged at 500 g for 5 min at 4 °C. Cells were re-suspended in 1% BSA/PBS, and 500 μl suspension was prepared, having a concentration of 1×106 cells/ml. The suspension was incubated for 30 min at room temperature and divided into two tubes. One served as negative control to which for RANKL a mouse IgG (15 μg/ml; Chemicon International, Billerica, USA) and for PAR-2 a mouse IgG coupled to phycoerythrin (IgG-PE: 20 μg/ml; R&D Systems) was added and the other was incubated with either a mouse anti-human RANKL antibody (15 μg/ml; R&D Systems) or a mouse anti-human PAR-2-phycoerythrin (PE) (20 μg/ml; SAM11, Santa Cruz Biotechnology, Santa Cruz, CA, USA) respectively, for 45 min at 4 °C. For RANKL, after this period, cells were washed and a goat anti-mouse FITC-conjugated secondary antibody (7.5 μg/ml; R&D Systems) was added for another 30 min at 4 °C. Cells were then washed in PBS, re-suspended in PBS, and analyzed using flow cytometry (FACSCalibur, BD Bioscience, Mississauga, ON, Canada). The negative control sample was used to determine background fluorescence and compared to that of the sample incubated with the specific antibody. The level of fluorescence was measured by a FACScan using the CellQuest program (BD Bioscience), calculated as the mean fluorescent intensity (MFI) of positive cells. Data were expressed in MFI for PAR-2 basal level and in fold of control for RANKL and PAR-2 modulation.
Resorptive activity determination
Resorptive activity was determined by using the BD BioCoat Osteologic Bone Cell Culture System (BD Bioscience) as previously described [20]. In brief, on a synthetic substrate, human peripheral blood mononuclear cells (PBMC; 100,000 cells/well) were cultured in DMEM 10% FCS supplemented with M-CSF (25 ng/ml) (R&D Systems) for 5 days at 37 °C, in order for the PBMC to differentiate into pre-osteoclasts [26]. After this period, human OA osteoblasts (10,000 cells/well) were added and incubated for 24 h. Fresh culture medium DMEM/10%FCS containing M-CSF (25 ng/ml) and PAR-2-AP at 100 μM or 400 μM was then added before incubation at 37 °C for 4 weeks. Media was changed every 3 days. At the end of the incubation period, cells were bleached (6% NaOCl, 5.2% NaCl), extensively washed in sterilized water, and stained with Von Kossa as described by the manufacturer’s instructions. The resorptive activity was quantitated by the measurement of the resorption pits with a light microscope (Leitz Orthoplan; Leica Inc., St. Laurent, QC, Canada) using the Bioquant software (Bioquant Osteo II, v8.00.20, Nashville, TN, USA). Results were expressed as the mean resorbed surface per total surface.
Signalling pathways determination
The levels of the phosphorylated MAP kinases Erk1/2, p38 and JNK were analyzed by the Cellular Activation of Signalling ELISA (CASE™; Super Array Bioscience Corporation, Frederick, MD, USA) and NF-κB p65 by a specific ELISA (Assay Designs, Ann Arbor, MI, USA).
Protein determination
Human OPG (Medicorp, Montreal, QC, Canada), IL-6, MMP-1, MMP-9 (all from R&D Systems) and MMP-13 (Millipore Corporation, Nepean, ON, Canada) production were determined in the culture media by specific ELISAs according to the manufacturer’s instructions.
Statistical analysis
Values are expressed as mean±SEM. Statistical analysis was performed using unpaired or paired Student’s t-test when appropriate, and p values ≤0.05 were considered significant.
Results
PAR-2 expression and production
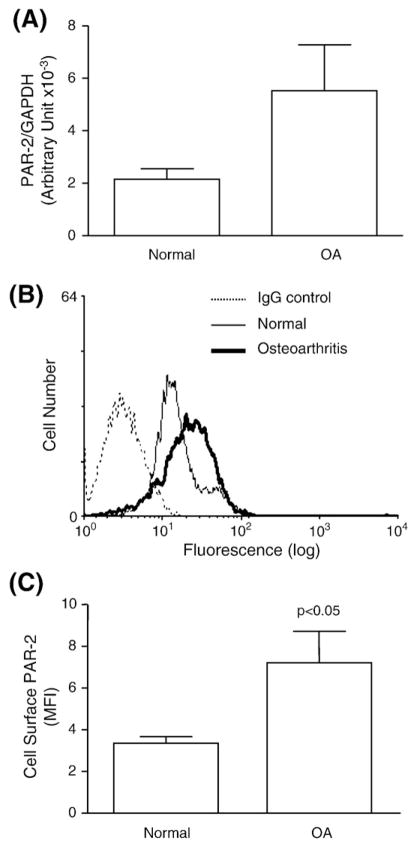
We first investigated whether PAR-2 is expressed on human subchondral bone osteoblasts, comparing normal to OA. Data showed (Fig. 1A) that PAR-2 was expressed on both cell types and that the OA osteoblast (n=8) gene expression level was higher than the normal (n=4); however, this did not quite reach statistical significance.
Fig. 1.
Proteinase-activated receptor 2 (PAR-2) (A) gene expression and (B, C) protein production. (A) Histogram of mRNA expression in normal (n=4) and OA (n=8) subchondral bone osteoblasts. (B) A flow cytometry representative profile of the PAR-2 specific antibody showing the pattern of a mouse IgG as control for background fluorescence and normal and OA human subchondral bone osteoblasts. (C) Level of the cell surface PAR-2, as determined by flow cytometry, in normal (n=5) and OA (n=6) osteoblasts. The p value indicates the comparison of normal and OA osteoblasts using the Student’s t-test.
We further determined PAR-2 protein levels by flow cytometry (Fig. 1B) and data revealed that the PAR-2 production was significantly enhanced in OA (n=6) osteoblasts compared to normal (n=5) (p<0.05) (Fig. 1C).
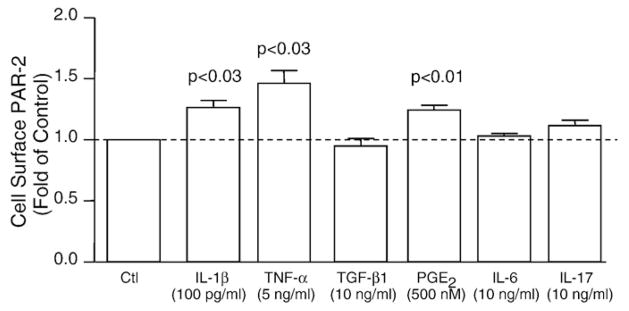
Modulation of PAR-2 production
Since PAR-2 was present and its levels enhanced in human OA subchondral bone osteoblasts, we further investigated on these cells, as they are the focus of this study, factors that could be responsible for its up-regulation. Human OA subchondral bone osteoblasts (n=4) were treated with factors involved in alterations of this tissue, IL-1β, TNF-α, TGF-β1, PGE2, IL-6 and IL-17, and PAR-2 synthesis was determined. Data, as illustrated in Fig. 2, revealed that PAR-2 was significantly up-regulated by the pro-inflammatory cytokines IL-1β (p<0.03) and TNF-α (p<0.03), and the inflammatory mediator PGE2 (p<0.01). No effect was found with TGF-β1, IL-6 or IL-17.
Fig. 2.
PAR-2 synthesis modulation, as determined by flow cytometry, in human OA subchondral bone osteoblasts (n=4). Cells were treated with IL-1β, TNF-α, TGF-β1, PGE2, IL-6 and IL-17 for 72 h. Data are expressed as fold of control in which the control was assigned a value of 1. P values indicate the comparison with the untreated (Ctl) cells using the paired Student’s t-test.
PAR-2 functional consequences
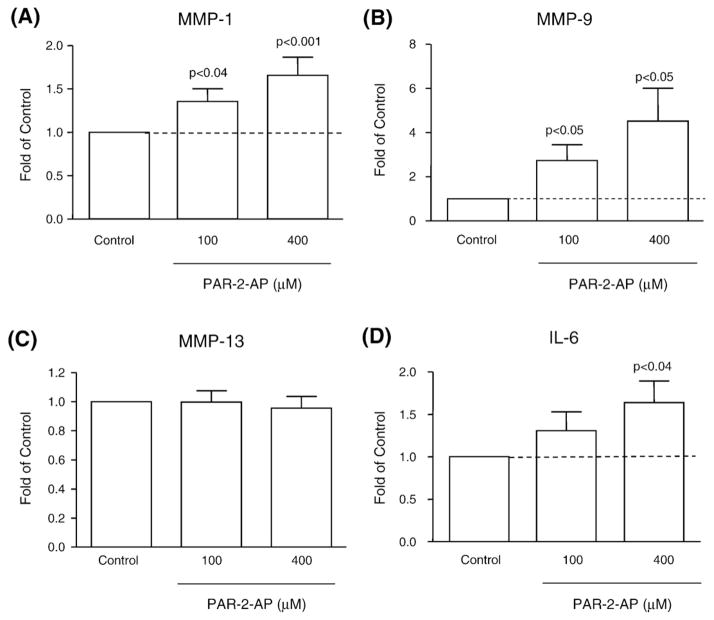
We further investigated on the OA osteoblasts (n=9–12) upon activation of PAR-2 by its specific agonist SLIGKV-NH2 (PAR-2-AP) at 100 and 400 μM, the modulation of various remodelling factors known to be involved in pathophysiological processes in bone. These factors included MMP-1, MMP-9, and MMP-13, the pro-inflammatory cytokine IL-6, and the factors involved in bone resorption, OPG and RANKL.
Data revealed that PAR-2 activation significantly increased in a dose-dependent manner MMP-1 (PAR-2-AP 100 μM, p <0.04; 400 μM, p<0.001), MMP-9 (p<0.05, both concentrations), and IL-6 with significance reached at 400 μM (p<0.04) (Figs. 3A, B, and D). The level of MMP-13 production was not affected by the activation of PAR-2 (Fig. 3C).
Fig. 3.
Production of the metalloproteases (A) MMP-1, (B) MMP-9, and (C) MMP-13, and (D) the pro-inflammatory cytokine IL-6 following specific PAR-2 activation. OA subchondral bone osteoblasts (n=9–12) were incubated for 72 h in the absence (Control) or presence of PAR-2-AP at 100 and 400 μM. Data are expressed as fold of control in which the control was assigned a value of 1. P values indicate the comparison with the untreated (Control) cells using the paired Student’s t-test.
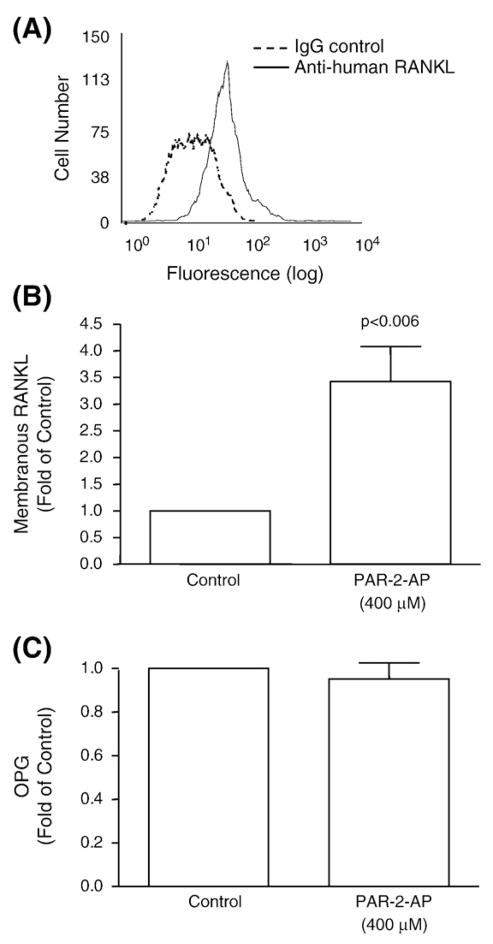
Interestingly, the membranous RANKL (Figs. 4A, B), but not OPG (Fig. 4C), was also significantly up-regulated (p<0.006) by PAR-2 activation in OA osteoblasts (n=10). Complementary experiments were performed in which we looked at the level of membranous RANKL comparing normal (n=3) and OA (n=10). Data showed, as expected, a significantly (p<0.02) higher level of membranous RANKL in OA with a mean fluorescent intensity of 127.5±8.9 and 79.5±13.6 for OA and normal osteoblasts, respectively (data not shown). Although data (Fig. 4B) showed that in OA osteoblasts, PAR-2 activation significantly induced the membranous RANKL level, this was not the case for the normal (n=4) osteoblasts (data not shown). On the other hand, the OPG level was not statistically different between normal (n=3) and OA (n=10) osteoblasts; values of 12.9± 4.3 pg/106 cells and 18.4±1.5 pg/106 cells were recorded respectively (data not shown). In normal (n=4) (data not shown) as with the OA cells (Fig. 4C), PAR-2 activation did not affect the production of OPG.
Fig. 4.
(A, B) Membranous RANKL and (C) OPG production levels from OA subchondral bone osteoblasts. (A) A flow cytometry representative profile of a RANKL specific antibody showing the pattern of a mouse IgG as control for background fluorescence and human OA subchondral bone osteoblasts. Level of (B) membranous RANKL (n=10) in human OA subchondral bone osteoblasts and (C) OPG (n=10) production as determined by a specific ELISA assay in OA human subchondral bone osteoblasts. Cells were incubated in the absence (Control) or presence of PAR-2-AP at 400 μM for 72 h. Data are expressed as fold of control in which the control was assigned a value of 1. The p value indicates the comparison with the untreated (Control) cells using the paired Student’s t-test.
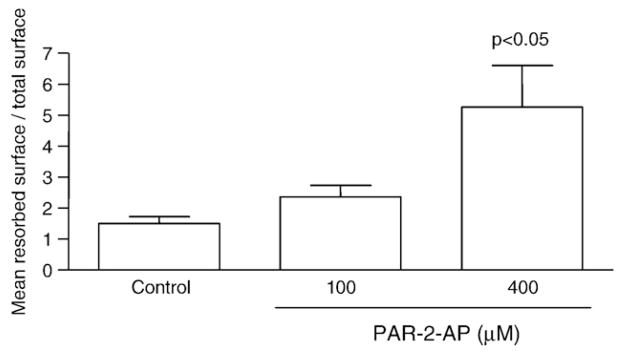
Since the above data on OA osteoblasts suggest a pattern of bone resorption, we further investigated the effect of PAR-2 activation on the resorptive properties induced by these osteoblasts (n=5) by using a co-culture model of human PBMC and OA subchondral bone osteoblasts. PAR-2-AP at 100 and 400 μM enhanced bone resorption, and statistical significance was reached at 400 μM (p<0.05) (Fig. 5).
Fig. 5.
Resorptive activity of human OA osteoblasts (n=5) co-incubated with human PBMC, on a synthetic substrate, in the absence (Control) or presence of PAR-2-AP at 100 and 400 μM for 4 weeks in DMEM/FCS supplemented with M-CSF (25 ng/ml) as described in Materials and methods. Data are expressed as the mean resorbed surface over the total surface. The p value indicates the comparison with the untreated (Control) cells using the paired Student’s t-test.
PAR-2-induced signalling pathways
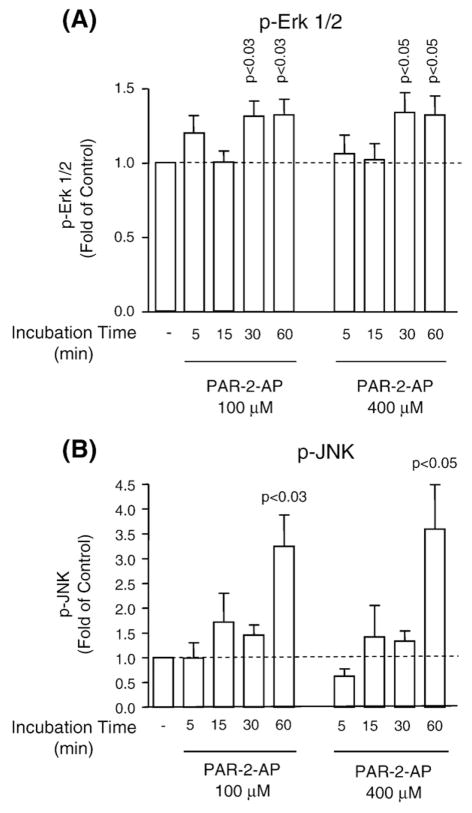
In OA osteoblasts, the effect of PAR-2 activation on the phosphorylated levels of three MAP kinases, namely Erk1/2 (n=7), p38 (n=5), and JNK (n=5), and on NF-κB (n=4) was determined. Activation of PAR-2 yielded a significant increase in the phosphorylation of Erk1/2 at 30 min and JNK at 60 min; this is true for both concentrations used (Fig. 6). The other signalling pathways, namely the phosphorylated p38 and NF-κB p65, were not significantly modulated following PAR-2 activation (data not shown).
Fig. 6.
Signalling pathways induced by specific PAR-2 activation. Time and dose profiles of PAR-2 signalling phosphorylated (A) Erk 1/2 (p-Erk 1/2) (n=7) and (B) JNK (p-JNK) (n=5) in OA human subchondral bone osteoblasts. Cells were incubated in the absence (−) or presence of PAR-2-AP at 100 and 400 μM for 0 (−) to 60 min. Data are expressed as fold of control in which the control was assigned a value of 1. P values indicate the comparison with the untreated (−) cells using the paired Student’s t-test.
Discussion
To date, few studies have reported the implication of PAR-2 in human OA. It has been shown in the literature that PAR-2 is expressed and produced in higher levels in OA chondrocytes [4,5], and that its activation in OA cartilage up-regulates some catabolic and pro-inflammatory pathways involved in the progression of the disease [5]. To our knowledge, only one study has reported that PAR-2 was expressed in osteoblast-like cells from rats [27]. In the present study, we showed that this receptor was present in human subchondral bone osteoblasts, up-regulated in OA, and that its activation might affect the course of pathological processes by inducing the levels of catabolic factors including MMP-1, MMP-9, IL-6, and the membranous RANKL, as well as increasing the resorptive activity of the osteoblasts. Evidence suggests that the fate of OA subchondral bone is not only related to the stiffening of this tissue but also to a resorption process. Thus, the catabolic effect of PAR-2 observed on human OA osteoblasts could contribute to and thus explain the resorptive remodelling process occurring in this tissue. These findings on the resorptive properties of PAR-2 are in agreement with data reporting the involvement of PAR-2 activity in periodontitis, a disease characterized by bone loss [28,29], as well as with in vivo studies reporting the presence of indicators of subchondral bone resorption in human OA patients and in some animal models of OA [12,13,30–32].
Data from the present study showed that PAR-2 production was significantly elevated in OA subchondral bone osteoblasts compared to normal, and following its specific activation, factors involved in the abnormal metabolism of these cells, including some MMPs, IL-6, and RANKL, were up-regulated. In turn, these factors were found to be implicated in the resorption of bone matrix (see below), which concurs with the finding that PAR-2 significantly increased the capacity of osteoblasts to induce resorptive activity.
The data showing the significant enhancement of MMP-1 and MMP-9 synthesis following PAR-2 activation are of importance as they concur with the findings that these two MMPs are elevated in OA subchondral bone and their levels are linked to the bone resorption process in this tissue [33–35]. The fact that the level of MMP-1, but not MMP-13, was up-regulated following PAR-2 activation is not surprising since MMP-1 was shown to be a key enzyme in the processing of collagen type I, the main collagen type in bone matrix [36]. Moreover, several studies suggest that in adults, MMP-13 is a specific enzyme of OA cartilage [37] even though its expression/production has also been found on osteoblastic cells [38,39]. The elevation of MMP-9 also agrees with the data showing that, in rats, activation of PAR-2 triggers periodontitis along with an up-regulation of MMP-9 levels [28]. Although the effect of MMP-9 on human bone resorption is now well established and one of its important roles in this process is to facilitate the migration of osteoclasts [40,41], recent literature has also reported its ability to act on native collagen in a manner similar to that of the collagenases [42]. Furthermore, exposure of osteoblasts to bone-resorbing cytokines such as IL-1β and TNF-α induces enhanced expression of some MMPs including MMP-9 [43–45].
In this study we also showed that the pro-inflammatory cytokine IL-6 and RANKL were significantly up-regulated following PAR-2 activation. Both factors, IL-6 and RANKL, were reported to promote osteoclast formation and activity, thereby potentiating osteoclastogenesis [46,47]. Hence, IL-6 secreted by human osteoblasts could promote bone resorption either directly on human pre-osteoclast cells [48,49] or indirectly via increasing the level of RANKL [20,50] and/or having a synergy [51]. In either case, this cytokine and RANKL act as potent inducers of osteoclast formation.
The results showing that PAR-2 in both cell types studied, normal and OA osteoblasts, demonstrated no effect on the OPG production, are of importance, as the OPG/RANKL ratio plays a crucial role in orientating the pathophysiological evolution of bone remodelling. Interestingly, abnormal OPG and RANKL levels favouring increased RANKL were recently reported in human OA subchondral bone [20,52]. Thus, one can hypothesize that the increased RANKL in this human tissue could be due, at least in part, to an increase in PAR-2 activation.
Further investigation of the modulatory factors of PAR-2 production in human OA osteoblasts showed that the pro-inflammatory cytokines IL-1β and TNF-α, as well as PGE2, significantly enhanced the PAR-2 protein synthesis. Such an effect by these factors, especially IL-1β and TNF-α, was not surprising as this was previously reported to occur in OA chondrocytes as well as in other cell types [4,5,53,54]. The finding that PAR-2 significantly increased the resorptive activity of the osteoblasts also concurs with this data, as these three factors are considered potent bone-resorbing agents [55–61]. Thus, the increased level of PAR-2 in OA subchondral bone compared to normal may be associated with an increased level of these factors during the progression of the disease.
The PAR-2-mediated functional consequence in human OA cells implicates the activation of the MAP kinases Erk1/2 and JNK. These two MAP kinase signalling cascades were found to play a crucial role in the response of osteoblasts to a variety of stimuli, particularly to those activated downstream of several G-protein-coupled receptors [62–64]. More specifically, our data are in agreement with those showing that the Erk1/2 pathway was preferentially employed following PAR-2 activation in the induction of MMP-9 in bronchial and airway epithelial cells [65,66] and of MMP-1 in human OA chondrocytes [5]. Moreover, PAR-2 was also shown to induce IL-6 levels through both Erk1/2 and JNK in endometriotic stromal cells and in human blood eosinophils [67,68]. Although little is known about the signalling pathway of PAR-2-inducing RANKL production, Tsubaki et al. [69] recently reported the requirement of Erk1/2 for the up-regulation of RANKL expression in osteoblasts.
Bone remodelling involves a close coupling of bone resorption and formation. Our data showed that abnormal PAR-2 activity may lead to an imbalance in bone remodelling, favouring bone resorption. In vivo activation of PAR-2 occurs through the involvement of a proteolytic cleavage. According to the literature, enzymes from the serine dependent protease family appear to be responsible for the activation of this receptor [1,2]. However, although some serine proteases are known to activate PARs, the endogenous activator of PAR-2 in joint tissues is yet to be identified. In OA tissues, and particularly in the subchondral bone, the predominant serine protease that is up-regulated is the urokinase (uPA), which belongs to the plasminogen activator/plasmin system [15,23]. Moreover, data from studies using an OA dog model showed that in vivo treatment that reduces subchondral bone resorption was associated with a reduction in the level of the uPA [70,71]. Although very speculative, it is tempting to hypothesize that in vivo activation of PAR-2 in humans occurs via the uPA. Studies are currently underway to validate this hypothesis.
In conclusion, this study is the first to report that PAR-2 is likely to be involved in the abnormal remodelling process that occurs in human OA subchondral bone. PAR-2 activation can induce the production of inflammatory mediators and MMPs as well as RANKL in subchondral bone, providing a critical link between inflammation and tissue remodelling and destruction. Although the initiating event of OA has yet to be identified, there is now strong evidence showing that subchondral bone alterations are closely associated with a resorptive activity at an early stage of the disease [13,20,33,70]. The catabolic involvement of PAR-2 activation during human OA appears to be present in the major articular tissues including cartilage [4,5], synovial membrane [72,73] and, from the present study, the subchondral bone. Hence, PAR-2 is an interesting candidate for the development of therapeutic approaches to this disease. Inhibition of the production and/or activation of this receptor may represent a novel therapeutic alternative for OA, targeting not only the cartilage but also the subchondral bone.
Supplementary Material
Acknowledgments
We thank François Mineau, François-Cyril Jolicoeur and Changshan Geng for their exceptional technical support and Virginia Wallis for her assistance in manuscript preparation. The authors also thank Dr. Marika Sarfati, Dr. Guy Delespesse and Manuel Rubio from the Immunoregulation and Allergy Research Unit at the University of Montreal Research Centre for the use of their flow cytometry apparatus. This study was supported by grants from the Research Centre of the University of Montreal Hospital Centre and by the Canadian Institutes of Health Research (CIHR) (#MOP-86478). Ms. Nathalie Amiable was a recipient of a doctoral bursary from the MENTOR/CIHR and Dr. Steeve Kwan Tat received a post-doctoral fellowship from ESCEO/Amgen and MENTOR/CIHR.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bone.2009.02.015.
References
- 1.Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–82. [PubMed] [Google Scholar]
- 2.Hollenberg MD, Compton SJ International Union of Pharmacology. XXVIII. Proteinase-activated receptors. Pharmacol Rev. 2002;54:203–17. doi: 10.1124/pr.54.2.203. [DOI] [PubMed] [Google Scholar]
- 3.Ferrell WR, Lockhart JC, Kelso EB, Dunning L, Plevin R, Meek SE, et al. Essential role for proteinase-activated receptor-2 in arthritis. J Clin Invest. 2003;111:35–41. doi: 10.1172/JCI16913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiang Y, Masuko-Hongo K, Sekine T, Nakamura H, Yudoh K, Nishioka K, et al. Expression of proteinase-activated receptors (PAR)-2 in articular chondrocytes is modulated by IL-1beta, TNF-alpha and TGF-beta. Osteoarthr Cartil. 2006;14:1163–73. doi: 10.1016/j.joca.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Boileau C, Amiable N, Martel-Pelletier J, Fahmi H, Duval N, Pelletier JP. Activation of proteinase-activated receptor 2 in human osteoarthritic cartilage upregulates catabolic and proinflammatory pathways capable of inducing cartilage degradation: a basic science study. Arthritis Res Ther. 2007;9:R121. doi: 10.1186/ar2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Busso N, Frasnelli M, Feifel R, Cenni B, Steinhoff M, Hamilton J, et al. Evaluation of protease-activated receptor 2 in murine models of arthritis. Arthritis Rheum. 2007;56:101–7. doi: 10.1002/art.22312. [DOI] [PubMed] [Google Scholar]
- 7.Kelso EB, Ferrell WR, Lockhart JC, Elias-Jones I, Hembrough T, Dunning L, et al. Expression and proinflammatory role of proteinase-activated receptor 2 in rheumatoid synovium: ex vivo studies using a novel proteinase-activated receptor 2 antagonist. Arthritis Rheum. 2007;56:765–71. doi: 10.1002/art.22423. [DOI] [PubMed] [Google Scholar]
- 8.Felson DT, McLaughlin S, Goggins J, LaValley MP, Gale ME, Totterman S, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139:330–6. doi: 10.7326/0003-4819-139-5_part_1-200309020-00008. [DOI] [PubMed] [Google Scholar]
- 9.Martel-Pelletier J, Lajeunesse D, Reboul P, Pelletier JP. The role of subchondral bone in osteoarthritis. In: Sharma L, Berenbaum F, editors. Osteoarthritis: A Companion to Rheumatology. Philadelphia, USA: MosbyElsevier; 2007. pp. 15–32. [Google Scholar]
- 10.Raynauld JP, Martel-Pelletier J, Berthiaume MJ, Abram F, Choquette D, Haraoui B, et al. Correlation between bone lesion changes and cartilage volume loss in patients with osteoarthritis of the knee as assessed by quantitative magnetic resonance imaging over a 24-month period. Ann Rheum Dis. 2008;67:683–8. doi: 10.1136/ard.2007.073023. [DOI] [PubMed] [Google Scholar]
- 11.Hayami T, Pickarski M, Wesolowski GA, McLane J, Bone A, Destefano J, et al. The role of subchondral bone remodeling in osteoarthritis: reduction of cartilage degeneration and prevention of osteophyte formation by alendronate in the rat anterior cruciate ligament transection model. Arthritis Rheum. 2004;50:1193–206. doi: 10.1002/art.20124. [DOI] [PubMed] [Google Scholar]
- 12.Pelletier JP, Boileau C, Brunet J, Boily M, Lajeunesse D, Reboul P, et al. The inhibition of subchondral bone resorption in the early phase of experimental dog osteoarthritis by licofelone is associated with a reduction in the synthesis of MMP-13 and cathepsin K. Bone. 2004;34:527–38. doi: 10.1016/j.bone.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 13.Hayami T, Pickarski M, Zhuo Y, Wesolowski GA, Rodan GA, Duong le T. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone. 2006;38:234–43. doi: 10.1016/j.bone.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Brandt KD, Myers SL, Burr D, Albrecht M. Osteoarthritic changes in canine articular cartilage, subchondral bone, and synovium fifty-four months after transection of the anterior cruciate ligament. Arthritis Rheum. 1991;34:1560–70. doi: 10.1002/art.1780341214. [DOI] [PubMed] [Google Scholar]
- 15.Hilal G, Martel-Pelletier J, Pelletier JP, Duval N, Lajeunesse D. Abnormal regulation of urokinase plasminogen activator by insulin-like growth factor 1 in human osteoarthritic subchondral osteoblasts. Arthritis Rheum. 1999;42:2112–22. doi: 10.1002/1529-0131(199910)42:10<2112::AID-ANR11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 16.Hilal G, Massicotte F, Martel-Pelletier J, Fernandes JC, Pelletier JP, Lajeunesse D. Endogenous prostaglandin E2 and insulin-like growth factor 1 can modulate the levels of parathyroid hormone receptor in human osteoarthritic osteoblasts. J Bone Miner Res. 2001;16:713–21. doi: 10.1359/jbmr.2001.16.4.713. [DOI] [PubMed] [Google Scholar]
- 17.Massicotte F, Lajeunesse D, Benderdour M, Pelletier JP, Hilal G, Duval N, et al. Can altered production of interleukin-1beta, interleukin-6, transforming growth factor-beta and prostaglandin E(2) by isolated human subchondral osteoblasts identify two subgroups of osteoarthritic patients. Osteoarthr Cartil. 2002;10:491–500. doi: 10.1053/joca.2002.0528. [DOI] [PubMed] [Google Scholar]
- 18.Paredes Y, Massicotte F, Pelletier JP, Martel-Pelletier J, Laufer S, Lajeunesse D. Study of the role of leukotriene B4 in abnormal function of human subchondral osteoarthritis osteoblasts: effects of cyclooxygenase and/or 5-lipoxygenase inhibition. Arthritis Rheum. 2002;46:1804–12. doi: 10.1002/art.10357. [DOI] [PubMed] [Google Scholar]
- 19.Massicotte F, Aubry I, Martel-Pelletier J, Pelletier JP, Fernandes J, Lajeunesse D. Abnormal insulin-like growth factor 1 signaling in human osteoarthritic subchondral bone osteoblasts. Arthritis Res Ther. 2006;8:R177. doi: 10.1186/ar2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwan Tat S, Pelletier JP, Lajeunesse D, Fahmi H, Lavigne M, Martel-Pelletier J. The differential expression of osteoprotegerin (OPG) and receptor activator of nuclear factor kappaB ligand (RANKL) in human osteoarthritic subchondral bone osteoblasts is an indicator of the metabolic state of these disease cells. Clin Exp Rheumatol. 2008;26:295–304. [PMC free article] [PubMed] [Google Scholar]
- 21.Tat SK, Pelletier JP, Lajeunesse D, Fahmi H, Duval N, Martel-Pelletier J. Differential modulation of RANKL isoforms by human osteoarthritic subchondral bone osteoblasts: influence of osteotropic factors. Bone. 2008;43:284–91. doi: 10.1016/j.bone.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 23.Hilal G, Martel-Pelletier J, Pelletier JP, Ranger P, Lajeunesse D. Osteoblast-like cells from human subchondral osteoarthritic bone demonstrate an altered phenotype in vitro: possible role in subchondral bone sclerosis. Arthritis Rheum. 1998;41:891–9. doi: 10.1002/1529-0131(199805)41:5<891::AID-ART17>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 24.Chi L, Li Y, Stehno-Bittel L, Gao J, Morrison DC, Stechschulte DJ, et al. Interleukin-6 production by endothelial cells via stimulation of protease-activated receptors is amplified by endotoxin and tumor necrosis factor-alpha. J Interferon Cytokine Res. 2001;21:231–40. doi: 10.1089/107999001750169871. [DOI] [PubMed] [Google Scholar]
- 25.Lin KW, Park J, Crews AL, Li Y, Adler KB. Protease-activated receptor-2 (PAR-2) is a weak enhancer of mucin secretion by human bronchial epithelial cells in vitro. Int J Biochem Cell Biol. 2008;40:1379–88. doi: 10.1016/j.biocel.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dempster DW, Hughes-Begos CE, Plavetic-Chee K, Brandao-Burch A, Cosman F, Nieves J, et al. Normal human osteoclasts formed from peripheral blood monocytes express PTH type 1 receptors and are stimulated by PTH in the absence of osteoblasts. J Cell Biochem. 2005;95:139–48. doi: 10.1002/jcb.20388. [DOI] [PubMed] [Google Scholar]
- 27.Abraham LA, Chinni C, Jenkins AL, Lourbakos A, Ally N, Pike RN, et al. Expression of protease-activated receptor-2 by osteoblasts. Bone. 2000;26:7–14. doi: 10.1016/s8756-3282(99)00237-9. [DOI] [PubMed] [Google Scholar]
- 28.Holzhausen M, Spolidorio LC, Vergnolle N. Proteinase-activated receptor-2 (PAR2) agonist causes periodontitis in rats. J Dent Res. 2005;84:154–9. doi: 10.1177/154405910508400209. [DOI] [PubMed] [Google Scholar]
- 29.Holzhausen M, Spolidorio LC, Ellen RP, Jobin MC, Steinhoff M, Andrade-Gordon P, et al. Protease-activated receptor-2 activation: a major role in the pathogenesis of Porphyromonas gingivalis infection. Am J Pathol. 2006;168:1189–99. doi: 10.2353/ajpath.2006.050658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharif M, George E, Dieppe PA. Correlation between synovial fluid markers of cartilage and bone turnover and scintigraphic scan abnormalities in osteoarthritis of the knee. Arthritis Rheum. 1995;38:78–81. doi: 10.1002/art.1780380112. [DOI] [PubMed] [Google Scholar]
- 31.Pastoureau PC, Chomel AC, Bonnet J. Evidence of early subchondral bone changes in the meniscectomized guinea pig. A densitometric study using dual-energy X-ray absorptiometry subregional analysis. Osteoarthr Cartil. 1999;7:466–73. doi: 10.1053/joca.1999.0241. [DOI] [PubMed] [Google Scholar]
- 32.Bettica P, Cline G, Hart DJ, Meyer J, Spector TD. Evidence for increased bone resorption in patients with progressive knee osteoarthritis: longitudinal results from the Chingford study. Arthritis Rheum. 2002;46:3178–84. doi: 10.1002/art.10630. [DOI] [PubMed] [Google Scholar]
- 33.Hulejova H, Baresova V, Klezl Z, Polanska M, Adam M, Senolt L. Increased level of cytokines and matrix metalloproteinases in osteoarthritic subchondral bone. Cytokine. 2007;38:151–6. doi: 10.1016/j.cyto.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Logar DB, Komadina R, Prezelj J, Ostanek B, Trost Z, Marc J. Expression of bone resorption genes in osteoarthritis and in osteoporosis. J Bone Miner Metab. 2007;25:219–25. doi: 10.1007/s00774-007-0753-0. [DOI] [PubMed] [Google Scholar]
- 35.Shibakawa A, Yudoh K, Masuko-Hongo K, Kato T, Nishioka K, Nakamura H. The role of subchondral bone resorption pits in osteoarthritis: MMP production by cells derived from bone marrow. Osteoarthr Cartil. 2005;13:679–87. doi: 10.1016/j.joca.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Sasaki K, Takagi M, Konttinen YT, Sasaki A, Tamaki Y, Ogino T, et al. Upregulation of matrix metalloproteinase (MMP)-1 and its activator MMP-3 of human osteoblast by uniaxial cyclic stimulation. J Biomed Mater Res, B Appl Biomater. 2007;80:491–8. doi: 10.1002/jbm.b.30622. [DOI] [PubMed] [Google Scholar]
- 37.Reboul P, Pelletier JP, Tardif G, Cloutier JM, Martel-Pelletier J. The new collagenase, collagenase-3, is expressed and synthesized by human chondrocytes but not by synoviocytes. A role in osteoarthritis. J Clin Invest. 1996;97:2011–9. doi: 10.1172/JCI118636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rifas L, Arackal S. T cells regulate the expression of matrix metalloproteinase in human osteoblasts via a dual mitogen-activated protein kinase mechanism. Arthritis Rheum. 2003;48:993–1001. doi: 10.1002/art.10872. [DOI] [PubMed] [Google Scholar]
- 39.Haeusler G, Walter I, Helmreich M, Egerbacher M. Localization of matrix metalloproteinases, (MMPs) their tissue inhibitors, and vascular endothelial growth factor (VEGF) in growth plates of children and adolescents indicates a role for MMPs in human postnatal growth and skeletal maturation. Calcif Tissue Int. 2005;76:326–35. doi: 10.1007/s00223-004-0161-6. [DOI] [PubMed] [Google Scholar]
- 40.Delaisse JM, Engsig MT, Everts V, del Carmen Ovejero M, Ferreras M, Lund L, et al. Proteinases in bone resorption: obvious and less obvious roles. Clin Chim Acta. 2000;291:223–34. doi: 10.1016/s0009-8981(99)00230-2. [DOI] [PubMed] [Google Scholar]
- 41.Engsig MT, Chen QJ, Vu TH, Pedersen AC, Therkidsen B, Lund LR, et al. Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. J Cell Biol. 2000;151:879–89. doi: 10.1083/jcb.151.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bigg HF, Rowan AD, Barker MD, Cawston TE. Activity of matrix metalloproteinase-9 against native collagen types I and III. FEBS J. 2007;274:1246–55. doi: 10.1111/j.1742-4658.2007.05669.x. [DOI] [PubMed] [Google Scholar]
- 43.Lorenzo JA, Pilbeam CC, Kalinowski JF, Hibbs MS. Production of both 92- and 72-kDa gelatinases by bone cells. Matrix. 1992;12:282–90. doi: 10.1016/s0934-8832(11)80080-6. [DOI] [PubMed] [Google Scholar]
- 44.Uchida M, Shima M, Shimoaka T, Fujieda A, Obara K, Suzuki H, et al. Regulation of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) by bone resorptive factors in osteoblastic cells. J Cell Physiol. 2000;185:207–14. doi: 10.1002/1097-4652(200011)185:2<207::AID-JCP5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 45.Panagakos FS, Kumar S. Modulation of proteases and their inhibitors in immortal human osteoblast-like cells by tumor necrosis factor-alpha in vitro. Inflammation. 1994;18:243–65. doi: 10.1007/BF01534267. [DOI] [PubMed] [Google Scholar]
- 46.Kwan Tat S, Padrines M, Theoleyre S, Heymann D, Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004;15:49–60. doi: 10.1016/j.cytogfr.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 47.Kearns AE, Khosla S, Kostenuik PJ. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev. 2008;29:155–92. doi: 10.1210/er.2007-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishimi Y, Miyaura C, Jin CH, Akatsu T, Abe E, Nakamura Y, et al. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol. 1990;145:3297–303. [PubMed] [Google Scholar]
- 49.Kudo O, Sabokbar A, Pocock A, Itonaga I, Fujikawa Y, Athanasou NA. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone. 2003;32:1–7. doi: 10.1016/s8756-3282(02)00915-8. [DOI] [PubMed] [Google Scholar]
- 50.Palmqvist P, Persson E, Conaway HH, Lerner UH. IL-6, leukemia inhibitory factor, and oncostatin M stimulate bone resorption and regulate the expression of receptor activator of NF-kappa B ligand, osteoprotegerin, and receptor activator of NF-kappa B in mouse calvariae. J Immunol. 2002;169:3353–62. doi: 10.4049/jimmunol.169.6.3353. [DOI] [PubMed] [Google Scholar]
- 51.Devlin RD, Reddy SV, Savino R, Ciliberto G, Roodman GD. IL-6 mediates the effects of IL-1 or TNF, but not PTHrP or 1,25(OH)2D3, on osteoclast-like cell formation in normal human bone marrow cultures. J Bone Miner Res. 1998;13:393–9. doi: 10.1359/jbmr.1998.13.3.393. [DOI] [PubMed] [Google Scholar]
- 52.Sakao K, Takahashi KA, Mazda O, Arai Y, Tonomura H, Inoue A, et al. Enhanced expression of interleukin-6, matrix metalloproteinase-13, and receptor activator of NF-kappaB ligand in cells derived from osteoarthritic subchondral bone. J Orthop Sci. 2008;13:202–10. doi: 10.1007/s00776-008-1227-5. [DOI] [PubMed] [Google Scholar]
- 53.Abe K, Aslam A, Walls AF, Sato T, Inoue H. Up-regulation of protease-activated receptor-2 by bFGF in cultured human synovial fibroblasts. Life Sci. 2006;79:898–904. doi: 10.1016/j.lfs.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 54.Ritchie E, Saka M, Mackenzie C, Drummond R, Wheeler-Jones C, Kanke T, et al. Cytokine upregulation of proteinase-activated-receptors 2 and 4 expression mediated by p38 MAP kinase and inhibitory kappa B kinase beta in human endothelial cells. Br J Pharmacol. 2007;150:1044–54. doi: 10.1038/sj.bjp.0707150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomson BM, Mundy GR, Chambers TJ. Tumor necrosis factors alpha and beta induce osteoblastic cells to stimulate osteoclastic bone resorption. J Immunol. 1987;138:775–9. [PubMed] [Google Scholar]
- 56.Konig A, Muhlbauer RC, Fleisch H. Tumor necrosis factor alpha and interleukin-1 stimulate bone resorption in vivo as measured by urinary. J Bone Miner Res. 1988;3:621–7. doi: 10.1002/jbmr.5650030607. [DOI] [PubMed] [Google Scholar]
- 57.Akatsu T, Takahashi N, Udagawa N, Imamura K, Yamaguchi A, Sato K, et al. Role of prostaglandins in interleukin-1-induced bone resorption in mice in vitro. J Bone Miner Res. 1991;6:183–9. doi: 10.1002/jbmr.5650060212. [DOI] [PubMed] [Google Scholar]
- 58.Amano S, Naganuma K, Kawata Y, Kawakami K, Kitano S, Hanazawa S. Prostaglandin E2 stimulates osteoclast formation via endogenous IL-1 beta expressed through protein kinase A. J Immunol. 1996;156:1931–6. [PubMed] [Google Scholar]
- 59.Li X, Okada Y, Pilbeam CC, Lorenzo JA, Kennedy CR, Breyer RM, et al. Knockout of the murine prostaglandin EP2 receptor impairs osteoclastogenesis in vitro. Endocrinology. 2000;141:2054–61. doi: 10.1210/endo.141.6.7518. [DOI] [PubMed] [Google Scholar]
- 60.Suzawa T, Miyaura C, Inada M, Maruyama T, Sugimoto Y, Ushikubi F, et al. The role of prostaglandin E receptor subtypes (EP1, EP2, EP3, and EP4) in bone resorption: an analysis using specific agonists for the respective EPs. Endocrinology. 2000;141:1554–9. doi: 10.1210/endo.141.4.7405. [DOI] [PubMed] [Google Scholar]
- 61.Tomita M, Li X, Okada Y, Woodiel FN, Young RN, Pilbeam CC, et al. Effects of selective prostaglandin EP4 receptor antagonist on osteoclast formation and bone resorption in vitro. Bone. 2002;30:159–63. doi: 10.1016/s8756-3282(01)00688-3. [DOI] [PubMed] [Google Scholar]
- 62.Ahmed I, Gesty-Palmer D, Drezner MK, Luttrell LM. Transactivation of the epidermal growth factor receptor mediates parathyroid hormone and prostaglandin F2 alpha-stimulated mitogen-activated protein kinase activation in cultured transgenic murine osteoblasts. Mol Endocrinol. 2003;17:1607–21. doi: 10.1210/me.2002-0040. [DOI] [PubMed] [Google Scholar]
- 63.Miyahara T, Katoh T, Watanabe M, Mikami Y, Uchida S, Hosoe M, et al. Involvement of mitogen-activated protein kinases and protein kinase C in cadmium-induced prostaglandin E2 production in primary mouse osteoblastic cells. Toxicology. 2004;200:159–67. doi: 10.1016/j.tox.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 64.Lee SJ, Kim MS, Park JY, Woo JS, Kim YK. 15-Deoxy-delta 12,14-prostaglandin J2 induces apoptosis via JNK-mediated mitochondrial pathway in osteoblastic cells. Toxicology. 2008;248:121–9. doi: 10.1016/j.tox.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 65.Vliagoftis H, Schwingshackl A, Milne CD, Duszyk M, Hollenberg MD, Wallace JL, et al. Proteinase-activated receptor-2-mediated matrix metalloproteinase-9 release from airway epithelial cells. J Allergy Clin Immunol. 2000;106:537–45. doi: 10.1067/mai.2000.109058. [DOI] [PubMed] [Google Scholar]
- 66.Page K, Hughes VS, Bennett GW, Wong HR. German cockroach proteases regulate matrix metalloproteinase-9 in human bronchial epithelial cells. Allergy. 2006;61:988–95. doi: 10.1111/j.1398-9995.2006.01103.x. [DOI] [PubMed] [Google Scholar]
- 67.Temkin V, Kantor B, Weg V, Hartman ML, Levi-Schaffer F. Tryptase activates the mitogen-activated protein kinase/activator protein-1 pathway in human peripheral blood eosinophils, causing cytokine production and release. J Immunol. 2002;169:2662–9. doi: 10.4049/jimmunol.169.5.2662. [DOI] [PubMed] [Google Scholar]
- 68.Hirota Y, Osuga Y, Hirata T, Harada M, Morimoto C, Yoshino O, et al. Activation of protease-activated receptor 2 stimulates proliferation and interleukin (IL)-6 and IL-8 secretion of endometriotic stromal cells. Hum Reprod. 2005;20:3547–53. doi: 10.1093/humrep/dei255. [DOI] [PubMed] [Google Scholar]
- 69.Tsubaki M, Kato C, Manno M, Ogaki M, Satou T, Itoh T, et al. Macrophage inflammatory protein-1alpha (MIP-1alpha) enhances a receptor activator of nuclear factor kappaB ligand (RANKL) expression in mouse bone marrow stromal cells and osteoblasts through MAPK and PI3K/Akt pathways. Mol Cell Biochem. 2007;304:53–60. doi: 10.1007/s11010-007-9485-7. [DOI] [PubMed] [Google Scholar]
- 70.Pelletier JP, Lajeunesse D, Jovanovic DV, Lascau-Coman V, Jolicoeur FC, Hilal G, et al. Carprofen simultaneously reduces progression of morphological changes in cartilage and subchondral bone in experimental dog osteoarthritis. J Rheumatol. 2000;27:2893–902. [PubMed] [Google Scholar]
- 71.Lajeunesse D, Martel-Pelletier J, Fernandes JC, Laufer S, Pelletier JP. Treatment with licofelone prevents abnormal subchondral bone cell metabolism in experimental dog osteoarthritis. Ann Rheum Dis. 2004;63:78–83. doi: 10.1136/ard.2002.003624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pelletier JP, Boileau C, Mineau F, Geng C, Boily M, Martel-Pelletier J. Upregulation of proteinase-activated receptor (PAR)-2 in human osteoarthritic tissues: a new pathway for the mediation of joint destruction. Osteoarthr Cartil. 2005;13:S19. abstract. [Google Scholar]
- 73.Nakano S, Mishiro T, Takahara S, Yokoi H, Hamada D, Yukata K, et al. Distinct expression of mast cell tryptase and protease activated receptor-2 in synovia of rheumatoid arthritis and osteoarthritis. Clin Rheumatol. 2007;26:1284–92. doi: 10.1007/s10067-006-0495-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.