Abstract
The global human immunodeficiency virus (HIV)epidemic is characterized by significant genetic diversity in circulating viruses. We have recently characterized a group of viruses that form a distinct sub-subtype within the subtype A radiation, which we have designated HIV type 1 (HIV-1) sub-subtype A, circulating in West Africa. A prospective study of a cohort of female sex workers (FSW) in Dakar, Senegal over an 18-year period indicated that an A3-specific sequence in the C2-V3 region of the env gene was found in 46 HIV-1-infected women. HIV-1 sub-subtype A3 appeared in the FSW population as early as 1988 and continued to be transmitted as of 2001. We also found that HIV-1 A3 is not confined to the FSW cohort in Senegal but is also circulating in the general population in Dakar. Furthermore, analyses of viral sequences from a few other West and Central African countries also demonstrated evidence of HIV-1 A3 sequence in isolates from HIV-1-infected people in Ivory Coast, Nigeria, Niger, Guinea Bissau, Benin, and Equatorial Guinea. Overall, because of the evidence of sub-subtype A3 in the general population in Senegal, as well as in a few neighboring West and Central African countries, along with the increasing incidence of infection with A3-containing viruses in the Dakar high-risk FSW population, we feel that HIV-1 sub-subtype A3 viruses are important to distinguish and monitor.
Molecular surveillance has revealed significant heterogeneity in the prevalence and geographic distribution of various subtypes worldwide (5, 35, 36). For example, subtype B viruses account for an estimated 12.3% of cases globally, but infections with this subtype are primarily seen in the Americas, Western Europe, and Australia (35). Conversely, subtype C viruses were estimated to have caused >47% of the worldwide infections, with the highest incidence in southern African countries, Ethiopia, and India (35). As with the nonrecombinant subtypes, circulating recombinant forms (CRFs) also show founder effects and uneven geographic distributions. For example, CRF01_AE appears to be the cause of a high proportion of infections in Southeast Asia and seems to be largely confined to that area of the world with relatively little spread into other populations (35). In contrast, CRF02_AG contributes to a major number of new infections in West Africa and appears to be associated with rapidly spreading epidemics in West African countries (3, 6, 7, 28, 31, 35, 37). The underlying causes of the varied geographic distributions are most likely founder effects with other contributing factors, such as human population movements, unregulated commercial sex work, and intravenous drug use.
Over the course of an 18-year prospective study monitoring a cohort of female sex workers (FSWs) in Dakar, Senegal, we found that the most prevalent circulating strain in the cohort is CRF02_AG, as seen in other parts of West Africa (2, 6, 7, 28, 31, 35, 37). However, recent work conducted in our lab revealed that a unique group of viruses within the human immunodeficiency virus type 1 (HIV-1) subtype A radiation is also present in the FSW study population (40, 42). Through phylogenetic analyses, we have shown that this subcluster of viruses can be classified as HIV-1 sub-subtype A3 (30). The goal of the current investigation was to understand the distribution and dynamics of HIV-1 A3 in the Dakar FSW cohort. We examined the molecular epidemiology of sub-subtype A3 in Senegal, as well as other West and Central African countries. This investigation revealed that there were 46 women in the cohort with the A3 sequence in the C2-V3 region of the env gene. Furthermore, analyses of GenBank-submitted sequences from other West African countries revealed evidence of A3 sequence in isolates from Ivory Coast, Nigeria, Niger, Guinea Bissau, Benin, and Equatorial Guinea. Our findings also indicate that sub-subtype A3 viruses entered the high-risk population of Dakar FSWs as early as 1988, with continued transmission.
MATERIALS AND METHODS
Study population and sample collection.
Since 1985, we have conducted a prospective study of registered FSWs in Dakar, Senegal; blood samples and questionnaire data were collected after obtaining informed consent. The specific details of the study recruitment procedures and methods have been described elsewhere (21, 22). Serostatus was determined by immunoblot on whole virus lysates and recombinant envelope peptides and by diagnostic HIV-1 and/or HIV-2 PCR. The time of infection for women who converted to HIV-positive serostatus while in the study was estimated as the midpoint between their last seronegative and first seropositive samples. HIV-1-positive person-years of observation was calculated as described previously (22).
DNA amplification and sequencing.
Proviral DNA was extracted from peripheral blood mononuclear cells by using a kit from Qiagen, Inc. (Chatsworth, Calif.). The C2-V3 env region (350 bp) for all of our samples was amplified by nested PCR with primers and reaction conditions that have been described previously (22, 41, 48). The PCR products were purified and directly sequenced by using the second-round primers. In cases in which poor data or highly heterogeneous sequences were obtained from direct sequencing, purified products were cloned into the pCR2.1 vector (T/A Cloning; Invitrogen, San Diego, Calif.). Sequences for both strands of DNA were determined by dye terminator cycle sequencing with Taq polymerase (Perkin-Elmer Applied Biosystems Division, Foster City, Calif.) and an automatic sequencer (ABI 373; Perkin-Elmer Applied Biosystems Division).
Sequence analysis and statistical methods.
Multiple alignments of all of the consensus sequences to reference sequences from all of the major subtypes and sub-subtypes (A1, A2, A3, B, C, D, F1, F2, G, H, J, and K), as well as CRF02_AG, were performed by using the ClustalX package (45). Manual adjustments, when necessary, were made by using the MacClade version 4.05 program (Sinauer Associates, Inc., Sunderland, Mass.). For reference A3 sequences, we used AY521629 (DDI579), AY521630 (DDJ360), and AY521631 (DDJ369) (30). Phylogenetic analyses were performed, using the SEQBOOT, DNADIST, NEIGHBOR, and CONSENSE programs in the Phylip version 3.6 package (11). Neighbor-joining trees were generated by using the F84 model of substitution and a transition/transversion ratio of 1.42 (24). Alignments were gap stripped prior to analyses. Trees were visualized by using NJPlot (38).
In order to determine whether there was evidence of A3 sequence in any other regions of the world, a BLAST (http://www.ncbi.nlm.gov/BLAST) search was conducted with reference A3 sequences. Once a subset of sequences was obtained, more detailed phylogenetic analyses were performed. Neighbor-joining trees, using an F84 model of evolution and a transition/transversion ratio of 1.42 were generated. For these trees, all major subtype, sub-subtype, and CRF02_AG reference sequences were included in the analyses.
Sequence information.
The sequences have been deposited into the GenBank database under accession numbers AY646116 to AY646151.
RESULTS
From 1987 through 2001, 3,681 women were enrolled in the Dakar FSW cohort, 491 (13.3%) of whom were or became infected with HIV-1. Of the HIV-1-infected in the cohort, 175 (35.6%) were seroincident with HIV-1 only and 253 (51.5%) were seroprevalent with HIV-1 only; the remaining subjects were dually infected with HIV-1 and HIV-2.
Epidemiology of HIV-1 A3 in Senegalese FSW cohort.
Of the HIV-1-infected women, including those that were dually infected, we identified 301 (61%) women whose HIV-1 sequences were subtype A in the env. Of the 301 women, 46 (21%) had sequences that clustered distinctly from CRF02_AG as well as HIV-1 sub-subtypes A1 and A2, as HIV-1 sub-subtype A3 viruses. Overall, 9.4% of the HIV-1 infections in the Dakar FSW were attributed to HIV-1 A3. Of the 46 women whose viral sequences clustered as sub-subtype A3 (30), 26 (56.5%) were seroincident with HIV-1 only, 2 (4.3%) were seroincident and dually infected (Table 1), 14 (30.4%) were seroprevalent and infected with HIV-1 only, and the remaining 4 (8.7%) were seroprevalent and dually infected (Table 2).
TABLE 1.
Seroincident subjects in Senegalese FSW cohort with A3 sequence in the C2-V3 region (env)
| Subject | Serohistory | Date (yr):
|
|
|---|---|---|---|
| Enrolled in cohort | Diagnosed as HIV-1+ | ||
| DDA360 | HIV-1 only | 1988 | 1991 |
| DDA738 | HIV-1 only | 1988 | 1991 |
| DDI578 | HIV-1 only | 1985 | 1992 |
| DDA502 | HIV-1 only | 1986 | 1992 |
| DDB231 | HIV-1 only | 1990 | 1993 |
| DDI579 | HIV-1 only | 1987 | 1994 |
| DDB266 | HIV-1 only | 1995 | 1995 |
| DDB645 | HIV-1 only | 1988 | 1997 |
| DDI509 | HIV-1 only | 1989 | 1997 |
| DDF709 | HIV-1 only | 1990 | 1997 |
| DDB754 | HIV-1 only | 1993 | 1997 |
| DDI569 | HIV-1 only | 1990 | 1998 |
| DDI582 | HIV-1 only | 1994 | 1998 |
| DDI568 | HIV-1 only | 1988 | 1999 |
| DDI570 | HIV-1 only | 1990 | 1999 |
| DDI989 | HIV-1 only | 1996 | 1999 |
| DDI601 | HIV-1 only | 1998 | 1999 |
| DDJ351 | HIV-1 only | 1986 | 2000 |
| DDJ321 | HIV-1 only | 1988 | 2000 |
| DDJ201 | HIV-1 only | 1997 | 2000 |
| DDJ338 | HIV-1 only | 1985 | 2001 |
| DDJ452 | HIV-1 only | 1987 | 2001 |
| DDI998 | HIV-1 only | 1991 | 2001 |
| DDJ200 | HIV-1 only | 1995 | 2001 |
| DDI963 | HIV-1 only | 2000 | 2001 |
| DDI983 | HIV-1 only | 2001 | 2001 |
| DDA482 | HIV-1 and HIV-2 | 1985 | 1992 |
| DDB252 | HIV-1 and HIV-2 | 1993 | 1994 |
TABLE 2.
Seroprevalent subjects in Senegalese FSW cohort with A3 sequence in the C2-V3 region (env)
| Subject | Serohistory | Yr enrolled in cohort |
|---|---|---|
| DD170 | HIV-1 only | 1988 |
| DD737 | HIV-1 only | 1993 |
| DDB107 | HIV-1 only | 1993 |
| DDA399 | HIV-1 only | 1993 |
| DDA467 | HIV-1 only | 1993 |
| DDC571 | HIV-1 only | 1995 |
| DDH188 | HIV-1 only | 1995 |
| DDH189 | HIV-1 only | 1996 |
| DDH394 | HIV-1 only | 1998 |
| DDI944 | HIV-1 only | 1998 |
| DDJ371 | HIV-1 only | 1998 |
| DDJ426 | HIV-1 only | 2000 |
| DDJ330 | HIV-1 only | 2000 |
| DDI979 | HIV-1 only | 2001 |
| DDB85 | HIV-1 and HIV-2 | 1989 |
| DD935 | HIV-1 and HIV-2 | 1989 |
| DDB501 | HIV-1 and HIV-2 | 1992 |
| DDE303 | HIV-1 and HIV-2 | 1997 |
A time-lapse graph was generated to examine the appearance and prevalence of A3 in the cohort (Fig. 1a). This graph included all of the seroincident subjects in the Senegalese FSW cohort who had been identified as infected with either A3 or CRF02_AG. For each subject, we plotted first bleed date, seroconversion date, and last bleed date. The data indicated that the first evidence of HIV-1 seroconversion with A3 occurred in 1991. However, when examining study entry dates for seroprevalent subjects (Table 2), we found evidence of A3 infection as early as 1988. Overall, the annual incidence of A3 has steadily increased from 0% in the time period of 1987 through 1989 to an average of 0.67% in the time period of 1999 through 2001 in this FSW population (Fig. 1b).
FIG. 1.
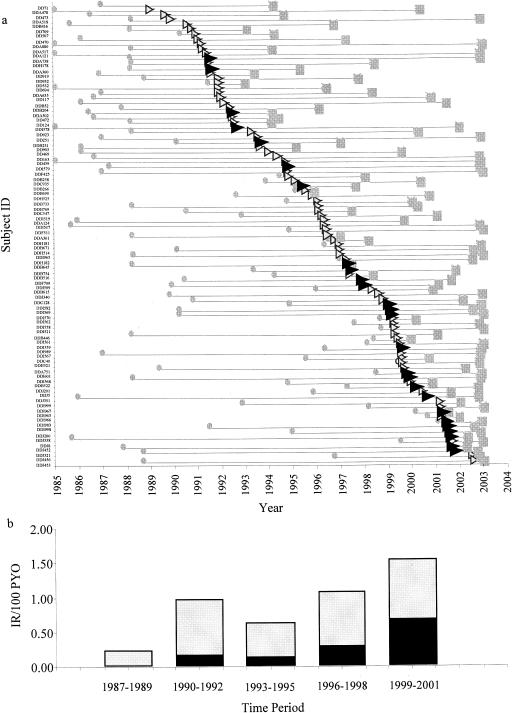
(a) Distribution of HIV-1 CRF02_AG and A3 infections by year of seroconversion among Senegalese FSWs that were seroincident in the cohort. For each subject, the first bleed date, the time of seroconversion to HIV-1-positive status, and the last bleed date are plotted. The position of the triangles for each subject corresponds to the time of seroconversion. The shading and sizes of the triangles correspond to the HIV-1A sub-subtype or CRF with which the individual was infected:  , first bleed date; ▸, seroconversion date for HIV-1 A3-infected subjects; ▹, seroconversion date for HIV-1 CRF02_AG-infected subjects;
, first bleed date; ▸, seroconversion date for HIV-1 A3-infected subjects; ▹, seroconversion date for HIV-1 CRF02_AG-infected subjects;  , last bleed date. (b) Annual incidence of HIV-1A env sub-subtypes in the Senegalese FSW cohort. Incidence rate per 100 person-years of observation (PYO) of each of the subtype A env viruses is plotted in 3-year intervals. The y axis indicates the incidence rate per 100 person-years of observation, and the x axis indicates the time period to which the values correspond. Shading of rectangles corresponds to HIV-1 sub-subtype or CRF with which the individual was infected: ▪, A3; □, CRF02_AG.
, last bleed date. (b) Annual incidence of HIV-1A env sub-subtypes in the Senegalese FSW cohort. Incidence rate per 100 person-years of observation (PYO) of each of the subtype A env viruses is plotted in 3-year intervals. The y axis indicates the incidence rate per 100 person-years of observation, and the x axis indicates the time period to which the values correspond. Shading of rectangles corresponds to HIV-1 sub-subtype or CRF with which the individual was infected: ▪, A3; □, CRF02_AG.
Kaplan-Meier analyses were conducted to determine whether there was any difference between women in our cohort infected with A3 and those infected with CRF02_AG in terms of progression to AIDS. We did not find a statistically significant difference between the two groups (data not shown). Interestingly, nonparametric univariate analyses indicated that women infected with A3 had a longer time to HIV-1 infection from the date at which they self-reported starting commercial sex work than did those women infected with CRF02_AG (12.9 ± 9.4 versus 7.4 ± 6.2, respectively; P = 0.02). We also found that, on average, women infected with A3 were older at time of seroconversion than those infected with CRF02_AG (39.7 versus 35.4, respectively; P = 0.04). There was no statistically significant difference between those infected with A3 and those infected with CRF02_AG with respect to age at which sex work began (27.4 versus 27.9, respectively; P = 0.67). There were no statistically significant differences between the two groups with regard to nationality, religion, ethnicity, or level of education.
Evidence of HIV-1 A3 in other West and Central African populations.
In order to determine whether A3 was specific to the high-risk population in Dakar, we obtained GenBank-submitted sequences from a study conducted by Toure-Kane et al. in Senegal (46) that looked at sequences from HIV-1-infected people attending a hospital in Dakar, Senegal. The sequences, which had previously been designated “A′” (A prime) by Toure-Kane et al. (AJ272646, AJ272648, AJ272656, AJ272659, AJ272669, and AJ272680) (46), clustered with our reference A3 sequences. In addition, we wanted to examine whether there was evidence of spread of A3 to other neighboring African countries (3, 4, 9, 14, 26 27, 33, 34, 43, 44). We first conducted a BLAST search to identify a subset of GenBank submitted sequences, which might have originally been classified as subtype A, that would potentially cluster with sub-subtype A3 reference sequences. Phylogenetic analyses indicated that a few of the previously characterized sequences from Ivory Coast (AF000450, AF000453, AF000456, AF000466, and AF000475) (9), Equatorial Guinea (AF530014) (34), Niger (AJ429851, AJ429856, AJ429866, AJ429869, and AJ429875) (26), Guinea-Bissau (AF178170, AF178172, AF178184, and AF178195) (3), Nigeria (AF069932, AJ389774, AY653735, and AY653736) (29, 37), and Benin (U61862 and U61866) (14) clustered with sub-subtype A3 viruses identified in the Dakar FSW cohort (Fig. 2). Finally, we also found evidence of A3 in West Brittany, France (AF461904) (47). Although this sequence had previously been characterized as sub-subtype A1, we found that it clustered with our A3 reference sequences, but with a relatively low bootstrap value (data not shown).
FIG. 2.
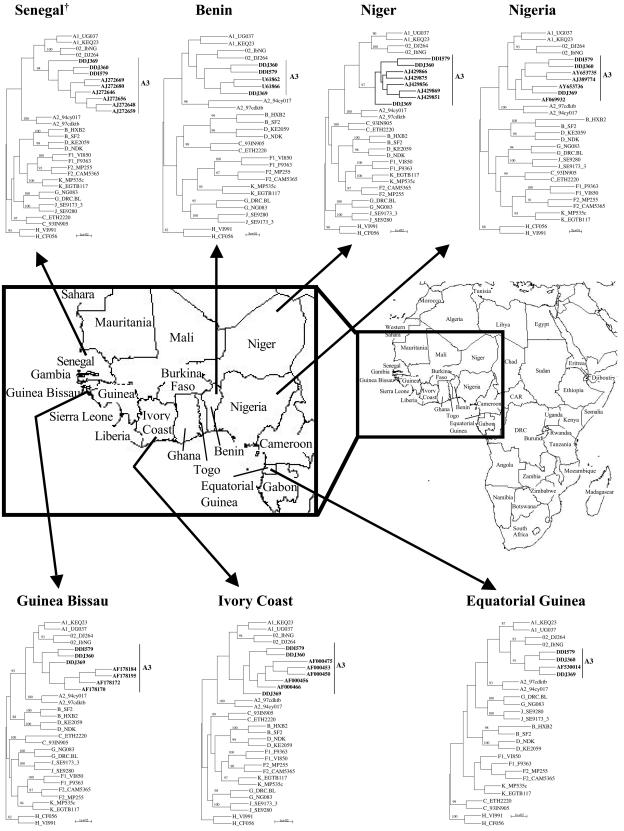
Evaluation of sequences from studies conducted on other cohorts in Senegal, as well as those in other countries in West Africa. Evidence of sequences clustering with A3 viruses identified in our Senegalese FSW cohort was found in another Senegalese cohort (46), as well as in the Ivory Coast (9), Nigeria (29, 37), Niger (26), Guinea-Bissau (3), Benin (14), and Equatorial Guinea (34). Phylogenetic trees including GenBank-submitted sequences from each of these respective locations are shown. All trees were generated by using an F84 model of evolution, a transition/transversion ratio of 1.42 and references from all published subtypes and sub-subtypes (A1, A2, B-D, F1, F2, G, H, J, and K), as well CRF02_AG. For reference A3 sequences, AY521629 (DDI579), AY521630 (DDJ360), and AY521631 (DDJ369) were used. Only bootstrap values of >80 are indicated at the major branch points. The A3 sequences are in boldface. Arrows link phylogenetic trees with the appropriate sequences to their corresponding geographic sources.
DISCUSSION
We have determined that 9.4% of the HIV-1-infected women in the Senegalese FSW cohort are infected sub-subtype A3. We have shown that HIV-1 A3 has been circulating in the Dakar FSW study population from as early as 1988 and that the incidence of HIV-1 infections due to A3 in this cohort is increasing. Our analyses also indicate that there is evidence of HIV-1 A3 in other populations, including those in some neighboring West and Central African countries.
Although we are uncertain as to when A3 was actually introduced into the cohort, the time-lapse graph indicates that the A3 sub-subtype has been newly infecting our population since 1991. Furthermore, our records indicate that a seroprevalent A3-infected subject first entered the cohort in 1988, which dates sub-subtype A3 to the early part of the HIV-1 epidemic in Senegal. Because this first case of A3 in our population was seroprevalent, her date of seroconversion could have been much earlier, so it is possible that A3 has been present in the population for even longer. Regarding the origin of sub-subtype A3, it is also interesting that we have identified sequences from a few other West and Central African countries that cluster with A3 viruses found in the Senegalese FSW cohort. This finding was not surprising given that Senegal has important trade and travel links with other parts of West Africa (20, 46). It had been suggested that HIV-1 originally entered the Senegalese population through these routes via migrant workers (20). In addition, the evidence of A3 sequence in West Brittany, France, was also initially surprising and was the first indication of this virus in Europe. Vallet et al. (47) suggest that the presence of non-B subtype sequences in northwest France is likely due to either African immigrants in the region or returning fishermen who had traveled to west African countries. Therefore, as seen in West Africa, the transmission of HIV-1 from Africa to northwest France was not unlikely.
Understanding the genetic diversity and molecular epidemiology of the various circulating viral strains is important for a number of reasons. First of all, these studies can help us evaluate the patterns of disease spread and provide us with a tool for tracking the virus. As we have shown, there is evidence of A3 not only in various countries of West and Central Africa but also in northwest France. Second, characterizing the epidemiology of the different viruses assists in studies regarding biological characteristics of these viruses and the biologic consequences of these viral interactions. Although a number of studies have shown associations between viral genotype and disease phenotype (12, 13, 15-19, 22, 25, 32, 39), some cross-sectional studies have not been able to corroborate these findings (1, 10, 23). Several studies have indicated a slower disease progression for CRF02_AG or subtype A-infected individuals relative to other non-A subtypes (17, 18, 22). Furthermore, in a study that teased out the distinctions between subtype A viruses, Sarr et al. (42) uncovered different levels of interaction with HIV-2. These authors found that the in vivo interaction between HIV-1 and HIV-2 is influenced by HIV-1 subtype and that the prevalence of A3 viruses (previously referred to as HIV-1 subtype A subcluster 2 viruses) was significantly higher in dually infected individuals compared to women who were singly infected with HIV-1 (42). This suggested that the potential protective effect of HIV-2 would be less against A3 viruses relative to the predominant CRF02_AG. It is possible that future studies that similarly make more accurate subtype and sub-subtype distinctions will better clarify findings regarding the association between viral genotype and biological phenotype. Finally, the further characterization of the predominant HIV-1 subtypes, sub-subtypes, and circulating recombinant forms in a given population will enhance our understanding of viral diversity critical to the informed design of interventions, therapies, and vaccines (8). Although the importance of matching a vaccine candidate to regional circulating strains is yet unclear, incorporation of local strains might maximize the efficacy of a potential vaccine candidate (8).
We have characterized and described a new sub-subtype of HIV-1 termed A3. We have found evidence of sub-subtype A3 in Senegal, as well as in other neighboring West and Central African countries. In addition, we have identified the presence of A3 in northwest France but note that this case is likely traceable to West Africa. We have documented the presence of A3 in the Senegalese high-risk populations in the late 1980s, with significant spread within that population to the present time. Continued monitoring and future molecular epidemiologic studies will elucidate the role of HIV-1 sub-subtype A3 in the global epidemic. It will also allow the study of viral variation and its impact on the distribution, dynamics, and consequences of virus infection in humans.
Acknowledgments
We thank Abdoulaye Dieng Sarr, Christopher Mulins, Ibrahima Traoré, Mamadou Ciré Dia, and Ousmane Diouf for continued assistance and support on this project.
This study was supported in part by U.S. Army grant DAMD 17-95-C-5005 and by NIH grants AI43879 and AI467274.
REFERENCES
- 1.Aleus, A., K. Lidman, A. Bjorkman, J. Giesecke, and J. Albert. 1999. Similar rate of disease progression among individuals infected with HIV-1 genetic subtypes A-D. AIDS 13:901-907. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, J., A. Rodrigo, G. Learn, A. Madan, C. Delahunty, M. Coon, M. Girard, S. Osmanov, L. Hood, and J. Mullins. 2000. Testing the hypothesis of a recombinant origin of human immunodeficiency virus type 1 subtype E. J. Virol. 74:10752-10765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson, S., H. Norrgren, F. Dias, G. Biberfeld, and J. Albert. 1999. Molecular characterization of human immunodeficiency virus (HIV)-1 and -2 individuals from Guinea-Bissau with single or dual infections: predominance of a distinct HIV-1 subtype A/G recombinant in West Africa. Virology 267:312-330. [DOI] [PubMed] [Google Scholar]
- 4.Bikandou, B., J. Takehisa, I. Mboudjeka, E. Ido, T. Kuwata, Y. Miyazaki, H. Moriyama, Y. Harada, Y. Taniguchi, H. Ichimura, M. Ikeda, P. J. Ndolo, M. Y. Nzoukoudi, R. M'Vouenze, M. M'Pandi, H. J. Parra, P. M'Pele, and M. Hayami. 2000. Genetic subtypes of HIV type 1 in Republic of Congo. AIDS Res. Hum. Retrovir. 16:613-619. [DOI] [PubMed] [Google Scholar]
- 5.Burke, D., and F. McCutchan. 1997. Global distribution of human immunodeficiency virus-1 clades, p. 119-126. In T. Vincent, J. DeVita, S. Hellman, and S. Rosenberg (ed.), AIDS: biology, diagnosis, treatment, and preventions, 4th ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 6.Carr, J., T. Laukkanen, M. Salminen, J. Albert, A. Alaeus, B. Kim, E. Sanders-Buell, D. Birx, and F. McCutchan. 1999. Characterization of subtype A HIV-1 from Africa by full genome sequencing. AIDS 13:18189-18226. [DOI] [PubMed] [Google Scholar]
- 7.Cornelissen, M., R. vandenBurg, F. Zorgdrager, and J. Goudsmit. 2000. Spread of distinct human immunodeficiency virus type 1 AG recombinant lineages in Africa. J. Gen. Virol. 81:515-523. [DOI] [PubMed] [Google Scholar]
- 8.Ellenberger, D. L., B. Li, L D. Lupo, S. M. Owen, J. Nkengasong, M. S. Kadio-Morokro, J. Smith, H. Robinson, M. Ackers, A. Greenberg, T. Folks, and S. Butera. 2002. Generation of a consensus sequence from prevalent and incident HIV-1 infections in West Africa to guide AIDS vaccine development. Virology 302:156-163. [DOI] [PubMed] [Google Scholar]
- 9.Ellenberger, D. L., D. Pieniazek, O. Nkengasong, C.-C. Luo, S. Devare, C. Maurice, M. Janini, A. Ramos, C. Fridlund, D. J. Hu, I.-M. Coulibaly, E. Ekpini, S. Z. Wiktor, A. E. Greenberg, G. Shochetman, and M. A. Rayfield. 1999. Genetic analysis of human immunodeficiency virus in Abidjan, Ivory Coast, reveals predominance of HIV type 1 subtype A and introduction of subtype G. AIDS Res. Hum. Retrovir. 15:3-9. [DOI] [PubMed] [Google Scholar]
- 10.Eshleman, S. H., L. A. Guay, T. Fleming, A. Mwatha, M. Mracna, G. Becker-Pergola, P. Musoke, F. Mmiro, and J. B. Jackson. 2002. Survival of Ugandan infants with subtype A and D HIV-1 infection (HIVNET 012). J. Acquir. Immune Defic. Syndr. 31:327-330. [DOI] [PubMed] [Google Scholar]
- 11.Felsenstein, J. 1992. PHYLIP (Phylogenetic Inference Package), version 3.5c ed. Department of Genetics, University of Washington, Seattle, Wash.
- 12.Harmelen, J., R. Wood, M. Lambrick, E. Rybicki, A. Williamson, and C. Williamson. 1997. An association between HIV-1 subtypes and mode of transmission in Cape Town, South Africa. AIDS 11:81-87. [DOI] [PubMed] [Google Scholar]
- 13.Herring, B. L., Y. C. Ge, B. Wang, M. Ratnamohan, F. Zheng, A. L. Cunningham, N. K. Saksena, and D. E Dwyer. 2003. Segregation of human immunodeficiency virus type 1 subtypes by risk factor in Australia. J. Clin. Microbiol. 41:4600-4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heyndrickx, L., W. Janssens, M. Alary, K. Fransen, K. Vereecken, S. Coppens, B. Willems, N. Davo, A. Guedeme, E. Baganizi, J. Joly, and G. Van der Groen. 1996. Genetic variability of HIV type 1 in Benin. AIDS Res. Hum. Retrovir. 12:1495-1497. [DOI] [PubMed] [Google Scholar]
- 15.Hu, D. J., S. Vanichseni, T. D. Mastro, S. Raktham, N. L. Young, P. A. Mock S. Subbarao, B. S. Parekh, L. Srisuwanvilai, R. Sutthent, C. Wasi, W. Heneine, and K. Choopanya. 2001. Viral load differences in early infection with two HIV-1 subtypes. AIDS 15:683-691. [DOI] [PubMed] [Google Scholar]
- 16.Hudgens, M. G., I. M. Longini, Jr., S. Vanichseni, D. J. Hu, D. Kitayaporn, P. A. Mock, M. E. Halloran, G. A. Satten, K. Choopanya, and T. D. Mastro. 2002. Subtype-specific transmission probabilities for human immunodeficiency virus type 1 among injecting drug users in Bangkok, Thailand. Am. J. Epidemiol. 155:159-168. [DOI] [PubMed] [Google Scholar]
- 17.Kaleebu, P., N. French, C. Mahe, D. Yirrell, C. Watera, F. Lyagoba, J. Nakiyingi, A. Rutebemberwa, D. Morgan, J. Weber, C. Gilks, and J. Whitworth. 2002. Effect of human immunodeficiency virus (HIV) type 1 envelope subtypes A and D on disease progression in a large cohort of HIV-1-positive persons in Uganda. J. Infect. Dis. 185:1244-1250. [DOI] [PubMed] [Google Scholar]
- 18.Kaleebu, P., A. Ross, D. Morgan, D. Yirrell, J. Oram, A. Rutebemberwa, F. Lyagoba, L. Hamilton, B. Biryahwaho, and J. Whitworth. 2001. Relationship between HIV-1 env subtypes A and D and disease progression in a rural Ugandan cohort. AIDS 15:293-299. [DOI] [PubMed] [Google Scholar]
- 19.Kalish, M. L., B. T. Korber, S. Pillai, K. E. Robbins, Y. S. Leo, A Saekhou, I. Verghese, P. Gerrish, C. L. Goh, D. Lupo, B. H. Tan, T. M. Brown, and R. Chan. 2002. The sequential introduction of HIV-1 subtype B and CRF01_AE in Singapore by sexual transmission: accelerated V3 region evolution in a subpopulation of Asian CRF01 viruses. Virology 304:311-329. [DOI] [PubMed] [Google Scholar]
- 20.Kane, F., M. Alary, I. Ndoye, A. M. Coll, S. Mboup, A. Gueye, P. J. Kanki, and J. R. Joly. 1993. Temporary expatriation is related to HIV-1 infection in rural Senegal. AIDS 7:1261-265. [DOI] [PubMed] [Google Scholar]
- 21.Kanki, P., S. Mboup, R. Marlink, K. Travers, C. C. Hsieh, A. Gueye, C. Boye, J. L. Sankale, C. Donnelly, W. Leisenring, T. Siby, I. Thior, M. Dia, E. Gueye, I. Ndoye, and M. Essex. 1992. Prevalence and risk determinants of human immunodeficiency virus type 2 (HIV-2) and human immunodeficiency virus type 1 (HIV-1) in West African female prostitutes. Am. J. Epidemiol. 136:895-907. [DOI] [PubMed] [Google Scholar]
- 22.Kanki, P. J., D. J. Hamel, J. L. Sankale, C. Hsieh, I. Thior, F. Barin, S A. Woodcock, A. Gueye-Ndiaye, E. Zhang, M. Montano, T. Siby, R. Marlink, N. D. I., M. E. Essex, and S. Mboup. 1999. Human immunodeficiency virus type 1 subtypes differ in disease progression. J. Infect. Dis. 179:68-73. [DOI] [PubMed] [Google Scholar]
- 23.Laurent C., A. Bourgeois, M. A. Faye, R. Mougnutou, M. Seydi, M. Gueye, F. Liegeois, C. T. Kane, C. Butel, J. Mbuagbaw, L. Zekeng, S. Mboup, E. Mpoudi-Ngole, M. Peeters, and E. Delaporte. 2002. No difference in clinical progression between patients infected with the predominant human immunodeficiency virus type 1 circulating recombinant form (CRF) 02_AG strain and patients not infected with CRF02_AG, in Western and West-Central Africa: a four-year prospective multicenter study. J. Infect. Dis. 18:486-492. [DOI] [PubMed] [Google Scholar]
- 24.Leitner, T., S. Kumar, and J. Albert. 1997. Tempo and mode of nucleotide substitutions in gag and env gene fragments in human immunodeficiency virus type 1 populations with a known transmission history. J. Virol. 71:4761-4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liitsola, K., P. Holmstrom, T. Laukkanen, H. Brummer-Korvenkontio, P. Leinikki, and M. O. Salminen. 2000. Analysis of HIV-1 genetic subtypes in Finland reveals good correlation between molecular and epidemiological data. Scand. J. Infect. Dis. 32:475-480. [DOI] [PubMed] [Google Scholar]
- 26.Mamadou, S., C. Montavon, A. Ben, A. Djibo, S. Rabiou, S. Mboup, E. Delaporte, and M. Peeters. 2002. Predominance of CRF02-AG and CRF06-cpx in Niger, West Africa. AIDS Res. Hum. Retrovir. 18:723-726. [DOI] [PubMed] [Google Scholar]
- 27.Mboudjeka, I., B. Bikandou, L. Zekeng, J. Takehisa, Y. Harada, Y. Yamaguchi-Kabata, Y. Taniguchi, E. Ido, L. Kaptue, P. M'pelle, H. Parra, M. Ikeda, M. Hayami, and T. Miura. 1999. Genetic diversity of HIV-1 group M from Cameroon and Republic of Congo. Arch. Virol. 144:2291-2311. [DOI] [PubMed] [Google Scholar]
- 28.McCutchan, F. E. 2000. Understanding the genetic diversity of HIV-1. AIDS 14:S31-S44. [PubMed] [Google Scholar]
- 29.McCutchan, F. E., J. K. Carr, M. Bajani, E. Sanders-Buell, T. O. Harry, T. C. Stoeckli, K. E. Robbins, W. Gashau, A. Nasidi, W. Janssens, and M. L. Kalish. 1999. Subtype G and multiple forms of A/G intersubtype recombinant human immunodeficiency virus type 1 in Nigeria. Virology 254:226-234. [DOI] [PubMed] [Google Scholar]
- 30.Meloni, S. T., B. Kim, J.-L. Sankalé, D. J. Hamel, S. Tovanabutra, S. Mboup, F. E. McCutchan, and P. J. Kanki. 2004. Distinct human immunodeficiency virus type 1 subtype A virus circulating in West Africa: sub-subtype A3. J. Virol. 78:12438-12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montavon, C., C. Toure-kane, F. Liegeois, E. Mpoudi, A. Bourgeois, L. Vergne, J. L. Perret, A. Boumah, E. Saman, and S. Mboup. 2000. Most env and gag subtype A HIV-1 viruses circulating in West and West Central Africa are similar to the prototype AG recombinant virus IBNG. J. Acquir. Immune Defic. Syndr. 23:363-374. [DOI] [PubMed] [Google Scholar]
- 32.Neilson, J. R., G. C. John, J. K. Carr, P. Lewis, J. K. Kreiss, S. Jackson, R. W. Nduati, D. Mbori-Ngacha, D. D. Panteleeff, S. Bodrug, C. Giachetti, M. A. Bott, B. A. Richardson, J. Bwayo, J. Ndinya-Achola, and J. Overbaugh. 1999. Subtypes of human immunodeficiency virus type 1 and disease stage among women in Nairobi, Kenya. J. Virol. 73:4393-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nkengasong, J. N., C. C. Luo, L. Abouya, D. Pieniazek, C. Maurice, M. Sassan-Morokro, D. Ellenberger, D. J. Hu, C. P. Pau, T. Dobbs, R. Respess, D. Coulibaly, I. M. Coulibaly, S. Z. Wiktor, A. E. Greenberg, and M. Rayfield. 2000. Distribution of HIV-1 subtypes among HIV-seropositive patients in the interior of Cote d'Ivoire. J. Acquir. Immune Defic. Syndr. 23:430-436. [DOI] [PubMed] [Google Scholar]
- 34.Ortiz, M., I. Sanchez, M. P. Gonzalez, M. I. Leon, N. Abeso, E. Asumu, and A. Garcia-Saiz. 2001. Molecular epidemiology of HIV type 1 subtypes in Equatorial Guinea. AIDS Res. Hum. Retrovir. 17:851-855. [DOI] [PubMed] [Google Scholar]
- 35.Osmanov, S., C. Pattou, N. Walker, B. Schwardlander, and J. Esparza. 2002. Estimated global distribution and regional spread of HIV-1 genetic subtypes in the year 2000. J. Acquir. Immune Defic. Syndr. 29:184-190. [DOI] [PubMed] [Google Scholar]
- 36.Peeters, M. 2000. Recombinant HIV sequences: their role in the global epidemic, p. I39-I54. In C. Kuiken, B. Foley, B. Hahn, B. Korber, F. McCutchan, P. Marx, J. Mellors, J. Mullins, J. Sidroski, and J. Wolinsky (ed.), Human retroviruses and AIDS 2000: a compilation and analysis of nucleic acid and amino acid sequences. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, N.Mex.
- 37.Peeters, M., E. Esu-Williams, L. Vergne, C. Montavon, C. Mulanga-Kabeya, T. Harry, A. Ibironke, D. Lesage, D. Patrel, ad E. Delaporte. 2000. Predominance of subtype A and G HIV type 1 in Nigeria, with geographical differences in their distribution. AIDS Res. Hum. Retrovir. 16:315-325. [DOI] [PubMed] [Google Scholar]
- 38.Perriere, G., and M. Gouy. 1996. WWW-Query: an on-line retrieval system for biological sequence banks. Biochimie 78:364-369. [DOI] [PubMed] [Google Scholar]
- 39.Renjifo, B., W. Fawzi, D. Mwakagile, D. Hunter, G. Msamanga, D. Spiegelman, M. Garland, C. Kagoma, A. Kim, B. Chaplin, E. Hertzmark, and M. Essex. 2001. Differences in perinatal transmission among human immunodeficiency virus type 1 genotypes. J. Hum. Virol. 4:16-25. [PubMed] [Google Scholar]
- 40.Sankale, J., D. Hamel, A. Woolsey, I. Traore, M. Dia, A. Gueye-Ndiaye, M. Essex, S. Mboup, and P. Kanki. 2000. Molecular evolution of human immunodeficiency virus type 1 subtype A in Senegal: 1988-1997. J. Hum. Virol. 3:157-164. [PubMed] [Google Scholar]
- 41.Sankale, J.-L., S. Mboup, M. E. Essex, and P. J. Kanki. 1998. Genetic characterization of viral quasispecies in blood and cervical secretions of HIV-1- and HIV-2-infected women. AIDS Res. Hum. Retrovir. 14:1473-1481. [DOI] [PubMed] [Google Scholar]
- 42.Sarr, A. D., J. L. Sankale, D. J. Hamel, K. U. Travers, A. Gueye-Ndiaye, M. Essex, S. Mboup, and P. J. Kanki. 2000. Interaction with human immunodeficiency virus (HIV) type 2 predicts HIV type 1 genotype. Virology 268:402-410. [DOI] [PubMed] [Google Scholar]
- 43.Takehisa, J., L. Zekeng, E. Ido, I. Mboudjeka, H. Moriyama, T. Miura, M. Yamashita, L. G. Gurtler, M. Hayami, and L. Kaptue. 1998. Various types of HIV mixed infections in Cameroon. Virology 245:1-10. [DOI] [PubMed] [Google Scholar]
- 44.Tebit, D., L. Zekeng, L. Kaptue, M. Salminen, H. Krausslich, and O. Herchenroder. 2002. Genotypic and phenotypic analysis of HIV type 1 primary isolates from western Cameroon. AIDS Res. Hum. Retrovir. 18:39-48. [DOI] [PubMed] [Google Scholar]
- 45.Thompson, J., T. Gibson, F. Plewniak, F. Jeanmougin, and D. Higgins. 1997. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toure-Kane, C., C. Montavon, M. A. Faye, P. M. Gueye, P. S. Sow, I. Ndoye, A. Gaye-Diallo, E. Delaporte, M. Peeters, and S. Mboup. 2000. Identification of all HIV type 1 group subtypes in Senegal, a country with low and stable seroprevalence. AIDS Res. Hum. Retrovir. 16:603-609. [DOI] [PubMed] [Google Scholar]
- 47.Vallet, S., M. C. Legrand-Quillien, C. Roger, V. Bellein, P. Perfezou, L. de Saint-Martin, M. Garre, F. Brun-Vezinet, and B. Picard. 2002. HIV-1 genetic diversity in Western Brittany, France. FEMS Immunol. Med. Microbiol. 34:65-71. [DOI] [PubMed] [Google Scholar]
- 48.Wolinsky, S. M., C. M. Wike, B. T. M. Korber, C. Hutto, W. P. Parks, L. L. Rosenblum, K. J. Kunstman, M. R. Furtado, and J. L. Munoz. 1992. Selective transmission of human immunodeficiency virus type-1 variants from mothers to infants. Science 255:1134-1137. [DOI] [PubMed] [Google Scholar]


