Abstract
Objective
Bone tissue in osteoarthritis (OA) is composed of abundant undermineralized osteoid matrix. The aim of this study was to investigate the mechanisms responsible for this abnormal matrix, using in vitro OA subchondral osteoblasts.
Methods
Primary normal and OA osteoblasts were prepared from tibial plateaus. Phenotype was determined by alkaline phosphatase activity, and osteocalcin, osteopontin, prostaglandin E2 (PGE2), and transforming growth factor β1 (TGFβ1) were assessed by enzyme-linked immunosorbent assay. Expression of COL1A1 and COL1A2 was determined by real-time polymerase chain reaction. The production of type I collagen was determined by the release of its C-terminal propeptide and Western blot analysis. In vitro mineralization was evaluated by alizarin red staining. Inhibition of TGFβ1 expression was performed using a small interfering RNA technique.
Results
Mineralization of OA osteoblasts was reduced compared with mineralization of normal osteoblasts, even in the presence of bone morphogenetic protein 2 (BMP-2). Alkaline phosphatase and osteocalcin levels were elevated in OA osteoblasts compared with normal osteoblasts, whereas osteopontin levels were similar. The COL1A1-to-COL1A2 messenger RNA ratio was 3-fold higher in OA osteoblasts compared with normal osteoblasts, and the production of collagen by OA osteoblasts was increased. Because TGFβ1 inhibits BMP-2–dependent mineralization, and because TGFβ1 levels are ~4-fold higher in OA osteoblasts than in normal osteoblasts, inhibiting TGFβ1 levels in OA osteoblasts corrected the abnormal COL1A1-to-COL1A2 ratio and increased alizarin red staining.
Conclusion
Elevated TGFβ1 levels in OA osteoblasts are responsible, in part, for the abnormal ratio of COL1A1 to COL1A2 and for the abnormal production of mature type I collagen. This abnormal COL1A1-to-COL1A2 ratio generates a matrix that blunts mineralization in OA osteoblasts.
Osteoarthritis (OA) is a leading cause of morbidity in the aging population and is characterized by cartilage degradation and loss, inflammation of the synovium, formation of osteophytes, and bone sclerosis. The etiology of this disease remains elusive. The joint is now viewed as an organ, and OA is considered to be a disease of this organ. Recent data indicate a key role of subchondral bone tissue in the onset and/or progression of OA (1–3). Thus, understanding the mechanisms leading to bone sclerosis could be of utmost importance in the treatment of OA, because bone tissue sclerosis in OA increases stress to the overlying cartilage (4). Bone sclerosis was believed to explain elevated bone mineral density (BMD) in patients with OA; however, increased BMD does not appear to reflect elevated material tissue density (5,6) and does not reflect mechanical properties of OA bone tissue (7,8). Moreover, microfocal computed tomography analysis of human OA bone tissue indicated abnormal structure and organization of this tissue (8).
A key role of alteration of the subchondral bone tissue architecture in the progressive destruction of articular cartilage (as in OA) was recently described in the Brittle IV (Brtl) mouse model of osteogenesis imperfect a via a specific type I collagen knock in (9). Hence, the observation that bone sclerosis in OA subchondral bone tissue may be attributable to abnormal collagen deposition in vivo is likely correct (2,10). Indeed, because type I collagen levels are elevated in the trabecular bone of the femoral heads of patients with OA, this should lead to an increase in mineralization (11); however, this tissue is hypomineralized (2,5,12). Type I collagen is composed of a heterotrimer of α1 and α2 chains at an average ratio of 2.4:1 in normal subchondral bone, yet this ratio varied from 4:1 to 17:1 in in vivo OA bone tissue (10). Coupled to the reduction in crosslinks observed in OA bone tissue (2) and the overhydroxylation of lysine in collagen fibrils (10), this could explain a reduction in bone mineralization. However, whether the alterations of collagen production observed in in vivo OA subchondral bone are attributable to abnormal cell metabolism or systemic regulation remains unresolved.
Our group (1,13–16) and other investigators (17,18) previously showed that osteoblasts from OA patients are abnormal and show altered phenotypic characteristics. Moreover, OA osteoblasts may produce factor(s) that can promote glycosaminoglycan release from normal cartilage in vitro (3,17) or down-regulate aggrecan from chondrocytes (18). Bone tissue from patients with OA also produce collagen and collagenase(s), albeit at very variable levels (2). The factors produced by OA osteoblasts that affect either collagen turnover and/or promote glycosaminoglycan release from normal cartilage remain elusive. Our group (16,19,20) and other investigators (3,18) have shown that cytokine and growth factor synthesis by OA osteoblasts is similar to that by normal osteoblasts in most cases. However, we recently reported that interleukin-6 (IL-6) and prostaglandin E2 (PGE2) production by OA osteoblasts can discriminate 2 subgroups of patients, low OA and high OA (16), whereas we could not distinguish these patients in terms of disease activity, duration, and/or medication use. Osteoblasts from the same patients showed elevated levels of transforming growth factor β1 (TGFβ1) (16), and the expression of TGFβ1 is increased in OA bone tissue compared with normal tissue (21). We also showed that OA osteoblasts produced variable levels of leukotriene B4 (22), which could also differentiate the 2 subgroups of patients.
In the present study, we observed reduced in vitro mineralization of OA osteoblasts compared with normal osteoblasts. This was not corrected in the presence of bone morphogenetic protein 2 (BMP-2) regardless of the endogenous PGE2 levels in OA osteoblasts and their expression of the α1 chain of type I collagen, which was significantly increased. Correcting the elevated production of endogenous TGFβ1 observed in these cells corrected, in part, both abnormal mineralization and the production of type I collagen.
PATIENTS AND METHODS
Patients and clinical parameters
Tibial plateaus were obtained from patients with OA who were undergoing total knee replacement surgery and the tissue samples were prepared as previously described (1,14–16). The study group comprised 84 patients (34 men and 50 women; mean ± SD age 70.3 ± 8.5 years), all of whom had OA according to the recognized clinical criteria of the American College of Rheumatology (23). No patients had received medication that would interfere with bone metabolism, including corticosteroids, for 6 months before surgery. A total of 16 subchondral bone specimens of tibial plateaus from normal individuals (10 men and 6 women; mean ± SD age 63.8 ± 16.9 years) were collected at autopsy, within 12 hours of death. These individuals had not been receiving any medication that could interfere with bone metabolism, they did not have any bone metabolic disease, and no abnormal macroscopic cartilage changes were observed. All human materials were acquired following signed consent from patients undergoing knee surgery (or by their relatives for the autopsy specimens), following the Centre Hospitalier de l’Université de Montréal ethics committee guidelines.
Preparation of primary subchondral bone cell culture
Isolation of the subchondral bone plate and the cell cultures were performed as previously described (1,15,24,25). Briefly, the overlaying cartilage was first removed from tibial plateaus, and the trabecular bone tissue was dissected away from the subchondral bone plate. The subchondral bone plates of the mediotibial plateaus were dissected out, as previously described (9). All manipulations were performed under a magnifying microscope to ensure complete removal of cartilage and trabecular bone.
Subchondral bone specimens were cut into small pieces that were washed 3 times in serum-free medium to remove any bone marrow. These bone pieces were then used for sequential digestion in the presence of 1 mg/ml type I collagenase (Sigma, St. Louis, MO) in BGJb medium (Sigma) without serum at 37°C for 20, 20, and 240 minutes. The digested bone pieces were again washed 3 times in serum-free medium and then cultured in the same medium containing 20% fetal bovine serum (FBS; Wisent, St. Bruno, Quebec, Canada). This medium was replaced every 2 days until cells were observed in the petri dishes. At this point, the culture medium was replaced with fresh medium containing 10% FBS.
At confluence, which typically took 4–6 weeks, cells were passaged only once at 25,000 cells/cm2 and grown for 5 days in Ham’s F-12/Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich, Oakville, Ontario, Canada) containing 10% FBS. Confluent cells were then incubated in the presence or absence of 1,25-dihydroxyvitamin D3 (1,25[OH]2D3) (50 nM) for 48 hours for the determination of biomarkers or in presence of 0.5% bovine serum albumin (BSA) for the determination of prostaglandins, cytokines, and collagen. Supernatants were collected at the end of the incubation period and kept at −80°C prior to performance of the assays.
Cells were either prepared for sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) separation or reverse transcription–polymerase chain reaction (RT-PCR) experiments. Cells prepared for SDS-PAGE separation were lysed with radioimmunoprecipitation assay buffer as previously described (20) and kept at −80°C prior to performance of the assay. Protein determination was performed using the bicinchoninic acid method (26).
Phenotypic characterization of human subchondral osteoblast cell cultures
The phenotypic features of osteoblasts were determined by evaluating 1,25(OH)2D3-dependent alkaline phosphatase activity and osteocalcin and osteopontin release. Alkaline phosphatase activity in cell aliquots was determined by substrate hydrolysis using p-nitrophenyl phosphate. Osteocalcin release was determined in cell supernatants using an enzyme immunoassay, as previously described (1,16). Osteopontin levels were determined using a selective enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN). The specificity of the ELISA for osteopontin is 100%, and the mean sensitivity is 0.011 ng/ml. Collagen synthesis was determined as the de novo release of the carboxy-terminal peptide fragment of type I collagen in conditioned medium from confluent normal and OA osteoblasts. The carboxy-terminal peptide fragment was determined using a selective ELISA (Cedarlane, Hornby, Ontario, Canada).
Identification of potential mesenchymal stem cells (MSCs) or bone osteal macrophages in our osteoblast preparations was performed using complementary approaches. First, cartilage and subchondral bone specimens from tibial plateaus were processed for immunohistochemical analysis, fixed in TissuFix #2 (Chaptec, Montreal, Quebec, Canada) for 24 hours, decalcified with EDTA, and embedded in paraffin. Serial sections (5 μm) of paraffin-embedded specimens were placed on Superfrost Plus slides (Fisher Scientific, Nepean, Ontario, Canada), deparaffinized in toluene, rehydrated in a reverse graded series of ethanol, and heated in citrate buffer (10 mM, pH 6.0) at 68°C for 20 minutes. The specimens were subsequently washed in phosphate buffered saline (PBS), incubated in 0.3% Triton X-100 for 20 minutes, and placed in 3% hydrogen peroxide/PBS for 15 minutes. Slides were further incubated with a blocking serum (Vectastain ABC Kit; Vector, Burlingame, CA) for 60 minutes, after which they were blotted and overlaid with the primary antibody against STRO-1 (1:50, mouse monoclonal; R&D Systems) for the identification of MSCs or the primary antibody against CD68 (1:50, mouse monoclonal; DakoCytomation, Glostrup, Denmark) for the detection of macrophages, for 18 hours at 4°C in a humidified chamber. Each slide was washed 3 times in PBS (pH 7.4) and stained using the avidin–biotin complex method (Vectastain ABC Kit), which entails incubation in the presence of the biotin-conjugated secondary antibody for 45 minutes at room temperature followed by the addition of avidin–biotin–peroxidase complex for 45 minutes. All incubations were carried out in a humidified chamber at room temperature, and the color was developed with 3,3′-diaminobenzidine (Dako, Mississauga, Ontario, Canada) containing hydrogen peroxide. The slides were counterstained with hematoxylin and eosin.
Second, we performed a flow cytometry analysis of the cell surface antigen STRO-1, using a protocol described by Neumann et al (27). Briefly, cells were incubated for 15 minutes with the primary STRO-1 antibody as described above, washed with PBS/1% BSA, and stained with a fluophore-labeled donkey anti-mouse secondary antibody (Invitrogen, Burlington, Ontario, Canada). Staining of cell-surface antigens was analyzed using the FACSCanto II system equipped with FACSDiva version 6 software (Becton Dickinson, Palo Alto, CA).
Last, we performed a series of PCR assays in normal and OA osteoblasts to detect the expression of CD73 (SH3, 5′-nucleotidase) and CD105 (endoglin, TGFβ receptor), 2 markers of MSCs and osteoprogenitor cells, using selective primer sets (Table 1). We also assessed whether our preparations of normal and OA osteoblasts contained macrophages, using PCR assays to detect the expression of 2 specific cell-surface receptors of macrophages, EMR1 and CSF-1R, using selective primer sets (Table 1).
Table 1.
Primers and amplicon size
| Gene | Accession no. | Primers, 5′ to 3′ | Amplicon size, bp |
|---|---|---|---|
| GAPDH | BC026907.1 | Forward: CAGAACATCATCCCTGCCTCT Reverse: GCTTGACAAAGTGGTCGTTGAG |
318 |
| COL1A1 | NM_000088.2 | Forward: AGAGGTTTCAGTGGTTTGGA Reverse: CCAGGAGCACCATTGGCACC |
409 |
| COL1A2 | NM_000089.3 | Forward: GGACACAATGGATTGCAAGG Reverse: TAACCACTGCTCCACTCTGG |
461 |
| TGFβ1 | NM_000660 | Forward: GCGTGCTAATGGTGGAAAC Reverse: GCTGAGGTATCGCCAGGAA |
221 |
| EMR1 | NM_001974 | Forward: CTGACCTGGACCTTGTGGAT Reverse: TGAGCAGACAGTGGATGAGG |
238 |
| CSF1R | NM_005211 | Forward: TCCCAGTGATAGAGCCCAGT Reverse: GGAAGGTAGCGTTGTTGGTG |
171 |
| CD105 | NM_001114753 | Forward: GCCAGCATTGTCTCACTTCA Reverse: CTTGTCACCCCTGTCCTCTG |
249 |
| CD73 | NM_002526 | Forward: CCGAAAACCTGGAGACAGAG Reverse: CGACCTTCAACTGCTGGATA |
249 |
Preparation of SaOS-2 cells
SaOS-2 cells (American Type Culture Collection, Rockville, MD) were grown in DMEM containing 10% FBS and passaged once weekly at a ratio of 1:6. At confluence, cells were fed with BGJb medium (Sigma-Aldrich) containing 10% FBS, 50 μg/ml β-glycerophosphate, and 50 μg/ml ascorbic acid, to induce mineralization. At specific time points after reaching confluence, cells were used to assess for COL1A1 and COL1A2 expression or were prepared for alizarin red staining.
RT-PCR assays
For RT-PCR assays, total cellular RNA was extracted with TRIzol reagent (Invitrogen) according to the manufacturer’s specifications and treated with the DNA-free DNase Treatment & Removal kit (Ambion, Austin, TX) to ensure complete removal of chromosomal DNA. The RNA was quantitated using the RiboGreen RNA Quantification kit (Molecular Probes, Eugene, OR). The RT reactions were primed with random hexamers with 1 μg of total RNA in a 100-μl final reaction volume, followed by PCR amplification as previously described (14), using 20 pmoles of each specific PCR primer. Oligonucleotide primers used in the PCR amplification are shown in Table 1. Amplification of all messenger RNA (mRNA) species was performed separately from GAPDH mRNA amplification to avoid substrate depletion. Real-time quantification (quantitative RT-PCR) of COL1A1, COL1A2, and GAPDH mRNA was performed in the Rotor-Gene RG-3000A thermal cycler (Corbett Research, Mortlake, New South Wales, Australia) with 2× QuantiTect SYBR Green PCR Master Mix (Qiagen, Mississauga, Ontario, Canada) according to the manufacturer’s specifications.
Briefly, 100 ng of the complementary DNA obtained from the RT reactions were amplified in a total volume of 25 μl consisting of 1× Master Mix, 0.5 unit uracil-N-glycosylase (UNG; Epicentre Technologies, Madison, WI), and the gene-specific primers (Table 1) were added at a final concentration of 200 nM. The tubes were first incubated for 2 minutes at 50°C (UNG reaction), then at 95°C for 15 minutes (UNG inactivation and polymerase activation), followed by 40 cycles consisting of denaturation (94°C for 15 seconds), annealing (60°C for 30 seconds), extension (72°C for 30 seconds), and data acquisition (77°C for 15 seconds) steps. The data were given as Ct values. The standard curves were generated with the same plasmids as the target sequences, and Ct values were converted to the number of molecules. Data were calculated as the ratio of the number of molecules of the target gene:number of molecules of GAPDH. The primer efficiencies for the test genes were the same as those observed for the GAPDH gene.
Western immunoblotting
The cell extracts were loaded on polyacrylamide gels and separated by SDS-PAGE under reducing condition (28). Loading of the protein was adjusted according to the cellular protein concentration. The proteins were electrophoretically transferred onto polyvinylidene difluoride membranes (Boehringer Mannheim, Penzberg, Germany), and immunoblotting was performed as described in the manual for the ECL Plus Western blotting detection system (Amersham Pharmacia Biotech, Baie d’Urfé, Quebec, Canada), using rabbit anti-human type I collagen antibodies at a dilution of 1:7,500 (Cedarlane) and rabbit anti-human actin at a dilution of 1:10,000 (Sigma-Aldrich) as primary antibodies and goat anti-rabbit IgG as secondary antibodies at a dilution of 1:20,000 (Upstate Biotechnology, Lake Placid, NY). Densitometry analysis of Western blot films was performed using the public domain NIH Image program developed with the Scion Image 1.63 program (29).
Evaluation of mineralization
Confluent cells were incubated in BGJb medium containing 10% FBS, 50 μg/ml ascorbic acid, and 50 μg/ml β-glycerophosphate. This medium was changed every 2 days until day 30 or as individual conditions indicated. Normal and OA osteoblasts were treated (or were not treated) with 10 ng/ml BMP-2 (R&D Systems) beginning on day 2 until day 30. Mineralization of cell cultures was evaluated by alizarin red staining (30) and von Kossa’s staining (31). Quantification of alizarin red staining was also performed, following the extraction procedure described by Gregory et al (30).
Evaluation of PGE2 and TGFβ1
PGE2 and TGFβ1 levels were determined in conditioned medium of normal and OA osteoblasts containing 0.5% BSA. Total TGFβ1 levels were determined using highly specific Quantikine ELISAs (R&D Systems). The sensitivity of the assay was 7 pg/ml. PGE2 was assessed using a highly specific ELISA from Cayman Chemical (Ann Arbor, MI), and the sensitivity was 15 pg/ml. Determinations were performed in triplicate for each cell culture preparation.
Inhibition of TGFβ1 in OA osteoblasts by small interfering RNA (siRNA)
We used an siRNA technique to transiently inhibit TGFβ1 expression in OA osteoblasts. Small interfering RNAs (4 different siRNA constructs are provided by the manufacturer in the same sample) were obtained from Dharmacon (Lafayette, CO), and preparation was performed according to the manufacturer’s recommendations. Briefly, OA osteoblasts were split at 100,000 cells/ml. TGFβ1 siRNA (a mixture of 4 constructs) or scramble RNA (basal condition) was added to OA osteoblasts at a final concentration of 100 ng/ml, with 6 μl HiPerFect (Qiagen) per 100 μl total volume in BGJb medium without serum for 1 hour on day 0 and day 3. Cells were then fed BGJb medium with 10% FBS containing 50 μg/ml ascorbic acid and 2 mM β-glycerophosphate, in the presence or absence of 10 ng/ml BMP-2, every other day for 28 days, to perform both alizarin red staining or quantitative RT-PCR for TGFβ1, COL1A1, COL1A2, and GAPDH, as described above.
Transfection experiments
Transient transfection of human OA osteoblasts was carried out using the Nucleofector system (Amaxa, Gaithersburg, MD) and a protocol modified for transfecting osteoblasts. Briefly, cells were trypsinized, and 1 × 106 cells per reaction were centrifuged at 1,000 rpm for 10 minutes. After resuspension in the provided transfection solution, 1 μg of DNA was subjected to electroporation, using the provided cuvettes and the D-24 program. Cells were recovered in pre-warmed low-calcium culture medium (without serum) and left to recover at 37°C for 15 minutes. Cells were then replated in 35-mm dishes with BGJb medium containing 10% FBS. Optimal conditions were first determined using a construct containing green fluorescent protein (basal condition) such that 60–80% transfection efficiency was obtained for at least 72 hours (data not shown). Using the optimized conditions for transfection, 2 TGFβ1 short hairpin RNA (shRNA) constructs (Origen, Rockville, MD) were tested for their effect on mineralization, as described above, after 28 days of continuous treatment with BMP-2. In parallel experiments, cells treated with the empty vector or shTGFβ1 were used at specific time points as indicated and extracted with TRIzol reagent to prepare for quantitative RT-PCR, as described above.
Statistical analysis
All quantitative data are expressed as the mean ± SEM. The data were analyzed by Student’s t-test. P values less than 0.05 were considered significant.
RESULTS
Phenotypic characterization of osteoblasts
In normal osteoblasts, the mean ± SEM levels of alkaline phosphatase and osteocalcin were 543.3 ± 105.7 nmoles/mg protein/30 minutes and 129.6 ± 20.2 ng/mg protein, respectively. These levels were increased in OA osteoblasts, as previously described (1,14,15), and reached values of 1,704.2 ± 135.6 nmoles/mg protein/30 minutes (P < 0.0001 versus normal) and 288.5 ± 29.0 nmoles/mg protein (P < 0.0001 versus normal) for alkaline phosphatase and osteocalcin, respectively. Osteopontin levels were slightly higher in OA osteoblasts compared with normal osteoblasts, reaching mean ± SEM levels of 449.7 ± 149.9 and 328.9 ± 122.1 ng/mg protein, respectively (P not significant [NS]). PGE2 levels were 853.5 ± 106.4 pmoles/mg protein in normal osteoblasts (n = 16) and reached 641.4 ± 53.5 in the subgroup of OA osteoblasts producing low levels of PGE2 (n = 54; P NS versus normal) and 6,364.6 ± 796.1 in the subgroup producing high levels of PGE2 (n = 30; P < 0.0001 versus normal).
No differences were noted for the values for alkaline phosphatase, osteocalcin, or osteopontin between the 2 OA osteoblast subgroups, as previously reported (16). Subchondral osteoblast preparations were a homogeneous population and were positive for CD73 and CD105. Both CD73 and CD105 levels were not significantly different between normal osteoblasts and the 2 OA osteoblast subgroups (additional information is available from the corresponding author). Immunohistochemical detection of the stromal cell marker STRO-1, an early indicator of MSCs, was performed and showed a similar scattered distribution in all tissues of the joints (additional information is available from the corresponding author); no significant differences were noted between normal and OA specimens. Moreover, STRO-1 levels, as determined by flow cytometry, were low in OA osteoblast preparations (n = 4; mean ± SEM 9.98 ± 2.78%) (additional information is available from the corresponding author).
In vitro mineralization potential
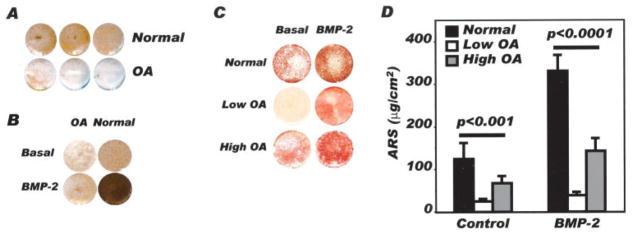
To determine whether altered bone mineralization in OA bone tissue may be attributable to a cellular defect, systemic regulation, or both, we incubated confluent normal and OA osteoblasts for 28 days in a culture medium that promotes mineralization, with and without BMP-2, and determined their mineralization potential by alizarin red staining and von Kossa’s staining. First, von Kossa’s staining was lower in OA osteoblasts under basal conditions (Figure 1A). This was not corrected by the addition of BMP-2, contrary to what was observed in normal osteoblasts (Figure 1B), indicating that basal and BMP-2–stimulated mineralization of OA osteoblasts were reduced in vitro and in vivo (2). However, not all OA osteoblasts showed similar levels. Both subgroups of OA osteoblasts (low and high, as identified by their endogenous PGE2 levels) showed reduced alizarin red staining compared with normal osteoblasts (Figure 1C), yet, again, the response of OA osteoblasts to BMP-2 was blunted compared with that of normal osteoblasts. Indeed, quantification of alizarin red staining showed that mineralization was 2-fold to 3-fold lower in OA osteoblasts compared with normal osteoblasts, in the presence or absence of BMP-2 (Figure 1D), regardless of the subgroup of OA osteoblasts.
Figure 1.
Evaluation of in vitro mineralization of normal and osteoarthritis (OA) osteoblasts. Confluent normal and OA osteoblasts were incubated in BGJb medium containing 10% fetal bovine serum, 50 μg/ml ascorbic acid, and 50 μg/ml β-glycerophosphate for 30 days, in the presence or absence of 10 ng/ml bone morphogenetic protein 2 (BMP-2). Mineralization of cell cultures was evaluated by either von Kossa’s staining or alizarin red staining (ARS). A, Representative von Kossa’s staining in normal and OA osteoblast cultures (n = 3 separate individuals per group). B, Representative von Kossa’s staining in 1 normal and 1 OA osteoblast following treatment with or without 10 ng/ml BMP-2, from day 2 until day 30 (results are representative of 4 separate experiments). C, Representative alizarin red staining following treatment of normal osteoblasts, OA osteoblasts producing low levels of prostaglandin E2 (PGE2), and OA osteoblasts producing high levels of PGE2, with or without 10 ng/ml BMP-2. D, Quantification of alizarin red staining according to the method described by Gregory et al (30). Values are the mean and SEM results from 6 normal, 21 low OA, and 14 high OA preparations.
Because Chang et al (32) recently suggested that osteal tissue macrophages, intercalated throughout human bone lining tissues, could regulate osteoblast functions in vitro, we assessed the presence of macrophages in subchondral bone tissues and in in vitro osteoblasts. A few macrophages could be detected in subchondral bone tissue, using immunohistochemical detection of CD68, but were mostly located at the base of the subchondral bone plate and within the trabecular bone space (additional information is available from the corresponding author). No significant differences were observed between normal and OA specimens. Moreover, using real-time PCR assays, we failed to detect significant differences in the levels of either CSF-1R or EMR1, 2 markers of macrophages, between normal and OA osteoblasts (additional information is available from the corresponding author).
Type I collagen expression
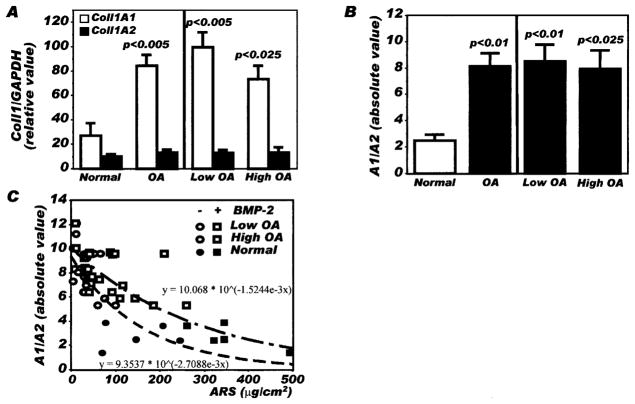
Blunted mineralization could not be attributed to reduced production of type I collagen in OA osteoblasts. As shown in Figure 2A, a 3.4-fold higher expression of COL1A1 mRNA was observed in OA osteoblasts compared with normal osteoblasts, using quantitative RT-PCR (P < 0.005), whereas the expression of COL1A2 mRNA chains was similar between OA and normal osteoblasts. This increase in COL1A1 expression in OA osteoblasts without any significant changes in COL1A2 expression led to a significant increase in the ratio of COL1A1 to COL1A2 (8.17 ± 0.95 in OA osteoblasts compared with 2.49 ± 0.46 in normal osteoblasts; P < 0.006) (Figure 2B).
Figure 2.
Expression of type I collagen α1 and α2 chains in normal and osteoarthritis (OA) osteoblasts by real-time polymerase chain reaction (PCR). Confluent osteoblasts were lysed in TRIzol, and RNA was extracted as described in Patients and Methods. RNA was reverse transcribed followed by PCR amplification of cDNA using specific primers. Plasmid DNAs containing the target gene sequences were used to generate the standard curves for COL1A1, COL1A2, and GAPDH. The value for each sample was calculated as the ratio of the number of molecules of the target gene:number of molecules of GAPDH. A, Expression of COL1A1 and COL1A2 under basal conditions. B, Ratio of COL1A1 to COL1A2 (A1/A2) under basal conditions in normal osteoblasts, total OA osteoblast preparations, and the subgroups of OA osteoblasts producing low levels of prostaglandin E2 (PGE2) and those producing high levels of PGE2. C, Relationship between alizarin red staining (ARS) and the COL1A1-to-COL1A2 ratio in normal osteoblasts, OA osteoblasts producing low levels of PGE2, and OA osteoblasts producing high levels of PGE2, treated or not treated with bone morphogenetic protein 2 (BMP-2). Values are the mean and SEM results from 8 normal osteoblasts, 14 total OA osteoblasts, 6 OA osteoblasts producing low levels of PGE2, and 8 OA osteoblasts producing high levels of PGE2.
When we separated the OA osteoblast subgroups according to low or high endogenous production of PGE2, no significant differences were noted in mRNA levels (Figure 2A) or for the ratio of COL1A1 to COL1A2 between osteoblasts producing high levels of PGE2 (8.50 ± 1.30) and those producing high levels of PGE2 (7.92 ± 1.42) (Figure 2B). When we plotted the COL1A1-to-COL1A2 ratio of osteoblasts as a function of the quantification of alizarin red staining, we observed that the results for OA osteoblasts formed a cluster of high COL1A1-to-COL1A2 ratios with low alizarin red staining values both under basal conditions and after BMP-2 stimulation, whereas normal osteoblasts showed lower COL1A1-to-COL1A2 ratios and elevated alizarin red staining values (Figure 2C). Normal and OA osteoblasts did not produce type II collagen, as assessed by quantitative RT-PCR (results not shown), as reported previously (16).
Type I collagen production
The de novo synthesis of type I collagen was increased 42 ± 7% (mean ± SD) in OA osteoblasts compared with normal osteoblasts (P < 0.001) (Figure 3A), in agreement with the abnormal COL1A1 expression. Moreover, Western blot analysis also indicated that the expression of α1 chains was increased in OA osteoblasts compared with normal osteoblasts (Figure 3B). Using the NIH Image program with the Scion Image 1.63 program, we determined that the mean ± SD ratio of α1 to α2 chains under these conditions was 1.52 ± 0.26 (n = 6) for normal and 2.92 ± 0.35 (n = 8) for OA osteoblasts (P < 0.01). Moreover, no differences in the ratio were observed between the subgroups of OA osteoblasts producing low levels of PGE2 (3.02 ± 0.50; n = 5) and those producing high levels of PGE2 (2.76 ± 0.53; n = 3). Normal and OA osteoblasts also produced very low levels of type III collagen (data not shown).
Figure 3.
Type I collagen production by normal and OA osteoblasts. Confluent osteoblasts were incubated for the last 48 hours of culture in Ham’s F-12/Dulbecco’s modified Eagle’s medium containing 0.5% bovine serum albumin. A, Culture medium was collected for the determination of collagen synthesis as the de novo release of the carboxy-terminal peptide fragment (CICP) of type I collagen, which reflects true collagen synthesis. The release of CICP was determined using a very selective enzyme-linked immunosorbent assay. Values are the mean and SD results from 9 normal and 22 OA osteoblast cultures (n = 14 OA osteoblasts producing low levels of PGE2 and 8 OA osteoblasts producing high levels of PGE2). B, Western blotting for type I collagen production by osteoblasts in 2 normal and 5 OA osteoblasts was performed. Cells lysed in radioimmunoprecipitation assay buffer and 25 μg of total protein were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Western blotting was performed with a polyclonal antibody that detects type I collagen α1 and α2 chains. Western blot analysis of actin was performed to demonstrate equivalent loading between samples. The results shown are representative of 7 normal and 10 OA osteoblast preparations (6 with low production of PGE2 and 4 with high production of PGE2). See Figure 2 for other definitions.
Mineralization of SaOS-2 cells and collagen expression
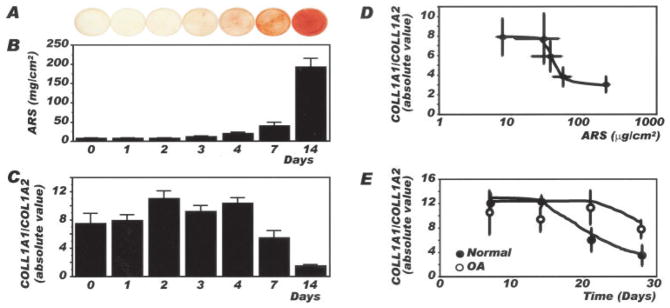
A relationship between the COL1A1-to-COL1A2 ratio and alizarin red staining was also studied using the human osteosarcoma cell model SaOS-2. As-shown in Figures 4A and B, alizarin red staining increased as a function of the length of time in culture, from day 0 to day 14 postconfluence. This increase in mineralization was accompanied by a reduction in COL1A1 expression without any net changes in COL1A2 expression, which led to a decrease in the COL1A1-to-COL1A2 ratio (Figure 4C) concomitant with the increase in alizarin red staining. Hence, when the COL1A1-to-COL1A2 ratio was reported as a function of the number of days in culture (results not shown) or alizarin red staining, a progressive and biphasic decrease in the COL1A1-to-COL1A2 ratio was observed with increasing alizarin red staining in SaOS-2 cells (Figure 4D). We then evaluated the evolution of the COL1A1-to-COL1A2 ratio in normal and OA osteoblasts. As illustrated in Figure 4E, the ratio of COL1A1 to COL1A2 was similar and high in normal and OA osteoblasts on days 7 and 14 postconfluence. This ratio was progressively reduced in normal osteoblasts, beginning after day 14 postconfluence until day 28, showing a biphasic effect as a function of time. In contrast, it decreased at a slower rate in OA osteoblasts compared with normal osteoblasts after 14 days and remained more elevated than normal until day 28 postconfluence.
Figure 4.
Relationship between mineralization and the COL1A1-to-COL1A2 ratio in SaOS-2 cells and normal and osteoarthritis (OA) osteoblasts. Confluent SaOS-2 cells were incubated for 0, 1, 2, 3, 4, 7, or 14 days in BGJb medium containing 10% fetal bovine serum, 50 μg/ml ascorbic acid, and 50 μg/ml β-glycerophosphate. Confluent normal and OA osteoblasts were incubated in the same medium in the presence of 10 ng/ml bone morphogenetic protein 2 from day 2 until day 7, day 14, day 21 or day 28 postconfluence, when cells were lysed in TRIzol for the determination of COL1A1 and COL1A2 expression. COL1A1 and COL1A2 expression was determined by real-time polymerase chain reaction, as described in Figure 2. A, Alizarin red staining (ARS) as a function of time in culture in SaOS-2 cells in the presence of mineralization medium. B, Quantification of alizarin red staining. C, Ratio of COL1A1 to COL1A2 expression. Values in B and C are the mean and SEM results from 3–7 different cell preparations per day. D, Ratio of COL1A1 to COL1A2 expression in SaOS-2 cells as a function of alizarin red staining. E, Ratio of COL1A1 to COL1A2 expression in normal and OA osteoblasts as a function of time in culture. Values in D and E are the mean ± SEM results from 3–8 normal osteoblast preparations and 8 OA osteoblast preparations.
Potential role of TGFβ1 in abnormal mineralization of osteoblasts
Previous studies suggested that TGFβ could prevent BMP-2–dependent mineralization in vitro in several cell types (33). Because our group previously demonstrated that OA osteoblasts have elevated TGFβ1 levels (16), we tested whether the presence of this growth factor would interfere with the action of BMP-2. As shown in Figure 5A, TGFβ1 inhibited basal and BMP-2–stimulated mineralization in normal osteoblasts. BMP-2 stimulated mineralization of normal osteoblasts (mean ± SEM 374.2 ± 101.2% versus basal; P < 0.01), while TGFβ1 fully prevented the stimulating action of BMP-2 (57.4 ± 21.9% of basal value; P < 0.01 versus BMP-2 alone). Figure 5B shows the values of TGFβ1 measured in conditioned medium of normal osteoblasts, OA osteoblasts producing low levels of PGE2, and OA osteoblasts producing high levels of PGE2. Both subgroups of OA osteoblasts produced significantly elevated TGFβ1 levels compared with normal osteoblasts (3–5-fold higher), yet these levels were not significantly different between the 2 OA osteoblast subgroups. We questioned whether reducing TGFβ1 levels might correct, at least in part, the abnormal mineralization of OA osteoblasts. Using shRNA inhibition techniques, we demonstrated that TGFβ1 expression could be reduced in OA osteoblasts. Indeed, TGFβ1 inhibition reached ~90% after 3 days of treatment, and this inhibition was maintained between 50% and 65% in OA osteoblasts until day 28 postconfluence, using 2 different plasmids (Figure 5C). This reduction in TGFβ1 mRNA levels was accompanied by a reduction in COL1A1 expression, leading to a significant reduction (approximately half) of the COL1A1-to-COL1A2 ratio (Figure 5D). Reducing TGFβ1 levels increased alizarin red staining of these cells, which increased ~40% under BMP-2 stimulation (Figure 5E).
Figure 5.
Effect of transforming growth factor β1 (TGFβ1) on bone morphogenetic protein 2 (BMP-2)–induced alizarin red staining (ARS). A, Representative alizarin red staining for confluent normal osteoblasts (n = 5 separate experiments) incubated as described in Figure 1. B, TGFβ1 levels in normal and osteoarthritis (OA) osteoblasts as measured by selective enzyme-linked immunosorbent assay. Values are the mean and SEM results from 8 normal osteoblast preparations, 14 preparations of osteoblasts producing low levels of prostaglandin E2 (PGE2), and 14 preparations of OA osteoblasts producing high levels of PGE2. C, TGFβ1 mRNA levels, as determined by quantitative polymerase chain reaction, under basal conditions and following inhibition of TGFβ1 expression with short hairpin RNA (shRNA) plasmids. Values are the mean and SEM results from 7 OA osteoblast preparations. D, COL1A1- to -COL1A2 ratio of OA osteoblasts under basal conditions or following inhibition of TGFβ1 expression with shRNA. Values are the mean and SEM results from 7 OA osteoblast preparations. E, Top, Representative alizarin red staining of OA osteoblasts treated or not treated with TGFβ1 shRNA. Bottom, Quantification of alizarin red staining following BMP-2 treatment in OA osteoblasts treated or not treated with TGFβ1 shRNA. Values are the mean and SEM results from 6 preparations.
DISCUSSION
The mechanisms responsible for the involvement of subchondral bone tissue in the progression and/or initiation of OA remain elusive. It is now clear, from both animal models and human studies, that bone is altered in OA, even at sites not involved in mechanical loading, therefore limiting the impact of this aspect on disease onset (34–37). Moreover, the idea that bone mineral density is increased in patients with OA compared with age-matched individuals, protecting them from osteoporosis and/or fractures, has to be reviewed with current findings that OA bone tissue is sclerotic mainly due to an abundant osteoid matrix that fails to mineralize normally in vivo (2,6,38,39). Therefore, we elected to examine the cellular causes for this abnormal deposition of an abundant osteoid matrix.
Our results indicate that type I collagen production in OA osteoblasts is increased compared with that in normal osteoblasts and may be responsible for abnormal mineralization. This is attributable, first, to a direct effect on the expression of COL1A1 chains in OA osteoblasts. Coupled with no significant increases in COL1A2 expression, this resulted in an altered ratio of α1 to α2 chains that was higher in OA osteoblasts than in normal osteoblasts. A similar increase in expression of the α1 chain of type I collagen has been reported in ex vivo OA bone explants (21,39).
Our results for normal osteoblasts in vitro are consistent with the expected in vivo ratio of α1 to α2 chains of ~2.4 (10,40). Moreover, the α1-to-α2 ratio observed in vitro with OA osteoblasts is similar to the ratio recently reported by Bailey et al with bone explants from the femoral heads of patients with OA (10). These results would thus indicate that our cell culture system reflects closely the in vivo situation for collagen synthesis, as it does for the other cell markers we previously reported (1,14–16). Our data also indicated that the variation in the COL1A1-to-COL1A2 ratio was mostly attributable to elevated COL1A1 expression, because COL1A2 levels did not vary significantly between normal and OA osteoblasts.
Using the osteoblast-like SaOS-2 cells, we observed that COL1A2 expression in these cells also did not vary with time when cells were exposed to a mineralization medium, whereas COL1A1 expression, which was initially high, progressively declined, leading to a decrease in the COL1A1-to-COL1A2 ratio concomitant with an increase in alizarin red staining. Interestingly, the relationship between the COL1A1-to-COL1A2 ratio and time (results not shown) or between alizarin red staining levels in SaOS-2 cells and time in primary human osteoblasts showed a similar pattern, indicating that as the COL1A1-to-COL1A2 ratio changed with time, so did alizarin red staining. In addition, this abnormal COL1A1-to-COL1A2 ratio was similar in OA osteoblasts producing either low or high levels of endogenous PGE2, indicating that their basic mineralization defect remains similar. This defect in type I collagen composition and ultimately tridimensional structure would then be similar to the recently described abnormal subchondral bone architecture in the Brtl mouse that leads to rapidly progressive OA-like characteristics (9).
Second, and as expected, the basal synthesis of type I collagen, as measured by release of the carboxy-terminal propeptide of type I collagen, was enhanced in OA osteoblasts compared with normal osteoblasts. A similar increase in type I collagen production by OA osteoblasts compared with normal osteoblasts has been previously reported (41). We also observed an elevated ratio of α1 to α2 chains compared with normal, using Western blot analysis, which was reminiscent of our observations at the expression level. If the situation we observed in vitro is similar in vivo, this would translate into more type I collagen being layed down with an imbalance of α1 to α2 chains that would retard mineralization. Indeed, although more abundant, this collagen matrix would not mineralize properly, which would lead to a less mineralized subchondral bone tissue. Third, the decrease in mineralization observed in vitro under basal conditions indicates that a cellular defect is responsible for this abnormal mineral deposition, a situation that is not fully corrected by the potent osteogenesis stimulator BMP-2 (42,43).
The hypothesis that abnormal mineralization of OA osteoblasts could be linked with altered functioning of osteal tissue macrophages is also unlikely. Indeed, a recent study indicated that osteal tissue macrophages intercalated throughout human bone lining tissues could regulate osteoblast function and mineralization in vitro and in vivo (32). However, macrophages are very sparsely distributed in subchondral bone tissue as compared with trabecular bone tissue; moreover, isolated osteoblasts, both normal and OA, had little CSF-1R and EMR1 expression, suggesting low levels of contaminating osteal tissue macrophages in our in vitro preparations, which could have explained abnormal mineralization. Therefore, altered distribution or the presence of variable levels of macrophages could not explain the observed low mineralization levels in OA osteoblasts.
The increase in the level of early (type I collagen and alkaline phosphatase) and late (osteocalcin) markers, together with a reduced capacity to mineralize, suggests that OA osteoblasts progress into cell differentiation yet are stopped at a possible point necessary to reach full differentiation into mature osteoblasts laying a mineralized matrix. The possibility that this abnormal mineralization is attributable to the production of other collagens is unlikely, because we failed to detect elevated levels of type III collagen in OA osteoblasts, and we previously showed (using RT-PCR) that our OA osteoblast cell cultures do not express type II collagen (16). Although we observed a slight increase in the level of osteopontin in OA osteoblasts, this failed to reach significance, thereby precluding the possibility that osteopontin could play a key role. In addition, the possibility that our cell culture preparations could be representing MSCs at different stages of differentiation is also an unlikely explanation for the altered mineralization. The levels of markers for MSCs, CD73 and CD105 (44,45), were not significantly different between normal and OA osteoblasts, and we observed low levels of STRO-1–positive cells. STRO-1 is considered a marker of early MSCs (45,46), and indeed, as the level of STRO-1 decreases in in vitro osteoblast cell cultures, the alkaline phosphatase level goes up (46). Therefore, our phenotype and STRO-1 data for OA osteoblasts would indicate that these cells are fully differentiated osteoblasts that should mineralize normally, which is not the case.
A key element possibly involved in abnormal mineralization in OA osteoblasts is their elevated production of TGFβ1 in vitro (16) as well as in ex vivo OA bone explants (47). Indeed, elevated TGFβ1 levels inhibit in vitro mineralization in other cell systems, either directly or via the inhibition of BMP-2–induced mineralization (33,48). TGFβ1 is a potent inducer of osteophytes in OA bone tissue, whereas it decreases cartilage repair (49), and TGFβ1 injections in mouse knees result in OA-like features (50). Using microarray gene expression profiling of OA bone explants, Hopwood et al suggested that altered bone remodeling in OA may be linked with abnormal TGFβ/BMP signaling (21). Interestingly, in the current study, BMP-2–stimulated mineralization of OA osteoblasts was reduced compared with that in normal osteoblasts, and TGFβ1 reduced mineralization of normal osteoblasts.
We observed elevated TGFβ1 levels in both low and high OA osteoblasts that otherwise showed blunted mineralization of OA osteoblasts. Hence, our observation that siRNA- or shRNA-induced inhibition of endogenous TGFβ1 levels in OA osteoblasts leads to reduced TGFβ1 expression while also leading to a reduction in COL1A1 expression and the COL1A1-to-COL1A2 ratio and to an increase in mineralization following BMP-2 stimulation provides evidence for a key role for TGFβ1 in abnormal mineralization of OA osteoblasts. TGFβ1 has been shown to directly regulate fibrosis in other cell systems (51–53), a situation linked with activation of TGFβ receptor 1 (activin receptor–like kinase 1) and downstream Smad3 effectors (53), leading to Sp-1 transcription factor induction (52) and increased COL1A1 expression thereof. Moreover, a silencing RNA technique similar to that used in the current study has been employed to reduce lung fibrosis (54).
Thus, these data collectively demonstrate that the elevated production of endogenous TGFβ1 by OA osteoblasts is responsible for their abnormal production of type I collagen and possibly abnormal subchondral bone tissue architecture. What triggers the elevation of TGFβ1 levels in OA osteoblasts remains elusive. A recent study by Falanga et al suggested that hypoxia-induced fibrosis of skin fibroblasts occurred via up-regulation of TGFβ1 levels in these cells (55). In in vivo OA bone tissue, hypoxia may be possible, whereas this situation seems unlikely in our in vitro setting, unless up-regulation of TGFβ1 expression remains following an in vivo hypoxic stress. Another potential stimulator of TGFβ1 levels could be leptin. Indeed, Dumond et al showed that leptin can stimulate TGFβ1 expression in rat chondrocytes (56), and our group recently reported that OA osteoblasts express more leptin than do normal osteoblasts (57).
Regardless of what triggers up-regulation of TGFβ1 levels, our observations of abnormal type I collagen levels and the ratio of α1 to α2 chains in OA osteoblasts are reminiscent of those made with the Brtl mouse, which shows spontaneous and progressive OA (9), and further support the key role played by subchondral bone tissue in OA onset and progression. An intriguing observation is the slightly variable level of mineralization in OA osteoblasts with either low or high PGE2 levels (16). Indeed, although all OA osteoblasts had lower levels of mineralization compared with normal osteoblasts, OA osteoblasts with the highest PGE2 levels mineralized slightly better than did OA osteoblasts with low levels of PGE2. This would then suggest that elevated PGE2 levels could have a positive impact on these cells and directly affect the mineralization process in vitro. However, this hypothesis will require further experiments to be fully appreciated.
In conclusion, we showed abnormal expression and synthesis of type I collagen in OA subchondral osteoblasts coupled with low mineralization, which mimics the in vivo situation. We further demonstrated that this is linked with abnormal production of TGFβ1 by these cells, further suggesting that an abnormal cellular defect of OA osteoblasts is responsible for the observed in vivo situation.
Acknowledgments
Dr. Lajeunesse’s work was supported by grant MOP-49501 from the Canadian Institutes for Health Research (CIHR) and grant TAS-0089 from the Arthritis Society of Canada/CIHR.
We thank Dr. Christelle Boileau for her help with the immunohistochemical analyses of STRO-1 and CD68 in normal and OA specimens. We also thank Dr. Rafick Terra for his help with the flow cytometry analyses of STRO-1 in OA osteoblasts.
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Lajeunesse had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Couchourel, Aubry, Delalandre, Lavigne, Martel-Pelletier, Pelletier, Lajeunesse.
Acquisition of data. Couchourel, Aubry, Delalandre, Lavigne, Martel-Pelletier, Pelletier, Lajeunesse.
Analysis and interpretation of data. Couchourel, Aubry, Delalandre, Lavigne, Martel-Pelletier, Pelletier, Lajeunesse.
Immunohistochemical analysis. Martel-Pelletier.
Patient evaluation. Pelletier.
References
- 1.Hilal G, Martel-Pelletier J, Pelletier JP, Ranger P, Lajeunesse D. Osteoblast-like cells from human subchondral osteoarthritic bone demonstrate an altered phenotype in vitro: possible role in subchondral bone sclerosis. Arthritis Rheum. 1998;41:891–9. doi: 10.1002/1529-0131(199805)41:5<891::AID-ART17>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 2.Mansell JP, Bailey AJ. Abnormal cancellous bone collagen metabolism in osteoarthritis. J Clin Invest. 1998;101:1596–603. doi: 10.1172/JCI867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Westacott CI, Webb GR, Warnock MG, Sims JV, Elson CJ. Alteration of cartilage metabolism by cells from osteoarthritic bone. Arthritis Rheum. 1998;40:1282–91. doi: 10.1002/1529-0131(199707)40:7<1282::AID-ART13>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 4.Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop. 1986;213:34–40. [PubMed] [Google Scholar]
- 5.Li B, Aspden RM. Composition and mechanical properties of cancellous bone from the femoral head of patients with osteoporosis or osteoarthritis. J Bone Miner Res. 1997;12:641–51. doi: 10.1359/jbmr.1997.12.4.641. [DOI] [PubMed] [Google Scholar]
- 6.Li B, Aspden RM. Mechanical and material properties of the subchondral bone plate from the femoral head of patients with osteoarthritis or osteoporosis. Ann Rheum Dis. 1997;56:247–54. doi: 10.1136/ard.56.4.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding M, Danielsen CC, Hvid I. Bone density does not reflect mechanical properties in early-stage arthrosis. Acta Orthop Scand. 2001;72:181–5. doi: 10.1080/000164701317323444. [DOI] [PubMed] [Google Scholar]
- 8.Ding M, Odgaard A, Hvid I. Changes in the three-dimensional microstructure of human tibial cancellous bone in early osteoarthritis. J Bone Joint Surg Br. 2003;85:906–12. [PubMed] [Google Scholar]
- 9.Blair-Levy JM, Watts CE, Fiorientino NM, Dimitriadis EK, Marini JC, Lipsky PE. A type I collagen defect leads to rapidly progressive osteoarthritis in a mouse model. Arthritis Rheum. 2008;58:1096–106. doi: 10.1002/art.23277. [DOI] [PubMed] [Google Scholar]
- 10.Bailey AJ, Sims TJ, Knott L. Phenotypic expression of osteoblast collagen in osteoarthritic bone: production of type I homotrimer. Int J Biochem Cell Biol. 2002;34:176–82. doi: 10.1016/s1357-2725(01)00107-8. [DOI] [PubMed] [Google Scholar]
- 11.Mansell JP, Tarlton JF, Bailey AJ. Biochemical evidence for altered subchondral bone collagen metabolism in osteoarthritis of the hip. Br J Rheumatol. 1997;36:16–9. doi: 10.1093/rheumatology/36.1.16. [DOI] [PubMed] [Google Scholar]
- 12.Mkukuma LD, Imrie CT, Skakle JM, Hukins DW, Aspden RM. Thermal stability and structure of cancellous bone mineral from the femoral head of patients with osteoarthritis or osteoporosis. Ann Rheum Dis. 2005;64:222–5. doi: 10.1136/ard.2004.021329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lajeunesse D, Hilal G, Pelletier JP, Martel-Pelletier J. Subchondral bone morphological and biochemical alterations in osteoarthritis. Osteoarthritis Cartilage. 1999;7:321–2. doi: 10.1053/joca.1998.0180. [DOI] [PubMed] [Google Scholar]
- 14.Hilal G, Massicotte F, Martel-Pelletier J, Fernandes JC, Pelletier JP, Lajeunesse D. Endogenous prostaglandin E2 and insulin-like growth factor 1 can modulate the levels of parathyroid hormone receptor in human osteoarthritic osteoblasts. J Bone Miner Res. 2001;16:713–21. doi: 10.1359/jbmr.2001.16.4.713. [DOI] [PubMed] [Google Scholar]
- 15.Hilal G, Martel-Pelletier J, Pelletier JP, Duval N, Lajeunesse D. Abnormal regulation of urokinase plasminogen activator by insulin-like growth factor 1 in human osteoarthritic subchondral osteoblasts. Arthritis Rheum. 1999;42:2112–22. doi: 10.1002/1529-0131(199910)42:10<2112::AID-ANR11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 16.Massicotte F, Lajeunesse D, Benderdour M, Pelletier JP, Hilal G, Duval N, et al. Can altered production of interleukin 1β, interleukin-6, transforming growth factor-β and prostaglandin E2 by isolated human subchondral osteoblasts identify two subgroups of osteoarthritic patients. Osteoarthritis Cartilage. 2002;10:491–500. doi: 10.1053/joca.2002.0528. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez C, Deberg MA, Piccardi N, Msika P, Reginster JY, Henrotin YE. Subchondral bone osteoblasts induce phenotypic changes in human osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2005;13:988–97. doi: 10.1016/j.joca.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez C, Deberg MA, Piccardi N, Msika P, Reginster JY, Henrotin YE. Osteoblasts from the sclerotic subchondral bone downregulate aggrecan but upregulate metalloproteinases expression by chondrocytes: this effect is mimicked by interleukin-6, -1β and oncostatin M pre-treated non-sclerotic osteoblasts. Osteoarthritis Cartilage. 2005;13:979–87. doi: 10.1016/j.joca.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Massicotte F, Fernandes JC, Martel-Pelletier J, Pelletier JP, Lajeunesse D. Modulation of insulin-like growth factor 1 levels in human osteoarthritic subchondral bone osteoblasts. Bone. 2006;38:333–41. doi: 10.1016/j.bone.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Massicotte F, Aubry I, Martel-Pelletier J, Pelletier JP, Fernandes J, Lajeunesse D. Abnormal insulin-like growth factor 1 signaling in human osteoarthritic subchondral bone osteoblasts. Arthritis Res Ther. 2006;8:R177. doi: 10.1186/ar2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopwood B, Tsykin A, Findlay DM, Fazzalari NL. Microarray gene expression profiling of osteoarthritic bone suggests altered bone remodelling, WNT and transforming growth factor-β/bone morphogenetic protein signalling. Arthritis Res Ther. 2007;9:R100. doi: 10.1186/ar2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paredes Y, Massicotte F, Pelletier JP, Martel-Pelletier J, Laufer S, Lajeunesse D. Study of role of leukotriene B4 in abnormal function of human subchondral osteoarthritis osteoblasts: effects of cyclooxygenase and/or 5-lipoxygenase inhibition. Arthritis Rheum. 2002;46:1804–12. doi: 10.1002/art.10357. [DOI] [PubMed] [Google Scholar]
- 23.Altman RD, Asch E, Bloch DA, Bole G, Borenstein D, Brandt KD, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 24.Lajeunesse D, Busque L, Menard P, Brunette MG, Bonny Y. Demonstration of an osteoblast defect in two cases of human malignant osteopetrosis: correction of the phenotype after bone marrow transplant. J Clin Invest. 1996;98:1835–42. doi: 10.1172/JCI118984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lajeunesse D, Kiebzak GM, Frondoza C, Sacktor B. Regulation of osteocalcin secretion by human primary bone cells and by the human osteosarcoma cell line MG-63. Bone Miner. 1991;14:237–50. doi: 10.1016/0169-6009(91)90025-u. [DOI] [PubMed] [Google Scholar]
- 26.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 27.Neumann K, Dehne T, Endres M, Erggelet C, Kaps C, Ringe J, et al. Chondrogenic differentiation capacity of human mesenchymal progenitor cells derived from subchondral cortico-spongious bone. J Orthop Res. 2008;26:1–8. doi: 10.1002/jor.20635. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli UK. Cleavage of structure proteins during assembly of the head of the bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Research Services Branch. Public domain NIH Image program. http://rsb.info.nih.gov/nih-image/
- 30.Gregory CA, Gunn WG, Peister A, Prockop DJ. An alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004;329:77–84. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Franceschi RT, Iyer BS, Cui Y. Effects of ascorbic acid on collagen matrix formation and osteoblast differentiation in murine MC3T3-E1 cells. J Bone Miner Res. 1994;9:843–54. doi: 10.1002/jbmr.5650090610. [DOI] [PubMed] [Google Scholar]
- 32.Chang MK, Raggatt LZ, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K, et al. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol. 2008;181:1232–44. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- 33.Lee MH, Kim YJ, Kim HJ, Park HD, Kang AR, Kyung HM, et al. BMP-2-induced Runx2 expression is mediated by Dlx5, and TGF-β 1 opposes the BMP-2-induced osteoblast differentiation by suppression of Dlx5 expression. J Biol Chem. 2003;278:34387–94. doi: 10.1074/jbc.M211386200. [DOI] [PubMed] [Google Scholar]
- 34.Carlson CS, Loeser RF, Jayo MJ, Weaver DS, Adams MR, Jerome CP. Osteoarthritis in cynomolgus macaques: a primate model of naturally occurring disease. J Orthop Res. 1994;12:331–9. doi: 10.1002/jor.1100120305. [DOI] [PubMed] [Google Scholar]
- 35.Carlson CS, Loeser RF, Purser CB, Gardin JF, Jerome CP. Osteoarthritis in cynomolgus macaques. III. Effects of age, gender, and subchondral bone thickness on the severity of disease. J Bone Miner Res. 1996;11:1209–17. doi: 10.1002/jbmr.5650110904. [DOI] [PubMed] [Google Scholar]
- 36.Dieppe P, Cushnaghan J, Young P, Kirwan J. Prediction of the progression of joint space narrowing in osteoarthritis of the knee by bone scintigraphy. Ann Rheum Dis. 1993;52:557–63. doi: 10.1136/ard.52.8.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Billingham ME, Meijers MH, Mahwinney B, Malcolm A. Spontaneous osteoarthritis in guinea pigs: cartilage degeneration is preceded by loss of subchondral trabecular bone [abstract] J Rheum Suppl. 1996;1:104. [Google Scholar]
- 38.Li B, Marshall D, Roe M, Aspden RM. The electron microscope appearance of the subchondral bone plate in the human femoral head in osteoarthritis and osteoporosis. J Anat. 1999;195:101–10. doi: 10.1046/j.1469-7580.1999.19510101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Truong LH, Kuliwaba JS, Tsangari H, Fazzalari NL. Differential gene expression of bone anabolic factors and trabecular bone architectural changes in the proximal femoral shaft of primary hip osteoarthritis patients. Arthritis Res Ther. 2006;8:R188. doi: 10.1186/ar2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viguet-Carrin S, Garnero P, Delmas PD. The role of collagen in bone strength. Osteoporos Int. 2006;17:319–36. doi: 10.1007/s00198-005-2035-9. [DOI] [PubMed] [Google Scholar]
- 41.Lisignoli G, Toneguzzi S, Piacentini A, Cristino S, Grassi F, Cavallo C, et al. CXCL12 (SDF-1) and CXCL13 (BCA-1) chemokines significantly induce proliferation and collagen type I expression in osteoblasts from osteoarthritis patients. J Cell Physiol. 2006;206:78–85. doi: 10.1002/jcp.20435. [DOI] [PubMed] [Google Scholar]
- 42.Hay E, Hott M, Graulet AM, Lomri A, Marie PJ. Effects of bone morphogenetic protein-2 on human neonatal calvaria cell differentiation. J Cell Biochem. 1999;72:81–93. doi: 10.1002/(sici)1097-4644(19990101)72:1<81::aid-jcb9>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 43.Yamagiwa H, Endo N, Tokunaga K, Hayami T, Hatano H, Takahashi HE. In vivo bone-forming capacity of human bone marrow-derived stromal cells is stimulated by recombinant human bone morphogenetic protein-2. J Bone Miner Metab. 2001;19:20–8. doi: 10.1007/s007740170056. [DOI] [PubMed] [Google Scholar]
- 44.Goff LA, Boucher S, Ricupero CL, Fenstermacher S, Swerdel M, Chase LG, et al. Differentiating human multipotent mesenchymal stromal cells regulate microRNAs: prediction of microRNA regulation by PDGF during osteogenesis. Exp Hematol. 2008;36:1354–69. doi: 10.1016/j.exphem.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ho AD, Wagner W, Franke W. Heterogeneity of mesenchymal stromal cell preparations. Cytotherapy. 2008;10:320–30. doi: 10.1080/14653240802217011. [DOI] [PubMed] [Google Scholar]
- 46.Gronthos S, Zannettino AC, Graves SE, Ohta S, Hay SJ, Simmons PJ. Differential cell surface expression of the STRO-1 and alkaline phosphatase antigens on discrete developmental stages in primary cultures of human bone cells. J Bone Miner Res. 1999;14:47–56. doi: 10.1359/jbmr.1999.14.1.47. [DOI] [PubMed] [Google Scholar]
- 47.Martel-Pelletier J, Hilal G, Pelletier JP, Ranger P, Lajeunesse D. Evidence for increased metabolic activity in human osteoarthritic subchondral bone explants [abstract] Arthritis Rheum. 1997;40(Suppl 9):S182. [Google Scholar]
- 48.Lee MH, Kwon TG, Park HS, Wozney JM, Ryoo HM. BMP-2-induced osterix expression is mediated by Dlx5 but is independent of Runx2. Biochem Biophys Res Commun. 2003;309:689–94. doi: 10.1016/j.bbrc.2003.08.058. [DOI] [PubMed] [Google Scholar]
- 49.Scharstuhl A, Glansbeek HL, van Beuningen HM, Vitters EL, van der Kraan PM, van den Berg WB. Inhibition of endogenous TGF-β during experimental osteoarthritis prevents osteophyte formation and impairs cartilage repair. J Immunol. 2002;169:507–14. doi: 10.4049/jimmunol.169.1.507. [DOI] [PubMed] [Google Scholar]
- 50.Van Beuningen HM, Glansbeek HL, van der Kraan PM, van den Berg WB. Osteoarthritis-like changes in the murine knee joint resulting from intra-articular transforming growth factor-beta injections. Osteoarthritis Cartilage. 2000;8:25–33. doi: 10.1053/joca.1999.0267. [DOI] [PubMed] [Google Scholar]
- 51.Cutroneo KR. How is type I procollagen synthesis regulated at the gene level during tissue fibrosis. J Cell Biochem. 2003;90:1–5. doi: 10.1002/jcb.10599. [DOI] [PubMed] [Google Scholar]
- 52.Ghosh AK, Mori Y, Dowling E, Varga J. Trichostatin A blocks TGF-β-induced collagen gene expression in skin fibroblasts: involvement of Sp1. Biochem Biophys Res Commun. 2007;354:420–6. doi: 10.1016/j.bbrc.2006.12.204. [DOI] [PubMed] [Google Scholar]
- 53.Jinnin M, Ihn H, Tamaki K. Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-β1-induced extracellular matrix expression. Mol Pharmacol. 2006;69:597–607. doi: 10.1124/mol.105.017483. [DOI] [PubMed] [Google Scholar]
- 54.Lok CN, Ehrlich HP, White SL, Buttolph TR, Cutroneo KR, Chiu JF. Oligodeoxynucleotide decoy therapy blocks type 1 procollagen transcription and the prolyl hydroxylase β subunit translation. J Cell Biochem. 2008;103:1066–75. doi: 10.1002/jcb.21477. [DOI] [PubMed] [Google Scholar]
- 55.Falanga V, Zhou L, Yufit T. Low oxygen tension stimulates collagen synthesis and COL1A1 transcription through the action of TGF-β1. J Cell Physiol. 2002;191:42–50. doi: 10.1002/jcp.10065. [DOI] [PubMed] [Google Scholar]
- 56.Dumond H, Presle N, Terlain B, Mainard D, Loeuille D, Netter P, et al. Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum. 2003;48:3118–29. doi: 10.1002/art.11303. [DOI] [PubMed] [Google Scholar]
- 57.Lajeunesse D, Aoulad Aissa M, Delalandre A, Fernandes J. Increased expression and production of leptin by subchondral osteoblasts from osteoarthritic patients could play a role in cartilage degradation [abstract] Arthritis Rheum. 2005;52(Suppl 9):S44. [Google Scholar]